Abstract
Mu opioid receptor (MOR) signaling in the nucleus accumbens (NAcc) elicits marked increases in the consumption of palatable tastants. However, the mechanism and circuitry underlying this effect are not fully understood. Multiple downstream target regions have been implicated in mediating this effect but the role of the ventral pallidum (VP), a primary target of NAcc efferents, has not been well defined. To probe the mechanisms underlying increased consumption, we identified behavioral changes in licking patterns following NAcc MOR stimulation. Because the temporal structure of licking reflects the physiological substrates modulating consumption, these measures provide a useful tool in dissecting the cause of increased consumption following NAcc MOR stimulation. Next, we used a combination of pharmacological inactivation and lesions to define the role of the VP in hyperphagia following infusion of the MOR-specific agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) into the NAcc. In agreement with previous studies, results from lick microstructure analysis suggest that NAcc MOR stimulation augments intake through a palatability-driven mechanism. Our results also demonstrate an important role for the VP in normal feeding behavior: pharmacological inactivation of the VP suppresses baseline and NAcc DAMGO-induced consumption. However, this interaction does not occur through a serial circuit requiring direct projections from the NAcc to the VP. Rather, our results indicate that NAcc and VP circuits converge on a common downstream target that regulates food intake.
Keywords: opioids, feeding, palatability, lick microstructure, ventral pallidum, nucleus accumbens
In omnivorous diets (such as those of rats and humans), preferred foods are typically those which are fatty and/or sweet and represent an efficient source of calories because of their energy density. Physiological mechanisms geared toward exploiting these rich energy resources through hyperphagia provide an obvious survival advantage when food resources are uncertain and scarce, and when foraging may have its own dangers, such as increased risk of predation. But mechanisms promoting hyperphagia are a health liability for many humans living in contemporary Western societies, where food resources are abundant, ubiquitous, and cheap (Hill and Peters, 1998). In these industrialized nations, obesity and its attendant ills (e.g., heart disease, diabetes, and hypertension) are markedly elevated (World Health Organization, 2000), and palatability-driven consumption is likely an important contributor (Zheng and Berthoud, 2008). Thus, understanding neural signaling mechanisms underlying palatable food intake has widespread clinical relevance.
Stimulation of mu opioid receptors (MOR) in the ventral striatum has remarkable effects on food intake. Infusion of the MOR-specific agonist [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) into the nucleus accumbens (NAcc) elicits ravenous feeding, even in sated rats (Bakshi and Kelley, 1993a, Ragnauth et al., 2000, MacDonald et al., 2003, Will et al., 2006). Following NAcc DAMGO infusion, sated rats can consume more than 100 kcal in an hour – intake that approaches the animal’s daily ad lib consumption (Zhang et al., 1998, Berner et al., 2008).
Feeding caused by MOR stimulation is notable for its specificity for energy dense foods. While having little effect upon consumption of non-preferred tastants (like standard rat chow), MOR stimulation in the NAcc causes avid consumption of preferred, highly palatable foods (Zhang and Kelley, 2002). The specificity of the feeding elicited by NAcc DAMGO for highly reinforcing foods is consistent with the notion that MOR signaling increases feeding through effects determined by the sensory qualities of the consumed substance (Kelley et al., 2002). MOR-stimulated feeding can occur independent of post-consummatory effects, as consumption of palatable non-caloric saccharin and a dilute salt solution are both elevated by this manipulation (Zhang and Kelley, 2002). This finding dovetails with previous studies demonstrating that NAcc opioid signaling increases positive taste reactivity displays (Pecina and Berridge, 2000, 2005), and that systemic opioid manipulations alter feeding by way of palatability mechanisms acting through orosensory cues (Levine and Billington, 2004).
However, opioid effects on consumption need not be confined to palatability-based mechanisms. Manipulations of opioid signaling can affect appetitive drive independent of any orosensory cues. For instance, blockade of endogenous opioid signaling decreases operant responding for drug rewards under extinction conditions (when no drug reward is present), demonstrating that endogenous opioids contribute to appetitive mechanisms in certain contexts (Ciccocioppo et al., 2003, Burattini et al., 2008). In addition, genetic deletion of the MOR disrupts meal-entrained anticipatory locomotion (Kas et al., 2004), a behavior that likely subserves foraging in natural environments. Both of these findings point to a potential role for opioid signaling in the control of appetitive behaviors which precede feeding.
Lick microstructure analysis offers a quantitative means of probing the disparate factors that could contribute to DAMGO-induced hyperphagia. Studies establishing this approach have documented the effects of systematic manipulations of palatability, satiety, and other variables on the temporal pattern of licking occurring during consumption of a liquid meal (e.g., Davis and Smith, 1992, Smith, 1996, Spector et al., 1998). For example, increased palatability (achieved, for instance, by increasing sucrose concentration) causes characteristic changes in consumption, such as an elevated initial lick rate and prolonged bursts of licking (Davis and Perez, 1993). These behavioral signatures of modulators of consumption provide a valuable tool for dissecting the physiological substrates of pharmacologically induced changes in feeding. In the present study, we use lick microstructure analysis to explore changes underlying NAcc DAMGO-induced feeding.
Neural circuits downstream of NAcc DAMGO signaling were first explored extensively in elegant studies by Kelley et al, which demonstrate that NAcc opioid signaling ramifies widely from the NAcc to engage multiple downstream targets implicated in motivation and food intake, including the lateral hypothalamus, the ventral tegmental area, and the dorsomedial hypothalamus (Will et al., 2003, Zheng et al., 2007). One brain region that has not been extensively studied in this regard, however, is the ventral pallidum (VP).
The VP is a primary target of NAcc efferents (Groenewegen et al., 1999), and it has a well-documented role in gustatory and more generally in hedonic processing (Pecina et al., 2006, Mickiewicz et al., 2008). Neural firing in VP neurons encodes the reinforcing value of palatable sucrose and salt solutions, and tracks the changing value of the latter when it is increased through salt deprivation (Tindell et al., 2004, Tindell et al., 2006). Pharmacological inactivation or lesion of the VP strongly increases aversive taste reactivity to and consumption of palatable solutions; conversely blockade of GABA signaling through VP bicuculline infusion increases palatable saccharin consumption (Cromwell and Berridge, 1993, Shimura et al., 2006). NAcc inputs to the VP powerfully modulate VP firing (Chrobak and Napier, 1993), and a functional role for the NAcc-VP circuit in mediating locomotion caused by opioidergic stimulation of the NAcc has been defined (Austin and Kalivas, 1989). Moreover, recent investigations have proposed a role for the NAcc-VP connection in an opioid-mediated reciprocal processing network (Smith and Berridge, 2007).
These data provide evidence of an important role for the VP in gustatory processing, and of the importance of the NAcc-VP circuit in motivated behaviors, including food intake. Consequently, we hypothesized that feeding induced by NAcc MOR stimulation requires a direct and intact link with the VP for behavioral expression. We addressed this question by combining reversible pharmacological inactivation of the VP with NAcc DAMGO infusion. In addition, we extended our lick microstructure analysis to investigation of the effects of VP inactivation on feeding.
Experimental Procedures
Subjects
All procedures used were approved by the University of California, San Francisco, Animal Care and Use Committee. Male Long-Evans rats (300–450 g; Harlan, Indianapolis, IN) were used in all experiments. Rats were allowed ad libitum access to food and water throughout the experimental period. Four groups of rats were used in these experiments. Treatments for each group are summarized in Table 1. Briefly, in group 1 (n=14), the effects of NAcc DAMGO injection +/− pharmacological inactivation of the VP on high fat chow intake were tested. Bilateral cannulae were implanted in the NAcc and the VP in these rats. In group 2 (n=22), a similar paradigm was used in a microstructural analysis of licking in rats consuming 0.15 M sucrose. In group 3 (n=4), bilateral NAcc cannulae were implanted, and unilateral excitotoxic lesion of the VP was performed. These rats were used to distinguish between two possible mechanisms for interaction between NAcc and VP signaling: serial signaling, in which the behavioral effects of NAcc DAMGO require direct communication with the downstream VP, or convergent signaling, in which NAcc DAMGO effects do not require direct VP connectivity, but are modulated by convergence of NAcc and VP afferents on a common downstream target. High fat chow intake was measured in this group. The fourth group (n=5) was used to investigate the effects of VP muscimol on locomotion, summarized below in Locomotor Activity.
Table 1.
Summary of experimental groups
| Group | Paradigm | N (# rats) | NAcc manipulation | VP manipulation | Food |
|---|---|---|---|---|---|
| 1 | Bilateral NAcc DAMGO ± VP inactivation | 14 | Bilateral DAMGO: 0, 250 ng/μl | Bilateral muscimol: 0, 25, 100 ng/μl | High fat chow |
| 2 | Bilateral NAcc DAMGO ± VP inactivation (lick microstructure) | 22 | Bilateral DAMGO: 0, 50 ng/μPl | Bilateral muscimol: 0, 25, 100 ng/μl | 0.12 M sucrose |
| 3 | Unilateral NAcc DAMGO ± unilateral VP lesion | 4 (6*) | Unilateral DAMGO ipsi- or contralateral to VP lesion: 0, 250 ng/μl | Unilateral excitotoxic lesion | High fat chow |
| 4 | Locomotion | 5 | - | Bilateral muscimol: 0, 25, 100 ng/μl | - |
Cannula implantation: groups 1–3
Surgical anesthesia was induced and maintained with isoflurane (5% and 3% in O2, respectively). 24 gauge 16 mm stainless steel cannulae were implanted in the NAcc (anterioposterior +1.4, mediolateral +/− 0.75, dorsoventral −6.5) and VP (anterioposterior −0.2, mediolateral +/− 2.4, dorsoventral −6.8). Medetomidine (1 mg/kg IP; Domitor; Pfizer, NY, NY) was administered for post-surgical analgesia.
Excitotoxic lesion: group 3
Unilateral infusion of 1 microliter of the excitotoxin quinolinic acid (QA: 15 μg/μL) was used to lesion the VP (anterioposterior −0.2, mediolateral +/− 2.5, dorsoventral −8.2). QA was infused over 5 minutes, and the injector left in place an additional 5 minutes following infusion. Lesions were randomly divided between left and right hemispheres across rats.
