Abstract
Interleukin (IL)-18 stimulates T helper 1 (Th1) and Th2-mediated immune responses and has been shown to modulate acute graft-versus-host disease (aGvHD). It is still unknown whether increased IL-18 levels during aGvHD are of host or donor origin and how the absence of IL-18 impacts migration and expansion of conventional CD4+CD25− (Tconv) and CD4+CD25+ regulatory (Treg) T cells in vivo. By utilizing IL-18 gene deficient donor versus recipient animals we found that the major cytokine production during the early phase of aGVHD induction was recipient derived, while donor hematopoietic cells contributed significantly less. By generating IL-18−/− luciferase transgenic mice we were able to investigate the impact of IL-18 on Tconv and Treg expansion and trafficking with in vivo bioluminescence imaging. While migration to secondary lymphoid organs was not significantly impacted by the absence of host IL-18, Tconv but not Treg expansion increased significantly. Absence of host IL-18 production translated into lower IFN-γ levels in the early phase after transplantation. We conclude that host derived IL-18 is a major factor for IFN-γ production that may have a protective effect on CD4+ mediated aGvHD, but is non-essential for Treg expansion in an allogeneic environment.
Keywords: Acute graft-versus-host disease, regulatory T cells, Interleukin-18
INTRODUCTION
The production of proinflammatory cytokines, such as IL-1, IL-2, IL-12, IL-18, TNF and IFN-γ, is a key feature of acute graft-versus-host disease (aGvHD) and the balance between T helper 1 (Th1) and Th2 cytokine production determines end organ damage [1]. While TNF was demonstrated to affect regulatory T cell (Treg) function [2], the impact of IL-18, which is increased in human and murine aGvHD [3, 4], on Treg is not yet defined. IL-18R engagement leads to MyD88 signaling followed by activation of TNF-receptor-associated factor and NFκB [5] which enhances the production of IFN-γ [6, 7]. Interestingly, IFN-γ and other Th1 cytokines have been shown to be involved in mechanisms that are protective against aGvHD [8–12].
Cell types that are relevant in GvHD with reported ability to produce IL-18 include macrophages, Kupffer cells, dendritic cells, T-cells, intestinal epithelial cells and keratinocytes [4, 13–15]. Itoi and colleges have shown that IL-18 levels were only slightly elevated when Caspase-1-deficient recipients were engrafted with wildtype H-2 disparate splenocytes [4]. Although this indicates that IL-18 secretion during GvHD is dependant on host caspase-1, there is currently no information on the relative contribution of host versus donor to IL-18 cytokine levels and whether host or donor IL-18 is critical for GvHD induction and promotion. Increased levels of serum IL-18 and IL-18(R)α receptor expression on T lymphocytes were found in patients that developed GvHD after aHCT [3, 16]. In murine studies IL-18 was demonstrated to have a differential impact on CD4 as compared to CD8 mediated GvHD [17], to be critical for Fas mediated donor T-cell apoptosis [18] and to reduce GvHD severity when administered to the donor prior to transplantation [19]. Another study confirmed that IL-18 neutralization increased T cell expansion but did not influence GVHD severity, which may be explained by the use of a different model with non-irradiated recipients and the difference in IL-18 neutralization by a soluble IL-18 binding protein [20]. CD4+CD25+ regulatory T cells (Treg) have been demonstrated to play a major role in modulating GvHD [21, 22]. The relevance of IL-18 for this cell population has so far only been investigated in a model of oral tolerance induction, a study that indicated that IL-18 is essential for the induction of antigen-specific regulatory T cells, that were defined by CD25 expression and the production of TGF-β [23].
In the present study we investigate the kinetics of IL-18Rα expression on Treg and Tconv as well as the origin and the relevance of IL-18 in the early phase after transplantation for Treg and Tconv expansion by utilizing cytokine deficient donor or recipient animals.
MATERIALS AND METHODS
Mice
C57B/6 (H-2kb), IL-18−/− (H-2kb), FVB/N (H-2kq), and Balb/c (H-2kd) mice were purchased from Jackson Laboratory (Bar Harbor, ME) or Charles River Laboratory (Wilmington, MA). Mice were used between 6 and 12 weeks of age. Only male to male or female to female combinations were used for transplant experiments. The luciferase-expressing (luc+) transgenic FVB/N line was generated as previously described [24]. luc+ offspring of the transgenic founder line FVB-L2G85 were backcrossed onto the C57B/6 background (F8) and then crossed with IL-18−/− animals, both on C57B/6 background. All animal protocols were approved by the University Committee on Use and Care of Laboratory Animals at Stanford University.
Generation of luciferase-(luc+) transgenic IL-18−/− mice
In order to track Treg and Tconv in the absence of IL-18 production by donor derived hematopoetic cells, we generated luciferase transgenic IL-18−/− animals. The luciferase-expressing (luc+) transgenic line [24] was backcrossed onto the C57B/6 background (F8) and then crossed with IL-18−/− animals (C57B/6). IL-18 gene deficiency was verified by RT-PCR (Fig. 4A) and luciferase expression was monitored by bioluminescence imaging (Fig. 4B). The employed luc+/IL-18−/− mice were bred for more than 10 generations on the C57B/6 background.
Figure 4. Generation of IL-18−/− luciferase transgenic C56Bl/6 mice.
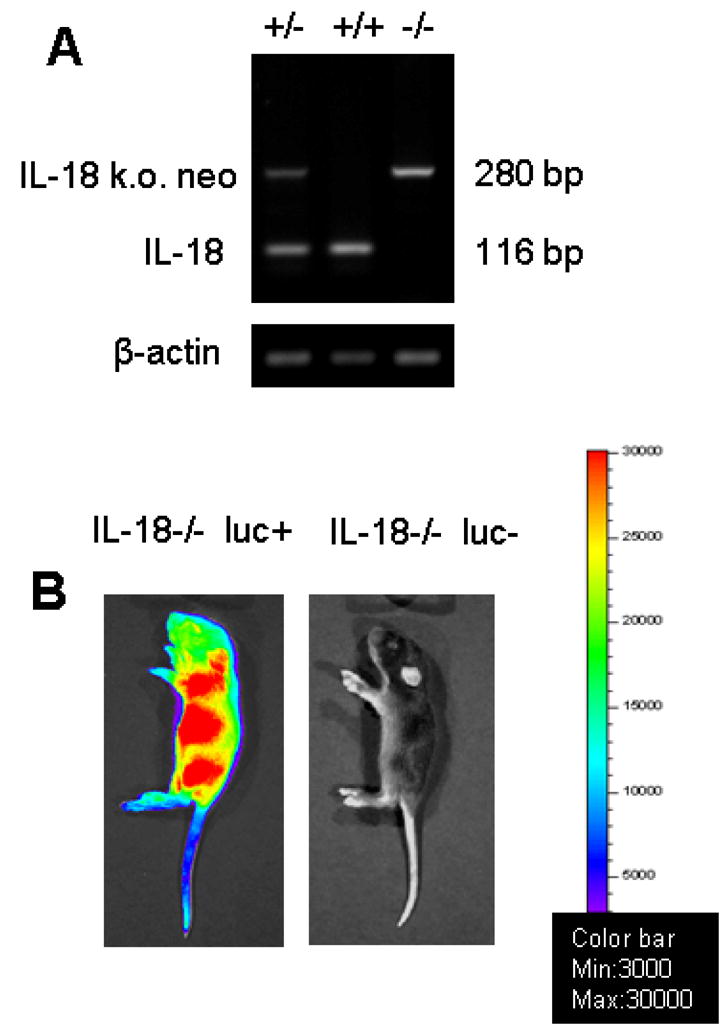
A. Genomic DNA was isolated from wt, heterozygote or homozygote IL-18−/− C56Bl/6 mice and amplified by RT PCR with IL-18 and IL-18−/− neo cassette specific primers. No IL-18 gene product (116 bp) was detected in IL-18−/− C56Bl/6 that had been crossed on the luc background.
