Abstract
Background
There is increasing interest in hormone replacement therapy to improve health and quality of life (QoL) of older men with age-related decline in hormone levels. This paper reports the preliminary development and evaluation of the psychometric properties of a new individualised questionnaire, the A-RHDQoL, measuring perceived impact of age-related hormonal decline on QoL of older men. A-RHDQoL design was based on the HDQoL for people with growth hormone (GH) deficiency and the ADDQoL (for diabetes).
Methods
Internal consistency reliability and some aspects of validity of the A-RHDQoL were investigated in a cross-sectional survey of 128 older men (age range: 64 – 80 yrs), being screened for inclusion in a trial of GH and testosterone (T) replacement, and who completed the A-RHDQoL once. Respondents rated personally applicable life domains for importance and impact of their hormonal decline. A single overview item measured present QoL. Serum levels of Insulin-like Growth Factor-I and total T were measured.
Results
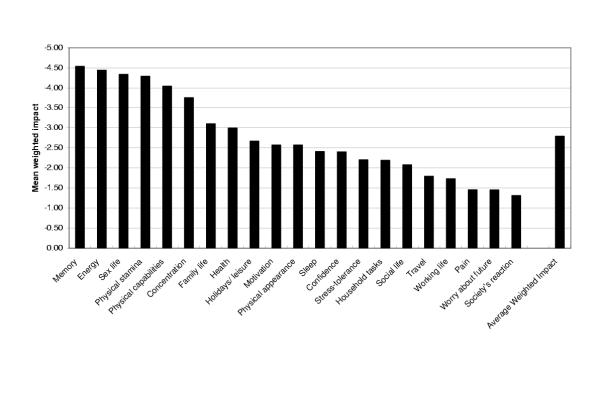
Of the 24 A-RHDQoL domains, 21 were rated as relevant and important for older men. All domains were perceived as negatively impacted by hormonal decline. The most negatively impacted domains were: memory (-4.54 ± 3.02), energy (-4.44 ± 2.49), sex life (-4.34 ± 3.08) and physical stamina (-4.29 ± 2.41), (maximum range -9 to +9). The shorter 21-domain A-RHDQoL had high internal consistency reliability (Cronbach's alpha coefficient = 0.935, N = 103) and applicable domains could be weighted and summed into an overall Average Weighted Impact score. The questionnaire was acceptable to the majority of respondents and content validity was good. The single overview item measuring present QoL correlated significantly with total T levels [r = 0.26, p <0.01, N = 114].
Conclusion
The new 21-item A-RHDQoL is an individualised questionnaire measuring perceived impact of age-related hormonal decline on the QoL of older men. The internal consistency reliability and content validity of the A-RHDQoL are established, but the measure is at an early stage of its development and its sensitivity to change and other psychometric properties need now to be evaluated in clinical trials of hormone replacement in older men.
Keywords: A-RHDQoL, quality of life, age-related hormonal decline
Background
People aged over 60 now constitute about 20% of the population in more developed regions of the world; by 2050 they will probably account for 33% [1]. However, a proportion of the additional years gained with increasing life expectancy are associated with increasing poor health and disability. Common changes affecting older men include a general decrease in well-being, work capacity, reduced muscle mass and strength, reduced virility, libido and sexual activity, increased central adiposity, atherosclerosis, impaired cognitive performance and sleep disturbances. Many of these changes resemble phenomena found in well-recognised hormone deficiencies such as hypogonadism and adult growth hormone deficiency (GHD) [2]. Growth hormone (GH) secretion declines in adulthood with a parallel fall in Insulin-like Growth Factor-I (IGF-I), such that 30% of older men (over 60 years) have been found to be GH deficient compared to healthy young adults [3]. Levels of testosterone (T) also fall as men get older (1–2% per year after 30 years of age) [4]. In patients with organic GHD, GH replacement improves body composition, muscle strength, bone mass and sense of well-being [5] as well as cardiovascular risk factors [6]. Androgen replacement improves body composition [7] and sexual function [8]. Hormone replacement therapy has been available to post-menopausal women for some time. The question has now arisen whether older men might also benefit from hormone replacement to help reduce and prevent frailty [9] and prolong the capacity for independent living [10].
In older people GH treatment improves muscle mass and strength [11] and bone mineral density [12]. T treatment given to healthy older men increases bone mineral density [13], increases lean body mass and decreases body fat [14]. Combined GH and sex steroid administration improves body composition, muscle strength (marginally) and maximum oxygen uptake during exercise [15,16]. Higher bioavailable T levels have been associated with improved cognitive function [17], and reduced depression [18]. However, side effects such as carpal tunnel syndrome, arthralgia and hyperglycaemia are common with GH therapy, and T administration may also have side effects, e.g. gynaecomastia and skin irritation with T patches [19].
These and many other studies indicate the increasing interest in determining whether normalisation of hormone levels in older men leads to improvements in both physical and psychological functioning. Few studies, however, have measured quality of life (QoL), or the patient reported outcomes of health status and psychological well-being. The Short-Form Health Survey, (SF-36), [20], a health status measure, found a significant increase in physical functioning following T supplementation in one study [21] but no significant effects in other studies [22,23]. The Psychological General Well-being Index (PGWB) [24], a measure of psychological well-being, did not find any significant effects after 8 weeks T supplementation [23]. As pointed out elsewhere [25], such generic measures of health status and well-being may not be sufficiently sensitive, nor are they measures of QoL, although often described as such. However, QoL is an important outcome of clinical trials and with increasing research on hormone replacement in older men there is an urgent need for a measure of QoL for use in this field.
The Age-Related Hormone Deficiency-Dependent Quality of Life Questionnaire (A-RHDQoL) is a new individualised questionnaire designed to measure the QoL of older men with age-related hormonal decline. This paper concerns the evaluation of the psychometric properties of this questionnaire in a sample of older men who had volunteered for a trial of GH and T replacement therapy at St Thomas' Hospital, London and were being screened for their suitability for inclusion in the trial. The Guy's and St Thomas' Hospital Trust Research Ethics Committee gave approval for the study (EC 99/154).