Measuring high fat chow intake: groups 1 and 3
Rats were habituated to daily 2 hour sessions of high fat chow consumption. Standard dry rat chow (5% fat; Purina Mills, St. Louis, MO) and high fat chow (36% fat; Bio-Serv; Frenchtown, NJ) were provided in the home cage during these sessions. Water was freely available throughout the session. Both normal and high fat chow consumption were measured at hourly intervals.
Measuring sucrose intake: group 2
Rats were habituated to daily 2 hour sessions of 0.15 M sucrose consumption. Sessions took place in operant chambers (Med Associates; Georgia, VT) equipped with a single lick spout. Licks were detected by interruption of an attached photobeam, time-stamped and stored on an attached PC for off-line analysis.
Drug infusion – group 1
DAMGO (0.5μl of 250 ng/μl solution bilaterally) or control saline were infused into the NAcc. Two doses of the GABA agonist muscimol (0.5 μl of high dose of 100 ng/μl; or low dose of 25 ng/μl) or control saline were infused bilaterally into the VP. Thus a total of six drug combinations (NAcc saline/DAMGO x VP saline/low muscimol/high muscimol) were tested in each rat using a Latin-square design. For brevity, we use ‘control saline’ to refer the NAcc saline + VP saline condition in the Results and Discussion section. For other drug combinations, we use a ‘NAcc drug + VP drug’ convention. Drug infusion occurred at 0.25 μl/minute, and injectors were left in place for an additional minute following infusion. For all injection targets, injectors extended 1 mm beyond the cannula tip. Infusions took place on alternate days with an intervening rest day. In this and all other experiments, the order in which drug infusion took place was randomized across days.
Drug infusion – group 2
Two doses of NAcc DAMGO (0 or 50 ng/μl) in combination with three doses of VP muscimol (0, 25, or 100 ng/μl) were tested. Thus, a total of 6 drug combinations were tested in a Latin square design. The dose of DAMGO used in these sucrose consumption experiments was considerably (5-fold) lower than that used in high fat chow consumption experiments, because higher concentrations of DAMGO cause a transient suppression of sucrose intake.
Drug infusion – group 3
DAMGO (0.5μl of 250 ng/μl solution unilateral) was infused either ipsi- or contralateral to a unilateral lesion of the VP. The side of the DAMGO infusion was randomized, with each rat receiving one infusion into each NAcc.
Measures of licking microstructure
Meals were defined by interlick intervals (ILI) greater than 10 minutes (Davis and Perez, 1993, Spector et al., 1998). The termination of each meal was taken as the onset of an ILI > 10 minutes. Shorter epochs of consumption, termed bursts, were defined by ILI ≥ 1 second. Termination of a burst was defined by the onset of an ILI > 1 second. Meal duration and meal size were measured from the first lick of a meal to the last lick of that meal. Meal duration refers to the time elapsed from first to last lick, while the meal size refers to the number of licks occurring in the meal. The latency to initiate a meal was calculated as the time elapsed from the onset of the session until the first of three consecutive (ILI < 1 s) licks for the first meal of the session.
Burst duration and burst size, similar to meal parameters, refer to the time spanned by a burst, and the number of licks in that burst, respectively. To exclude very short bursts, potentially caused by incidental contact with the lick spout, only bursts of 3 or more licks were considered. Pauses, the intervals between bursts, were defined as intervals for which the ILI was > 1 second. The mean pause duration was calculated as the meal duration minus the cumulative burst duration, divided by the number of pauses, which is equal to burst number – 1 (Baird et al., 2006).
Lick rate was defined as the number of licks per second. To study post-ingestive effects on consumption, we analyzed declining lick rates over the course of the sessions for two drug conditions - control saline infusions into the NAcc and VP; and NAcc DAMGO in combination with VP saline. The mean lick rate for each time point was used for this analysis. The effects of VP muscimol infusion were not considered in this analysis as they rapidly and sharply curtailed intake, limiting the impact of any subsequent post-ingestive suppressive effects on intake. We also analyzed lick rate by temporally dividing meals into thirds for each drug condition. This analysis has the advantage of excluding the contribution of rats that are not actively ingesting. Consumption during meal thirds for NAcc DAMGO vs saline was analyzed with 2-way repeated measures ANOVA.
Interpreting measures of lick microstructure
Lick microstructure analysis is useful in distinguishing between appetitive/motivational and consummatory processes. Latency to initiate meal, and the number of bursts and meal duration reflect motivational processes and are, for instance, decreased and increased, respectively, by increased motivation arising from food deprivation (Davis and Perez, 1993, Spector et al., 1998).
Consummatory mechanisms include the repertoire of behaviors that arise from direct sensory/motor interaction with the ingestate, and encompass taste processing (i.e., palatability or aversiveness) as well as mechanisms related to satiety. Changes in taste palatability are reflected in burst size and initial lick rate. Increasing palatability by increasing the sweetness of a taste solution results in longer bursts and faster initial lick rates (Davis and Perez, 1993, Spector et al., 1998). Decreases in satiety are manifest in a general elevation of consumption measures (increased number of bursts, increases in meal size and meal duration) but also in slower changes in the rate at which consumption declines late in a meal (Smith, 1998). The negative slope of declining consumption is attenuated when satiety mechanisms are suppressed, as animals maintain higher intake rates for a longer duration under these conditions.
Locomotor activity
Rats (n=5) were implanted with bilateral cannulae directed at the VP (anterioposterior −0.2, mediolateral +/− 2.4, dorsoventral −6.8). After a one week recovery period, rats were habituated to a locomotor chamber (35 × 55 × 40 cm, with equally spaced grid marks on the floor) by placement in the chamber on two consecutive days for 30 minute sessions. Subsequently, muscimol (high dose of 100 ng/μl or low dose of 25 ng/μl) or saline was infused bilaterally (0.5 μl volume) into the VP. Each rat received each drug in a randomized Latin-square design. Rats were immediately placed in the chamber, where locomotion was videotaped over a 2 hour session. Locomotor measures of rearing and grid crossing were quantified in 10 minute bins by an observer blind to drug treatment.
Statistical analysis
One way repeated measures ANOVA were used to compare consumption across drug conditions for both high fat and sucrose consumption. The Pearson product moment test was used to assess the correlations in licking with burst size in the licking microstructure analysis.
Histology
Rats were deeply anesthetized with sodium pentobarbital (40mg/kg) and perfused with 10% formaldehyde. Cannula positions were identified in cresyl violet stained tissue sections cut in the coronal plane.
Results
High fat chow intake
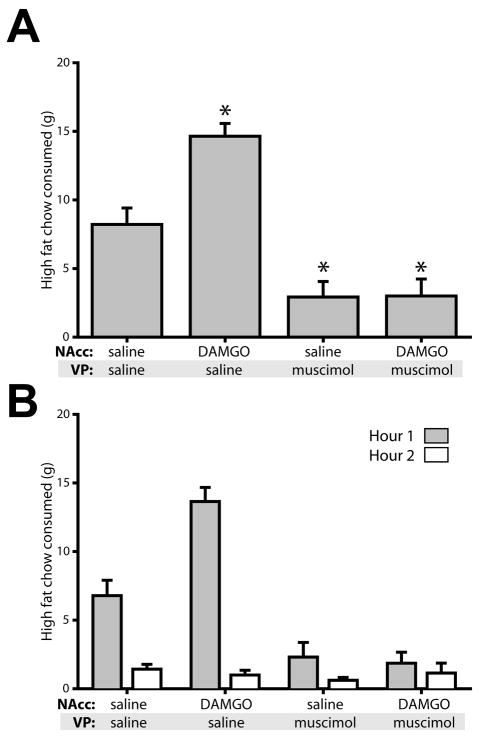
Drug infusion significantly affected high fat chow intake (F(3,13)=39.0, p<0.001). DAMGO infusion into the NAcc more than doubled consumption over the two hour session (Figure 1A). This effect occurred mainly in the first hour of consumption, with only minor consumption in the second hour (Figure 1B). Muscimol infusion into the VP greatly reduced baseline consumption and this blockade of intake persisted when DAMGO was concurrently infused into the NAcc (both p<0.01 relative to saline control).
Figure 1.
Pharmacological inactivation of the VP blocks feeding potentiated by NAcc DAMGO, but also reduces baseline consumption. (A) Consumption of high fat chow (mean ± SEM) was elevated by NAcc DAMGO relative to control. Infusion of the GABA agonist muscimol blocked this effect, but also had strong effects on baseline levels of consumption, which were significantly reduced. NAcc DAMGO concentration, 250 ng/μl; VP muscimol concentration, 100 ng/μl. Asterisks indicated significant posthoc differences (P<0.001) from control (saline in NAcc and VP). (B) Timeline of drug effects. Shaded bar shows high fat chow consumption in the first hour; open bars show consumption in the second hour. N = 14 rats for data in Figures 1–2.
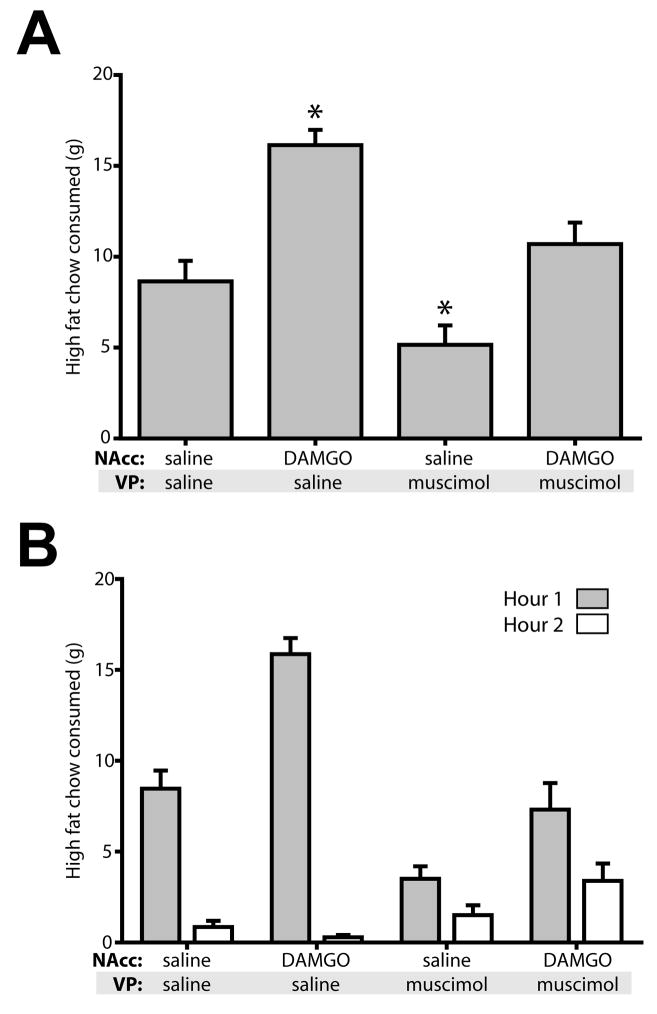
The 100 ng/μl dose of VP muscimol decreased baseline consumption, making it difficult to dissociate specific effects of this manipulation in blocking DAMGO-induced increases in intake versus general suppressive effects on consumption. To explore this issue further, we tested the effects of a four-fold lower dose of muscimol. Similar to the high dose, the low dose of VP muscimol significantly decreased high fat chow consumption (F(3,13)=20.14, p<0.001; Figure 2). Bilateral infusion of 25 ng/μl muscimol into the VP attenuated food intake to 60% of control levels, a smaller decrease than seen with the higher muscimol dose, but still significant (p<0.05). Concurrent infusion of NAcc DAMGO significantly elevated consumption (p = 0.001, relative to NAcc saline + VP muscimol), but did not restore the high levels of consumption seen after NAcc DAMGO + VP saline infusion (p<0.001, comparing NAcc DAMGO +/− VP muscimol).
