Abstract
Mesenchymal stem cells (MSCs) are widely distributed throughout the body. Despite intensive studies on the immunosuppressive effect of MSCs, little is known about whether MSCs affect lymphocyte apoptosis. We investigated the effect of MSCs on the spontaneous death of lymphocytes and found that MSCs inhibit the apoptosis of splenocytes and thymocytes as well as purified T and B cells. The protective effect of MSCs was absent when lymphocytes were not in contact with MSCs, indicating that the anti-apoptotic effect is exerted through direct interaction between MSCs and lymphocytes. Interestingly, this anti-apoptotic effect could be inhibited by neutralization of IL-6. Consequently, we found that of the expression of IL-6 by MSCs was augmented by contact with lymphocytes. Taken together, these results demonstrate that IL-6 plays an important role in the inhibition of lymphocyte apoptosis by MSCs.
Keywords: Apoptosis, lymphocytes, Cytokines, Mesenchymal Stem Cells
Introduction
Mesenchymal stem cells (MSCs), also known as mesenchymal progenitor cells, are adult stem cells that have been identified in various tissues, including bone marrow, placenta, neurons, cartilage, fat, skin and intestine. MSCs are capable of differentiating into lineage-specific cells and it is believed that these cells are critical in replenishing their respective adult tissues to maintain cellular homeostasis. The most studied MSCs are those derived from bone marrow, and it has been proposed that all stem cells in adult tissues originate from bone marrow. MSCs hold great promise for use in regenerative medicine in the treatment of degenerative diseases, pathological tissue damage, cancer, and autoimmune disorders. Furthermore, unlike embryonic stem cells, there are few ethical issues associated with clinical use of MSCs. Recently, several studies have shown that MSCs have strong immunosuppressive functions both in vitro and in vivo [1–4], eliciting great enthusiasm for their use as a potential therapy for autoimmune disorders [5] and transplant rejection. In fact, clinical trials with MSCs have achieved great success in treating severe graft versus host disease in patients [6, 7].
Recent studies have also revealed that MSCs are capable of promoting the survival of surrounding cells. It is believed that the supportive effect of bone marrow stromal cells on the hematopoietic process is in fact mediated by MSCs, especially for the survival and development of B lymphocytes [8, 9]. MSCs also promote the survival of tumor cells [10–12]. While the immunosuppressive properties of MSCs have been studied extensively, little is known about their influence on lymphocyte apoptosis. Apoptosis is critical in regulating the development and survival of immune cells, as well as in mediating their effector function. By default, lymphocytes must defend the body while avoiding reactivity to self-antigens. Lymphocytes turnover very quickly in comparison to other cell types, and are easily induced to undergo apoptosis. The negative selection of thymocytes occurs through apoptosis, [13, 14] and apoptosis mediates peripheral lymphocyte contraction after antigen-induced expansion[15, 16]. In the current study, we found that MSCs inhibit lymphocyte apoptosis, specifically the spontaneous death of splenocytes and thymocytes, as well as purified T and B cells. Surprisingly, this inhibitory effect required direct interaction between lymphocytes and MSCs, and depended on the induction of IL-6 expression by MSCs. Our findings reveal a novel mechanism by which MSCs provide survival signals to other cells.
Methods and Materials
Reagents, antibodies and mice
Antibodies against IL-6, and isotype controls were from eBioscience (San Diego, CA). Mouse T cell and B cell isolation kits were purchased from Miltenyi Biotech (Bergisch Gladbach, Germany). C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME) were kept under specific pathogen-free conditions in an American Association for Accreditation of Laboratory Animal Care-accredited animal facility at the Robert Wood Johnson Medical School.