B. The constitutive expression of the luc transgene is monitored by bioluminescence imaging. Presented is a representative homozygote IL-18−/− C56Bl/6 mouse with (left) or without (right) the luc transgene.
Flow cytometric cell purification and analysis
The following antibodies were used for flow cytometric analysis: unconjugated anti-CD16/32 (2.4G2), CD4 (RM4-5), CD8α (53-6.7), CD25 (PC61), CD11c (M1/70), CD45R/B220 (RA3-6B2), H-2Kq (KH114), H-2Kd (34-2-12) from BD Pharmingen (San Diego, CA) and eBiosciences (San Diego, CA). Foxp3 staining was performed using the intracellular Foxp3 staining kit (Ab: FJK-16s) as described in the manufacturers instructions (eBioscience, San Diego, CA). Goat anti-murine IL-18Rα(R&D Systems) staining was followed by SP-biotinylated mouse anti–goat IgG (H plus L) diluted in PBS (Jackson ImmunoResearch Laboratories) for 20 min. Cells were subsequently washed twice in PBS and then stained with streptavidin-APC (BD PharMingen). Staining was performed in the presence of purified anti-CD16/32 at saturation to block unspecific staining. Propidium iodide (Sigma, St Louis, MO) was added prior to analysis to exclude dead cells. All analytical flow cytometry was done on a modified dual laser LSRScan (BD Immunocytometry Systems, San Diego, CA) in the Shared FACS Facility, Center for Molecular and Genetic Medicine at Stanford using FlowJo software (TreeStar, Ashland, OR) for data analysis.
Cell isolation and sorting
Single cell-suspensions from cervical lymph nodes (cLN), axillary lymph nodes (aLN), inguinal lymph nodes (iLN), mesenteric lymph nodes (mLN) and spleens were enriched for CD25+ cells after sequential staining with anti-CD25 PE (BD PharMingen) and anti-PE magnetic beads using the autoMACS system (POSSEL program, Miltenyi Biotec, Auburn, CA). CD25+ cells were then stained with anti-CD4 APC and sorted on a MoFlow cell sorter (Becton Dickinson, Mountain View, CA) for the CD25high population (15–20% of the enriched CD25+ cells) or for CD4+CD25− cells. The CD4+CD25+ population was routinely greater than 95% Foxp3+. T-cell–depleted bone marrow (TCD BM) was obtained through negative depletion using anti-CD4 and anti-CD8 magnetic beads (Miltenyi Biotech). For isolation of lymphocytes from the liver, the portal vein was perfused with PBS, the organ was removed and homogenized and the lymphocytes were separated by Ficoll gradient centrifugation.
Real time quantitative PCR for Foxp3
Total RNA was isolated from fresh cell pellets using the RNeasy MiniKit (Qiagen, Valencia, CA) and genomic DNA was eliminated by digestion with a modified proprietary Dnase (DNA-free; Ambion, Austin, TX). 500ng total RNA was mixed with dT16 primer in a volume of 11 μl, incubated at 65° C for 10 min and immediately placed on ice. Following addition of 100 units Superscript II reverse transcriptase (GIBCO, Carlsbad, CA) reverse transcription was performed for 2 h at 42° C in 1x RT reaction buffer (GIBCO, Carlsbad CA), 10 μM DTT, 500 μM dNTP (Amersham Biosciences, Pittsburgh, PA) with 2.5 μM dT16 primer in a volume of 20μl PCR reactions were performed in a final volume of 20μl with cDNA prepared from 20ng RNA and a final concentration of 1x SYBR®Green PCR Master Mix (ABI, Foster City, CA, USA) and 200nM of each primer (sequences: FoxP3 forward, GGAGCCGCAAGCTAAAAGC; FoxP3 reverse, TGCCTTCGTGCCCACTG; GAPDH forward, GTCCTGAAGTATGTCGTGGAGTCTAC; and GAPDH reverse, GGCCCCGGCCTTCTC). The reaction was run in an ABI 7700 Sequence Detection System with the following cycling conditions: 50° C for 2 min, 94°C for 10min, then 40 cycles of 94° C for 15 sec and 60° C for 60 sec. For each gene a standard curve was prepared and triplicate measurements were performed for each sample.
GVHD model
Acute GVHD was induced as described previously [25]. Briefly, recipients were given 5 × 106 TCD-BM cells after lethal irradiation with 800 cGy. To induce aGvHD the following numbers of CD4+ T cells were given: 8 × 105 (C57B/6→Balb/c), 2 × 106 (FVB/N→C57Bl/6), 2 × 106 (Balb/c→C57B/6). For Treg trafficking studies in the FVB/N→C57Bl/6 model, 5 × 105 CD4+CD25high luc+ Treg were injected on d0. Transplanted mice were housed in autoclaved cages and kept on antibiotic water (Sulfomethoxazole Trimethoprim, Schein Pharmaceutical, Corona, CA).
In vivo Bioluminescence imaging (BLI)
In vivo bioluminescence imaging (BLI) was performed as previously described [26]. Briefly, mice were injected intraperitoneally with luciferin (10 μg/g bodyweight). Ten minutes later mice were imaged using an IVIS200 charge-coupled device (CCD) imaging system (Xenogen, Alameda, CA) for 5 minutes. Imaging data were analyzed and quantified with Living Image Software (Xenogen) and IgorProCarbon (WaveMetrics, Lake Oswego, OR).