Figure 2.
A four-fold lower dose of VP muscimol reduces baseline consumption. (A) As for the higher muscimol dose shown in Figure 1, VP inactivation with a lower dose of muscimol attenuated NAcc DAMGO effects, but also significantly reduced baseline feeding. NAcc DAMGO concentration, 250 ng/μl; VP muscimol concentration, 25 ng/μl. Asterisks indicated significant posthoc differences (P<0.05) from control (saline in NAcc and VP). (B) Time course of feeding. Low dose muscimol had the most pronounced effects in the first hour, with some recovery of feeding in the second hour.
Microstructure of licking during sucrose consumption
Overall effects on sucrose intake
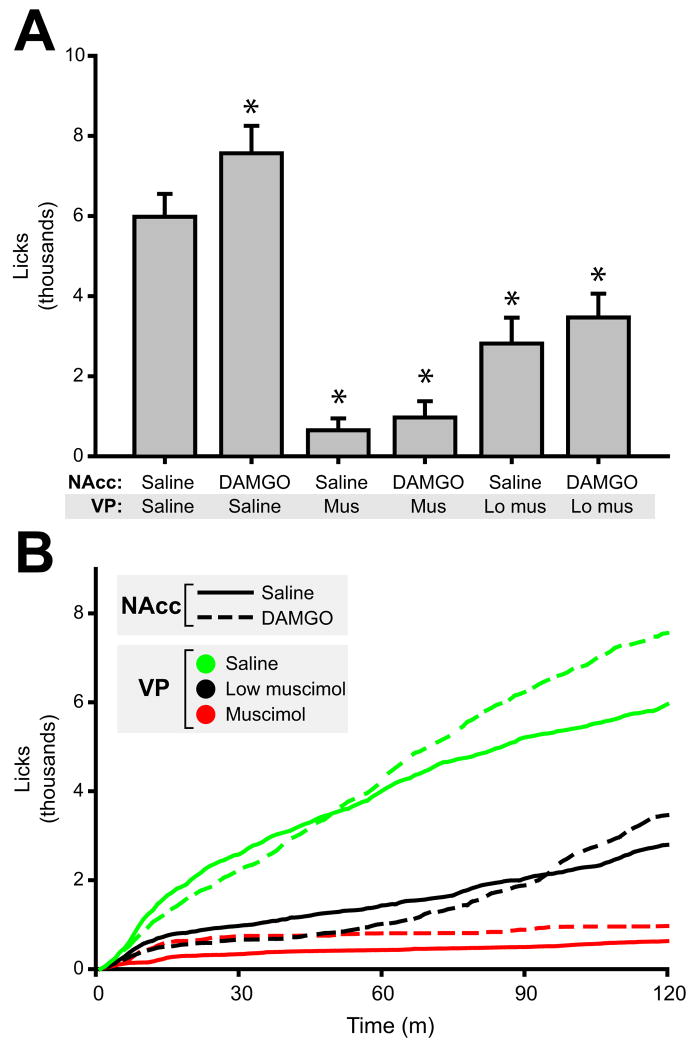
NAcc DAMGO infusion during sucrose consumption significantly elevated sucrose intake (Figure 3A: main effect of drug on lick number, F(5,21)=37.1, p<0.001; NAcc DAMGO significantly higher than saline control, p<0.05). This increase was smaller than that observed for high fat chow intake (27% versus 82% increase). NAcc DAMGO infusion during sucrose intake caused an early and transient suppression of licking (visible in cumulative plots of licking, Figure 3B) that was not seen during high fat chow consumption. Because this suppression was dose dependent (data not shown), a dose of 50 ng/μl DAMGO was used in sucrose consumption experiments.
Figure 3.
NAcc DAMGO elevates sucrose consumption. (A) Stimulation of NAcc MORs increased mean (± SEM) 0.15 M sucrose consumption significantly. However, DAMGO effects were blocked with concurrent pharmacological inactivation of the VP. Both doses of muscimol infused into the VP had strong effects on baseline consumption of sucrose. NAcc DAMGO dose = 50 ng/μl; low VP muscimol dose = 25 ng/μl; high VP muscimol dose = 100 ng/μl. Asterisks indicated significant posthoc differences (P<0.05) from control (saline in NAcc and VP). (B) Time course of feeding. Solid lines indicate NAcc saline infusion; broken lines indicate NAcc DAMGO infusion. Colors indicate VP infusion: green = saline, black = low muscimol dose, red = high muscimol dose. Note that NAcc DAMGO (with VP saline infusion) caused a transient suppression of sucrose consumption (first ~45 minutes), followed by elevated rates of intake thereafter. N = 22 rats for data shown in Figures 3 – 7.
Infusion of VP muscimol at both high (100 ng/μl) and low (25 ng/μl) concentrations attenuated sucrose consumption in a dose dependent fashion (both p<0.001 relative to control NAcc + VP saline infusion). Concurrent DAMGO infusion did not increase consumption for either VP muscimol dose (both p≫ 0.05).
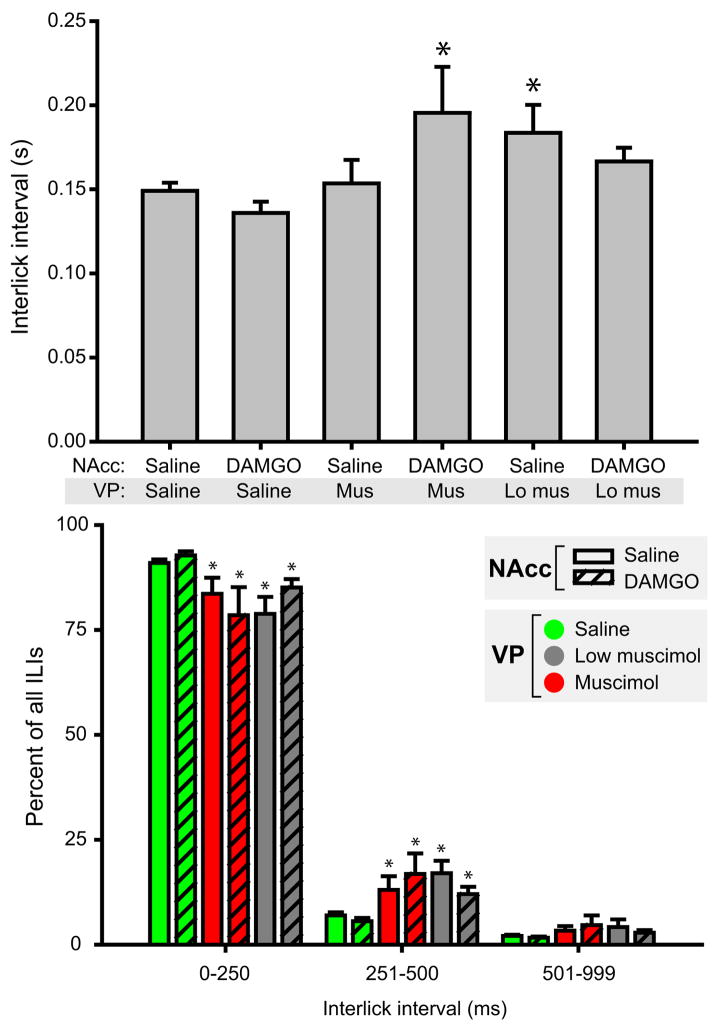
Lick microstructure: Interlick Intervals (ILI)
Following infusion of control saline, ILI within lick bursts averaged 149 ± 5 ms (mean ± SEM; 6.7 licks/s). Infusion of VP muscimol, with or without simultaneous NAcc DAMGO infusion, slowed licking rates and increased the mean ILI (Figure 4A; main effect of drug condition, F(5,21)=4.7, p<0.001). This increase relative to saline control was significant for NAcc saline + VP low muscimol infusions and NAcc DAMGO + VP muscimol infusion (both p<0.05 relative to control). NAcc DAMGO alone had no effect on ILI relative to control infusions (p≫0.05).
Figure 4.
VP muscimol disrupts lick microstructure. (A) Mean (±SEM) interlick intervals occurring during consummatory bursts are shown. Relative to the control treatment (saline in both NAcc and VP), the mean interlick interval was elevated for NAcc DAMGO + VP muscimol treatment and NAcc saline + VP low muscimol treatment (asterisks, p<0.05). Overall, there was a significant elevation in ILIs comparing muscimol treatments to control saline (collapsing across DAMGO treatments, which had no effect). (B) Binned interlick interval times. Muscimol treatment shifted ILIs toward higher values. The percentage of ILIs occurring in the 0–250 ms bin was significantly decreased for muscimol treatments relative to saline control, and significantly elevated in the 251–500 ms bin (asterisks, p<0.05).
