Abstract
Introduction:
Waterpipe tobacco smoking is increasing in popularity though the toxicant exposure and effects associated with this tobacco use method are not well understood.
Methods:
Sixty-one waterpipe tobacco smokers (56 males; mean age ± SD, 30.9 ± 9.5 years; mean number of weekly waterpipe smoking episodes, 7.8 ± 5.7; mean duration of waterpipe smoking 8.5 ± 6.1 years) abstained from smoking for at least 24 hr and then smoked tobacco from a waterpipe ad libitum in a laboratory. Before and after smoking, expired-air carbon monoxide (CO) and subjective effects were assessed; puff topography was measured during smoking.
Results:
The mean waterpipe use episode duration was 33.1 ± 13.1 min. Expired-air CO increased significantly from a mean of 4.0 ± 1.7 before to 35.5 ± 32.7 after smoking. On average, participants took 169 ± 100 puffs, with a mean puff volume of 511 ± 333 ml. Urge to smoke, restlessness, craving, and other tobacco abstinence symptoms were reduced significantly after smoking, while ratings of dizzy, lightheaded, and other direct effects of nicotine increased.
Discussion:
Expired-air CO and puff topography data indicate that, relative to a single cigarette, a single waterpipe tobacco smoking episode is associated with greater smoke exposure. Abstinent waterpipe tobacco smokers report symptoms similar to those reported by abstinent cigarette smokers, and these symptoms are reduced by subsequent waterpipe tobacco smoking. Taken together, these data are consistent with the notion that waterpipe tobacco smoking is likely associated with the risk of tobacco/nicotine dependence.
Introduction
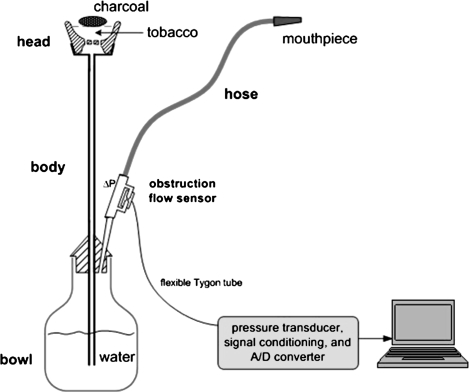
A tobacco waterpipe (also known as hookah, narghile, and shisha) is a device that allows tobacco smoke to pass through water prior to user inhalation (Figure 1). Until recently, waterpipes were associated with Middle Eastern societies (e.g., Maziak, Ward, Soweid, & Eissenberg, 2004; Knishkowy & Amitai, 2005), but surveys from different geographic regions suggest that waterpipe tobacco smoking is increasing in popularity globally, especially among young people (e.g., Eissenberg, Ward, Smith-Simone, & Maziak, 2008; Jackson & Aveyard, 2008; World Health Organization, 2005). These surveys indicate that waterpipe tobacco smoking is often practiced in social settings and is a pleasurable pastime experienced in the company of friends and family (Hammal, Mock, Ward, Eissenberg, & Maziak, 2008; Smith-Simone, Maziak, Ward, & Eissenberg, 2008). While many waterpipe tobacco smokers perceive that the behavior is less addictive than cigarette smoking (e.g., Smith-Simone et al., 2008), waterpipe tobacco smoke contains nicotine (Saleh & Shihadeh, 2008; Shihadeh, 2003) that is delivered to smokers in pharmacologically active doses (Shafagoj, Mohammed, & Hadidi, 2002). Indeed, a recent review shows that daily waterpipe use is associated with nicotine absorption rate equivalent to smoking 10 cigarettes/day (Neergaard, Singh, Job, & Montgomery, 2007). Interestingly, waterpipe tobacco smokers report behaviors that are indicative of tobacco/nicotine dependence, including repeated self-administration despite known risks, behavioral adaptations to insure access, and failed quit attempts (Maziak, 2008; Maziak, Eissenberg, Ward, & 2005; Salameh, Waked, & Aoun, 2008; Ward et al., 2005).
Figure 1.
