Abstract
Objective
To investigate the association between hyperprolactinemia and variants of the dopamine D2 receptor (DRD2) gene in children and adolescents in long-term treatment with risperidone.
Methods
Medically healthy 7–17-year-old patients chronically treated with risperidone but receiving no other antipsychotics were selected for a cross-sectional evaluation. Four DRD2 variants were genotyped and prolactin concentration was measured. Medication history was obtained from the medical record. The effect of the TaqIA variants of the DRD2 on the risk of risperidone-induced hyperprolactinemia was the primary outcome measure.
Results
Hyperprolactinemia was present in 50% of 107 patients (87% males) treated with risperidone for an average of 2.9 years. Age, stage of sexual development, and the dose of risperidone independently predicted a higher prolactin concentration, whereas the dose of psychostimulants was negatively correlated with it. However, these four predictors became nonsignificant when risperidone serum concentration was entered into the model. Adverse events potentially related to hyperprolactinemia were more common in participants with elevated prolactin concentration in girls (45%) compared with boys (10%). After controlling for risperidone concentration and the dose of psychostimulants, the TaqIA A1 and the A-241G alleles were associated with higher prolactin concentration, whereas the -141C Ins/Del AQ1and C957T variants had no significant effect. In addition, adverse events potentially related to hyperprolactinemia were four times more common in TaqIA A1 allele carriers.
Conclusion
Prolactin concentration is closely related to central DRD2 blockade, as reflected by risperidone serum concentration. Furthermore, the TaqIA and A-241G variants of the DRD2 gene could be useful in predicting the emergence of hyperprolactinemia and its potential adverse events.
Keywords: adolescents, antipsychotics, children, dopamine receptors, DRD2, genetic variants, hyperprolactinemia, risperidone, TaqIA
Introduction
Antipsychotic medications induce hyperprolactinemia by blocking the dopamine D2 receptors (DRD2) in the anterior pituitary [1,2]. This, in turn, can cause galactorrhea and suppress gonadotropin production, leading to amenorrhea, decreased libido, and infertility [2]. Acutely, these adverse events (AEs) potentially interfere with medication adherence and quality of life. Over more extended treatments, hyperprolactinemia may lead to decreased bone mineral density[1-3].
Risperidone is one of the antipsychotics most likely to cause hyperprolactinemia with up to 71% of the children developing this AE acutely and more than 30% continuing to have it, a year or more later [4,5].
In adults, sex, menopausal status, and antipsychotic type and dose are associated with prolactin concentration [2]. In addition, genetic factors, such as variants of the DRD2 gene, seem to influence the propensity for antipsychotic-induced hyperprolactinemia. For example, in women with schizophrenia treated with nemonapride or bromperidol, the A1 allele of the TaqIA variant of the DRD2 gene was associated with a higher prolactin concentration and with a higher ratio of change in prolactin by medication serum concentration [6,7]. Similarly, Young et al. [8] described higher prolactin concentration in TaqIA A1 allele carriers receiving antipsychotics.
The interest in the DRD2 gene stems from the identification of several variants that might alter its function. For example, neuroimaging and postmortem receptor binding studies have revealed that carriers of the TaqIA A1 allele have a lower DRD2 density in the basal ganglia [9-11]. Furthermore, this allele results in a glycine to lysine substitution in a gene located 9.5λkb downstream of the DRD2 gene and is associated with increased striatal L-amino acid decarboxylase activity [12,13]. In addition, the C957T and -141C Ins/Del represent other DRD2 gene variants linked to decreased messenger RNA stability and DRD2 synthesis or to reduced gene transcription, respectively [14,15]. Thus, findings across different lines of research converge to suggest a functional role for several DRD2 variants. This, in turn, might translate into a differential propensity for response to treatment or emergence of AEs.
In this study, we evaluated the association between the TaqIA, C957T, -141C Ins/Del, and A-241G variants of the DRD2 gene and prolactin concentration in youths treated chronically with risperidone. As studies have most consistently linked the TaqIA variants to alteration in DRD2 density and to antipsychotic-induced hyperprolactinemia in adults with psychosis, our primary hypothesis was that hyperprolactinemia would be more common in the A1 allele carriers. We aimed to replicate and extend findings from studies conducted in adults because of the differential drug metabolism and tolerability of children and adolescents, the active brain development during growth, and the incomplete maturation of the dopaminergic system and its potential susceptibility to long-lasting disruption after a brief postnatal exposure to drugs [16,17]. Finally, the downstream effect of hyperprolactinemia on bone mineralization in children and adolescent might be more dramatic, compared with adults, and possibly irreversible [18,19].
Patients and methods
Participants
Participants, 7–17 years old, treated with risperidone for 6 months or more, irrespective of diagnosis or indication, were selected from psychiatric outpatient clinics. Concomitant treatment with psychotropic medications, such as psychostimulants and antidepressants, was allowed except for antipsychotics other than risperidone. Medical conditions that could interfere with normal pituitary function led to exclusion as did metabolic, autoimmune, and hormonal diseases, except for minor thyroid stimulating hormone elevation [20]. Pregnant females and those receiving hormonal contraception were not eligible.
Procedures
This study was approved by the local institutional review board. Assent was obtained from children and consent from adolescents and all parents or legal guardians.
Race and ethnicity were based on self-report and the psychiatric diagnoses on chart review. Treatment information was extracted from the medical and psychiatric records. All dosages of psychostimulants were expressed in methylphenidate equivalents for amphetamines (x2) [21]. To evaluate the validity of the psychiatric diagnoses made by the participants’ mental healthcare providers, a best-estimate diagnosis was generated in 49 participants based on a combination of a review of the psychiatric record and a standardized interview of the parent and the youth (if 11 years old or older) using the National Institute of Mental Health Diagnostic Interview Schedule for Children [22]. Agreement between the clinical diagnosis and the research-generated best-estimate diagnosis was compared, using κ statistic [23], for five diagnostic categories: attention deficit hyperactivity disorder, disruptive behavior disorder, pervasive developmental disorder, tic disorder, and mood disorder. The κ values were 0.63, 0.66, 0.73, 0.61, and 0.51, respectively, reflecting moderate to substantial agreement. The lower agreement for mood disorders likely reflects the transient nature of this disorder, in contrast to the others, making it less likely for the symptoms to remain present upon selection.
Pubertal stage was evaluated by a physical exam and self-assessment [24]. During this exam, before any laboratory results became available, the participants and their parent(s) were queried about the presence of AEs potentially related to hyperprolactinemia (Table 1).