MSC culture
Bone marrow was flushed out of tibia and femur bones isolated from 6 – 10 week old C57BL/6 mice. Cells were filtered through 40 μm nylon mesh, and cultured in α-MEM medium supplemented with 10% FBS, 2 mM glutamine, 100U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA). After 24 h., all non-adherent cells were removed. The medium was replenished every three days. MSC clones were generated by limiting dilution. Clone #12 was used for all clonal experiments. As reported in other studies, these cells were found to express MHC class I (Kb and Db) and Sca-1, but were negative for MHC class II, CD11b, CD31 and CD34. These MSCs were demonstrated to be capable of differentiating into adipocytes and osteocytes under appropriate differentiation conditions [17], indicating their pluripotency. Cells were always used before the 20th passage.
Apoptosis assay by DNA content analysis
Lymphocytes were cultured with MSCs at a 30:1 ratio (lymphocytes:MSCs) in 24 well plates, unless otherwise stated. DNA content analysis was done as previously described [18]. Briefly, cells were harvested and washed once with PBS. The cell pellets were then resuspended in staining buffer consisting of PBS containing 0.2% saponin, 50 ug/ml propidium iodide, and 10 ug/ml RNase A. Following a thirty-minute incubation at 37°C in dark, the samples were analyzed by flow cytometry using a FACScan flow cytometer (BD Immunocytometry Systems, San Jose, CA) with Cellquest software.
Real time PCR
Total RNA was isolated from cell pellets using an RNeasy Mini Kit (QIAGEN, Germany). Genomic DNA was removed from total RNA prior to cDNA synthesis using the RNase-free DNase Set for DNase digestion during RNA purification (QIAGEN). First-strand cDNA synthesis was performed for each RNA sample using Sensiscript RT Kit (QIAGEN). Random hexamers were used to prime cDNA synthesis. Messenger RNA expression was determined by real-time PCR using SYBR Green Master Mix (Applied Biosystems, Foster City, CA). Nucleotide sequences of specific primers were as follows:
IL-6, forward 5′-GAGGATACCACTCCCAACAGACC-3′,
reverse 5′-AAGTGCATCATCGTTGTTCATACA-3′. Thermocycling conditions included an initial hold at 50°C for 2 min., then 95°C for 10 min, followed by a 2-step PCR program of 95°C for 15 sec. and 60°C for 60 sec. for 40 cycles. Data were collected and quantitatively analyzed on an Mx 4000 system (Stratagene). Total RNA in each sample was normalized on endogenous β-actin expression level. All values are expressed as fold increase relative to the expression of β-actin
Microbead-based Cytokine Assay
