Abstract
Angiogenesis plays a critical role in many normal physiological processes as well as in tumor neovascularization associated with cancer progression. Among various animal model systems designed to study the mechanisms underlying angiogenesis, chick embryo models have been useful tools in analyzing the angiogenic potential of purified factors and intact cells. The chorioallantoic membrane (CAM), a specialized, highly vascularized tissue of the avian embryo, serves as an ideal indicator of the anti- or pro-angiogenic properties of test compounds. In this chapter, we describe a number basic chick embryo CAM models of angiogenesis. A special emphasis is on the model system employing three-dimensional (3D) collagen grafts planted on the CAM, referred herein as onplants. This collagen onplant model allows for unambiguous quantification of angiogenesis and also for in-depth analysis of the cellular and biochemical mechanisms by which specific cells of different origin or purified effector molecules induce or inhibit the angiogenic process.
1. Introduction
Angiogenesis is a progressive, multistep physiological process by which new blood vessels are generated from pre-existing vasculature. Adult vasculature is maintained mostly in an angiostatic state that must be switched off to allow for new blood vessel formation. This angiogenic switch is a part of normal physiologic responses, for example to tissue injury, as well as a critical step in the pathology of tumor progression. It is commonly accepted that specific mechanisms underlining the angiogenic switch involve a selective remodeling of the extracellular matrix (ECM) by proteolytic enzymes and the induction, generation or release of angiogenic growth factors, which induce endothelium sprouting, followed by reorganization and formation of new blood vessels.
During cancer progression, the newly formed tumor-associated blood vessels serve first as feeding/nurturing tubes for a growing tumor and next, as conduits for dissemination of tumor cells that escaped from an established primary tumor. Therefore, control of tumor angiogenesis has became a central issue in the fight against cancer progression since anticancer therapy could be ineffective once tumor cells reach favored secondary organs and generate metastatic foci.
To analyze the mechanisms underlying normal and pathological angiogenesis, numerous in vivo angiogenic assays have been established employing different species of laboratory animals, including mammals (mouse, rat, hamster, and rabbit), birds (chicken and quail), and fish (mainly zebra fish). In this chapter, we will focus on major models of angiogenesis in the chick embryo. The use of chick embryo models for angiogenic studies is facilitated by the existence in avian species of a specialized respiratory tissue, named the chorioallantoic membrane (CAM) that allows for gas exchange between the embryo and the atmosphere surrounding the egg and in effect performs the function of a lung during embryonic life (Romanoff, 1960).
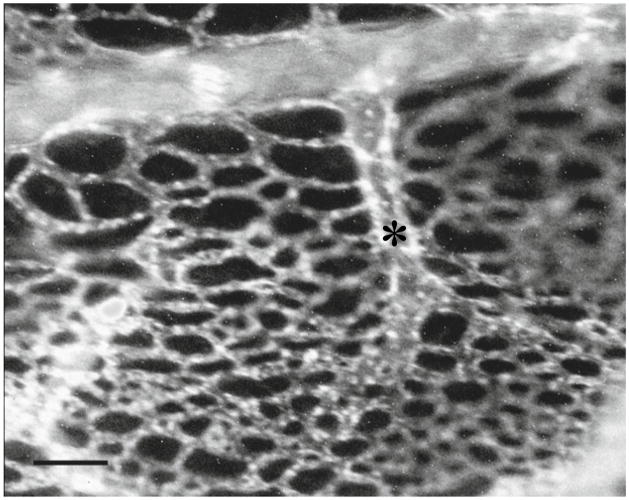
In the chick embryo, the chorioallantois is formed between days 4 and 5 of development, when the outer mesodermal layer of the allantois fuses with the mesodermal lining of the chorion, and a network of blood vessels is gradually formed between the two layers. The central portion of the CAM is fully developed by day 8 to 10 at which time it becomes capable of sustaining tissue grafts, while the outskirts of the CAM are still developing and expanding until the CAM fully envelopes the embryo at day 12 of incubation. Histologically, the CAM consists of three germ layers, that is, ectoderm, mesoderm, and endoderm. The ectoderm faces the shell membrane and is underlined by the respiratory capillary plexus, which starts to form between days 5 and 6 of embryonic development by both angiogenesis and vasculogenesis (Melkonian et al., 2002). This capillary plexus is very dense and appears as a honeycomb network of tiny capillaries originating from terminal capillaries (Fig. 2.1). The mesoderm of the chorioallantois is a collagen-rich embryonic connective tissue transversed by blood vessels belonging to the arteriolar and venous systems. The mesoderm is underlined with a thin endoderm layer, which separates the CAM from the allantoic cavity (Romanoff, 1960; Tufan and Satiroglu-Tufan, 2005).
Figure 2.1.
Ectoderm capillary plexus. Chick embryos on day 12 of development were injected intravenously with rhodamin-conjugated Lens culinaris agglutinin (0.1 ml of 1 mg/ml solution in PBS per embryo), which specifically binds to the chick endothelium. After 30 min of incubation at 37 °C, the embryos were sacrificed and the CAMs were detached from the shell membrane and visualized with a fluorescent microscope equipped with a digital camera. The image shows a terminal capillary (*), which radiates into the honeycomb-like network of capillaries constituting the ectoderm plexus. Bar, 50 μm.
Until day 11 or 12 of chick embryo development, the blood vessel system of the CAM is highly angiogenic, that is, undergoing maturation through a constant generation of new blood vessels as well as establishment of new blood vessel anastomoses. Therefore, between day 8 and day 10, the developing CAM vasculature is ready to sprout in response to additional proangiogenic stimuli and, in turn, is very responsive to antiangiogenic factors. This feature renders the chick embryo CAM models well suited for experimental validation of pro- and anti-angiogenic compounds. The response of the CAM to angiogenic stimuli is relatively rapid and most assays require only 3 to 5 days. In addition, recent modifications of the originally described assays readily allow for quantitation of the angiogenic process, revolutionizing the use of CAM assays in angiogenesis studies. In addition, the chick embryo is naturally immunoincompetent until embryonic day 17, thus allowing for grafting of cells of different species origin, such as human tumor cells, and therefore providing a useful tool for analysis of the proangiogenic potential of test cells.
