Abstract
The mortality and incidence of chronic obstructive pulmonary disease (COPD) and coronary heart disease increase with age. Despite the clear evidence of beta blockers (BBs) effectiveness, there is a general reluctance to use them in patients with COPD due to a perceived contraindication and fear of inducing adverse reactions and bronchspasm. BBs are well tolerated in patients with cardiac disease and concomitant COPD with no evidence of worsening of respiratory symptoms or FEV1, and the safety of BBs in patients with COPD has been demonstrated, but their use in this group of patients remains low. The cumulative evidence from trials and meta-analysis indicates that cardioselective BBs should not be withheld in patients with reactive airway disease or COPD.
Patients with COPD have a high incidence of cardiac events necessitating careful consideration of prophylactic treatment. The benefits of beta blockade in this group appear to outweigh any potential risk of side effects according to the available evidence.
In this article, we will discuss the use of BBs in patients with COPD and review the evidence for their use and safety in this group of patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death and is estimated to rise to be the third most common cause of death worldwide by 2020 (Rielly et al 2005). Many patients with COPD have concomitant conditions, mostly coronary artery disease (CAD), that require the use of beta-blockers (BBs). However, despite the clear evidence of BBs effectiveness, there is a general reluctance to use them in patients with COPD due to a perceived contraindication and fear of inducing adverse reactions and bronchspasm (Kennedy and Rosenson 1995; Viskin and Barron 1996; Gottlieb et al 1998; Chafin et al 1999). It is a common practice of physicians to consider COPD as contraindication to the use of BBs, based mainly on anecdotal evidence and case reports citing acute bronchospasm following the administration of BBs (Tattersfield 1986, 1990; Belli and Topol 1995; Craig et al 1996; Kendall 1997).
The most common comorbid conditions associated with withholding BBs in elderly patients after myocardial infarction (MI) are COPD and asthma (Heller et al 2000), while peripheral arterial and bronchial problems are reported to be the leading side effects (Frishman et al 1979; Frishman 1998). On the other hand, many patients are diagnosed and treated for COPD with no objective evidence, such as pulmonary function tests or specialist assessment, to confirm the diagnosis, as recommended by most thoracic societies. This may indicate that a significant number of patient are deprived the prognostic benefits of using BBs (Chen et al 2001).
BBs are well tolerated in patients with cardiac disease and concomitant COPD with no evidence of worsening of respiratory symptoms or FEV1 (Formgren 1976; George et al 1983; Quan et al 1983; Krauss et al 1984; Falliers et al 1985; Dorrow, Bethge et al 1986; Mooss et al 1994) and the safety of BBs in patients with COPD has been demonstrated, but their use in this group of patients remains low (Salpeter et al 2001, 2002a, 2002b, 2003). The cumulative evidence from trials and meta-analysis indicates that cardioselective BBs should not be withheld in patients with reactive airway disease or COPD (Salpeter et al 2001, 2002a, 2002b, 2003). Patients with COPD have a high incidence of cardiac events necessitating careful consideration of prophylactic treatment. The benefits of beta blockade in this group appear to outweigh any potential risk of side effects according to the available evidence. For instance, BBs are well tolerated by the large majority of patients with heart failure, even in those with comorbid conditions such as diabetes mellitus, COPD, and peripheral vascular disease (HFSA 2006).
In this article, we will discuss the use of BBs in patients with COPD and review the evidence for their use and safety in this group of patients.
Beta-blockers
Beta-blockers were originally designed by the Nobel Prize winner Sir James Black to counteract the adverse effects of adrenergic stimulation. He demonstrated that, by blocking the cardiac ß-receptors, these agents could cause inhibitory effects on the sinus node (chronotropic effect), atrioventricular node (dromotropic effect), and on myocardial contractility (inotropic effect).
There are 3 types of β receptors. β1-Adrenoceptors are situated in the cardiac sarcolemma. If activated, they lead to an increase in the rate and force of myocardial contraction (positive inotropic effect) by opening the calcium channels. On the other hand, β2-Adrenoceptors are found mainly in bronchial and vascular smooth muscles. If activated, they cause broncho- and vaso-dilatation. There are, however, sizable populations of β2-Adrenoceptors in the myocardium, of about 20%–25%, which leads to the cardiac effects of any β2-Adrenoceptors stimulation. There is a relative up-regulation of these receptors to about 50% in heart failure. The role of β3-Adrenoceptors in the heart is not yet fully identified and accepted (Kalinowski et al 2003).