Histopathology
Tissues were fixed with 10% formalin, embedded in paraffin and sections of 5 μm thickness were mounted on positively charged precleaned microscope slides (Superfrost/Plus; Fisher Scientific, Hampton, NH). Hematoxylin/eosin (H/E) staining of paraffin-embedded tissue sections was performed according to standard protocols. Tissues from small bowel, large bowel and liver were evaluated by an experienced pathologist (N.K.) according to a previously published histopathology scoring system [27]. Evaluation of the stained tissue sections was performed on a Nikon microscope (Eclipse, TE 300; Melville, NY). Standard magnifications were 200x/numerical aperture 0.45 and 400x/numerical aperture 0.60. Microscopic photos were obtained using a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI).
In vitro Treg/Tconc activation culture
Treg or Tconv cells (C57Bl/6) were incubated with irradiated (30Gy) CD11c+ cells derived from Balb/c mice. Each cell type in a concentration of 2×105 per flat bottom well. After 48 hours cells were collected, washed and analyzed by FACS for IL-18Rα surface expression in combination with CD4 and intracellular Foxp3 staining.
ELISA for IL-18 and IFN-γ
Serum was collected from Balb/c recipients on days 1, 2, 3, 5 and 8 after transplantation. ELISA Assays were performed according to the manufacturer’s instructions (R&D systems Minneapolis, MN). Briefly, samples were diluted 1:2 to 1:5, and the cytokine was captured by the specific primary mAb pre-coated on the microplate and detected by horseradish peroxidase labeled secondary mAbs. Plates were read at 450 nm using a microplate reader (model Spectra Max 190; Bio-Rad Labs, CA). Recombinant murine cytokines (BD PharMingen) were used as standards. Samples and standards were run in duplicate, and the sensitivity of the assays was 16 to 20 pg/mL for each cytokine, depending on the sample dilution.
Statistical analysis
Differences in animal survival (Kaplan-Meier survival curves) were analyzed by log-rank test. Differences in mean fluorescence intensity (MFI), lymphocyte counts, Foxp3 RNA expression, thymidine incorporation, proliferation of luc transgenic T-cells and serum cytokine levels were analyzed using the two tailed Student’s t test. Error bars indicate the standard deviation from the geometric mean. A p-value <0.05 was considered statistically significant.
RESULTS
CD4 mediated aGvHD severity is enhanced in IL-18 deficient hosts
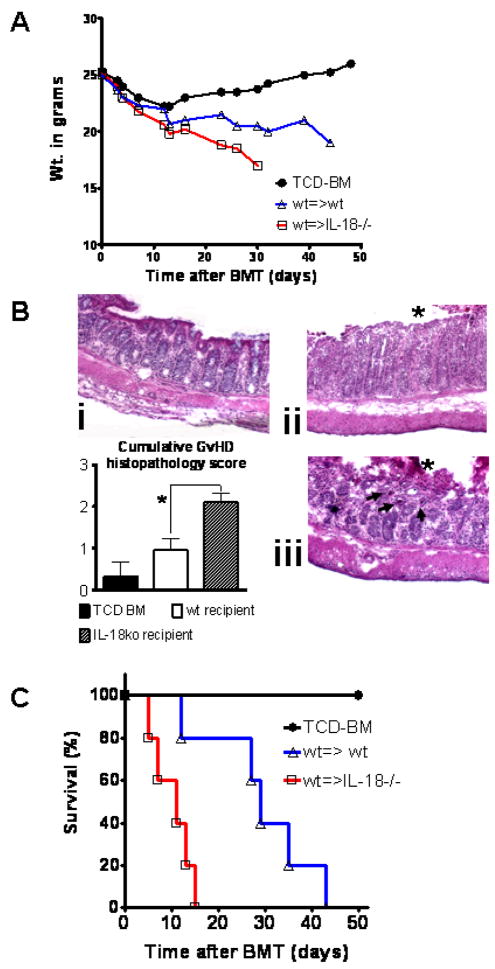
To investigate the impact of IL-18 deficiency of the host on aGvHD severity in our model, CD4 Tconv (H-2kq) were transplanted into lethally irradiated C57Bl/6 recipients that were either wt or IL-18 deficient. Interestingly, IL-18 deficiency of the host led to increased aGvHD severity as assessed by weight loss (Fig. 1A) and more severe histopathological GvHD damage of the colon with multiple crypt abscesses when the host was IL-18 deficient (Fig. 1B). Histopathology scoring from small bowel, large bowel and liver tissue samples harvested on day 10 after BMT from 5 animals per group, demonstrated the highest GvHD severity (*p<0.05) in IL-18 deficient recipients (Fig. 1B). This translated into a more aggressive course of GvHD in the wt→IL-18−/− combination as compared to the wt→wt combination (Fig. 1C).
Figure 1. IL-18 deficiency of the recipient enhances graft-versus-host disease severity.
A. C57Bl/6 wt or IL18−/− mice were given 5 × 106 TCD-BM cells and 2 × 106 CD4+ T cells (both H-2kq) after lethal irradiation with 800 cGy.
Weight change of mice receiving TCD-BM (●, n=15), with T cells from wt donors transplanted into wt recipients (△, n=15) or with T cells from wt donors transplanted into IL18−/− recipients (□, n=15).
B. Ten days after transplantation, mice from the indicated group were sacrificed for histological examination. Representative colon sections stained by conventional H&E of mice receiving TCD-BM (i), wt→wt (ii) or wt→IL-18 deficient recipients (iii) are shown. GvHD tissue damage manifests as crypt abscess (arrow) and mucosal denudation (asterix). IL-18 deficiency of the host leads to more severe histopathological GvHD damage of the colon with multiple crypt abscesses. Magnification is x200. Cumulative histopathology scoring from small bowel, large bowel and liver tissue samples harvested on day 10 after BMT from 3 representative animals per group is shown (*p<0.05).
C. Survival of mice receiving TCD-BM alone (●, n= 5) with T cells from wt donors transplanted into IL-18 deficient (□, n= 5) or wt (△, n= 5) recipients. Percentage survival of C57Bl/6 recipients is significantly reduced when IL-18−/− as compared to wt recipients are used (□ versus △, p=0.027). Survival data from one of three independent experiments is shown.