2. Overview of CAM Angiogenesis Models
Several CAM angiogenic assays have been introduced since almost a century ago when rat Jensen sarcoma cells, implanted into the CAM on the day 6 of incubation, were demonstrated to develop large tumors showing signs of tumor-induced angiogenesis (Murphy, 1913). All modifications of the original angiogenic assay in the chick embryo involve grafting of test material onto developing CAM. The grafting is often performed through a window cut in the egg shell over the CAM. The angiogenic material is usually introduced in the form of small disks soaked in angiogenic factors or small pieces of polymerized materials such as gelatin sponges or biologically inert synthetic polymers, containing either purified angiogenic factors or impregnated with tumor cells. Another less traumatic way of introducing angiogenic material onto the CAM involves the use of shell-less embryos grown ex ovo, which makes the CAM more accessible for repetitive manipulations, for quantitation of angiogenesis, and for direct visualization of the angiogenic process under a stereoscope. Below, we will briefly describe major CAM angiogenic assays employing both in-shell and shell-less chick embryos and different sources of angiogenic factors such as purified molecules and tumor cells.
2.1. CAM filter disk assays
The factor-containing disks placed directly on the CAM are widely used in validation of the pro- and anti-angiogenic properties of test compounds (Beckers et al., 1997; Brooks et al., 1998; Eliceiri et al., 1999; Han et al., 2001; Hood et al., 2003; Miller et al., 2004; Murugesan et al., 2007; Sahni et al., 2006). The filter disks can be cut from nitrocellulose membrane or Whatman paper. The CAM is prepared for grafting by making an air sac, usually on day 3 of egg incubation, by sucking out approximately 3 ml of albumen with a syringe. This procedure allows for the development of the intact, noninjured CAM, which becomes experimentally accessible between day 8 and 9 through a window cut in the egg shell. The fact that the CAM develops intact is an important issue since any injury to the CAM provides potent hypertrophic or atrophic reactions that may alter the angiogenic potential of test material. However, the egg shell dust generated while making the window to access the CAM might serve as inflammatory stimuli and cause a change in the angiogenesis readout. An alternative method of separating the CAM from the shell membrane involves opening a small window in the egg shell at days 7 to 10 of embryonal development above the CAM after removing 2 to 3 ml of albumen or after creating a false air sac (Brooks et al., 1994; Hood et al., 2003).
Filter disks are used to confine the test material to a defined area of the CAM. The disks are pre-soaked in test compounds and usually are dried before grafting on the CAM. Following disk application, the window in the egg shell is closed with a piece of tape or glass and the eggs are placed back into the incubator. The available CAM area under the window is only enough for the application of one disk per embryo, thus dictating the use of large groups of animals to get reliable, quantitative readouts. In this assay, quantitation is performed usually 3 days after implantation and involves counting the number of CAM vessels in the area of filter disk. In response to proangiogenic stimuli, the newly formed blood vessels appear converging toward the disk in a wheel-spoke pattern. Inhibition of angiogenesis by antiangiogenic compounds results in the lack of new blood vessel formation and sometimes in disappearance of pre-existing vessel networks. Angiogenesis levels can be also determined by counting branch points in the vessels adjacent to the disks.
Time course of new blood vessel formation in the CAM can be readily performed by analyzing the images taken at different time points after grafting of the disk using a stereoscope equipped with a digital camera. More sophisticated techniques have been designed recently to perform reliable quantitative evaluation of vascular density, endothelial proliferation, and protein expression in response to angiogenic agents released from the filter disks to the underlying CAM (Miller et al., 2004). These techniques include in ovo cell proliferation, layered expression scanning to visualize the protein of interest, and fluorescent confocal microscopy of new blood vessel formation in the CAM at the site of filter application. The major disadvantage of this angiogenic assay is that introducing a disk alone, even without growth factors, can induce a high angiogenic response, therefore obscuring any proangiogenic properties of the test compounds. Thus, more commonly filter disk assays are employed for evaluation of antiangiogenic potential of test materials since the diminishment or lack of vascular convergence towards the disk is easier to appreciate and quantify (Ribatti et al., 2000).
2.2. CAM assays employing various gelated materials
A number of CAM angiogenesis assays are based on testing purified factors and intact cells incorporated into gelated materials such as methylcellulose, Matrigel, or sodium alginate. Although disks containing gelated material impose the same mechanical injury as filter disks directly soaked in test compounds, the use of gels allows a slow, but efficient release of anti- or pro-angiogenic factors into the underlying CAM tissue.
Preparation of methylcellulose disks involves spreading and drying of the factor-containing mixtures on Teflon surfaces (Yang and Moses, 1990), glass surfaces (Ribatti et al., 1995), or parafilm (Hagedorn et al., 2004) before their application on the CAMs of in-shell or shell-less individual embryos (Struman et al., 1999). Otherwise, methylcellulose mixture can be dried on nylon meshes, which provide a support for the disks (Cao et al., 1998). Three to 5 days after disk implantation, the CAMs are examined by stereomicroscope for new blood vessel formation or inhibition of angiogenesis within the field of the implanted disks.
Matrigel mixtures can be distributed in small volumes directly onto the CAM where rapid polymerization occurs. Alternatively, defined aliquots of Matrigel supplemented with test compounds can be pre-gelated at 37 °C on nylon meshes and then placed onto the CAM (Vazquez et al., 1999; Watanabe et al., 2004). Qualitative and quantitative variations in the growth factors intrinsically present in different preparations of Matrigel poses a serious concern. Therefore, the use of Matrigel with reduced amounts of growth factors is recommended in order to negate lot-to-lot variations.
Slow release of purified growth factors or angiogenic factors produced by test cells can be achieved by incorporating the test components into alginate pellet (Riboldi et al., 2005). The pellets are prepared by mixing a solution of sodium alginate with cells or purified molecules to achieve desirable final concentrations, followed by a dropwise releasing of the mixture into a CaCl2-contating solution. The calcium ions cause immediate gelling of the alginate droplets, which, after washing, can be implanted onto the CAM.
New models are constantly introduced aiming to improve various aspects of in-shell CAM angiogenesis models. One of such models is a cylinder model designed to assess the vascularization potential of engineered tissues (Borges et al., 2003). In this model, cell-containing matrices are applied within specially constructed plastic cylinders, allowing for continual observation of graft vascularization using a light microscope. This model requires a variety of specialized technical devices and the experimental setup is complex. In addition, it lacks a straightforward approach to quantify the implant-induced angiogenic response.