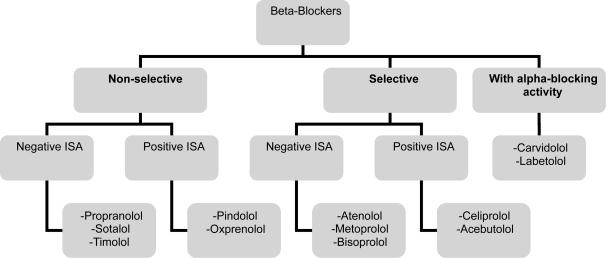
Beta-blockers are classified into three generations (Figure 1). The first generation agents (such as Propranolol, Sotalol, Timolol, and Nadolol), are nonselective and block β1 and β2 receptors. Blocking β1-receptors affects the heart rate, conduction and contractility, while blocking β2-receptors, tends to cause smooth muscle contraction, therefore, bronchospasm in predisposed individuals. The second-generation agents or the cardioselective agents (such as Atenolol, Bisoprolol, Celiprolol, and Metoprolol) block β1-receptors in low doses but are capable of blocking β2-receptors in higher doses. This selective mode of action makes the use of these agents more suitable in patients with chronic lung disease (Wellstien et al 1987) or those with insulin-requiring diabetes mellitus. Cardioselectivity varies between agents with the Bisoprolol among the most selective. The third generation agents have vasodilatory properties. There action is either selective (Nebivolol) or nonselective (Carvidolol and Labetolol). The vasodilatory properties are mediated either by nitric oxide release as for Nebivolol or Carvidolol (Kalinowski et al 2003) or by added alpha-adrenergic blockade as in Labetolol and Carvidolol. A third vasodilatory mechanism, as in Pindolol and Acebutolol, acts via β2-intrinsic sympathomimetic activity (ISA). These beta-blockers therefore have the capacity to stimulate as well as to block adrenergic receptors and tend to cause less bradycardia than the other beta-blockers and may cause less coldness of the extremities.
Figure 1.
Beta blockers classification.
Abbreviations: ISA, intrinsic sympathomimetic activity.
Beta-blockers are used extensively and have a proven morbidity and mortality benefits in the management of patients with cardiac disease (Frishman 1984; The IPPPSH Collaborative Group 1985; Wadworth et al 1991; Stienbeck et al 1992; Mangano et al 1996; Doughty et al 1997; JNC VI 1997; Lechat et al 1998; Freemantle et al 1999; Heidenreich et al 1999; Poldermans et al 1999). They are a standard therapy for hypertension, angina, unstable angina, post myocardial infarction, tachyarrhythmias, and congestive heart failure (The MERIT-HF Study Group 2000; Packer et al 2001). BBs also reduce mortality in the perioperative period. Despite the evidence, their use remains low in patients with COPD.
Beta-blockers use in COPD
Many patients with COPD have concomitant conditions such as CAD (coexists in up to 27% of COPD patients (Karoli and Rebrov 2005)) that require the use of BBs. BBs are often avoided in these patients (Kennedy and Rosenson 1995; Viskin and Barron 1996; Gottlieb et al 1998; Chafin et al 1999) because of fear of bronchospasm and possible adverse reactions, despite the known cardiovascular mortality and morbidity benefit (Frishman et al 1984; The IPPPSH Collaborative Group 1985; Wadworth et al 1991; Stienbeck et al 1992; Mangono et al 1996; Doughty et al 1997, JNC VI 1997; Lechat et al 1998; Freemantle et al 1999; Heidenreich et al 1999; Polderman et al 1999). This is mainly based on anecdotal evidence and case reports citing acute bronchospasm following the administration of beta-blocker (Tattersfield 1986, 1990; O’Malley et al 1991; Belli and Topol 1995; Craig et al 1996; Kendall 1997).
COPD patients are at greater risk of ischaemic heart disease than asthmatics, so would benefit from the use of BBs. On the other hand, they also have more severe airway obstruction, so may be more sensitive to small changes in FEV1 due to beta-blockade.