To understand the effects of VP muscimol infusion on the distribution of ILIs, we binned ILIs for each drug condition into 0–250 ms, 251–500 ms, and 501–999 ms bins. Under normal conditions, the majority of ILIs fall into the first of these categories - rats typically lick at ~ 7 Hz, yielding a mean ILI of approximately 140 ms. For both drug conditions in which saline was infused into the VP (+ NAcc saline/DAMGO), more than 90% of ILIs were <250 ms in duration (Figure 4B). Infusion of either muscimol dose into the VP significantly changed the distribution of ILIs (χ2 (10) = 140.1, p<0.001), decreasing the percentage of short ILIs, and approximately doubling the percentage of ILIs in the 251–500 ms range (7 ± 1% for saline control vs. a maximum of 17 ± 3 % for NAcc saline + VP low muscimol). Thus VP muscimol infusion resulted in decreased rates of intake in part by causing the insertion of brief interruptions in ongoing bursts of licking.
Meal parameters
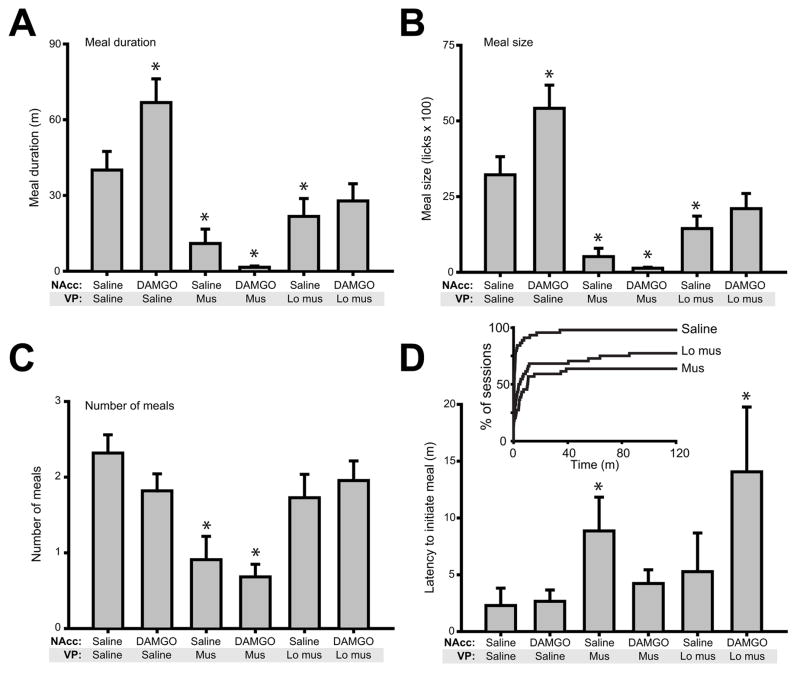
NAcc DAMGO infusion prolonged meal duration and size (Figure 5A–B), increasing both by > 60%. There was a main effect of drug condition (F(5,21) = 16.4, p <0.001), and a significant increase in meal duration following NAcc DAMGO relative to saline control (p=0.001). In contrast, VP muscimol infusion dose-dependently decreased meal duration (both p<0.05 relative to control saline). In the presence of either dose of VP muscimol, NAcc DAMGO failed to increase meal duration (both p≫0.05).
Figure 5.
Meal parameters. (A) NAcc DAMGO increased meal duration, while VP inactivation dramatically decreased meal duration and blocked DAMGO effects. (B) Meal size closely tracked meal duration, with DAMGO induced increases and muscimol induced decreases. (C) Muscimol treatment significantly decreased the number of meals consumed relative to saline treatment. (D) Muscimol inactivation of the VP increased latency to meal initiation. (Inset) Muscimol infusion blocked consumption completely in a subset of rats. The x axis shows time of meal initiation in the session; the y axis shows the percentage of all sessions for each drug condition. Note that low muscimol and muscimol treatments blocked all licking in 16% and 32% of sessions, respectively. For A–D, asterisks indicate significant difference (p<0.05) relative to control condition (NAcc saline + VP saline).
Muscimol infusion decreased not only meal duration, but at the higher dose, also sharply curtailed the number of sucrose meals that were initiated (Figure 5C; main effect of drug, F(5,21)=7.2, p<0.001). Following NAcc saline +VP high muscimol infusion, rats averaged slightly less than 1 meal over the experimental session (p<0.001, comparing this drug condition with control saline infusion) - reflecting the absence of any consumption in 11 of 22 rats. For both VP muscimol concentrations, concurrent NAcc DAMGO administration did not change meal counts (p≫0.05). When paired with VP saline, NAcc DAMGO infusion had no effect on meal count relative to saline control, in contrast to the pronounced effects on meal duration. The absence of an increase in meal count following NAcc DAMGO is perhaps not surprising given the pronounced increase in meal duration caused by this manipulation.
Analysis of latency to initiate meals demonstrated a main effect of drug condition (Figure 5D; F(5,21)=2.8, p<0.05). Posthoc analyses showed significant increases in latency relative to control saline for two conditions: NAcc saline + VP muscimol and NAcc DAMGO+VP muscimol (both p<0.01). However, it should be noted that we did not include in these calculations data from rats which failed to initiate any consumption, which occurred frequently following infusions of VP muscimol: this included 16 of 44 sessions after high muscimol infusion; 7 of 44 sessions after low muscimol dose infusion; and 0 of 44 sessions after VP saline infusion (Figure 5B, inset; significantly different, χ2(2)= 20.3, p <0.001)
These data suggest partially overlapping mechanisms occur following NAcc DAMGO and VP muscimol infusion, albeit working in opposite directions. After both manipulations, measures of consumption were affected, with total consumption and meal duration changing (in opposite directions). However, appetitive behavior was also suppressed by VP muscimol infusion, evidenced by decreased meal count and elevated meal initiation latency, and the failure of many rats to initiate consumption at all.
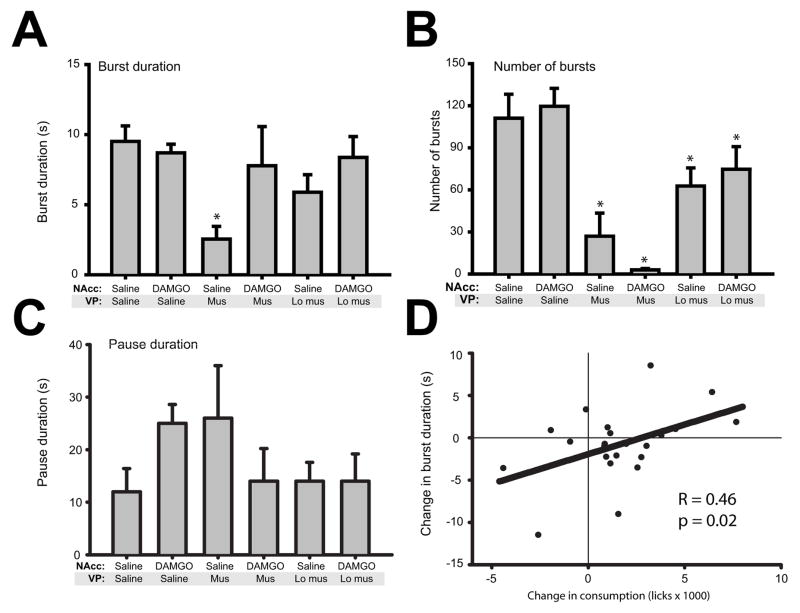
Burst and pause parameters
Somewhat surprisingly, given potent effects in increasing meal duration, we found that NAcc DAMGO administration had little effect on burst length when compared to control saline infusion (Figure 6A). One-way ANOVA showed a main effect of drug condition on burst duration (F(5,21)=3.6, p<0.01). However, posthoc tests showed this was due to significant decreases in burst duration for the NAcc saline + VP muscimol condition (p<0.001, relative to saline control). When paired with VP muscimol infusion (either dose), NAcc DAMGO had no significant effects on burst duration (both p≫0.05).
Figure 6.
Burst parameters. (A) Surprisingly, NAcc DAMGO infusion had no effect on burst duration, a correlate of palatability. VP inactivation decreased burst duration. (B) VP inactivation strongly reduced the number of bursts initiated. NAcc DAMGO infusion had no significant effects on the number of bursts. (C) Pause duration was not significantly altered by any treatment condition. For each graph, asterisks indicate significant difference (p<0.05) relative to control condition (NAcc saline + VP saline). (D) Though mean burst size was not elevated after NAcc DAMGO infusion, there was a significant positive correlation between DAMGO induced increases in consumption (x-axis, relative to saline condition) and increased burst duration (y-axis). Thus, DAMGO-induced consumption occurred in large part through longer bursts.
DAMGO-induced increases in meal duration could arise through a number of mechanisms beyond simple increases in burst duration: the number of bursts might increase, or the duration of pauses - brief interruptions of consumption - might decline. We analyzed each of these possibilities for all drug conditions (Figure 6B–C). Again, we were surprised to find that infusion of NAcc DAMGO did not alter any of these of parameters relative to saline control in a consistent fashion. For all measures, NAcc DAMGO+VP saline measures were very similar to those measured for control saline (all p≫0.05). How, then, might DAMGO increase meal duration in the absence of any effects on burst/pause measures? We reasoned that variability in individual rats’ responses to DAMGO might underlie the apparent absence of an effect on burst size. To explore this possibility, we analyzed the correlation between DAMGO-induced changes in overall consumption with changes in burst duration (Figure 6D). This analysis showed a significant correlation, such that DAMGO-induced increases in consumption were correlated with increased burst duration (r=0.46, p=0.02). No such correlation existed for pause duration or burst number (all p≫0.05; data not shown). Thus, DAMGO-induced increases in consumption appear to be expressed through increases in burst duration.
In contrast to NAcc DAMGO, VP muscimol infusion potently decreased the number of bursts (Figure 6B; main effect of drug, F (5,21) = 3.2, p<0.05), but had no significant effect on pause duration (Figure 6C; main effect of drug, F(5,21)=0.8, p=0.55).
