Abstract
Here we report the immunological characterization of lipid-polymer hybrid nanoparticles (NPs) and propose a method to control the levels of complement activation induced by these NPs. This method consists of the highly specific modification of the NP surface with methoxyl, carboxyl, and amine groups. Hybrid NPs with methoxyl surface groups induced the lowest complement activation, whereas the NPs with amine surface groups induced the highest activation. All possible combinations among carboxyl, amine, and methoxyl groups also activated the complement system to a certain extent. All types of NPs activated the complement system primarily via the alternative pathway rather than the lectin pathway The classical pathway was activated to a very small extent by the NPs with carboxyl and amine surface groups. Human serum and plasma protein binding studies showed that these NPs had different protein binding patterns. Studies of both complement activation and coagulation activation suggested that NPs with methoxyl surface groups might be an ideal candidate for drug delivery applications, since they are not likely to cause any immunological adverse reaction in the human body.
1. Introduction
Lipid-polymer hybrid nanoparticles (NPs) hold great promise as a drug delivery vehicle in the treatment of various diseases. The NP is comprised of a hydrophobic polymeric core and a hydrophilic polymeric shell separated by a lipid monolayer (Fig. 1A). [1] This type of hybrid NP combines the merits of both liposomes and polymeric NPs, two of the most popular drug delivery vehicles approved for clinical use. It has been shown in vitro that lipid-polymer hybrid NPs have the advantages of carrying poorly water-soluble drugs with high encapsulation and loading yields, tunable and sustained drug release profile, excellent serum stability, and differential targeting of cells. In addition to potential applications in drug delivery, the hybrid NPs can also be used as novel adjuvants for vaccination.
Figure 1.
A) Schematic illustration of lipid-polymer hybrid nanoparticle (NP) bearing various surface functional groups. The NP is comprised of a poly (D, L-lactide-co-glycolide) (PLGA) core, a polyethylene glycol (PEG) shell and a lipid monolayer at the interface of the core and the shell. The NP surface can be functionalized with one or a combination of carboxyl, methoxyl and amine groups. B) Size and surface ζ potential of functionalized lipid-polymer hybrid NPs. The size of these NPs is similar between each other. Carboxyl and methoxyl-ends lipid-polymer NPs have negative charge whereas amine-ends lipid-polymer NPs have positive charge.
However, the immunological properties of these lipid-polymer hybrid NPs have not yet been systematically investigated. If these NPs are to be utilized for systemic drug delivery applications or as adjuvants for vaccination, we must know more about their immunocompatibility characteristics such as complement system activation, plasma and serum protein binding properties, and coagulation cascade activation.
The complement system, part of the innate immune system, is a biochemical cascade in blood for the recognition and clearance of foreign materials [2]. It consists of over 20 small proteins and protein fragments. These proteins normally circulate in the blood as inactive zymogens. When they are stimulated by a trigger or activator, the complement system is activated, resulting in response and activation of the cell-killing membrane attack complex. The complement system can be activated via three different pathways: the classical pathway, the alternative pathway, and the lectin pathway. In the classical pathway, the activation is triggered when protein C1q recognises activators and binds to their surface mainly via charges or hydrophobic interactions [2–3]. The alternative pathway is activated by the covalent binding of C3b to the hydroxyl or amino groups on the pathogen surface, and subsequent events leading to complement activation are analogous to those in the classical pathway [2][4]. In the lectin pathway the mannan-binding lectin (MBL) protein binds to activators through an interaction with neutral sugar residues (e.g. mannose) [2]. Similarly L-ficolin can also initiate the lectin pathway, but we know little about its recognition specificity.
Because reducing the complement system activation of a polymer’s surface is very important for improving its blood compatibility, this subject has attracted much attention. Especially in the 1980s and 1990s, there are many reports of various methods to diminish the activation of the complement system. However, most methods involved complicated organic synthesis [5–10] or other procedures impractical for translational research. The study of protein binding to a material surface is also highly relevant to its immunocompatibility [11]. Bound proteins can initiate thrombosis on the material surface by interacting with platelets or activating the intrinsic clotting cascade[11]. Though there are many studies of human plasma protein binding to various nanomaterials [12–22], only one paper in 1998 examined the binding of human plasma proteins to polymeric NPs with different surface characteristics [23]. Martin et al. reported the binding of human plasma proteins to five types of latex particles (diameter: 89 ± 35 nm to 660 ± 36 nm) surface-functionalized with a hydroxyl group, an ammonium group, and a combination of the two groups. Martin et al. found remarkable differences of the types and quantity of human plasma proteins that bind to those latex particles, complement system activation experiments were not mentioned.
Here we report our systematic investigation of the immunocompatibility properties of lipid-polymer hybrid NPs by measuring their complement system activation, human plasma protein binding properties, and coagulation system activation at various surface chemistry circumstances. In addition, though the focus of this work is the immunocompatibility properties of lipid-polymer hybrid NPs, this study also provides a methodology to tune the surface chemistry composition of new nanotherapeutic designs to screen for optimal nano-platforms that have minimal immunogenicity.