Supernatants from cultures of MSC or MSCs co-cultured with splenocytes were collected at the indicated time points, and IL-6 was assayed using Bio-plex (Bio-Rad, Hercules, CA) according to the product instruction.
Results
MSCs inhibit the spontaneous apoptosis of lymphocytes
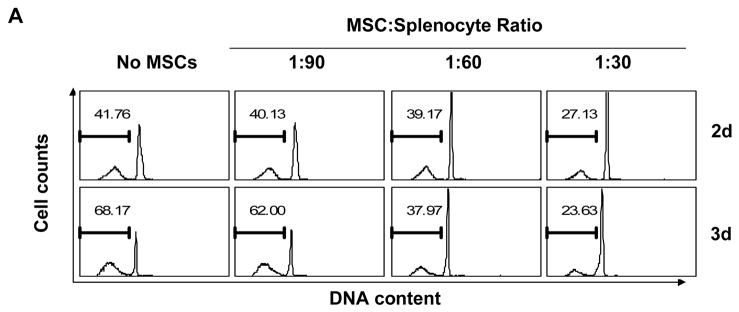
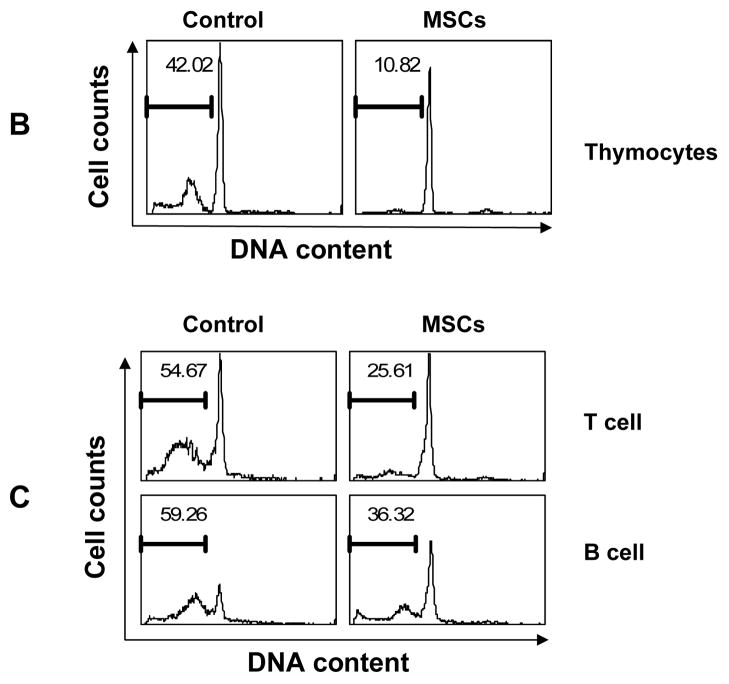
In our studies of MSCs, we have noticed that various cell types exhibit improved survival when co-cultured with MSCs. To systematically examine whether MSCs affect apoptosis, we employed mouse lymphocytes, which undergo rapid apoptosis in vitro. We co-cultured MSCs with freshly isolated splenocytes for two or three days, and determined apoptosis by detection of hypodiploid DNA content. MSCs strongly inhibited spontaneous splenocyte apoptosis, with the anti-apoptotic effect being proportional to the number of MSCs added (Fig. 1A). Significant inhibition was observed even at a ratio of 1:90 (MSCs:splenocytes). MSCs also inhibited the spontaneous apoptosis of thymocytes (Fig. 1B).
Figure 1. MSCs inhibit spontaneous lymphocyte apoptosis.
(A) Splenocytes were co-cultured with MSCs at a ratio of 30:1, 60:1 or 90:1 (splenocytes:MSCs), harvested at the indicated time points, then permeabilized and stained with propidium iodide to reveal hypodiploid peaks characteristic of apoptotic cells. (B) Thymocytes were co-cultured with MSCs at a 30:1 ratio for 2 days, and similarly analyzed for DNA content. (C) Purified T cells and B cells, prepared from splenocytes by immunoaffinity magnetic separation, were co-cultured with MSCs (30:1) for 2.5 days and DNA content determined. Values indicate percentage of cells with hypodiploid DNA content as detected by flow cytometry. Data shown are representative of four experiments.
Since preparations of splenocytes and thymocytes are mixtures of several cell types and contain some non-lymphocytic cells, it is possible that the effect of MSCs on one subgroup of splenocytes requires the presence of another population. To rule out this possibility, we next determined whether purified lymphocytes are similarly protected by MSCs. Accordingly, T cells and B cells were purified from whole splenocytes using immunoaffinity sorting with magnetic beads (Miltenyi Biotech) and then cultured with MSCs. As shown in Fig. 1C, purified T cells and B cells were protected by MSCs to the same extent as were bulk splenocytes. These results suggest that the anti-apoptotic effect of MSCs is independent of other cell types and is exerted on lymphocytes directly. In addition, we found that the anti-apoptotic function of MSCs is not MHC-restricted, since splenocytes from C57BL/6 and BALB/c mice were equally well protected from apoptosis by C57BL/6-derived MSCs (data not shown).
