Abstract
Rationale
Patterns of drug self-administration are often highly regular, with a consistent pause following each self-injection. This pausing might occur because the animal has learned that additional injections are not reinforcing once the drug effect has reached a certain level, possibly due to the reinforcement system reaching full capacity. Thus, interoceptive effects of the drug might function as a discriminative stimulus, signaling when additional drug will be reinforcing and when it will not.
Objective
To emulate this hypothetical stimulus-control aspect of drug self-administration using a schedule of food reinforcement.
Methods
Rats’ nose-poke responses produced food only when a cue light was present. No drug was administered at any time. However, the state of the light stimulus was determined by calculating what the whole-body drug level would have been if each response in the session had produced a drug injection (delivered during a 5-s timeout). The light was only presented while this virtual drug level was below a specific threshold. A range of doses of cocaine and remifentanil were emulated, using parameters based on previous self-administration experiments.
Results
Response patterns were highly regular, dose-dependent, and remarkably similar to actual drug self-administration.
Conclusion
These results support a stimulus control account of regulated drug intake, in which rats learn to discriminate when the level of drug effect has fallen to a point where another self-injection will be reinforcing.
Keywords: Self-administration, Incentive Learning, Titration, Cocaine, Remifentanil, Food
A prominent characteristic of intravenous drug self-administration is that it tends to occur in highly regular patterns. When each response delivers a fixed dose of a reinforcing drug, there tends to be a consistent pause following each injection. This phenomenon of regulated drug intake has been observed numerous times with a variety of drugs of abuse in rodents, nonhuman primates, and humans (Sughondhabirom et al. 2005), but the question of why it occurs continues to be the subject of debate (Lynch and Carroll, 2001; Panlilio et al. 2003; Tsibulsky and Norman 1999).
There is evidence that animals tend to self-administer an injection whenever the level of drug effect drops below a certain point (Yokel and Pickens 1974), termed the “satiety threshold” (Tsibulsky and Norman 1999), “trigger point” (Ranaldi et al. 1999), or “set point” (Ahmed and Koob 1998, 1999, 2005). For example, Tsibusky and Norman (1999) showed that the durations of post-injection pauses are well described by a pharmacokinetic/pharmacodynamic model in which the rat self-administers the next injection whenever its whole-body level of cocaine drops below a certain point. The terms “satiety” and “satiation,” which have been used to describe this process, imply that it might involve drive reduction, in which the motivation to receive the drug is satisfied as long as the drug level is above the threshold (Wise 1987). In this state of satiety, the need state has been removed, so the animal is no longer motivated to self-administer the drug (Ahmed and Koob 2005). However, an alternative possibility is described by the related term, “saturation” (Ranaldi et al. 1999); the reward system might become saturated when the drug level is above the threshold. In this state, the animal is still motivated, but additional drug administration is not reinforcing because the reward system has reached its full capacity.
This scenario — in which taking an additional injection is sometimes reinforcing and sometimes not — provides the basis for a stimulus control hypothesis of regulated drug intake. According to this hypothesis, the level of drug effect serves as an interoceptive discriminative stimulus that is differentially associated with reinforcement. When the drug level drops below the threshold, it signals that another injection will be reinforcing, and thereby occasions the self-administration response. When the drug level is above the threshold, it signals a period of nonreinforcement (extinction), so responding ceases. Thus, this stimulus-control hypothesis is an incentive-based account in which the animal learns to respond when it detects that the drug level has fallen below the threshold. This contrasts with a homeostasis-based drive-reduction account, in which responding ceases when drug levels are above the threshold because a need state has been satisfied.
To evaluate the stimulus-control hypothesis, we created a schedule of food reinforcement to emulate the differential reinforcement that might be inherent in drug reinforcement. To the extent that the behavior under this emulation schedule is similar to drug self-administration, the stimulus-control hypothesis is plausible as an account of regulated drug intake. This emulation procedure was accomplished by modeling the interoceptive drug-level stimulus with an exteroceptive stimulus, a cue light. Responding produced food pellets when the light was on, but not when it was off. No actual drug was administered, but each response — in both light-on and light-off periods — was recorded as producing a “virtual” drug injection. By tracking the virtual drug level throughout the session, it was possible to present the light and deliver food pellets only when the virtual drug level was below a specific threshold. This procedure was used to emulate a range of doses of cocaine and remifentanil, based on parameter values obtained in our earlier study of regulated drug intake (Panlilio et al. 2003), and the resulting dose-effect functions were subjected to the same battery of analyses used in the earlier study.
Methods
Subjects
Ten experimentally-naive, male, Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA), weighing 380–425 g, were individually housed with free access to water. Food was restricted to approximately 15 g/day to maintain stable body weights. Lights in the cage room were on from 1800–0600 hrs (reversed light cycle), and experiments were conducted between 0900–1500 hrs. The facilities were fully accredited by AAALAC, and all procedures were approved by the ACUC of the NIDA-IRP and followed the guidelines of the National Research Council (1996).
Apparatus
Ten experimental chambers, each with two nose-poke holes and a food trough, were controlled by MED-Associates (St. Albans, VT) software. These were the same chambers used for cocaine and remifentanil self-administration in our earlier study (Panlilio et al. 2003). A dim, green LED served as a houselight, and a shielded light bulb (type 1820, 24V) on the wall above the nose-poke holes served as the cue light.