Methods
The A-RHDQoL
Design of the A-RHDQoL was influenced by work on the Schedule for the Evaluation of Individual Quality of Life (SEIQoL) [26], the Audit of Diabetes-Dependent Quality of Life (ADDQoL) [27,28] and subsequent adaptations of the ADDQoL for people with macular degeneration (MacDQoL) [29], and with adult GHD, (the HDQoL) [30,31]. The HDQoL was found to have high internal consistency reliability, and to be sensitive to change and to sub-group differences in adult GHD [30,32,33]. Although patients were involved in generating versions of this instrument for these disorders (through interviews and solicited written comments), time constraints prevented qualitative research with older men with age-related hormonal decline. Superficial adjustments were therefore made to the HDQoL to make it suitable for these older men. Nineteen of the 24 A-RHDQoL life domains are in the latest version of the HDQoL, and include domains of family, social, working and sex life, physical aspects such as physical capabilities, appearance, stamina, sleep and pain, and psychological aspects such as confidence and motivation. Additional domains of health, fertility, concentration, household tasks, and society's reaction were derived from the MacDQoL, and from a review of the literature as well as discussions with health professionals. (The Results section provides details of the final selection of items following psychometric analyses). At the end of the questionnaire there is a 'free comments' section in which respondents are asked if there are any other ways in which they perceive age-related decline in hormone levels to affect their QoL. This open section allows for the addition of further domains to the questionnaire in the future as part of its continuing development.
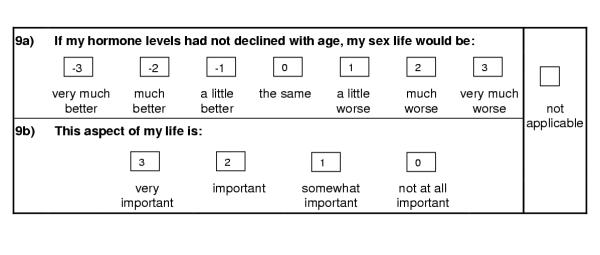
The first two items in the questionnaire provide an overview of the respondent's QoL. Question I asks respondents to rate their present QoL on a 7-point scale from 'as good as it could possibly be' to 'as bad as it could possibly be'. Question II asks them to rate what their QoL would be if they did not have age-related decline in hormone levels, on a 7-point scale from 'very much better' to 'very much worse', providing a global measure of impact of age-related hormonal decline on QoL. Like the ADDQoL and its related questionnaires, the A-RHDQoL is individualised. It takes into account the relevance for the individual of each aspect of life or domain covered in the questionnaire, by giving men the opportunity to indicate whether a particular domain is not applicable (N/A), specifically in questions concerning work, family, sex life, fertility, and pain. The impact of age-related decline in hormone levels on each relevant domain is then assessed as well as its perceived importance to the individual. Each domain is introduced by the hypothetical statement: If my hormone levels had not declined with age, my [domain] would be...... and is followed by a 7-point Likert scale from 'very much better' to 'very much worse' (the impact rating). Respondents then rate how important that domain is to them on a 4-point Likert scale from 'very important' to 'not at all important'. Owing to time restrictions only a small pilot study could be conducted (N = 3) but this found the questionnaire acceptable to respondents and no additional domains were suggested.
Scoring
The domain impact ratings are scored from -3 to +3 as shown in Fig. 1. Importance ratings are scored from 0 (not at all important) to 3 (very important). A weighted domain impact score is obtained by multiplying the domain's impact rating by the corresponding importance rating. For example, if a respondent indicated that a domain would be much better (score -2) if he did not have age-related hormonal decline, and that the domain was very important to him (score 3), the weighted domain score would be -6. An overall score for the questionnaire, the A-RHDQoL Average Weighted Impact score (A-RHDQoL AWI) is obtained by summing all applicable weighted domain scores, before dividing by the number of domains applicable to the individual. Weighted domain scores and A-RHDQoL AWI range from -9 to +9 (maximum negative to maximum positive impact of age-related hormonal decline on the individual domain or on overall QoL). The number of applicable domains excludes all domains marked N/A. The overview items are not weighted by importance ratings, but Question II – impact on QoL (QII) is scored in the same way as the domains, from -3 to + 3. Question I – present QoL (QI), however, is scored +3 to -3 from 'as good as it could possibly be' to 'as bad as it could possibly be', so that a higher score indicates better QoL.
Figure 1.
Example of an A-RHDQoL domain item and scoring
Other questionnaires used in the study
Two well-established generic questionnaires were also completed in the screening study: the SF-36 [20], and the 12-item Well-being Questionnaire (W-BQ12) [34,35]. They were selected to provide additional data on health status and well-being and, although their data were used to investigate correlations with A-RHDQoL variables, high correlations were not expected as neither measures QoL. The SF-36 has 8 subscales to measure Bodily Pain, General Health, Mental Health, Physical Functioning, Role-Emotional, Role-Physical, Social Functioning and Vitality. Scores on the SF-36 range from 0 to 100 (poor to good health status). The W-BQ12 has 3 subscales to measure Negative Well-being, Energy and Positive Well-being and a combined General Well-being scale. Subscale scores range from 0 to 12 (higher scores indicating increased mood of the subscale label) and the total score ranges from 0 to 36 (a higher score indicates better well-being).
Recruitment
The participants were older men who had volunteered for a 6-month randomised controlled trial of GH and T replacement (HRT trial) at St Thomas' Hospital, London, in response to advertisements and articles in the national and local press, and were undergoing screening for inclusion in this trial. The advertisements stated that the trial was to investigate whether GH and T treatment would improve general well-being, strength, exercise tolerance and cholesterol levels in older men. Volunteers completed a pre-screening questionnaire to indicate whether they fitted the inclusion and exclusion criteria for participation in the clinical trial. The inclusion criteria were male sex, and age 65–80 years. Exclusion criteria were clinically significant pulmonary, cardiac, hepatic, renal, psychiatric or progressive neurological disease, uncontrolled hypertension and/ or diabetes, any evidence of active malignancy or a history of prostate cancer, any history of pituitary disease, obesity (body mass index > 30 kg/m2), taking corticosteroids, appetite suppressants drugs or other drugs that affect bone metabolism.