A schematic depiction of a waterpipe with topography unit attached. The head (fired clay), body (metal or wood), water bowl (metal or glass), and corrugated hose (leather or nylon stretched over a wound flexible wire coil support) are the primary elements. Tobacco is loaded into the head, where several large holes in the base allow the smoke to pass into the central conduit of the body that leads to the water bowl. When flavored tobacco known as ma’assel is used, as shown here, a relatively deep (ca. 3 cm) head is filled with 10–20 g of a flavored tobacco mixture and covered with an aluminum foil sheet that is perforated for air passage. Burning coals are placed on top of the aluminum foil. When a smoker inhales through the hose, smoke bubbles into the water bowl from the body.
One factor that contributes to failed quit attempts in cigarette smokers is an aversive tobacco abstinence syndrome, which includes urges to smoke, anxiety, restlessness, and difficulty concentrating (Hughes, Higgins, & Hatsukami, 1990; John, Meyer, Hapke, Rumpf, & Schumann, 2004). Abstinence-induced symptoms that are reduced by tobacco use are key indicators of tobacco/nicotine dependence among cigarette smokers (e.g., Buchhalter, Acosta, Evans, Breland, & Eissenberg, 2005; Hughes & Hatsukami, 1986) but have not been studied systematically in waterpipe users. Smokers’ puff topography (e.g. puff number, volume, and duration) also may be related to dependence, as it predicts exposure to nicotine and other toxicants (e.g., carbon monoxide [CO]), as well as cessation in cigarette smokers (Breland, Kleykamp, & Eissenberg, 2006; Djordjevic, Stellman, & Zang, 2000; Kassel et al., 2007; Strasser, Pickworth, Patterson, & Lerman, 2004; Zacny & Stitzer, 1988). Waterpipe puff topography measurement has been limited (Shihadeh, Antonios, & Azar, 2005; Shihadeh, Azar, Antonios, & Haddad, 2004) but suggests that smokers may inhale 100 or more times the amount of smoke inhaled when a single cigarette is smoked (see World Health Organization, 2005). Inhalation of this volume of nicotine-containing smoke may explain reports of dependence among waterpipe tobacco smokers (e.g., Salameh et al., 2008; Maziak, Ward, & Eissenberg, 2004). This clinical laboratory study was designed to examine waterpipe smokers’ puff topography and its relationship to exposure to one tobacco smoke toxicant, CO, and also to investigate the effects of waterpipe tobacco smoking on tobacco abstinence symptoms in waterpipe tobacco smokers. We hypothesized that topography and CO level would be correlated and that waterpipe tobacco smokers’ abstinence symptoms would be reduced by smoking.
Methods
Subjects and design
This study was approved by the Institutional Review Board of the University of Memphis and Syrian Society Against Cancer and included 61 waterpipe smokers (18–56 years) from Aleppo, Syria, who reported using a waterpipe to smoke tobacco at least three times per week and not smoking cigarettes in the past year. Participants were recruited by brochure and word-of-mouth, provided written informed consent, were generally healthy, and, before completing the study's single waterpipe smoking episode, abstained from smoking for at least 24 hr as verified by expired-air carbon monoxide (CO) of <7 parts per million (ppm; the abstinence period was implemented to standardize recent smoke exposure and thus reduce variability on study outcomes).
Each participant provided demographic and tobacco use information and then was familiarized with the assessment procedures. Prior to the waterpipe use episode, expired-air CO was assessed and participants responded to all subjective effect measures (see below). Participants then were provided with a waterpipe (15 cm diameter, 61 cm height, 2.4 kg weight, 750 ml watervolume, and a leather hose), traditional kiln charcoal (i.e., not quick lighting disks), aluminum foil, and their preferred tobacco (all participants used a type of sweetened and flavored waterpipe tobacco known as ma’assel). Participants were invited to load the waterpipe and smoke it ad libitum (mean loaded tobacco ± SD 8.4 ± 2.6 g, mean consumed tobacco during session 4.1 ± 5.6 g). Puff topography parameters were measured continuously during the smoking session using a portable topography unit attached to the waterpipe hose (see below and Figure 1). At the end of smoking session, participants responded to all subjective questionnaires, and CO was measured 5 min after the last puff.
Outcome measures
Expired-air CO.
Expired-air CO was measured before and 5 min after waterpipe use via BreathCO monitor (Vitalograph, Lenexa, KS).
Puff topography.