2.3. Gelatin sponge CAM assay
One of the major limitations of the filter disk angiogenesis assays is that the support material does not allow for a proper maintenance of tumor cell inoculums. This problem is overcome in a modification of the CAM angiogenic assay employing gelatin sponges impregnated with tumor cells. The sponge assay usually involves the use of small pieces of polymerized gelatin pre-soaked with test ingredients or filled with tumor cells (Ribatti et al., 2000, 2001, 2006). Gelatin sponges can be implanted on the growing CAM on day 8 of embryonic development. Since the sponges firmly adhere to the CAM surface, the test substances or cell suspensions are confined to the site of administration. The in-shell chick embryos are prepared for the sponge CAM assay in a similar way as described for the filter disk assay (Section 2.1). Similarly, the number of converging blood vessels or branch points is determined at the end of the experiment. Therefore, the same readout limitation of the filter disk assay is applicable to the CAM sponge assay, that is, the ambiguity in the level of new versus pre-existing vessel convergence. However, despite this limitation, the sponge assay is regarded as more advanced and reliable compared to the filter disk CAM assay. Furthermore, the implantation of gelatin sponges is regarded to be better tolerated because it causes less nonspecific inflammatory reaction than filer disk grafting. In addition, new truly angiogenic blood vessels growing vertically into the sponge can be quantified by morphometric evaluation of histologic CAM sections (Ribatti et al., 2000). Recently, the gelatin sponge CAM assay was used to demonstrate the role of aquaporin-1 in tumor-induced angiogenesis (Camerino et al., 2006).
2.4. CAM collagen onplant model
Many of the disadvantages of the above-described methods have been negated with the introduction of a shell-less modification of the CAM angiogenesis assay, employing viable embryos grown ex ovo and grafted with 3D gridded collagen onplants. These modifications led to a major breakthrough in quantitation of the CAM angiogenesis methods. This method will be described in detail because it offers many practical advantages over filter disk, Matrigel, and sponge CAM assays (see Sections 2.1 to 2.3) and allows for unambiguous scoring of new blood vessels, and also direct imaging, in situ analysis, dissection, and ex vivo biochemical analysis. The method was first introduced by Judah Folkman and colleagues in 1994 (Nguyen et al., 1994) and is based on the scoring of new capillary CAM blood vessels grown vertically from the pre-existing vasculature into a pepsin-solubilized bovine dermal collagen (vitrogen) through two parallel nylon meshes. Angiogenesis was induced either by purified basic fibroblast growth factor, bFGF or FGF-2, or by tumor cells incorporated into gels. New blood vessels are induced to grow upright into collagen gels from the underlying CAM, and therefore can be clearly discriminated from the background vascular network, providing a clear advantage for angiogenesis scoring. This represents a modification of the CAM angiogenesis method to offer a straightforward approach for quantifying angiogenesis (Nguyen et al., 1994).
Our modification of this assay (Seandel et al., 2001) involves the use of 3D grafts, each of which consists of two nylon grid meshes embedded into native, nonpepsinized, type I fibrillar collagen. As a support for angiogenic blood vessels, collagen is superior to filter disks, sponges and even Matrigel because collagen is the natural major protein component of stromal tissue where angiogenesis occurs (Tufan and Satiroglu-Tufan, 2005). Neutralized collagen in solution is premixed with defined amounts of factors and/or cells to be tested for their pro- or anti-angiogenic properties. The collagen mixture is distributed over the grid meshes in a small, defined volume (usually 30 μl per onplant) and allowed to polymerize in a 37 °C thermostat. Polymerization occurs within minutes, which ensures that the incorporated cells are distributed evenly throughout the 3D collagen and not settled at the bottom of the onplants. Additional 30 to 45 min of incubation allow the collagen to completely solidify, after which time the collagen onplants are placed with forceps on the CAMs of 10-day-old shell-less embryos incubated ex ovo in a stationary incubator. This method of embryo preparation excludes the proinflammatory effects of shell dust induced by making a shell window. Moreover, since the entire CAM in the shell-less embryos develops and expands facing the top of a culture dish, large areas (about 30 to 40 cm2) are available for the onplant grafting. Thus, several grafts (onplants) can be placed on the CAM of each embryo (routinely from 4 to 7), which greatly improves statistical power of the assay, allowing 20 to 42 individual angiogenic determinations when only 5 to 6 embryos are used for a given variable.
After receiving onplants, the embryos are returned immediately to the incubator for an additional 3 to 4 days. During this period of incubation, the embryos are readily available for repeated viewing under the light stereoscope or for intravital microscopy since the CAM is relatively thin, varying between 50 and 110 μm in thickness (Reizis et al., 2005). In addition, pharmacological intervention is simple: The grafts can be treated directly with chemicals of choice, or the embryos can be systemically treated by intravenous inoculation through the allantoic vein, which is clearly visible and accessible on the expanded CAM, or more locally treated through delivery of chemicals under the CAM into the allantoic cavity. This ability to readily modulate the angiogenic response in shell-less embryos provides an invaluable tool to analyze biochemically the mechanisms involved in angiogenesis induced by factors or cells originally incorporated into the collagen mixture.
Within 2 to 4 days of incubation, collagen onplants are infiltrated with newly formed blood vessels making an anastomosized tubular network filled with circulating blood. The blood vessels, which are visualized with a stereomicroscope above the lower nylon mesh, are regarded as angiogenic since only newly formed vessels infiltrate the collagen graft from the pre-existing CAM vasculature, which is located below the nylon mesh of the graft. Importantly, during scoring of angiogenesis, the ectoderm capillary plexus and the mesodermal vascular network are off the focus plane of the viewer, and therefore do not interfere with angiogenic scoring. Since the meshes incorporated into collagen are gridded, angiogenesis can be quantified as a ratio of the grid areas filled with distinct blood vessels over the total number of grid areas observed in an individual onplant. This approach makes the CAM onplant assay unambiguous in determination of the angiogenic response induced in each individual onplant, each individual embryo and, finally, in each set of embryos designated for a certain variable.
