Abstract
A common procedure for studying the ability of animals to time is the peak procedure. With the peak procedure animals are first trained on a fixed interval schedule (i.e., 30 seconds). After the animals have been well trained on the fixed interval schedule, probe trials are introduced. On probe trials the stimulus is presented longer (i.e., 90 seconds) and the animal does not receive reinforcement for responding. When animals are first presented with probe trials responding remains flat following the point that reinforcement normally occurs on fixed interval trials. The descending slope that eventually emerges is acquired with experience with probe trials. The present experiments manipulated the percentage of probe trials compared to FI trials across groups of rats. It was hypothesized that the descending limb of peak responding would be acquired more quickly when there were many probe trials per session as this might facilitate extinction of responding beyond the interval that reinforcement normally occurs. It was found, however, that acquisition of peak responding occurred best when there were few probe trials per session.
Introduction
Catania (1970) established the peak procedure to study the timing ability of animals. The peak procedure involves two trial types – fixed interval (FI) trials and probe trials. On FI trials the first response following presentation of a stimulus for a fixed amount of time (i.e., 30 seconds) results in reinforcement. On probe trials the stimulus is presented longer (i.e., 90 seconds) and the animal does not receive reinforcement for responding. Examination of the pattern of responding that occurs after the animals have had considerable experience with probe trials reveals that animals tend to increase responding until the time that reinforcement would normally occur on FI trials and then decrease responding as the probe trial extends. This pattern of results has been taken as an indication of accurate assessment of the passage of time (Church, 1978; Gibbon, 1977, Roberts & Church, 1978).
In the early work with the peak procedure researchers were interested in how various parameters might affect the location of peak responding. One parameter that Catania (1970) examined was the proportion of reinforcement. Catania trained pigeons first with 90% fixed interval trials and 10% probe trials. After forty-five 60 trial sessions, he then switched the proportions such that 10% of the trials were fixed interval trials, and 90% of the trials were probe trials. Catania discussed the results of the last session of training on each procedure. Catania found that when there were many reinforced FI trials and few nonreinforced probe trials animals responded at a higher rate and the curve that emerged on probe trials was steeper than when there were few reinforced FI trials and many nonreinforced probe trials. Regardless, however, of the proportion of FI trials to probe trials, peak responding on probe trials occurred at the time that reinforcement normally occurred on FI trials. This was an important finding. The pigeons timed accurately despite differences in reinforcement density that produced dramatic effects on response rate.
S. Roberts (1981) performed a similar manipulation with rats, however, he examined the effect of the proportion of reinforcement simultaneously rather than successively. The rats were trained that one stimulus (e.g., a light) was associated with a peak procedure that had a high rate of reinforcement (80% fixed interval trials and 20% probe trials). A second stimulus (e.g., a tone) was associated with a peak procedure that had a low rate of reinforcement (20% fixed interval trials and 80% probe trials). S. Roberts (like Catania) found that when the probability of reinforcement was low responding was reduced, however, the rats’ peak responding remained accurate.
The relative symmetry of the response distributions that occur following experience with probe trials is described in many models of animal timing to be the result of a single timing mechanism which controls both the ascending and descending response patterns (Gibbon, 1977; Killeen & Fetterman, 1988; Staddon &Higa, 1999). However, if there was a single mechanism (such as the internal clock) controlling responding one would predict that peak responding should occur when probe trials are first introduced. Kirkpatrick-Steger, Miller, Betti, and Wasserman (1996) found that when probe trials were first introduced responding remained asymptotic through the 90 second interval. The descending slope emerged only later, as experience with probe trials increased. Thus, it is possible that the ascending and descending slopes are the result of different processes. The rising slope of the timing function may be the result of the internal clock (or some other timing mechanism) however, responding on the falling slope may (at least in part) be the result of extinction due to experience with nonreinforced probe trials.
One model of animal timing that predicts that experience with nonreinforced probe trials will affect acquisition of peak responding is the learning to time (LeT) model posited by Machado (1997). In his model responding on FI trials is the result of a series of behavioral states that are activated sequentially and are each to some degree associated with operant responding. Those behavioral states that are closest to reinforcement become the most likely to elicit responding. Machado points out that an animal that had been trained only with fixed interval trials should (when first tested with a longer duration probe trial) continue responding at asymptote, because the behavioral state that normally precedes reinforcement would remain active and (presumably) those behavioral states that follow have not acquired inhibitory strength. Following experience with probe trials, the behavioral states that occur beyond the FI duration will undergo extinction and responding will gradually decrease as a trial extends beyond the duration experienced on FI trials.
The present experiments examined further whether extinction of responding beyond the FI duration contributes to the descending response pattern that occurs following experience with the peak procedure. This was accomplished by manipulating the percentage of probe trials compared to FI trials across three groups of rats. A similar manipulation was used by Catania and S. Roberts to examine the shape of the response distributions that occur following training on the peak procedure. Neither of these seminal works, however, examined the development of peak responding. If extinction during nonreinforced probe trials contributes to the descending slope, one would expect that rats trained with higher proportions of probe trials should acquire peak responding more quickly because experience with nonreinforcement should facilitate extinction of responding beyond the FI period.