Puff topography was assessed using validated equipment (Shihadeh et al., 2004, 2005). Briefly, a differential pressure obstruction flow sensor was integrated into the waterpipe hose, and inhalation-induced pressure changes were converted to voltage signals, amplified, digitized, and sampled. Previously calibrated software converted digital signals to airflow (standard milliliters per seconds) and integrated these data to produce measures of puff volume, duration, number, and interpuff interval (IPI).
Subjective effects.
Participants used a computer keyboard and mouse to respond to three subjective measures that were adapted for waterpipe smokers and were translated to Arabic and back translated to check for consistency. The Hughes–Hatsukami scale (adapted from Buchhalter et al., 2005) consists of 11 items: urges to smoke, irritability/frustration/anger, anxious, difficulty concentrating, restlessness, hunger, impatient, craving a cigarette/nicotine, drowsiness, depression/feeling blue, and desire for sweets. These items are presented as Visual Analog Scales (VASs) with a word or a phrase centered above a horizontal line anchored on the left with not at all and on the right with extremely. Participants respond to each item by moving a computer mouse-controlled cursor to any point on the line and clicking a mouse button, thus producing a vertical mark on the horizontal line, which can be further adjusted as necessary. The score for each scale is the distance of the vertical mark from the left anchor, expressed as a percentage of total line length. The brief version of the Tiffany–Drobes Questionnaire on Smoking Urges (QSU-brief, adapted from Cox, Tiffany, & Christen, 2001) consists of 10 items that are rated on a scale ranging from 0 (strongly disagree) to 6 (strongly agree). The 10 items form two factors: Factor I (intention to smoke) and Factor II (anticipation of relief from withdrawal). The direct effects of nicotine scale (adapted from Kleykamp, Jennings, Sams, Weaver, & Eissenberg, 2008) consist of 10 VAS items: nauseous, dizzy, lightheaded, nervous, sweaty, headache, excessive salivation, heart pounding, confused, and weak. The items are presented as VAS and are calculated the same way as with the Hughes–Hatsukami scale (above).
Data analysis
Because the first several puffs of a waterpipe use episode are used to heat the tobacco with the charcoal and represent an atypical pattern of puffing, the first minute of each puff topography record was not included in any subsequent analysis. In addition, puffs of less than 0.3-s duration were discarded. The primary analysis involved a within-subject analysis comparing pre- and postexhaled CO levels and questionnaire scores using paired t tests. In addition, Pearson correlation coefficient was calculated (log transformed data to deal with outliers) for the relation between puff topography parameters and CO boost (calculated by subtracting presmoking CO level from postsmoking CO level).
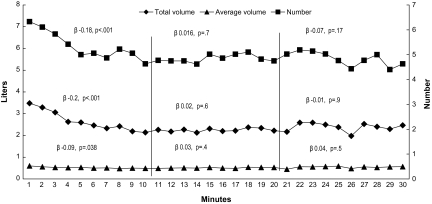
In addition, average puff parameters (frequency, puff volume, and total smoke volume) for each minute were calculated for all participants to create a time–puff plot demonstrating the collective puff dynamics throughout the smoking session. Because the mean duration of waterpipe smoking was 33 min in our sample and in order to maximize the number of participants included in the analysis, we analyzed puffing patterns of participants for 30 min. Linear regression analysis was used to analyze changes in average puffing parameters during the first, second, and third 10-min segments of the 30-min period because examination of the data suggested different puffing dynamics during these periods (see Figure 2). In this regression analysis, puff frequency, puff volume, and total smoke volume per minute were the dependent variables, while time was the independent variable.
Figure 2.
Mean total smoke volume (diamonds), puff volume (triangles), and puff number (squares) for each minute of the first 30 min of the waterpipe tobacco smoking session. Standardized linear regression coefficients (β) and their p value for the relation between session time and average puffing parameters for each 10-min portion are shown, together with the number of participants’ data analyzed in each portion.