Factor analyses ideally require a minimum of 5 respondents per questionnaire item [36] thus analyses of the 24-item A-RHDQoL required a minimum of 120 completed questionnaires. However, screening for the HRT trial had already started before the full 24-item questionnaire was ready for use, and only 64 completed A-RHDQoL questionnaires were obtained at screening. Volunteers who had not completed this questionnaire at screening were later approached by mailshot (i.e. by post). The questionnaire was enclosed with a letter in which the volunteers were informed that they would not be invited to participate in the HRT trial because their hormonal levels were insufficiently low. (These volunteers would have exhibited similar age-related hormonal decline to some of the 64 volunteers in the initial screening, who completed the questionnaire, but who were also not invited to participate in the trial, as a result of insufficient hormonal decline). Finally, the baseline data were used from HRT trial participants who had not completed the A-RHDQoL at the screening stage. There were thus 3 methods of recruitment:
(1) Volunteers at initial screening (N = 64)
(2) Post-screening mailshot of volunteers (N = 12)
(3) HRT trial participants, baseline data (N = 53);
Total number of recruits = 129.
Statistical analyses
The 'Not Applicable' response option and loss of data
None of the data from any respondent who selected a N/A response option would normally be included in factor and reliability analyses, because N/A responses are treated as missing by the statistical package used in these analyses (SPSS for Windows, Release 9.0). Furthermore, if the SPSS default of listwise deletion of missings is used, all cases that have any missing values across all 24 items are lost to analysis, so considerable data could be lost. As will be seen below, between 3 to 40% of volunteers chose a N/A option. N/A responses were therefore set to zero for one set of analyses, with pairwise deletion of missings as in the original development of the ADDQoL [27]. (Pairwise deletion minimises loss of data because only cases with missing values for one or both of a pair of variables in a correlation are excluded from the analysis, however, this can result in a set of coefficients based on a varying number of cases). Results obtained from a second set of analyses, when N/A responses were coded as missing with listwise deletion of missings, are also reported where relevant.
Normality issues
Normality of distributions was determined through investigation of histograms and z (skew) scores, where z (skew) scores ≥ ± 2.58 are indicative of non-normality [37]. The A-RHDQoL is not a questionnaire where a normal spread of scores and normal distributions would be expected. The bi-polar scale allows for some respondents to have positive scores, indicating their perception that hormonal decline had some positive effects on their lives, but these were expected to be uncommon. Item data were transformed as near to normality as possible. The assumption was made that if the reliability of the scale was high, and the number of respondents sufficiently great, then a robust factor analysis should override the problem of non-normality.
Internal consistency reliability
Cronbach's alpha coefficient of internal consistency reliability was determined. An alpha of 0.8 was taken as the minimum acceptable [38], but 0.9 was preferable as it is considered by some to be the minimum for measures of differences between individuals [39]. Acceptable item-total correlations were those >0.2 [40].
Factor structure
This was explored by Principal Components Analysis with Varimax rotation, (a rotation that minimises the correlation between components). Salient loadings were taken as ≥ 0.4. This is higher than the recommended minimum 0.3 [41], erring on the side of caution in an effort to reduce the risk of spurious loadings that owed their origin to non-normality of item distributions, and also to avoid double loadings.
Correlations
Correlations were run on relationships between A-RHDQoL variables and Body Mass Index (BMI), age, levels of total T and IGF-I (the marker for GH levels), as well as SF-36 and W-BQ12 subscales. It was not appropriate to consider the correlations with SF-36 and W-BQ12 questionnaires as measures of concurrent validity as the A-RHDQoL measures different experiences (QoL and perceived impact of age-related hormonal decline on QoL) from these measures of health status and well-being. Small to moderate correlations only were anticipated, though significance was likely with this sample size. Correlations were investigated using Pearson's r for parametric, and Spearman's rho for non-parametric variables.
'Familywise' error in multiple tests
The Bonferroni correction for familywise error was adopted (i.e. alpha was set initially to 0.05/n where n was the number of variables within a 'family' and then the Holm's sequential Bonferroni procedure for multiple tests was applied [42] whereby the family size reduces with each significant result. For example if 3 similar statistical tests were performed on A-RHDQoL AWI score and the A-RHDQoL overview items, QI and QII, (regarded as one 'family'), the minimum significance value of each sequential significant result would be 0.017, 0.025, and 0.05. Correlations of A-RHDQoL AWI and the 2 overview items with the 3 subscales and total score of the W-BQ12 (12 pairs of correlations) would require sequential significance values of 0.0042, 0.0045, 0.005 etc. Correlations with the 8 subscales of the SF-36 (24 pairs of correlations) would require sequential significance values of 0.0021, 0.0022, 0.0023 etc.
Results
A-RHDQoL completion rates
One of the 129 returned questionnaires had no items completed and this was not included in analyses. Completion rates of the 128 completed A-RHDQoL questionnaires were as follows: impact ratings (98.1%), importance ratings (97.2%), indicating good acceptability to respondents.
The study sample characteristics
The average age of the sample was 70.2 years, and the mean age of leaving full-time education was 17.3 years. (See Table 1 for sample characteristics). Three volunteers reported stable disabilities (caused by blindness in one eye, osteoarthritis, loss of finger respectively). Forty-two (32.8%) reported no illnesses. Frequencies of reported illnesses were: osteoarthritis or other bone problem (19), hypertension (18), cardiovascular disease (10), benign prostate disease (9), bronchial disorder (9), digestive system disorder (6), skin disorder (3), and non-prostate cancer (3). None of these were considered severe enough to preclude invitation to screening for inclusion in the clinical trial.
Table 1.