Lymphocyte protection by MSCs requires direct interaction
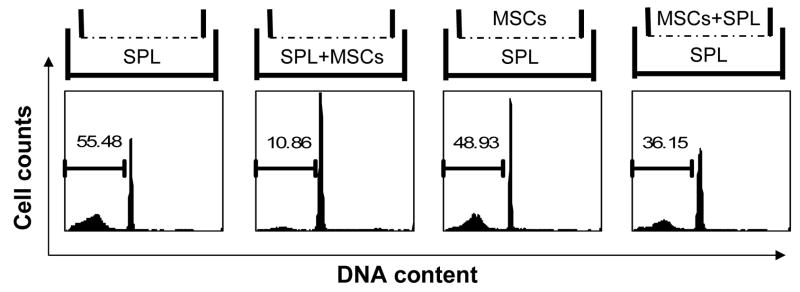
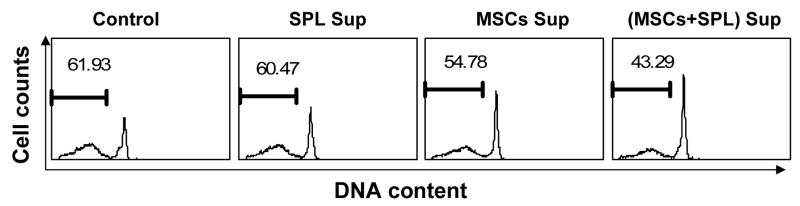
To understand the mechanism by which MSCs inhibit lymphocyte apoptosis, we first asked whether this effect is mediated by soluble factor(s) secreted by MSCs or if it requires cell-cell contact between splenocytes and MSCs. To distinguish between these two mechanisms, we cultured cells using the Transwell system, in which a permeable membrane separates two different chambers of a well. When MSCs and lymphocytes were co-cultured in the upper and lower chambers of the Transwell, the anti-apoptotic effect of MSCs was largely diminished (Fig. 2A), demonstrating that direct contact between splenocytes and MSCs is necessary for optimal inhibition of cell death. We reasoned that three possible scenarios may explain this result: (1) inhibition of apoptosis is dependent on direct cell-cell contact between MSCs and lymphocytes; (2) the anti-apoptotic factor(s) secreted by MSCs are effective only at close range; or, (3) MSCs produce soluble survival factors only after direct contact with lymphocytes. To distinguish between these possibilities, Transwell cultures were again employed. Lymphocytes and MSCs were added together in the upper chamber, with lymphocytes alone in the lower chamber (Fig. 2A). Spontaneous apoptosis of lymphocytes in the lower chamber was found to be inhibited when the upper chamber contained the MSC + splenocytes co-culture, but not when it contained MSCs alone (Fig. 2A). To further verify the above results, supernatant was harvested from cultures of MSCs grown alone or with splenocytes. Fresh splenocytes were then supplemented with 50 % supernatant and the effect on spontaneous apoptosis determined. As shown in Fig. 2B, supernatant from the MSC + splenocyte co-culture significantly inhibited splenocyte apoptosis, while supernatant from MSCs alone had limited effect. This result is consistent with that obtained using the Transwell system. These data indicate that cell-cell contact between MSCs and lymphocytes is required for MSCs to secrete the soluble survival factor(s). It should be noted that although this soluble factor(s) inhibited lymphocyte apoptosis, protection was incomplete, suggesting that other non-soluble factors may also be involved.
Figure 2. Direct interaction is important for the inhibition of lymphocyte apoptosis by MSCs.
(A) Splenocytes and MSCs were cultured in 6-well Transwell plates in which upper and lower chambers were separated by a permeable membrane (0.4 μm pores). Splenocytes (4.5×106) and MSCs (1.5×105) were added to the upper and/or lower chambers in the configurations depicted in the upper panels, at a 30:1 ratio. After 60 hours, splenocytes were analyzed to detect apoptotic cells. Data shown are representative of three independent experiments. (B) MSCs and splenocytes were cultured alone or together, and supernatant medium collected after 3 days. Subsequently, cultures of fresh splenocytes were supplemented with 50% supernatant, and the percentage of cells undergoing spontaneous apoptosis after 60 h determined. Cultures used to generate supernatants are indicated [SPL Sup = splenocytes alone, MSC Sup = MSCs alone, (MSC + SPL) Sup = co-culture of MSCs and splenocytes]. Data shown are representative of two separate experiments.