Procedures
Experimental sessions were conducted 5 days/week for 2.5 hours or until 100 pellets were delivered, whichever came first. Responses in the inactive nose-poke hole had no scheduled effect. During the first two sessions, responses in the active hole immediately produced a 45-mg food pellet (F0021; Bio-Serv, Frenchtown, NJ). During these two sessions, the cue light remained on throughout the session, but responses had no scheduled effect if they followed a reinforced response by less than 5 s. All rats received 100 pellets during these initial sessions.
During all subsequent sessions, the emulation schedule was in effect. A response in the presence of the cue light caused the cue light to turn off for a 5 s timeout period, and a food pellet was delivered 2.5 s into this period. This delay between the response and delivery of the food pellet was intended to mimic the delivery of a drug injection over several seconds. No actual drug was given at any time during the study. However, each time a food pellet was delivered, the computer calculated what the drug level would have been if a drug injection had been delivered. If this virtual drug level was greater than the designated threshold, the light remained off at the end of the 5-s timeout; during this period, responses did not produce food pellets, but they increased the virtual drug level and were followed by an unsignaled 5-s “timeout” in which further responses did not increase the virtual drug level. The light was not presented again until the virtual drug level fell to the threshold. This cycle was repeated until the end of the session.
Virtual drug levels were calculated using the equation, Bn=(Bn-1 + D) · e−KT, where Bn = drug level at the current time, Bn-1 = drug level at the time of the previous calculation, D= amount of drug delivered since the previous calculation, K= elimination rate constant (halflife/0.693), and T=time since the previous calculation. During each session, there was a fixed virtual dose of cocaine (30, 100, 300, or 1000μg/kg/injection) or remifentanil (1,4,16, or 32 μg/kg/injection). Each rat was tested at each of the four virtual doses of a drug in counterbalanced order, for four consecutive sessions at each dose. Data were analyzed for the last session under each dose. During initial training, all 10 rats were trained with cocaine parameters (halflife = 492 s; threshold =1720 μg/kg) reported by Tsibulsky and Norman (1999). Then, the rats were divided into two groups of 5; one group was trained with cocaine parameters (halflife = 762 s; threshold =1190 μg/kg) and one with remifentanil parameters (halflife = 42 s; threshold = 1.01 μg/kg) reported by Panlilio et al. (2003). These parameters were derived in the previous studies through nonlinear regression based on the mean latency at each unit dose (see Tsibulsky and Norman 1999 for details). Since the major features of behavior under the emulation schedule were similar under the initial training parameters and the Panlilio et al. (2003) cocaine parameters, the initial training data are not presented here.
Data Analysis
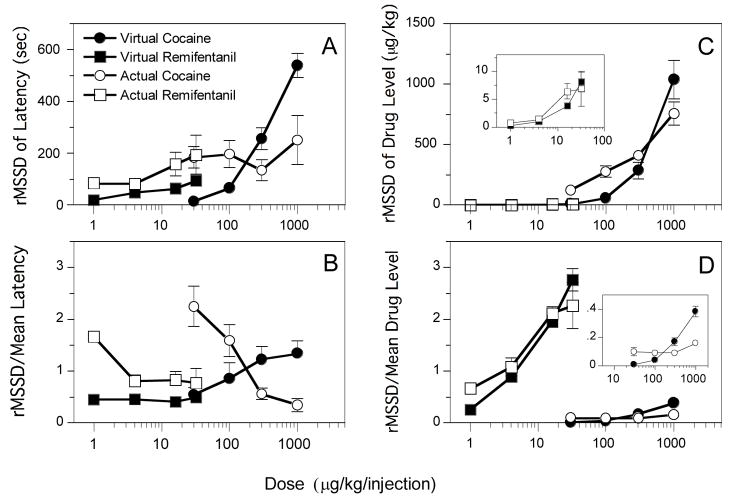
The same analyses that were applied to the data in Figs. 1–6 of the study by Panlilio et al. (2003) were applied to the data in Figs. 1–6, respectively, of the present study. For comparison, data from Panlilio et al. (2003) for the corresponding doses of actual cocaine and remifentanil are included in the present figures. Because there was a brief “loading” phase during which virtual cocaine levels steadily increased and virtual injections were spaced more closely in time than in the remainder of the session, data from the first 500 s of the session were not included in the analyses or figures, except for Figs. 1 and 3, which represent whole sessions, and Figs. 7B and C, which represent responding at the beginning of the session. All measures (except those in Fig. 7) were based on virtual injections without regard to whether a food pellet was delivered. For further discussion of the root mean square of successive deviations (rMSSD, a measure of within-subject variability), autocorrelation (a measure of how each latency is affected by the preceding latency), and whole-body drug level measures, see Panlilio et al. (2003). All figures that show group data depict mean ± s.e.m., but in many cases the error bars are covered by the symbol.
