Abstract
TNFR2 is predominantly expressed by a subset of human and mouse CD4+CD25+FoxP3+ Tregs. In this study, we characterized the phenotype and function of TNFR2+ Tregs in peripheral lymphoid tissues of normal and tumor bearing C57BL/6 mice. We found that TNFR2 was expressed on 30%−40% of peripheral activated/memory subset of Tregs that were most highly suppressive. In contrast, TNFR2− Tregs exhibited the phenotype of naïve cells and only had minimal suppressive activity. Although not typically considered to be Tregs, CD4+CD25−TNFR2+ cells nevertheless possessed moderate suppressive activity. Strikingly, the suppressive activity of TNFR2+ Tregs was considerably more potent than that of reportedly highly suppressive CD103+ Tregs. In the Lewis lung carcinoma model, more highly suppressive TNFR2+ Tregs accumulated intratumorally than in the periphery. Thus, TNFR2 identifies a unique subset of mouse Tregs with an activated/memory phenotype and maximal suppressive activity which may account for tumor-infiltrating lymphocyte mediated immune evasion by tumor.
Keywords: T cell, cytokine receptor, tolerance/suppression/anergy
Introduction
TNF is a pleiotropic cytokine that is a major participant in the initiation and orchestration of inflammation and immunity (1). TNF is also implicated in inflammation-associated cancers, produced either by tumor cells and/or by infiltrating leukocytes (2). TNF mediates its biological functions through its receptors: TNFR1 and TNFR2. TNFR1 through its death domain mediates the cytotoxic effect while TNFR2 is largely confined to cells of the immune system and its biological role is still not fully understood (1). TNF has been shown to have proinflammatory effects, nevertheless, increasing evidence reveals TNF also has unexpected immunosuppressive effects and this may be based on the capacity of TNF in concert with IL-2 to activate and expand mouse CD4+CD25+ T regulatory cells (Tregs) (3), presumably by interacting with TNFR2 which is preferentially expressed by both human and mouse Tregs (3, 4). Thus, up-regulation of Treg activity by TNF may mediate the delayed immunosuppressive effect of TNF on inflammatory responses and the expansion of Tregs in the tumor microenvironment. Consequently, we hypothesized that TNFR2 expression may identify the more functionally active Tregs in tumor-infiltrating lymphocytes (TILs).
Materials and Methods
Mice, cells and reagents
Female wild type C57BL/6 mice, 8 to 12 wk old, were provided by the Animal Production Area of the NCI (Frederick, MD). NCI-Frederick is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council; 1996; National Academy Press; Washington, D.C.). C57BL/6-derived Lewis lung carcinoma (LLC) cell line was obtained from ATCC. Antibodies purchased from BD Biosciences (San Diego, CA) consisted of anti-CD3 (145−2C11), CD4 (GK1.5), anti-CD25 (PC61), CD45RB (16A), CD62L (MEL-14), CD44 (IM4), CD69 (H1.2F3), CD103 (M290), TNFR2 (TR75−89), CTLA4 (UC10−4F10−11) and CD16/CD32 (2.4G2). Anti CD3e (145−2C11), Foxp3 Staining Set (FJK-16s), GITR (DTA-1) Abs were purchased from eBioscience (San Diego, CA).
Mouse tumor inoculation and separation of tumor infiltrating lymphocytes (TILs)
C57BL/6 mice were inoculated subcutaneously in the right rear flank with 100,000 LLC cells. After two weeks, tumors were excised, minced and digested in RPMI 1640 supplemented with 1 mg/ml collagenase IV, 0.1 mg/ml DNase I.
Cell purification, in vitro culture and proliferation assay
CD4 subsets were purified from spleen and lymph nodes (inguinal, axillary and mesenteric regions) or TILs using Cytomation MoFlo cytometer (Fort Collins, CO), yielding a purity of ∼98% for both subsets. For in vitro assays of inhibition of proliferation by Treg, CFSE-labelled (2 μM, 8 min at room temperature) responder cells (5×104 cells/well) were seeded in a U-bottom 96-well plate with 2×105 cells/well of APCs (T cell-depleted, irradiated spleen cells) plus 0.5 μg/ml of functional grade anti-CD3e antibody. Subsets of CD4 cells were added to the wells at the desired ratio. After 48 h, CFSE dilution was determined with FACS.