The trial evidence
The use and effectiveness of BBs therapy after MI for elderly patients with COPD or asthma was evaluated (Chen et al 2001). The study was rationalised by the fact that patients with COPD and asthma have largely been excluded from clinical trials of BBs therapy for AMI. The authors used the data from the Cooperative Cardiovascular Project (Marciniak et al 1998) to examine the relationship between discharge use of BBs and one-year mortality in patients with COPD or asthma who were divided into three groups: those who are not using beta-agonists, those who are using beta-agonists, and those with severe disease (on prednisone or previous hospitalization) in comparison with patients free from COPD or asthma. Of 54,962 patients without contraindications to beta-blockers, patients with COPD or asthma (20%) were significantly less likely to be prescribed beta-blockers at discharge after AMI. Patients with COPD or asthma who were not on beta-agonist had lower one-year mortality if they were on BBs. This mortality benefit was not found among patients using beta-agonists or those with severe COPD or asthma.
Large meta-analyses were published by Salpeter et al (2001, 2002a, 2002b) where randomized, blinded, placebo-controlled trials that studied the effects of cardioselective BBs on FEV1, symptoms, and the use of inhaled β2-agonists in patients with reactive airway disease were selected, of which, there were 19 single dose treatment studies and 10 continued treatment studies. The outcomes measures were the change in FEV1 from baseline, the number of patients with respiratory symptoms, and the use of inhaled β2-agonists with active treatment compared with placebo. The results were that no significant treatment effect in terms of FEV1 was found in patients with concomitant COPD, whether single doses (change in FEV1, −5.28% [CI, −10.03% to −0.54%]) or continued treatment (change in FEV1, 1.07% [CI, −3.3% to 5.44%]) was used. The conclusion was that cardioselective BBs do not produce clinically significant adverse respiratory effects in patients with mild to moderate reactive airway disease, and that they should not be withheld from these patients. The studies were not designed to make recommendations about people with significant chronic airway obstruction.
A similar review on a similar cohort of patients 11 single dose treatment studies (Schanning and Vilsvik 1976; Beil and Ulmer 1977; McGavin and Williams 1978; Perks et al 1978; Sinclair 1979; Anderson et al 1980; Sorbini et al 1982; Von Wichert 1982; Adam et al 1984; Dorow, Bethge et al 1986; Macquin-Mavier et al 1988) and 8 continued treatment studies (Tivenius 1976; Wunderlich et al 1980; Ranchod et al 1982; Buttand et al 1983; Fenster et al 1983; Lammers et al 1985; Dorow, Clauzel et al 1986; Fogari et al 1990) was also published by the previous group (Salpeter et al 2003). This demonstrated that cardioselective BBs produced no significant change in FEV1 or respiratory symptoms compared to placebo and did not significantly affect the FEV1 treatment response to β2-agonists. Subgroup analysis revealed no significant change in results for those participants with severe COPD or for those with a reversible obstructive component. The conclusion was again that cardioselective BBs given to COPD patients do not produce a significant reduction in airway function or an increase in the incidence of COPD exacerbations.
A retrospective study (Egred et al 2005) assessed the use of BBs in patients with COPD admitted with acute coronary syndrome (ACS). Only 54% were discharged on BBs. A diagnosis of “COPD” was the most common cause for withholding BBs and only 62% of patients with COPD have been reviewed by a chest physician or had a previous pulmonary function test. Of these COPD patients, only 16% were prescribed BBs and many patients with a diagnosis of COPD have no objective evidence to support this diagnosis. The conclusion was that, these patients are being denied the prognostic benefits of BBs when presenting with ACS, and the recommendation was that before withholding BBs, COPD and reversibility should be ascertained by pulmonary function testing and that the overall use of BBs was sub-optimal in this setting.
The use of BBs in congestive heart failure patients with COPD and/or asthma was also assessed in a retrospective analysis (Peters et al 2004). One thousand sixty seven patients with CHF were included and reviewed over 18 months period. Medications, non-routine office visits, emergency room visits, and hospitalizations for respiratory events were assessed. Of the 1067 patients, 19.6% had obstructive pulmonary disease (OLD): 5.9% asthma, 11.2% COPD and 2.5% asthma/COPD. Only 35.9% of the patients with OLD were on BBs half of which were cardioselective. The use of BBs did not result in any increased respiratory events, respiratory encounters, emergency room visits, or hospitalizations. The result showed that the long-term use of BBs did not increase the risk of respiratory complications and there was no difference in outcomes with the use of cardioselective or noncardioselective BBs and the conclusion was that cardio-selective BBs without intrinsic sympathomimetic activity are preferred until future studies resolve this issue.
Most of the evidence presented is meta-analysis; therefore, it has many limitations like any other meta-analysis (Ionnidis and Lau 1999). It only reports on published literature and is therefore subject to publication bias. A few studies did not have placebo controls, and many did not provide standard deviations for FEV1 treatment effects. In addition, most of the studies were of short duration. It is possible that a longer study period may be required in order to detect clinically important side effects of BBs.