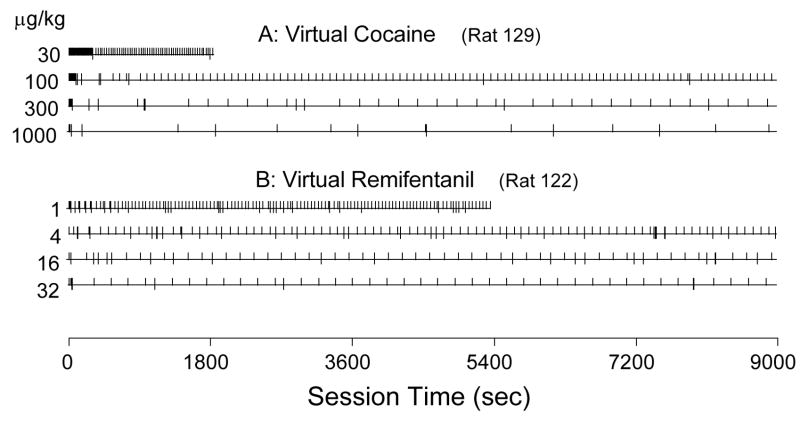
Fig. 1.
Representative event records for (A) Rat #129 under the virtual cocaine parameters; and (B) Rat #122 under the virtual remifentanil parameters. Each horizontal line represents the record for a complete session. Each vertical mark above the horizontal line represents one response that was counted as a virtual drug injection. Each vertical mark extending both above and below the line indicates that the response was counted as a virtual injection but did not produce a food pellet. At the lowest virtual dose of cocaine and remifentanil, the session was ended when 100 pellets had been delivered.
Fig. 6.
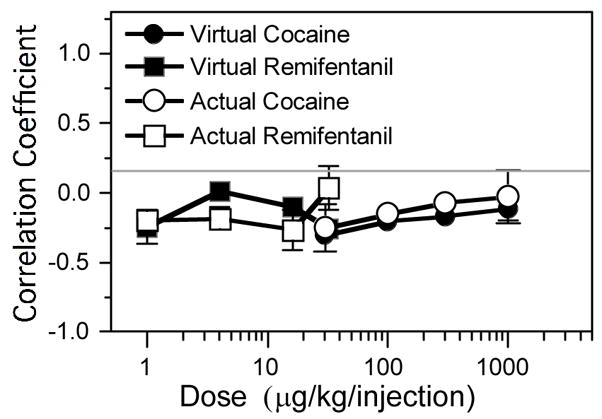
Autocorrelation of sequential latencies for actual cocaine (open circles), virtual cocaine (filled circles), actual remifentanil (open squares), and virtual remifentanil (filled squares). These values were significantly less than zero for virtual doses (μg/kg) of 30 and 100 for virtual cocaine and 1 and 32 for virtual remifentanil.
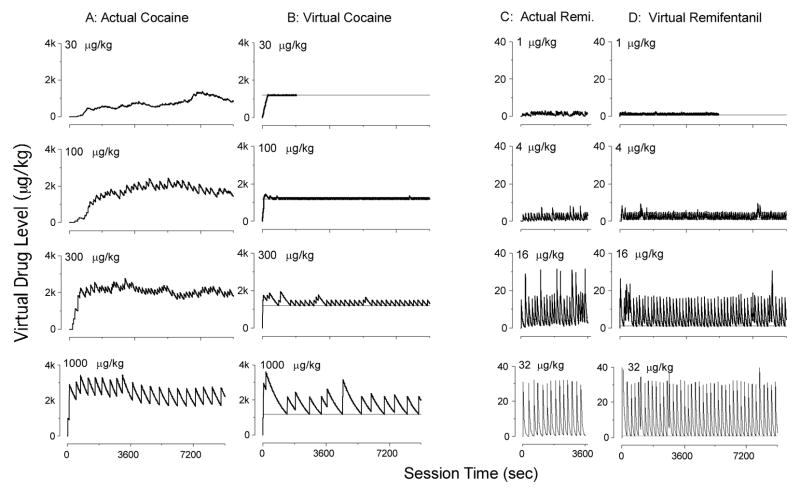
Fig. 3.
Virtual drug levels during whole sessions of (A) actual cocaine, (B) virtual cocaine, (C) actual remifentanil, and (D) virtual remifentanil self-administration. Data in B and D are from the same sessions shown in Fig. 1. Horizontal lines in B and D represent value of threshold. Sessions for actual remifentanil were limited to 3600 s. At the lowest virtual dose of cocaine and remifentanil, the session was ended when 100 pellets had been delivered.
Fig. 7.
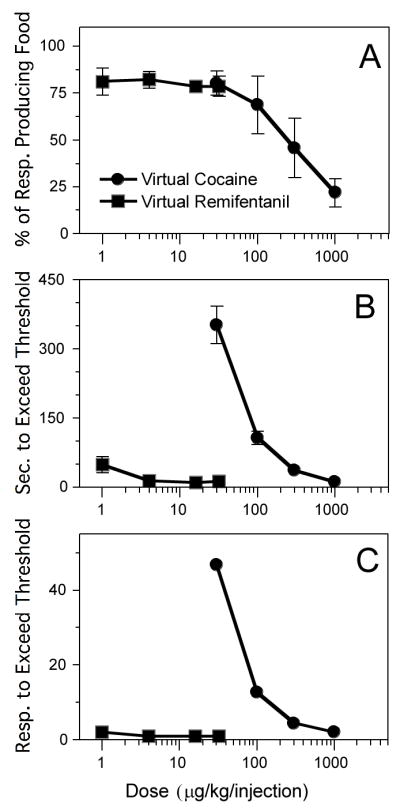
A. Percentage of responses that produced a food pellet, as a function of virtual dose (μg/kg) of cocaine (filled circles) and virtual remifentanil (filled squares). Within the virtual cocaine curve, all pairs of virtual doses were significantly different from each other except 30 vs. 100, 100 vs. 300, and 300 vs. 1000. Virtual dose did not significantly affect this measure for remifentanil. B. Number of seconds before the threshold was first exceeded in the session. Within the virtual cocaine curve, all pairs were significantly different from each other except 100 vs. 300 and 300 vs. 1000. Virtual dose did not significantly affect this measure for remifentanil. C. Number of responses before the threshold was first exceeded in the session. Within the virtual cocaine curve, all pairs were significantly different from each other. Virtual dose did not significantly affect this measure for remifentanil.