Flow Cytometry
After blocking FcR, cells were incubated with appropriately diluted antibodies. Data were acquired on a FACSort (BD Biosciences, Mountain View, CA) and data analysis was conducted using CellQuest software (BD Biosciences).
Results and Discussion
Phenotype and distribution of mouse TNFR2+ Tregs
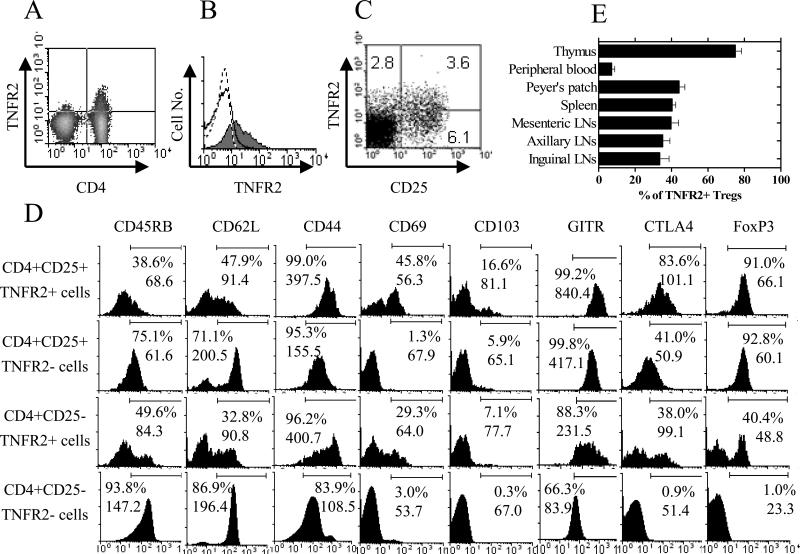
As shown for normal C57BL/6 (B6) mice in Fig 1A, the expression of TNFR2 was largely restricted to splenic CD4+ cells. Similar results were observed by LN cells (data not shown). CD4+CD25+ T cells were the primary cell type expressing TNFR2, while fewer CD4+CD25− T cells expressed this receptor (Fig 1B). The proportions of CD25+TNFR2+ cells, CD25+TNFR2− cells and CD25−TNFR2+ splenic CD4 cells were 3.6%, 6.1% and 2.8%, respectively (Fig 1C).
Figure 1. Phenotypic characterization and distribution of normal C57BL/6 mouse CD4+CD25+TNFR2+ Tregs.
(A) Splenocytes were stained with anti CD4 and TNFR2 Abs and analyzed by FACS. (B) Splenocytes were stained with anti CD3, CD4, CD25 and TNFR2 Abs. The expression of TNFR2 by CD4+CD25+ cells (grey histogram) and CD4+CD25− cells (solid line histogram) was analyzed by FACS, gating on CD3+ cells. Dashed line represents isotype control. (C) Splenocytes were stained with anti CD3, CD4, CD25 and TNFR2. Expression of CD25 and TNFR2 was analyzed with FACS by gating on CD3+CD4+ cells. (D) Splenocytes were stained with anti CD4, CD25, TNFR2 and CD45RB, or CD62L, or CD44, or CD69, or CD103, or GITR, or CTLA-4, or FoxP3 Abs. CTLA-4 and FoxP3 were stained intracellularly. Expression of phenotypic markers was analyzed with FACS by gating on indicated CD4 subsets. (E) Cells from indicated lymphoid tissues and peripheral blood were stained with anti CD3 (or CD8 for thymocytes), CD4, CD25 and TNFR2 Abs. The percentage of TNFR2+ cells was analyzed with FACS by gating on CD3+CD4+CD25+ population (except for thymocytes which were gated on CD8−CD4+CD25+ cells). Error bars indicate SE derived from 3 mice (n=3). Numbers in the quadrants represent percentage of positive staining cells (%).The numbers in the histograms show percentage of positive cells (%) and MFI. Data shown are representatives of at least 3 separate experiments with similar results.