Demographic and other sample characteristics
| Mean ± S.D. | |
| Age (years) | 70.17 ± 3.69 |
| Age at leaving full-time education | 17.25 ± 3.24 |
| Body Mass Index (kg/m2) | 26.24 ± 3.07 |
| Total T levels (nmol/l)* | 15.59 ± 5.58 |
| IGF-I levels (nmol/l)** | 15.26 ± 4.95 |
*Total T reference range for young males: 10 – 30 nmol/l **IGF-I reference range for young males: 12.8 – 62.2 nmol/l
Initial analyses to show that the 3 recruitment samples could be treated as one
Preliminary analyses were undertaken to check that there were no significant differences caused by the 3 recruitment procedures, using methods adopted in the development of the ADDQoL [27]. Item scores on the A-RHDQoL (brought as close as possible to normality) were first converted to z scores for each of the 3 subgroups and then recombined. All items on the questionnaire were forced onto one factor in Principal Components Analyses of recombined z scores and raw weighted scores. Loadings thus obtained were then compared by regression analysis. Regression analysis showed no significant difference between the loadings of standardized and raw scores. The correlation of 0.993 was close to a perfect 1, the constant (0.053) did not differ significantly from zero (t = 3.72, df = 23, p < 0.01) and the slope of the regression line (0.914) did not differ significantly from 1, (t = 39.8, df = 23, p < 0.0001). Thus the initial analyses demonstrated that the 3 recruitment subgroups could be treated as one for the purposes of reliability and factor analyses, (for which a larger N is desirable).
Deletion of 3 items from the 24-item scale
On the basis of early frequency, reliability and forced 1-factor analyses, 3 domain items (fertility, finances and depend) were deleted from the scale for the following reasons:
• Severe non-normality of distributions (fertility; finances; depend).
• A high proportion of volunteers regarded the domain as either not applicable [fertility (39.8%)] or not important [fertility (56.3%)].
• A high proportion of volunteers perceived age-related hormonal decline as having no impact on the domain [fertility (74.0%); finances (79.4%); depend (59.5%)].
• Items did not contribute to overall scale reliability in a reliability analysis where Cronbach's alpha was 0.933 (N = 100, N/A responses coded as zero): ['alpha if item deleted' for fertility and depend was 0.936 and 0.934 respectively].
• Unsatisfactory item-total correlation: [fertility (0.126)].
• A low and unsatisfactory loading on a forced 1-factor analysis [fertility (0.051); depend (0.288)].
Analysis of the 21-item A-RHDQoL
Descriptive statistics
Domains perceived to be most severely (and negatively) impacted by age-related hormonal decline were: memory (-4.54 ± 3.02), energy (-4.44 ± 2.49), sex life (-4.34 ± 3.08) and physical stamina (-4.29 ± 2.41), (maximum range -9 to +9). The least impacted domains were: society's reaction (-1.31 ± 1.91), worry about future (-1.45 ± 2.21), and bodily pain (-1.46 ± 2.05). (See Fig. 2). The A-RHDQoL AWI score was -2.79 ± 1.63, indicating an overall negative perceived impact of hormonal decline on QoL, although the mean for overview item QII – impact on QoL was less negative (-1.5 ± 1.05, score range -3 to 2). The men perceived their current QoL to be between good and very good (mean QI – present QoL was 1.51 ± 0.84, score range -2 to +3). Percentages of volunteers choosing the N/A response option were: work (34%), bodily pain (19%), family (8%) and sex life (3%).
Figure 2.
Mean weighted impact scores of A-RHDQoL domains and Average Weighted Impact score (N = 128)
Non-normality, found in 14/21 weighted item scores, was dealt with by reducing outlier scores and then conducting reflect and log transformations, leaving 5 items with a small degree of non-normality [z (skew) ranging from 3.0 to 5.9].
Reliability analyses
Reliability analyses showed the 21-item A-RHDQoL to have high internal consistency reliability: Cronbach's alpha was 0.935, N = 103 (N/A coded as zero), or 0.923, N = 55 (N/A coded as missing). All item-total correlations were satisfactory (>0.2), the lowest being work (0.358). The only items to detract slightly from the overall scale alpha were sleep and work (overall alpha with either item deleted was 0.936, N/A coded as zero). (See Table 2).
Table 2.
Reliability of the 21-item A-RHDQoL (N/A responses coded as zero)
| A-RHDQoL domain | Scale mean if item deleted | Scale variance if item deleted | Corrected item-total correlation | Alpha if item deleted |
| 1. Family life* | 21.86 | 1.13 | 0.68 | .9310 |
| 2. Social life | 21.89 | 1.15 | 0.62 | .9322 |
| 3. Work* | 21.92 | 1.21 | 0.36 | .9359 |
| 4. Health | 21.85 | 1.13 | 0.72 | .9304 |
| 5. Physical appearance | 21.86 | 1.17 | 0.55 | .9332 |
| 6. Physical capabilities | 21.81 | 1.13 | 0.74 | .9301 |
| 7. Physical stamina | 21.81 | 1.15 | 0.63 | .9320 |
| 8. Energy | 21.80 | 1.14 | 0.70 | .9309 |
| 9. Sex life* | 21.82 | 1.14 | 0.53 | .9342 |
| 10. Sleep | 21.88 | 1.16 | 0.43 | .9364 |
| 11. Bodily pain* | 21.91 | 1.19 | 0.45 | .9348 |
| 12. Stress-tolerance | 21.88 | 1.13 | 0.64 | .9318 |
| 13. Memory | 21.81 | 1.11 | 0.69 | .9308 |
| 14. Concentration | 21.83 | 1.11 | 0.76 | .9295 |
| 15. Travel | 21.89 | 1.15 | 0.69 | .9312 |
| 16. Holidays & leisure | 21.87 | 1.13 | 0.67 | .9312 |
| 17. Household tasks | 21.88 | 1.14 | 0.64 | .9317 |
| 18. Confidence | 21.87 | 1.13 | 0.72 | .9303 |
| 19. Motivation | 21.87 | 1.13 | 0.70 | .9306 |
| 20. Society's reaction | 21.90 | 1.18 | 0.50 | .9341 |
| 21. Worry about future | 21.90 | 1.16 | 0.56 | .9332 |
Cronbach's alpha for 21-item A-RHDQoL = 0.935, (N = 103). Standardized item alpha = 0.936.
Note: If reliability and factor analyses were run with N/A coded as missing only 55 cases were available for analysis because any volunteer responding N/A in any of the 4 items with N/A response options would be lost to analysis. This increased to 103 when N/A was coded as zero, still short of the full number of participants owing to missing data in other variables without N/A response options.