2. Materials and Methods
2.1 Preparation of the nanoparticles
Poly (D, L-lactide-co-glycolide)-Lipid poly (ethylene glycol) nanoparticles (PLGA-Lipid-PEG NPs) were prepared following a protocol described previously [1]. Briefly, PLGA polymer was dissolved in acetonitrile at a concentration of 2 mg/mL. The lipid lecithin (Alfa Aesar) was mixed with 1, 2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-N-[Carboxy (Polyethylene Glycol)2000] (DSPE-PEG-COOH)(Avanti Polar Lipids) at a molar ratio of 8.5:1.5 and dissolved in 4% ethanol aqueous solution. The total lipid weight (lecithin + DSPE-PEG-COOH) was 15% of the PLGA polymer. The lipid solution was preheated at 65 °C for 3 minutes, and the PLGA solution was added dropwise under gentle stirring. The mixed solution was vortexed vigorously for 3 minutes followed by gentle stirring for 2 hours at room temperature. Finally the nanoparticles were washed three times using an Amicon Ultra-4 centrifugal filter (Millipore, Billerica, MA) with a molecular weight cutoff of 10K Da. The same procedures were used to prepare the hybrid nanoparticles with other surface functional groups or a mixture of surface groups.
2.2 Characterization of the lipid-polymer hybrid NPs
The NPs were suspended in 1X Phosphate Buffer Saline (PBS, pH=7.4). The hydrodynamic diameter (nm) of the nanoparticles and their polydispersity index were determined from three repeated experiments using dynamic light scattering with a Zeta Pals instrument (Brookhaven, USA) at 25 °C. The Zeta potential (mV) of the nanoparticles was calculated from three repeated measurements on a Zetasizer 2000 (Malvern Instruments, UK).
2.3 Complement System Activation studies
To assess complement system activation of the lipid-polymer hybrid nanoparticles in vitro, three complement split products (SC5b-9, Bb, and 4cd) were analyzed using enzyme-linked immunosorbent assay kits from Quidel Corp. (San Diego, CA, USA). SC5b-9 is the S-protein-bound form of the terminal complex, a sensitive biomarker of C5a formation via both the classical and the alternative pathways. Bb is the proteolytically active fragment of factor B, a biomarker of complement system activation via the alternative pathway. C4d measures the amount of the C4d-containing activation fragments of C4 (C4b, iC4b, and C4d), a biomarker of complement system activation via the classical pathway. The nanoparticles were incubated with human serum at a volume ratio of 1:5 in a shaking incubator (80 rpm) at 37°C for 1 hour. After incubation the reaction was stopped by adding 60 times volume of PBS solution containing 0.05 wt% Tween-20 protein stabilizers and 0.035 wt% ProClin 300. Complement system activation of the nanoparticles was assayed following manufacturer’s instructions. Zymosan was used as a positive control for the alternative pathway and terminal cascade. Complement system activation via the lectin pathway was assessed using the ALPCO Diagnostics kit (Salem, MA, USA).
To assess the genuine activation of the complement system via the alternative pathway, human serum was diluted 1:1 by volume with Mg-EGTA buffer as described in [12]. The diluted human serum was incubated with lipid-polymer nanoparticles and incubated for 1 hr at 37°C in a shaking incubator. The reaction was stopped as described above.
The experiments described above were carried out in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975, as revised in 1983.
2.4 Protein binding studies
Lipid-polymer hybrid NPs were incubated with the diluted (1:1 volume) human serum or human plasma under gentle shaking of 80 rpm at 37°C for 1 hour. After incubation the NPs were spun down using a microcentrifuge at 13,000 rpm for 15 min, and the supernatant was removed. The same procedure was repeated 5 times. Next the NPs were reduced by 25 mM DTT and heated for 10 min at 95° C. Then the NP samples were loaded and run through a gradient gel (4–12%) at 150 V for 70 min. The gel was stained with coomassie blue, and the bands were analyzed by mass spectrometry (MIT Proteomics core facility).
2.5 Coagulation studies
Lipid-polymer hybrid NPs with methoxyl surface group (NP-OCH3) were incubated with fresh human citrated plasma under gentle shaking (80 rpm) at 37 °C for 30 minutes. As negative controls, NPs were incubated with human plasma and 1X PBS buffer in parallel. Triplicate samples were prepared for each condition. After incubation, the samples were measured for Prothrombin (PT), Activated Partial Thromboplastin Time (APTT), and Thrombin Time (TT). These measurements were done by the Clinical Lab, Hematology section, at Brigham and Women’s Hospital.
3. Results
3.1 Preparation and characterization of lipid-polymer hybrid NPs
Lipid-polymer hybrid NPs were prepared using a modified nanoprecipitation method as described in section 2.1. As shown in Fig. 1A, the hybrid NPs are comprised of three components: a hydrophobic poly(D,L-lactide-co-glycolide) (PLGA) core, a hydrophilic poly(ethylene glycol) (PEG) shell, and a soybean phosphatidylcholine (lecithin) monolayer at the interface of the hydrophobic core and hydrophilic shell. The PEG molecule is covalently attached to 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE-PEG), which is interspersed throughout the lecithin monolayer By changing the end group of DSPE-PEG, the NPs can be modified with different surface functional groups such as carboxyls, amines, and methoxyls. In the present study, the NPs containing PLGA-lecithin-PEG-COOH, PLGA-lecithin-PEG-NH2 and PLGA-lecithin-PEG-OCH3 are referred to as NP-COOH, NP-NH2, and NP-OCH3, respectively.
Dynamic light scattering (DLS) was used to characterize NP hydrodynamic size (diameter, nm) and surface zeta potential (mV) in each preparation. As shown in Fig. 1B, the average NP size remained in the same range (89–110 nm) under our particular experimental conditions, regardless of the surface functional groups. However, the surface zeta potential of NP-COOH, NP-NH2, and NP-OCH3 are −25±1 mV, 10±2 mV, and −9±1 mV, respectively. All three NPs were well-dispersed in 1X PBS buffer.