Results
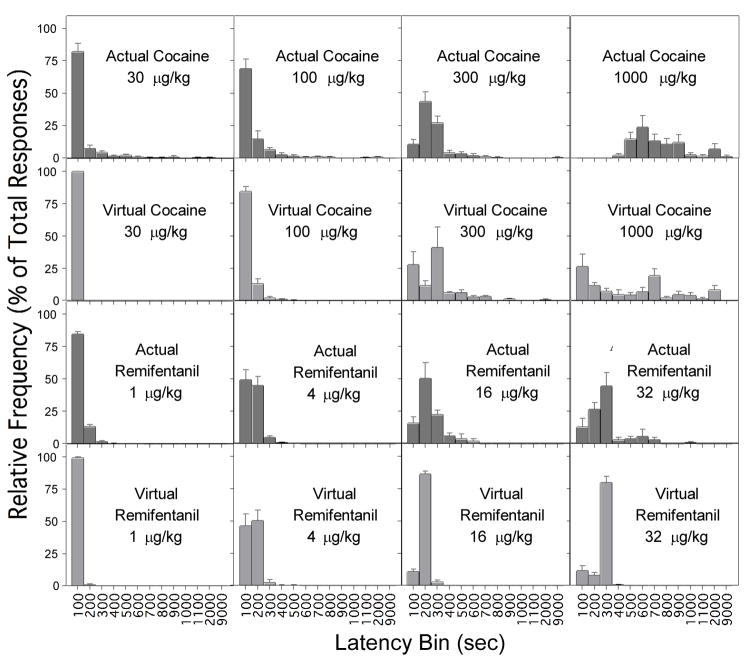
Visual inspection of event records (Fig. 1) indicated that response patterns under the emulation schedule were similar to those typically seen with actual drug self-administration, with responses tending to occur at regular temporal intervals, which were longer at higher virtual doses. As with actual drug self-administration, frequency distributions (Fig. 2) of latencies under the emulation schedule showed a well-defined peak at the lower doses and a less-defined peak, progressively further to the right, at the higher doses. A noticeable difference between actual and virtual self-administration was that the highest dose of actual cocaine produced no latencies less than 300 s, but this dose of virtual cocaine produced a bimodal distribution with one of the modes at the bin for latencies of 100 s and less.
Fig. 2.
Frequency distributions of response latencies for actual cocaine (upper row), virtual cocaine (second row), actual remifentanil (third row), and virtual remifentanil (bottom row). Data for actual cocaine and actual remifentanil shown in Figs. 2–6 are from the study of Panlilio et al. (2003).
Over the course of the session (see Fig. 3), both actual and virtual drug levels showed a loading phase for cocaine but not remifentanil. This phase was longer at the lower doses of virtual cocaine than the higher doses, but it was shorter than at the same nominal doses of actual cocaine. For both cocaine and remifentanil, once the threshold was reached, the virtual drug level did not drop substantially below the threshold for the remainder of the session. Thus, rats trained with the emulation schedule showed a “maintenance phase” like that typically observed with actual drug self-administration. More prominently than in the parallel study with actual drug reinforcement (Panlilio et al. 2003), but consistent with some other studies (e.g., Yokel and Pickens 1974; see also Ahmed and Koob 2005), higher virtual doses of cocaine tended to produce an “overshoot” of the drug level during the transition from the loading phase to the maintenance phase.
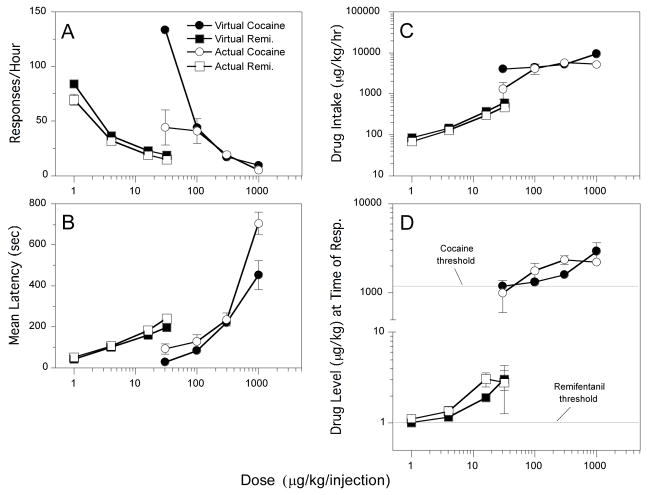
Dose-response functions (Fig. 4A) for virtual cocaine and remifentanil under the emulation schedule resemble the descending limb of curves typically obtained with actual drug self-administration. Response rates were dose-dependent for virtual cocaine [F(3,12)=1712.4, P<.0001] and virtual remifentanil [F(3,12)=686.9, P<.0001]. Latency curves (Fig. 4B) also closely resembled the typical functions obtained with actual drug self-administration, with latency increasing as a function of virtual dose for cocaine [F(3,12)=32.2, P<.0001] and remifentanil [F(3,12)=218.3, P<.0001]. Overall, these response and latency curves were remarkably similar to the actual cocaine and remifentanil curves, except for some separation at 30 and 1000 μg/kg of cocaine.