Lick rate over the course of consumption
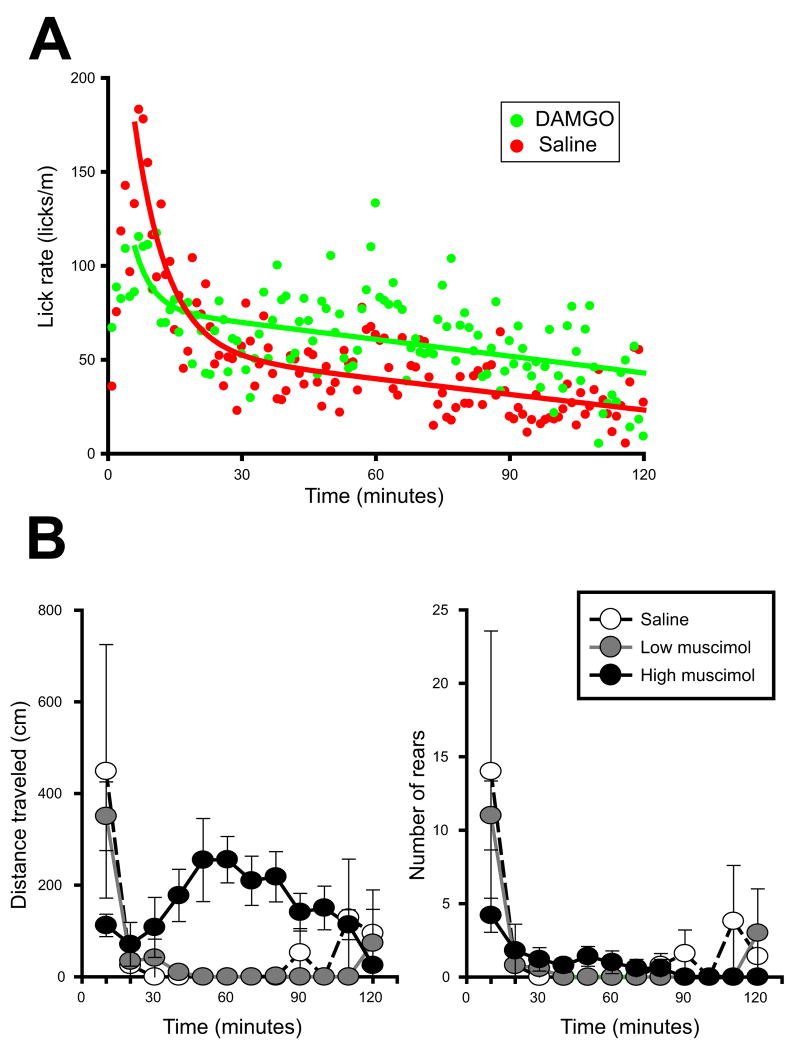
To complete our analysis of licking patterns, we investigated the decline in lick rate over the course of the session, a measure that reflects the impact of post-ingestive factors. Following NAcc saline infusion, rats showed an early peak in consumption which rapidly decreased to a much lower but sustained level of licking (Figure 7). Relative to previous reports, lick rates peaked somewhat later than expected (at ~ 6 minutes rather than in the first minute). Because rats were placed in the operant chambers immediately after drug infusion, this delay was likely due to a short period of grooming which typically follows handling. Rats injection with NAcc DAMGO + VP saline showed a smaller initial peak in consumption, but stabilized their consumption thereafter with high ongoing rates of licking.
Figure 7.
(A) NAcc DAMGO does not alter the rate at which licking declines. Suppression of satiation is associated a decreased rate of declining intake in later stages of consumption. DAMGO caused a transient suppression of intake relative to saline control (first 30 minutes); however, the rate at which licking declined thereafter was nearly identical for saline and DAMGO conditions. (B) Locomotor effects of VP muscimol. The low dose of muscimol (25 ng/μl) did not alter the magnitude nor pattern of locomotion tested in an open field (p≫0.05 compared to saline). The high dose of muscimol (100 ng/μl) attenuated the initial locomotor response, but elevated locomotion thereafter. Neither dose of muscimol significantly altered rearing.
To quantitatively analyze lick rates, we fit each of these mean lick rates with the sum of an exponential (accounting for the early and steep decline in lick rates) and a linear fit (reflecting steadily declining lick rates later in the session). We compared the slopes of the linear component of each fit to determine if drug conditions affected the decline in lick rates. For the two drug conditions, these were nearly identical (−0.28 vs. −0.30 licks/m2 for NAcc saline vs. DAMGO). These data indicated NAcc DAMGO elevated overall ingestion rates but did not decrease the rate at which consumption declined during the session. Because these curves average contributions from all rats (including those that have stopped ingesting), we also analyzed ingestion rate across meal thirds. NAcc saline vs DAMGO did not significantly differ in this analysis (F(1, 21)=0.60, p=0.45; data not shown), confirming that DAMGO did not substantially alter the rate of declining ingestion late in the meal.
Effects of VP muscimol on locomotion
Bilateral infusion of low dose muscimol (25 ng/μl) had no had no significant effects on locomotion – the magnitude and timing of locomotion after infusion of this dose did not differ from that after saline infusion into the VP (p>0.05, Figure 7B, left panel). The high dose of muscimol (100 ng/μl) significantly altered locomotor patterns, in particular attenuating the initial locomotor response, but increasing locomotion for the remainder of the session (main effect of drug dose, F(4, 8)=11.7, p<0.004; high dose muscimol significantly different from saline and low dose, both p<0.01). Neither dose of muscimol significantly altered rearing (Figure 7B, right panel).
Unilateral DAMGO + VP lesion
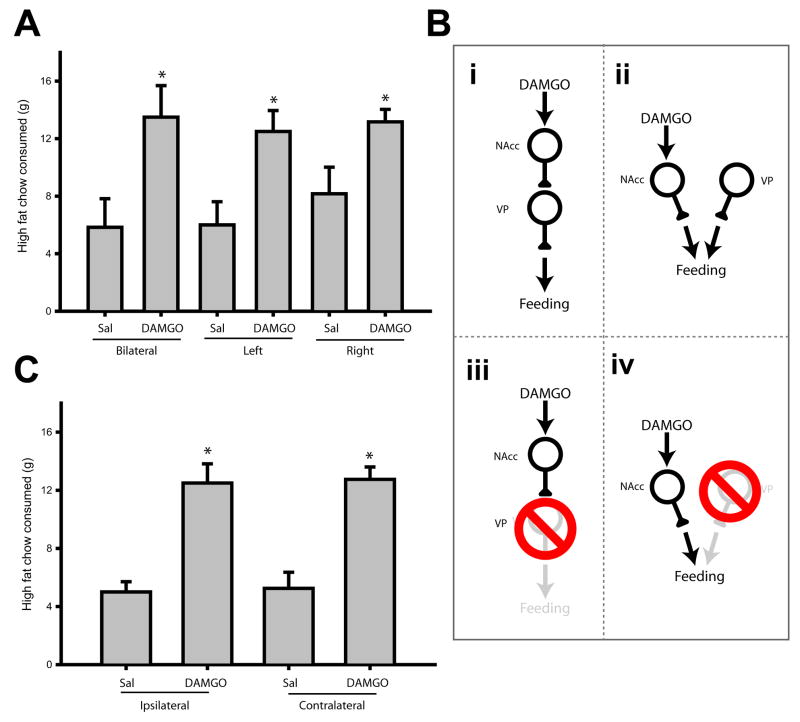
Bilateral infusions demonstrated that VP muscimol blocked DAMGO-induced increases in feeding, but these experiments could not reveal the connectivity between these brain regions underlying this interaction. However, in pilot studies we found that the effects of unilateral NAcc DAMGO infusion were identical to those of bilateral infusion of the drug (Figure 8A; F(5,5)=4.3, p<0.01). Bilateral and left hemisphere infusion of DAMGO significantly increased consumption relative to saline infusions (both p<0.05). There was a trend for right hemisphere infusion of DAMGO to increase consumption, but this effect just missed significance (p=0.052, compared to right saline infusion). This was likely due to a slightly increased baseline feeding rate following right hemisphere saline infusion, rather than a diminution of the DAMGO effect, because the magnitude of consumption after bilateral, left or right DAMGO infusion did not differ (all p≫0.05).
Figure 8.
VP function is not required for NAcc DAMGO induced hyperphagia. (A) Bilateral infusion of NAcc DAMGO (250 ng/μl) more than doubled high fat chow consumption in sated rats (left two bars; n = 6 rats). Similarly elevated levels of consumption occurred after unilateral infusion of DAMGO in either hemisphere (“left” and “right”). Asterisks indicate significant differences (p<0.05) relative to control condition (bilateral saline). (B) Bilateral VP inactivation blocked NAcc DAMGO effects. This effect could arise through serial (i) or convergent (ii) connectivity. If neural signaling caused by NAcc DAMGO were relayed through an obligatory synapse in the VP, lesion of the VP ipsilateral to NAcc DAMGO infusion should block the increase in feeding (iii). On the other hand, if NAcc and VP efferents converge downstream, the VP ipsilateral to NAcc DAMGO infusion may not be required for MOR-stimulated increases in consumption (iv). (C) NAcc DAMGO ipsilateral and contralateral to VP lesion was equally effective in increasing food intake (n = 4 rats). Asterisks indicate significant difference (p<0.001) relative to control condition (ipsilateral saline). These data support the circuit diagram shown in (B-iv).
The effectiveness of unilateral DAMGO infusion, in combination with the predominantly ipsilateral routing of NAcc efferents to the VP, allowed us to distinguish between two possible ways in which NAcc DAMGO and VP muscimol might interact. Feeding elicited by NAcc DAMGO could require direct, serial communication with the downstream VP for behavioral expression (Figure 8B-i). Alternately, changes in neural activity caused by NAcc DAMGO could be routed through an alternate neural target for behavioral expression, and converge with VP efferents at some common downstream target (Figure 8B-ii). We combined unilateral infusion of NAcc DAMGO with unilateral VP lesion to distinguish between these possibilities. If a serial NAcc-VP connection were required, infusion of NAcc DAMGO ipsilateral to the lesion would be expected not to increase consumption (Figure 8B-iii). On the other hand, if instead NAcc and VP efferents converge, ipsilateral NAcc DAMGO infusion would result in a robust increase in food intake, despite the presence of the VP lesion (Figure 8B-iv).
We found that unilateral VP lesion had no effect on hyperphagia induced by NAcc DAMGO (Figure 8C). The magnitude of feeding elicited by NAcc DAMGO infused ipsilateral to the VP lesion site was indistinguishable from that occurring after contralateral infusion (overall effect of drug, F(3,9)=17.7, p< 0.001; saline infusions significantly different from DAMGO infusion, p< 0.05; no difference within drug treatment groups, p > 0.05). This result provides strong evidence against the necessity of a serial NAcc-VP connection for DAMGO induced hyperphagia.