3.2 Human serum complement system activation by lipid-polymer hybrid NPs
After incubation with human serum, SC5b-9 concentration was measured to evaluate the complement system activation of these NPs. As shown in Fig. 2A, it was found that lipid-polymer hybrid NPs with an amine surface group (NP-NH2) more effectively activate the complement system than NPs with carboxyl (NP-COOH) or methoxyl (NP-OCH3) surface groups. However, all three NP formulations activate the complement system slightly more than human serum (negative control) but significantly less than Zymosan (positive control), a well-known complement system activator of the alternative pathway. Further studies found that these NPs activate the alternative pathway (Fig. 2B) but not the lectin pathway (data not shown here). As compared to human serum without any NPs (negative control), negligible complement system activation via the classical pathway was noted for NP-COOH and NP-NH2, and NP-OCH3 did not activate the classical pathway at all (Fig. 2C).
Figure 2.
Human serum complement system activation of lipid-polymer hybrid NPs surface functionalized with carboxyl (NP-COOH), methoxyl (NP-OCH3) and amine (NP-NH2) groups, respectively. (A) Complement system activation at the terminal cascade. Bars represent the mean concentration of SC5b-9, a sensitive biomarker of C5a formation via both the alternative pathway and the classical pathway. The results were obtained from three independent experiments with a 95% confidential interval. (B) Complement system activation via the alternative pathway. Bb is the proteolytically active fragment of factor B, a biomarker of the complement system activation via the alternative pathway. (C) Complement system activation via the classical pathway. C4d measures the amount of C4d-containing activation fragments of C4, a biomarker of the complement system activation via the classical pathway.
Complement components C6-8 precursors are proteins that participate in the formation of the membrane attack complex, which leads to complement activation. The activation of the human serum complement system via the classical pathway takes place when C1q, the recognition subunit of the C1 complex, binds to complement activators. C1 binds to target ligands via the globular domains or head groups, thereby triggering the activation of C1r and C1s, the proteases associated with C1q [3]. Since we found that binding occurred in the presence of Ca2+ ions (Section 2.3), C1q would present as a C1 complex (C1qr2s2) in serum. Thus it appears that the entire C1 binds to the hybrid NPs, activating C1s, which in turn cleaves C4 and C2 to produce C4b. C4b then binds to complement activators via covalent bonds. It is possible that C4b covalently binds to the carboxyl and amine groups on the NP surface. That would lead to the formation of the C4b2a complex on the NP surface, resulting in C3 activation. The final consequence of these processes is the activation of the complement system via the classical pathway, as shown in Fig. 2C.
Fig. 2B shows that NP-NH2 activates the alternative pathway considerably more effectively than NP-COOH or NP-OCH3. In particular, NP-OCH3 induced the lowest (negligible) activation of the alternative pathway. Further studies found that Factor H, a main downregulator of the alternative pathway, bound less effectively to NP-COOH than to NP-NH2 or NP-OCH3 (Figs. 3B–4B and Table 1). Immunologically, less amount of factor H binding induces higher level of complement system activation, consistent with the data in Fig. 2A–B and Table 1. One possible reason is that factor H and C3b compete for binding to the NPs, thereby affecting NP complement activation. The high level of complement activation of NP-NH2 suggests C3b binds more strongly than Factor H to NPs. This idea is supported by the fact that C3b covalently binds to NH2 and OH groups on the surface of the activator [4]. In addition, the binding of other complement proteins such as C6 (Table 1) also indicates that the presence of amine groups on the NP surface contributes to the high level of complement activation.
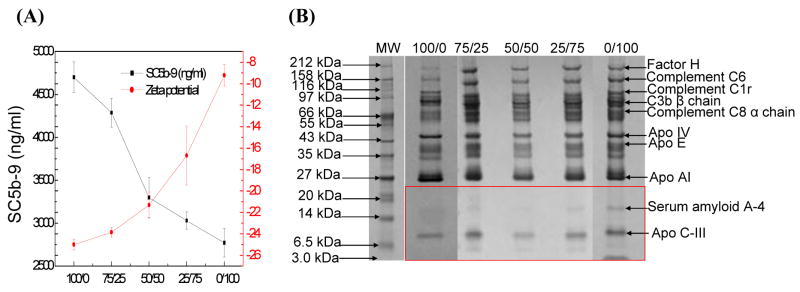
Figure 3.
Human serum complement system activation of lipid-polymer hybrid NPs with a mixture of carboxyl and methoxyl surface groups. (A) SC5b-9 concentration and NP surface zeta potential plotted as a function of carboxyl group and methoxyl group molar ratio (COOH/OCH3). (B) Gel electrophoresis results of human serum proteins binding to the hybrid nanoparticles at various COOH/OCH3 molar ratios.
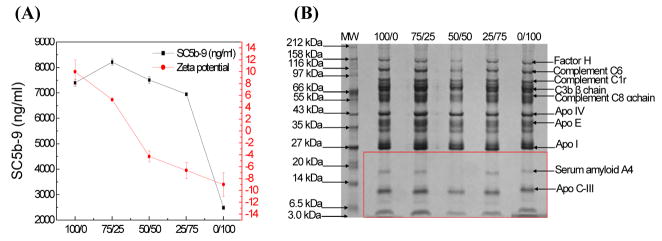
Figure 4.