Table 1.
Adverse events potentially related to hyperprolactinemia induced by risperidone
| Boys (n=88) | Girls (n=11) | Tanner stages n at I/II/III/IV/V |
A1 carrier statea (%) |
Hyperprolactinemiab (%) |
|
|---|---|---|---|---|---|
| λGynecomastia, n (%) |
7 (8) | — | 0/2/1/2/2 | 100 | 100 |
| Breast tenderness |
3 (3) | 2 (22) | 2/1/1/1/0 | 80 | 100 boys, 0 girls |
| Galactorrhea | 0 | 1 (11) | 0/0/0/0/1 | 0 | 100 |
| Menarche | — | 4 (44) | 0/0/1/2/1 | 25 | 75 |
| Irregular cycles | — | 3 (33) | 0/0/1/1/1 | 33 | 67 |
| Amenorrhea | — | 0 | 0/0/0/0/0 | 0 | 0 |
| Menorrhagia | — | 0 | 0/0/0/0/0 | 0 | 0 |
| Oligomenorrhea | — | 2 (22) | 0/0/0/1/1 | 0 | 100 |
| ≥1 Adverse events |
9 (10) | 5 (56) | 2/2/2/4/4 | 71 | 100 boys, 60 girls |
A1 carrier state reflects the percentage of patients with each of the adverse events who carried at least one A1 allele of the TaqIA variants.
Hyperprolactinemia reflects the percentage of patients with each of the adverse events who also had hyperprolactinemia, defined as a prolactin level of greater than 18.4λng/ml in males and greater than 24.1λng/ml in females.
In 83% of the participants, a morning fasting blood sample was obtained to measure thyroid stimulating hormone, prolactin, and trough risperidone and 9-OH-risperidone serum concentrations (referred to as risperidone concentration, henceforth). In the remaining participants, a nonfasting blood sample was collected. Prolactin was measured by electrochemiluminescence immunoassay. On the basis of the upper range of normal of this assay, hyperprolactinemia was defined as a prolactin level of greater than 18.4λng/ml in males and greater than 24.1λng/ml in females. In our work, reported elsewhere [3], we have found prolactin concentrations above these cutoffs to negatively affect bone mineral density in boys in this sample.
Genotyping for the TaqIA, C957T, -141C Ins/Del, and A-241G variants of the DRD2 gene was done using polymerase chain reaction (PCR) and sequencing primers that were designed with Pyrosequencing SNP Primer Design Version 1.01 software (http://www.pyrosequencing.com). PCR reactions were done in a 30λμl volume with 1.5λmmol/l Mg2+, 10λpmol of each forward and reverse primer, according to the following specifications: initial denaturation time of 5λmin at 95°C followed by 45 cycles of 95°C for 30λs, primer specific annealing temperatures for 30λs, 72°C for 30λs, and a final extension of 10λmin at 72°C. Genotyping was done with Pyrosequencing Technology [25]. PCR products were visualized by electrophoresis on 1.8% agarose gels stained with ethidium bromide before pyrosequencing.
Statistical analysis
Prolactin values were log transformed to normalize the data residuals. InitiallyAQ2, multiple linear regression was used to investigate, in the total sample (n=107), whereas age, stage of sexual development, sex, treatment with selective serotonin reuptake inhibitors (SSRIs), dose of psychostimulants and risperidone per kilogram of body weight, and duration of risperidone treatment independently predicted prolactin concentration. Then, to investigate the role of the different DRD2 variants in risperidone-induced hyperprolactinemia, we entered each genetic group, individually, in the model while controlling for the significant covariates identified in the initial step. Finally, we used logistic regression to test for the association of hyperprolactinemia, defined categorically as described earlier, with the TaqIA A1 carrier state, controlling for the same significant independent covariates [26]. The adjusted odds ratios (OR) for hyperprolactinemia along with 95% confidence intervals (CI) were then computed.
Owing to racial/ethnic differences in the distribution of the DRD2 variants [15], we restricted the genetic analysis to nonHispanic Caucasians (n=90). The homozygous wild genotypes for the TaqIA, -141 Ins/Del, and A-241G variants were uncommon (≤3%, see Results). Consequently, a recessive mode of inheritance was used in the analyses. In contrast, both a recessive and a codominant mode were tested for the C957T variant.
As our primary hypothesis was concerned with the TaqIA variants of the DRD2 gene, we divided the sample based on A1 allele carrier state. Though not statistically significant, fewer TaqIA A1 allele carriers received psychostimulants (Table 2), which can suppress prolactin release because of their dopamine-agonist activity [27]. To test whether this potential confound affected our results, we matched the two TaqIA genetic groups on the number of patients treated with psychostimulants and, in those treated, on the daily dose of psychostimulants per kilogram of body weight.
Table 2.
Demographic, clinical, and laboratory characteristics of nonHispanic Caucasians in the two TaqIA genetic groups
| Characteristics | A1A1/A1A2 genotypes (N=33) |
A2A2 genotype (N=57) |
Statistical analysis | P value |
|---|---|---|---|---|
| λAge, mean±SD, years |
12.5±2.5 | 11.8±3.0 | t=0.6, d.f.=88 | P=0.5 |
| Male, n (%) | 30 (91) | 50 (88) | Fisher’s Exact | P=0.7 |
| Pubertal status, % at Tanner stage I, II, III, IV, V |
27/21/12/27/12 | 35/19/9/26/11 | Wilcoxon S=1571 | P=0.5 |
| Psychiatric diagnoses |
||||
| Attention deficit hyperactivity disorder, n (%) |
23 (70) | 53 (93) | Fisher’s Exact | bP<0.006 |
| Disruptive behavior disorder, n (%) |
21 (64) | 37 (65) | Fisher’s Exact | P=1.0 |
| Mood disorder, n (%) |
9 (27) | 13 (23) | Fisher’s Exact | P=0.6 |
| Anxiety disorder, n (%) |
11 (33) | 22 (39) | Fisher’s Exact | P=0.7 |
| Tic disorder, n (%) |
8 (24) | 10 (18) | Fisher’s Exact | P=0.6 |
| Pervasive developmental disorder, n (%) |
8 (24) | 11 (19) | Fisher’s Exact | P=0.6 |
| Psychotic disorder, n (%) |
1 (3) | 1 (2) | Fisher’s Exact | P=1.0 |
| Pharmacotherapy | ||||
| Risperidone dose, median (quartiles), (mg/kg/d) |
0.03 (0.02–0.04) | 0.02 (0.01–0.04) | Wilcoxon S=1658 | P=0.2 |
| Risperidone treatment duration, median (quartiles), years |
3.1 (1.5–5.0) | 2.9 (1.2–3.9) | Wilcox on S=1575 | P=0.5 |
| Risperidone level, median (quartiles), ng/ml |
8.3 (6.2–16.8) | 7.3 (4.3–14.1) | Wilcoxon S=1281 | P=0.2 |
| Psychostimulants, n (%) |
17 (52) | 40 (70) | Fisher’s Exact | cP=0.1 |
| Psychostimulants dose, mean±SD , (mg/kg/d) |
1.4±0.6 | 1.2±0.4 | t=1.8, d.f.=55 | cP=0.08 |
| Prolactin concentration |
||||
| Prolactin, median (quartiles), ng/ml |
24.0 (17.8–37.8) | 17.0 (12.8–26.1) | Wilcoxon S=1769 | bP=0.03 |
| Elevated prolactin, n (%)a |
22 (67) | 24 (42) | Fisher’s Exact | bP=0.03 |
| Prolactin >25.7 λng/ml, n (%) |
16 (49) | 15 (26) | Fisher’s Exact | bP=0.04 |
Defined as a prolactin level of greater than 18.4λng/ml in males and greater than 24.1λng/ml in females.
