Abstract
Chronic obstructive pulmonary disease (COPD) is associated with smoking but other etiological factors contribute. Chlamydia pneumoniae is an obligate intracellular bacterium causing both acute and chronic respiratory tract infections. Studies have revealed an association between chronic C. pneumoniae infection and COPD, asthma and lung cancer but there have been difficulties detecting C. pneumoniae in the bronchial tree. Cytospin slides prepared from bronchoalveolar lavage (BAL) fluid from 14 patients with COPD, 10 healthy smokers (S) and 7 non smokers (NS) were analyzed with a fluorescein isothiocyanate labeled monoclonal antibody to C. pneumoniae. Lung tissue from 24 patients with advanced emphysema who had undergone lung volume reduction surgery (LVRS) was examined with immunohistochemistry for C. pneumoniae. Archived serum samples for detection of specific C. pneumoniae antibodies by microimmunofluorescence were available for 30 of the BAL subjects and 11 of LVRS patients. C. pneumoniae elementary body like structures were found in 29% of cytospin specimens from COPD patients, 14% of NS and 10% of HS. C. pneumoniae was detected in lung tissue in 8%. COPD patients had higher titres of IgG and IgA than NS and S. There was no association between occurrence of C. pneumoniae in BAL fluid and antibody titres. In conclusion, the assays used for detection of C. pneumoniae in lung tissue are feasible, and could be adapted in adequately powered studies to further confirm an association between C. pneumoniae infection and COPD.
Keywords: Chlamydia pneumoniae, COPD, lung tissue, bronchoalveolar lavage, cytospin
Introduction
Chronic obstructive pulmonary disease (COPD) has a significant impact on burden of illness, and WHO predict that by 2020 COPD will be the 5th most prevalent disease worldwide and the 3rd most common cause of death (Lopez et al 1998; Michaud et al 2001). Tobacco smoking is the major cause of COPD. However, since non-smokers can develop COPD and lung function deterioration continues rapidly in some patients despite smoking cessation, other etiological factors than smoking seem to contribute to the development of the disease. It is likely that both genetic and acquired host factors as well as environmental exposures, either separately or in combination may contribute to the development of the disease.
Chlamydia pneumoniae, an obligate intracellular bacterium with a unique biphasic replicative cycle, is an established cause of acute and chronic upper and lower respiratory tract infections. During the replicative cycle, C. pneumoniae presents as infectious elementary bodies and metabolically active intracellular reticulate bodies. Release of pro-inflammatory cytokines in response to a chronic C. pneumoniae infection could potentially amplify inflammation and damage of the lungs caused by cigarette smoke. Several studies have demonstrated persistent elevated titres of specific IgA antibodies to C. pneumoniae in patients with COPD (von Hertzen et al 1996; von Hertzen et al 1997), and we recently reported that serology suggestive of chronic C. pneumoniae infection was an independent risk factor for the development of COPD (Brandén et al 2005). Further, macrophages in lung tissue from patients with COPD have been reported to stain positive for C. pneumoniae LPS (Rupp et al 2004), and C. pneumoniae has also been detected in lung tissue from subjects with COPD (Wu et al 2000).
The two major components of airways obstruction in COPD are small airways disease and emphysema, which both to a large extent are irreversible and cause permanent reduced lung function. Samples from these peripheral compartments of the lung can be obtained by bronchoalveolar lavage (BAL), and the present study was conducted to evaluate the usefulness of BAL fluid cytospin preparations for the detection of C. pneumoniae. Further, we examined lung tissue from patients with advanced emphysema undergoing lung volume reduction surgery (LVRS) in order to evaluate the association between C. pneumoniae and severe COPD.
Material and methods
Study population
The investigated subjects were recruited in two separate studies. Both were approved by the Ethics Committee, Karolinska University Hospital, and informed consent was given by all patients.
Study A
Study A was performed April 2000 to June 2004, and included 31 subjects; 14 with stable clinical COPD without any exacerbations for the last 3 months, and as controls 10 healthy smokers and 7 non-smokers with normal chest radiographs (Table 1). They consist of a sub-group of subjects from a previous study (Lofdahl et al 2005) where cytospin preparations from BAL were available. All subjects underwent spirometry and bronchoscopy with BAL, and the cells were concentrated on glass slides using a cytospin procedure. Analysis of specific C. pneumoniae antibodies in serum taken prior to bronchos-copy was performed in all subjects except one.
Table 1.