Experiment 1
All rats were initially trained on a 30 second FI schedule and were then tested with probe trials. For a third of the rats 10% of their test trials were probe trials, for a third of the rats 25% of their test trials were probe trials, and for a third of the rats 50% of their test trials were probe trials. If the ascending and descending slopes that occur with the peak procedure are the result of a single process one would expect that acquisition of peak responding would not differ across these groups. However, if the descending slopes are the result of extinction due to experience with nonreinforced probe trials one would expect that acquisition of peak responding would occur more rapidly when there are many nonreinforced trials (the 50% probe trial group) than when there are fewer nonreinforced trials (the 10% probe trial group).
Materials and Methods
Subjects
Eighteen experimentally naïve male Sprague Dawley rats obtained from Charles River Laboratories, Raleigh, North Carolina were maintained on a 12:12 hour light cycle and received ad lib water. The rats were 120 days old when they began training and were maintained at 85% of their free feeding weights throughout the duration of the experiment. All procedures were approved by the institutional animal care and use committee of Purdue University, and were conducted in accordance with the ethical guidelines of the American Psychological Association.
Apparatus
Six identical Med Associates (St. Albans Vermont) operant chambers (ENV-008) served as the apparatuses during the experiment. Each operant chamber measured 24 cm from side to side, 21 cm tall, and 30.5 cm front to back. The floor was a stainless steel grid (ENV-005) that consisted of nineteen 0.5 cm diameter rods. Two circular 2.5 cm in diameter 100 mA signal lights (ENV-221M) were located 5 cm from the top and 2.5 cm in from each side of the front panel. The signal light lenses were flat and translucent white. 6.4 cm below each signal light was a 2 mm thick response lever (ENV-110M) that was 4.8 cm wide and protruded 1.9 cm in from the face panel. The tension was set at 25 grams for the response levers. Only the left signal light and left response lever were used in the present study. A 5 × 5 cm pellet receptacle (ENV-200R2M) located in the center of the face panel 2.5 cm from the floor of the operant chamber received 45 mg Noyes pellets from a circular modular pellet dispenser (ENV-203M). A 1.3 cm in diameter 100 mA houselight (ENV-215M) was located at the back of the operant chamber 1.3 cm from the top of the operant chamber. The house light was contained in a stainless steel housing that projected light toward the ceiling of the operant chamber. The operant chambers were contained in wooden boxes with internal measurements of 55 cm front to back, 33 cm top to bottom, and 37 cm side to side. The boxes were covered with blankets to limit external light and noise. The operant chambers were connected to a med associates interface and were controlled by personal computer using MED-PC notation.
Procedure
Pretraining
The rats were initially trained with a variation of the autoshaping procedure to press the left lever. With this procedure the left signal light was lit for 5 s and then a food pellet was released. A variable intertrial interval (ITI; mean 45 s) separated presentations of the light. For all rats the houselight was lit during the ITI. If the rats pressed the left lever at any time (during presentation of the signal light or during the ITI) they received reinforcement. They received 60 “autoshaping” trials per session. After the rats were reliably pressing the lever they were successively placed on a fixed ratio 1 (FR 1), an FR 5, an FR 10, and finally a Variable Ratio 5 (VR 5) schedule. Each trial was separated by the lit variable ITI. During this phase of pretraining only appropriate responding resulted in reinforcement (e.g., lever presses during the ITI did not result in reinforcement). After reliable responding on the VR 5 schedule was obtained (mean 5.44 sessions with a standard deviation of 2.85), the rats received FI 30 training.
FI 30 training
The rats experienced sixty FI 30 trials per session for 15 sessions. The signal light was lit and the first response to the lever following 30 seconds darkened the signal light, turned on the houselight, and provided access to a food pellet. The houselight then remained lit throughout the variable ITI (mean 45 s). Following 15 sessions of FI 30 training the rats were randomly divided into three groups and tested with probe trials.
Probe trial testing
All rats continued to receive 60 trials per session during probe trial testing, however, the proportion of FI trials to probe trials varied across the three groups. Group 10% Probes received 54 FI trials and 6 probe trials per session. Group 25% Probes received 45 FI trials and 15 probe trials per session. Group 50% Probes received 30 FI trials and 30 probe trials per session. Group 50% Probes was tested for 10 sessions, group 25% Probes was tested for 20 sessions, and group 10% Probes was tested for 50 sessions. This ensured that at the end of the experiment all rats had experienced 300 probe trials.