Statistically significant findings.
Statistically significant events.
Differences across the two TaqIA genetic groups were compared using Student’s t-test for continuous variables and Fisher’s Exact test for categorical ones. The Wilcoxon’s rank sum test was used whenever the t-test assumption of normality was violated. The McNemar’s test was used to compare the rate of hyperprolactinemia and the Wilcoxon’s signed rank test to compare prolactin concentration across the two matched TaqIA genetic groups. All tests were two tailed with the statistical significance set at α=0.05. All analyses were conducted using SAS version 9.1.3 (SAS Institute Inc, Cary North Carolina, USA).
Results
A total of 109 participants were enrolled. Two boys, one from each TaqIA genetic group, had a risperidone concentration less than 0.5λng/ml and were excluded from further analyses because of suspected medication nonadherence. In the remaining 107 participants (87% males), although externalizing disorders were the most prevalent psychiatric conditions, comorbidity was common (Table 2). Risperidone was administered to target irritability and aggression in 76% (n=81) of the sample, tic disorder in 10% (n=11), severe impulsivity in 8% (n=8), sleep problems in 3% (n=3), obsessive compulsive disorder in 2% (n=2), and mood symptoms in one child. The dose prescribed is consistent with published trials [28]. In addition to risperidone, participants were most often taking psychostimulants (65%), α2-agonists (52%), and SSRIs (32%), concurrently.
The median prolactin level was 18.7λng/ml (interquartile range: 13.4–28.5) with 50% of the participants exhibiting hyperprolactinemia as defined earlier. With a cutoff of 25.7λng/ml, regardless of sex, age, or pubertal status [29], 34% (n=36) of the sample had hyperprolactinemia.
Using multiple linear regression, we tested whether stage of sexual development, sex, SSRI treatment, dose of psychostimulants and risperidone per kilogram of body weight, or duration of risperidone treatment were associated with prolactin concentration in the entire sample (n=107). We found that the dose of risperidone (partial R2=0.1, P=0.0009) and Tanner stage (partial R2=0.07, P<0.003) positively predicted prolactin concentration, whereas the dose of psychostimulants (partial R2=0.04, P<0.03) was negatively associated with it. None of the other covariates were significant. When age was substituted for Tanner stage, comparable results were found with age accounting for 3.5% of the variance in prolactin concentration (partial R2=0.035, P<0.04). When risperidone concentration was substituted for the dose, it accounted for 39% (P<0.0001) of the variance in prolactin concentration with Tanner stage (or age) and the dose of psychostimulants becoming nonsignificant. Similar results were found when these analyses were restricted to males (females were a small proportion of the total sample).
As illustrated in Table 1, of 99 participants queried, 14% reported AEs potentially related to hyperprolactinemia. Of the 11 queried girls, five (45%) reported at least one AE compared with 10% (n=9) of boys (Fisher’s Exact P=0.008). Although most participants did not report any AE potentially related to hyperprolactinemia, 23% (n=12) of those with hyperprolactinemia endorsed at least one AE compared with 4% (n=2) of those without (Fisher’s Exact P=0.01). In fact, all the males and the three girls who reported at least one AE had hyperprolactinemia (Table 1).
Owing to racial/ethnic differences in the distribution of the different DRD2 variants [15], we restricted the genetic analyses to nonHispanic Caucasians who formed 84% (n=90) of the sample. The genotype frequencies were as follows: TaqIA A2A2 63% (n=57), A1A2 33% (n=30), and A1A1 3% (n=3); C957T CC 24% (n=22), CT 44% (n=40), and TT 31% (n=28); -141 Ins/Del Ins/Ins 77% (n=69), Ins/Del 22% (n=20), and Del/Del 1% (n=1); and A-241G AA 87% (n=78), AG 13% (n=12), and GG 0%. All were in Hardy—Weinberg equilibrium. Using the Haploview program [30] (Fig. 1), TaqIA and -141C Ins/Del were found to be incomplete, but AQ3nonsignificant (D’1, R2=0.04, LOD AQ4score 0.79), linkage disequilibrium (LD). Significant LD was detected between C957T and TaqIA (D’0.76, R2=0.17, LOD score 3.39) and C957T and -141C Ins/Del (D’0.86, R2=0.12, LOD score 2.69). A-241G was not in LD with any of the three other variants.
Fig. 1.
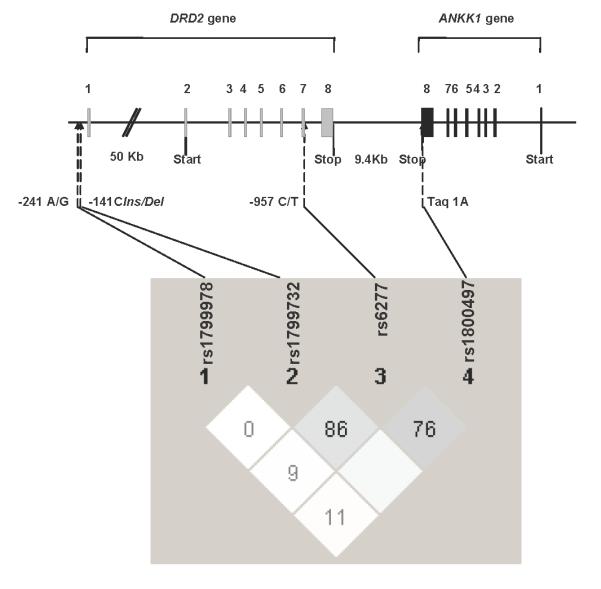
Pairwise linkage disequilibrium (LD) analysis across the four DRD2 variants. Light grey: LD (moderate-to-high D’) between C957T and TaqIA and between C957T and -141C Ins/Del. White: almost no significant LD between the variants. Empty white: complete, but nonsignificant, LD between TaqIA and -141C Ins/Del.