Results
This study involved 61 waterpipe users (56 males; mean age ± SD 30.9 ± 9.5 years; mean number of weekly waterpipe smoking episodes 7.8 ± 5.7; mean duration of waterpipe smoking 8.5 ± 6.1 years). While data from all 61 participants are available for subjective effect and CO measures, technical issues led to a failure to collect complete topography data from five participants. As displayed in Table 1, average pre- and postsmoking expired-air CO was 4 ± 1.7 and 35.5 ± 32.7 ppm, respectively (i.e., a CO boost of 31.5 ± 32.7 ppm; p < .001). As Table 2 shows, on average, sessions lasted 33.1 ± 13.1 min, during which participants took a mean of 169 ± 100 puffs. Mean puff duration was 3.2 ± 1.2 s, mean puff volume was 511 ± 333 ml, mean IPI was 12.6 ± 5.9 s, and the mean total puff volume for the entire session was 79.1 ± 54.5 L. Standardized regression coefficient for the relation between smoking session time (minutes) and average puff parameters per minute was significant for total smoke volume, puff number, and puff volume in the first 10 min (β −.2, p < .001; −.18, p < .001; −.09, p = .038, respectively), while no relation was significant for the second 10-min portion of the session (Figure 2). Expired-air CO was strongly correlated with all puff parameters (Table 2) but most so with total volume of smoke inhaled per session (r = .7).
Table 1.
Scores of subjective questionnaires and physical measurements pre–post waterpipe use after 24-hr abstinence (N = 61)
| Measure | Presmoking; M ± SD | Postsmoking; M ± SD | Difference (post–pre) | Paired t | p* |
| CO | 4.0 ± 1.7 | 35.5 ± 32.7 | 31.5 | −7.550 | .000 |
| Hughes–Hatsukami scale | |||||
| 1—Urges to smoke | 39.5 ± 33.2 | 22.0 ± 30.2 | −17.5 | 4.047 | .000 |
| 2—Irritability/frustration/anger | 21.8 ± 29.6 | 16.9 ± 24.4 | −4.9 | 1.088 | .281 |
| 3—Anxious | 26.5 ± 28.0 | 19.3 ± 26.2 | −7.1 | 1.844 | .070 |
| 4—Difficulty concentrating | 21.7 ± 27.5 | 19.4 ± 25.2 | −2.3 | 0.529 | .599 |
| 5—Restlessness | 31.4 ± 32.3 | 19.3 ± 24.2 | −12.1 | 2.854 | .006 |
| 6—Hunger | 27.4 ± 30.3 | 29.7 ± 31.4 | 2.3 | −0.562 | .576 |
| 7—Impatient | 30.8 ± 34.0 | 22.4 ± 28.9 | −8.4 | 1.747 | .086 |
| 8—Craving a waterpipe/nicotine | 36.7 ± 35.8 | 18.0 ± 25.8 | −18.8 | 4.415 | .000 |
| 9—Drowsiness | 18.1 ± 23.7 | 24.4 ± 26.3 | 6.3 | −1.857 | .068 |
| 10—Depression/feeling blue | 21.6 ± 30.9 | 21.8 ± 27.2 | 0.2 | −0.053 | .958 |
| 11—Desire for sweets | 35.7 ± 36.4 | 36.8 ± 35.2 | 1.1 | −0.257 | .798 |
| Tiffany–Drobes QSU-brief | |||||
| Factor I—Intention to smoke | 2.97 ± 2.23 | 0.93 ± 1.71 | −2.03 | 7.041 | .000 |
| Factor II—Anticipation of relief from withdrawal | 1.90 ± 2.31 | 0.80 ± 1.63 | −1.10 | 3.962 | .000 |
| The direct effect of nicotine scale | |||||
| 1—Nauseous | 8.5 ± 20.3 | 21.6 ± 27.2 | 13.10 | −4.355 | .000 |
| 2—Dizzy | 9.6 ± 18.8 | 27.9 ± 31.2 | 18.25 | −4.854 | .000 |
| 3—Lightheaded | 18.5 ± 27.8 | 27.9 ± 30.7 | 9.39 | −2.370 | .021 |
| 4—Nervous | 21.0 ± 27.0 | 18.5 ± 23.5 | −2.54 | 0.781 | .438 |
| 5—Sweaty | 12.6 ± 22.5 | 17.3 ± 26.3 | 4.66 | −1.233 | .223 |
| 6—Headache | 17.7 ± 26.2 | 22.2 ± 29.3 | 4.46 | −1.099 | .276 |
| 7—Excessive salivation | 15.7 ± 21.3 | 18.7 ± 27.5 | 2.92 | −1.002 | .320 |
| 8—Heart pounding | 25.0 ± 27.5 | 23.7 ± 26.9 | −1.31 | 0.452 | .653 |
| 9—Confused | 15.2 ± 22.2 | 22.0 ± 28.9 | 6.82 | −2.411 | .019 |
| 10—Weak | 20.0 ± 29.6 | 24.9 ± 28.6 | 4.89 | −1.327 | .190 |
Note. CO, carbon monoxide; QSU, Questionnaire on Smoking Urges. p value for the difference in pre–post scores according to the paired t test (t test for dependent samples).
