Abstract
Recently, angiogenesis and pulmonary vascular remodeling in COPD has been investigated. It has been hypothesized that endothelial dysfunction might be an initiating event that promotes vessel remodeling in COPD.
Inflammatory tissue- a pivotal pathological feature of COPD- often hypoxic, can induce angiogenesis through upregulation of factors such as VEGF or FGF and regulators of angiogenesis such as chemokines (CXC family), acting either as angiogenic or angiostatic. Angiopoietins are distinct molecules that act in association with VEGF at different stages of angiogenic process. The regulation of angiogenesis is determined by a dual, yet opposing balance of angiogenic and angiostatic factors that promote or inhibit neovascularization, respectively, not yet elucidated in detail in COPD.
Recent studies suggested an increased expression of VEGF in pulmonary muscular arteries of patients with moderate COPD and also in smokers with normal lung function. This was also associated with enlargement of the arterial wall. However, in patients with severe emphysema, the expression of VEGF tended to be low, despite intense vascular remodelling. Furthermore, it has been suggested that VEGF might be involved in the pathogenesis of emphysema through apoptotic mechanisms. Experimental studies showed that the lung microvascular endothelial cells (including the alveolar septal capillary cells) are particularly vulnerable and dependent on VEGF for their survival. Apoptosis of endothelial, leading to the loss of capillaries may well be a central mechanism in patients with emphysema and muscle wasting.
This review article summarizes the current knowledge regarding the contribution of vascular remodeling, as well as the pathogenetic and therapeutic implications of pivotal angiogenic mediators, in COPD.
Keywords: angiogenic, angiostatic, growth factors, CXC chemokines, CC chemokines, COPD
Definitions – implication of angiogenesis in the pathogenesis of lung diseases
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease state characterised by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases, primarily caused by cigarette smoking. Although COPD affects the lungs, it also produces significant systemic consequences (Celli et al 2004; Pauwels and Rabe 2004).
The disease ranks among the top five leading causes of death and it is estimated that it will be in the mortality top three by 2020. The main risk factor is tobacco smoking which is associated with 80% of COPD cases. However, only 15%−20% of the smokers develop COPD, pointing to other susceptibility factors (Siafakas and Tzortzaki 2002). The cause of response variability of the lung to tobacco exposure remains largely unclear. Research in the past two decades exhibited pathological features of COPD patients comprising lung tissue remodeling-like changes in mucosal tissue, fiber types and/or fibrosis, pulmonary and systemic inflammation, lung vascular remodeling, and angiogenesis (Jeffery 2001a, 2004).
Angiogenesis is the growth of new blood vessels from existing ones, whereas microvascular remodeling involves structural alterations-usually enlargement-of arterioles, capillaries or venules, without the formation of new vessels (McDonald 2001; Walsh and Pearson 2001). Both types of change in the microvasculature result from endothelial cell proliferation and often occur together, but they represent different phenomena and responses to different stimuli. In addition, they are instrumental phenomena under physiologic and pathologic conditions. Physiologic conditions include embryogenesis, growth, and tissue repair after injury and the female reproductive cycle.
The literature on angiogenesis and vascular remodeling in human airway disease is relatively sparse, while the mechanisms and consequences of the changes are just beginning to be elucidated. In a model of chronic airway inflammation produced by Mycoplasma pulmonis infection on the airways of mice or rats (McDonald 1999; McDonald 2001), angiogenesis and microvascular remodeling create vessels that mediate leukocyte influx and leak plasma proteins into the airway mucosa. These vascular changes are driven by the immune response to the organisms.
Interestingly, angiogenesis and microvascular remodeling are elements of the tissue remodeling in tumors (Strieter et al 2004). A role for angiogenesis in enhancing tumor growth is now widely accepted, and a variety of anti-angiogenic agents are in clinical development. Furthermore, angiogenesis may contribute to the pathogenesis of interstitial lung diseases (Tzouvelekis et al 2006). Recent theories implicate recurrent injurious exposure, imbalance that shifts Th1/Th2 equilibrium towards Th2 immunity and angiogenesis in the pathogenesis of pulmonary fibrosis, both in human and experimental studies (Antoniou et al 2006; Tzouvelekis et al 2006).
Angiogenesis is an important event both in the development of allergic inflammation and in the pathophysiology of tissue remodeling in atopic diseases (Li and Wilson 1997; Redington et al 2001; Hoshino et al 2001a, 2001b, Asai et al 2002; Lee et al 2004). Previous studies suggested an increased number of bronchial vessels in asthma where increased collagen IV staining, a marker of new vessels, was seen in bronchial biopsies of asthmatic airways compared with controls (Orsida et al 1999; Orsida et al 2001). Subsequent studies by the same group and by Salvato and coworkers have confirmed the presence of angiogenesis in the bronchial circulation in asthma (Salvato 2001). Recent research showed that an imbalance in favor of proangiogenic factors leads to the abnormal growth of new blood vessels in asthma. This may then contribute to swelling and stiffening of the airway wall and hence affect airway obstruction.
While in asthma angiogenesis is well documented, only a few studies have directly shown that angiogenesis and vascular remodeling occur in COPD. The structures most affected, in COPD, are the airways (thickening, fibrosis, loss of alveolar-bronchiolar attachments, and stenosis) and the alveolar component [lung parenchyma (in emphysema)]. Table 1 summarizes human studies investigating angiogenesis and vascular remodeling in COPD.
Table 1.