As shown in Table 2, there were no demographic or clinical differences between the two TaqIA genetic groups except for patients with the A2A2 genotype being more likely to have attention deficit hyperactivity disorder (P<0.006).
Prolactin concentration was higher in the TaqIA A1 allele carriers (P=0.03). Furthermore, whether defined based on the laboratory upper normal limit for males and females or using a single cutoff of prolactin concentration greater than 25.7λng/ml [29], the rate of hyperprolactinemia was 1.5–2 times higher in the A1 allele carriers compared with participants with the A2A2 genotype (P≤0.04). We found similar results when we matched the two genetic groups on the daily dose of psychostimulant per kilogram of body weight. In fact, the median prolactin concentration was 24.0λng/ml in the A1 allele carriers and 16.2 in the A2A2 group (Wilcoxon’s signed rank S=114, P=0.04) and the rate of hyperprolactinemia was 65% (n=22) in the A1 allele carriers versus 34% (n=11) in the A2A2 group (McNemar’s S=7.1, d.f.=1, P=0.01).
We then used multiple linear regression to predict prolactin concentration adjusting for the TaqIA genetic group as well as the significant covariates identified earlier (i.e. age or Tanner stage, the dose of risperidone or its serum concentration, and the dose of psychostimulants). As expected, the daily dose of risperidone per kilogram of body weight increased and that of psychostimulants decreased prolactin concentration. We also found a trend for prolactin to increase with age (F(1,85)=2.7, P=0.1) and with the TaqIA A1 allele carrier state [adjusted least squares means of log prolactin 3.2λng/ml in A1 allele carriers versus 2.9 in noncarriers, β=0.24, F(1,85)=3.2, P<0.08]. This genetic effect accounted for 2.8% of the variance in log prolactin concentration (ΔR2=0.028). Similar results were obtained when the stage of sexual development, instead of age, was included in the model. When the risperidone concentration was substituted for the oral dose, the TaqIA effect became significant (β=0.24, F(1,78)=4.3, P=0.04, ΔR2=0.030) while the opposite was true for the dose of psychostimulants (F(1,78)=2.5, P=0.1). However, neither age nor Tanner stage significantly contributed to the model (P>0.4). Finally, we used logistic regression to compare the rate of hyperprolactinemia across the two TaqIA genetic groups while controlling for the concentration of risperidone and the dose of psychostimulants per kilogram of body weight. We found that the adjusted odds of developing hyperprolactinemia for A1 allele carriers were 3.1 times (OR: 95% CI: 1.1–9.7, Wald χ2=4.0, P<0.05) greater than for participants with the A2A2 genotype. The risperidone concentration also increased the probability of hyperprolactinemia (Wald χ2=14.8, P<0.0001), whereas the dose of psychostimulants had no effect (P=0.3). When the analyses were restricted to boys, the pattern of the results remained unchanged.
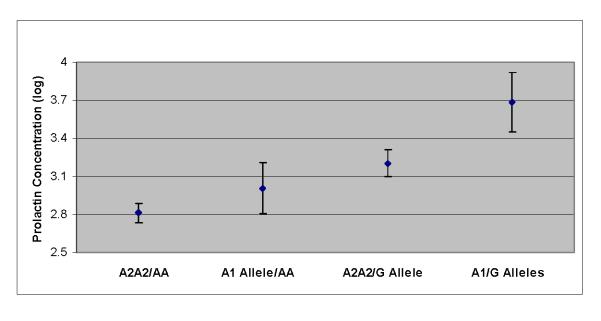
Next, we explored the effects of the -141C Ins/Del, C957T, or A-241G variants on prolactin concentration using multiple linear regression and controlling for the serum concentration of risperidone and the dose of psychostimulants per kilogram of body weight. Although the former continued to have a strong positive effect on prolactin, neither psychostimulants nor the -141C Ins/Del variant had a significant effect (P>0.1 and P=0.6, respectively). When C957T was substituted for -141C Ins/Del, we again found a strong effect of risperidone concentration (P<0.001) but no effect of psychostimulants (P>0.2). In addition, there was a trend for the three genotypes to predict prolactin, with the CT genotype having the highest concentration (F(2,77)=2.6, P=0.08, ΔR2=0.037). With a recessive mode of transmission, C957T had no significant effect (P=0.4). In contrast, after controlling for the serum concentration of risperidone (F(1,78)=52.2, P<0.0001) and the dose of psychostimulants (F(1,78)=5.6, P=0.02), having the AA genotype of the A-241G variant was associated with a strong protective effect (β=-0.53, F(1,78)=10.0, P=0.002). This genetic effect accounted for 6.6% of the variance in prolactin concentration (ΔR2=0.066). When both TaqIA and A-241G genetic groups were introduced into the regression model predicting prolactin, both independently contributed to the model [β=0.23, F(1,77)=4.1, P<0.05, ΔR2=0.026 and β=-0.51, F(1,77)=9.7, P<0.003, ΔR2=0.062, respectively] in addition to risperidone serum concentration and the dose of psychostimulants. Finally, after controlling for the concentration of risperidone and dose of psychostimulants, we found a synergistic effect of the TaqIA and A-241G variants on prolactin concentration (F(3,76)=5.1, P=0.003) (Fig. 2). In fact, compared with those participants with the TaqIA A2A2/-241AA joint genotype, participants with the A1 allele/AA genotype experienced a mean increase in prolactin (log transformed) of 7% (n=28, t=1.5, P=0.1) whereas those with the A2A2/-241G allele genotype experienced a 14% mean increase in prolactin (n=7, t=1.8, P=0.07). In participants with the A1/-241G alleles joint genotype, the mean prolactin concentration was 31% higher (n=5, t=3.6, P=0.0006).
Fig. 2.