Characteristics of the population, presence of C. pneumoniae in cytospin from bronchoalveolar lavage and antibody titres against C. pneumoniae in patients with COPD, healthy smokers and non-smokers (Study A)
| COPD N = 14 | Smokers N = 10 | Non-smokers N = 7 | ||
|---|---|---|---|---|
| Females | N | 6 | 7 | 4 |
| Age (Years) | Mean (SD) | 56.5 (3.7) | 54.0 (8.4) | 57.7 (5.0) |
| Pack-years | Mean (SD) | 37.1 (7.0) | 40.6 (24.1) | 0.0 (0.0) |
| FEV1 (% predicted) | Mean (SD) | 54.4 (9.8)b | 100.5 (11.2) | 108.9 (9.0) |
| Reversibility (% predicted) | Mean (SD) | 5.3 (4.6) | 3.1 (2.3) | 3.1 (2.8) |
| BAL recovery (%) | Mean (SD) | 36.2 (15.7)b | 57.2 (13.3) | 65.1 (11.3) |
| C. pneumoniaea in cytospin from BAL | N (%) | 4 (29) | 1 (10) | 1 (14) |
Elementary body like structures.
p < 0.001 vs smokers and non-smokers.
Study B
Study B included 24 patients (15 females and 9 males), who had undergone LVRS for severe emphysema between January 1995 and May 2001. Six patients were homozygote for α1-antitrypsin deficiency (4 females and 2 males), 3 were heterozygote (1 female and 2 males) and the other 15 had normal enzyme levels. The mean age was 61.6 years (SD 7.7). All were ex-smokers except one homozygote female. Information on the smoking habits was based on medical records, and the information was not sufficient to enable calculations in three subjects. Mean smoking pack-years (PY; product of years smoking and the number of cigarettes smoked per day divided by 20) 27.5 (SD 17.2). Spirometries were performed in all, and mean forced expired volume in one second (FEV1) was 23.8 (SD 4.8) % predicted. Reversibility was tested in all except two, and demonstrated in mean 3.3 (SD 3.4) % predicted. C. pneumoniae serology was available for 11 of the patients. Samples were collected taken at time of surgery in 7 patients, and 2–7 years post surgery in 4 patients.
Lung function tests
Lung function was determined by the use of dynamic spirometers, in Study A Vitalograph Compact (Vitalo-graph Ltd, Buckingham, UK) and in Study B Gould 2400 (Gould Electronics, Netherlands). The procedures were performed in a standardized manner, and the results for forced vital capacity (FVC), vital capacity (VC) and forced expiratory volume in one second (FEV1) were expressed as percentage of predicted values (Quanjer 1983; Quanjer et al 1993). Reversibility of airways obstruction was tested by measuring FEV1 before and 10 min after inhalation of bronchodilators; in Study A one mg terbutaline (Bricanyl Turbuhaler; AstraZeneca) and in Study B nebulisation of 5 mg salbutamol (Ventoline; GlaxoSmithKline) and 0.25 mg ipratropium bromide (Atrovent; Boehringer Ingelheim), and was calculated as improvement of FEV1 in percentage of predicted FEV1.
Bronchoscopy and bronchoalveolar lavage (BAL)
Bronchoscopy was performed on an outpatient basis after overnight fasting. Following pre-medication with morphin-hyoscine i.m. 45 min prior to the investigation, the bronchoscope (Olympus F Type P30, Olympus Optical Co, Ltd, Tokyo, Japan) was inserted nasally after topical anesthesia with lignocaine (Xylocain; AstraZeneca, Södertälje, Sweden). BAL was performed by wedging the bronchoscope in a sub-segment of the middle lobe. In one of the COPD patients BAL was performed in one of the basal sub-segments of the right lower lobe due to difficulties in wedging the bronchoscope in the middle lobe. Five aliquots of 50 ml of sterile phosphate buffered saline (PBS) adjusted to 37 °C were instilled and gently suctioned back with a negative pressure of −40 to −50 mm Hg. Dwell time was kept to a minimum as recommended by the European Respiratory Society task force (Haslam et al 1999). The procedure was interrupted if the recovery after 150–200 ml of instilled fluid was <35 ml or in case of persistent cough or hypoxia to <90% oxygen saturation despite appropriate oxygen supplement. The BAL fluid was collected in a silicone treated bottle kept on ice, which was immediately transported to the laboratory. The fluid was filtered through a Dacron membrane (Millipore, Cork, Ireland) and centrifuged onto microscope slides at 400x g for 10 min at 4 °C, then frozen at −80 °C.