Dealing with missing data
To assess the effects of missing data on the measure's reliability, reliability analyses were run sequentially deleting the strongest item each time, (i.e. deleting the item having the lowest 'alpha if item deleted' and therefore contributing most to the internal consistency reliability of the scale) [43]. Calculation of overall A-RHDQoL AWI score was reliable at alpha = 0.9 with up to 4 items of missing data and reliable at alpha = 0.8 with up to 10 items of missing data, whether N/A responses were coded as zero or missing. Thus even if a completed questionnaire has some missing data, the overall A-RHDQoL AWI score can still be calculated, and that respondent's available data not lost to analysis.
Factor analyses
Unforced factor analysis
An unforced Principal Components Analysis of the 21-item A-RHDQoL, with Varimax rotation, produced 5 components with eigen values >1 that accounted for 66.7% of the total variance if N/A responses were coded as zero, (or 71.0% of the variance if N/A responses were coded as missing). Several items double loaded and there was no readily interpretable pattern of factor loadings (details not shown). The scree plot indicated only 1 strong factor.
Forced 1-factor analysis
Loadings of the 21-item A-RHDQoL, with N/A responses coded as zero, found that all items had satisfactory loadings (>0.4) except work (0.378). (See Table 3). When N/A responses were coded as missing, all loadings were satisfactory except worry about future (0.396), but work loaded at 0.624 (full details not supplied). Regression analysis found a moderately high correlation of 0.71 between the 2 sets of loadings (N/A responses coded as zero or missing). This result supported the calculation of the overall A-RHDQoL AWI score.
Table 3.
Forced 1-factor loadings of the 21-item A-RHDQoL (N/A responses coded as zero)
| 1. Family life* | 0.725 |
| 2. Social life | 0.689 |
| 3. Work* | 0.378 |
| 4. Health | 0.749 |
| 5. Physical appearance | 0.592 |
| 6. Physical capabilities | 0.770 |
| 7. Physical stamina | 0.684 |
| 8. Energy | 0.742 |
| 9. Sex life* | 0.555 |
| 10. Sleep | 0.491 |
| 11. Bodily pain* | 0.470 |
| 12. Stress-tolerance | 0.644 |
| 13. Memory | 0.671 |
| 14. Concentration | 0.730 |
| 15. Travel | 0.682 |
| 16. Holidays & leisure | 0.709 |
| 17. Household tasks | 0.650 |
| 18. Confidence | 0.727 |
| 19. Motivation | 0.715 |
| 20. Society's reaction | 0.543 |
| 21. Worry about future | 0.579 |
Loadings in bold are satisfactory (≥ 0.4). *item has N/A response option.
Note: If work were excluded from the forced 1-factor analysis, all items loaded satisfactorily and in excess of 0.46. Cronbach's alpha increased by 0.001 to 0.936 and the only item to detract from alpha was sleep (alpha with item deleted = 0.937)
Correlations
Biomedical variables
The overview item QI – present QoL showed a small significant correlation with total T levels (r = 0.26, p < 0.01, N = 114), indicating deteriorating QoL with decreasing T levels. There were no other significant correlations (following Bonferroni corrections) between QoL scores and hormone levels. Correlations with total T levels that approached significance were: overview item QII – impact on QoL (r = 0.21, p = 0.026), and domain bodily pain (rho = 0.27, p = 0.009) (indicating a tendency for greater perceived impact of hormonal decline on QoL, and greater perceived impact on bodily pain with decreasing T levels). The only correlation with IGF-I levels that approached significance was for the domain stress-tolerance (r = 0.22, p = 0.022) (indicating a tendency for greater perceived impact on tolerance of stress with decreasing IGF-I levels). There were no significant correlations with BMI or age.
SF-36 and W-BQ12 variables
Moderately strong positive correlations were found between A-RHDQoL AWI and all 3 W-BQ12 subscales and W-BQ12 General Well-being total, and with all SF-36 subscales except Bodily Pain. These indicated that perceived overall impact of age-related hormonal decline on QoL was inversely related to well-being and health status as expected. Overview item QI – present QoL correlated with W-BQ12 General Well-being total and all W-BQ12 subscales except Negative Well-being, and with all SF-36 subscales except Bodily Pain. Overview item QII – impact on QoL correlated with W-BQ12 General Well-being total but with fewer subscales (WBQ-12 Positive Well-being and Energy, and SF-36 General Health and Vitality). (See Table 4). Some correlations were undertaken of A-RHDQoL domains with subscales measuring similar quantities: A-RHDQoL domain social life correlated with SF-36 Social Functioning (rho = 0.24, p < 0.01), domain pain with SF-36 Bodily Pain (rho = 0.43, p < 0.001), domain physical capabilities with SF-36 Physical Functioning (rho = 0.26, p < 0.01), domain energy with SF-36 Vitality (rho = 0.25, p < 0.01) and with W-BQ12 Energy (r = 0.37, p < 0.001). These indicated less perceived impact of the domain on QoL with improving health status or well-being as expected.
Table 4.
Correlations of the A-RHDQoL with SF-36 and W-BQ12 variables
| A-RHDQoL | |||
| Average Weighted Impact | Overview item QI -present QoL | Overview item QII -impact on QoL | |
| WBQ-12 | |||
| Positive Well-being | 0.41** | 0.47** | 0.32** |
| Energy | 0.43** | 0.36** | 0.37** |
| Negative Well-being† | -0.23* | -0.20 | -0.13 |
| General Well-being total | 0.40** | 0.48** | 0.34** |
| SF-36 | |||
| Bodily Pain | 0.23 | 0.17 | 0.08 |
| General Health | 0.33** | 0.36** | 0.26** |
| Mental Health | 0.30** | 0.41** | 0.20 |
| Physical Functioning | 0.31** | 0.32** | 0.17 |
| Role-Emotional | 0.25** | 0.39** | 0.06 |
| Role-Physical | 0.29** | 0.27** | 0.06 |
| Social Functioning | 0.33** | 0.32** | 0.21 |
| Vitality | 0.30** | 0.46** | 0.28** |
Significant at **p < 0.01 and *p < 0.05 (2-tailed) after Bonferroni corrections. Correlations of all SF-36 subscales, W-BQ12 Negative Well-being and General Well-being total were Spearman's rho (non-parametric data), and those of W-BQ12 Positive Well-being and Energy were Pearson's r (parametric data). N: range 112 – 125. †A high score for W-BQ12 subscales indicates more of the subscale mood, (hence the negative correlations between Negative Well-being and A-RHDQoL variables).