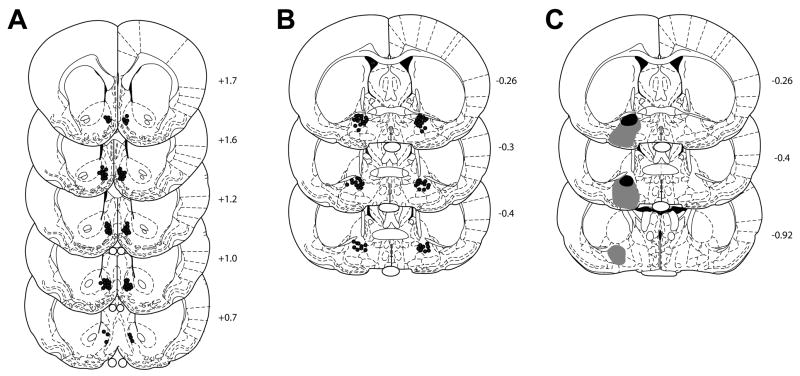
Infusion and lesion sites
NAcc and VP infusion sites are shown in Figure 9A–B. Most NAcc infusions sites were in the medial shell region. VP lesion boundaries are shown in Figure 9C (black – minimum area of damage; gray – maximum area).
Figure 9.
Cannula and lesion placements. (A) NAcc cannulae placements were largely confined to the shell region, approaching the border between shell and core in some places. Anterioposterior position (relative to bregma) is shown to the right of each section. (B) VP cannulae placements. (C) VP lesions were large, extending from just ventral to the anterior commissure to below the VP’s ventral border in some cases. Black region indicates minimum area ablated; gray indicates maximum lesion area.
Discussion
Lick microstructure: Effects of NAcc DAMGO
Consistent with previous reports, NAcc DAMGO infusion significantly elevated consumption of a palatable sucrose solution (Zhang and Kelley, 1997). This increase occurred through a delayed elevation of licking rates (Figure 3). Lick rates were slightly suppressed by MOR stimulation over the first ~45 minutes following infusion, but then increased to consumption levels that exceeded those following control saline infusion.
Increased sucrose intake occurred principally through changes in measures associated with consummatory, rather than appetitive, factors. Meal duration and meal size were both significantly increased (by more than 60%) after NAcc DAMGO infusion relative to saline control (Figure 5A–B). Notably, MOR stimulation did not alter the latency to initiate the first meal (Figure 5D), nor, despite increased meal duration, did it alter the number of meals initiated (Figure 5C). Changes in meal number and the latency to initiate a meal are both associated with altered appetitive factors, and the absence of a NAcc DAMGO effect on these measures suggests action instead through consummatory mechanisms.
Our initial analysis of burst duration yielded surprising results. Given the overall elevation of consumption, we anticipated increased burst duration but found no evidence of a NAcc DAMGO effect on this measure (Figure 6A). Indeed, there was if anything a slight reduction in the mean burst duration following DAMGO infusion. The number of bursts as well as the duration of pauses were also unchanged (Figure 6B–C).
A substantial body of previous work supports the notion that NAcc DAMGO increases intake chiefly through elevating palatability, changes in which are typically associated with alterations in burst duration. DAMGO infusion had variable effects on sucrose ingestion (unlike effects on consumption of a high fat chow, which was reliably elevated; we discuss this difference at more length below in NAcc DAMGO: Fats vs. carbohydrates). To determine if DAMGO effects on burst duration covaried with overall effects on consumption, we performed a correlation of these two intake measures (Figure 6D). This analysis showed a direct relationship between increased consumption and burst duration: the more potent the effects of DAMGO in increasing sucrose intake, the greater the increase in burst duration during the session. This finding suggests that DAMGO-induced increases in intake do indeed arise through increased palatability.
Our results are consistent with previous studies supporting a palatability-based mechanism for NAcc MOR signaling. Manipulations of NAcc opioids rather specifically affect palatable food consumption (Evans and Vaccarino, 1990, Zhang and Kelley, 1997); blockade of endogenous NAcc MOR signaling decreases palatable food intake (Bodnar et al., 1995, Ward et al., 2006); NAcc MOR signaling increases positive taste reactivity displays (Pecina and Berridge, 2000, 2005, Smith and Berridge, 2007); and NAcc DAMGO elevates consumption of non-caloric palatable saccharin and salt solutions (Zhang and Kelley, 2002), demonstrating that hyperphagia can occur independent of post-ingestive feedback. These effects of NAcc DAMGO are also generally consistent with the results of systemic opioid manipulations, which indicate preferential effects on palatability, rather than metabolically-driven feeding (Giraudo et al., 1993, Levine and Billington, 2004). This accumulation of evidence provides strong convergent support for a palatability-based mechanism underlying the ingestive effect of NAcc MOR stimulation.
The mechanism through which this elevation in palatability is achieved remains poorly understood. However, it is likely to be expressed at least in part through suppression of satiety mechanisms – indeed, palatability is widely conceived of as driving excess food intake by overriding metabolically-driven satiety circuits (Saper et al., 2002, Zheng and Berthoud, 2007, 2008). Our data provide a mixed picture of the extent to which NAcc DAMGO suppresses satiety signaling. A manipulation such as sham feeding, which removes negative post-ingestive feedback, elevates overall ingestion rates, meal duration, burst counts, and decrease the rate at which ingestion declines (Davis and Smith, 1990, Schneider et al., 1990). NAcc DAMGO infusion resulted in two of these four changes in our study (increased meal duration and ingestion rate). We did not detect an increase in burst count, perhaps due to the variability of DAMGO effects on individual rats, which tended to obscure burst-related changes (Figure 6). NAcc MOR signaling had an overall effect of shifting ingestion rate vertically, rather than changing the slope of declining ingestion. This may be related to the rather weak effects of DAMGO on sucrose intake (in our hands), relative to high fat chow. In other studies using solid chow, MOR stimulation in the NAcc has been shown to decrease the rate at which intake declines (Bakshi and Kelley, 1993b). Thus our data, while not unequivocal, provide support for the notion that NAcc DAMGO infusion increases palatability which in turn causes food intake to rise through suppression of satiety signaling.
Lick microstructure: Effects of VP muscimol
Pharmacological inactivation of the VP strongly decreased consumption. Most measures of intake reflected this hypophagia. Latency to initiate consumption was increased; early lick rates were suppressed; meal size and duration were decreased, as were burst size and duration, and the microstructure of licking within bursts was altered as well, with longer interlick intervals (>250 ms) becoming more common. Nearly all changes associated with VP inactivation occurred independent of the presence or absence of DAMGO in the NAcc, suggesting that the former manipulation more powerfully regulates motor outputs controlling feeding.
These results are in many ways reminiscent of changes that occur following conditioned taste aversion (CTA) or consumption of an aversively bitter quinine solution (Hsiao and Fan, 1993, Spector and St John, 1998, Baird et al., 2005) and suggest that the effects of VP inactivation arise at least in part through decreased hedonic value of the tastant. Similar to VP inactivation, CTA and quinine consumption are characterized by a broad decrease in variables associated with consumption, many of which reflect decreased palatability of the tastant. All three manipulations result in decreased burst duration and decreased initial intake rates (in the first minute of consumption). Both of these measures directly correlate with the hedonic value of the tastant – increasing with increasing palatability, and decreasing with increasing aversiveness.
Our results support a significant role for the VP in gustatory processing, consistent with other studies of the ventral pallidum. Most relevant in this regard are results from Shimura et al (2006) showing that VP muscimol infusion dramatically increased negative taste reactivity displays and decreased positive taste reactivity displays in response to saccharin infusion. These authors also showed that VP muscimol decreased saccharin intake, and demonstrated that VP infusion of the GABA antagonist bicuculline increased intake of saccharin. Further evidence comes from studies performed by Kent Berridge and colleagues. Cromwell and Berridge showed that lesion of the caudal VP increased aversive taste reactivity to sucrose infusion (Cromwell and Berridge, 1993). Subsequently, Tindell and Berridge showed that the reward value of a reinforcing taste solution (sucrose or salt) was encoded by VP neurons, and these neurons flexibly encoded changing reward value after the salt solution was made more rewarding through sodium depletion (Tindell et al., 2004, Tindell et al., 2006). These studies and our own suggest a precise relationship between GABAergic signaling in the VP and consumption – increasing inhibition blocks feeding, while decreasing inhibition increases food intake. Moreover, they demonstrate that electrophysiological correlates of gustatory reward are encoded in VP neural discharge. Together, these studies suggest an important role for the VP in processing gustatory reward information.
Feeding behavior following VP inactivation did differ in some measures from that occurring after CTA or during quinine ingestion (Hsiao and Fan, 1993, Spector and St John, 1998, Baird et al., 2005). Notably, burst count was substantially elevated in both of the latter contexts, but was dramatically decreased by the high dose of muscimol in our study (~80% reduction relative to VP saline infusion, Figure 6B). Meal duration was also increased during quinine consumption, and only modestly reduced following CTA. In contrast, meal durations were much shorter after VP inactivation in our study (Figure 5A).
These distinctions may point to a fundamental difference in appetitive processes occurring during our task compared to those occurring during consumption of an aversive substance. To ensure consumption, rats were water deprived in both the CTA and quinine studies, while rats in our studies always had ad lib access to food and water. Thus, rats in the CTA and quinine studies were highly motivated to consume, despite the aversive nature of the tastant; in contrast, VP inactivation in our study is likely to have led to a decrease in motivation, perhaps independent of the increased tastant aversiveness associated with VP inactivation (which would itself be expected to lead to a decline in motivation, but only subsequent to sampling the tastant). The increased latency to initiate a meal and decreased burst count following VP inactivation is consistent with this idea (Figures 5D, 6B). Similar changes occur following manipulations that decrease appetitive motivation, such as prefeeding to satiety (Davis and Perez, 1993, Spector et al., 1998). Supporting a role for the VP in appetitive processing, McFarland and Kalivas (2001) found that pharmacological inactivation of the VP suppressed reinstatement of food- and cocaine- directed behavior. Other investigators have noted that infusion of the GABA antagonist bicuculline into the VP increased not just locomotion, but also food carrying behaviors, suggesting a possible role in foraging (Stratford et al., 1999, Smith and Berridge, 2005). Thus, it seems likely that neural activity in the VP contributes to both the appetitive and consummatory phases of food-directed behaviors.