Recent human studies investigating angiogenesis and vascular remodeling in COPD
| Investigator (year) | Tissue samples Sample size | Studied parameters |
|---|---|---|
| Kranenburg et al (2005) | Central and peripheral lung tissues 14 patients-14 controls | VEGF |
| FLT-1 | ||
| KDR/Flk-1 | ||
| Hashimoto et al (2005) | Lung specimens 11 patients-8 controls | VEGF |
| bFGF | ||
| Calabrese et al (2006) | Bronchial specimens 18 patients-8 controls | VEGF |
| Integrin αvβ3 | ||
| Santos et al (2003) | Lung specimens-pulmonary muscular arteries 19 nonsmokers-21 smokers-28 moderateCOPD-severe emphysema | VEGF |
| Peinado et al (2006) | Pulmonary arteries from lung specimens 9 COPD patients-6 controls | VEGF |
| VEGFR2 |
Angiogenesis in COPD
Airway component
Kranenburg and colleagues showed that COPD is associated with increased expression of vascular endothelial growth factor (VEGF) in the bronchial, bronchiolar, and alveolar epithelium and in bronchiolar macrophages, as well as, airway smooth muscle and vascular smooth muscle cells in both the bronchiolar and alveolar regions (Kranenburg et al 2005a). The authors postulated that VEGF and its receptor system may contribute to the maintenance of endothelial and epithelial cell viability in response to injury. On the contrary, a previous report, only in emphysematous patients, showed that VEGF and its receptor VEGF-R2 were decreased in total lung extracts of emphysematous lungs (Kasahara et al 2000). In addition, the involvement of bronchial vasculature in the airway remodeling occurring in symptomatic patients smokers with normal lung function and with COPD has been recently investigated (Calabrese et al 2006). In order to evaluate whether an angiogenic process occurs in the airways of symptomatic smokers with and without COPD, the authors have also investigated, by immunohistochemistry, the number of integrin αvβ3 positive vessels. Recent knowledge suggests that VEGF and integrin αvβ3 may collaborate to vessel formation (Byzova et al 2000, Liu et al 2003). This study showed an increase in bronchial vascularity, expressed in terms of both number of vessels and vascular area, compared to healthy non-smokers in the central airways of symptomatic smokers with normal lung function and with moderate COPD (Calabrese et al 2006). The increased αvβ3 expression detected on bronchial vessels associated to the higher VEGF expression suggests that these two molecules could cooperate in the angiogenetic process occurring in the airways of symptomatic smokers with normal lung function and with COPD. Furthemore, Hashimoto and colleagues compared small and large airways in Asthma and COPD, and found that the number of vessels in the medium and small airways in patients with asthma showed a greater increase than in patients with COPD and control subjects, while the vascular area in the small airways was increased in COPD patients versus control subjects (Hashimoto et al 1997).
Alveolar component
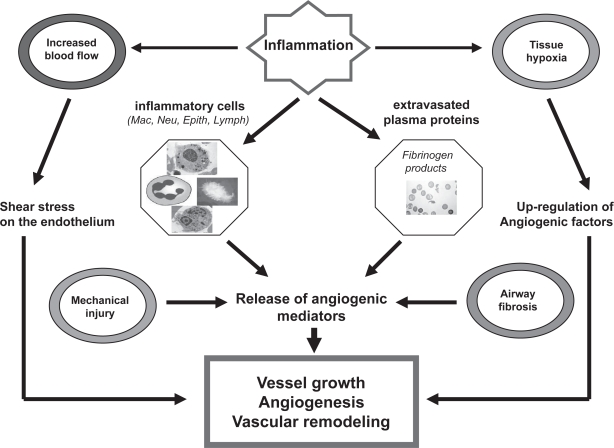
Inflammation- a pivotal pathological feature of COPD- may promote angiogenesis in a number of ways (Walsh and Rearson 2001; Risau 1997) (Figure 1). Firstly, airway inflammation in COPD is characterised by an influx of inflammatory cells –predominantly neutrophils, macrophages and CD8+ T lymphocytes- in the lumen and wall of the bronchial and bronchiolar airways and parenchyma (Jeffery 2001a, 2001b, 2004; Saetta et al 2001). Secondly, several studies have reported a thickened bronchiolar wall and airway remodeling with peribronchiolar fibrosis, an increase in airway smooth muscle mass, and emphysema (Cosio et al 1980; Lang et al 1994; Jeffery 2001b). Additionally, inflammatory tissue is often hypoxic, and hypoxia can induce angiogenesis through upregulation of factors such as VEGF or Fibroblast growth factor (FGF). Extravasated plasma proteins such as fibrinogen products may stimulate neovascularisation (Jeffery 1998, 2001b). Inflammatory cells such as macrophages, lymphocytes, mast cells and fibroblasts, and the angiogenic mediators they produce, can stimulate vessel growth. Many proinflammatory cytokines, such as tumour necrosis factor (TNF)-α, may have angiogenic activity in addition to proinflammatory activity (Strieter et al 2004; Tzouvelekis et al 2006). Increased blood flow itself may stimulate angiogenesis through shear stresses on the endothelium (Figure 1). Inflammation also may upregulate the expression of angiogenic growth factors (VEGF, FGF) and regulators of angiogenesis such as chemokines (CXC family), acting either as angiogenic or angiostatic factors (Tzouvelekis et al 2006). Growth factors like epidermal growth factor (EGF), FGF and VEGF stimulate tissue repair and vascular remodeling seen in COPD. Angiopoietins are distinct molecules that act in association with VEGF at different stages of angiogenic processes in several biological systems (McDonald 1999; Ribatti et al 2000; Yancopoulos et al 2000). The regulation of angiogenesis is determined by a dual, yet opposing balance of angiogenic and angiostatic factors that promote or inhibit neovascularization, respectively, not yet elucidated in detail in COPD.
Figure 1.
Angiogenetic process in COPD. The angiogenetic pathway is stimulated when inflamed, hypoxic, or injured tissues produce and release different angiogenetic promoters. Loss of the pulmonary vascular bed has been suggested to lead to the formation of new vessels comprising lung tissue remodeling-like changes in mucosal tissue, fiber types and/or fibrosis, pulmonary and systemic inflammation, lung vascular remodeling, and angiogenesis.
In addition to studying VEGF expression in bronchial and bronchiolar walls, Kranenburg and coworkers also investigated VEGF staining in the alveolar spaces and pulmonary vessels (Kranenburg et al 2005a). The authors found that the epithelial and endothelial cells in the alveolar spaces and the distal airways were intensively positive for VEGF in patients with COPD suggesting a paradoxical role for this angiogenic factor (Knox et al 2005; Postma and Times 2006). This role seems different between bronchi and air spaces in COPD – a protective one in the alveolus but a detrimental function in the bronchi and bronchioles.