IL-18Rα expression on Tconv and Treg cells is upregulated in the presence of alloantigen
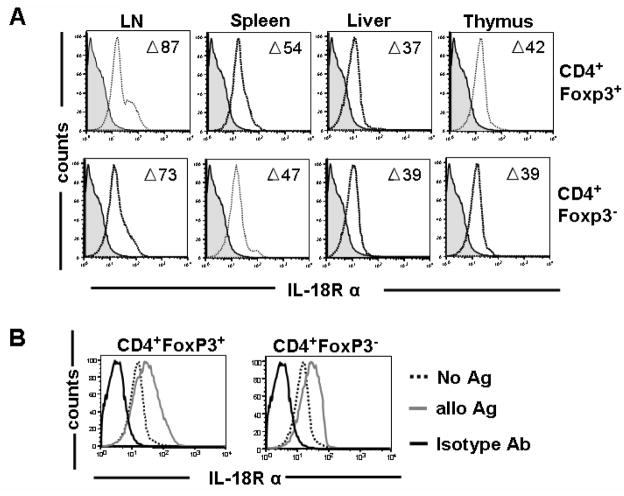
Based on the observation that the absence of IL-18 led to a more severe course of aGvHD we aimed to study the relevance of IL-18 for Treg which have been shown to promote transplantation tolerance in models of aGvHD [21] and solid organ transplantation [28]. Therefore, in a first step IL-18Rα surface expression was evaluated on conventional CD4+ Tcells (Tconv) and Treg cells from mesenteric (mLN), cervical (cLN), axillary (aLN) and mesenteric (mLN) lymph nodes, spleen, liver and thymus as shown for the respective experiments. Gating on CD4+Foxp3+ versus CD4+Foxp3− demonstrated comparable expression of IL-18Rα on Treg and Tconv cells (Fig 2A). Data are presented as the difference (Δ) between the mean fluorescence intensity (MFI) of the positive stain (anti–IL-18Rα) and the MFI of the negative control (goat Ig alone). Interestingly IL-18Rα surface expression differed with respect to the origin of the cell population being the highest in the lymph nodes (73 and 87) and lowest in liver derived T cells (37 and 39). Activation for 48h with allogeneic APCs led to an upregulation of the IL-18Rα on both Tconv and Treg (Fig 2B) suggesting that IL-18 signaling may be relevant during alloantigen driven T cell responses.
Figure 2. Expression of IL-18Rα on regulatory and conventional T cells.
A. The indicated lymphoid organs and the liver were harvested from naïve C57B6 mice. Upper row: CD4+Foxp3+ cells, lower row: CD4+Foxp3− cells. Open histogram: anti-IL-18Rα Ab staining, filled histogram: isotype control staining. One representative of 4 independent experiments is shown. (LN=pooled mesenteric, cervical, axillary and inguinal lymph nodes). Numbers refer to the difference (Δ) between the MFI of the positive stain and the MFI of the isotype (negative) stain.
B. CD4+CD25+ or CD4+CD25− cells (H-2kb) derived from the spleen, were activated by co-culture with irradiated (30 Gy) CD11c+ APCs (H-2kd) for 48 hours and stained for 18Rα and intracellular Foxp3. The presented histograms display gating on CD4+Foxp3+ cells or CD4+Foxp3− cells. Mean fluorescence intensity for 18Rα surface expression increases significantly in both Treg and Tconv during alloantigen activation (MFI no Ag vs alloAg: 65.3±2 vs 127.4±7, p<0.05 and 69.5±3 vs 108.6±2.1, p<0.05, respectively). Solid black line: isotype control.
Intact Treg development and function in the absence of IL-18
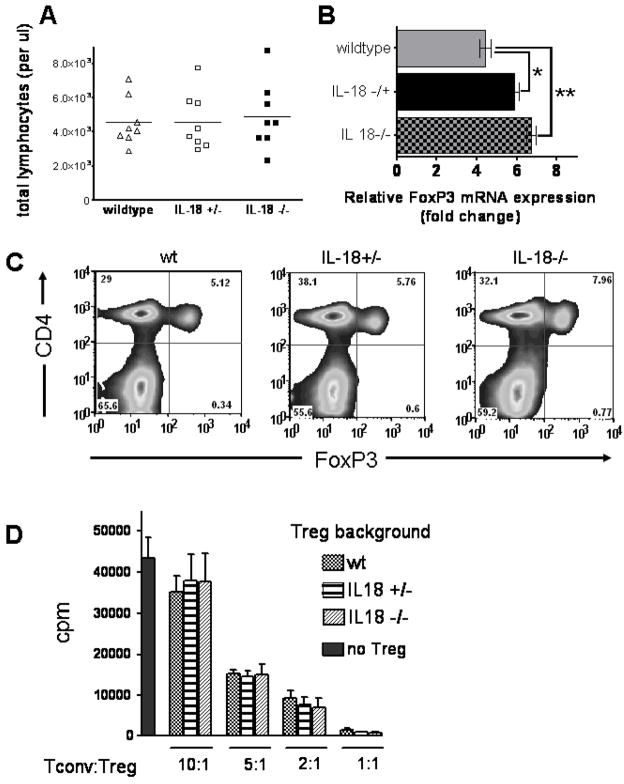
To study the relevance of intact IL-18 production for the development of Treg we employed mice with a disrupted IL-18 gene which have been previously described [29]. Total lymphocyte counts in IL-18−/− and IL-18−/+ mice were comparable with wildtype animals (Fig. 3A). Foxp3 expression within the CD4+ cell population was comparable in IL-18 deficient as compared to wildtype animals as quantified by real-time PCR (Fig. 3B) and intracellular Foxp3 protein analysis by FACS (Fig. 3C). To further investigate if there was a functional difference, Treg from IL-18−/− and wildtype mice were compared with respect to their capability to suppress alloantigen driven T cell proliferation. Importantly, there were no significant differences with respect to suppressor function by Treg derived from IL-18−/−, IL-18−/+ or wildtype mice (Fig. 3D). These data indicate that Treg that have developed in the absence of IL-18 are functionally suppressive. Furthermore, these findings are in line with the observation that IL-18 deficient mice display a defect in NK cell function but no signs of autoimmunity [29].
Figure 3. CD4+Foxp3+ T cells develop normal and are functionally suppressive in the absence of IL-18.
A. The absolute numbers of lymphocytes in the peripheral blood of wt, heterozygote or homozygote IL-18−/− C56Bl/6 mice were determined. Each data point represents an individual animal. No significant difference between the groups.
B. Relative Foxp3 mRNA expression level in CD4 T cells isolated from the spleens of wt, heterozygote or homozygote IL-18−/− C56Bl/6 mice (*p=0.24, **p=0.09).
C. Frequency of CD4+Foxp3+ cells isolated from the spleens of wt, heterozygote or homozygote IL-18−/− C56Bl/6 mice.
D. Treg isolated from the spleens of wt, heterozygote or homozygote IL-18−/− C56Bl/6 mice were used to suppress alloantigen driven proliferation of CD4+CD25− cells (H-2kb) after 72 hours. Stimulator cells were irradiated (30 Gy) CD11c+ APCs (H-2kd). Thymidine incorporation of conventional T cells is significantly reduced when Treg are included in the culture at different ratios. One proliferation analysis of 3 independent experiments is shown.