Tregs are heterogeneous and consist of activated/memory as well as naïve cells. As shown in Fig 1D, TNFR2+ Treg cells were CD45RBlo, CD62Llo, CD44hi and expressed relatively high levels of CD69, CD103, GITR and CTLA-4, indicative of the memory and activated phenotype. In contrast, naïve Tregs were CD45RBhi, CD62Lhi, CD44lo and expressed relative low levels of CD69, CD103, GITR and CTLA-4, and were TNFR2−. CD4+CD25−TNFR2+ cells contained fewer CD45RBhi and CD62Lhi, and more CD44hi and CD69+ cells, compared with CD4+CD25+TNFR2− cells. Furthermore, TNFR2 showed a closer relationship than CD25 to the subset of memory and activated mouse peripheral FoxP3+ Treg cells. Both TNFR2+ and TNFR2− subsets of CD4+CD25+ cells expressed comparably high levels of FoxP3. However, the intensity of FoxP3 expression was consistently higher in CD4+CD25+TNFR2+ cells than in CD4+CD25+TNFR2− cells. Although only about 1% of CD4+CD25−TNFR2− cells were FoxP3+ cells, 25∼40% of CD4+CD25−TNFR2+ cells expressed FoxP3 (Fig 1D).
In normal C57BL/6 mice, ∼80% of CD8−CD4+CD25+ thymocytes were TNFR2+, which resembles the reported high TNFR2 expression by human thymic Tregs (4). In peripheral LNs, spleen and Peyer's patches, 30%∼40% of CD4+CD25+ cells expressed TNFR2. In contrast, in mouse peripheral blood, less than 10% of CD4+CD25+ cells were TNFR2+ (Fig 1E).
Mouse TNFR2+ Tregs are the most highly suppressive cells
Upon stimulation with APC and anti-CD3, only CD4+CD25−TNFR2− T cells were readily activated to proliferate and produce IFNγ, whereas CD4+CD25+TNFR2+ and CD4+CD25+TNFR2− cells were unreactive. Interestingly, TNFR2-expressing CD4+CD25− T cells were also non-responsive to TCR stimulation (data not shown).
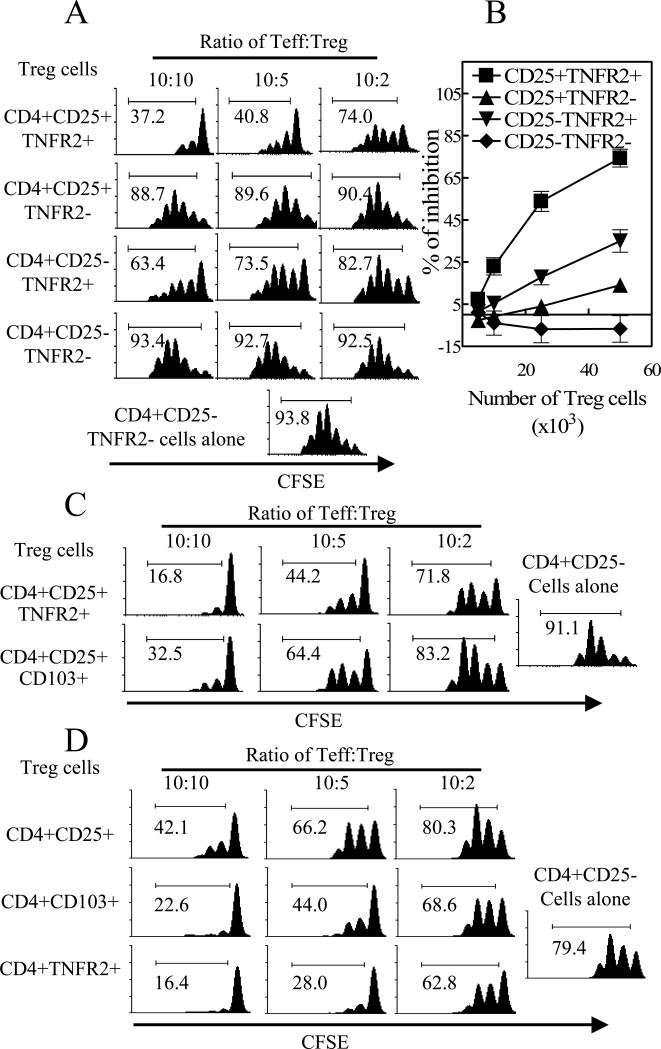
Next, we examined the suppressive potential of these CD4 subsets. Flow-sorted CD4 subsets were co-cultured as shown in Fig 2A. As we expected, CD4+CD25+TNFR2+ T cells were potent suppressor cells and inhibited replication of responder CD4+CD25-TNFR2− T cells. Surprisingly, CD4+CD25+TNFR2−, comprising the majority of classical Tregs (60−70% of CD4+CD25+FoxP3+ T cells), had only minimal suppressive activity. Although not usually considered to be Tregs, TNFR2-expressing