Adjusted least squares mean of prolactin concentration (log transformed) as a function of the TaqIA and A-241G joint genotypes of the dopamine D2 receptor gene in children and adolescents in long-term treatment with risperidone.
Finally, after controlling for hyperprolactinemia status (Wald χ2=4.1, P=0.04), the odds of developing at least one AE potentially related to hyperprolactinemia was 4.4 times greater in TaqIA A1 allele carriers compared with A2A2 participants (OR: 95% CI: 1.1–76.0, Wald χ2=4.0, P<0.05). However, although hyperprolactinemia status continued to increase the risk for AE (Wald χ2=5.0, P<0.03), the A-241G variants did not affect this risk, likely because of the small number of A-241G allele carriers (n=11). Similar analyses were not conducted with the other variants, owing to their lack of effect on prolactin concentration, or with the joint genotypes because of the small size of the different genetic subgroups.
Discussion
To our knowledge, this is the largest study to investigate the role of several variants of the DRD2 gene in chronic antipsychotic-induced hyperprolactinemia in children and adolescents. Prolonged hyperprolactinemia may interfere with bone mineralization [3], which, in turn, could increase lifetime morbidity and mortality, particularly when it develops during childhood and adolescence, a period of accelerated bone mass accrual [18,19]. After an average of 3 years of risperidone treatment, 34–50% of the sample exhibited hyperprolactinemia. Moreover, the TaqIA A1 and A-241G alleles were associated with higher prolactin concentrations, with the A1/-241G alleles joint genotype resulting in a 31% higher concentration compared with the genotype without either alleles. Furthermore, the TaqIA A1 carrier state also increased the risk for hyperprolactinemia-related AEs.
Before discussing the results, we review the study limitations. First, the sample is heterogenous, reflecting the variety of psychiatric conditions where risperidone is prescribed. In addition, the diagnoses were based on chart review and prolactin measurements were conducted cross-sectionally, in a naturalistically treated sample. When we compared the chart-based diagnoses to best-estimate research diagnoses made in nearly half the sample, there was moderate-to-substantial agreement. This is likely because of the fact that more than 90% of our participants were selected from a teaching hospital where AQ5DSM-based diagnoses are generated after an extensive evaluation and are reviewed systematically on a regular basis. As our overall aim was to investigate the safety of long-term treatment with risperidone in children and adolescents, we designed this study to reduce the risk of patients discontinuing the medication because of acute AEs or lack of efficacy. High attrition is a characteristic of most long-term psychiatric studies[4,31,32]. The possible impact of attrition on the role of the DRD2 variants in hyperprolactinemia is unknown but should not detract from the significance of our findings. In fact, our data implicate the TaqIA and the A-241G variants in risperidone-induced hyperprolactinemia specifically during long-term treatment. Furthermore, attrition did not seem to selectively affect a particular genotype as the allele frequencies remained in Hardy—Weinberg equilibrium, at rates comparable with those observed in the other short-term pediatric placebo-controlled study [4]. Another shortcoming of this study is the unequal, though not statistically significant, proportion of participants receiving psychostimulants across the two TaqIA genetic groups. Owing to their dopamine-agonist activity, this difference could explain the lower rate of hyperprolactinemia in the A2A2 participants [27]. In contrast, in those treated with psychostimulants, the A1 allele carriers tended to receive a higher dose, potentially attenuating the effect of this allele on prolactin (Table 2). We addressed this confound by matching the two genetic groups on the number of patients treated with psychostimulants and, in those treated, on the daily dose of psychostimulants. We also controlled for the daily dose of psychostimulants in the other analyses. In fact, although psychostimulants did predict prolactin concentration in the model that included the dose of risperidone, this effect disappeared when the serum concentration of risperidone was substituted for the oral dose. In contrast, the genetic effect persisted. Although we did not correct for multiple comparisons because our primary analysis involving the TaqIA variant was hypothesis-driven, the highly significant finding of the A-241G variants withstands a Bonferroni correction (α=0.05/4=0.013). Another challenge intrinsic to longitudinal studies relates to medication nonadherence, a common problem in chronic diseases. We tried to minimize this effect by excluding patients with undetectable risperidone concentration. Furthermore, we confirmed that our findings applied to boys but could not do the same with girls or with ethnic/racial minority groups because of small sample sizes. In addition, because of the low frequency of certain genotypes, we could not test a codominant mode of inheritance for three of the four genotypes. Therefore, our findings require replication in a larger and more racially/ethnically diverse sample. This would also allow testing the role other genes might have in antipsychotic-induced hyperprolactinemia, such as genes transcribing alternative dopaminergic receptors or membrane drug transporters. As the contribution of additional genetic variants is clarified, a more comprehensive model for antipsychotic-induced hyperprolactinemia will be developed.
Several studies have suggested that the rate of risperidone-induced hyperprolactinemia decreases over time but remains significant [4,5,33-35]. Consistent with these reports, a sizeable proportion of our sample continued to have hyperprolactinemia. Owing to the wide range of duration of exposure to risperidone (05–8.3 years), it is possible that this AE will resolve in some of our participants. We, however, found no correlation between the total duration of risperidone treatment and prolactin (Spearman’s r=0.07, n=107), suggesting that after an initial period, the concentration stabilizes regardless of the total duration of maintenance treatment.
Fourteen percent of the sample endorsed at least one AE potentially related to hyperprolactinemia. This rate is higher than those reported in two large pediatric longitudinal studies [4,5], possibly because of the older age of our participants and their longer treatment with risperidone. In addition, we explicitly asked about these AEs, which might have prompted patients to endorse symptoms they may not have reported spontaneously. Moreover, participants with hyperprolactinemia were six times more likely to endorse an AE.
Owing to the potential clinical significance of our findings, however, some clarifications are necessary. The reliable and valid assessment of these symptoms in children is challenging [4,5]. For example, in those patients who complained of gynecomastia and were examined by a physician, no increase in breast tissue was noted. Two factors could lead to the endorsement of AEs that do not necessarily result from hyperprolactinemia. First, some of these symptoms occur during normal development [5]. In fact, more than 40% of males could develop pubertal gynecomastia [36] and a sizeable number of females exhibit menstrual irregularities during the first 2 years after menarche [37]. As such developmental changes usually normalize within 2 years of the onset of puberty, longitudinal follow-up is paramount. This is being pursued by our group in a follow-up study currently underway. Second, excessive weight, often associated with risperidone [24] makes the accurate diagnosis of gynecomastia more challenging because of the increase in the adipose tissue component of the breast [36]. Interestingly, however, BMI z-score did not vary across the two TaqIA genetic groups (t=0.2, d.f.=88, P=0.8) even though only A1 allele carriers reported gynecomastia. We also found no correlation between BMI z-score and prolactin (Spearman’s r=0.04). Finally, querying about libido is also of unclear reliability in this age group. In total, these challenges, which do not necessarily apply to adults, add to the complexity of the decision-making process in children when evaluating the clinical implications of hyperprolactinemia.