Human serum complement system activation of lipid-polymer hybrid nanoparticles with a mixture of carboxyl and amine surface groups. (A) SC5b-9 concentration and NP surface zeta potential plotted as a function of carboxyl group and amine group molar ratio (COOH/NH2). (B) Gel electrophoresis results of human serum proteins binding to the hybrid nanoparticles at various COOH/NH2 molar ratios.
Table 1.
Quantification of human serum proteins binding to lipid-polymer hybrid NPs with different surface functional groups. The protein binding density, characteristic of integrated density value (IDV), was obtained by using the software of the FluorCHEM quantitative imaging system (Alpha Innotech). For clarity reason, the IDVs of each protein binding to NP-COOH, NP-NH2 and NP-OCH3 nanoparticles, respectively, were normalized to the IDV of NP-OCH3 nanoparticles. Smaller IDV number represents higher amount of protein binding to the NPs.
| Human serum proteins | COOH | CH3O | NH2 |
|---|---|---|---|
| Amount of protein bound | |||
| Factor H | 0.98 | 1 | 0.94 |
| Complement component C6 precursor | 1.02 | 1 | 0.86 |
| Complement C1r subcomponent precursor | 0.89 | 1 | 0.89 |
| C3b β chain | 0.97 | 1 | 0.98 |
| Complement component C8α chain precursor | 1.02 | 1 | 0.91 |
| Apolipoprotein IV | 0.72 | 1 | 0.87 |
| Apolipoprotein E | 0.80 | 1 | 0.84 |
| Apolipoprotein AI | 1.05 | 1 | 1.06 |
| Serum amyloid A-4 protein precursor | 0 | 1 | 0.92 |
| Apolipoprotein C-III | 1.01 | 1 | 0.95 |
To investigate whether alternative pathway activation occurs independently and not as a consequence of classical pathway activation, we used the Bb plus Quidel immuno assay kit. The hybrid NPs were incubated with human serum in the presence of Mg2+ but not Ca2+, as described in section 2.3. The absence of Ca2+ in this assay prevents classical pathway activation, because the recognition complex of the classical pathway, C1qr2s2, is dissociated and becomes inactive. The results confirm that alternative pathway activation occurs independently and not as a consequence of classical pathway activation (data not shown). Since alternative pathway activation relies on C3b binding to the activators, we expected that this protein would bind to the surface of the hybrid NPs. Fig. 3B shows C3b β chain (~75 kDa) on the NP surface (see next section for more details).
Figs. 3–5 demonstrates human serum complement system activation of lipid-polymer hybrid NPs with a combination of surface functional groups, including carboxyl and methoxyl (NP-COOH/OCH3), carboxyl and amine (NP-COOH/NH2), and amine and methoxyl (NP-NH2/OCH3). For NP-COOH/OCH3 (Fig. 3A–B), more methoxyl groups on the NP surface decrease the zeta potential (less negative) while reducing the level of complement activation of the NPs. For example, when the molar ratio of COOH/OCH3(Fig. 3B) varied from 75/25 to 50/50 and to 25/75, the NP surface zeta potential was approximately −23 mV, −21 mV, and −17 mV, respectively, whereas the SC5b-9 concentration (Fig. 3A) decreased from 4287 ng/ml to 3298 ng/ml and to 3030 ng/ml. For NP-COOH/NH2 (Fig. 4A–B), different tendencies of NP surface charge and complement activation were found. More amine groups on the NP surface result in higher NP surface zeta potential (Fig. 4A) and higher complement activation (Fig. 4B). For NP-NH2/OCH3 (Fig. 5A–B), we found that more methoxyl groups on the NP surface led to lower NP surface zeta potential (Fig. 5A) and lower complement activation (Fig. 5B). These results indicate that NPs with a combination of carboxyl, methoxyl, and amine groups produced different levels of complement activation, probably resulting from the different physico-chemical characteristics of the NPs. Table 2–4 showed that the amount of factor H and C3b β chain proteins binding to the NP surface was correlated with the levels of complement activation of the NPs. For example, factor H bound less effectively to the NP-COOH/NH2 with a COOH/NH2molar ratio of 75/25 (Table 3) than to the NP-COOH/OCH3 with a COOH/OCH3 molar ratio of 75/25 (Table 2). The highest level of C3bβ chain was found on the surface of NP-NH2/OCH3 (Table 4), which more complement activation than either NP-COOH/OCH3 (Table 2) or NP-COOH/NH2 (Table 3).
Figure 5.
Human serum complement system activation of lipid-polymer hybrid NPs with a mixture of amine and methoxyl surface groups. (A) SC5b-9 concentration and NP surface zeta potential plotted as a function of amine group and methoxyl group molar ratio (NH2/OCH3). (B) Gel electrophoresis results of human serum proteins binding to the hybrid NPs at various NH2/OCH3 molar ratios.
Table 2.
Quantification of human serum proteins binding to lipid-polymer hybrid NPs with a mixture of carboxyl and methoxyl surface groups (NP-COOH/OCH3). The numbers of 75/25, 50/50 and 25/75 represent the molar ratios of COOH/OCH3.
| Human serum proteins | 75/25 | 50/50 | 25/75 |
|---|---|---|---|
| Amount of protein bound | |||
| Factor H | 0.73 | 1 | 0.91 |
| Complement component C6 precursor | 0.72 | 1 | 0.85 |
| Complement C1r subcomponent precursor | 0.70 | 1 | 0.80 |
| C3b β chain | 0.80 | 1 | 0.71 |
| Complement component C8α chain precursor | 0.68 | 1 | 0.95 |
| Apolipoprotein IV | 0.85 | 1 | 0.83 |
| Apolipoprotein E | 0.69 | 1 | 0.80 |
| Apolipoprotein AI | 1.20 | 1 | 0.95 |
| Serum amyloid A-4 protein precursor | - | 1 | - |
| Apolipoprotein C-III | 0.77 | 1 | 0.86 |
Table 4.