CD4+CD25− T cells had moderate and consistently more potent suppressive capacity than CD4+CD25+TNFR2− cells. Due to technical difficulty of sorting FoxP3+ cells from normal C57BL/6 mice, we have not examined whether FoxP3+ cells present in CD4+CD25−TNFR2+ subset are solely responsible for the suppressive activity. We very carefully repeated this experiment and obtained highly consistent results (Fig 2B). Furthermore, Balb/c mouse TNFR2+ Tregs also had a memory/activated phenotype and were upto10 fold more suppressive than TNFR2- Tregs (data not shown).
Figure 2. CD4+CD25+TNFR2+ T cells were highly potent suppressor cells.
Flow-sorted CD4+CD25−TNFR2− or CD4+CD25− T cells (5×104 cells/well) were labeled with CFSE and cultured alone or co-cultured with indicated number or ratio of flow-sorted CD4 subsets from spleen and LNs of normal C57BL/6 mice. The percentage of CFSE-diluted cells was shown in the histograms. (A), (C) and (D) show a representative data of at least 3 separate experiments with similar results and (B) shows summary of percent inhibition of replication (%) from 6 separate experiments.
TNFR2 is a better phenotypic marker of highly suppressive cells than CD103
It has been reported that CD103 expression can define the most potent suppressive subset of CD4+CD25+ Tregs (5). Since CD4+CD25+TNFR2+ T cells expressed the highest level of CD103, it is possible that the expression of CD103 may more accurately predict the suppressive potential of CD4 subsets. We confirmed the previous report (5) that CD4+CD25+CD103+ T cells were more potent suppressors than CD4+CD25+CD103− T cells (data not shown). However, the inhibition of proliferation by CD103+ Tregs was significantly less potent than by TNFR2+ Tregs, as shown by both CFSE-dilution assay (Fig 2C) and [3H] thymidine incorporation assay and also in the inhibition of cytokine production (P<0.01∼0.05, data not shown). Furthermore, CD4+TNFR2+ T cells exhibited more suppressive effects than CD4+CD103+ T cells, and both of these subsets were more suppressive than the more heterogeneous CD4+CD25+ T cells (Fig 2D). Consequently, TNFR2 is superior to both CD103 and CD25 in defining functional CD4 T suppressor cells.