The feeding impairments we observed after VP inactivation are unlikely to have resulted from nonspecific motoric impairments. Low dose (25 ng/ml) muscimol had no significant effects on motor behavior relative to saline control, but had pronounced effects on overall consumption and lick microstructure parameters (Figures 2–6). Similar results were obtained by Shimura et al (2006) who reported a temporal dissociation of the locomotor and feeding effects of VP muscimol (at higher doses than those used in this study). Nonetheless, our results with high dose muscimol, which altered locomotor patterns, suggest caution is warranted in interpreting the effects of VP muscimol infusion, particularly given previous reports of the effects of VP inactivation on locomotor behavior (Mogenson and Nielsen, 1983, Austin and Kalivas, 1989). As we note in the preceding paragraph, however, locomotor effects resulting from manipulations of VP GABA signaling may not reflect ‘non-specific’ motor impairments but rather specific deficits in food-directed appetitive behavior, such as foraging.
Analyzing the NAcc-VP circuit
Our initial experiments (Figures 1–2) provided tentative support for serial transmission of NAcc MOR signaling through the VP before continuing to motor output. Pharmacological inactivation of the VP blocked (high muscimol dose) or attenuated (low dose) NAcc DAMGO effects on feeding. However, this experiment did not permit dissociation of the latter effect from baseline effects on food consumption, which was decreased after both doses of muscimol.
Pilot experiments (Figure 8a) showed that unilateral stimulation of NAcc MOR was sufficient to elicit hyperphagia. This manipulation increased consumption to levels that were indistinguishable from feeding produced by bilateral DAMGO infusion. Anatomically, NAcc efferents project overwhelmingly to ipsilateral targets (Nauta et al., 1978; D. Scott Zahm, personal communication). There is also functional evidence for primarily ipsilateral downstream effects of pharmacological manipulations of NAcc activity (Stratford, 2005). The robust effects of unilateral DAMGO infusion, combined with the ipsilateral projection pattern of NAcc efferents, enabled a direct test of the necessity of the VP for increased consumption following NAcc MOR stimulation. We first unilaterally lesioned the VP, and then assayed the effects of ipsi- and contralateral NAcc DAMGO on high fat chow consumption, reasoning that if NAcc efferents made an obligatory synapse in the VP, NAcc DAMGO infusions ipsi- but not contralateral to the lesion would be ineffective in eliciting hyperphagia. To our knowledge, this experimental approach is novel, but it echoes asymmetric ‘disconnection’ experiments which have been widely used to identify circuit substrates of many behaviors (Parkinson et al., 2000, Christakou et al., 2001, Ambroggi et al., 2008).
Despite large VP lesions, ipsilateral DAMGO infusion reliably increased food intake at levels that matched the consumption produced by contralateral (and bilateral) infusion (Figure 8C). This result argues strongly against a role for the VP in mediating feeding produced by NAcc MOR stimulation. This result is somewhat surprising, given strong evidence that both the NAcc and the VP participate in taste hedonics, and the anatomical connections linking the two nuclei. Indeed, previous behavioral studies of NAcc and VP function have emphasized the contributions of the VP in a NAcc-VP serial circuit in generating behaviors. This is true for locomotion elicited by pharmacological stimulation of the NAcc (Mogenson and Nielsen, 1983, Swerdlow et al., 1984, Austin and Kalivas, 1989), as well as reinstatement of food and cocaine (McFarland and Kalivas, 2001). The latter study, in particular, showed directly that a serial link between the NAcc and VP was critical, as only asymmetric pharmacological inactivations (i.e., those in opposite hemispheres) of nodes in this circuit interrupted reinstatement.
However, a recent study of feeding reported results consistent with our data, demonstrating NAcc-generated feeding independent of the downstream VP. Testing serial opioid neurotransmission in the NAcc-VP circuit, Smith et al found that blockade of VP opioid receptors had no effect on feeding caused by NAcc DAMGO infusion (Smith and Berridge, 2007). This result fits well with our own, in establishing the functional independence of the NAcc from the VP with respect to DAMGO-induced food intake.
It is interesting to note that Smith and Berridge found that VP naltrexone infusion did block increases in positive taste reactivity caused by NAcc DAMGO infusion. This anatomical dissociation of circuits underlying feeding (perhaps due to appetitive changes) and taste hedonics raises the possibility that the lick patterns induced by NAcc DAMGO could, with finer anatomical mapping, show a similar dissociation of appetitive and consummatory factors.
If not the VP, what target circuits are required for NAcc DAMGO to elevate feeding? An obvious candidate is the LH, as there is a direct projection from the NAcc shell to the LH (Heimer et al., 1991), and considerable evidence supports a role for the NAcc-LH circuit in controlling feeding (Stratford and Kelley, 1999, Will et al., 2003, Zheng et al., 2003, Baldo et al., 2004, Zheng et al., 2007). However, this circuit is unlikely to completely account for DAMGO-induced feeding. A striking finding from the initial study which mapped the boundaries of NAcc DAMGO effects was how extensive the anatomical substrate for this effect is, as the full mediolateral extent of the ventral striatum produces hyperphagia upon MOR stimulation (Zhang and Kelley, 2000). This range extends from the NAcc shell abutting the lateral septum to the ventrolateral striatum adjacent to the dorsal endopiriform nucleus, with more lateral sites producing the largest effects after DAMGO infusion (sensitive sites different slightly after mapping with morphine, but show a similar range; Bakshi and Kelley, 1993b). However, connections between the NAcc and the LH are confined to the medial shell, a small portion of the striatal territory that supports MOR-driven hyperphagia (Groenewegen and Russchen, 1984). Thus it seems unlikely that signaling through the LH could fully account for DAMGO-induced hyperphagia, particularly that arising from sites in the core and ventrolateral striatum.
The connectivity of the NAcc core resembles that of the striatum more than that of the NAcc shell, and shares with the dorsal striatum projections to the substantia nigra arising from direct and indirect pathways (Alheid, 2003). Striatal circuits are importantly involved in generating reward-directed behaviors (e.g., Hikosaka et al., 2006), and there is preliminary evidence supporting a role for these circuits in palatability-driven food ingestion (Harrold et al., 2002). It will be interesting in future experiments to determine if these basal ganglia circuits are required for NAcc DAMGO-induced hyperphagia.
NAcc DAMGO: Fats vs. carbohydrates
Previous work has shown that hyperphagia elicited by NAcc MOR-stimulation is strongly macronutrient specific, with a much more pronounced effect evident for fat vs. carbohydrate consumption. This is true even in rats which have a baseline preference for carbohydrates, providing a clear dissociation of NAcc DAMGO effects on macronutrient vs. preference (Zhang et al., 1998). We found a similar bias in our experiments. NAcc DAMGO reliably and strongly increased consumption of high fat chow (e.g., in Figure 1, 13/14 rats showed DAMGO-induced increases in consumption), but had smaller and less consistent effects on sucrose consumption (e.g., variability in effects shown in Figure 6D, where 11/22 showed clear increases). The temporal profile and dose-responsiveness of DAMGO-induced hyperphagia was also very different for sucrose vs. high fat chow. While a high dose of DAMGO (250 ng/μl) reliably elicited robust high fat chow consumption at short latency, the same dose caused a transient suppression of sucrose intake, before elevating consumption levels modestly (data not shown).
A preferential effect of NAcc opioid signaling in promoting fat relative to carbohydrate consumption is consistent with the systemic effects of opioid agonists, which have been reported to preferentially increase fat consumption (Marks-Kaufman and Kanarek, 1980, Marks-Kaufman et al., 1985, Marks-Kaufman and Kanarek, 1990; but see Evans and Vaccarino, 1990, Gosnell et al., 1990). However, the natural context and physiological mechanisms underlying opioid-mediated fat consumption remain poorly understood. One intriguing possibility is that opioid-mediated fat intake may be part of an integrated physiological response to stress; in this scenario, stress-induced opioid release would promote high fat food intake, has been proposed to lead to a down-regulation of the hypothalamic-pituitary axis stress response (Dallman et al., 2005, Adam and Epel, 2007). This proposal is particularly interesting in light of evidence that stressors cause endogenous opioid release in the NAcc (Bertrand et al., 1997, Lapeyre et al., 2001).
Finally, despite the powerful effects of NAcc opioids on fat intake, it should be noted that endogenous opioid effects on food intake can be dissociated from macronutrient content. Opioids can increase food intake solely as a function of a learned preference (Taha et al., 2006, Woolley et al., 2007, Cottone et al., 2008). Rats trained in a successive contrast paradigm learn to suppress consumption of a palatable food (0.12 M sucrose) when it is followed by presentation of a more preferred substance (0.6 M sucrose). In contrast, rats presented sequentially with 0.12 M sucrose and water avidly consume 0.12 M sucrose. Blockade of opioid signaling markedly reduces consumption of the more dilute sucrose solution in the latter group (0.12 M → 0 M sequence), but has little effect on consumption of that solution in the former group (0.12 M → 0.6 M sequence). This result demonstrates that opioid signaling evoked by a particular reinforcer is plastic, context-dependent, and can be attenuated with training in the contrast paradigm. It also demonstrates a strong effect of endogenous opioids on food intake exclusively as a function of preference, with macronutrient content held constant.
Conclusions
Our findings advance understanding of the mechanisms underlying opioid-induced food intake. Consistent with previous studies, we find that NAcc DAMGO increases tastant palatability, leading to increased feeding at least in part through suppression of satiety mechanisms. NAcc DAMGO did not alter appetitive factors. In contrast, pharmacological inactivation of the VP blocked food intake through decreased appetitive and consummatory mechanisms. Consummatory mechanisms altered by VP inactivation included decreased palatability. Finally, our data argue against a role for a direct connection to the ipsilateral VP in mediating NAcc DAMGO effects on food intake.
Acknowledgments
This work was supported by grants from the State of California, The Wheeler Center for Neurobiology of Addiction, and the DOD (H.L.F.); NARSAD and NIMH (S.T.). We gratefully acknowledge M. Krause and J.P. Baird for their critical comments on this work.