Quantification of human serum proteins binding to lipid-polymer hybrid NPs with a mixture of amine and methoxyl surface groups (NP-NH2/OCH3). The numbers of 75/25, 50/50 and 25/75 represent the molar ratios of NH2/OCH3.
| Human serum proteins | 75/25 | 50/50 | 25/75 |
|---|---|---|---|
| Amount of protein bound | |||
| Factor H | 0.73 | 1 | 0.80 |
| Complement component C6 precursor | 0.53 | 1 | 0.80 |
| Complement C1r subcomponent precursor | 0.92 | 1 | 0.87 |
| C3b β chain | 0.48 | 1 | 0.91 |
| Complement component C8α chain precursor | 0.47 | 1 | 0.76 |
| Apolipoprotein IV | 0.69 | 1 | 1.00 |
| Apolipoprotein E | 0.48 | 1 | 0.93 |
| Apolipoprotein AI | 0.42 | 1 | 0.92 |
| Serum amyloid A-4 protein precursor | 1.16 | 1 | 1.21 |
| Apolipoprotein C-III | 0.59 | 1 | 0.74 |
Table 3.
Quantification of human serum proteins binding to lipid-polymer hybrid NPs with a mixture of carboxyl and amine surface groups (NP-COOH/NH2). The numbers of 75/25, 50/50 and 25/75 represent the molar ratios of COOH/NH2.
| Human serum proteins | 75/25 | 50/50 | 25/75 |
|---|---|---|---|
| Amount of protein bound | |||
| Factor H | 0.82 | 1 | 1 |
| Complement component C6 precursor | 0.72 | 1 | 0.87 |
| Complement C1r subcomponent precursor | 0.79 | 1 | 1 |
| C3b β chain | 0.58 | 1 | 0.98 |
| Complement component C8α chain precursor | 0.72 | 1 | 1.04 |
| Apolipoprotein IV | 0.66 | 1 | 0.83 |
| Apolipoprotein E | 0.78 | 1 | 1.21 |
| Apolipoprotein AI | 2.08 | 1 | 3.71 |
| Serum amyloid A-4 protein precursor | - | 1 | - |
| Apolipoprotein C-III | 0.85 | 1 | 0.96 |
3.3 Human serum and plasma proteins binding to lipid polymer hybrid NPs
We studied human serum and plasma protein binding to hybrid NPs with different surface functional groups. Interestingly, we found that serum amyloid A-4 protein precursor preferentially bound to NPs with surface amine and/or methoxyl groups, such as NP-OCH3 (Fig. 3B), NP-NH2 (Fig. 4B) and NP-NH2/OCH3 (Fig. 5B) The protein precursor did not bind to NPs with carboxyl groups on the surface such as NP-COOH, NP-COOH/OCH3, and NP-COOH/NH2. Together these observations indicate that binding of the serum amyloid A-4 protein to the NPs might be related to surface charge. This protein has been found abundantly in the brains of schizofrenic patients. It would be interesting and worthwhile to further elucidate the interaction and binding mechanisms of amyloid A-4 and NPs.
Determining how human plasma proteins bind to the lipid-polymer hybrid NPs is also important, because it is closely related to the in vivo circulation half-life and biodistribution of NPs. We found that human plasma and human serum had similar adsorption patterns on the hybrid NPs, except for the binding of fibrinogen (Figs. 3–5B and 6). Fibrinogen α chain bound less effectively to NP-COOH than to NP-OCH3 or NP-NH2 (Table 5). It was also found that human plasma proteins with low molecular weight (such as the apolipoprotein family) bound to the hybrid NPs (Figs. 6A–C). Fig. 6B shows that a small amount of factor H and complement proteins C6, C8, and C1r bound to the NP-NH2/OCH3, but there was little binding of apolipoprotein AI, serum amyloid A-4, or apolipoprotein C-III. However, all these proteins bound to NP-COOH/NH2 with obvious absorption patterns (Fig. 6C) and to a lesser extent to NP-COOH/OCH3 (Fig. 6A).
Figure 6.
Gel electrophoresis results of human plasma proteins binding to the lipid-polymer hybrid NPs with a mixture of surface functional groups: (A) COOH/OCH3, (B) NH2/OCH3, and (C) COOH/NH2.
Table 5.
Quantification of human plasma proteins binding to lipid-polymer hybrid NPs with different surface functional groups.