As reported here and elsewhere, several clinical factors correlate with prolactin concentration during antipsychotic treatment [2]. For example, we found that prolactin rises with age or sexual development, likely signaling the maturation of the central dopaminergic system and the hypothalamic-pituitary-gonadal axis [38-40]. In addition, psychostimulants exerted a suppressing effect on prolactin but only before the risperidone concentration was taken into account. Once the latter was entered into the regression model, it alone explained 39% of the variance in the prolactin concentration with the other potential covariates becoming nonsignificant. This probably reflects the more proximal nature of the serum concentration as a marker of DRD2 blockade compared with the oral dose.
Though sexual development (or age), dose of psychostimulants, and dose of risperidone (or serum concentration) are all closely and independently correlated with prolactin, more than 60% of the variance in prolactin concentration remains unaccounted for. This suggests that genetic factors may contribute to one’s susceptibility to antipsychotic-induced hyperprolactinemia. In fact, as predicted, compared with the A2A2 genetic group, the TaqIA A1 allele carriers were two to three times more likely to develop hyperprolactinemia, after controlling for the dose of risperidone (or its serum concentration) and of psychostimulants. In contrast, the AA genotype of the A-241G variants exerted a protective effect. As a result, prolactin concentration gradually escalated with the different possible genotype combinations.
Although, to our knowledge, this is the first study to evaluate the role of the A-241G variants in antipsychotic-related hyperprolactinemia, three studies have identified a link between the TaqIA A1 allele and increase in prolactin in adults with schizophrenia treated with antipsychotics [6-8]. Furthermore, in healthy Chinese-Canadian men, Aklillu et al. [41] found carriers of this variant to predict a faster increase in prolactin after a single dose of perphenazine. In contrast, no association was found between TaqIA variants and risperidone-induced hyperprolactinemia in institutionalized Japanese adults with schizophrenia or in children with autism [4,42]. Nonetheless, our findings partially agree with data from this latter study where C957T and -141C Ins/Del were also found not to affect prolactin concentration. Possible factors that could explain the partial divergence in the results include our enrolment of a more heterogenous clinical group of older age, receiving other psychotropics concomitantly with risperidone. For example, 32% of our participants were prepubertal compared with 81% in the study by Anderson et al. [4]. In addition, we focus on long-term prolactin concentration, whereas they restricted their genetic analysis to the change in prolactin at week 8 after the onset of risperidone treatment [4]. As the vast majority of children develops risperidone-induced hyperprolactinemia acutely [5], it might be argued that genetic factors are more critically implicated in the long-term risk for this AE to emerge where more interindividual variability is observed. Furthermore, in most of our participants, prolactin was measured in the morning, whereas Anderson et al. [4] collected a nonfasting sample between 9λ:λ00 and 15λ:λ00λh. Finally, we control for dose of risperidone and its serum concentration in the analysis. This is most important as these two variables account for a large part of the variance in prolactin concentration and can mask the smaller genetic effect.
The TaqIA locus has been proposed as a potential marker for antipsychotic-induced hyperprolactinemia because of the reduced DRD2 density in A1 allele carriers [10,43,44]. As a result, when treated with a DRD2 antagonist, these individuals may have a relatively higher receptor occupancy at a given drug serum concentration, thereby increasing their propensity for hyperprolactinemia [10,43,44]. Although most studies suggest that the A1 allele carrier state is associated with lower DRD2 density, less research has involved the other variants. The Ins/Ins genotype of the -141C Ins/Del variant has been associated with higher DRD2 promoter activity; however, one imaging study reported a paradoxically lower DRD2 density in participants with this genotype and another one was negative [9,14,45]. In contrast, the 957T allele has been linked to reduced mRNA stability, translating into lower DRD2 receptor availability [15,46]. However, neither the C957T nor -141C Ins/Del variants were found to influence the DRD2 gene expression in a recent study [47]. Finally, the A-241G had the largest effect on prolactin concentration in this study. This could not be explained by an association with the TaqIA variant as they are not in LD. Both variants independently predicted prolactin concentration when simultaneously introduced into the regression model, and they had a synergistic effect in the joint genotypes analysis. Although Arinami et al. [14] failed to show an effect of the A-241G variants on DRD2 gene promoter activity, the A-241G allele has been associated with a faster response to antipsychotic treatment in adults with schizophrenia [48]. This finding could be seen as consistent with our results as a lower DRD2 density would be associated with a relatively larger receptor occupancy, resulting in a better response to treatment and higher prolactin concentration [6,48]. We have started administering rating scales to measure clinical response and intend to investigate whether hyperprolactinemia and response to treatment are associated with the DRD2 genotypes. Nonetheless, our finding should be considered preliminary because of the relatively small number of G allele carriers and the exploratory nature of the investigation.
Equally significant, after controlling for hyperprolactinemia, the TaqIA A1 allele carrier state was also associated with a higher rate of AEs potentially related to the hormonal abnormality. If our findings are replicated and as further work is conducted to identify genetic predictors of AEs, it might eventually become possible to more specifically tailor treatments to match each patient’s strengths and vulnerabilities.
Conclusion
The last decade has seen a dramatic increase in the use of antipsychotic medications in children [49]. Although these medications have received FDA’s approval for pediatric use in acute psychosis, mania, and irritability and aggression in autistic disorder, they are frequently prescribed off-label to target symptoms such as irritability and insomnia [50,51]. Owing to their potential to cause weight gain, metabolic, and hormonal abnormalities, clinicians must prescribe them judiciously, especially in children and adolescents, and only after nonpharmacological interventions as well as more benign psychotropics have been tried [52-54]. When unavoidable, close monitoring of patients who receive antipsychotics is critical [24,29].