In the recent publication of the Heart Failure Society of America (HFSA) on heart failure practice guidelines (HFSA 2006), BBs therapy is recommended in the great majority of patients with LV systolic dysfunction, even in the presence of concomitant COPD. They recommend that BBs are initiated at a low dose and uptitrated gradually in two weeks intervals. The guidelines also recommend that patients with difficulties in initiating, uptitrating, or maintaining BBs therapy to be referred to a physician with expertise in the management of these patients. This is to ensure that no patient is denied the benefits of BBs (HFSA 2006).
In this article, we presented a review of the data supporting the use of cardioselective BBs in patients with mild to moderate COPD. The evidence suggests that cardio-selective BBs are not only safe but also beneficial in patients with co-existing airways and coronary disease and can significantly improve prognosis. Due to the proven mortality benefit of BBs in numerous cardiac conditions, many of the other relative or absolute contraindications traditionally listed for BBs have been questioned, including impaired left ventricular function, peripheral vascular disease, and diabetes mellitus (Kjekshus et al 1990; Wichkmayr et al 1990; Radack and Deck 1991; Rosenson 1993; Jonas et al 1996; Gottlieb et al 1998; Lechat et al 1998).
Finally, although we believe physicians should feel more comfortable prescribing cardioselective BBs to COPD patients, we can not make a generalised recommendation that BBs can be used in all COPD patients. This is due to the fact that almost all of the evidence is from retrospective analysis of data available and more prospective studies are needed. Physicians should use their clinical judgment and use BBs where they think the benefits outweigh the risks.
We suggest that patients who are admitted with any cardiac condition requiring BBs and who have concomitant COPD should be tried on BBs. A safe approach is to initiate cardio-selective BBs at a low dose and titrate them up as tolerated during the hospital admission. Metoprolol is cardioselective BBs with short half-life and has been shown to be safe and effective in patients with COPD (Camsari et al 2003) and may be the BBs of choice to initiating therapy. This will allow close observation and assessment of tolerance of these medications and will ensure that these patients are not denied the prognostic benefits of a well-tolerated and effective treatment. It may be necessary to discontinue the drug in few patients due to bronchconstriction, but the potential benefit appears large enough to warrant this small risk.
Further randomised studies may be required to assess the use of the new BBs (Nebivolol) with its nitric oxide (NO) releasing property in patient with COPD. This will allow the assessment of weather it is safe and similarly will help define weather NO has any role to play in reducing the effect of BBs on bronchospasm.
A drug called Pimobendan which is a phosphodiesterase (PDE) III-inhibitor, with vasodilatory and bronchodilatory effects, has been used in two patients with heart failure who were unable to tolerate BBs. The use of Pimobendan allowed the start and maintenance of BBs without worsening COPD or heart failure with evidence of progressive decline in brain natriuretic peptide (BNP) (Shiga et al 2002). This may present an interesting way of dealing with BBs intolerant patients, where the bronchdilatory effects of Pimobendan would allow the initiation of BBs. This is only two case reports and the safety and efficacy of this drug has not been tested in a controlled manner.
Conclusion
BBs reduce mortality in patients with COPD and coexisting CAD and should be used whenever possible. Cardioselective BBs are safe in patients with COPD who have an indication for their use. Nonselective BBs are better avoided in general, except in patients with heart failure who may benefit from the use of Carvedilol. A short acting cardioselective BBs (such as metoprolol) should be started at a low dose and uptitrated slowly. Once Metoprolol is established, it can be changed to a once daily longer acting product such as Bisoprolol or Atenolol. In case of uncertainty or difficulty, specialist opinion should be sought to insure that these patients are not denied the prognostic benefit of BBs therapy.