| Human plasma proteins | COOH | CH3O | NH2 |
|---|---|---|---|
| Amount of protein bound | |||
| Factor H | 0.61 | 1 | 1.09 |
| Complement component C6 precursor | 1.10 | 1 | 1.17 |
| Complement C1r subcomponent precursor | 1.04 | 1 | 1.07 |
| Fibrinogen α chain | 0.83 | 1 | 1.18 |
| Fibrinogen β chain | 1.18 | 1 | 1.06 |
| Fibrinogen γ chain | 1.16 | 1 | 0.96 |
| Apolipoprotein IV | 1.31 | 1 | 1.27 |
| Apolipoprotein E | 1.22 | 1 | 1.05 |
| Apolipoprotein AI | 0.89 | 1 | 1.32 |
| Serum amyloid A-4 protein precursor | 1.06 | 1 | 1.03 |
| Apolipoprotein C-III | - | 1 | 1.03 |
3.4 Coagulation studies of lipid-polymer hybrid NPs
Fibrinogen is one of the most abundant proteins in human plasma and requires special attention, because it activates the coagulation cascade and plays a significant role in the opsonization process of the complement system. To investigate whether NPs change fibrinogen’s effect on human plasma clotting time, we chose NP-OCH3 for further immunocompatibility studies because the hybrid NPs with methoxyl surface groups induced the lowest level of complement activation. Coagulation assays including prothrombin time (PT), activated partial thromboplastin time (APTT), and thrombin time (TT) were conducted with these NPs. PT refers to the time that plasma takes to clot, which is a function of the extrinsic pathway; the reference range is usually 12–15s [24]. The PT assay measures the presence of factors II, V, VII, and X and fibrinogen. APTT measures the performance of the intrinsic and common coagulation pathways. Thrombin converts fibrinogen to an active form that assembles into fibrin and assures the success of the coagulation cascade. The results show that when human plasma was incubated either with or without NP-OCH3, the addition of NPs has no effect on clotting time. Only an insignificant change in APTT was noted in the presence of NP-OCH3. Overall these coagulation assays suggest that hybrid NPs have no effect on the coagulation system.
4. Discussion
4.1 NP preparation and characterization
The lipid-polymer hybrid NPs were prepared through a modified nanoprecipitation method combined with self-assembly. This makes NP synthesis scalable and reproducible for clinical manufacturing purposes. The hydrodynamic diameter of the NPs can be well-controlled within the range of 50–200 nm by tuning the intrinsic parameters of NP formulation. This size range would allow the NPs to be used clinically as drug delivery vehicles. In addition, these NPs are well-dispersed in and remain stable in 1X PBS buffer, human serum, and human plasma.
4.2 Complement system activation
In the 1990s, various types of pegylated polymeric NPs including poly (lactic-co-glycolic acid) (PLGA) [13] [15][16] [17] and poly (D, L-lactide) (PLA) [19][22] were assessed for complement activation. Those tests primarily involved hemolytic assays, a common and well-accepted method among the scientific community. However, the recently developed complement activation kits not only help to overcome some practical issues such as the partial hydrolysis that the NPs might undergo during the assay, but also provide highly specific quantitative analysis. For this reason Quidel Immunoassay kits were used.
It is well-known that the alternative pathway is activated when C3b protein is deposited onto an activator surface containing amine or hydroxyl groups. However, the mechanism of complement system activation via the alternative pathway is unpredictable, because it relies on both charge and neutral sugars on the surface. On the other hand, the main recognition unit of the classical pathway, C1q, usually binds to the activator surface through electrostatic and hydrophobic interactions [3].
Our results at least partially agree with published literature on complement system activation, since C3b covalently binds to the amine groups on the surface of NP-NH2. However we found that NP-COOH and NP-OCH3 activate the human serum complement system primarily via the alternative pathway. This disagrees with published findings that NP-COOH activates the classical pathway because of the negative charge on the NPs. The poor activation of the classical pathway suggests that, in competition with C1 complex or C1q to bind to the NP surface, C3b binds dominantly. Another possible rationale is that NP-COOH and NP-OCH3 are taken as hydrophilic surface by C1q, which prevents the binding of C1q to the NPs. Nevertheless, the levels of complement activation elicited these NPs are much lower than that produced by Zymosan. Furthermore, the complement activation products C3b and iC3b derived from the activation of C3 have an important medical role, because they act as adjuvants and opsonins.
There is extensive literature on complement system activation of other nanomaterials such as liposomes and polymeric NPs. It has been reported that positively charged liposomes preferentially activate the alternative pathway, that negatively charged liposomes are likely to activate the classical pathway, and that neutral liposomes are insensitive to complement system activation [25]. Vittaz et al. [22] reported that PLA-PEG NPs consume very low amounts of complement via the classical pathway when PEG coverage density is higher than 0.2 PEG molecule per nm2. However the PLA-PEO NPs consume very high amounts of complement via the classical pathway when the PEG coverage density is 0.19 PEG molecule per nm2.
Both the published literature and the results presented here indicate that several factors besides surface charge and charge density, such as topographical accessibility of complement proteins and excipients of the NP activators, determine complement system activation.
4.3 Serum and plasma protein binding
Over the last two decades, human serum and plasma protein binding to different nanomaterials [10–22] including polymeric latex NPs[23], PLGA NPs[22], and carbon nanotubes [12] has been extensively studied. Such studies have mainly focused on the apolipoprotein family (A-IV, E, A-I, C-III), albumin, and fibrinogen on the nanomaterials’ surfaces. However the binding of factor H has been observed only in chemically modified carbon nanotube systems [26] and in the hybrid NP systems reported in this study. Factor H is the main downregulator of the alternative pathway in plasma, which consists of 20 short consensus repeat domains (SCRs), each about 60 residues long [27]. The structure of FH modules 6–8 reveals multiple sulphated sugar-binding sites [27]. In addition, the carboxyl-terminal region (SCRs 19–20, FH 19–20) is unique configured to bind to C3b/C3d The heterogeneous intercalation of carboxyl, methoxyl, and amine groups on the surface of the lipid-polymer hybrid NPs allows us to control their surface chemistry and charge density, and in turn their human serum complement system activation. Human serum and plasma protein binding studies identified the binding of two key complement proteins, Factor H and C3b β chain, which play a pivotal role in the activation of the complement system via the alternative pathway.