IL-6 plays an important role
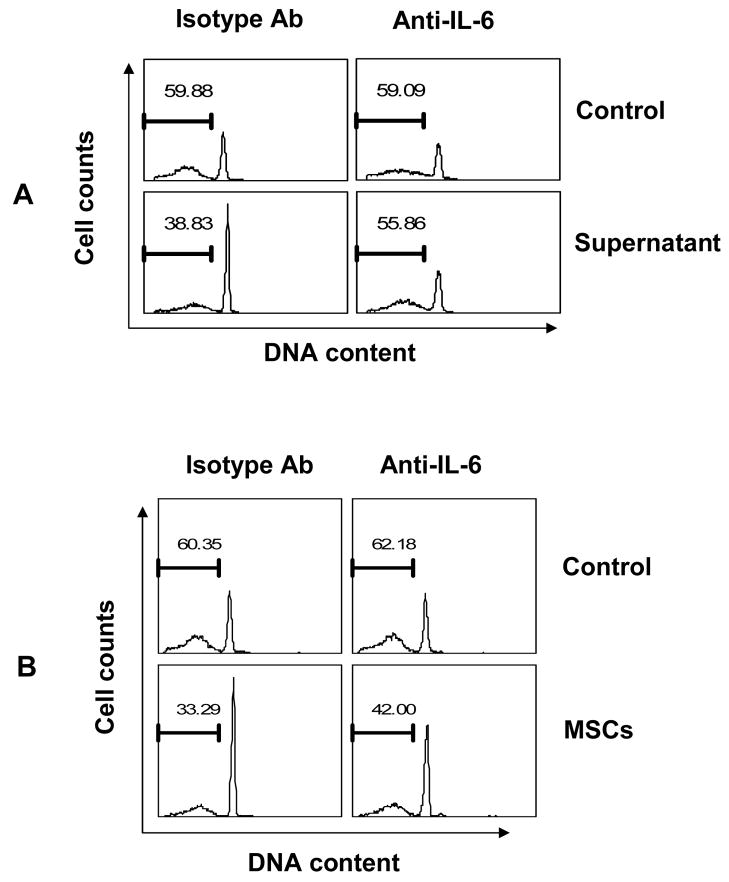
We have shown that soluble factor(s) are important in mediating the anti-apoptotic function of MSCs. To identify the culpable soluble factor(s), we employed neutralizing antibodies against several potential cytokines, such as IL-2 [19–21], IL-4[19, 22], IFNβ [23–26] and IL-6[21, 27–32] that have been shown to promote the survival of lymphocytes. As shown in Fig 3A, we found that neutralization of IL-6 largely eliminated the anti-apoptotic effect of supernatant from MSC + splenocyte co-culture, while the other antibodies had no significant effect (data not shown). Furthermore, anti-IL-6 also inhibited the protection of lymphocytes co-cultured directly with MSCs (Fig. 3B).
Figure 3. Neutralization of IL-6 blocked the anti-apoptotic function of MSCs.
(A) Cultures of splenocytes were supplemented with or without 50% supernatant from MSC + splenocyte co-culture, or (B) splenocytes were co-cultured directly with MSCs in the presence of antibody against IL-6 or an isotype control, and apoptosis determined after 60 h. Data are representative of three experiments.