Fig. 4.
A: Dose-response functions for actual cocaine (open circles), virtual cocaine (filled circles), actual remifentanil (open squares), and virtual remifentanil (filled squares). For virtual cocaine, all pairs of doses (μg/kg) differed significantly. For remifentanil, all pairs differed significantly except 16 vs. 32. B: Response latencies. Within the virtual cocaine curve and within the virtual remifentanil curve, all pairs of doses were significantly different from each other except 30 vs. 100 and 100 vs. 300 for virtual cocaine. C: Drug intake. Within the virtual cocaine curve and within the virtual remifentanil curve, all pairs of points were significantly different from each other except 300 vs. 1000 for virtual cocaine. D: Mean drug level at the time of response. Horizontal lines represent thresholds. Within the virtual cocaine curve and within the virtual remifentanil curve, all pairs of points were significantly different from each other except 100 vs. 1000 and 300 vs. 1000 for virtual cocaine and 1 vs. 32 and 4 vs 32 for virtual remifentanil.
Rate of drug intake (Fig. 4C) was also similar for virtual and actual drug self-administration, increasing as a function of virtual dose for cocaine [F(3,12)=7.9, P<.005] and remifentanil [F(3,12)=454.6, P<.0001]. Mean virtual drug levels at the time of response (Fig. 4D) were dose dependent for cocaine [F(3,12)=5.6, P<.05] and remifentanil [F(3,12)=6.9, P<.01] and were quite similar to the data from actual cocaine and remifentanil self-administration. Panlilio et al. (2003) used this measure as an estimate of the threshold drug level for each subject at each dose. Note that under the emulation schedule, where threshold and halflife were known parameters, the average virtual drug level at the time of response was higher than the threshold set by the schedule, and this difference increased as a function of the virtual dose.
Within-subject variability of response latencies was used to measure the consistency of response patterns. As with actual drug self-administration, absolute levels of variability in latencies increased as a function of virtual dose (Fig. 5A). This measure was significantly affected by virtual dose for cocaine [F(3,12)=65.3, P<.0001] and remifentanil [F(3,12)=8.5, P<.005]. To allow direct comparisons between conditions, within-subject variability in latencies was also scaled by the mean latency (Fig. 5B). This relative variability of latencies was dose-dependent for virtual cocaine [F(3,12)=6.9, P<.01] but not for virtual remifentanil [P>.88]. Neither the virtual remifentanil nor the virtual cocaine schedules produced the high levels of variability seen with actual remifentanil and cocaine at low doses. At higher doses, the virtual remifentanil schedule produced slightly more consistent response patterns than actual remifentanil, but the virtual cocaine schedule produced less consistent patterns than actual cocaine.
Fig. 5.
Within-subject measures of variability as a function of dose of actual cocaine (open circles), virtual cocaine (filled circles), actual remifentanil (open squares), and virtual remifentanil (filled squares). A: rMSSD of latencies. Within the virtual cocaine curve and within the virtual remifentanil curve, all pairs of doses (μg/kg) were significantly different from each other except 30 vs. 100 for virtual cocaine and 1 vs. 32 and 4 vs. 32 for virtual remifentanil. B: rMSSD of latencies scaled by mean latency. Within the virtual cocaine curve, all pairs were significantly different from each other except 30 vs. 300 and 300 vs 1000 for virtual cocaine. Dose did not significantly affect this measure for virtual remifentanil. C: rMSSD of drug level (μg/kg) at the time of response. Inset shows results for remifentanil with the y-axis expanded. Within the virtual cocaine curve and within the virtual remifentanil curve, all pairs of points were significantly different from each other except 30 vs. 100 and 30 vs. 300 for virtual cocaine and 1 vs. 4 and 4 vs. 16 for virtual remifentanil. D: Within-subject rMSSD of drug level at the time of response scaled by the mean latency. Inset shows results for cocaine with the scale of the y-axis expanded. Within the virtual cocaine curve and within the virtual remifentanil curve, all pairs of points were significantly different from each other except 30 vs. 100 for cocaine.
Within-subject variability of virtual drug levels at the time of response were also analyzed. As with actual drugs, absolute variability of this measure (Fig. 5C) increased as a function of dose for virtual cocaine [F(3,12)=39.1, P<.0001] and virtual remifentanil [F(3,12)=18.3, P<.0001], and this was also true of relative variability (Fig. 5D) for both virtual cocaine [F(3,12)=50.5, P<.0001] and virtual remifentanil [F(3,12)=62.9, P<.0001]. For both the absolute and relative variability measures of drug level at the time of response, the values for actual and virtual drugs were similar, except for the relative measure at the highest dose of cocaine. As with actual drug self-administration, (1) absolute variability of the drug level at the time of response was higher for virtual cocaine than virtual remifentanil, but (2) relative variability of this measure was lower for virtual cocaine than virtual remifentanil.