Vascular remodeling in COPD
COPD is associated with structural and functional changes in the pulmonary circulation that commence at an early stage. Pulmonary vascular remodeling leading to pulmonary hypertension and cor pulmonale is a characteristic feature of COPD (Wright et al 1983; Wilkinson et al 1988). Hypoxia has been classically considered the major pathogenic mechanism of these changes (Nilson et al 2004). However, structural abnormalities of pulmonary arteries are not exclusive of advanced COPD, as they have been shown also in patients with mild COPD without arterial hypoxemia and even in smokers with normal lung function (Barbera et al 1994). In agreement with this notion, recent studies suggest that the natural history of pulmonary hypertension in COPD might commence at moderate degrees of disease severity (Kessler et al 2001). In addition, previous observations have indicated that muscular and bronchiolar arteries have increased adventitial infiltration of CD8+ T lymphocytes and have intimal thickening that is correlated with the amount of total collagen deposition (Santos et al 2002). It has been demonstrated that VEGF could play a role in the pathogenesis of the intimal cell proliferation shown in pulmonary arteries of smokers and patients with mild COPD (Peinado et al 1999). This concept has promoted a growing interest in clarifying the role of VEGF at different stages of COPD. With this background in mind, the same group of investigators evaluated the expression of VEGF protein and mRNA in pulmonary arteries and lung tissue of smokers and patients with different degrees of COPD severity (Santos et al 2003). The results of this interesting study showed an increased expression of VEGF in pulmonary muscular arteries of patients with moderate COPD and also in smokers with normal lung function, as compared with nonsmokers, and that this expression is associated with the enlargement of the arterial wall. In contrast, in patients with severe emphysema, the immunohistochemical expression of VEGF in pulmonary arteries and its protein content in lung tissue tends to be low, despite intense vascular remodeling.
In order to further illuminate the pathobiology of pulmonary vascular remodeling in COPD, the same group conducted a recently reported study to investigate whether the presence of vascular progenitor cells is related to endothelial function or the expression of angiogenic factors (Peinado et al 2006). They demonstrated the presence of these cells in the endothelial surface and the intimal space of pulmonary arteries of patients with COPD (Peinado et al 2006). The number of progenitor cells was associated with the response to hypoxic stimulus but also with the enlargement of the arterial wall suggesting that these cells might be involved in the mechanisms of pulmonary vessel repair and remodeling in COPD (Peinado et al 2006).
Furthermore, it has been recently suggested that VEGF might be involved in the pathogenesis of emphysema through apoptotic mechanisms (Kasahara et al 2001). Several experimental strategies in mice (Tang et al 2004a, Petrache et al 2006) and rats showed that chronic cigarette smoking and administration of a VEGF receptor blocker (Kasahara et al 2000; Tuder et al 2003) caused lung cell apoptosis and significant airspace enlargement. It has been suggested that the lung microvascular endothelial cells (including the alveolar septal capillary cells) are particularly vulnerable and dependent on VEGF (Voelkel et al 2006) for their survival. Apoptosis of endothelial, leading to the loss of capillaries may well be a central mechanism in patients with emphysema and muscle wasting. For example, Tang and coworkers (Tang et al 2004b) demonstrated that skeletal muscle-specific conditional knockout of VEGF in mice caused endothelial cell apoptosis and capillary loss at the sites of VEGF knockout. These observations support the concept of capillary loss via endothelial cell apoptosis in animal models of emphysema (Yamato et al 1996; Plataki et al 2006).
Growth factors
Vascular endothelial growth factor (VEGF)
One of the pivotal proteins involved in vascular remodeling is VEGF. The VEGF family (VEGF-A to F) are heparin binding proteins and act via their affinity transmembrane receptors VEGFR1(Flt-1) and VEGFR-2 (KDR-Flk-1) (Cross and Claesson-Welsh 2001). VEGF promotes an array of responses in the endothelium including hyperpermeability, endothelial cell proliferation, and angiogenesis with new vessel tube formation in vivo (Cross and Claesson-Welsh 2001; Voelkel et al 2002). VEGF expression can be induced under a number of pathophysiological conditions including pulmonary hypoxia and pulmonary hypertension (Tuder et al 1995; Voelkel et al 2002). Both hypoxia and pulmonary hypertension are pathological features often seen in patients with advanced COPD (Pauwels and Rabe 2004). VEGF and its receptors are involved in many processes in COPD including bronchial wall remodeling, emphysema, and pulmonary hypertension.
A number of conditions relevant to COPD have been shown to increase VEGF expression and release including cigarette smoke (Ribatti et al 2000; Yancopoulos et al 2000), hypoxia (Ribatti et al 2000; Yancopoulos et al 2000) and cytokines such as TGF-β (Wen et al 2003). However, the significance of VEGF expression pattern in the bronchial vessels in COPD and its role to the pathology of this systemic disease is still not elucidated. An increased bronchial vasculature would increase inflammatory cell trafficking and exudation and transudation of mediators, particularly if vascular permeability is altered. The increased vasculature could also contribute to airway hyper responsiveness by supporting the enlarged airway smooth muscle mass which is a feature of COPD. Previous animal (Siafakas et al 2001) and human studies in COPD patients (Alexopoulou et al 2005) exhibited upregulation of VEGF levels in the diaphragm. This up regulation of VEGF expression in COPD compared with control subjects suggested an enhancement of angiogenesis in the cardinal respiratory muscle. A recent study by Kranenburg et al (2005) showed increased staining of VEGF in bronchial smooth muscle consistent with this hypothesis. The authors found increased VEGF expression and its receptors in 14 ex-smoking patients with COPD in comparison with 14-smoking healthy control subjects (Kranenburg et al 2005). TGF-β staining in the bronchiolar epithelium also correlated with VEGF in the same patients. They postulated that VEGF and its receptor system may contribute to the maintenance of endothelial and epithelial cell viability in response to injury suggesting that TGF-β and VEGF represents a complex molecule contributing to airway remodeling in COPD (Knox et al 2005).
Angiopoietin-1 (Ang-1)
The endothelial cell-specific growth factors, VEGF and Ang-1 have potent and clinically relevant actions on the microvasculature (Yancopoulos et al 2000; McDonald 2001). Ang1 is essential for the development of the vasculature but in a different way than VEGF. Mice lacking Ang1, or its tyrosine kinase receptor Tie–2, die because primitive endothelial cells tubes do not evolve into mature vessels (Alexopoulou et al 2005). The ability of Ang1 to block plasma leakage without producing angiogenesis may be therapeutically advantageous. This antileakage action of Ang1 offers a possible new strategy for reducing airway edema in COPD and asthma. Furthermore, because VEGF and Ang1 have additive effects in promoting angiogenesis but opposing effects on vascular permeability, they could be used together to avoid the formation of leaky vessels in therapeutic angiogenesis. The role of this novel mediator in the pathogenetic pathway of COPD remains to be further elucidated.