Abbreviations
- CTA
Conditioned taste aversion
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol]-enkephalin
- GABA
Gamma-aminobutyric acid
- ILI
Interlick interval
- MOR
Mu opioid receptor
- NAcc
Nucleus accumbens
- QA
Quinolinic acid
- VP
Ventral pallidum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Alheid GF. Extended amygdala and basal forebrain. Ann N Y Acad Sci. 2003;985:185–205. doi: 10.1111/j.1749-6632.2003.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Ishikawa A, Fields HL, Nicola SM. Basolateral amygdala neurons facilitate reward-seeking behavior by exciting nucleus accumbens neurons. Neuron. 2008;59:648–661. doi: 10.1016/j.neuron.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MC, Kalivas PW. Blockade of enkephalinergic and GABAergic mediated locomotion in the nucleus accumbens by muscimol in the ventral pallidum. Jpn J Pharmacol. 1989;50:487–490. doi: 10.1254/jjp.50.487. [DOI] [PubMed] [Google Scholar]
- Baird JP, St John SJ, Nguyen EA. Temporal and qualitative dynamics of conditioned taste aversion processing: combined generalization testing and licking microstructure analysis. Behav Neurosci. 2005;119:983–1003. doi: 10.1037/0735-7044.119.4.983. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993a;265:1253–1260. [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 1993b;111:207–214. doi: 10.1007/BF02245525. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- Berner LA, Avena NM, Hoebel BG. Bingeing, Self-restriction, and Increased Body Weight in Rats With Limited Access to a Sweet-fat Diet. Obesity (Silver Spring) 2008 doi: 10.1038/oby.2008.328. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Smadja C, Mauborgne A, Roques BP, Dauge V. Social interaction increases the extracellular levels of [Met]enkephalin in the nucleus accumbens of control but not of chronic mild stressed rats. Neuroscience. 1997;80:17–20. doi: 10.1016/s0306-4522(97)00136-x. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1995;700:205–212. doi: 10.1016/0006-8993(95)00957-r. [DOI] [PubMed] [Google Scholar]
- Burattini C, Burbassi S, Aicardi G, Cervo L. Effects of naltrexone on cocaine- and sucrose-seeking behaviour in response to associated stimuli in rats. Int J Neuropsychopharmacol. 2008;11:103–109. doi: 10.1017/S1461145707007705. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Functional disconnection of a prefrontal cortical-dorsal striatal system disrupts choice reaction time performance: implications for attentional function. Behav Neurosci. 2001;115:812–825. doi: 10.1037//0735-7044.115.4.812. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Napier TC. Opioid and GABA modulation of accumbens-evoked ventral pallidal activity. J Neural Transm Gen Sect. 1993;93:123–143. doi: 10.1007/BF01245342. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Reinstatement of ethanol-seeking behavior by drug cues following single versus multiple ethanol intoxication in the rat: effects of naltrexone. Psychopharmacology (Berl) 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008;33:524–535. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am J Physiol. 1993;264:R97–103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Learning to sham feed: behavioral adjustments to loss of physiological postingestional stimuli. Am J Physiol. 1990;259:R1228–1235. doi: 10.1152/ajpregu.1990.259.6.R1228. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106:217–228. [PubMed] [Google Scholar]
- Evans KR, Vaccarino FJ. Amphetamine- and morphine-induced feeding: evidence for involvement of reward mechanisms. Neurosci Biobehav Rev. 1990;14:9–22. doi: 10.1016/s0149-7634(05)80156-3. [DOI] [PubMed] [Google Scholar]
- Giraudo SQ, Grace MK, Welch CC, Billington CJ, Levine AS. Naloxone’s anorectic effect is dependent upon the relative palatability of food. Pharmacol Biochem Behav. 1993;46:917–921. doi: 10.1016/0091-3057(93)90222-f. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Krahn DD, Majchrzak MJ. The effects of morphine on diet selection are dependent upon baseline diet preferences. Pharmacol Biochem Behav. 1990;37:207–212. doi: 10.1016/0091-3057(90)90322-9. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Russchen FT. Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol. 1984;223:347–367. doi: 10.1002/cne.902230303. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Harrold JA, Elliott JC, King PJ, Widdowson PS, Williams G. Down-regulation of cannabinoid-1 (CB-1) receptors in specific extrahypothalamic regions of rats with dietary obesity: a role for endogenous cannabinoids in driving appetite for palatable food? Brain Res. 2002;952:232–238. doi: 10.1016/s0006-8993(02)03245-6. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- Hsiao S, Fan RJ. Additivity of taste-specific effects of sucrose and quinine: microstructural analysis of ingestive behavior in rats. Behav Neurosci. 1993;107:317–326. doi: 10.1037//0735-7044.107.2.317. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Lapeyre S, Mauborgne A, Becker C, Benoliel JJ, Cesselin F, Hamon M, Bourgoin S. Subcutaneous formalin enhances outflow of met-enkephalin- and cholecystokinin-like materials in the rat nucleus accumbens. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:399–406. doi: 10.1007/s002100000377. [DOI] [PubMed] [Google Scholar]
- Levine AS, Billington CJ. Opioids as agents of reward-related feeding: a consideration of the evidence. Physiol Behav. 2004;82:57–61. doi: 10.1016/j.physbeh.2004.04.032. [DOI] [PubMed] [Google Scholar]
- MacDonald AF, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the ventral tegmental area and in the nucleus accumbens shell region in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R999–R1004. doi: 10.1152/ajpregu.00271.2003. [DOI] [PubMed] [Google Scholar]
- Marks-Kaufman R, Kanarek RB. Morphine selectively influences macronutrient intake in the rat. Pharmacol Biochem Behav. 1980;12:427–430. doi: 10.1016/0091-3057(80)90048-9. [DOI] [PubMed] [Google Scholar]
- Marks-Kaufman R, Kanarek RB. Diet selection following a chronic morphine and naloxone regimen. Pharmacol Biochem Behav. 1990;35:665–669. doi: 10.1016/0091-3057(90)90305-2. [DOI] [PubMed] [Google Scholar]
- Marks-Kaufman R, Plager A, Kanarek RB. Central and peripheral contributions of endogenous opioid systems to nutrient selection in rats. Psychopharmacology (Berl) 1985;85:414–418. doi: 10.1007/BF00429656. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickiewicz AL, Dallimore JE, Napier TC. The Ventral Pallidum is Critically Involved in the Development and Expression of Morphine-Induced Sensitization. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.111. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Nielsen MA. Evidence that an accumbens to subpallidal GABAergic projection contributes to locomotor activity. Brain Res Bull. 1983;11:309–314. doi: 10.1016/0361-9230(83)90166-1. [DOI] [PubMed] [Google Scholar]
- Nauta WJ, Smith GP, Faull RL, Domesick VB. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience. 1978;3:385–401. doi: 10.1016/0306-4522(78)90041-6. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci. 2000;114:42–63. [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Smith KS, Berridge KC. Hedonic hot spots in the brain. Neuroscientist. 2006;12:500–511. doi: 10.1177/1073858406293154. [DOI] [PubMed] [Google Scholar]
- Ragnauth A, Moroz M, Bodnar RJ. Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res. 2000;876:76–87. doi: 10.1016/s0006-8993(00)02631-7. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- Schneider LH, Davis JD, Watson CA, Smith GP. Similar effect of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding. Eur J Pharmacol. 1990;186:61–70. doi: 10.1016/0014-2999(90)94060-b. [DOI] [PubMed] [Google Scholar]
- Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci. 2006;23:1596–1604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- Smith GP. The direct and indirect controls of meal size. Neurosci Biobehav Rev. 1996;20:41–46. doi: 10.1016/0149-7634(95)00038-g. [DOI] [PubMed] [Google Scholar]
- Smith GP. Satiation: From gut to brain. New York: 1998. [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose "liking" and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behav Neurosci. 1998;112:678–694. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Spector AC, St John SJ. Role of taste in the microstructure of quinine ingestion by rats. Am J Physiol. 1998;274:R1687–1703. doi: 10.1152/ajpregu.1998.274.6.R1687. [DOI] [PubMed] [Google Scholar]
- Stratford TR. Activation of feeding-related neural circuitry after unilateral injections of muscimol into the nucleus accumbens shell. Brain Res. 2005;1048:241–250. doi: 10.1016/j.brainres.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE. Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci. 1999;19:11040–11048. doi: 10.1523/JNEUROSCI.19-24-11040.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE, Simansky KJ. Blockade of GABAA receptors in the medial ventral pallidum elicits feeding in satiated rats. Brain Res. 1999;825:199–203. doi: 10.1016/s0006-8993(99)01239-1. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Swanson LW, Koob GF. Substantia innominata: critical link in the behavioral expression of mesolimbic dopamine stimulation in the rat. Neurosci Lett. 1984;50:19–24. doi: 10.1016/0304-3940(84)90455-5. [DOI] [PubMed] [Google Scholar]
- Taha SA, Norsted E, Lee LS, Lang PD, Lee BS, Woolley JD, Fields HL. Endogenous opioids encode relative taste preference. Eur J Neurosci. 2006;24:1220–1226. doi: 10.1111/j.1460-9568.2006.04987.x. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24:1058–1069. doi: 10.1523/JNEUROSCI.1437-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tindell AJ, Smith KS, Pecina S, Berridge KC, Aldridge JW. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- Ward HG, Nicklous DM, Aloyo VJ, Simansky KJ. Mu-opioid receptor cellular function in the nucleus accumbens is essential for hedonically driven eating. Eur J Neurosci. 2006;23:1605–1613. doi: 10.1111/j.1460-9568.2006.04674.x. [DOI] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE. Nucleus accumbens mu-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci. 2003;23:2882–2888. doi: 10.1523/JNEUROSCI.23-07-02882.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Pratt WE, Kelley AE. Pharmacological characterization of high-fat feeding induced by opioid stimulation of the ventral striatum. Physiol Behav. 2006;89:226–234. doi: 10.1016/j.physbeh.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Lee BS, Taha SA, Fields HL. Nucleus accumbens opioid signaling conditions short-term flavor preferences. Neuroscience. 2007;146:19–30. doi: 10.1016/j.neuroscience.2007.01.005. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Obesity: preventing and managing the global epidemic. Geneva, Switzerland: 2000. [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132:350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- Zheng H, Berthoud HR. Eating for pleasure or calories. Curr Opin Pharmacol. 2007;7:607–612. doi: 10.1016/j.coph.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Berthoud HR. Neural systems controlling the drive to eat: mind versus metabolism. Physiology (Bethesda) 2008;23:75–83. doi: 10.1152/physiol.00047.2007. [DOI] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR. Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1436–1444. doi: 10.1152/ajpregu.00781.2002. [DOI] [PubMed] [Google Scholar]
- Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–11082. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]