Autocorrelations (Fig. 6) were performed on sequential latencies to determine whether the latency of a given response was affected by the latency of the previous response. This measure was used to determine whether sequential latencies were adjusted in a way that compensated for deviation of previous latencies from the average; this would be indicated by a negative correlation. As with actual drug self-administration, the correlations for cocaine and remifentanil were near zero or slightly negative. Unlike food-reinforced responding under a non-emulation schedule (Panlilio et al. 2003), none of the correlations in the present study were significantly positive.
About 80% of responses were effective in producing food pellets under all doses of virtual remifentanil (Fig. 7A). In contrast, the longer halflife of virtual cocaine made the requirements for obtaining food more stringent, with only 47 and 36% of responses producing food at the two highest doses, respectively. The percentage of responses that produced food was dose dependent for virtual cocaine [F(3,12)=12.0, P<.005], but not virtual remifentanil [P>.96]. The amount of time before the threshold was first surpassed within the session (Fig. 7B) was also dose dependent for virtual cocaine [F(3,12)=53.8, P<.0001], but not for remifentanil [P>.2]. The number of responses before the threshold was first surpassed within the session (Fig. 7C) was dose dependent for virtual cocaine [F(3,12)=1779.2, P<.0001]. For remifentanil, the threshold was always surpassed with 2 responses at the lowest virtual dose and 1 response at all other doses. This was because the ratio of unit dose to threshold was high for virtual remifentanil (ranging from 0.99–31.7), allowing the threshold to be rapidly exceeded. In contrast, this ratio was low for virtual cocaine (ranging from 0.03–0.84), requiring more time and responses to exceed the threshold during the loading phase.
Discussion
Virtual vs. actual drug self-administration
According to a stimulus control account of regulated drug intake, the animal responds when the level of interoceptive drug effect signals that another injection will be reinforcing. When this situation was emulated with a schedule of virtual drug self-administration, patterns of responding were strikingly similar to those obtained earlier with actual cocaine and remifentanil self-administration (Panlilio et al. 2003). When the same comprehensive battery of analyses was applied to virtual and actual drug self-administration, there were only a few differences.
Unlike actual drug self-administration, the emulation schedule did not produce an ascending limb in the dose-response curve, and the lowest dose of virtual cocaine produced much higher response rates than the same nominal dose of actual cocaine (Fig. 4A). The emulation schedule also did not replicate the high levels of variability in response patterns at lower doses of actual cocaine and remifentanil (Fig. 5B). These discrepancies are probably attributable to the fact that — in order to focus on the effects of stimulus control — no attempt was made to model reinforcement magnitude in the emulation schedule. The fact that low unit doses of actual drug are less reinforcing than higher doses probably accounts for the relatively low response rates and increased variability of response latencies seen at low doses with actual drug self-administration.
Another difference relates to responding under the highest dose of cocaine. At this dose, there were no response latencies less than 300 s under the actual cocaine schedule, but there were many latencies less than 300 s under the emulation schedule (Fig. 2). This difference led to lower mean response latencies (Fig. 4B) and higher levels of variability in response patterns (Fig. 5B) for virtual cocaine at this dose compared to actual cocaine. The lack of latencies less than 300 s following the highest dose of actual cocaine might be due to (1) the rats having learned to avoid aversively high drug levels; or (2) i.v. cocaine eliciting unconditioned responses, such as running and jumping (Tella 1996), that could disrupt ongoing operant responding (Pickens and Thompson 1968; Schindler et al. 2000). These aversive or disruptive effects might explain the cessation of responding immediately after the injection of a high dose, even though disruptive effects do not adequately account for the entire post-injection pause (as demonstrated by rats’ ability to perform intracranial self-stimulation during the post-injection pause; Wise et al. 1977).
Underlying vs. observed threshold
With the emulation schedule, the average drug level at the time of response (i.e., the observed threshold) was greater than the threshold set by the schedule, and this discrepancy increased as a function of the unit dose. This fact suggests that if there is truly an underlying threshold in actual drug self-administration studies, then it is lower than the observed threshold. The reason for this discrepancy is the tendency to sometimes respond too early, while the virtual drug level is still above threshold (see Figs. 1, 3, and 7A). This phenomenon is probably related to the early responding that occurs in other temporal reinforcement schedules, even when it delays or prevents reinforcement (e.g., Marcucella 1974). The factors that influence this early responding may contribute to the addiction-like dysregulation of drug intake that can occur when animals are given extended access to self-administered drugs (e.g., see Ahmed and Koob 1998, 1999; see also Panlilio et al. 2006). Such dysregulation has been attributed to a shift in the underlying threshold (Ahmed and Koob 2005), but the stimulus control hypothesis suggests that dysregulation may be due to an increased discrepancy between the underlying and observed thresholds when responding becomes more habitual, less sensitive to interoceptive cues, or more sensitive to environmental cues.