Fibroblast growth factors (FGFs)
In view of their important role in chronic inflammation, fibrosis, and repair of various tissues, including the lung, FGFs (Sato et al 1995), may well play a pivotal role in airway and vascular walls remodeling (Holgate 1997; Szebenyi and Fallon 1999). FGFs exert their biologic effects via binding to four high-affinity FGF transmembrane tyrosine-kinase receptors (FGFR) (Werner 1998). Distinct FGF subtypes bind with different affinity to the various FGFR. In the lung as well as in the vascular system, FGFs have been implicated in several pathologic conditions. FGF-1 and FGFR-1 were shown to be upregulated during the development of lung fibrosis (Barrios et al 1997). Moreover, vascular remodeling in response to increased blood pressure is associated with elevated levels of basic FGF (Singh et al 1998; Bryant et al 1999).
Recent investigations (Kranenburg et al 2002; Shute et al 2004; Kranenburg et al 2005b; Guddo et al 2006) support the notion that in patients with COPD, increased vascular expression of FGF-1, FGF-2, and FGFR-1 could participate in an autocrine and/or a complex growth factor–cytokine interactive manner in regulating the process of pulmonary vascular remodeling. These data further support the hypothesis that COPD is associated with pulmonary vascular remodeling and that the FGF–FGFR system contributes to the pathogenesis and severity of the disease (Kranenburg et al 2002; Shute et al 2004; Kranenburg et al 2005b; Guddo et al 2006).
Epidermal growth factor (EGF)
The epidermal growth factor and its receptor (EGFR) autocrine pathway contribute to a number of processes important to cell proliferation, apoptosis, and angiogenesis. EGF plays a critical role in airway mucus secretion from goblet cells and submucosal glands and appears to mediate the mucus secretory response to several secretagogues, including oxidative stress, cigarette smoke and inflammatory cytokines (de Boer et al 2006).
Chemokines
Selective leukocyte trafficking and recruitment is primarly regulated by a specific family of small proteins called “chemokines”. Over 50 different chemokines are now recognized, and they activate up to 20 different surface receptors (Rossi and Zlotnik 2000) playing a cardinal role in orchestrating inflammatory and immune cells to target organs (Olson and Ley 2002). Airway inflammation in COPD has been reported to consist mainly of CD68+ macrophages, elastase-positive neutrophils and CD8+ T cells. Physiologically, each of these cells is activated by a specific subset of chemokines.
Molecules that originally promote angiogenesis include members of the CXC chemokine family, characteristically heparin binding proteins which on structural level have four highly conserved cysteine amino acid residues, with the first two cysteines separated by one nonconserved amino acid residue. CXC chemokines display unique diverse roles in the regulation of angiogenesis resulting from dissimilarity in structure. Therefore, members that contain in the NH2-terminus a three amino-acid motif (ELR) such as IL-8/CXCL8, epithelial neutrophil activating protein (ENA)-78/CXCL5, growth- related genes (GROs, α,β,γ/CXCL1,2,3), granulocyte chemotactic protein (GCP)-2/CXCL6 and neutrophil activating protein (NAP)-2/CXCL7, originally promote angiogenesis (Strieter et al 2004). There are two candidate CXC chemokine receptors that mediate this effect: CXCR1 and CXCR2 (Strieter et al 2004). In the airway inflammation of COPD, neutrophils are mainly attracted by CXCL8 via CXCR1 and CXCR2. In addition, CXCR1 is involved in neutrophilic degranulation, protease release and oxidative stress. Hence, these chemokines are thought to be involved in the pathogenesis of COPD. There is particular interest in identifying chemokines in COPD, as small molecule chemokine receptor inhibitors are now in development for COPD therapy (Barnes 2002; Panina-Bordignon and D’Ambrosio 2003; Barnes and Stockley 2005; Barnes 2006).
By contrast, other members of the CXC chemokine family that do not contain the angiogenic ELR motif (ELR-) behave as potent inhibitors of angiogenesis. Platelet factor-4 (PF-4)/CXCL4 was the first chemokine described to inhibit aberrant angiogenesis. Furthermore, the angiostatic ELR-members of the CXC chemokine family include the interferon (IFN)-γ inducible protein (IP)-10/CXCL10, monokine induced by IFN-γ (MIG)-CXCR3 and IFN-γ Inducible T-cell-α chemoattractant (ITAC)/CXCL11. All three chemokines activate CXC chemokine receptor CXCR3, although I-TAC has the highest activity (Jeffery 2001a). Table 2 summarizes the studied angiogenic and angiostatic mediators in COPD. The role of cardinal angiogenic / angiostatic chemokines in the pathogenesis of COPD is described below.
Table 2.
List of studied angiogenic and angiostatic mediators in COPD
| Systematic name | Previous name | Chemokine receptor | Activity |
|---|---|---|---|
| CXC Chemokines | |||
| CXCL1 | GRO-α | CXCR2>CXCR1 | angiogenic |
| CXCL5 | ENA-78 | CXCR2 | angiogenic |
| CXCL8 | IL-8 | CXCR2, CXCR1 | angiogenic |
| CXCL9 | MIG | CXCR3 | angiostatic |
| CXCL10 | IP-10 | CXCR3 | angiostatic |
| CXCL11 | I-TAC | CXCR3 | angiostatic |
| CC Chemokines | |||
| CCL2 | MCP-1/Eotaxin | CCR2 | angiogenic |
| Growth factors | |||
| VEGF | Flt-1, KDR/Flk-1 | angiogenic | |
| bFGF | FGFR-1 | angiogenic | |
| Angiopoietin-1 | Tie-2 | angiogenic | |
| HGF | angiogenic | ||
| EGF | angiogenic | ||
IL-8/CXCL8
The CXC L8 is a potent, selective chemoattract of neutrophils and is present in high concentrations in induced sputum of patients with COPD (Keatings et al 1996; Yamamoto et al 1997). Furthermore, there is a good correlation between the levels of IL-8 and the degree of neutrophilia in sputum. IL-8 levels are also increased in BAL fluid of patients with COPD (Pesci et al 1998). The concentrations of IL-8 are significantly higher in smokers with emphysema than in matched smokers without airflow limitation, whereas the concentrations of other CXC chemokines in BAL do not appear to discriminate between these groups (Tanino et al 2002).