Within the A-RHDQoL
There was a moderately strong correlation between A-RHDQoL AWI and overview item QII – impact on QoL (r = 0.61, p < 0.001), and a weaker, but still significant, correlation between A-RHDQoL and overview item QI – present QoL (r = 0.28, p < 0.01).
Free comments section
In the free comments section at the end of the A-RHDQoL questionnaire, respondents are asked to list any other ways in which age-related hormonal decline affects their QoL that have not already been included in the questionnaire. Seventy-three men responded, but when comments were analysed, the men were emphasising or expanding on their responses to existing questionnaire domains. Areas that did not have a specific item in the questionnaire, but were mentioned by at least one respondent were:
• Loss of physical and muscle strength (N = 5)
• Participation in sports (N = 5)
• Getting overheated and sweaty at night (N = 1)
• Loss of hearing and sight (N = 1) and singing voice (N = 1)
• Depression (N = 1)
• Taking longer to recover from illnesses (N = 1)
• Taking longer to do things (N = 1)
• Stiffness (N = 1).
'Loss of physical strength' is adequately covered by domains of physical capabilities and physical stamina, and 'participation in sports' is covered by domains holidays/leisure and physical capabilities. They do not appear to warrant new domains in the questionnaire. Only one respondent mentioned each of the other areas, insufficient to suggest the need for new domains. This, taken with the evidence that all 21 domains were perceived as negatively impacted by age-related hormonal decline (see Fig. 2), supports the content validity of the A-RHDQoL.
Eighty-six men responded to the section asking for comments on the A-RHDQoL questionnaire. Seven men (8%) made positive comments, remarking that the questionnaire was satisfactory, that all relevant areas had been covered or that they had found it interesting. On the other hand 12 men (14%) responded that they had had difficulty answering the questionnaire as they had little knowledge of the effects of declining hormone levels on QoL. Comments included:
"My answers have been based on the conceptions (? misconceptions) I have gained from observing effects of HRT on my wife" (age 72).
"Answers to foregoing questions are very subjective because my understanding of the actual effect of a decline in hormone levels is nil" (age 68).
"I feel that some questions relating to hormone levels are difficult to answer unless one has specific knowledge of the subject. However, I have tried to answer to the best of my scant knowledge – plus a little guess work" (age 70).
"Different hormones have different effects (growth, sex, etc.). The questions, however, only use the generic term "hormone"" (age 67).
"Subjective answers on subjects I know little about" (age 70).
"It would have helped to know how hormones affect the body and mind" (age 68).
"How do I know how I would feel if things were different?" (Respondent aged 77 referring to the hypothetical questions in the A-RHDQoL).
As we were interested to measure subjective impressions only, these comments were not considered to be a major cause for concern.
Discussion
The A-RHDQoL is a new measure of the perceived impact of age-related hormonal decline on QoL of older men, and which is still under development. The questionnaire is individualised because respondents rate only those domains that are relevant to them, and both the impact of age-related hormonal decline on life domains and the importance of each domain to the individual are taken into account in scoring. Being bi-polar it assesses both positive and negative impact on QoL, although this resulted, as expected, in skewed data distributions because few men perceived any positive benefits of hormonal decline. There were very high completion rates indicating that the questionnaire had good acceptability to the great majority of respondents.
Early psychometric analyses indicated that the original 24-item A-RHDQoL, which had been adapted from the version for adult GHD, the HDQoL, would be improved by dropping 3 of the original items. Internal consistency reliability and item-total correlations were high and factor analyses highly supportive of the shorter 21-item A-RHDQoL. The work domain was marked N/A by one third of respondents, it detracted very little from overall scale reliability (by 0.001) and produced a loading slightly lower than the preferred 0.4 on a forced 1-factor analysis (with N/A responses coded as zero), but had an acceptable loading if N/A responses were coded as missing. It was decided to retain work in the questionnaire because it was reported as relevant and important to the majority or respondents, and internal consistency reliability was excellent and very little impaired. There was therefore sufficient evidence from factor analyses to conclude that the 21 items could be summed into a single total, the A-RHDQoL AWI score.
Useful information can be elicited from the single overview items on their own. On average, respondents' current QoL was perceived to be between good and very good (QI – present QoL). QII, concerning impact of age-related hormonal decline on QoL, could provide an approximate substitute for the full A-RHDQoL for some purposes, (e.g. when respondent burden is of particular concern), as the correlation between QII and overall A-RHDQoL AWI was moderately high (0.61). Furthermore, rich information can be obtained by analysing the individual domains separately, and this could prove useful in studies where there is interest in the perceived impact of age-related hormonal decline on specific aspects of life. For example, analysing the individual domains separately in the present study showed that age-related hormonal decline was reported as having the most negative impact on the men's memory, energy, sex life, and physical stamina.
Insufficient data were collected to support the construct validity of the A-RHDQoL with regard to sub-group differences. The small but significant positive correlation of overview item QI – present QoL with total T levels may be a preliminary indication of construct validity, but there is insufficient research evidence to date to support a link between T levels and QoL. The lack of correlations with age may reflect the narrow (15-year) age range in this study. Strong correlations of A-RHDQoL AWI with the SF-36 and W-BQ12 questionnaires were not expected as the A-RHDQoL measures neither health status nor well-being. The small to moderate correlations found suggested that the A-RHDQoL is measuring something related to, but markedly different from the SF-36 or W-BQ12, and that neither of those instruments provides a substitute for the A-RHDQoL. As expected, overview item QI – present QoL had generally higher correlations with the SF-36 and W-BQ12 subscales than A-RHDQoL AWI or overview item QII – impact on QoL. The highest correlations of A-RHDQoL AWI and overview items were found with W-BQ12 subscales and W-BQ12 General Well-being total, and these were in the expected direction of decreasing self-reported impact of age-related decline in hormone levels with increasing well-being. Lower but still significant correlations were found with SF-36 subscales indicating that health status is a smaller component of QoL than well-being.