References
- Adam WR, Meagher EJ, Barter CE. Labetalol, beta blockers, and acute deterioration of chronic airway obstruction. Clin Exp Hypertens A. 1982;4:1419–28. doi: 10.3109/10641968209060799. [DOI] [PubMed] [Google Scholar]
- Anderson G, Jariwalla AG, Al-Zaibak M. A comparison of oral metoprolol and propranolol in patients with chronic bronchitis. J Int Med Res. 1980;8:136–8. doi: 10.1177/030006058000800206. [DOI] [PubMed] [Google Scholar]
- Beil M, Ulmer WT. Effects of a new cardioselective betaadrenergic blocker (atenolol) on airway resistance in chronic obstructive disease. Arzneim-Florsch. 1977;27:419–22. [PubMed] [Google Scholar]
- Belli G, Topol EJ. Adjunctive pharmacologic strategies for acute MI. Contemp Intern Med. 1995;7:51–9. [PubMed] [Google Scholar]
- Butland RJ, Pang JA, Geddes DM. Effect of beta-adrenergic blockade on hyperventilation and exercise tolerance in emphysema. J Appl Physiol. 1983;54:1368–73. doi: 10.1152/jappl.1983.54.5.1368. [DOI] [PubMed] [Google Scholar]
- Camsari A, Arikan S, Candan A, et al. Metoprolol, a β-1 selective blocker, can be used safely in coronary artery disease patients with chronic obstructive pulmonary disease. Heart Vessels. 2003;18:188–92. doi: 10.1007/s00380-003-0706-z. [DOI] [PubMed] [Google Scholar]
- Chafin CC, Soberman JE, Demircan K, et al. Beta-blockers after myocardial infarction: do benefits ever outweigh risks in asthma? Cardiology. 1999;92:99–105. doi: 10.1159/000006955. [DOI] [PubMed] [Google Scholar]
- Chen J, Redford MJ, Wang Y, et al. Effectiveness of beta-blocker therapy after acute myocardial infarction in elderly patient with chronic obstructive airways disease or asthma. J Am Coll Cardiol. 2001;37:1950–6. doi: 10.1016/s0735-1097(01)01225-6. [DOI] [PubMed] [Google Scholar]
- Craig T, Richerson HB, Moeckli J. Problem drugs for the patient with asthma. Compr Ther. 1996;22:339–44. [PubMed] [Google Scholar]
- Dorow P, Bethge H, Tönnesmann U. Effects of single oral doses of bisobrolol and atenolol on airway function in nonasthmatic chronic obstructive lung disease and angina pectoris. Eur J Clin Pharmacol. 1986;31:143–7. doi: 10.1007/BF00606650. [DOI] [PubMed] [Google Scholar]
- Dorow P, Clauzel AM, Capone P, et al. A comparison of celiprolol and chlorthalidone in hypertensive patients with reversible bronchial obstruction. J Cardiovasc Pharmacol. 1986;8(Suppl 4):S102–4. doi: 10.1097/00005344-198608004-00022. [DOI] [PubMed] [Google Scholar]
- Doughty RN, Rodgers A, Sharpe N, et al. Effects of beta-blocker therapy on mortality in patients with heart failure A systematic overview of randomised controlled trials. Eur Heart J. 1997;18:560–5. doi: 10.1093/oxfordjournals.eurheartj.a015297. [DOI] [PubMed] [Google Scholar]
- Egred M, Shaw S, Mohammad B, et al. Under-use of beta-blockers in patients with ischaemic heart disease and concomitant chronic obstructive pulmonary disease. Q J Med. 2005;98:493–7. doi: 10.1093/qjmed/hci080. [DOI] [PubMed] [Google Scholar]
- Falliers CJ, Vrchota J, Blasucci DJ, et al. The effects of treatment with labetolol and hydrochlorothiazide on ventilatory function of asthmatic hypertensive patients with demonstrated bronchosensitivity to probranolol. J Clin Hypertens. 1985;1:70–9. [PubMed] [Google Scholar]
- Fenster PE, Hasan FM, Abraham T, et al. Effect of metoprolol on cardiac and pulmonary function in chronic obstructive pulmonary disease. Clin Cardiol. 1983;6:125–9. doi: 10.1002/clc.4960060305. [DOI] [PubMed] [Google Scholar]
- Fogari R, Zoppi A, Tettamanti F, et al. Comparative effects of celiprolol, propranolol, oxprenolol, and atenolol on respiratory function in hypertensive patients with chronic obstructive lung disease. Cardiovasc Drugs Ther. 1990;4:1145–9. doi: 10.1007/BF01856511. [DOI] [PubMed] [Google Scholar]