The cellular source of IL-8 in COPD is not completely clear and may be secreted by macrophages, neutrophils, and airway epithelial cells. It signals through two receptors: a low – affinity CXCR1 specific for IL-8 that is involved in neutrophil activation and a high affinity that is activated by two other major angiogenic CXC chemokines, such as GRO-α and ENA-78, which may also be increased in COPD (Traves et al 2002). Therefore, antagonism of CXCR2 may be a more effective strategy, while a monoclonal antibody to IL-8 has been developed and has been tested in COPD without reported success (Yang et al 1999). An inhibitor of CXCR2, such as SB225002, may prove to be particularly beneficial and is now entering in clinical trials (Hay and Sarau 2001; Widdowson 2004). These drugs may also be useful in exacerbations of COPD when CXCR2 are upregulated in the airways (Qiu et al 2003) and in mucus hypersecretion which may be mediated via CXCR2 in experimental virus-induced exacerbations (Miller et al 2003).
Growth-related oncogene-α (GRO-α/CXCL1)
It is another angiogenic chemokine implicated in the pathogenesis of COPD. GRO-α is secreted by alveolar macrophages and airway epithelial cells in response to stimulation with TNF-α and IL-17 (Jones and Chan 2002) and activates neutrophils, monocytes, basophils, and T lymphocytes via CXCR2. The concentrations of GRO-α have been found markedly elevated in induced sputum and BAL of patients with COPD compared with normal smokers and nonsmokers (Traves et al 2002). It is possible that the increased chemotactic response of monocytes to GRO-α is one of the mechanisms leading to increased numbers of alveolar macrophages in the lungs of patients with COPD (Retamales et al 2001) and could be one of the molecular mechanisms of susceptibility to cigarette smoking.
Epithelial cell-derived neutrophil-activating peptide-78 (ENA-78/CXCL5)
It is derived predominantly from epithelial cells and also activates CXCR2 (Imaizumi et al 1997), although monocytes do not appear to show an increased chemotactic response to this chemokine as they do to GRO-α (Traves et al 2004). ENA-78 is increased in BAL fluid of COPD patients compared with normal subjects, but no difference has been found between patients with emphysema and normal smokers (Traves et al 2004). A significant increase in expression of ENA-78 has been reported in epithelial cells during exacerbations of COPD (Qiu et al 2003).
CXC3 chemokines
The CXC3 chemokine family behaves as potent inhibitors of angiogenesis. T cells in peripheral airways of COPD patients showed increased expression of CXCR3. CXCR3 is expressed on T lymphocytes, particularly of the CD8+ subtype. There is increased expression of IP-10 by bronchiolar epithelial cells and airway smooth muscle cells, and this could therefore contribute to the accumulation of the CD8+ cells, which preferentially express CXCR3 (Chrysofakis et al 2004; Tzanakis et al 2004). It is of interest that interferon-γ stimulates dendritic cells to produce IP-10 and Mig, which enhance their ability to attract CD8+ cells (Barnes and Stockley 2005). Alveolar macrophages also have the capacity to produce IP-10 and Mig and thus attract CD8+ T cells (Barnes 2002, Barnes 2006). These data suggest that CXCR3 antagonists might also be useful as a therapeutic approach in COPD.
Monocyte chemoattractant protein or eotaxin (MCP-1/CCL2)
CC-chemokines are also involved in COPD. There is increased expression of monocyte chemotactic protein-1 and its receptor cysteine-cysteine receptor (CCR)2 in macrophages and epithelial cells from COPD patients and this may play a role in recruitment of blood monocytes to the lungs of COPD patients (Barnes 2002; Saetta et al 2002). This suggests that CCR2 antagonists may be of use and small molecule inhibitors are in clinical development (Rose et al 2003; Barnes 2004).
Conclusions
COPD is associated with structural and functional changes in the pulmonary circulation that commence at an early stage, as they have been shown also in patients with mild COPD without arterial hypoxemia and even in smokers with normal lung function. Angiogenesis and microvascular remodeling not only result in more or larger blood vessels in the airway mucosa but also in functionally abnormal.
Various cells (macrophages, lymphocytes, mast cells, fibroblasts, and their mediators) participating in the inflammatory process of COPD can stimulate vessel growth. Pro-inflammatory cytokines, such as tumour necrosis factor (TNF)-α, may also have angiogenic in addition to proinflammatory activity. Increased blood flow in COPD itself may stimulate angiogenesis through shear stresses on the endothelium. Growth factors like epidermal growth factor (EGF), FGF and VEGF stimulate tissue repair and vascular remodeling seen in COPD. However, the regulation of angiogenesis is determined by a dual, yet opposing balance of angiogenic and angiostatic factors that promote or inhibit neovascularization, respectively, not yet elucidated in detail in COPD.
Recently, it has been demonstrated that VEGF could play a role in the pathogenesis of the intimal cell proliferation shown in pulmonary arteries of smokers and patients with mild COPD. This concept has promoted a growing interest in clarifying the role of VEGF at different stages of COPD. An increased expression of VEGF in pulmonary muscular arteries of patients with moderate COPD and also in smokers with normal lung function, has been shown, and its expression was associated with the enlargement of the arterial wall. In contrast, in patients with severe emphysema, VEGF content tended to be low, despite intense vascular remodeling. Furthermore, it has been lately suggested that VEGF might be involved in the pathogenesis of emphysema through apoptotic mechanisms. Apoptosis of endothelial, leading to the loss of capillaries may well be a central mechanism in patients with emphysema and muscle wasting.