The respondents in this study were a self-selecting group who had answered advertisements placed in the national press for volunteers in a trial of GH and T replacement. It is probable that most of these men were aware that levels of these hormones decline with age, and perceived that hormone replacement therapy might be of benefit to their health and QoL. The great majority therefore had no difficulty in answering questions about the impact of declining hormone levels on various aspects of life. However, a minority of respondents (14%) reported (in the free comments section) problems in responding to the hypothetical items because they lacked knowledge of the effects of declining hormone levels on QoL. It is possible that some respondents associated the symptoms of declining hormonal levels with those of ageing, and similar item responses would have been obtained if the question stem "if my hormone levels had not declined with age....." had been replaced by "if I were younger....". It is also quite likely that the majority of men would be unaware that hormones might affect all the domains covered by the questionnaire, but that the questionnaire provided them with clues to an explanation of their symptoms of ageing. In other respects content validity was supported, as no new domains emerged from an analysis of the open section that invited comments.
Although the A-RHDQoL would be inappropriate for older men in the general population who were not aware of the hormonal decline that accompanies ageing, there is good evidence that it may be suitable for older men participating in trials of hormone replacement therapy, as they will be aware of the age-related decline in hormone levels. The A-RHDQoL is still under development and further studies are needed to assess construct validity (including sub-group differences), and test-retest reliability. The measure's sensitivity to change in the HRT trial that followed the screening process will be reported in due course. We recommend that the A-RHDQoL be used in conjunction with an established measure of health status (such as the SF-36) and another of well-being (such as the W-BQ12) to ensure that all areas of QoL and associated patient-reported outcomes of health status and well-being are covered.
Conclusion
The A-RHDQoL is a new individualised measure of the perceived impact of age-related hormonal decline on QoL, where the relevance and importance of life domains to an individual's QoL are taken into account in the scoring. Although it is still at an early stage of development, the 21-item questionnaire is performing well: it has good acceptability to the great majority of respondents, and excellent internal consistency reliability. No subscales emerged, but domains can be analysed separately if required and an overall score, the Average Weighted Impact score, calculated. Even at this present stage, the 21-item A-RHDQoL appears to be a useful tool and its construct validity (including sensitivity to subgroup differences), its test-retest reliability and sensitivity to change need now to be evaluated in clinical trials of hormone replacement in older men.
A-RHDQoL copyright
For access to and permission to use the A-RHDQoL, contact the copyright holder, Clare Bradley PhD, Professor of Health Psychology, Health Psychology Research, Royal Holloway, University of London, Egham, Surrey, TW20 0EX.
Authors' Contributions
MG, FM and PS conceived, designed and coordinated the screening study.
CM participated in the study design and coordination, contributed to questionnaire design, carried out the psychometric and statistical analyses of questionnaire data and drafted the manuscript.
CB led the design of the A-RHDQoL and contributed to the interpretation of psychometric analyses, decision-making regarding item selection, and manuscript preparation.
MG carried out biochemical measurements in the screening process for the subsequent HRT trial, and distributed A-RHDQoL, SF-36 and W-BQ12 questionnaires to older men invited to screening.
All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
C V McMillan was funded by a grant from the Guy's and St Thomas' Charitable Foundation. We also acknowledge the essential contributions of participants in the studies.
Contributor Information
Carolyn V McMillan, Email: c.mcmillan@rhul.ac.uk.
Clare Bradley, Email: c.bradley@rhul.ac.uk.
Manthos Giannoulis, Email: manthos.giannoulis@kcl.ac.uk.
Finbarr Martin, Email: finbarr.martin@gstt.sthames.nhs.uk.
Peter H Sönksen, Email: peter.sonksen@kcl.ac.uk.
References
- United Nations The world population prospects: the 2000 revision http://www.un.org/esa/population/publications/wpp2000/highlights.pdf (accessed June 2003)
- Hermann M, Berger P. Hormone replacement in the aging male? Exp Gerontol. 1999;34:923–933. doi: 10.1016/S0531-5565(99)00069-8. [DOI] [PubMed] [Google Scholar]
- Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth-hormone secretion in the adult population – Relation to age and adiposity. J Clin Invest. 1981;67:1361–1369. doi: 10.1172/JCI110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen A. Clinical review 24: Androgens in the aging male. J Clin Endocrinol Metab. 1991;73:221–224. doi: 10.1210/jcem-73-2-221. [DOI] [PubMed] [Google Scholar]
- Carroll PV, Christ ER, Growth Hormone Research Society Scientific Committee Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. J Clin Endocrinol Metab. 1998;83:382–395. doi: 10.1210/jcem.83.2.4594. [DOI] [PubMed] [Google Scholar]
- Colao A, di Somma C, Cuocolo A, Spinelli L, Tedesco N, Pivonello R, Bonaduce D, Salvatore M, Lombardi G. Improved cardiovascular risk factors and cardiac performance after 12 months of growth hormone (gh) replacement in young adult patients with gh deficiency. J Clin Endocrinol Metab. 2001;86:1874–1881. doi: 10.1210/jcem.86.5.7464. [DOI] [PubMed] [Google Scholar]
- Howell S, Shalet S. Testosterone deficiency and replacement. Horm Res. 2001;56:86–92. doi: 10.1159/000048142. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, Santanna J, Loh L, Lenrow DA, Holmes JH, Kapoor SC, Atkinson LE, Strom BL. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- Martin FC. Frailty and the somatopause. Growth Horm IGF Res. 1999;9:3–10. [PubMed] [Google Scholar]
- Anawalt BD, Merriam GR. Neuroendocrine aging in men. Andropause and somatopause. Endocrinol Metab Clin North Am. 2001;30:647–669. doi: 10.1016/s0889-8529(05)70206-1. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M, McHenry B. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab. 1996;81:3239–3243. doi: 10.1210/jcem.81.9.8784075. [DOI] [PubMed] [Google Scholar]
- Rudman D, Feller AG, Cohn L, Shetty KR, Rudman IW, Draper MW. Effects of human growth-hormone on body-composition in elderly men. Horm Res. 1991;36:73–81. doi: 10.1159/000182193. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH, Dlewati A, Staley J, Santanna J, Kapoor SC, Attie MF, Haddad JG, Jr, Strom BL. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:1966–1972. doi: 10.1210/jcem.84.6.5741. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2001;56:M266–272. doi: 10.1093/gerona/56.5.m266. [DOI] [PubMed] [Google Scholar]
- Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O'Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St Clair C, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- Giannoulis MG, McMillan CV, Bradley C, Breen L, Umpleby M, Sönksen PH, Martin F. Effects of growth hormone administration (by individually tailored dose titration) and/ or testosterone on body composition, physical performance and quality of life in healthy elderly men. Presentation at the Endocrine Society's 85th annual meeting. 2003.
- Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84:3681–3685. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Von Muhlen DG, Kritz-Silverstein D. Bioavailable testosterone and depressed mood in older men: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1999;84:573–577. doi: 10.1210/jcem.84.2.5495. [DOI] [PubMed] [Google Scholar]
- Wilson DE, Kaidbey K, Boike SC, Jorkasky DK. Use of topical corticosteroid pretreatment to reduce the incidence and severity of skin reactions associated with testosterone transdermal therapy. Clin Ther. 1998;20:299–306. doi: 10.1016/S0149-2918(98)80093-3. [DOI] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36). 1. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Bellantonio S, Gruman CA, Acosta RD, Prestwood KM. Effects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2002;57:M321–M325. doi: 10.1093/gerona/57.5.m321. [DOI] [PubMed] [Google Scholar]
- Reddy P, White CM, Dunn AB, Moyna NM, Thompson PD. The effect of testosterone on health-related quality of life in elderly males – a pilot study. J Clin Pharm Ther. 2000;25:421–426. doi: 10.1046/j.1365-2710.2000.00309.x. [DOI] [PubMed] [Google Scholar]
- Dupuy HJ. The Psychological General Well-being Index (PGWB) In: Wenger NK, Mattson ME, Furburg CD, Elinson J, editor. In Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. New York: Le Jacq Publishing Inc; 1984. [Google Scholar]
- Bradley C. Importance of differentiating health status from quality of life. Lancet. 2001;357:7–8. doi: 10.1016/S0140-6736(00)03562-5. [DOI] [PubMed] [Google Scholar]
- McGee HM, O'Boyle CA, Hickey A, O'Malley KM, Joyce CRB. Assessing the quality of life of the individual: The SEIQoL with a healthy and a gastroenterology unit population. Psychol Med. 1991;21:749–759. doi: 10.1017/s0033291700022388. [DOI] [PubMed] [Google Scholar]
- Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res. 1999;8:79–91. doi: 10.1023/a:1026485130100. [DOI] [PubMed] [Google Scholar]
- Bradley C, Speight J. Patient perceptions of diabetes and diabetes therapy: assessing quality of life. Diabetes Metab Res Rev. 2002;18:S64–S69. doi: 10.1002/dmrr.279. [DOI] [PubMed] [Google Scholar]
- Mitchell J, Bradley C. Design of the MacDQoL individualised measure of the impact of macular disease on quality of life. Qual Life Res. 2002;11:302. doi: 10.1023/B:QURE.0000031348.51292.4a. [DOI] [PubMed] [Google Scholar]
- McMillan C, Bradley C. A comparison of the psychometric properties of quality of life and related measures (GWBI, W-BQ12, NHP, SF-36 and HDQoL) for use in research into adult growth hormone deficiency (GHD) [abstract] Qual Life Res. 2000;9:1352. [Google Scholar]
- Bradley C. Achieving accessibility with quality: questionnaire measurement of condition-specific individualised quality of life. Proceedings of the British Psychological Society. 1999;7:143. [Google Scholar]
- McMillan CV, Bradley C, Gibney J, Healy ML, Russell-Jones DL, Sönksen PH. Psychological effects of withdrawal of growth hormone therapy from adults with growth hormone deficiency. Clin Endocrinol (Oxf) 2003;59:467–475. doi: 10.1046/j.1365-2265.2003.01870.x. [DOI] [PubMed] [Google Scholar]
- McMillan CV. PhD Thesis. Royal Holloway, University of London, Psychology Department; 2001. A psychometric evaluation of measures of quality of life and related health outcomes in adults with growth hormone deficiency. [Google Scholar]
- Bradley C. The Well-being Questionnaire. In: Bradley C, editor. In Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Chur Switzerland: Harwood Academic Publishers; 1994. pp. 89–109. [Google Scholar]
- Bradley C. The12-item Well-being Questionnaire: Origins, current stage of development, and availability. Diab Care. 2000;23:875. doi: 10.2337/diacare.23.6.875. [DOI] [PubMed] [Google Scholar]
- Tabachnik BG, Fidell LS. Using Multivariate Statistics. 2. New York: Harper and Row; 1989. [Google Scholar]
- Tabachnik BG, Fidell LS. Using Multivariate Statistics. 1. New York: Harper and Row; 1983. [Google Scholar]
- Todd C, Bradley C. Evaluating the design and development of psychological scales. In: Bradley C, editor. In Handbook of Psychology and Diabetes: A Guide to Psychological Measurement in Diabetes Research and Practice. Chur Switzerland: Harwood Academic Publishers; 1994. pp. 15–42. [Google Scholar]
- Nunnally JC, Bernstein IH. Psychometric Theory. 3. New York: McGraw-Hill, Inc; 1994. [Google Scholar]
- Kline P. A Handbook of Test Construction. London: Routledge; 1993. [Google Scholar]
- Kline P. An Easy Guide to Factor Analysis. London: Routledge; 1994. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- Mitchell J, Bradley C. Psychometric evaluation of the 12-item Well-being Questionnaire for use with people with macular disease. Qual Life Res. 2001;10:465–473. doi: 10.1023/A:1012540100613. [DOI] [PubMed] [Google Scholar]