Results and Discussion
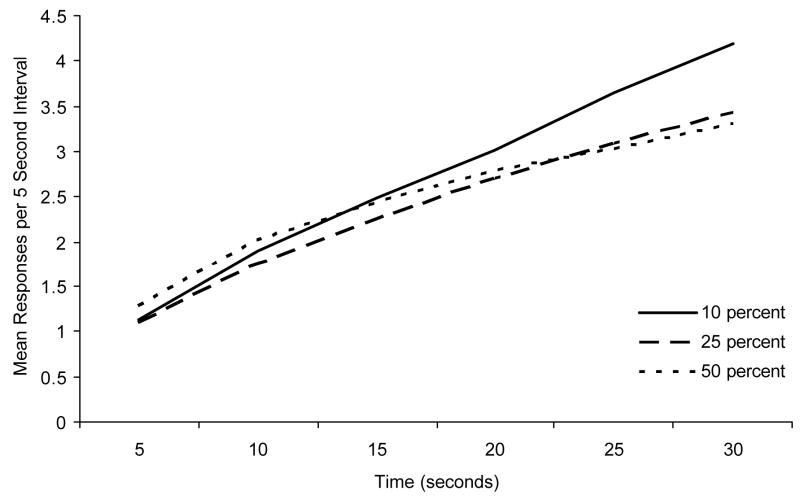
All statistical analyses used the .05 significance level. All post-hoc analyses were corrected with the Bonferroni procedure. The response data were collected in 1 second bins, but were collapsed into 5 second bins for ease of analysis. Examination of Figure 1 reveals that there was little difference among groups in responding across the last 5 sessions of FI training. It appears the 10% probe group may have responded somewhat more than the other groups near the latter portion of the interval, but a two-way (3 group × 6 interval) mixed groups analysis of variance (ANOVA) revealed only a significant main effect of interval, F (5, 75) = 46.80. The main effect of group and group × interval interaction each had F’s less than 1 (0.07 and 0.85, respectively). Thus, there was no indication of an initial difference in response rates among groups that could have affected the subsequent test data.
Figure 1.
Mean responses per 5 second bin across the last 5 sessions of training for each group as a function of time.
Because the number of test trials per session varied among groups, the data were analyzed on the basis of number of probe trials completed (300 probe trials for all rats) as well as number of test sessions completed (10 sessions of probe trials for all rats). Several indicators of peak responding were collected according to a variation of procedures used by Al-Ruwaitea, Al-Zahrani, Ho, Bradshaw, and Szabadi (1997) and Church, Miller, Meck, and Gibbon (1991). The peak time was defined as the interval in which maximum responding occurred. The ascending spread was defined as the point of maximum responding minus the point at which 70% of maximum responding first occurred. The descending spread was defined as the point at which 70% of maximum responding was permanently eliminated minus the point of maximum responding.
Analyses based on number of probe trials completed
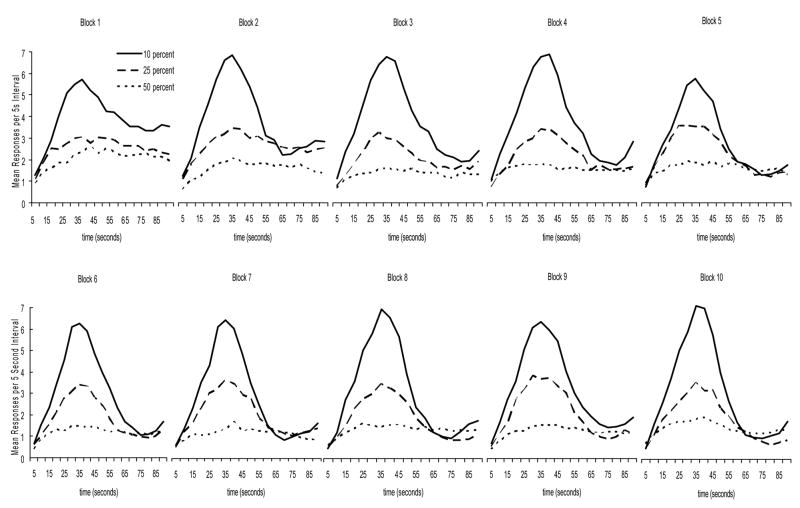
Figure 2 displays the 10 blocks (30 trials per block) of test for each group. It reveals that the proportion of fixed interval trials to probe trials had a dramatic effect on the rats’ performance. One affect of the manipulation was on response rate. The more nonreinforced probe trials that were introduced the less responding that occurred. It is also clear that the development of peak responding differed among the groups. Contrary to what was expected at the outset of the experiment, it appeared that the 10% group developed peak responding first, followed by the 25% group. Peak responding for the 50% group developed later than for either of the other groups (if it developed at all).
Figure 2.
Mean responding across the 10 blocks of test (30 probe trials per block) for each group as a function of interval.