Intrinsically, this method allows for a wide variety of technical modifications, including different support material (any gelating material vs. originally introduced native collagen, such as fibrinogen, noncleavable mutant collagen, or collagen impregnated with various ECM proteins), and use of combinations of very small and importantly, defined amounts of incorporated effector molecules, that is, nanogram quantities of purified growth factors, cytokines, inhibitors, or matrix-modifying molecules such as proteinases. In addition, various types of cells can be tested in the assay, including tumor cells or inflammatory cells, alone or in combination with each other or with defined chemicals.
After angiogenesis scoring, the onplants are available for a variety of analyses, including histological, immunohistochemical, and biochemical examinations. Histological analysis has previously demonstrated that collagen onplants are rapidly integrated into the CAM tissue. Twenty-four hours following grafting, the onplants tightly attach to the CAM, and by 48 hours they are already covered with the ectoderm and have become an integral part of the mesoderm. As soon as 2 h after grafting, inflammatory cells, first heterophils (avian analog of mammalian neutrophils) and then monocyte/macrophages, infiltrate the onplants. These cells deliver pro- and anti-inflammatory factors and cytokines, as well as important modifiers of the ECM, that is, matrix metalloproteinases (MMPs). Avian neutrophils (heterophils) were demonstrated to import MMP-9, a major gelatinase (Zijlstra et al., 2006), while monocyte/macrophages were associated with delivery of MMP-13, a potent collagenase (Zijlstra et al., 2004). The original observation of defined inflammatory cell influx into onplants allowed us to begin a systematic investigation of the role of inflammatory MMPs in angiogenesis.
3. Assessing the Role of Purified Effector Molecules and Cells in Angiogenesis Using CAM Collagen Onplant Model
The role of human MMPs, including that of the neutrophil MMP-9, was thoroughly analyzed using the CAM collagen onplant assay. Recently, we demonstrated that neutrophils appear to provide an important proangiogenic factor, that is, MMP-9 to sites of physiological and tumor-induced angiogenesis (Ardi et al., 2007). The proangiogenic activity of neutrophil MMP-9 was mechanistically linked to its TIMP-free status, activation, and catalytic activity.
Neutrophil MMP-9 is stored in secretory granules and released upon stimulation in a zymogen form (proMMP-9). Pro-MMP-9 purified from neutrophil granule contents was shown to constitute a distinctly potent proangiogenic moiety, inducing angiogenesis in the CAM collagen onplant assay at subnanogram levels. Uniquely, neutrophils produce MMP-9 as a TIMP-free proenzyme that is readily available for proteolytic activation. Both MMP-9 proenzyme activation and the catalytic activity of the activated enzyme were required to induce an angiogenic response. That the high angiogenic potency of neutrophil proMMP-9 is associated with its unique TIMP-free status, was confirmed when a purified stoichiometric complex of neutrophil proMMP-9 with TIMP-1 failed to induce angiogenesis. Recombinant human pro-MMP-9, operationally free of TIMP-1, also induced angiogenesis at subnanomolar levels, but lost its proangiogenic potential when stoichiometrically complexed with TIMP-1. Similar pro-MMP-9/TIMP-1 complexes that are naturally produced by human monocytic U937 cells and HT-1080 fibrosarcoma cells did not stimulate angiogenesis. These findings have provided the first biochemical evidence that infiltrating neutrophils, in contrast to other cell types, deliver a potent proangiogenic moiety, that is, the unencumbered TIMP-free MMP-9 (Ardi et al., 2007). The cellular and biochemical evidence supporting these conclusions was generated solely because the CAM collagen onplant method allowed for the direct addition of intact neutrophils, their crude released contents (releasate) and purified releasate components such as MMP-9.
When tumor cells are incorporated into collagen onplants as low as 1 × 104 cells per onplant, they can provide proangiogenic stimuli (e.g., HT-1080 fibrosarcoma, HEp-3 epidermoid carcinoma, PC-3 prostate carcinoma) or be essentially inert, that is, not inducing additional levels of angiogenesis beyond basal control levels (e.g., HeLa cells). If tumor-containing onplants are left for 6 to 7 days on the CAM, they produce small, confined, and well-vascularized tumors implicating angiogenesis as an essential part of primary tumor establishment. Combining tumor cells with an exogenous source of inflammatory cells or their products allows for a detailed study of the role of inflammation on tumor-induced angiogenesis. In this modification of the CAM-collagen onplant method, the influx into onplants of endogenous, host inflammatory cells could be suppressed by the treatment of embryos with anti-inflammatory drugs such as ibuprofen. The shell-less embryos bearing the onplants are easily manipulated, allowing direct addition of the anti-inflammatory drugs. Treatment of the embryos with anti-inflammatory drugs significantly inhibits the high levels of angiogenesis induced by various tumor cell lines. In addition, incorporation of various function-blocking antibodies into the onplants containing tumor cells also decreases angiogenesis levels, thus indicating the involvement of the antigens targeted by the antibodies in tumor-induced angiogenesis. All of the above-discussed examples illustrate the versatile nature of the collagen onplant assay in analyzing the mechanisms of angiogenesis under physiological and pathological conditions.
4. CAM Collagen Onplant Assay Protocol
Provided in the following section is a detailed angiogenesis protocol employing 3D collagen grafts planted on the CAM (onplants). This assay is performed using shell-free chick embryos, essentially as described in Seandel et al. (2001), Zijlstra et al. (2004), Zijlstra et al. (2006), and Ardi et al. (2007), with some modifications.