In conclusion, the pathobiology of angiogenesis and vascular remodeling in COPD is not fully understood. Because endothelium plays a key role in regulating cell growth in vessel wall, it has been hypothesized that endothelial dysfunction might be an initiating event that promotes vessel remodeling in COPD. Progenitor cells of bone marrow origin migrate to injured vessels, where they may contribute to endothelial maintenance and vessel remodeling through VEGF-related signals. Future studies should be focused in elucidating the role of progenitor cells in vascular changes occurring in patients with COPD. Advances in understanding the pathogenesis of COPD have the potential for identifying new therapeutic targets that could alter the natural history of the disease.
References
- Alexopoulou C, Mitrouska I, Arvanitis D, et al. Vascular-specific growth factor mRNA levels in the human diaphragm. Respiration. 2005;72:636–41. doi: 10.1159/000089580. [DOI] [PubMed] [Google Scholar]
- Antoniou KM, Tzouvelekis A, Alexandrakis MG, et al. Different angiogenic activity in idiopathic pulmonary fibrosis and pulmonary sarcoidosis. Chest. 2006;130:982–8. doi: 10.1378/chest.130.4.982. [DOI] [PubMed] [Google Scholar]
- Asai K, Kanazawa H, Otan K, et al. Imbalance between vascular endothelial growth factor and endostatin levels in induced sputum from asthmatic subjects. J Allergy Clin Immunol. 2002;110:571–5. doi: 10.1067/mai.2002.127797. [DOI] [PubMed] [Google Scholar]
- Barberà JA, Riverola A, Roca J, et al. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;149:423–9. doi: 10.1164/ajrccm.149.2.8306040. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Stockley RA. COPD: current therapeutic interventions and future approaches. Eur Respir J. 2005;25:1084–106. doi: 10.1183/09031936.05.00139104. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Mediators of chronic obstructive pulmonary disease. Phatmacol Rev. 2004;56:515–48. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New approaches to COPD. Eur Respir J. 2006;94:2–11. [Google Scholar]
- Barnes PJ. New treatments for COPD. Nat Rev Drug Discov. 2002;1:437–46. doi: 10.1038/nrd820. [DOI] [PubMed] [Google Scholar]
- Barrios R, Pardo A, Ramos C, et al. Upregulation of acidic fibroblast growth factor during development of experimental lung fibrosis. Am J Physiol. 1997;273:L451–8. doi: 10.1152/ajplung.1997.273.2.L451. [DOI] [PubMed] [Google Scholar]
- Bryant SR, Bjercke RJ, Erichsen DA, et al. Vascular remodeling in response to altered blood flow is mediated by fibroblast growth factor-2. Circ Res. 1999;84:323–8. doi: 10.1161/01.res.84.3.323. [DOI] [PubMed] [Google Scholar]
- Byzova TV, Goldman CK, Pampori N, et al. A mechanism for modulation of cellular responses to VEGF. Activation of the integrins. Mol Cell. 2000;6:851–60. [PubMed] [Google Scholar]
- Calabrese C, Bocchino V, Vatrella A, et al. Evidence of angiogenesis in bronchial biopsies of smokers with and without airway obstruction. Respir Med. 2006;100:1415–22. doi: 10.1016/j.rmed.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- Chrysofakis G, Tzanakis N, Kyriakou D, et al. Perforin expression and cytotoxic activity of sputum CD8+ lymphocytes in patients with COPD. Chest. 2004;125:71–6. doi: 10.1378/chest.125.1.71. [DOI] [PubMed] [Google Scholar]
- Cosio MG, Hale KA, Niewoehner DE. Morphologic and morphometric effects of prolonged cigarette smoking on the small airways. Am Rev Respir Dis. 1980;122:265–71. doi: 10.1164/arrd.1980.122.2.265. [DOI] [PubMed] [Google Scholar]
- Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–7. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- de Boer WI, Hau CM, van Schadewijk A, et al. Expression of epidermal growth factors and their receptors in the bronchial epithelium of subjects with chronic obstructive pulmonary disease. Am J Clin Pathol. 2006;125:184–92. doi: 10.1309/W1AX-KGT7-UA37-X257. [DOI] [PubMed] [Google Scholar]
- Guddo F, Vignola AM, Saetta M, et al. Upregulation of basic fibroblast growth factor in smokers with chronic bronchitis. Eur Respir J. 2006;27:957–63. doi: 10.1183/09031936.06.00057205. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Tanaka H, Abe S. Quantitative analysis of bronchial wall vascularity in the medium and small airways of patients with asthma and COPD. Chest. 2005;127:965–72. doi: 10.1378/chest.127.3.965. [DOI] [PubMed] [Google Scholar]
- Hay DWP, Sarau HM. Interleukin-8 receptor antagonists in pulmonary diseases. Curr Opin Pharmacol. 2001;1:242–7. doi: 10.1016/s1471-4892(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Holgate ST. Asthma: a dynamic disease of inflammation and repair. Ciba Found Symp. 1997;206:5–34. doi: 10.1002/9780470515334.ch2. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol. 2001;107:1034–8. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001;107:295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Albertine KH, Jicha DL, et al. Human endothelial cells synthesize ENA-78: relationship to IL-8 and to signaling of PMN adhesion. Am j Respir Cell Mol Biol. 1997;17:181–92. doi: 10.1165/ajrcmb.17.2.2818. [DOI] [PubMed] [Google Scholar]
- Jeffery PK.2001Lymphocytes, chronic bronchitis and chronic obstructive pulmonary disease Novartis Found Symp 234149–61.discussion 161–8. [PubMed] [Google Scholar]
- Jeffery PK. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2004;1:176–83. doi: 10.1513/pats.200402-009MS. [DOI] [PubMed] [Google Scholar]
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- Jeffery PK. Structural and inflammatory changes in COPD: a comparison with asthma. Thorax. 1998;53:129–36. doi: 10.1136/thx.53.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cel Mol Biol. 2002;26:748–53. doi: 10.1165/ajrcmb.26.6.4757. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, Tuder RM, Cool CD, et al. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163:737–44. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–19. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatings VM, Collins PD, Scott DM, et al. Differences in interleukin-8 and tumor necrosis factor-α in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–4. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- Kessler R, Faller M, Weitzenblum E, et al. Natural history of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:219–24. doi: 10.1164/ajrccm.164.2.2006129. [DOI] [PubMed] [Google Scholar]