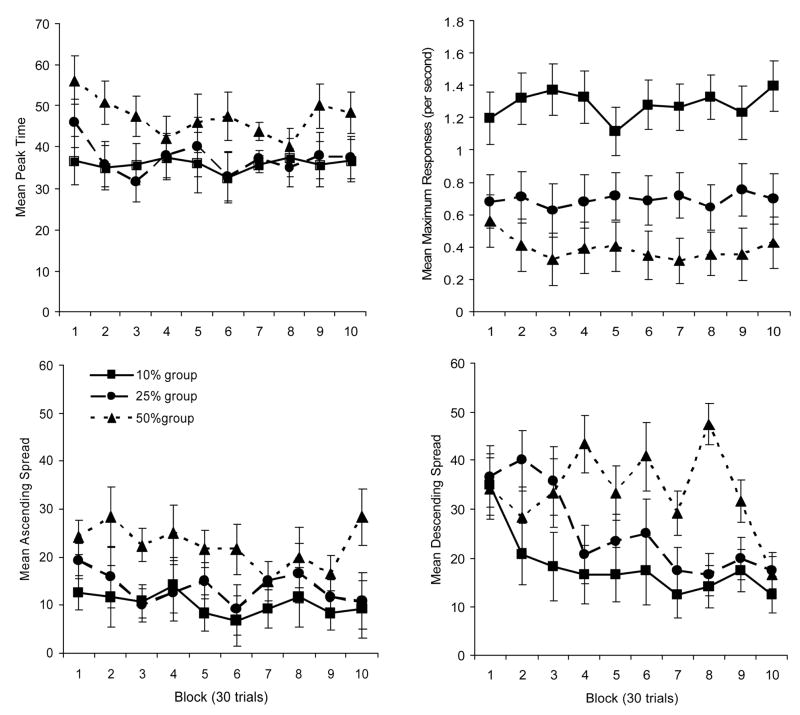
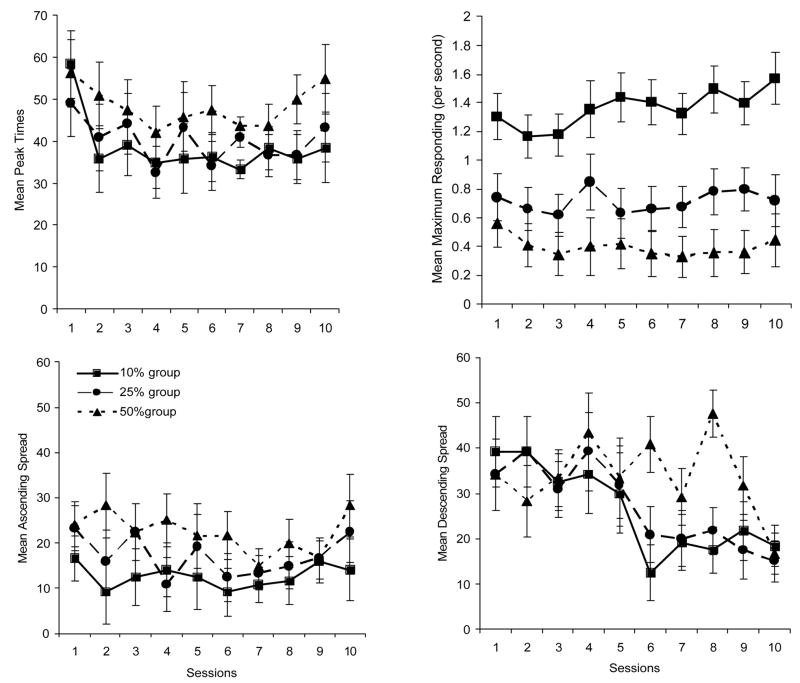
Figure 3 displays the mean points of peak responding as a function of group and block (top left graph) the mean maximum response rate as a function of group and block (top right graph) the mean ascending spread as a function of block and group (lower left graph) and the mean descending spread as a function of block and group (lower right graph).
Figure 3.
Mean points of peak responding as a function of group and block (top left graph). Mean maximum response rate as a function of group and block (top right graph). Mean ascending spread as a function of group and block (lower left graph). Mean descending spread as a function of group and block (lower right graph).
A 3 (group) × 10 (block) mixed groups Analysis of Variance (ANOVA) of the peak time data indicated a significant main effect of group F(2, 15) = 5.06. The main effect of block, and Group × Block interaction were not significant. Post-hoc analyses of the main effect of group indicated that the 50% group had peak times that occurred significantly later than the 10% group, but not the 25% group. The 10% and 25% group did not differ from one another.
A similar analysis was performed on the maximum response data (top right graph of Figure 3). Again the main effect of group was significant F(2, 15) = 10.42. The main effect of block, and the Block × Group interaction were not significant. Post-hoc analyses of the main effect of group indicated that the 10% group responded more than the 25% group and the 50% group, however, the 25% group and 50% group did not differ from one another.
Of particular interest were the analyses of the ascending and descending spreads. A 3 (group) × 10 (block) mixed groups ANOVA of the ascending spread data revealed a main effect of group F(2, 15) = 8.48. The main effect of block, and Block × Group interaction were not significant. Post-hoc analyses of the main effect of group revealed that the 50% group had a significantly wider ascending spread than both the 10% group and the 25% group. The 10% group and 25% group did not differ.
The ANOVA of the descending spread revealed a main effect of group F(2, 15) = 20.13, and a significant main effect of block, F(9, 135) = 2.87. The Group × Block interaction was not significant, though it was close (p = .057). The main effect of block seemed to be the result of the descending slope becoming narrower as training with probe trials continues. Post-hoc comparison of each block of test to one another indicated that Block 10 had a significantly narrower descending spread than occurred at Block 1 and Block 2. Post-hoc analysis of the main effect of group indicated that all groups differed from one another. The descending spreads were narrower as the proportion of probe trials per session decreased.