4.1. Preparation of chick embryos: Shell-less ex ovo cultures
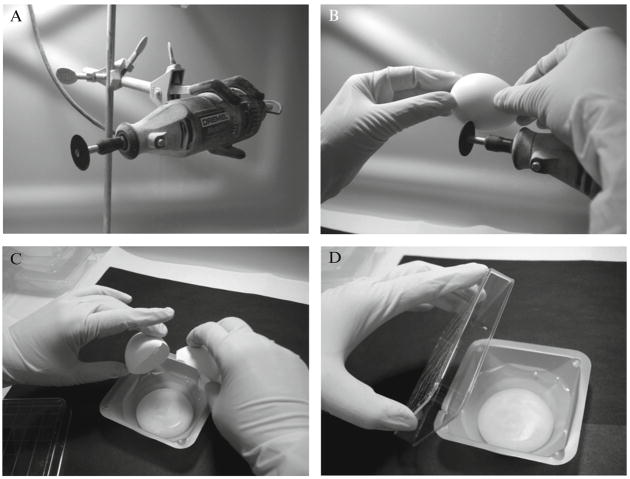
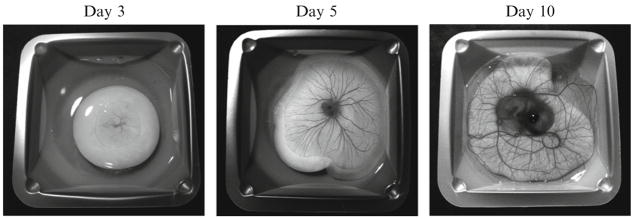
Fertilized COFAL-negative White Leghorn chicken eggs can be obtained from Charles River Labs (North Franklin, CT) or any other source providing specific pathogen-free animals. Upon arrival, the eggs should be placed either into a humidified refrigerator set at 7 to 10 °C for storage or into a rotary thermostat at 37.5 °C and 70 to 75% relative humidity for incubation. At day 3, the eggs are cleaned with 70% alcohol solution, and the egg shell is carefully cut using a wide wheel of a portable drill (e.g., Dremel) (Fig. 2.2A and B). The entire contents of the egg are transferred to plastic weigh boats (Fig. 2.2C). The boats should be pre-soaked in 70% ethanol and then dried under UV in the laminar hood to prevent microbial and particularly, fungal, contamination. The weigh boats with the egg contents are covered with square Petri dishes (Fig. 2.2D) and placed into the stationary incubator at 37.5 °C and 70 to 75% relative humidity. The embryos are allowed to develop for the next 7 days, at which time, (i.e., on embryonic day 10) the CAMs are developed enough to be able to sustain angiogenesis in collagen onplants (Fig. 2.3).
Figure 2.2.
Preparation of shell-less chick embryos. At day 3 of embryonal development, the shell of the eggs is cut using a portable drill (A). Three incisions are made in the shell with awide wheel (B), and the contents of the egg are transferred into sterilized weigh boats (C). The ex ovo cultures are covered with a square Petri dish and placed into a stationary incubator (D).
Figure 2.3.
Development of chick embryos in shell-less culture ex ovo. Sequential images depict a day-3 chick embryo immediately after cracking the egg and placing the contents into a dish and after 2 and 7 additional days of incubation ex ovo.
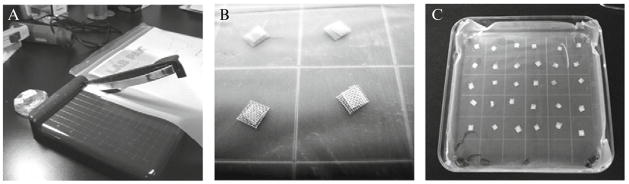
4.2. Preparation of grid meshes
Nitex nylon mesh with 180-μm grid size can be purchased from Sefar America, Inc. (Kansas City, MO). The layers are cut into 4 × 4-mm (lower mesh) and 3 × 3-mm (upper mesh) pieces with an Ingento paper cutter (Fig. 2.4A). Cut pieces are placed in glass Petri dishes and sterilized in an autoclave. The larger lower meshes are placed into a square plastic Petri dish layered with parafilm (sprayed with alcohol and air-dried), which facilitates adhesion of meshes. Lower meshes are then covered by the smaller upper meshes, making double-gridded sandwiches (Fig. 2.4B) ready to be embedded into a drop of collagen mixture (Fig. 2.4C).
Figure 2.4.
Preparation of meshes for collagen onplants. Nytex nylon meshes are cut into 4 × 4 mm and 3 × 3-mm pieces with a papercutter (A) and sterilized by autoclaving. Large pieces are distributed into a Petri dish layered with parafilm and then covered with smaller pieces, making sandwiches (B). Meshes designated for collagen onplants with one test variable are assembled in an individual dish (C).
4.3. Preparation of test effector molecules and cell suspensions
Effector molecules should be prepared and kept on ice (unless indicated otherwise) to be readily available for incorporation into the neutralized collagen solution. Cells such as inflammatory cells are isolated from proper sources, such as neutrophils from peripheral blood, and resuspended in PBS or serum-free culture medium. Tumor cells are harvested by the desirable method, such as enzymatic or nonenzymatic detachment of adherent cell layers, washed and resuspended in serum-free medium or PBS. Cell suspensions should be prepared at desirable concentrations and kept on ice. A 5- to 15-× concentrated stock solutions of ingredients will allow for incorporating defined quantities of purified molecules or cells in a small volume into the neutralized collagen, such as 0.1 to 0.2 ml per 1.0 to 1.2 ml of collagen.
4.4. Preparation of collagen onplants and angiogenesis scoring
Native, nonpepsinized, type I rat tail collagen (BD Biosciences, Bedford, MA) is used in most of the CAM collagen onplant assays. Collagen should be neutralized (see below) before mixing with the effector molecules or cells. All components should be kept on ice to prevent premature polymerization of the collagen mixture. Importantly, even on ice, collagen will polymerize within 1 to 2 h after neutralizing. Therefore, all components of the collagen mixture should be prepared in advance and kept handy on ice. It is also recommended that all plastic material, including tubes, serological pipettes, and plastic tips are precooled on ice or in the freezer.
The original commercial collagen is provided in acid solution and therefore should be first neutralized to achieve a suitable pH of 7.4 to 7.6. To neutralize collagen, 8 parts (by volume) of original collagen preparation are mixed with 1 part of 10× PBS or 10× Eagle’s minimal essential medium (EMEM) and 1 part of NaOH solution. If not indicated otherwise, we would recommend the use of 10× EMEM supplemented with phenol red, since the color of the final mixture would give a good indication of whether pH 7.4 to 7.6 is reached during neutralization. Therefore, collagen is first carefully, without vortexing or agitation, but rather swirling by hand, mixed with 10× EMEM. The mixture will become intensely yellow, indicating the acidic nature of the original collagen preparation. Then, 1 M NaOH solution is added dropwise to collagen/EMEM mixture until it reaches pH 7.4 to 7.6. If not all volume of 1 part is used, the rest is supplemented with sterile water. The concentration of collagen after neutralization is 80% of original concentration. Importantly, this concentration of neutralized collagen should be higher than the final concentration of collagen used in onplants (usually 2.0 to 2.1 mg/ml), allowing for addition of supplements and test molecules and/or cell suspensions. There is a considerable lot-to-lot variability in the original concentration of collagen. Therefore, the amount of collagen to be neutralized to prepare a desirable volume of mixture with the appropriate final concentration will depend on the original concentration of collagen. Taking into consideration a final volume of collagen solution, neutralized collagen can be supplemented with 10 to 25 mM HEPES buffer (pH 7.2 to 7.4) (Invitrogen, Carlsbad, CA) and, if desired, with 0.1 to 0.25 mg/ml BSA (Fraction V, Sigma). The collagen mixture should be further diluted with DMEM or PBS to 2.2 to 2.4 mg/ml, allowing for a final collagen concentration of 2.0 to 2.1 mg/ml after addition of test chemicals or cell suspensions.