Tumor infiltrating Tregs express higher TNFR2 level and are more suppressive
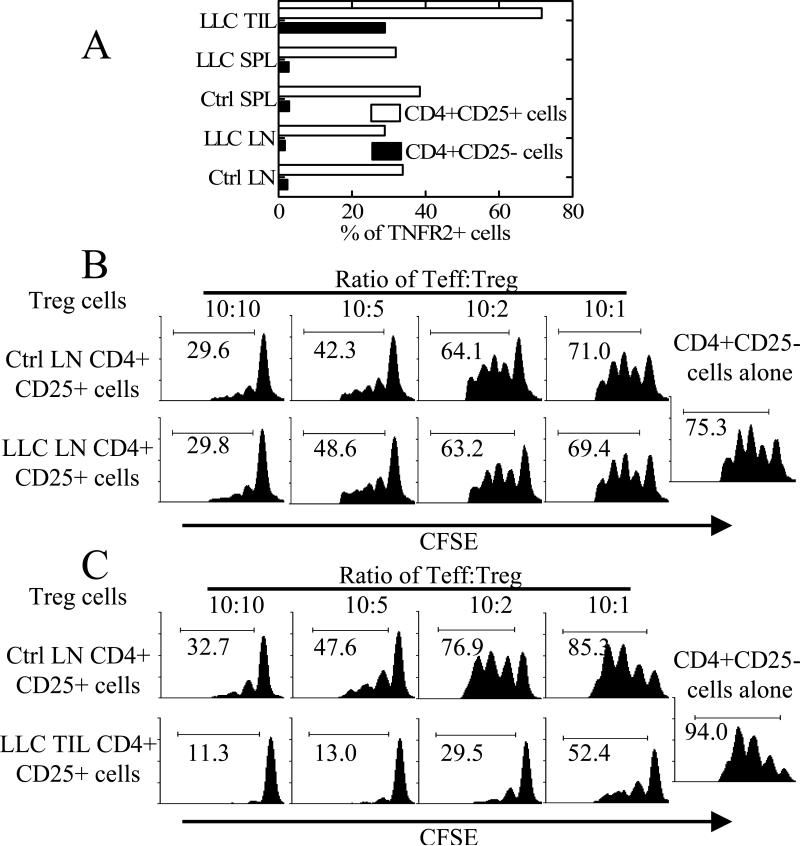
Tregs accumulate intratumorally and promote tumor growth by inhibiting anti-tumor immune response (6). Based on reports that TNFR2 is expressed by TILs isolated from several human solid tumors (7), we hypothesized that TNFR2+ Tregs with enhanced suppressive potential may be more prevalent in the tumor microenvironment. For this purpose, we examined the C57BL/6 mouse Lewis lung carcinoma (LLC) tumor model. As predicted, 75∼100% of CD4+CD25+ TILs expressed TNFR2. The proportion of TNFR2+ cells present in the subset of CD4+CD25− TILs was also increased over the low levels present in splenic and LN cells (Fig 3A). Similar results were also observed in the Balb/c mouse 4T1 breast tumor model (data not shown).
Figure 3. Highly suppressive TNFR2+ Tregs accumulated in the TILs of LLC mouse model.
(A) LN cells, spleen cells (SPL) and TILs from LLC tumor bearing mice were stained with CD4, CD25 and TNFR2. Expression of TNFR2 was analyzed with FACS by gating on CD4+CD25+ or CD4+CD25− cells from LLC bearing mouse TIL (LLC TIL) or spleen (LLC SPL) or LNs (LLC LN) or from tumor free C57BL/6 mouse spleen (Ctrl SPL) or LNs (Ctrl LN). (B-C) CD4+CD25− T cells from normal control mouse LNs (5×104 cells/well) were labeled with CFSE and cultured alone or co-cultured with indicated source of Tregs at desired ration. The percentage of CFSE-diluted cells was shown in the histograms. Data shown are representatives of 3 separate experiments with similar results.
We next tested the prediction that the inhibitory activity of LLC CD4+CD25+ TILs should be greater than that of peripheral LN CD4+CD25+ cells. As shown in Fig 3B, there was no difference in the suppressive potency of Tregs over various ratios of Teff:Treg from peripheral LNs of tumor bearing and control mice. In contrast, TIL CD4+CD25+ cells retrieved from LLC tumors exhibited far more potent suppressive activity than LN CD4+CD25+ cells from tumor-free mice (Fig 3C). Thus, the suppressive effects of peripheral LN Tregs and TIL Tregs correlated well with their level of TNFR2 expression. As shown in Fig. 3B-C, the potent inhibitory effect exerted by CD4+CD25+ TILs was not antigen specific, since both targeted responder cells and APCs were from tumor-free mice. The phenotype of TIL CD4+CD25+ cells, which were 75∼100% TNFR2+, resembled that of normal mouse LN TNFR2+ Tregs and were indicative of an activated/memory subset (data not shown).