Cocaine vs. remifentanil
With actual drug self-administration, the observed threshold was more precisely regulated with cocaine than with remifentanil (Panlilio et al. 2003; see also Zernig et al. 2007). This difference — which also occurred under the emulation schedule (Fig. 5D) — was surprising in the original study because the ultra-short halflife of remifentanil was specifically designed to allow precise titration of effect in the operating room. However, an explanation for this unexpected difference is suggested by (1) the high incidence of early responses under the emulation schedule; and (2) the fact that the rapid elimination of remifentanil allows safe unit doses to be many times higher than the threshold. When a response is made at the threshold, the threshold is surpassed by one unit dose. Thus, with remifentanil, there is a wider range of drug levels that must be passed through before the level returns to the threshold. This allows a wider range of possible values at which early responses can occur, increasing the variability of the observed threshold.
Nature of the discriminative stimulus
It is well established that the interoceptive discriminative-stimulus properties of drugs can control operant responding maintained by non-drug reinforcers in rats (Colpaert 1999) and that exteroceptive discriminative stimuli such as a cue light can exert precise control over drug self-administration responding (Panlilio et al. 1996, 1998, 2000b; Weiss et al. 2003). Therefore, it is reasonable to expect that interoceptive effects of self-administered drugs can control the self-administration response. Unfortunately, the specific mechanisms (e.g., receptors, sites, etc.) that underlie this stimulus control are not known (see Crespo et al. 2006). This process could involve direct detection or sensing of saturation of the reward system, or it could be based on secondary, correlated effects. For the sake of simplicity, the emulation schedule used here was based on a general pharmacokinetic model that makes few assumptions. But, it should be noted that the general emulation procedure can easily be modified to test specific hypotheses regarding mechanisms, as long as the pharmacokinetic/pharmacodynamic parameters can be specified.
The cue light of the present study differed from an actual drug cue in that the light was varied in a quantal fashion (on vs. off), but drug levels vary continuously. However, there is substantial evidence that the discriminative stimulus effects of drugs can be treated as a quantal dimension by the animal (Colpaert 1991, 1999). That is, different drug levels may be perceived as qualitatively different, much like we perceive the continuous visual spectrum as a set of qualitatively different colors. This possibility seems especially likely when considering values near a threshold. Thus, it is possible that levels of drug effect just below the threshold are perceived as being “off”, but values above the threshold are detected as “on.” This possibility, along with the similarity of the behavior under the virtual and actual drug schedules, suggests that the light cue in the emulation schedule provides an adequate model of an actual drug cue.
Drive reduction vs. incentive learning
According to a drive reduction hypothesis, the animal automatically stops seeking a reinforcer with which it has been sated. The non-emulation food schedule used by Panlilio et al. (2003) can be considered a test of whether drive reduction can produce regulated responding like that typically observed with drug self-administration. Rats were trained with a simple schedule in which the amount of food per reinforcement was varied between sessions (20–180 mg/delivery for 2.5 hr/session). These conditions did not produce a maintenance phase like that seen with drug self-administration. Over 90% of response latencies were 100 s or less, even under the largest reinforcer sizes, and there were no pauses lasting more than a few seconds until several hundred pellets had been received. Thus, food satiation did not produce regulated responding, even when the effects of satiation were not minimized as in the present study (where 45 mg food pellets were used at all virtual doses and the total number of pellets was limited to 100/session).
The ability of the emulation schedule to readily produce drug-like response patterns supports the stimulus control hypothesis. According to this hypothesis, drug seeking stops when the drug level is above threshold because the animal has learned that additional drug will not be reinforcing (i.e., that its incentive value is reduced). This hypothesis is consistent with research demonstrating “instrumental incentive learning” (Dickinson and Balleine 1994; Hutcheson et al. 2001). For example, Balleine (1992) showed that food satiation does not automatically decrease the rate of an operant response that was previously reinforced with food pellets. Satiation only reduces responding if the rat has previously experienced the pellets in a non-deprived state. Thus, the level of deprivation may come to serve as an interoceptive cue that signals when food will and will not be reinforcing.
Implications and further applications
One implication of the stimulus control hypothesis is that injections that occur when the drug level is already above threshold probably do not extend a period of “satisfaction,” but rather extend a period of non-reinforcement (extinction). In other words, maintaining the level above threshold is not in itself reinforcing or the goal of self-administration. Although it may be counterintuitive, this possibility is consistent with evidence that the onset of drug effect is critical for drug reinforcement, but duration of effect has little effect on reinforcement efficacy (Ko et al. 2002; Panlilio et al. 1998, 2000a).
The stimulus control hypothesis may complement other threshold-based models of regulated drug intake. For example, it could provide a more thorough account of how regulated drug intake is established and maintained within the model of Tsibulsky and Norman (1999; Norman and Tsibulsky 2006). The stimulus control hypothesis might also be incorporated into the model of Ahmed and Koob (2005), which attributes addiction-related increases in drug intake to alterations in the responsivity of the reward system. Although Ahmed and Koob (2005) assume the subject actively seeks a specific level of reward system responsivity and that this set point changes in addicted individuals, this phenomenon could potentially be described in terms of stimulus control.
Finally, the emulation schedule may be useful as a control condition for studies involving actual drug self-administration. Food-based control conditions have often been used to determine whether an experimental treatment selectively alters drug self-administration. However, a perennial problem is that food typically maintains much higher response rates than drug reinforcers when given under (ostensibly) the same schedule. The present results suggest that a simple food schedule is not ideal as a control condition for a drug reinforcement because it does not involve the same dynamics of differential reinforcement and stimulus control. In contrast, the emulation schedule could replicate both the response patterns and dynamics of actual drug self-administration.