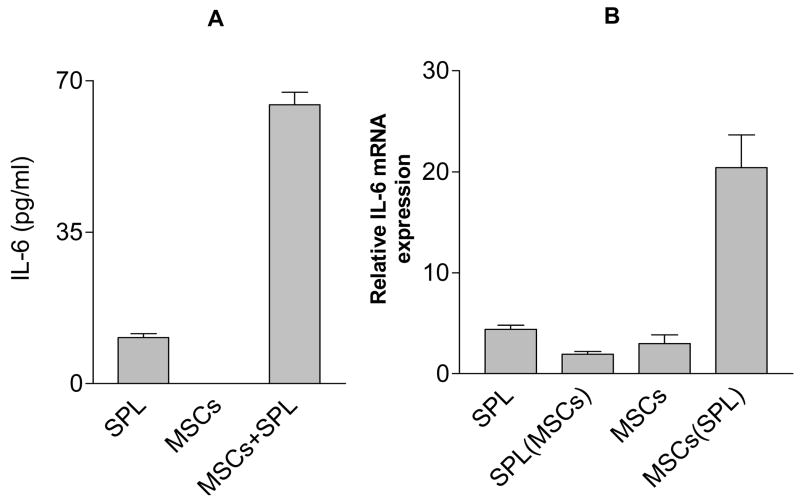
To confirm that IL-6 is indeed present in the culture supernatant, IL-6 protein was determined by Luminex microbead-based immunoassay. We found the amount of IL-6 was increased in supernatant from the MSC + splenocyte co-culture (Fig. 4A). To determine whether the increased IL-6 arises from MSCs or from splenocytes, IL-6 mRNA expression was measured in both MSCs and splenocytes separately harvested after mixed co-culture. As shown in Fig. 4B, IL-6 mRNA levels increased in MSCs after culture with splenocytes, while decreased in the co-cultured splenocytes. These data indicate that direct interaction between MSCs and lymphocytes is required to induce the expression of IL-6 by MSCs.
Figure 4. Direct interaction between splenocytes and MSCs induced the expression of IL-6 by MSCs.
(A) MSCs and splenocytes were cultured separately or together for 3 days and the amount of IL-6 in the supernatant measured using Bio-Plex analysis. (B) Splenocytes and MSCs were then harvested separately from either monoculture or the co-culture (MSCs are adherent) and expression of IL-6 mRNA was determined by real time PCR. Results shown as fold increase relative to β-actin. All values represent means ± SD of three separate samples. Data are representative of two independent experiments. SPL (MSC) = splenocytes from co-culture with MSCs; MSC (SPL) = MSCs from co-culture with splenocytes.
Discussion
MSCs have great potential for use in regenerative medicine. While various studies have focused on the immunosuppression and differentiation aspects of this unique cell population, very limited data is available on the ability of MSCs to inhibit apoptosis. In consistent with a recent report[33], our findings demonstrated that MSCs inhibit spontaneous lymphocyte apoptosis. Importantly, our findings further showed that the inhibition of lymphocyte apoptosis by MSCs requires direct interaction between MSCs and lymphocytes. Interestingly we demonstrate that one of the major anti-apoptotic factors produced by MSCs is IL-6. Also, the secretion of IL-6 by MSCs is also dependent on the direct interaction between MSCs and lymphocytes. Therefore, our study revealed a novel mechanism underlying the anti-apoptotic function of MSCs.
Our studies demonstrate a critical role for IL-6 in mediating the anti-apoptotic function of MSCs. Our evidence is that anti-IL-6 abrogated a major part of the protection from apoptosis provided by supernatant from co-cultures of MSCs + splenocytes. In addition, IL-6 expression is dramatically increased in MSCs after their contact with lymphocytes, as detected by real time quantitative PCR. Supernatants from of MSC + splenocyte co-cultures also contained large amounts of IL-6. While splenocytes alone also produced little IL-6, these cells actually showed decreased IL-6 expression after contact with MSCs, indicating that splenocytes are not the main source of IL-6 providing protection from apoptosis. We conclude that IL-6 secreted by MSCs is the major soluble factor, if not the only one, responsible for the anti-apoptotic effect of MSCs. It remains to be discovered how the interaction between lymphocytes and MSCs leads to increased IL-6 expression by MSCs.
We found that supernatant from co-cultures of MSCs and lymphocytes significantly protected lymphocytes from apoptosis. This effect, however, was less than that achieved by direct contact between lymphocytes and MSCs. Therefore, it is possible that non-soluble factors associated with MSCs also contribute to their ability to inhibit lymphocyte apoptosis.