Table 2.
Puff topography results and correlation with CO boost
| Correlation with CO boost |
|||
| Puff topography parameter | M ± SD | r | p |
| Total session time (s) | 1,985 ± 783 | .367 | .005 |
| Total puff time (s) | 506 ± 272 | .595 | .000 |
| Number of puffs | 169 ± 100 | .445 | .001 |
| Mean puff duration (s) | 3.21 ± 1.16 | .19 | .161 |
| Mean puff volume (L) | 0.511 ± 0.333 | .315 | .018 |
| Mean interpuff interval (s) | 12.56 ± 5.85 | −.255 | .058 |
| Mean flow rate (unit) | 9.19 ± 4.12 | .377 | .004 |
| Maximum flow rate (unit) | 24.51 ± 32.82 | .235 | .081 |
| Total smoke inhaled per session (L) | 79.1 ± 54.5 | .638 | .000 |
Note. CO, carbon monoxide.
In addition, significant postsmoking decreases were observed on several tobacco abstinence measures, including items of the Hughes–Hatsukami scale (i.e., urges to smoke, restlessness, and craving a waterpipe) and both factors of the QSU-brief. In contrast, increases were observed on several items of the direct effect of nicotine scale (i.e., nauseous, dizzy, lightheaded, and confused).
Discussion
The results of this study suggest that waterpipe users are exposed to high levels of at least one smoking-related toxicant, CO. Also, the study corroborated previous reports that waterpipe tobacco smokers’ topography differs considerably from cigarette smokers’ topography. With regard to CO exposure, the mean CO boost of 31.5 ppm in this study was similar to that observed elsewhere (see El-Nachef & Hammond, 2008) and is several times that typically observed in smoking a single cigarette (6 ppm, e.g., Breland et al., 2006). In cigarette smokers, puff topography patterns predict exposure to tobacco smoke toxicants (e.g., Djordjevic et al., 2000; Zacny & Stitzer, 1988), thus a reasonable inference is that the greater CO exposure observed in waterpipe tobacco smokers may reflect differences in topography (e.g., mean puff volume in cigarette smokers [see Breland et al., 2006] is an order of magnitude lower than that observed here). This inference is supported by the observation that CO levels were correlated with several topography parameters: about 50% of the variation in CO exposure in this study can be explained by the variability in total smoke inhaled (i.e., r2 = .46). Once waterpipe smoke toxicant content is analyzed fully, this relationship may be useful in understanding waterpipe user toxicant exposure. In addition, the observation that puff volume and number decreased over the course of the session (see Figure 2) may reflect titration of nicotine dose, though this hypothesis will require empirical investigation where topography and plasma nicotine level are collected concurrently.
With regard to subjective effects, scores were reduced following waterpipe tobacco smoking on some measures, despite the fact that the required abstinence period of 24 hr may not have been unusual for participants in this study who generally smoked no more than once per day on average. To the extent that waterpipe tobacco smokers are nicotine/tobacco dependent, longer deprivation periods may elicit more symptoms and/or higher symptom ratings on these measures, especially considering that, for cigarette smokers, some abstinence effects peak after 3 days (e.g., Gross & Stitzer, 1989; Hatsukami, Hughes, Pickens, & Svikis, 1984). In addition, several dimensions of the waterpipe dependence syndrome may have not been tapped by the measures used in this study as these measures were developed and validated mainly in cigarette smokers. Some of the distinctive features of dependence among waterpipe users have been documented, including those related to its social domain. In particular, an essential stage on the dependence path may involve a transition from social to individual patterns of use (Maziak, Ward, & Eissenberg, 2004). These factors were not measured in this study and await future systematic research.