The fact that there was not a main effect of block on the ascending spreads indicates that the ascending slope did not change as experience with probe trials increased. There is, however, a clear effect of experience on the descending spreads. As experience with probe trials increased the descending slopes became steeper and the spreads narrowed (producing peak responding). The interaction was only marginally significant, however, if one examines the lower right graph of Figure 3, it is clear that all groups started out on block 1 of test with very similar descending spreads. As experience with the probe trials increased the 10% group’s descending spread narrowed first, followed by the 25% group, which was followed by the 50% group. This pattern of results indicates what is clearly apparent in Figure 2. It was the 10% group that acquired peak responding first. The 25% group acquired peak responding somewhat later, and the 50% group acquired peak responding last.
Analyses based on number of test sessions completed
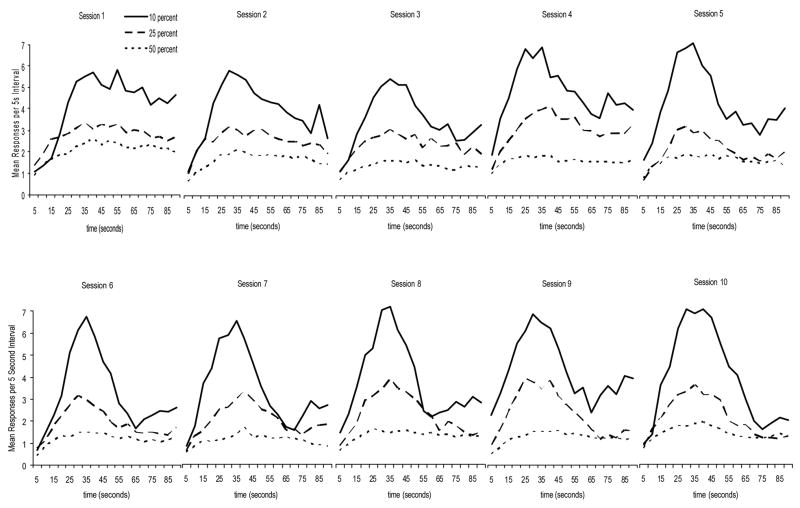
Because the 10% group had so few probe trials per session (6 per session) it is possible that the differences in acquisition of peak responding that were seen when the data were analyzed as a function of blocks of 30 test trials were the result of greater amounts of test sessions for the 10% group compared to the other groups. Blocks of 30 test trials represent 5 test sessions for the 10% group, 2 test sessions for the 25% group and a single test session for the 50% group. To rule out this explanation, the data were also analyzed as a function of session. Because the 50% group was tested for only 10 test sessions, the data were plotted for each group across their initial 10 sessions of test. In this analysis the 50% group had many more test trials (30 per session) than the 25% group (15 per session) and 10% group (6 per session). Thus, if the results presented above were simply the result of greater experience, one would expect that the 50% group would perform the best across the first 10 sessions, followed by the 25% group and then the 10% group. If, however, it is the proportion of probe trials to FI trials that is causing these effects, one would expect that the session data would be similar to the block data. Figure 4 presents the response data as a function of session. This Figure reveals a pattern of results similar to that found when the data were plotted as blocks of trials.
Figure 4.
Mean responding for the first 10 sessions of probe trial training for each group as a function of interval.
Figure 5 displays the mean points of peak responding as a function of group and session (top left graph) the mean maximum response rate as a function of group and session (top right graph) the mean ascending spread as a function of group and session (lower left graph) and the mean descending spread as a function of group and session (lower right graph).
Figure 5.
Mean points of peak responding as a function of group and session (top left graph). Mean maximum response rate as a function of group and session (top right graph). Mean ascending spread as a function of group and session (lower left graph). Mean descending spread as a function of group and session (lower right graph).
A 3 (group) × 10 (session) mixed factor ANOVA of the peak time data indicated a significant main effect of session F (9, 135) = 2.03. The main effect of group, and Group × Session interaction were not significant. Examination of the top right graph of Figure 5 indicates that the main effect of block is likely the result of the peak times decreasing as experience with probe trials increased. Pairwise post-hoc comparisons indicated that the only sessions that significantly differed from one another were sessions 1 and 4.
A similar analysis was performed on the maximum response data (top right graph of Figure 5). The main effect of session was significant, F(9, 135) = 2.71, as was the main effect of group, F(2, 15) = 10.67. The Session × Group interaction was not significant, though it was very close (p = .051). Post-hoc analysis of the main effect of group indicated that the 10% group responded more than the 25% group and the 50% group, however, the 25% group and 50% group did not differ from one another.