Before mixing with test material, equal volumes of collagen is distributed into small tubes kept on ice and then various test molecules or cell suspensions are added to the tubes and gently but efficiently mixed with the tube contents. The use of equal volumes of collagen and test solutions or cell suspensions throughout collagen mixture preparation is recommended since it minimizes inconsistency in final collagen concentrations between experimental variables. Test molecules and cell suspensions should be prepared at 5 to 15× concentrations to allow for desirable final concentrations in the onplants. For example, a convenient volume to mix is 1.0 ml of collagen solution at 2.4 mg/ml and 0.2 ml of 6× test component, providing a final 1.2-ml collagen mixture, which is enough for 40 onplants of 30 μl volume at a final concentration of collagen of 2.0 mg/ml and 1× of effector molecule. Similar considerations are applicable to incorporation of cells. For example, 1.1 ml of 2.18 mg/ml collagen solution could be mixed with 0.1 ml cell suspension prepared at 12.0 × 106 cells/ml to make 40 onplants, each containing 3 × 104 cells in 30 μl (1 × 106/ml) and collagen at a final concentration of 2.0 mg/ml.
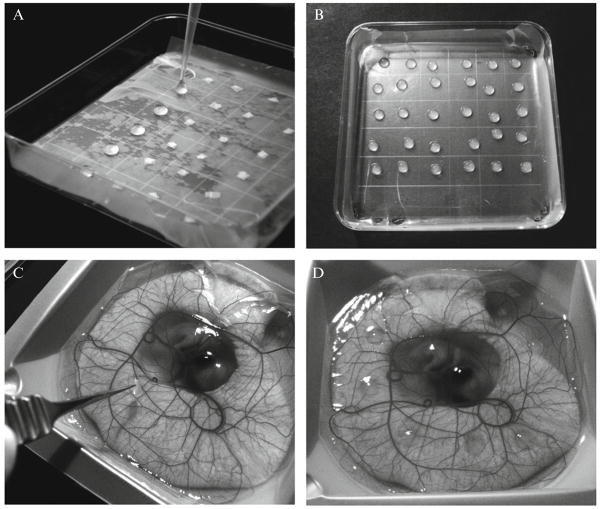
To assemble onplants, 30 μl of the final collagen mixture are placed atop two gridded nylon meshes (Fig. 2.5A). The collagen onplants containing the same test molecule or cells are allowed to polymerize at 37 °C in a Petri dish (Fig. 2.5B). After solidifying for 30 to 45 min, the onplants are lifted individually with fine-end forceps and placed on the CAM in areas containing fine vessel networks, between large blood vessels (Fig. 2.5C). The CAM should not be mechanically irritated during placement of onplants. Four to eight onplants per embryo are grafted onto the CAM of each 10-day-old shell-less embryo (Fig. 2.5D). After onplants are grafted on the CAM, the chick embryos are immediately returned to the incubator for 3 to 4 days. Groups of four to six embryos should be used for a given variable to provide enough data points for a reliable statistical analysis of angiogenesis differentials.
Figure 2.5.
Preparation of collagen onplants and grafting on the CAM. Collagen mixture with test cells or purified effector molecules is distributed at 30-μl volumes over the meshes (A). Collagen onplants are allowed to polymerize in a Petri dish (B) placed into 37 °C incubator for 30 to 45 min. Using fine forceps, individual onplants are placed onto the CAM of day-10 embryos in areas located between large blood vessels (C). Individual embryos usually receive six collagen onplants containing the same test compounds (D).
4.5. Treatment of shell-less embryos carrying onplants with test compounds
During incubation, onplants are readily available for pharmacological intervention. Test chemicals can be applied topically onto the onplants, or injected intravenously into one of large allantoic veins with a glass capillary. The latter procedure is quite complicated technically and can be substituted with the injection of test components under the CAM into the allantois cavity. However, the permeability of CAM endoderm for an individual effector molecule should be considered. Incorporation of 5% DMSO into the 0.1- to 0.2-ml inoculums might increase CAM permeability for the test compounds.
4.6. Angiogenesis scoring
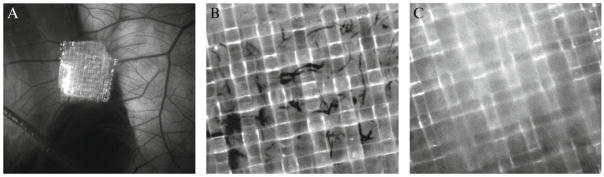
Angiogenesis is usually scored at 70 to 90 h after onplant grafting using a stereomicroscope (Fig. 2.6A). The plane of focus is chosen above the ectodermal capillary plexus and the network of pre-existing blood vessels. This assures that only newly formed, angiogenic vessels will be scored. Distinct blood-carrying vessels visualized in the grids of the upper mesh are regarded as angiogenic (Fig. 2.6B). Angiogenesis level is determined either as an angiogenic index, that is, ratio of angiogenic grids (number of grids containing blood vessels over the total number of grids scored), or as a fold difference between a variable (intact cells or cell components added into collagen) over control (collagen alone) (Fig. 2.6C).
Figure 2.6.
Scoring of angiogenesis in collagen onplants. Angiogenesis is scored with a stereomicroscope 70 to 90 h after onplant grafting. At that time point, the onplants appear more opaque as they become integrated into the CAM tissue (A). Newly formed blood vessels are scored in the focus plane of the upper mesh (B), that is, above vascular networks of the underlying CAM, including the ectoderm capillary plexus. In the absence of proangiogenic stimuli, control onplants have few if any angiogenic blood vessels (C).