IL-18 production of the donor is non-essential for aGvHD development
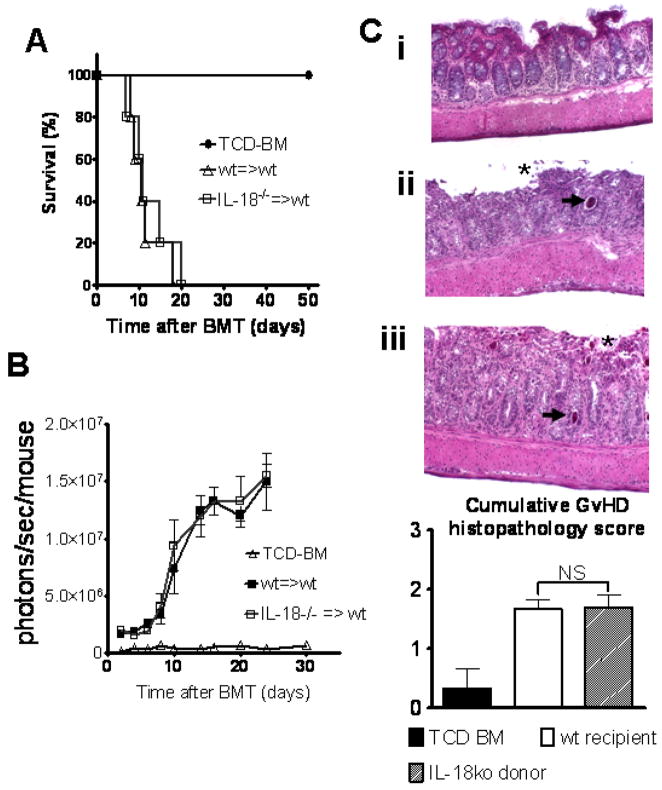
To study the impact of donor IL-18 on the course of aGvHD we employed TCD-BM and luc+ CD4 T cells from IL-18 deficient donors. Transplantation of luc+ IL-18−/− donor CD4 T cells into wt animals (C57Bl/6→Balb/c) resulted in a comparable course of aGvHD with no survival difference (Fig. 5A). The expansion kinetics and homing pattern of luc+ IL-18−/− and wt donor CD4 T cells was comparable (Fig. 5B). Histopathological analysis confirmed the survival data, indicating an intact colon mucosa in the TCD-BM group, while crypt abscesses and mucosal denudation as signs of aGvHD tissue damage to the intestinal tract were found to a comparable extend in recipients of wt and IL-18 deficient donor T cells (Fig. 5C). Cumulative histopathology scoring from small bowel, large bowel and liver tissue derived from 3 animals per group, demonstrated comparable GvHD severity in IL-18 deficient as compared to wt donors (Fig. 5C). These data indicate that donor IL-18 production does not impact aGvHD severity.
Figure 5. IL-18 deficiency of the donor does not impact CD4 T cell expansion and graft-versus-host disease severity.
A. Balb/c mice were given 5 × 106 TCD-BM cells and 8 × 105 CD4+ T cells (both H-2kb) after lethal irradiation with 800 cGy.
Survival of mice receiving wt TCD-BM alone (●, n= 5), with T cells from wt donors (△, n=5) or TCD-BM and T cells from IL-18 deficient donors (□, n=5). Percentage survival of Balb/c recipients is not different when IL-18−/− donors are used (△ versus □, NS). Survival data from one of three independent experiments is shown.
B. Expansion of luciferase labeled T cells was quantified in emitted photons over total body area at serial time points after BMT.
BLI signal intensity of mice receiving TCD-BM (●, n=5), with T cells from wt donors (△, n=5) or TCD-BM and T cells from IL-18 deficient donors (□, n=5).
C. Ten days after transplantation, mice from the indicated groups were sacrificed for histological examination. Representative colon sections stained by conventional H&E of mice receiving TCD-BM (i), with T cells from wt donors (ii) or IL-18 deficient donors (iii) are shown. GvHD tissue damage manifests as crypt abscess (arrow) and mucosal denudation (asterix). Comparable histopathological GvHD damage is seen when the donor is IL-18 deficient. Magnification is x200. Cumulative histopathology scoring from small bowel, large bowel and liver tissue samples harvested on day 10 after BMT from 3 representative animals per group is shown (NS=not significant).
Increased IL-18 levels during aGvHD are mainly host derived and absence of host IL-18 translates into lower IFN-γ production
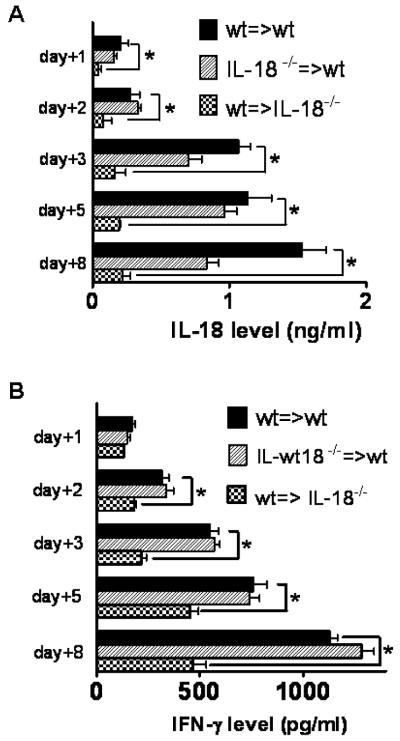
The observation that the course of CD4 T cell induced aGvHD was independent of donor IL-18 production prompted us to measure the IL-18 serum levels in the situation where either the donor or the recipient was deficient for the cytokine. Serum was collected at the different time points and was significantly reduced when the recipient was IL-18 deficient (Fig. 6A). Interestingly, donor contribution to the IL-18 production in the early phase after transplantation (days 1–5) was minimal as evidenced by IL-18 levels that were comparable to the wt→wt situation (Fig. 6A). At later time points (day 8) there was also a slight decrease of the cytokine when the donor was IL-18 deficient (Fig. 6A). Since IL-18 can induce IFN-γ production in T cells and NK cells [30], we investigated the levels of this cytokine in parallel. Interestingly, IFN-γ levels were significantly reduced when the recipient but not when the donor was IL18 deficient (Fig. 6B).
Figure 6. Host but not donor derived IL-18 is the major contributor to elevated IL-18 serum levels during the early phase of acute graft-versus-host disease.