A recent report showed that MSCs can also inhibit apoptosis in tumor cells [11]. Although the mechanism of MSC-mediated protection of tumor cells is not known, IL-6 may play a role here as well. We believe that MSCs might inhibit apoptosis in a wide range of cells. It would be interesting to discover whether apoptosis in different cell types is inhibited by MSCs through a common mechanism.
In conclusion, our findings indicated that MSCs inhibit apoptosis of lymphocytes, which requires direct interaction between MSCs and lymphocytes, and that soluble factors (mainly IL-6) secreted by MSCs after having cell-cell contact with lymphocytes play an important role in the anti-apoptotic function of MSCs.
Acknowledgments
The authors thank Arthur I. Roberts for his critique of the manuscript. This work was supported in part by New Jersey Science Commission (NJSTC-2042-014-84), USPHS grants (AI43384, AI057596), and the National Space Biomedical Research Institute (IIH00405), which is supported by the National Aeronautics and Space Administration through the Cooperative Agreement NCC 9-58.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28 (5):219–226. doi: 10.1016/j.it.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30 (1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 3.Rasmusson I. Immune modulation by mesenchymal stem cells. Exp Cell Res. 2006;312 (12):2169–2179. doi: 10.1016/j.yexcr.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Le Blanc K, Ringden O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11 (5):321–334. doi: 10.1016/j.bbmt.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Uccelli A, Zappia E, Benvenuto F, Frassoni F, Mancardi G. Stem cells in inflammatory demyelinating disorders: a dual role for immunosuppression and neuroprotection. Expert Opin Biol Ther. 2006;6 (1):17–22. doi: 10.1517/14712598.6.1.17. [DOI] [PubMed] [Google Scholar]
- 6.Ringden O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81 (10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363 (9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 8.Welch PA, Burrows PD, Namen A, Gillis S, Cooper MD. Bone marrow stromal cells and interleukin-7 induce coordinate expression of the BP-1/6C3 antigen and pre-B cell growth. International immunology. 1990;2 (8):697–705. doi: 10.1093/intimm/2.8.697. [DOI] [PubMed] [Google Scholar]
- 9.Dorshkind K, Landreth KS. Regulation of B cell differentiation by bone marrow stromal cells. International journal of cell cloning. 1992;10 (1):12–17. doi: 10.1002/stem.5530100104. [DOI] [PubMed] [Google Scholar]
- 10.Ame-Thomas P, Maby-El Hajjami H, Monvoisin C, et al. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109 (2):693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- 11.Ramasamy R, Lam EW, Soeiro I, et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21 (2):304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 12.Zhu W, Xu W, Jiang R, et al. Mesenchymal stem cells derived from bone marrow favor tumor cell growth in vivo. Experimental and molecular pathology. 2006;80 (3):267–274. doi: 10.1016/j.yexmp.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Siggs OM, Makaroff LE, Liston A. The why and how of thymocyte negative selection. Current opinion in immunology. 2006;18 (2):175–183. doi: 10.1016/j.coi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Green DR, Bissonnette RP, Glynn JM, Shi Y. Activation-induced apoptosis in lymphoid systems. Seminars in immunology. 1992;4 (6):379–388. [PubMed] [Google Scholar]
- 15.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 16.Badovinac VP, Harty JT. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunological reviews. 2006;211:67–80. doi: 10.1111/j.0105-2896.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 17.Xu G, Zhang L, Ren G, et al. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell research. 2007;17 (3):240–248. doi: 10.1038/cr.2007.4. [DOI] [PubMed] [Google Scholar]