4.7. Analysis of cellular and protein composition of onplants
At different time points after grafting, collagen onplants can be subjected to histological, immunohistochemical or biochemical analyses. For histological purposes, the onplants are excised from the CAM along with the underlying tissues with fine scissors and washed in PBS. For paraffin sectioning, the onplants are fixed in a proper fixative, such as Zn-formalin, and processed further following standard protocols (Fig. 2.7). For cryosectioning, the onplants should be carefully aligned in the cryomolds filled with the O.C.T. embedding compound (Tissue-Tek, Miles Laboratories, Naperille, IL), frozen on dry ice and kept at −70 to −80 °C until use. Tissue-staining protocols vary and depend on the antigen or molecule of interest and might require antigen retrieval. For biochemical purposes, the onplants are harvested with or without underlying CAM tissue, and immediately placed into the corresponding buffer, such as 5 to 10× SDS sample buffer for zymography or Western blot analyses, or extraction buffers, such as modified RIPA for ELISA analyses. Along with angiogenesis scoring, the immunohistological and biochemical analyses add valuable information about the cell origin or time of appearance or activation status of a particular molecule of interest.
Figure 2.7.
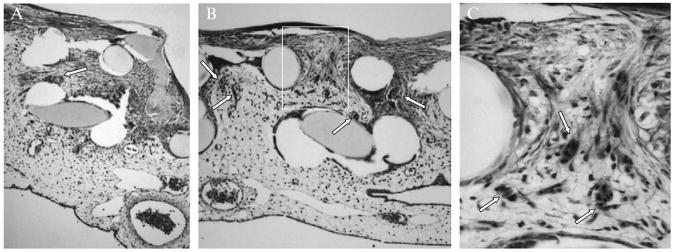
Histological analysis of collagen onplants for angiogenesis. After angiogenesis scoring, collagen onplants were excised from the CAM, fixed in Zn-formalin and processed for paraffin sectioning. Tissue sections were deparaffinized and stained by hematoxylineosin by standard procedure. Two rows of circular structures devoid of tissue represent the cross areas of lower and upper gridded meshes. Only a few blood vessels (arrows) grew into the control collagen onplant that was not supplemented with proangiogenic stimuli (A). In contrast, in the collagen onplant supplemented with a proangiogenic moiety, that is, recombinant MMP-9 (B), numerous angiogenic blood vessels could be identified between the meshes (arrows). Larger magnification of the boxed area (C) allows one to appreciate that angiogenic vessels are filled with blood (nucleated erythrocyes are stained dark pink with eosin) and lined with a continuous layer of endothelium.
5. Validation of CAM Angiogenesis Findings in Collagen Implant Mouse Model
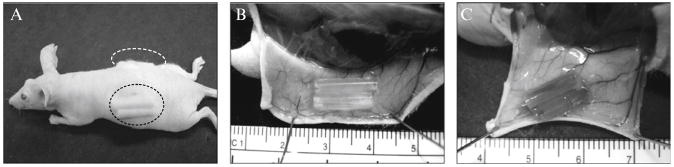
Findings generated with 3D collagen grafts in the avian angiogenesis model system can be validated in a mammalian model employing grafting of collagen-filled silicon tubes (angiotubes) under the skin of immunodeficient mice (Fig. 2.8A). Similar to the CAM assay, in this model system, collagen can be left nonsupplemented (Fig. 2.8B) or supplemented with different pro- and anti-angiogenic molecules as well as with tumor or inflammatory cells (Fig. 2.8C). Within 10 to 15 days after implantation, silicon tubes are infiltrated with newly formed angiogenic blood vessels originating from the pre-existing blood vessels converging towards the tube openings (Fig. 2.8C). Angiogenesis in individual tubes can be measured by hemoglobin content or by the level of endothelial cells (through indirect measurements of labeled ligand binding to endothelial cell–specific receptors).
Figure 2.8.
Mouse angiogenesis model system to study angiogenic potential of purified proteins and tumor cell variants selected in vivo (A). Inert silicon tubes were filled with native type I collagen and surgically inserted under the skin of immunodeficient mice (two per each side of the back). Twelve days later, the mice were sacrificed and tubes under the skin exposed and excised to determine levels of angiogenesis, such as by determining hemoglobin content in the lysed contents of the tubes. In addition, the levels of blood vessel convergence toward tube openings correlate well with the levels of angiogenesis in the implants. Thus, little convergence is observed toward control implants, which were not supplemented with any additional angiogenic factors (B). In contrast, when collagen mixture was supplemented with a highly disseminating variant of human HT-1080 fibrosarcoma, pre-existing blood vessels appear to converge toward tube openings and sprout into a fine network of angiogenic capillaries (C). Note that the tubes are filled with blood, which apparently leaks from the angiogenic capillaries that had grown into the tubes.
Acknowledgments
The authors would like to dedicate this chapter to the memory of Judah Folkman, a pioneer in the development of angiogenesis assays.