As above, of particular interest were the analyses of the ascending and descending spreads (see the lower left and lower right graphs of Figure 5, respectively). The ANOVA of the ascending spread data revealed a significant main effect of group F(2, 15) = 5.05, however, the main effect of session, and Block × Session interaction were not significant. Post-hoc analysis of the main effect of group revealed that the 50% group had a significantly wider ascending spread than the 10% group. The 50% group, however, did not differ from the 25% group and the 10% group did not differ from the 25% group.
The ANOVA of the descending spread revealed only a significant main effect of session F(9, 135) = 3.26, p < .001. This effect appears to be the result of the descending slopes becoming narrower as training with probe trials continued. Pairwise Post-hoc comparisons indicated that session 10 had a significantly narrower descending slope than occurred at session 1, 2, and 3.
Although there was not an effect of group when the descending spread was analyzed as a function of session, the general pattern of results was very similar to what was found when the data were analyzed as a function of block. Contrary to what was expected at the onset of the experiment the data indicated that when fewer probe trials were used the rats acquired peak responding more quickly. When one examines the ascending and descending spreads as in the lower graphs of Figures 3 and 5 it can be seen that the ascending spreads were quite similar as testing proceeded. It was the descending spreads that took time to develop and developed more quickly for those rats tested with fewer probe trials per session.
Experiment 2
Experiment 1 indicated that when there were fewer probe trials it was easier for rats to acquire peak responding. This effect seemed to be largely the result of differences among groups in the acquisition of the descending slope. When there were many nonreinforced probe trials the descending spreads were wider and seemed to require more experience to narrow. In experiment 2 a within subjects analysis of the effect of probe trial proportion was performed. The animals from Experiment 1 were tested for 5 sessions in each of the probe percentage conditions that they had not yet experienced. In this way the effect of proportion of probe trials on the ascending and descending spreads could be examined independently of acquisition.
Materials and Methods
Subjects
The eighteen rats that were used in Experiment 1 were maintained under identical conditions to that used in Experiment 1.
Apparatus
The same apparatuses used in Experiment 1 were used in Experiment 2.
Procedure
Immediately after completion of testing in Experiment 1 the rats were randomly assigned to one of the two remaining testing conditions. Thus, rats that had completed testing in the 50% group were randomly assigned to either the 10% condition or the 25% condition, rats that had completed testing in the 25% group were randomly assigned to either the 10% condition or the 50% condition, and rats that had completed testing in the 10% group were randomly assigned to either the 25% condition or 50% condition (with the restriction that equal numbers of rats were transferred to each new testing condition). The rats were tested for 5 sessions in the novel testing condition and then were tested for an additional 5 sessions in the remaining testing condition.
Results and Discussion
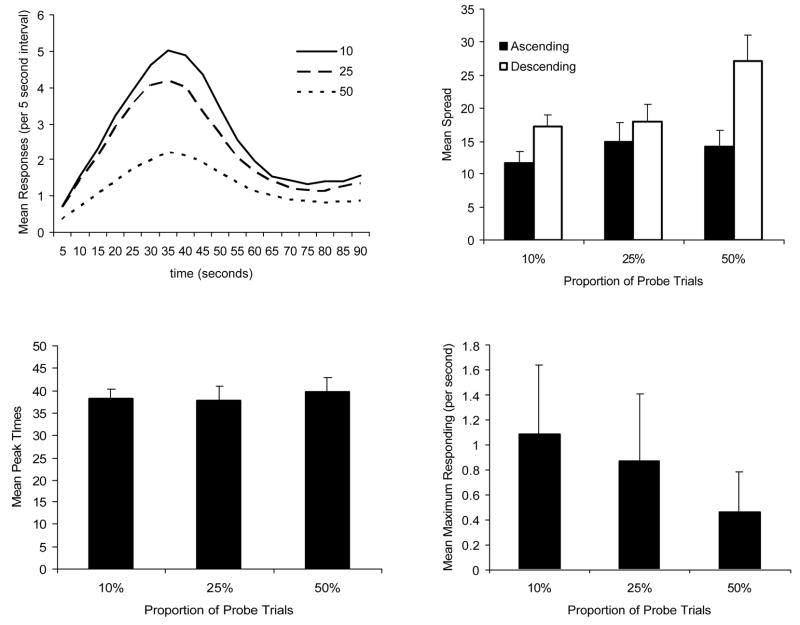
The data were collapsed across initial group and analyzed simply as a function of percent probe trial condition. Because some animals from each probe percentage condition were tested in that condition either first, second, or last - order of testing effects can be ruled out. The top left graph of Figure 6 presents the response data in 5 second bins. The results are similar to what was discovered in Experiment 1. The animals responded most when they were in the 10% probe condition, an intermediate amount when in the 25% probe condition, and least when in the 50% probe condition.
Figure 6.
Mean responding for each proportion of probe trial condition as a function of interval (top left graph). Mean ascending and descending spreads as a function of proportion of probe trials (top right graph). Mean peak times as a function of proportion of probe trials (bottom left graph). Mean maximum responding as a function of proportion of probe trials (bottom right graph).