Amine groups on the surface of the NPs induced high levels of human serum complement activation. However, a heterogeneous surface comprised of amine and methoxyl groups activated the complement system even more effectively. Our results clearly demonstrate that human serum factor H plays a very important role in complement activation, and that the binding of factor H strongly depended on the charge and charge density of the NPs. Although there is no strict correlation between the levels of complement activation and the amount of factor H binding to the NP surface, factor H barely bound to NP-COOH. In addition, the protein orientation of factor H on the NP surface might also affect activation of the complement system. Our results showed that factor H attached to NP-NH2/OCH3 with high affinity, but we cannot rule out the possibility that C3b and factor H might compete for binding sites. It is also likely that SCR7-8 and/or 19-20 of Factor H [27–28] bound to NP-COOH and NP-OCH3, because these domains have binding preference to negatively charged surfaces. Further studies should be carried out to investigate this possibility. It is important to mention that the binding of human plasma factor H to the lipid-polymer hybrid NPs was mediated by heparin in the present study. It is well-known that heparin binds to factor H.
The complete sequestration of human serum factor H to these NPs could switch off the activation of the complement system via the alternative pathway, thereby inhibiting complement activation.
4. 4 Coagulation studies
It has been reported that fibrinogen binds to receptors expressed by cells of the mononuclear phagocytic system [29]. However, results from coagulation studies of the lipid-polymer hybrid NPs suggest that the binding of fibrinogen does not play a crucial role in those phenomena. Thus, coagulation assays suggest that these NPs do not affect the coagulation cascade.
4.5 General comments
Here, studies of the immunocompatibility properties of lipid-polymer hybrid NPs with heterogeneous surface functional groups (carboxyl, methoxyl, and amine) demonstrated that the intercalation of various functional groups on the NP surface can precisely modulate the activation of the human serum complement system. This method constitutes a powerful tool that can help us understand the behavior of key complement proteins such as factor H and C3b at the nanoscale level. This method could be also applied to other NP systems to understand their complement activation properties.
5. Conclusions
Lipid-polymer hybrid NPs with heterogeneous surface functional groups (carboxyl, methoxyl, and amine) have been systematically investigated for their immunocompatibility. It was found that the NPs with methoxyl surface groups induced lower levels of complement activation compared the NPs with carboxyl or amine surface groups. Coagulation studies demonstrated that no hybrid NPs activated the coagulation cascade. Assays of both complement activation and coagulation provide evidence of the good biocompatibility of these NPs, suggesting their use in drug delivery to treat a myriad of diseases. In addition, our results reaffirm the idea that NP surface chemistry significantly affects human plasma and serum protein adsorption patterns. The heterogeneous intercalation of different surface functional groups on the NP surface plays an important role in modulation of the complement system activation by controlling the binding of key complement proteins such as Factor H and C3b.
A deeper understanding of how the key complement proteins interact with NPs will also help us design new colloidal drug carriers and find efficient adjuvants for novel vaccines.
Acknowledgments
This work was supported by National Institutes of Health Grants CA119349 and EB003647, and the David Koch-Prostate Cancer Foundation Award in Nanotherapeutics. We thank Dr. Hila Epstien-Barash, Gershon Golomb and Janos Szebeni for helping us familiarize with the use of Quidel Kits.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhang L, Chan JM, Gu FX, Rhee JW, Wang AZ, Radovic-Moreno AF, et al. Self-assembled lipid-polymer hybrid nanoparticles: A robust drug delivery platform. ACS Nano. 2008;2:1696–702. doi: 10.1021/nn800275r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Law SKA, Reid KBM. Complement. United Kingdom: IRL Press Oxford; 1995. pp. 43–5. [Google Scholar]
- 3.Kishore U, Reid KB. C1q: structure, function and receptors. Immunopharmacol. 2000;49:159–70. doi: 10.1016/s0162-3109(00)80301-x. [DOI] [PubMed] [Google Scholar]
- 4.Song WC, Sarrias MR, Lambris JD. Complement and innate immunity. Immunopharmacology. 2000;49:187–98. doi: 10.1016/s0162-3109(00)80303-3. [DOI] [PubMed] [Google Scholar]
- 5.Montdargent B, Labarre D, Jozefowicz M. Interactions of functionzalized polystyrene derivatives with the complement system in human serum. J Biomater Sci Polym Ed. 1991;2:25–35. doi: 10.1163/156856291x00034. [DOI] [PubMed] [Google Scholar]
- 6.Carreno MP, Labarre D, Jozefowicz M, Kazatchkine MD. The ability of sephadex to activate human complement is suppressed in specifically substituted function. Mol Immunol. 1988;25:165–71. doi: 10.1016/0161-5890(88)90064-8. [DOI] [PubMed] [Google Scholar]
- 7.Crepon B, Maillet F, Kazatchkine MD, Jozefonvicz J. Molecular weight dependency of the acquired anticomplementary and anticoagulant activities of specifically substituted dextrans. Biomaterials. 1987;8:248–53. doi: 10.1016/0142-9612(87)90111-6. [DOI] [PubMed] [Google Scholar]