A. Serum was collected from the indicated donor/recipient combinations. Wildtype (■) represents the mean IL-18 serum level derived from 5 animals of the C57Bl/6→Balb/c and 5 animals from the Balb/c→C57Bl/6 combination. Donor IL-18−/− (▨) represents the mean IL-18 serum level derived from 5 recipients of the C57Bl/6 IL-18−/−→Balb/c combination and recipient IL-18−/− ( ) represents the mean IL-18 serum level derived from 5 recipient animals of the Balb/c→C57Bl/6 IL-18−/−combinations. IL-18 serum levels are significantly lower when recipients are IL-18 deficient at all indicated time points (p<0.05). On day 8 the IL-18 serum level is significantly reduced in the donor IL-18 group as compared to the wt group (p<0.05).
) represents the mean IL-18 serum level derived from 5 recipient animals of the Balb/c→C57Bl/6 IL-18−/−combinations. IL-18 serum levels are significantly lower when recipients are IL-18 deficient at all indicated time points (p<0.05). On day 8 the IL-18 serum level is significantly reduced in the donor IL-18 group as compared to the wt group (p<0.05).
B. Serum was collected from the indicated donor/recipient combinations. Wildtype (■) represents the mean IFN-γ serum level derived from 5 animals of the C57Bl/6→Balb/c and 5 animals from the Balb/c→C57Bl/6 combinations. Donor IL-18−/− (▨) represents the mean IFN-γ serum level derived from 5 recipients of the C57Bl/6 IL-18−/−→Balb/c combination and recipient IL-18−/− ( ) represents the mean IFN-γ serum level derived from 5 recipient animals of the Balb/c→C57Bl/6 IL-18−/−combination. IFN-γ serum levels are significantly lower when recipients are IL-18 deficient at all indicated time points (p<0.05).
) represents the mean IFN-γ serum level derived from 5 recipient animals of the Balb/c→C57Bl/6 IL-18−/−combination. IFN-γ serum levels are significantly lower when recipients are IL-18 deficient at all indicated time points (p<0.05).
Absence of host IL-18 predicts increased CD4 Tconv but not Treg expansion
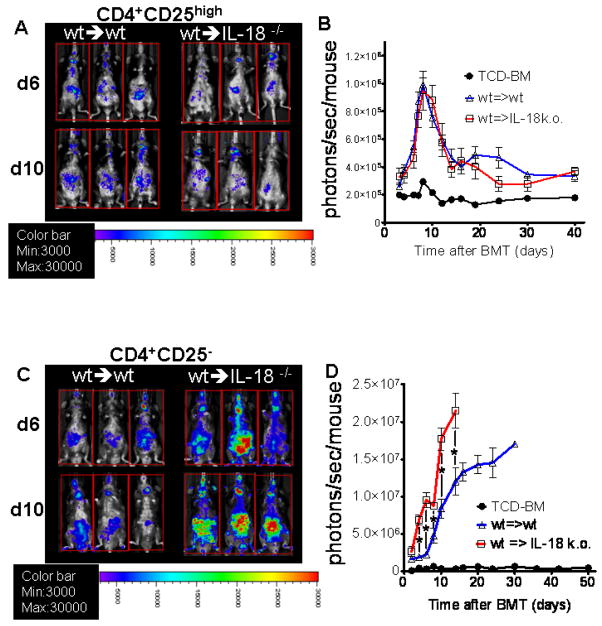
Based on the observation that the host has the major contribution to the IL-18 production during aGvHD we next investigated the expansion patterns of Treg and Tconv in the absence of host IL-18 production. Therefore, we transferred luc+ Treg (H-2kq) into lethally irradiated C57Bl/6 recipients that were either wt or IL-18 deficient. Treg expansion was comparable in the presence or absence of host IL-18 production as shown for 3 representative animals at two time points after BMT (Fig. 7A) and as quantified in photons over total body area at serial time points (Fig. 7B). To further evaluate the relevance of increased IL-18 levels during aGvHD for Treg expansion, luc+ CD4 Tconv or Treg (H-2kq) were transplanted into lethally irradiated C57Bl/6 recipients that were either wt or IL-18 deficient. Bioluminescence imaging at serial time points after BMT disclosed an increased expansion of luc+ CD4 Tconv cells in the absence of recipient IL-18 (Fig. 7C, D).
Figure 7. IL-18 deficiency of the recipient increases expansion CD4 T cell but not Treg cells.
A. C57Bl/6 wt or IL18−/− mice were given 5 × 106 TCD-BM cells and 2 × 106 luciferase transgenic (luc+) CD4+CD25high Treg cells (both H-2kq) after lethal irradiation with 800 cGy. Expansion of luc+ Treg cells is depicted in 3 representative recipients per group on days 6 and 10 after BMT.
B. Expansion of luciferase labeled Treg cells was quantified in emitted photons over total body area at serial time points after BMT.
BLI signal intensity of mice receiving TCD-BM (●, n=15), with T cells from wt donors transplanted into wt recipients (△, n=15) or with T cells from wt donors transplanted into IL18−/− recipients (□, n=15).
C. Expansion of luc+ Treg cells is depicted in 3 representative recipients per group on days 6 and 10 after BMT.
D. Expansion of luc+ Treg cells was quantified in emitted photons over total body area at serial time points after BMT.
BLI signal intensity of mice receiving TCD-BM (●, n=15), with Treg from wt donors transplanted into wt recipients (△, n=15) or with T cells from wt donors transplanted into IL18−/− recipients (□, n=15). T cell expansion signal is significantly higher in the IL18−/− recipients as compared to the wt recipients at the indicated time points (* p< 0.05).
DISCUSSION
Allogeneic BMT is a potentially curative therapeutic option for many malignant and non-malignant diseases. However, the beneficial effects of the donor immune system on engraftment and elimination of residual malignant cells are closely linked to the occurrence of aGvHD which leads to treatment related mortality. Treg have been shown to reduce the incidence and severity of aGvHD in rodent models [21, 22, 31] and increased Foxp3+ Treg cells in the hematopoietic graft were shown to predict a reduced risk for aGvHD in human transplant recipients [32]. Expansion and function of Treg relies on IL-2 [25, 33–35] while the role of other IL-2Rγ-chain dependent cytokines such as IL-7 and IL-15 are still under investigation [36]. Insight into the cytokine milieu that can favor Treg function may help to manipulate the function of this cell population. In this report we evaluated the role of IL-18 on aGvHD and the differential impact on Tconv and Treg expansion. Interestingly we found that expression of the IL-18Rα was similar in Treg and Tconv isolated from different organs. Since in the in vitro studies IL-18 from the stimulators and irradiated non-Treg responders was present we did not address the functional role of IL-18 on Treg suppressor function but showed that Treg which developed in the absence of IL-18 are equally suppressive as their wt counterparts.