- Knox AJ, Stocks J, Sutcliffe A. Angiogenesis and vascular endothelial growth factor in COPD. Thorax. 2005;60:88–9. doi: 10.1136/thx.2004.030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg AR, de Boer WI, Alagappan VKT, et al. Enhanced bronchial expression of vascular endothelial growth factor and receptors (Flk-1 and Flt-1) in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:106–13. doi: 10.1136/thx.2004.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg AR, De Boer WI, Van Krieken JH, et al. Enhanced expression of fibroblast growth factors and receptor FGFR-1 during vascular remodeling in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2002;27:517–25. doi: 10.1165/rcmb.4474. [DOI] [PubMed] [Google Scholar]
- Kranenburg AR, Willems-Widyastuti A, Mooi WJ, et al. Chronic obstructive pulmonary disease is associated with enhanced bronchial expression of FGF-1, FGF-2, and FGFR-1. J Pathol. 2005;206:28–38. doi: 10.1002/path.1748. [DOI] [PubMed] [Google Scholar]
- Lang MR, Fiaux GW, Gillooly M, et al. Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax. 1994;49:319–26. doi: 10.1136/thx.49.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Link H, Baluk P, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wilson JW. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–33. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Snyder R, Soma A, et al. VEGF-A and αvβ3 intergrin synergistically rescue angiogenesis via N-Ras and PI3-K signaling in human microvascular endothelial cells. FASEB J. 2003;17:1931–3. doi: 10.1096/fj.02-1171fje. [DOI] [PubMed] [Google Scholar]
- McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- McDonald DM. In: Experimental models of bronchial reactivity: effect of airway infections. Ogra PL, Mestecky J, Lamm ME, et al., editors. San Diego, California: Academic Press; 1999. pp. 1177–85. [Google Scholar]
- Miller AL, Strieter RM, Gruber AD, et al. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J Immunol. 2003;170:3348–56. doi: 10.4049/jimmunol.170.6.3348. [DOI] [PubMed] [Google Scholar]
- Nilsson I, Shibuya M, Wennstrom S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res. 2004;299:476–85. doi: 10.1016/j.yexcr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- Orsida BE, Li X, Hickey B, et al. Vascularity in asthmatic airways: relation to inhaled steroid dose. Thorax. 1999;54:289–95. doi: 10.1136/thx.54.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsida BE, Ward C, Li X, et al. Effect of a long-acting ß2-agonist over three months on airway wall vascular remodeling in asthma. Am J Respir Crit Care Med. 2001;164:117–21. doi: 10.1164/ajrccm.164.1.2006003. [DOI] [PubMed] [Google Scholar]
- Panina-Bordignon P, D’Ambrosio D. Chemokines and their receptors in asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2003;9:104–10. doi: 10.1097/00063198-200303000-00003. [DOI] [PubMed] [Google Scholar]
- Pauwels RA, Rabe KE. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364:613–20. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- Peinado VI, Barbera JA, Abate P, et al. Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1605–11. doi: 10.1164/ajrccm.159.5.9807059. [DOI] [PubMed] [Google Scholar]
- Peinado VI, Ramirez J, Roca J, et al. Identification of vascular progenitor cells in pulmonary arteries of patients with with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2006;34:257–63. doi: 10.1165/rcmb.2005-0255OC. [DOI] [PubMed] [Google Scholar]
- Pesci A, Balbi B, Majori M, et al. Inflammatory cells and meditaors in bronchial lavage of patients with chronic obstructive disease. Eur Respir J. 1998;12:380–6. doi: 10.1183/09031936.98.12020380. [DOI] [PubMed] [Google Scholar]
- Petrache I, Fijalkowska I, Zhen L, et al. An antiaptotic role for {alpha}1 - antitrypsin in the prevention of emphysema. Proc Am Thorac Soc. 2006;3:549. [Google Scholar]
- Plataki M, Tzortzaki E, Rytila P, et al. Apoptotic mechanisms in the pathogenesis of COPD. Internat J COPD. 2006;1:161–71. doi: 10.2147/copd.2006.1.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma DS, Timens W. Remodeling in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:434–39. doi: 10.1513/pats.200601-006AW. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Zhu J, Bandi V, et al. Biopsy neutrophilia, chemokine and receptor gene expression in severe exacerbations of COPD. Am J Respir Crit Care Med. 2003;168:968–75. doi: 10.1164/rccm.200208-794OC. [DOI] [PubMed] [Google Scholar]
- Redington AE, Roche WR, Madden J, et al. Basic fibroblast growth factor in asthma: measurement in bronchoalveolar lavage fluid basally and following allergen challenge. J Allergy Clin Immunol. 2001;107:384–7. doi: 10.1067/mai.2001.112268. [DOI] [PubMed] [Google Scholar]
- Retamales I, Elliot WM, Meshi B, et al. Amplification of inflammation in emphysema and its association with latent adenoviral infection. Am Respir Crit Care Med. 2001;164:469–73. doi: 10.1164/ajrccm.164.3.2007149. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Presta M. The discovery of angiogenic factors: a historical review. Gen Pharmacol. 2000;35:227–31. doi: 10.1016/s0306-3623(01)00112-4. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Rose CE, Jr, Sung SS, Fu SM. Significant involvement of CCL2 (MCP-1) in inflammation disorders of the lung. Microcirculation. 2003;10:273–88. doi: 10.1038/sj.mn.7800193. [DOI] [PubMed] [Google Scholar]
- Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- Saetta M, Mariani M, Panina-Bordignon P, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–9. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- Saetta M, Turato G, Maestrelli P, et al. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1304–9. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- Salvato G. Quantitative and morphological analysis of the vascular bed in bronchial biopsy specimens from asthmatic and non-asthmatic subjects. Thorax. 2001;56:902–6. doi: 10.1136/thorax.56.12.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos S, Peinado VI, Ramirez J, et al. Enhanced expression of vascular endothelial growth factor in pulmonary arteries of smokers and patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1250–6. doi: 10.1164/rccm.200210-1233OC. [DOI] [PubMed] [Google Scholar]