- 8.Montdargent B, Maillet F, Carreno MP, Jozefowicz M, Kazatchkine M, Labarre D. Regulation by sulphonate groups of complement activation induced by hydroxymethyl groups on polystyrene surfaces. Biomaterials. 1993;14:203–8. doi: 10.1016/0142-9612(93)90024-v. [DOI] [PubMed] [Google Scholar]
- 9.Mauzac M, Maillet F, Jozefonvicz J, Kazatchkine MD. Anticomplementary activity of dextran derivatives. Biomaterials. 1985;6:61–3. doi: 10.1016/0142-9612(85)90040-7. [DOI] [PubMed] [Google Scholar]
- 10.Carreno MP, Labarre D, Maillet F, Jozefowicz M, Kazatchkine MD. Regulation of the human alternative complement pathway: formation of a ternary complex between factor H, surface-bound C3b and Chemical groups on nonactivating surfaces. Eur J Immunol. 1989;19:2145–50. doi: 10.1002/eji.1830191126. [DOI] [PubMed] [Google Scholar]
- 11.Sadana A. Protein adsorption and inactivation on surfaces. Influence of heterogeneities Chem Rev. 1992;92:1799–818. [Google Scholar]
- 12.Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green MLH, Sim RB. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43:193–201. doi: 10.1016/j.molimm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Furtado Mosqueira VC, Legrand P, Gulik A, Bourdon O, Gref R, Labarre D, et al. Relationship between complement activation, cellular uptake and surface physicochemical aspects of novel PEG-modified nanocapsules. Biomaterials. 2001;22:2967–79. doi: 10.1016/s0142-9612(01)00043-6. [DOI] [PubMed] [Google Scholar]
- 14.Cedervall T, Lynch I, Lindman S, Berggard T, Thulin E, Nilsson H, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci USA. 2007;104:2050–5. doi: 10.1073/pnas.0608582104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blunk T, Luck M, Calvor A, Hochstrasser DF, Sanchez JC, Muller BW, et al. Kinetics of plasma protein adsorption of model particles for controlled drug delivery and drug targeting. Eur J Pharm Biopharm. 1996;42:262–8. [Google Scholar]
- 16.Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, et al. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids and Surfaces B. 2000;18:301–13. doi: 10.1016/s0927-7765(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 17.Peracchia MT, Harnisch S, Pinto-Alphandary H, Gulik A, Dedieu JC, Desmaele D, et al. Visualization of in vitro protein-rejection properties of PEGylated stealth polycyanoacrylate nanoparticles. Biomaterials. 1999;20:1269–75. doi: 10.1016/s0142-9612(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 18.Paulke BR, Moglich PM. Electrophoretic 3D-mobility profiles of latex particles with different surface groups. Langmuir. 1995;11:70–4. [Google Scholar]
- 19.AlleAaemann E, Gravel P, Leroux JC, Balant L, Gurny R. Kinetics of bloodcomponent adsorption on poly(D,L-lactic acid) nanoparticles: Evidence of complement C3 component involvement. J Biomed Mater Res. 1997;37:229–34. doi: 10.1002/(sici)1097-4636(199711)37:2<229::aid-jbm12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 20.Andersson J, Nilsson Ekdahl K, Lambris JD, Nilsson Bo. Binding of C3 fragments on top of adsorbed plasma proteins during complement activation on a model biomaterial surface. Biomaterials. 2005;26:1477–85. doi: 10.1016/j.biomaterials.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Chen S, Zheng J, Ratner BD, Jiang S. Protein adsorption on oligo(ethylene glycol)-terminated alkanethiolate self-assembled monolayers: The molecular basis for nonfouling behaviour. J Phys Chem B. 2005;109:2934–41. doi: 10.1021/jp0473321. [DOI] [PubMed] [Google Scholar]
- 22.Vittaz M, Bazile D, Spenlehauer G, Verrecchia T, Veillard M, Puisieux F, et al. Effect of PEO surface density on long-circulating PLA-PEO nanoparticles which are very low complement activators. Biomaterials. 1996;17:1575–81. doi: 10.1016/0142-9612(95)00322-3. [DOI] [PubMed] [Google Scholar]
- 23.Luck M, Paulke BR, Schroder W, Blunk T, Muller RH. Analysis of plasma protein adsorption on polymeric nanoparticles with different surface characteristics. J Biomed Mater Res. 1998;39:478–85. doi: 10.1002/(sici)1097-4636(19980305)39:3<478::aid-jbm19>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Beers MH. The merck manual of medical information. New Jersey: Whitehouse station; 2004. [Google Scholar]
- 25.Chonn A, Cullis PR, Devine DV. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146:4234–41. [PubMed] [Google Scholar]
- 26.Salvador-Morales C, Basiuk EV, Basiuk VA, Green MLH, Sim RB. Effect of covalent functionalization on the biocompatibility characteristics of multi-walled carbon nanotubes. J Nanosci Nanotechnol. 2008;8:2347–56. doi: 10.1166/jnn.2008.090. [DOI] [PubMed] [Google Scholar]
- 27.Jokiranta TS, Jaakola VP, Lehtinen MJ, Parepalo M, Meri S, Goldman A. Structure of complement factor H carboxyl-terminus reveals molecular basis of atypical haemolytic uremic syndrome. The EMBO. 2006;25:1784–94. doi: 10.1038/sj.emboj.7601052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hocking HG, Herbert AP, Kavanagh D, Soares DC, Ferreira VP, Pangburn MK, et al. Structure of the N-terminal region of complement factor H and conformational implications of disease-linked sequence variations. J Biol Chem. 2008;283:9475–87. doi: 10.1074/jbc.M709587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nielsen BL, Brown LR. The basis for colored silver-protein complex formation in stained polyacrylamide gels. Anal Biochem. 1984;141:311–5. doi: 10.1016/0003-2697(84)90047-2. [DOI] [PubMed] [Google Scholar]