Our findings add to the increasing body of evidence confirming the persistence of hyperprolactinemia in a sizeable number of children and adolescents in extended therapy with risperidone. They also suggest that variants of the DRD2 gene may contribute to this risk and to the emergence of AEs potentially related to hyperprolactinemia. Current guidelines do not recommend universal monitoring of prolactin concentration during antipsychotic treatment in children and adolescents [1]. However, measuring prolactin is indicated when galactorrhea or amenorrhea develop, especially when these AEs do not resolve with dose reduction or medication discontinuation [1]. In such cases, appropriate referrals are indicated.
Acknowledgements
The authors would like to thank the patients and their families for their commitment to this research, the child psychiatry staff, the research assistants, and the staff in the General Clinical Research Center. Dr Raymond Crowe offered critical feedback and Dr. Jennifer McWilliams assisted in data collection.
This study was funded by a 2005 Young Investigator Award to Chadi Calarge and by the National Institute of Health General Clinical Research Center Mechanism (RR00059).
Footnotes
Conflicts of interest: Dr Calarge has received research funds from the National Institute of Mental Health, the National Alliance for Research on Schizophrenia and Depression, Children’s Miracle Network, the Thrasher Research Fund, and the American Academy of Child and Adolescent Psychiatry.
Dr Ellingrod reports receiving royalties and consultation fees from Lexi Comp. She serves on an advisory board for Lilly Pharmaceuticals.
Dr Miller reports having received research funds from the National Institute of Mental Health and Pfizer, Inc.; and consulting and educational fees from Astra Zeneca Pharmaceuticals LP, Organon, and Pfizer Inc.
Dr Tansey, Dr. Schlechte, Ms. Acion, and Ms. Moline report no competing interests.
References
- 1.Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–791. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- 2.Kinon BJ, Gilmore JA, Liu H, Halbreich UM. Hyperprolactinemia in response to antipsychotic drugs: characterization across comparative clinical trials. Psychoneuroendocrinology. 2003;28(Suppl 2):69–82. doi: 10.1016/s0306-4530(02)00128-2. [DOI] [PubMed] [Google Scholar]
- 3.Calarge CA, Acion L, Kuperman S, Schlechte JA. Risperidone-induced hyperprolactinemia is negatively associated with bone mineral density in youth, in National Institute of Mental Health (NIMH) New Clinical Drug Evaluation Unit (NCDEU) Arizona; Phoenix: 2008. [Google Scholar]
- 4.Anderson GM, Scahill L, McCracken JT, McDougle CJ, Aman MG, Tierney E, et al. Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry. 2007;61:545–550. doi: 10.1016/j.biopsych.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Findling RL, Kusumakar V, Daneman D, Moshang T, De Smedt G, Binder C. Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry. 2003;64:1362–1369. doi: 10.4088/jcp.v64n1113. [DOI] [PubMed] [Google Scholar]
- 6.Mihara K, Kondo T, Suzuki A, Yasui N, Nagashima U, Ono S, et al. Prolactin response to nemonapride, a selective antagonist for D2 like dopamine receptors, in schizophrenic patients in relation to Taq1A polymorphism of DRD2 gene. Psychopharmacology (Berl) 2000;149:246–250. doi: 10.1007/s002139900364. [DOI] [PubMed] [Google Scholar]
- 7.Mihara K, Suzuki A, Kondo T, Yasui-Furukori N, Ono S, Otani K, et al. Relationship between Taq1 A dopamine D2 receptor (DRD2) polymorphism and prolactin response to bromperidol. Am J Med Genet. 2001;105:271–274. doi: 10.1002/ajmg.1303. [DOI] [PubMed] [Google Scholar]
- 8.Young RM, Lawford BR, Barnes M, Burton SC, Ritchie T, Ward WK, et al. Prolactin levels in antipsychotic treatment of patients with schizophrenia carrying the DRD2*A1 allele. Br J Psychiatry. 2004;185:147–151. doi: 10.1192/bjp.185.2.147. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 10.Pohjalainen T, Rinne JO, Nagren K, Lehikoinen P, Anttila K, Syvalahti EK, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- 11.Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem Res. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- 12.Dubertret C, Gouya L, Hanoun N, Deybach JC, Ades J, Hamon M, et al. The 3′ region of the DRD2 gene is involved in genetic susceptibility to schizophrenia. Schizophr Res. 2004;67:75–85. doi: 10.1016/s0920-9964(03)00220-2. [DOI] [PubMed] [Google Scholar]
- 13.Laakso A, Pohjalainen T, Bergman J, Kajander J, Haaparanta M, Solin O, et al. The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L-amino acid decarboxylase in healthy subjects. Pharmacogenet Genomics. 2005;15:387–391. doi: 10.1097/01213011-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet. 1997;6:577–582. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- 15.Duan J, Wainwright MS, Comeron JM, Saitou N, Sanders AR, Gelernter J, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Genet. 2003;12:205–216. doi: 10.1093/hmg/ddg055. [DOI] [PubMed] [Google Scholar]
- 16.Greenhill LL. Assessment of safety in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2003;42:625–626. doi: 10.1097/01.chi.0000046843.56865.a5. [DOI] [PubMed] [Google Scholar]
- 17.Moll GH, Hause S, Ruther E, Rothenberger A, Huether G. Early methylphenidate administration to young rats causes a persistent reduction in the density of striatal dopamine transporters. J Child Adolesc Psychopharmacol. 2001;11:15–24. doi: 10.1089/104454601750143366. [DOI] [PubMed] [Google Scholar]
- 18.M M. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. doi: 10.1001/jama.285.6.785. AQ6. [DOI] [PubMed] [Google Scholar]
- 19.Colao A, Di Somma C, Loche S, Di Sarno A, Klain M, Pivonello R, et al. Prolactinomas in adolescents: persistent bone loss after 2 years of prolactin normalization. Clin Endocrinol (Oxf) 2000;52:319–327. doi: 10.1046/j.1365-2265.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 20.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 21.Swanson JM, Elliott GR, Greenhill LL, Wigal T, Arnold LE, Vitiello B, et al. Effects of stimulant medication on growth rates across 3 years in the MTA follow-up. J Am Acad Child Adolesc Psychiatry. 2007;46:1015–1027. doi: 10.1097/chi.0b013e3180686d7e. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 24.Calarge CA, Acion L, Kuperman S, Tansey MJ, Schlechte JA. Metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol. doi: 10.1089/cap.2008.007. M; M:M—M (in press).AQ7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronaghi M. Pyrosequencing for SNP genotyping. Methods Mol Biol. 2003;212:189–195. doi: 10.1385/1-59259-327-5:189. [DOI] [PubMed] [Google Scholar]