Although other factors in the tumor microenvironment may also contribute to the activation of TIL Tregs, the presence of TNF in the inflammatory tumor site is most likely to contribute to up-regulating the number of functional Tregs by activating TNFR2 (3). It has been reported that TGFβ, a cytokine crucial for de novo generation of Tregs, is also able to induce TNF and TNFR2 expression on CD4 cells (8) and may therefore be responsible for the increase of TNFR2+ Tregs in the tumor. TNFR2 has also been reported to be a co-stimulator for antigen-driven T cell responses (1), thus, this receptor can also serve as a co-stimulator for the response of Treg to tumor antigen. Indeed, it has been shown that anti-TNF as well as anti-TNFR2 antibodies can inhibit proliferation of TNFR2-expressing TILs isolated from human solid tumors (7). Despite reports that anti-TNF therapy increases Treg activity in rheumatoid arthritis (9), our preliminary observations also indicate that anti-TNF therapy actually decreased the proportion of FoxP3+ Tregs in TILs of various mouse tumor models (our unpublished data). Thus blockade of TNF-TNFR2 interaction may result in down-regulation of Treg activity and this may provide the mechanistic basis for the reported therapeutic efficacy of anti-TNF therapy in cancer (2).
Several lines of evidence suggest that immunosuppressive action of TNF is mediated by TNFR2. For example, in experimental autoimmune encephalomyelitis (EAE) mouse model, TNFR1 deficient mice were completely resistant to induction of disease, while TNFR2 deficient mice exhibited more severe EAE (10). The immunosuppressive action of TNFR2-dependent effects of TNF is most compatible with our observations that TNF is a potent and selective activator of Tregs (3).
It was reported that CD103 expression defines a potent suppressive subset of Tregs (5) and CCR6 expression defines an effector/memory-like subset of Tregs (11). Expression of CD103 partially correlated with TNFR2 expression by Tregs (Fig. 1D), however, the reported low thymic level of CD103+ Tregs (5) as well as high level in peripheral blood of CCR6+ Tregs (11) is distinct from TNFR2+ Tregs in distribution. Furthermore, CD103 (integrin αEβ7) serves as a homing receptor for mucosa-seeking populations and has no clear relationship to the suppressive function of Tregs (5). In addition, while CD103 is only expressed by mouse Tregs, TNFR2 is also expressed by human Tregs (4) and correlates with their greater suppressive effects (our unpublished data). CCR6 is expressed by both memory type Tregs as well as T effector cells and serves to direct trafficking of CCR6+ cells to inflammatory sites. Although CCR6+ Tregs were described as effector/memory-like cells, CCR6+ Tregs and CCR6− Tregs do not show any differences in suppressive activity (11). Unlike CD103 and CCR6, TNFR2 is a functional receptor for TNF and therefore is likely to respond to TNF by activating and expanding Tregs at inflammatory sites (3). Nevertheless, TNFR2 knockout mice have a normal functional CD4+CD25+ Tregs. Thus TNFR2 is not necessary to maintain Treg activity, but it may play a crucial role in the activation and expansion of Tregs at inflammatory or tumor sites. Presumably this function of TNFR2 reflects down-regulatory effect of TNF, but not IL-1 and IL-6, on the inflammatory response (3).
Foxp3 is reported as both a specific marker and commitment factor for the Treg cell lineage (6). Both TNFR2+ and TNFR2− CD4+CD25+ T cells from normal C57BL/6 mice expressed comparable high level of FoxP3. Nevertheless, the suppressive function of FoxP3+TNFR2− cells is not appropriately “turned on” or is intrinsically deficient. Furthermore, CD4+CD25−TNFR2+ cells were more suppressive than CD4+CD25+TNFR2− cells, even though the former contained much less FoxP3+ cells than the latter. Thus, FoxP3 expression by resting mouse CD4 cells may not be sufficient to confer suppressive capacity. Our data suggest that expression of TNFR2 is more relevant to suppressive phenotype than expression of CD25 and even FoxP3.