As in Experiment 1, maximum responding, peak time, and the ascending and descending spreads were analyzed. There was no effect of proportion of probe trials on peak times F (2, 34) = 0.46 (see lower left graph of Figure 6), however, there was an effect of proportion of probe trials on maximum responding F (2, 34) = 41.36 (see lower right graph of Figure 6). Post-hoc analyses indicated that all groups differed from one another. When the rats were tested with 10% probes they responded the most, when they were tested with 25% probes they responded an intermediate amount, and when they were tested with 50% probes they responded the least.
A 2 (spread condition) × 3 (probe proportion) within subject ANOVA was used to analyze the spread data (top right graph of Figure 6). There was a significant main effect of spread condition, F (1, 17) = 5.86, a significant main effect of probe proportion, F (2, 34) = 8.57, and a significant Spread Condition × Probe Proportion interaction, F (2, 34) = 3.27. The interaction appeared to be the result of the ascending spread remaining similar across probe proportions, and the descending spreads getting wider as probe proportion increased. One-way ANOVAs of the ascending and descending spreads supported this observation. The effect of probe proportion was not significant for the ascending spread data, but was significant for the descending spread data, F (2, 34) = 41.36. Post-hoc analysis of the probe proportion effect on the descending spread indicated that when the rats were tested with 50% probes they had greater descending spreads than when they were tested with 10% probes or with 25% probes. The descending spreads did not differ when the rats were tested with 10% probes and 25% probes.
The results of Experiment 2 add to the findings of Experiment 1. Not only does having a large proportion of nonreinforced probe trials affect acquisition of peak responding, it also seems to affect the descending spread after the task has been well acquired. It is interesting that the proportion of probe trials has a greater affect on the descending spread than on the ascending spread. This seems to indicate that more than one process may be involved in the response functions that occur with the peak procedure. What that additional process might be will be discussed below.
General Discussion
In general the overall pattern of results from Experiments 1 and 2 indicate that the rats acquired peak responding fastest and performed best when fewer probe trials were included during test sessions. The effect on acquisition was particularly surprising. It had been hypothesized from the outset of these experiments that when there were more probe trials per session, the rats would have more experience with nonreinforced trials, and would acquire peak responding more quickly.
One issue that is clear from examining the pattern of results that developed as a result of manipulation of the proportion of probe trials to FI trials was that the more probe trials that were included the less responding in general that occurred. This is likely the result of the reduction in reinforcement that occurred when nonreinforced probe trials were introduced.
It is also clear that when many probe trials were introduced in Experiment 1 that acquisition of peak responding was affected. The more probe trials that were included per session, the more slowly acquisition of peak responding occurred. This is most apparent when one compares the performance of the 10% group to that of the 25% group. Although both of these groups acquired adequate peak responding, the 10% group acquired peak responding more quickly than the 25% group. This is readily apparent when one looks at the data as a function of session or as a function of block.
The fact that more probe trials per session disrupted acquisition of peak responding is difficult to reconcile with Machado’s LeT model. LeT predicts that the downward slope that occurs following the FI period on probe trials needs to be acquired through experience with nonreinforced probe trials. This process is likely a process of extinction. As the animals experience nonreinforcement on probe trials the behavioral states following the FI period will become inhibitory and should lead to a decline in responding. One might predict that having a larger proportion of nonreinforced probe trials per session would facilitate this extinction process. Apparently that is not the case.
The data from Experiment 1 indicated that when there were fewer probe trials per session rats acquired peak responding more quickly. The peak responding that developed in Experiment 1 seemed to depend on acquisition of the downward slope during probe trials. All of the animals were well trained on fixed interval 30 s trials prior to introduction of the 90 s probe trials, and thus, the ascending spreads during probe testing were fairly similar among groups whereas the descending spreads differed. This finding is an indication that more than one process may be involved in the response functions that occur with the peak procedure. Perhaps the upward slope is the function of a timing mechanism, but the downward slope may result from an additional process. The results of Experiment 1 seems to indicate that acquisition of the descending slope is not simply the result of extinction, or one would expect the 50% group (which had the most experience with extinction trials during a session) would have acquired peak responding the fastest.
Perhaps the descending slope originates from an active suppression of responding. As the animals learned about probe trials, they learned to actively suppress responding as the interval extended beyond 30 seconds. In support of this notion, Al-Ruwaitea, Al-Zahrani, Ho, Bradshaw, and Szabadi (1997) found that lesions of the ascending serotonin system affected acquisition of peak responding in rats. The ascending serotonin system has been implicated in the active inhibition of behaviors. Al-Ruwaitea et al., found that the ascending slopes were relatively unaffected by lesions to the ascending serotonin system. Responding, however, on the descending limb was elevated following lesions. This increased responding during the descending limb remained even after extensive training.
Of course if the descending slope that occurs following training with the peak procedure is the result of active suppression of responding by the rats, one might wonder why having fewer nonreinforced probe trials would facilitate this process. Perhaps when there are few probe trials against the back drop of many reinforced fixed interval trials those probe trials become more salient to the rat. This explanation is post-hoc, and one could certainly envision many nonreinforced probe trials being the more salient condition. It does appear, however, that the ascending and descending spreads can be affected independently, either by manipulation of testing procedures (as with the manipulation of proportion of probe trials described here), or by manipulation of the physiology of the rat (as with the manipulation of the serotonergic system described above).