- Formgren H. The effect of metoprolol and practolol on lung function and blood pressure in hypertensive asthmatics. Br J Clin Pharmacol. 1976;3:1007–14. doi: 10.1111/j.1365-2125.1976.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freemantle N, Cleland J, Young P, et al. Beta blockade after myocardial infarction: systematic review and meta regression analysis. Br Med J. 1999;318:1730–7. doi: 10.1136/bmj.318.7200.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishman W, Silverman R, Storm J, et al. Clinical pharmacology of the new beta blocking drugs: part-4. Adverse effects: choosing a beta adrenoreceptor blocker. Am Heart J. 1979;98:256–62. doi: 10.1016/0002-8703(79)90229-1. [DOI] [PubMed] [Google Scholar]
- Frishman WH. Beta adrenergic receptor blockers: adverse effects and drug interaction. Hypertension. 1998;11(Suppl II):1121–9. doi: 10.1161/01.hyp.11.3_pt_2.ii21. [DOI] [PubMed] [Google Scholar]
- Frishman WH, Furberg CD, Friedewald WT. Beta-adrenergic blockade for survivors of acute myocardial infarction. N Engl J Med. 1984;310:830–7. doi: 10.1056/NEJM198403293101306. [DOI] [PubMed] [Google Scholar]
- George RB, Manocha K, Burford JG, et al. Effects of labetalol in hypertensive patients with chronic obstructive pulmonary disease. Chest. 1983;83:457–60. doi: 10.1378/chest.83.3.457. [DOI] [PubMed] [Google Scholar]
- Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low risk patients after myocardial infarction. N Engl J Med. 1998;339:489–97. doi: 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]
- Heidenreich PA, McDonald KM, Hastie T, et al. Meta-analysis of trials comparing betablockers, calcium antagonists, and nitrates for stable angina. JAMA. 1999;281:1927–36. doi: 10.1001/jama.281.20.1927. [DOI] [PubMed] [Google Scholar]
- Heller DA, Ahern FM, Kozak M. Changes in rates of beta-blocker use between 1994 and 1997 among elderly survivors pf myocardial infarction. Am Heart J. 2000;140:663–71. doi: 10.1067/mhj.2000.109650. [DOI] [PubMed] [Google Scholar]
- [HFSA] Heart Failure Society of America Executive Summary: HFSA 2006 comprehensive heart failure practice guidelines. J Card Fail. 2006;12:10–38. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ionnidis JP, Lau J. Pooling research results: benefits and limitations of meta-analysis. Jt Comm J Qual Improv. 1999;25:462–9. doi: 10.1016/s1070-3241(16)30460-6. [DOI] [PubMed] [Google Scholar]
- JNC VI The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–46. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- Jonas M, Reicher-Reiss H, Boyko V, et al. Usefulness of betablocker therapy in patients with non-insulin-dependent diabetes mellitus and coronary artery disease. Am J Cardiol. 1996;77:1273–7. doi: 10.1016/s0002-9149(96)00191-9. [DOI] [PubMed] [Google Scholar]
- Kalinowski L, Dobruchi LW, Szczepanska-Konkel M, et al. Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 2003;107:2747–52. doi: 10.1161/01.CIR.0000066912.58385.DE. [DOI] [PubMed] [Google Scholar]
- Karoli NA, Rebrov AP. Chronic obstructive lung disease and coronary heart disease. Klin Med (Mosk) 2005;83:72–6. [PubMed] [Google Scholar]
- Kendall MJ. Clinical relevance of pharmacokinetic differences between beta blockers. Am J Cardiol. 1997;80:15J–19J. doi: 10.1016/s0002-9149(97)00833-3. [DOI] [PubMed] [Google Scholar]
- Kennedy HI, Rosenson RS. Physician use of beta-adrenergic blocking therapy: a changing perspective [editorial] J Am Coll Cardiol. 1995;26:547–52. doi: 10.1016/0735-1097(95)80035-f. [DOI] [PubMed] [Google Scholar]
- Kjekshus J, Gilpin E, Cali G, et al. Diabetic patients and beta-blockers after acute myocardial infarction. Eur Heart J. 1990;11:43–50. doi: 10.1093/oxfordjournals.eurheartj.a059591. [DOI] [PubMed] [Google Scholar]