Apparently, TGFβ and IL-10 did not contribute to the in vitro suppressive activity of TNFR2+ Tregs, because neutralizing antibodies against TGFβ and IL-10 failed to attenuate activity of TNFR2+ Treg (data not shown). Soluble TNFR2 potentially shed by TNFR2+ Tregs is also unlikely to influence Treg activity, since the level of soluble TNFR2 in the supernatant of co-cultures containing TNFR2+ Tregs was not increased in our in vitro Treg function assay (48 h, data not shown). CTLA4 expression is only detected in the Treg compartment of CD4 cells in normal mice and its expression is implicated in the suppressive function of Tregs (6). This suppressive molecule was expressed by >80% of TNFR2+ Tregs, which is in agreement with the potent suppressive capacity of this subset of Tregs. Intriguingly, the superior suppressive activity of CD4+CD25−TNFR2+ cells over CD4+CD25+TNFR2− cells correlate directly with the intensity of CTLA4 expression by these two subsets (Fig 1D). TNFR2 just like CTLA4 is selectively expressed by functional Tregs rather than Teffs, even in the absence of CD25 and FoxP3.
Taking together, our data demonstrate that TNFR2 expression defines a unique subset of mouse Tregs with an activated/memory phenotype and highly potent suppressive activity. In mouse tumor models, the proportion of tumor infiltrating suppressive TNFR2+ Tregs was dramatically increased, suggesting therapeutic value by targeting TNFR2+ Tregs in tumor immunotherapy.
Abbreviations used in this paper
- LLC
Lewis lung carcinoma
- Teff
CD4+CD25-T effector cells
- TILs
tumor infiltrating lymphocytes
- Tregs
CD4+CD25+ T regulatory cells
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. This Research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Additional funding provided by a DFG frant Ma760/16-1.
References
- 1.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 2.Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 2003;4:565–573. doi: 10.1016/s1470-2045(03)01196-3. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Baumel M, Mannel DN, Howard OM, Oppenheim JJ. Interaction of TNF with TNF Receptor Type 2 Promotes Expansion and Function of Mouse CD4+CD25+ T Regulatory Cells. J Immunol. 2007;179:154–161. doi: 10.4049/jimmunol.179.1.154. [DOI] [PubMed] [Google Scholar]
- 4.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehmann J, Huehn J, de la Rosa M, Maszyna F, Kretschmer U, Krenn V, Brunner M, Scheffold A, Hamann A. Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25- regulatory T cells. Proc Natl Acad Sci U S A. 2002;99:13031–13036. doi: 10.1073/pnas.192162899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 7.Trentin L, Zambello R, Bulian P, Cerutti A, Enthammer C, Cassatella M, Nitti D, Lise M, Agostini C, Semenzato G. Tumour-infiltrating lymphocytes bear the 75 kDa tumour necrosis factor receptor. Br J Cancer. 1995;71:240–245. doi: 10.1038/bjc.1995.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray JD, Liu T, Huynh N, Horwitz DA. Transforming growth factor beta enhances the expression of CD154 (CD40L) and production of tumor necrosis factor alpha by human T lymphocytes. Immunol Lett. 2001;78:83–88. doi: 10.1016/s0165-2478(01)00233-4. [DOI] [PubMed] [Google Scholar]
- 9.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suvannavejh GC, Lee HO, Padilla J, Dal Canto MC, Barrett TA, Miller SD. Divergent roles for p55 and p75 tumor necrosis factor receptors in the pathogenesis of MOG(35−55)-induced experimental autoimmune encephalomyelitis. Cell Immunol. 2000;205:24–33. doi: 10.1006/cimm.2000.1706. [DOI] [PubMed] [Google Scholar]
- 11.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877–2886. doi: 10.1182/blood-2004-07-2505. [DOI] [PubMed] [Google Scholar]