Conclusion
Response patterns under the emulation schedule closely mirrored those of actual drug self-administration. Only a few differences were observed, and these can be explained in a straightforward manner. This similarity suggests that the reinforcement contingencies explicitly imposed by the emulation schedule might provide a reasonably accurate model of the contingencies that are implicit in actual drug self-administration.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute on Drug Abuse. Thanks to Jonathan Katz, who commented on the manuscript, and to Roy Wise, Serge Ahmed, and Vladimir Tsibulsky for sharing their views of regulated drug intake.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Transition to drug addiction: a negative reinforcement model based on an allostatic decrease in reward function. Psychopharmacology. 2005;180:473–90. doi: 10.1007/s00213-005-2180-z. [DOI] [PubMed] [Google Scholar]
- Crespo JA, Panlilio LV, Schindler CW, Sturm K, Saria A, Zernig G. Peri-response pharmacokinetics of remifentanil during a self-administration session indicates that neither blood nor brain levels are titrated. Ann N Y Acad Sci. 2006;1074:497–504. doi: 10.1196/annals.1369.050. [DOI] [PubMed] [Google Scholar]
- Balleine B. Instrumental performance following a shift in primary motivation depends on incentive learning. J Exp Psychol Anim Behav Process. 1992;18:236–50. [PubMed] [Google Scholar]
- Colpaert FC. The discriminative response: an elementary particle of behavior. Behav Pharmacol. 1991;2:283–286. [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Anim Learn Behav. 1994;22:1–18. [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4:943–7. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Ko MC, Terner J, Hursh S, Woods JH, Winger G. Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther. 2002;301:698–704. doi: 10.1124/jpet.301.2.698. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Exp Clin Psychopharmacology. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for care and use of laboratory animals. National Academy Press; Washington: 1996. [Google Scholar]
- Marcucella H. Signalled reinforcement in differential-reinforcement-of-low rate schedules. J Exp Anal Behav. 1974;22:381–390. doi: 10.1901/jeab.1974.22-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology. 1998;137:253–8. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Katz JL, Pickens RW, Schindler CW. Variability of drug self-administration in rats. Psychopharmacology. 2003;167:9–19. doi: 10.1007/s00213-002-1366-x. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. Cocaine self-administration under variable-dose schedules in squirrel monkeys. Pharmacol Biochem Beh. 2006;84:235–243. doi: 10.1016/j.pbb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Cocaine self-administration increased by compounding discriminative stimuli. Psychopharmacology. 1996;125:202–208. doi: 10.1007/BF02247329. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Motivational effects of compounding discriminative stimuli associated with food and cocaine. Psychopharmacology. 1998;136:70–4. doi: 10.1007/s002130050540. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW. Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology. 2000a;150:61–6. doi: 10.1007/s002130000415. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Effects of compounding drug-related stimuli: Escalation of heroin self-administration. J Exp Anal Behav. 2000b;73:211–224. doi: 10.1901/jeab.2000.73-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens R, Thompson R. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Ranaldi R, Pocock D, Zereik R, Wise RA. Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous D-amphetamine self-administration. J Neurosci. 1999;19:4102–4109. doi: 10.1523/JNEUROSCI.19-10-04102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Ma JD, Goldberg SR. Conditioned suppression with cocaine as the unconditioned stimulus. Pharmacol Biochem Behav. 2000;65:83–9. doi: 10.1016/s0091-3057(99)00176-8. [DOI] [PubMed] [Google Scholar]
- Sughondhabirom A, Jain D, Gueorguieva R, Coric V, Berman R, Lynch WJ, Self D, Jatlow P, Malison RT. A paradigm to investigate the self-regulation of cocaine administration in humans. Psychopharmacology. 2005;180:436–46. doi: 10.1007/s00213-005-2192-8. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: A quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Weiss SJ, Kearns DN, Cohn SI, Schindler CW, Panlilio LV. Stimulus control of cocaine self-administration. J Exp Anal Behav. 2003;79:111–35. doi: 10.1901/jeab.2003.79-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Intravenous self-administration: a special case of positive reinforcement. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag; New York: 1987. pp. 117–141. [Google Scholar]
- Wise RA, Yokel RA, Hansson PA, Gerber GJ. Concurrent intracranial self-stimulation and amphetamine self-administration in rats. Pharmacol Biochem Behav. 1977;7:459–6. doi: 10.1016/0091-3057(77)90214-3. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Pickens RW. Drug level of d- and l-amphetamine during intravenous self-administration. Psychopharmacologia. 1974;34:255–264. doi: 10.1007/BF00421966. [DOI] [PubMed] [Google Scholar]
- Zernig G, Ahmed SH, Cardinal RN, Morgan D, Acquas E, Foltin RW, Vezina P, Negus SS, Crespo JA, Stockl P, Grubinger P, Madlung E, Haring C, Kurz M, Saria A. Explaining the escalation of drug use in substance dependence: models and appropriate animal laboratory tests. Pharmacology. 2007;80:65–119. doi: 10.1159/000103923. [DOI] [PubMed] [Google Scholar]