The groups in the present studies differed not only in the number of probe trials that were presented each session and the ratio of probe trials to FI trials during test, they also differed in the absolute number of FI trials experienced. All groups experienced the same number of FI trials in original training, but after probe trials were introduced the 10% group experienced more FI trials than the 25% group which experienced more FI trials than the 50% group. It is possible that this increased experience with FI trials helped the 10% group and 25% group maintain peak responding. The fact that the ascending spread was increased in the 50% group in Experiment 1 supports this notion.
The poor performance of the rats when they were tested in the 50% condition is somewhat puzzling given that researchers have used a 50% proportion of probe trials to fixed interval trials with success in the past (see for example, Buhusi & Meck, 2002; Bushusi, Perera, & Meck, 2005; Buhusi, Sasaki, & Meck, 2002). One difference in procedure of the current study is that the panel light was used as the to-be-timed stimulus, and the house light was lit during the ITI. In the past when researchers have used rats as subjects they have often used the houselight or a sound stimulus as the to-be-timed stimulus and darkened the chamber during the ITI. The houselight vs. dark chamber discrimination may be somewhat easier for the rats than the panel light vs. houselight discrimination. In any case, the choice of stimuli for the current experiments may have been fortuitous, as the difficulty of the discrimination may have allowed the affect of the proportion of probe trials to become apparent.
The results of the present experiments make it clear that the proportion of nonreinforced probe trials that occur during a training session affects not only the amount of responding that occurs, but also the acquisition of peak responding. Contrary to what was expected at the outset of the experiments the fewer probe trials that were introduced, the more rapidly peak responding was acquired. Because the acquisition of peak responding depended on the acquisition of the falling slope on probe trials it appears that more than one process may be involved in the response functions that occur when animals are tested with the peak procedure. The initial rising slope is likely the function of a timing mechanism, however, the falling slope seems to develop as a function of experience with nonreinforced probe trials. Interestingly, when fewer nonreinforced probe trials were introduced acquisition of the falling slope occurred most quickly.
Acknowledgments
These experiments were supported by Grant 1R03MH068264-01A1 from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Ruwaitea ASA, Al-Zahrani SSA, Ho MY, Bradshaw CM, Szabadi E. 5-hydroxytryptamine and interval timing. In: Bradshaw CM, Szabadi E, editors. Time and behaviour: Psychological and neurobehavioural analyses. Elsevier Science B.V.; Amsterdam: 1997. pp. 517–571. [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav Neurosci. 2002;116:291–297. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Perera D, Meck WH. Memory for timing visual and auditory signals in albino and pigmented rats. J Exp Psychol Anim Behav Process. 2005;31:18–30. doi: 10.1037/0097-7403.31.1.18. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Sasaki A, Meck WH. Temporal integration as a function of signal and gap intensity in rats (Rattus norvegicus) and pigeons (Columba livia) J Comp Psych. 2002;116:381–390. doi: 10.1037/0735-7036.116.4.381. [DOI] [PubMed] [Google Scholar]
- Catania AC. Reinforcement schedules and psychophysical judgements: A study of some temporal properties of behavior. In: Schoenfeld WN, editor. The theory of reinforcement schedules. Appleton-Century-Croft; New York: 1970. pp. 1–42. [Google Scholar]
- Church RM. The internal clock. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Erlbaum/Hillsdale; NJ: 1978. pp. 277–310. [Google Scholar]
- Church RM, Miller KD, Meck WH, Gibbon J. Symmetrical and asymmetrical sources of variance in temporal generalizations. Anim Learn Behav. 1991;19:207–214. [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychol Rev. 1977;84:279–325. [Google Scholar]
- Killeen PR, Fetterman JG. A behavioral theory of timing. Psychol Rev. 1988;95:274–295. doi: 10.1037/0033-295x.95.2.274. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick-Steger K, Miller SS, Betti CA, Wasserman EA. Cyclic Responding by pigeons on the peak timing procedure. J Exp Psychol Anim Behav Process. 1996;22:447–460. doi: 10.1037//0097-7403.22.4.447. [DOI] [PubMed] [Google Scholar]
- Machado A. Learning the temporal dynamics of behavior. Psychol Rev. 1997;104:243–262. doi: 10.1037/0033-295x.104.2.241. [DOI] [PubMed] [Google Scholar]
- Roberts S. Isolation of an internal clock. J Exp Psychol Anim Behav Process. 1981;7:242–268. [PubMed] [Google Scholar]
- Roberts S, Church RM. Control of an internal clock. J Exp Psychol Anim Behav Process. 1978;4:318–337. [Google Scholar]
- Staddon JER, Higa JJ. Time and memory: Towards a pacemaker-free theory of interval timing. J Exp Anal Behav. 1999;71:288–91. doi: 10.1901/jeab.1999.71-215. [DOI] [PMC free article] [PubMed] [Google Scholar]