The recruitment method and laboratory environment of this study may limit generalizability. Word-of-mouth was the primary recruitment method; thus, the sample may represent a subpopulation of waterpipe smokers in Aleppo. Waterpipe tobacco smoking is often associated with other activities that were not permitted in the laboratory (e.g., eating and drinking) that may influence puff topography and thus toxicant exposure and other effects. In addition, the 24-hr abstinence requirement in this study, instituted to standardize recent tobacco exposure, may have influenced topography results. While the topography data reported here are consistent with those reported in other studies where no abstinence was required (e.g., Shihadeh et al., 2004), a greater understanding of the effects of waterpipe tobacco smoking may benefit from studies with multiple conditions in which presession abstinence duration is varied systematically. Also, the subjective questionnaires used in this study were not validated for waterpipe users or Arabic speakers; thus, items may have been misperceived or responses may have biased in some manner. However, the influence of these potential effects was reduced by translating and back translating the items and then pilot testing them with local waterpipe smokers. Thus, while these questionnaires await the necessary validation studies, there is reason to be confident in their sensitivity in assessing some of the dimensions of the waterpipe-related dependence syndrome.
Conclusions
Waterpipe users are exposed to at least one smoke toxicant, CO. The strong correlation between puff topography parameters and exposure to CO may help predict exposure to other smoke toxicants. In some waterpipe tobacco smokers, waterpipe smoking can attenuate craving, urges to smoke, and restlessness. Systematic manipulation and assessment of the effects of presmoking abstinence periods, a nonabstinence control condition, and the use of measures that tap waterpipe-unique dependence domains are warranted to assess the full spectrum of waterpipe effects.
Funding
This study was supported by grants from the U.S. National Institutes of Health (DA024876 and CA120142) and Research for International Tobacco Control, a secretariat of the International Development Research Centre (Canada).
Declaration of Interests
None declared.
Supplementary Material
References
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8:727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: The role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. Journal of the National Cancer Institute. 2000;92:106–111. doi: 10.1093/jnci/92.2.106. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Ward KD, Smith-Simone S, Maziak W. Waterpipe tobacco smoking on a U.S. College campus: Prevalence and correlates. Journal of Adolescent Health. 2008;42:526–529. doi: 10.1016/j.jadohealth.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Nachef WN, Hammond SK. Exhaled carbon monoxide with waterpipe use in US students. Journal of the American Medical Association. 2008;299:36–38. doi: 10.1001/jama.2007.6. [DOI] [PubMed] [Google Scholar]
- Gross J, Stitzer ML. Nicotine replacement: Ten week effects on tobacco withdrawal symptoms. Psychopharmacology. 1989;98:334–341. doi: 10.1007/BF00451684. [DOI] [PubMed] [Google Scholar]
- Hammal F, Mock J, Ward KD, Eissenberg T, Maziak W. A pleasure among friends: How narghile (waterpipe) smoking differs from cigarette smoking in Syria. Tobacco Control. 2008;17:e3. doi: 10.1136/tc.2007.020529. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Hughes JR, Pickens RW, Svikis D. Tobacco withdrawal symptoms: An experimental analysis. Psychopharmacology. 1984;84:231–236. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami D. Effects of abstinence from tobacco: A critical review. In: Kozlowski LT, Annis HM, Cappell HD, Glaser FB, Goodstadt MS, Israel Y, Kalant H, Sellers EM, Vingilis ER, editors. Research advances in alcohol and drug problems. New York: Plenum Press; 1990. pp. 317–384. [Google Scholar]