- Santos S, Peinado VI, Ramirez J, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–8. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–4. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Shute JK, Solic N, Shimizu J, et al. Epithelial expression and release of FGF-2 from heparan sulphate binding sites in bronchial tissue in asthma. Thorax. 2004;59:557–62. doi: 10.1136/thx.2002.002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siafakas NM, Jordan M, Wagner H, et al. Diaphragmatic angiogenic growth factor mRNA responses to increased ventilation caused by hypoxia and hypercapnia. Eur Respir J. 2001;17:681–7. doi: 10.1183/09031936.01.17406810. [DOI] [PubMed] [Google Scholar]
- Siafakas NM, Tzortzaki EG. Few smokers develop COPD. Why. Respir Med. 2002;96:615–24. doi: 10.1053/rmed.2002.1318. [DOI] [PubMed] [Google Scholar]
- Singh TM, Abe KY, Sasaki T, et al. Basic fibroblast growth factor expression precedes flow-induced arterial enlargement. J Surg Res. 1998;77:165–73. doi: 10.1006/jsre.1998.5376. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Belperio JA, Burdick MD, et al. CXC chemokines: angiogenesis, immunoangiostasis, and metastases in lung cancer. Ann N Y Acad Sci. 2004;1028:351–60. doi: 10.1196/annals.1322.041. [DOI] [PubMed] [Google Scholar]
- Szebenyi G, Fallon JF. Fibroblast growth factors as multifunctional signaling factors. Int Rev Cytol. 1999;185:45–106. doi: 10.1016/s0074-7696(08)60149-7. [DOI] [PubMed] [Google Scholar]
- Tang K, Breen EC, Gerber HP, et al. Capillary regression in vascular endothelial growth factor–deficient skeletal muscle. Physiol Genomics. 2004;18:63–9. doi: 10.1152/physiolgenomics.00023.2004. [DOI] [PubMed] [Google Scholar]
- Tang K, Rossiter HB, Wagner PD, et al. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol. 2004;97:1559–66. doi: 10.1152/japplphysiol.00221.2004. [DOI] [PubMed] [Google Scholar]
- Tanino M, Betsuyaku T, Takeyabu K, et al. Increased levels of interleukin-8 in BAL fluid from smokers susceptible to pulmonary emphysema. Thorax. 2002;57:405–11. doi: 10.1136/thorax.57.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traves SL, Culpitt S, Russell REK, et al. Increased levels of the chemokines GRO-α and MCP-1 in sputum samples from patients with COPD. Thorax. 2002;57:590–5. doi: 10.1136/thorax.57.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traves SL, Smith SJ, Barnes PJ, et al. Specific CXC but not CC chemokines cause elevated monocyte migration in COPD: a role for CXCR2. J Leukoc Biol. 2004;76:441–50. doi: 10.1189/jlb.1003495. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Flook BE, Voelkel NF. Increased gene expression for VEGF and the VEGF receptors KDR/Flk and Flt in lungs exposed to acute or to chronic hypoxia. Modulation of gene expression by nitric oxide. J Clin Invest. 1995;95:1798–807. doi: 10.1172/JCI117858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuder RM, Zhen L, Cho CY, et al. Oxidative stress and apoptosis interact and cause emphysema due to vascular endothelial growth factor receptor blockade. Am J Respir Cell Mol Biol. 2003;29:88–97. doi: 10.1165/rcmb.2002-0228OC. [DOI] [PubMed] [Google Scholar]
- Tzanakis N, Chrysofakis G, Tsoumakidou M, et al. Induced sputum CD8+ T-lymphocyte subpopulations in chronic obstructive pulmonary disease. Respir Med. 2004;98:57–65. doi: 10.1016/j.rmed.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Tzouvelekis A, Anevlavis S, Bouros D. Angiogenesis in interstitial lung diseases: a pathogenetic hallmark or a bystander. Respir Res. 2006;7:82. doi: 10.1186/1465-9921-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelkel NF, Cool C, Taraceviene-Stewart L, et al. Janus face of vascular endothelial growth factor: the obligatory survival factor for lung vascular endothelium controls precapillary artery remodeling in severe pulmonary hypertension. Crit Care Med. 2002;30:S251–6. doi: 10.1097/00003246-200205001-00013. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–21. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- Walsh DA, Pearson CI. Angiogenesis in the pathogenesis of inflammatory joint and lung diseases. Arthritis Res. 2001;3:147. doi: 10.1186/ar292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen FQ, Liu X, Manda W, et al. TH2 Cytokine-enhanced and TGF-beta-enhanced vascular endothelial growth factor production by cultured human airway smooth muscle cells is attenuated by IFN-gamma and corticosteroids. J Allergy Clin Immunol. 2003;111:1307–18. doi: 10.1067/mai.2003.1455. [DOI] [PubMed] [Google Scholar]
- Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev. 1998;9:153–65. doi: 10.1016/s1359-6101(98)00010-0. [DOI] [PubMed] [Google Scholar]
- Widdowson KL, Elliott JD, Veber DF, et al. Evaluation of potent and selective small-molecule antagonists for the CXCR2 chemokine receptor. J Med Chem. 2004;47:1319–21. doi: 10.1021/jm034248l. [DOI] [PubMed] [Google Scholar]
- Wilkinson M, Langhorne CA, Heath D, et al. A pathophysiological study of 10 cases of hypoxic cor pulmonale. Q J Med. 1998;66:65–85. [PubMed] [Google Scholar]
- Wright JL, Lawson L, Pare PD, et al. The structure and function of the pulmonary vasculature in mild chronic obstructive pulmonary disease: the effect of oxygen and exercise. Am Rev Respir Dis. 1983;128:702–7. doi: 10.1164/arrd.1983.128.4.702. [DOI] [PubMed] [Google Scholar]
- Yamamoto C, Yoneda T, Yoshikawa M, et al. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest. 1997;112:505–10. doi: 10.1378/chest.112.2.505. [DOI] [PubMed] [Google Scholar]
- Yamato H, Sun JP, Churg A, et al. Cigarette smoke–induced emphysema in guinea pigs is associated with diffusely decreased capillary density and capillary narrowing. Lab Invest. 1996;75:211–19. [PubMed] [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–8. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- Yang XD, Corvalan JR, Wang P, et al. Fully human anti-interleukin-8 monoclonal antibodies: potential therapeutics for the treatment of inflammatory disease states. J Leukoc Biol. 1999;66:401–10. doi: 10.1002/jlb.66.3.401. [DOI] [PubMed] [Google Scholar]