- Krauss S, Spitz E, Krauss A, et al. Treatment of hypertension in mild asthmatic patients with atenolol. Angiology. 1984;35:773–8. doi: 10.1177/000331978403501204. [DOI] [PubMed] [Google Scholar]
- Lammers JWJ, Folgerin HTM, van Herwaarden CLA. Ventilatory effects of long-term treatment with pindolol and metoprolol in hypertensive patients with chronic obstructive lung disease. J Clin Pharmac. 1985;20:205–10. doi: 10.1111/j.1365-2125.1985.tb05062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechat P, Packer M, Chalon S, et al. Clinical effects of beta-adrenergic blockade in chronic heart failure: a meta-analysis of placebo-controlled, randomised trials. Circulation. 1998;98:1184–91. doi: 10.1161/01.cir.98.12.1184. [DOI] [PubMed] [Google Scholar]
- Macquin-Mavier I, Roudot-Thoraval F, Clerici C, et al. Comparative effects of bisoprolol and acebutolol in smokers with airway obstruction. Br J Clin Pharmacol. 1988;26:279–84. doi: 10.1111/j.1365-2125.1988.tb05278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangano D, Layug EL, Wallace A, et al. Atenolol reduced mortality and cardiovascular events after noncardiac surgery. N Engl J Med. 1996;335:1713–20. doi: 10.1056/NEJM199612053352301. [DOI] [PubMed] [Google Scholar]
- Marciniak TA, Ellerbeck EF, Radford MJ, et al. Improving the quality of care for medicare patients with acute myocardial infarction: results from the Cooperative Cardiovasular Project. JAMA. 1998;279:1351–7. doi: 10.1001/jama.279.17.1351. [DOI] [PubMed] [Google Scholar]
- McGavin CR, Williams IP. The effects of oral propranolol and metoprolol on lung function and exercise performance in chronic airways obstruction. Br J Dis Chest. 1978;72:327–32. [PubMed] [Google Scholar]
- Mooss AN, Hilleman DE, Mohiuddin SM, et al. Safety of esmolol in patients with acute myocardial infarction treated with thrombolytic therapy who had relative contraindications to beta-blocker therapy. Ann Pharmacother. 1994;28:701–3. doi: 10.1177/106002809402800601. [DOI] [PubMed] [Google Scholar]
- O’Malley K, Cox JP, O’Brien E. Choice of drug treatment for elderly hypertensive patients. Am J Med. 1991;90(Suppl 3A):275–335. doi: 10.1016/0002-9343(91)90433-x. [DOI] [PubMed] [Google Scholar]
- Packer M, Coats A, Fowler M, et al. COPERNICUS: Effect of carvedilol on survival in severe chronic heart failure. NEJM. 2001;344:1651–8. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- Perks WH, Chatterjee SS, Croxson RS, et al. Comparison of atenolol and oxprenolol in patients with angina or hypertension and co-existent chronic airways obstruction. Br J Clin Pharmacol. 1978;5:101–6. doi: 10.1111/j.1365-2125.1978.tb01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JI, Gomez-Dinger PL, Freeman GL, et al. Use of beta blockers in congestive heart failure (CHF) patients with COPD and/or asthma. Chest. 2004;126:921S. [Google Scholar]
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341:1789–92. doi: 10.1056/NEJM199912093412402. [DOI] [PubMed] [Google Scholar]
- Quan SF, Fenster PE, Hanson CD, et al. Suppression of atrial ectopy with intravenous metoprolol in chronic obstructive pulmonary disease patients. J Clin Pharmacol. 1983;23:341–7. doi: 10.1002/j.1552-4604.1983.tb02746.x. [DOI] [PubMed] [Google Scholar]
- Radack K, Deck C. Beta-adrenergic blocker therapy does not worsen intermittent claudication in subjects with peripheral arterial disease: a meta-analysis of randomized controlled trials. Arch Intern Med. 1991;151:1769–76. [PubMed] [Google Scholar]
- Ranchod A, Keeton GR, Benatar SR. The effect of betablockers on ventilatory function in chronic bronchitis. S Afr Med J. 1982;61:423–4. [PubMed] [Google Scholar]
- Reilly JJ, Silverman EK, Shapiro SD.2005Chronic obstructive pulmonary diseaseIn Harrison’s principles of internal medicine textbook.1547–9.