Our in vivo data indicate that ontogeny, function and homeostasis of Treg remain intact in the absence of IL-18 in healthy mice. In a model of oral tolerance induction, IL-18 deficient recipients did not develop Treg in Peyer’s patches after feeding with BLG [23]. The observation of Tsuji et al. that IL-18 was relevant for Treg mediated oral tolerance induction could be due to the requirement of the cytokine for Treg in the situation where an immune response requires regulation [23]. We therefore investigated the relevance of IL-18 for Treg and Tconv during aGvHD as an aberrant immune response. Our observation that donor deficiency for IL-18 did not affect CD4 T cell expansion and the course of aGvHD made us hypothesize that production of IL-18 by the host may be more relevant for the previously reported increased IL-18 levels after BMT [3, 4].
Indeed we found IL-18 levels to be significantly decreased when the recipient but not the donor animals were IL-18 deficient. This suggested that in the early phase after transplantation residual host hematopoietic cells such as macrophages, Kupffer cells and dendritic cells, that can survival the first week after the myeloablative irradiation, produce significant amounts of IL-18. Other recipient type cells with known ability to produce IL-18 are intestinal epithelial cells and keratinocytes [4, 13–15]. These recipient cells may secrete IL-18 triggered by tissue damage due to the conditioning regimen. Interestingly, IL-18 in contrast to other cytokines is stored as biologically inactive precursor (pro-IL-18) and is secreted when appropriate cleaving enzymes are present [37, 38]. Previous studies have demonstrated the involvement of a caspase-1-like molecule and caspase-1 for cleavage of IL-18 [4, 39]. Lipopolysaccharide (LPS), that enhances aGvHD severity and leaks into the blood stream after conditioning induced bowel wall damage [40], has been shown to induce caspase-1 which is critical in the process of IL-18 secretion [41]. Our observation that increased IL-18 serum levels after BMT are host derived is compatible with the finding that caspase-1-deficient recipients engrafted with wildtype H-2 disparate splenocytes displayed decreased IL-18 production [4]. Our study is the first to delineate the relative contribution of the host on increased IL-18 cytokine levels during the early phase after BMT that has a functional impact on the aGvHD course. With respect to the clinical situation, the manipulation of GvHD through the cytokine Il-18 may be difficult to achieve based on its opposing effects on CD4 as compared to CD8 T cells [17] and due to the fact that human GvHD is not restricted to either subset as it can be studied in murine models with selective MHC class I or class II differences.
Since IL-18 has been demonstrated to induce IFN-γ production [7], a cytokine with known relevance for downmodulation of aGvHD in the early phase after BMT [8] and for long-term allograft survival [42] we studied the impact of host IL-18 deficiency on IFN-γ secretion. We found that reduced IL-18 production in IL-18 deficient recipients was paralleled by diminished IFN-γ serum levels. This finding may explain why the absence of host IL-18 resulted in more aggressive aGvHD, since IFN-γ was shown to induce death of activated donor CD4 cells [17]. IL-18 may mediate its effects not only directly by augmentation of IFN-γ production [43, 44] but also by increasing the expression of the IL-12R complex, thereby enhancing the effects of IL-12 [45]. Interestingly, IL-12 has been demonstrated to be critical for tolerance induction in solid organ transplantation [46] and to reduce aGvHD [12]. A recent in vivo study demonstrated a unique role for IFN-γ in the functional activity of alloantigen-reactive Treg cells during the development of operational tolerance to donor skin allografts [47]. In our studies the absence of host IL-18 was paralleled by reduced IFN-γ and we did not find any change in Treg proliferation under these conditions. Further studies are needed to address the role of reduced IFN-γ levels on Treg suppressor function in the BMT model.
Since the expansion kinetics of Treg were independent of host IL-18 the protective effect of host IL-18 on aGvHD was not through immunoregulation by increased Treg expansion. In contrast to data from the oral tolerance model [23], we did not find an essential role of IL-18 for the induction of Treg cells which may be due to model specific differences.
We conclude that Treg and Tconv express comparable levels of IL-18Rα which is upregulated in response to alloantigen. Absence of host but not donor IL-18 production translated into lower IFN-γ levels in the early phase after transplantation and resulted in a more rapid course of aGvHD. Host derived IL-18 has a protective effect in CD4+ mediated aGvHD, possibly through increased IFN-γ production. IL-18 is non-essential for Treg expansion in an allogeneic environment however downmodulates CD4+ Tconv expansion. In the absence of IL-18 increased CD4+ T cell proliferation results in more aggressive aGvHD.
Acknowledgments
The authors are grateful to Ruby M. Wong for expert assistance with statistical analysis and to the members of the Negrin laboratory for helpful discussion. This study was supported by grants from the National Institute of Health (NIH; RO1 CA0800065 and P01 HL075462 to R.S.N.), the Small Animal Imaging Resource Program (SAIRP grant number R24CA92862) and an In Vivo Cellular Molecular Imaging Center (ICMIC) grant (P50 CA114747). R.Z. is supported by the Dr. Mildred-Scheel-Stiftung, Germany.