- 18.Wang R, Shi YF. A simplified protocol for apoptosis assay by DNA content analysis. BioTechniques. 2002;(Suppl):88–91. [PubMed] [Google Scholar]
- 19.Zubiaga AM, Munoz E, Huber BT. IL-4 and IL-2 selectively rescue Th cell subsets from glucocorticoid-induced apoptosis. J Immunol. 1992;149 (1):107–112. [PubMed] [Google Scholar]
- 20.Mor F, Cohen IR. IL-2 rescues antigen-specific T cells from radiation or dexamethasone-induced apoptosis. Correlation with induction of Bcl-2. J Immunol. 1996;156 (2):515–522. [PubMed] [Google Scholar]
- 21.Pajusto M, Ihalainen N, Pelkonen J, Tarkkanen J, Mattila PS. Human in vivo-activated CD45R0(+) CD4(+) T cells are susceptible to spontaneous apoptosis that can be inhibited by the chemokine CXCL12 and IL-2, -6, -7, and -15. Eur J Immunol. 2004;34 (10):2771–2780. doi: 10.1002/eji.200324761. [DOI] [PubMed] [Google Scholar]
- 22.Zamorano J, Mora AL, Boothby M, Keegan AD. NF-kappa B activation plays an important role in the IL-4-induced protection from apoptosis. International immunology. 2001;13 (12):1479–1487. doi: 10.1093/intimm/13.12.1479. [DOI] [PubMed] [Google Scholar]
- 23.Su L, David M. Inhibition of B cell receptor-mediated apoptosis by IFN. J Immunol. 1999;162 (11):6317–6321. [PubMed] [Google Scholar]
- 24.Scheel-Toellner D, Pilling D, Akbar AN, et al. Inhibition of T cell apoptosis by IFN-beta rapidly reverses nuclear translocation of protein kinase C-delta. Eur J Immunol. 1999;29 (8):2603–2612. doi: 10.1002/(SICI)1521-4141(199908)29:08<2603::AID-IMMU2603>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Pilling D, Akbar AN, Girdlestone J, et al. Interferon-beta mediates stromal cell rescue of T cells from apoptosis. Eur J Immunol. 1999;29 (3):1041–1050. doi: 10.1002/(SICI)1521-4141(199903)29:03<1041::AID-IMMU1041>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Chang L, Crowston JG, Sabin CA, Khaw PT, Akbar AN. Human tenon’s fibroblast-produced ifnbeta and the prevention of t-cell apoptosis. Investigative ophthalmology & visual science. 2001;42 (7):1531–1538. [PubMed] [Google Scholar]
- 27.Curnow SJ, Scheel-Toellner D, Jenkinson W, et al. Inhibition of T cell apoptosis in the aqueous humor of patients with uveitis by IL-6/soluble IL-6 receptor trans-signaling. J Immunol. 2004;173 (8):5290–5297. doi: 10.4049/jimmunol.173.8.5290. [DOI] [PubMed] [Google Scholar]
- 28.Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues resting mouse T cells from apoptosis. J Immunol. 1997;158 (12):5791–5796. [PubMed] [Google Scholar]
- 29.Ito H, Hirotani T, Yamamoto M, Ogawa H, Kishimoto T. Anti-IL-6 receptor monoclonal antibody inhibits leukocyte recruitment and promotes T-cell apoptosis in a murine model of Crohn’s disease. Journal of gastroenterology. 2002;37 (Suppl 14):56–61. doi: 10.1007/BF03326415. [DOI] [PubMed] [Google Scholar]
- 30.Mudter J, Wirtz S, Galle PR, Neurath MF. A new model of chronic colitis in SCID mice induced by adoptive transfer of CD62L+ CD4+ T cells: insights into the regulatory role of interleukin-6 on apoptosis. Pathobiology. 2002;70 (3):170–176. doi: 10.1159/000068150. [DOI] [PubMed] [Google Scholar]
- 31.Kurita-Ochiai T, Ochiai K, Suzuki N, Otsuka K, Fukushima K. Human gingival fibroblasts rescue butyric acid-induced T-cell apoptosis. Infection and immunity. 2002;70 (5):2361–2367. doi: 10.1128/IAI.70.5.2361-2367.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nature medicine. 2000;6 (5):583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 33.Benvenuto F, Ferrari S, Gerdoni E, et al. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells. 2007;25 (7):1753–1760. doi: 10.1634/stemcells.2007-0068. [DOI] [PubMed] [Google Scholar]