References
- Ardi VC, Kupriyanova TA, Deryugina TA, Quigley JP. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc Natl Acad Sci USA. 2007;104:20262–20267. doi: 10.1073/pnas.0706438104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers M, Gladis-Villanueva M, Hamann W, Schmutzler W, Zwadlo-Klarwasser G. The use of the chorio-allantoic membrane of the chick embryo as test for anti-inflammatory activity. Inflamm Res. 1997;46:S29–S30. [PubMed] [Google Scholar]
- Borges J, Tegtmeier FT, Padron NT, Mueller MC, Lang EM, Stark GB. Chorioallantoic membrane angiogenesis model for tissue engineering: A new twist on a classic model. Tissue Eng. 2003;9:441–450. doi: 10.1089/107632703322066624. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- Camerino GM, Nicchia GP, Dinardo MM, Ribatti D, Svelto M, Frigeri A. In vivo silencing of aquaporin-1 by RNA interference inhibits angiogenesis in the chick embryo chorioallantoic membrane assay. Cell Mol Biol (Noisy-le-grand) 2006;52:51–56. [PubMed] [Google Scholar]
- Cao Y, Linden P, Farnebo J, Cao R, Eriksson A, Kumar V, Qi JH, Claesson-Welsh L, Alitalo K. Vascular endothelial growth factor C induces angiogenesis in vivo. Proc Natl Acad Sci USA. 1998;95:14389–14394. doi: 10.1073/pnas.95.24.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Balke M, Schmidt A, Bloch W, Kurz H, Javerzat S, Rousseau B, Wilting J, Bikfalvi A. VEGF coordinates interaction of pericytes and endothelial cells during vasculogenesis and experimental angiogenesis. Dev Dyn. 2004;230:23–33. doi: 10.1002/dvdy.20020. [DOI] [PubMed] [Google Scholar]
- Han Z, Ni J, Smits P, Underhill CB, Zie B, Chen Y, Liu N, Tylzanowski P, Parmelee D, Feng P, Ding I, Gao F, et al. Extracellular matrix protein 1 (ECM1) has angiogenic properties and is expressed by breast tumor cells. FASEB J. 2001;15:988–994. doi: 10.1096/fj.99-0934com. [DOI] [PubMed] [Google Scholar]
- Hood JD, Frausto R, Kiosses WB, Schwartz MA, Cheresh DA. Differential alphav integrin-mediated Ras-ERK signaling during two pathways of angiogenesis. J Cell Biol. 2003;162:933–943. doi: 10.1083/jcb.200304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkonian G, Munoz N, Chung J, Tong C, Marr R, Talbot P. Capillary plexus development in the day five to day six chick chorioallantoic membrane is inhibited by cytochalasin D and suramin. J Exp Zool. 2002;292:241–254. doi: 10.1002/jez.10014. [DOI] [PubMed] [Google Scholar]
- Miller WJ, Kayton ML, Patton A, O’Connor S, He M, Vu H, Baibakov G, Lorang D, Knezevic V, Kohn E, Alexander HR, Stirling D, et al. A novel technique for quantifying changes in vascular density, endothelial cell proliferation and protein expression in response to modulators of angiogenesis using the chick chorioallantoic membrane (CAM) assay. J Transl Med. 2004;2:4. doi: 10.1186/1479-5876-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JB. Transplantability of tissues to the embryo of foreign species. Its bearing on questions of tissue specificity and tumor immunity J Exp Med. 1913;17:482–493. doi: 10.1084/jem.17.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan S, Mousa SA, O’Connor J, Lincoln DW, 2nd, Linhardt RJ. Carbon inhibits vascular endothelial growth factor- and fibroblast growth factor-promoted angiogenesis. FEBS Lett. 2007;581:1157–1160. doi: 10.1016/j.febslet.2007.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Shing Y, Folkman J. Quantitation of angiogenesis and anti-angiogenesis in the chick embryo chorioallantoic membrane. Microvasc Res. 1994;47:31–40. doi: 10.1006/mvre.1994.1003. [DOI] [PubMed] [Google Scholar]
- Reizis A, Hammel I, Ar A. Regional and developmental variations of blood vessel morphometry in the chick embryo chorioallantoic membrane. J Exp Biol. 2005;208:2483–2488. doi: 10.1242/jeb.01662. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A, Presta M. The gelatin sponge-chorioallantoic membrane assay. Nat Protoc. 2006;1:85–91. doi: 10.1038/nprot.2006.13. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A, Roncali L, Burri PH, Djonov V. Chorioallantoic membrane capillary bed: A useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat Rec. 2001;264:317–324. doi: 10.1002/ar.10021. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Urbinati C, Nico B, Rusnati M, Roncali L, Presta M. Endogenous basic fibroblast growth factor is implicated in the vascularization of the chick embryo chorioallantoic membrane. Dev Biol. 1995;170:39–49. doi: 10.1006/dbio.1995.1193. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Roncali L, Dammacco F. The chick embryo chorioal-lantoic membrane as a model for in vivo research on anti-angiogenesis. Curr Pharm Biotechnol. 2000;1:73–82. doi: 10.2174/1389201003379040. [DOI] [PubMed] [Google Scholar]
- Riboldi E, Musso T, Moroni E, Urbinati C, Bernasconi S, Rusnati M, Adorini L, Presta M, Sozzani S. Cutting edge: Proangiogenic properties of alternatively activated dendritic cells. J Immunol. 2005;175:2788–2792. doi: 10.4049/jimmunol.175.5.2788. [DOI] [PubMed] [Google Scholar]
- Romanoff AL. The Avian Embryo. New York: Macmillan, New York; 1960. [Google Scholar]
- Sahni A, Khorana AA, Baggs RB, Peng H, Francis CW. FGF-2 binding to fibrin(ogen) is required for augmented angiogenesis. Blood. 2006;107:126–131. doi: 10.1182/blood-2005-06-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seandel M, Noack-Kunnmann K, Zhu D, Aimes RT, Quigley JP. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97:2323–2332. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- Struman I, Bentzien F, Lee H, Mainfroid V, D’Angelo G, Goffin V, Weiner RI, Martial JA. Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: an efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA. 1999;96:1246–1251. doi: 10.1073/pnas.96.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufan AC, Satiroglu-Tufan NL. The chick embryo chorioallantoic membrane as a model system for the study of tumor angiogenesis, invasion and development of anti-angiogenic agents. Curr Cancer Drug Targets. 2005;5:249–266. doi: 10.2174/1568009054064624. [DOI] [PubMed] [Google Scholar]
- Vazquez F, Hastings G, Ortega MA, Lane TF, Oikemus S, Lombardo M, Iruela-Arispe ML. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999;274:23349–23357. doi: 10.1074/jbc.274.33.23349. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Hasegawa Y, Yamashita H, Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, Sonoda H, Sato Y. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest. 2004;114:898–907. doi: 10.1172/JCI21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang EY, Moses HL. Transforming growth factor beta 1–induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J Cell Biol. 1990;111:731–741. doi: 10.1083/jcb.111.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra A, Aimes RT, Zhu D, Regazzoni K, Kupriyanova T, Seandel M, Deryugina EI, Quigley JP. Collagenolysis-dependent angiogenesis mediated by matrix metalloproteinase-13 (collagenase-3) J Biol Chem. 2004;279:27633–27645. doi: 10.1074/jbc.M313617200. [DOI] [PubMed] [Google Scholar]
- Zijlstra A, Seandel M, Kupriyanova TA, Partridge JJ, Madsen MA, Hahn-Dantona EA, Quigley JP, Deryugina EI. Proangiogenic role of neutrophil-like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood. 2006;107:317–327. doi: 10.1182/blood-2005-04-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]