Footnotes
Competing interests statement: The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105:1289–1298. doi: 10.1172/JCI7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujimori Y, Takatsuka H, Takemoto Y, et al. Elevated interleukin (IL)-18 levels during acute graft-versus-host disease after allogeneic bone marrow transplantation. Brit J Haematol. 2000;109:4373–4380. doi: 10.1046/j.1365-2141.2000.02095.x. [DOI] [PubMed] [Google Scholar]
- 4.Itoi H, Fujimori Y, Tsutsui H, et al. Fas ligand-induced caspase-1-dependent accumulation of interleukin-18 in mice with acute graft-versus-host disease. Blood. 2001;98:235–241. doi: 10.1182/blood.v98.1.235. [DOI] [PubMed] [Google Scholar]
- 5.Adachi O, Kawai T, Takeda K, et al. Targeted disruption of the MyD88 gene results in loss of IL-1 and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 6.Bazan J, Timans J, Kastelein R. A newly defined interleukin-1? Nature. 1996;379:591–559. doi: 10.1038/379591a0. [DOI] [PubMed] [Google Scholar]
- 7.Okamura H, Tsutsui H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 8.Yang YG, Dey B, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102:2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8(+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97:2923–2931. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 10.Sykes M, Romick ML, Hoyles KA, Sachs DH. Interleukin 2 prevents graft-versus-host disease while preserving the graft-versus-leukemia effect of allogeneic T cells. Proc Natl Acad Sci U S A. 1990;87:5633–5637. doi: 10.1073/pnas.87.15.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brok H, Heidt PJ, van der Meide PH, Zurcher C, Vossen JM. Interferon-gamma prevents graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Immunol. 1993;151:6451–6459. [PubMed] [Google Scholar]
- 12.Dey B, Yang YG, Szot GL, Pearson DA, Sykes M. Interleukin-12 inhibits graft-versus-host disease through an Fas-mediated mechanism associated with alterations in donor T-cell activation and expansion. Blood. 1998;91:3315–3322. [PubMed] [Google Scholar]
- 13.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both th1 and th2 responses. Ann Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 14.Swain SL. Interleukin 18: tipping the balance towards a T helper cell 1 response. J Exp Med. 2001;194:11–14. doi: 10.1084/jem.194.3.f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy P, Ferrara JL. Role of interleukin-18 in acute graft-versus-host disease. J Lab Clin Med. 2003;141:365–371. doi: 10.1016/S0022-2143(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 16.Fujimori Y, Yoshimoto T, Matsui K, et al. Increased expression of interleukin 18 receptor on T lymphocytes in patients with acute graft-versus-host disease after allogeneic bone marrow transplantation. J Interferon Cytokine Res. 2002;22:751–759. doi: 10.1089/107999002320271332. [DOI] [PubMed] [Google Scholar]
- 17.Min C, Maeda Y, Lowler K, Liu C, Clouthier S, Lofthus D, Weisiger E, Ferrara JL, Reddy P. Paradoxical effects of interleukin-18 on the severity of acute graft-versus-host disease mediated by CD4+ and CD8+ T-cell subsets after experimental allogeneic bone marrow transplantation. Blood. 2004;104:3393–3399. doi: 10.1182/blood-2004-02-0763. [DOI] [PubMed] [Google Scholar]
- 18.Reddy P, Teshima T, Kukuruga M, et al. Interleukin-18 regulates acute graft-versus-host disease by enhancing Fas-mediated donor T cell apoptosis. J Exp Med. 2001;104:1433–1440. doi: 10.1084/jem.194.10.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy P, Teshima T, Hildebrandt G, Williams DL, Liu C, Cooke KR, Ferrara JL. Pretreatment of donors with interleukin-18 attenuates acute graft-versus-host disease via STAT6 and preserves graft-versus-leukemia effects. Blood. 2003;101:2877–2885. doi: 10.1182/blood-2002-08-2566. [DOI] [PubMed] [Google Scholar]
- 20.Arnold D, Wasem C, Juillard P, et al. IL-18-independent cytotoxic T lymphocyte activation and IFN-[gamma] production during experimental acute graft-versus-host disease. Int Immunol. 2002;14:503. doi: 10.1093/intimm/14.5.503. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor P, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji N, Nowak B. IL-18 and Antigen-Specific CD4+ Regulatory T Cells in Peyer’s Patches. Ann NY Acad Sci. 2004;1029:413–415. doi: 10.1196/annals.1309.049. [DOI] [PubMed] [Google Scholar]
- 24.Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, Weissman IL, Contag CH. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A. 2004;101:221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, Contag CH, Negrin RS. Inhibition of CD4+CD25+ regulatory T cell function by calcineurin dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeiser R, Nguyen VH, Hou JZ, Beilhack A, Zambricki EA, Buess M, Contag CH, Negrin RS. Early CD30 signaling is critical for adoptively transferred CD4+CD25+ regulatory T cells in prevention of acute graft versus host disease. Blood. 2007;109:2225–2233. doi: 10.1182/blood-2006-07-038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lerner K, Kao GF, Storb R, Buckner CD, Clift RA, Thomas ED. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant Proc. 1974;6:367–371. [PubMed] [Google Scholar]
- 28.Dai Z, Li Q, Wang Y, Gao G, Diggs LS, Tellides G, Lakkis FG. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest. 2004;113:310–317. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 30.Shi F, Takeda K, Akira S, Sarvetnick N, Ljunggren HG. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. J Immunol. 2000;165:3099–3104. doi: 10.4049/jimmunol.165.6.3099. [DOI] [PubMed] [Google Scholar]
- 31.Trenado A, Charlotte F, Fisson S, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezvani K, Mielke S, Ahmadzadeh M, Kilical Y, Savani BN, Zeilah J, Keyvanfar K, Montero A, Hensel N, Kurlander R, Barrett AJ. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108:1291–1297. doi: 10.1182/blood-2006-02-003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thornton A, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 34.Malek T, Yu A, Vincek V, et al. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–170. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, Long LM, Bernstein D, Hill BJ, Douek DC, Berzofsky JA, Carter CS, Read EJ, Helman LJ, Mackall CL. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 36.Peffault de Latour R, Dujardin HC, Mishellany F, et al. Ontogeny, function, and peripheral homeostasis of regulatory T cells in the absence of interleukin-7. Blood. 2006;108:2300–2306. doi: 10.1182/blood-2006-04-017947. [DOI] [PubMed] [Google Scholar]
- 37.Tsutsui H, Matsui K, Okamura H, Nakanishi K. Pathophysiological roles of interleukin-18 for inflammatory liver diseases. Immunol Rev. 2000;174:192–209. doi: 10.1034/j.1600-0528.2002.017418.x. [DOI] [PubMed] [Google Scholar]
- 38.Nakanishi K, Yoshimoto Y, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 39.Tsutsui H, Kayagaki N, Kuida K, et al. Caspase-1-independent, Fas/Fas ligand-mediated IL-18 secretion from macrophages causes acute liver injury in mice. Immunity. 1999;11:359–367. doi: 10.1016/s1074-7613(00)80111-9. [DOI] [PubMed] [Google Scholar]
- 40.Cooke K, Gerbitz A, Crawford JM, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;102:1581–1589. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghayur T, Banerjee S, Hugunin M, et al. Caspase-1 processes IFN- -inducing factor and regulates LPS-induced IFN- production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 42.Konieczny B, et al. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T-cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 43.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN- -inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 44.Tomura M, Maruo S, Mu J, Zhou ZY, Ahn HJ, Hamaoka T, Okamura H, Nakanishi K, Clark S, Kurimoto M, Fujiwara H. Differential capacities of CD4+, CD8+, and CD4−CD8− T cell subsets to express IL-18 receptor and produce IFN- in response to IL-18. 1998;160:3759–3765. [PubMed] [Google Scholar]
- 45.Chang JT, Segal BM, Nakanishi K, Okamura H, Shevach EM. The costimulatory effect of IL-18 on the induction of antigen-specific IFN- production by resting T cells is IL-12 dependent and is mediated by up-regulation of the IL-12 receptor β2 subunit. Eur J Immunol. 2000;30:1113–1119. doi: 10.1002/(SICI)1521-4141(200004)30:4<1113::AID-IMMU1113>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 46.Verma ND, Boyd R, Robinson C, Plain KM, Tran GT, Hall BM. Interleukin-12p70 prolongs allograft survival by induction of Interferon gamma and nitric oxide production. Tranplantation. 2006;82:1324–1333. doi: 10.1097/01.tp.0000239519.56358.c1. [DOI] [PubMed] [Google Scholar]
- 47.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–1935. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]