- Jackson D, Aveyard P. Waterpipe smoking in students: Prevalence, risk factors, symptoms of addiction, and smoke intake. Evidence from one British university. BMC Public Health. 2008;8:174. doi: 10.1186/1471-2458-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John U, Meyer C, Hapke U, Rumpf HJ, Schumann A. Nicotine dependence, quit attempts, and quitting among smokers in a regional population sample from a country with a high prevalence of tobacco smoking. Preventive Medicine. 2004;38:35–38. doi: 10.1016/j.ypmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Greenstein JE, Evatt DP, Wardle MC, Yates MC, Veilleux JC, et al. Smoking topography in response to denicotinized and high-yield nicotine cigarettes in adolescent smokers. Journal of Adolescent Health. 2007;40:54–60. doi: 10.1016/j.jadohealth.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Jennings JM, Sams C, Weaver MF, Eissenberg T. The influence of transdermal nicotine on tobacco/nicotine abstinence and the effects of a concurrently administered cigarette in women and men. Experimental and Clinical Psychopharmacology. 2008;16:99–112. doi: 10.1037/1064-1297.16.2.99. [DOI] [PubMed] [Google Scholar]
- Knishkowy B, Amitai Y. Water-pipe (narghile) smoking: An emerging health risk behavior. Pediatrics. 2005;116:e113–e119. doi: 10.1542/peds.2004-2173. [DOI] [PubMed] [Google Scholar]
- Maziak W, Eissenberg T, Ward KD. Patterns of waterpipe use and dependence: Implications for intervention development. Pharmacology Biochemistry and Behavior. 2005;80:173–179. doi: 10.1016/j.pbb.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Maziak W. The waterpipe: Time for action. Addiction. 2008;103:1763–1767. doi: 10.1111/j.1360-0443.2008.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maziak W, Ward KD, Eissenberg T. Factors related to frequency of narghile (waterpipe) use: The first insights on tobacco dependence in narghile users. Drug and Alcohol Dependence. 2004;76:101–106. doi: 10.1016/j.drugalcdep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Maziak W, Ward KD, Soweid RA, Eissenberg T. Tobacco smoking using a waterpipe: A re-emerging strain in a global epidemic. Tobacco Control. 2004;13:327–333. doi: 10.1136/tc.2004.008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neergaard J, Singh P, Job J, Montgomery S. Waterpipe smoking and nicotine exposure: A review of the current evidence. Nicotine & Tobacco Research. 2007;9:987–994. doi: 10.1080/14622200701591591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh P, Waked M, Aoun Z. Waterpipe smoking: Construction and validation of the Lebanon Waterpipe Dependence Scale (LWDS-11) Nicotine & Tobacco Research. 2008;10:149–158. doi: 10.1080/14622200701767753. [DOI] [PubMed] [Google Scholar]
- Saleh R, Shihadeh A. Elevated toxicant yields with narghile waterpipes smoked using a plastic hose. Food and Chemical Toxicology. 2008;46:1461–1466. doi: 10.1016/j.fct.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Shafagoj YA, Mohammed FI, Hadidi KA. Hubble-bubble (waterpipe) smoking: Levels of nicotine and cotinine in plasma, saliva and urine. International Journal of Clinical Pharmacology and Therapeutics. 2002;40:249–255. doi: 10.5414/cpp40249. [DOI] [PubMed] [Google Scholar]
- Shihadeh A. Investigation of mainstream smoke aerosol of the argileh waterpipe. Food and Chemical Toxicology. 2003;41:143–152. doi: 10.1016/s0278-6915(02)00220-x. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Antonios C, Azar S. A portable, low-resistance puff topography instrument for pulsating, high-flow smoking devices. Behavioral Research Methods. 2005;37:186–191. doi: 10.3758/bf03206414. [DOI] [PubMed] [Google Scholar]
- Shihadeh A, Azar S, Antonios C, Haddad A. Towards a topographical model of narghile waterpipe cafe smoking: A pilot study in a high socioeconomic status neighborhood of Beirut, Lebanon. Pharmacology, Biochemistry, and Behavior. 2004;79:75–82. doi: 10.1016/j.pbb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Smith-Simone S, Maziak W, Ward KD, Eissenberg T. Waterpipe tobacco smoking: Knowledge, attitudes, beliefs, and behavior in two U.S. samples. Nicotine & Tobacco Research. 2008;10:393–398. doi: 10.1080/14622200701825023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Pickworth WB, Patterson F, Lerman C. Smoking topography predicts abstinence following treatment with nicotine replacement therapy. Cancer Epidemiology Biomarkers & Prevention. 2004;13:1800–1804. [PubMed] [Google Scholar]
- Ward KD, Hammal F, VanderWeg MW, Eissenberg T, Asfar T, Rastam S, et al. Are waterpipe users interested in quitting? Nicotine & Tobacco Research. 2005;7:149–156. doi: 10.1080/14622200412331328402. [DOI] [PubMed] [Google Scholar]
- World Health Organization. TobReg Advisory Note. Waterpipe tobacco smoking: Health effects, research needs and recommended actions by regulators. Geneva, Switzerland: World Health Organization; 2005. [Google Scholar]
- Zacny JP, Stitzer ML. Cigarette brand-switching: Effects on smoke exposure and smoking behavior. Journal of Pharmacology and Experimental Therapeutics. 1988;246:619–627. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.