- Rosenson RS. The truth about beta-blocker adverse effects F depression, claudication, and lipids. J Ambulatory Monit. 1993;6:163–71. [Google Scholar]
- Salpeter S, Ormiston T, Salpeter E. The Cochrane Library. Oxford: Update Software; 2001. Cardioselective beta-blocker use in patients with reversible airway disease (Cochrane Review) p. 2. [DOI] [PubMed] [Google Scholar]
- Salpeter S, Ormiston T, Salpeter E. The Cochrane Library. Oxford: Update Software; 2002a. Cardioselective beta-blocker use in patients with COPD (Cochrane Review) p. 2. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Ormiston TM, Salpeter EE. Cardioselective β-blockers in patients with reactive airway disease: a meta analysis. Ann Intern Med. 2002b;137:715–25. doi: 10.7326/0003-4819-137-9-200211050-00035. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Ormiston TM, Salpeter EE, et al. Cardioselective β-blockers for chronic obstructive pulmonary disease: a meta analysis. Resp Med. 2003;97:1094–101. doi: 10.1016/s0954-6111(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Schanning J, Vilsvik JS. Beta1-blocker (Practolol) and exercise in patients with chronic obstructive lung disease. Acta Med Scand. 1976;199:61–4. doi: 10.1111/j.0954-6820.1976.tb06691.x. [DOI] [PubMed] [Google Scholar]
- Shiga T, Wakaumi M, Yajima T, et al. Beta-blocker therapy combined with low dose pimobendan in patients with idiopathic dilated cardiomyopathy and chronic obstructive pulmonary disease: report on two cases. Cardiovasc Drugs Ther. 2002;16(3):259–63. doi: 10.1023/a:1020608724335. [DOI] [PubMed] [Google Scholar]
- Sinclair DJ. Comparison of effects of propranolol and metoprolol on airways obstruction in chronic bronchitis. Br Med J. 1979;1:168. doi: 10.1136/bmj.1.6157.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbini CA, Grassi V, Tantucci C, et al. Acute effects of oral metoptolol on ventilatory function in patients with chronic obstructive lung disease. Acta Therap. 1982;8:5–16. [Google Scholar]
- Stienbeck G, Anderson D, Bach P, et al. A comparison of electrophysiologically guided antiarrhythmic drug therapy with beta-blocker therapy in patients with symptomatic, sustained ventricular tachyarrhythmias. N Engl J Med. 1992;327:987–92. doi: 10.1056/NEJM199210013271404. [DOI] [PubMed] [Google Scholar]
- Tattersfield AE. Beta adrenergic antagonists and respiratory disease. J Cardiovasc Pharmacol. 1986;8(Suppl 4):S35–9. doi: 10.1097/00005344-198608004-00007. [DOI] [PubMed] [Google Scholar]
- Tattersfield AE. Respiratory function in the elderly and the effects of beta blockade. Cardiovasc Drugs Ther. 1990;4:1229–32. doi: 10.1007/BF00114225. [DOI] [PubMed] [Google Scholar]
- The IPPPSH Collaborative Group Cardiovascular risk and risk factors in a randomised trial of treatment based on the beta-blocker oxprenolol: the international prospective primary prevention study in hypertension (IPPPSH) J Hypertension. 1985;3:379–92. doi: 10.1097/00004872-198508000-00011. [DOI] [PubMed] [Google Scholar]
- The MERIT-HF Study Group Effects of controlled-release metoprolol on total mortality, hospitalization, and well-being in patients with heart failure. JAMA. 2000;283:1295–302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- Tivenius L. Effects of multiple doses of metoprolol and propranolol on ventilatory function in patients with chronic obstructive lung disease. Scand J Respir Dis. 1976;57(4):190–6. [PubMed] [Google Scholar]
- Viskin S, Barron HV. Beta blockers prevent cardiac death following a myocardial infarction: so why so many infarct survivors discharged without beta blockers? [Editorial] Am J Cardiol. 1996;78:821–2. doi: 10.1016/s0002-9149(96)00428-6. [DOI] [PubMed] [Google Scholar]
- Von Wichert P. Reversibility of bronchospasm in airway obstruction. Am Heart J. 1982;104:446–50. doi: 10.1016/0002-8703(82)90138-7. [DOI] [PubMed] [Google Scholar]
- Wadworth AN, Murdoch D, Brogden RN. Atenolol. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular disorders. Drugs. 1991;42:468–510. doi: 10.2165/00003495-199142030-00007. [DOI] [PubMed] [Google Scholar]
- Wellstein A, Palm D, Belz G, et al. Reduction of exercise tachycardia in man after propranolol, atenolol, bisoprolol in comarison to beta-adrenoceptor occupancy. Eur Heart J. 1987;8(Suppl M):3–8. doi: 10.1093/eurheartj/8.suppl_m.3. [DOI] [PubMed] [Google Scholar]
- Wicklmayr M, Rett K, Dietze G, et al. Effects of beta-blocking agents on insulin secretion and glucose disposal. Horm Metab Res. 1990;22:29–33. [PubMed] [Google Scholar]
- Wunderlich J, Macha HN, Wudicke H, et al. Beta-adrenoceptor blockers and terbutaline in patients with chronic obstructive lung disease. Effects and interaction after oral administration. Chest. 1980;78:714–20. doi: 10.1378/chest.78.5.714. [DOI] [PubMed] [Google Scholar]