- 26.Agresti A. Categorical data analysis. John Wiley & Sons, Inc.; Hoboken, NJ: 2002. [Google Scholar]
- 27.Shaywitz BA, Shaywitz SE, Sebrechts MM, Anderson GM, Cohen DJ, Jatlow P, et al. Growth hormone and prolactin response to methylphenidate in children with attention deficit disorder. Life Sci. 1990;46:625–633. doi: 10.1016/0024-3205(90)90131-a. [DOI] [PubMed] [Google Scholar]
- 28.Aman MG, De Smedt G, Derivan A, Lyons B, Findling RL. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry. 2002;159:1337–1346. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- 29.Correll CU, Mughal T, Dudas M, Malhotra AK, Kane JM. Effect of atypical antipsychotics on prolactin levels and reproductive functioning in children and adolescents, in am acad child adolesc psychiatry. San Diego, CA: 2006. M. AQ8. [Google Scholar]
- 30.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 31.Correll CU, Nyilas M, Aurang C, Johnson B, Jin N, Marcus R, et al. Long-term safety and tolerability of aripiprazole in children (10–17) with mania, in American Academy of Child and Adolescent Psychiatry. Boston, MA: 2007. M. [Google Scholar]
- 32.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 33.Staller J. The effect of long-term antipsychotic treatment on prolactin. J Child Adolesc Psychopharmacol. 2006;16:317–326. doi: 10.1089/cap.2006.16.317. [DOI] [PubMed] [Google Scholar]
- 34.Troost PW, Lahuis BE, Hermans MH, Buitelaar JK, van Engeland H, Scahill L, et al. Prolactin release in children treated with risperidone: impact and role of CYP2D6 metabolism. J Clin Psychopharmacol. 2007;27:52–57. doi: 10.1097/JCP.0b013e31802e68d5. [DOI] [PubMed] [Google Scholar]
- 35.Stevens JR, Kymissis PI, Baker AJ. Elevated prolactin levels in male youths treated with risperidone and quetiapine. J Child Adolesc Psychopharmacol. 2005;15:893–900. doi: 10.1089/cap.2005.15.893. [DOI] [PubMed] [Google Scholar]
- 36.Styne DM. The Testes, Disorders of Sexual Differentiation and Puberty in the Male. In: Sperling MA, editor. Pediatric Endocrinology. Sauders; Philadelphia, PA: 2002. pp. 565–628. [Google Scholar]
- 37.Rosenfield RL. Puberty in the Female and its Disorders. In: Sperling MA, editor. Pediatric Endocrinology. Sauders; Philadelphia, PA: 2002. pp. 455–518. [Google Scholar]
- 38.Anderson GM, Cohen DJ. Neurochemistry of Childhood Psychiatric Disorders. In: Lewis M, editor. Child and Adolescent Psychiatry, A Comprehensive Textbook. Lippincott Williams & Wilkins; Philadelphia, PA: 2002. pp. 46–59. [Google Scholar]
- 39.Cummins TK, Anand KJ, Nemeroff CB. Developmental Psychoneuroendocrinology. In: Lewis M, editor. Child and Adolescent Psychiatry, A Comprehensive Textbook. Lippincott Williams & Wilkins; Philadelphia, PA: 2002. pp. 93–119. [Google Scholar]
- 40.Felt B, Jimenez E, Smith J, Calatroni A, Kaciroti N, Wheatcroft G, et al. Iron deficiency in infancy predicts altered serum prolactin response 10 years later. Pediatr Res. 2006;60:513–517. doi: 10.1203/01.PDR.0000242848.45999.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aklillu E, Kalow W, Endrenyi L, Harper P, Miura J, Ozdemir V. CYP2D6 and DRD2 genes differentially impact pharmacodynamic sensitivity and time course of prolactin response to perphenazine. Pharmacogenet Genomics. 2007;17:989–993. doi: 10.1097/FPC.0b013e3282f01aa3. [DOI] [PubMed] [Google Scholar]
- 42.Yasui-Furukori N, Saito M, Tsuchimine S, Nakagami T, Sato Y, Sugawara N, et al. Association between dopamine-related polymorphisms and plasma concentrations of prolactin during risperidone treatment in schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1491–1495. doi: 10.1016/j.pnpbp.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 43.Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48:648–54. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- 44.Nordstrom AL, Farde L. Plasma prolactin and central D2 receptor occupancy in antipsychotic drug-treated patients. J Clin Psychopharmacol. 1998;18:305–310. doi: 10.1097/00004714-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Pohjalainen T, Nagren K, Syvalahti EK, Hietala J. The dopamine D2 receptor 5′-flanking variant, -141C Ins/Del, is not associated with reduced dopamine D2 receptor density in vivo. Pharmacogenetics. 1999;9:505–509. [PubMed] [Google Scholar]
- 46.Hirvonen M, Laakso A, Nagren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of the dopamine D2 receptor (DRD2) gene affects striatal DRD2 availability in vivo. Mol Psychiatry. 2004;9:1060–1061. doi: 10.1038/sj.mp.4001561. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lencz T, Robinson DG, Xu K, Ekholm J, Sevy S, Gunduz-Bruce H, et al. DRD2 promoter region variation as a predictor of sustained response to antipsychotic medication in first-episode schizophrenia patients. Am J Psychiatry. 2006;163:529–531. doi: 10.1176/appi.ajp.163.3.529. [DOI] [PubMed] [Google Scholar]
- 49.Olfson M, Blanco C, Liu L, Moreno C, Laje G. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry. 2006;63:679–685. doi: 10.1001/archpsyc.63.6.679. [DOI] [PubMed] [Google Scholar]
- 50.FDA FDA approves the first drug to treat irritability associated with autism, risperdal. 2006. [PubMed]
- 51.FDA drug approved for two psychiatric conditions in children and adolescents. 2007.
- 52.Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, et al. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry. 2006;45:1284–1293. doi: 10.1097/01.chi.0000235077.32661.61. [DOI] [PubMed] [Google Scholar]
- 53.Pappadopulos E, Macintyre Ii JC, Crismon ML, Findling RL, Malone RP, Derivan A, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part II. J Am Acad Child Adolesc Psychiatry. 2003;42:145–161. doi: 10.1097/00004583-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Schur SB, Sikich L, Findling RL, Malone RP, Crismon ML, Derivan A, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part I. J Am Acad Child Adolesc Psychiatry. 2003;42:132–144. doi: 10.1097/00004583-200302000-00008. a review. [DOI] [PubMed] [Google Scholar]