Lung volume reduction surgery
Surgery was performed by median sternotomy except for a few cases in which thoracotomy was done. The most damaged areas were removed with an ambition to reduce the lung volume with 30%.
Detection of C. pneumoniae in study A
Detection of C. pneumoniae was performed by direct fluorescence antibody technique (DFA) on cytospin preparations of BAL fluid. The cytospin specimens on glass microscope slides were fixed in cold acetone for 15 minutes and dried in air. Twenty-five microliters of a monoclonal antibody directed against C. pneumoniae (Imagen™ Chlamydia pneumoniae, DAKO Ltd, Cambridgeshire, UK) was overlaid on each specimen, and slides were incubated at 37 °C for 30 min in a moist chamber. Positive and negative control slides were included in all runs. Slides provided in the Imagen kit containing C. pneumoniae infected cells served as positive controls, and C. pneumoniae infected cells incubated with a fluorescein isothiocyanate labeled adenovirus monoclonal antibody were used as negative controls. Washing with gentle agitation was done with PBS pH 7.5 for 5 minutes, after which the slides were dried in air, mounted, coverslipped and examined immediately using an UV microscope (Olympus B × 40) at 500x magnification. The presence of bright apple-green fluorescing round bodies concomitant in size with elementary bodies were considered as possible positive findings for C. pneumoniae.
Detection of C. pneumoniae in study B
Immunohistochemistry (IHC) for C. pneumoniae was performed with the patient identity blinded. Formalin fixed lung tissue was embedded in paraffin and cut at a thickness of 4 μm. The sections were placed on glass slides and deparaffinized according to standard procedure including clearing with xylene × 2, and hydrated through a series of alcohols with decreasing concentrations. The sections were overlaid with 0.5% pepsin (Sigma Chemical Co, St. Louis, Mo) to open up the cells and incubated for 15 minutes at 37 °C. After washing with PBS-Tween 20 (PBST) at pH 7.6 × 2 for 5 minutes, peroxidase blocking reagent (DAKO Corporation, Carpinteria, CA) was overlaid, and the slides were incubated for 15 minutes at room temperature in a moist chamber. Washing was done twice for 5 minutes in PBST and universal blocking solution (Dako Diagnostics Canada Inc, Mississauga, ON) was overlaid and incubated for 15 min at room temperature. The slides were gently drained and a 1:5 dilution of mouse monoclonal antibody to Chlamydia pneumoniae RR-402 (DakoCytomation Ltd, Ely, UK) in antibody diluting buffer (DAKO Diagnostics Canada Inc., Mississauga, ON) was added. Slides were incubated overnight at 4 °C and then washed twice with PBST. StreptABComplex/HRP Duet, Mouse/Rabbit (Dako A/S, Glostrup, Denmark) was used for the streptavidin/biotin reaction using a biotinylated goat antibody to mouse and rabbit immunoglobulin, streptavidin and a biotinylated horseradish peroxidase. Finally, DAKO AEC Substrate System (DAKO Corporation, Carpinteria, CA) was used for visualization of the red coloured Chlamydia antigen in the tissue sections.
Tissue sections previously obtained from patients with lung cancer and proven C. pneumoniae infection were used as positive controls. As negative controls an antibody to mouse IgG3 (NeoMarkers, Fremont, CA), the same class of antibody as the RR-402 monoclonal, was used in all runs.
Serology for C. pneumoniae
C. pneumoniae antibodies were analyzed by microimmunofluorescence (MIF) at an accredited clinical microbiology laboratory using a previously standardized procedure (Gnarpe, Naas et al 2000; Gnarpe, Sparr et al 2000). All sera were separated from erythrocytes upon arrival at the laboratory and frozen at −20 °C until tested. Sera were diluted 1:32 with PBS at pH 7.4 and tested for IgG and IgA antibodies using 21-well antigen slides with elementary bodies of C. psittaci, C.trachomatis and C. pneumoniae in each test well (LabSystems Oy; Helsinki, Finland).
Sera found to be positive in a 1/64 screening dilution for IgG were re-diluted and tested in doubling dilutions to endpoint. All positive sera were retested and sera positive for IgA were absorbed with Gullsorb (Gull Laboratories Ltd, USA) to remove IgG before dilution, and then tested in doubling dilutions. Serum dilutions were incubated with antigens for 14–16 h at 4 °C at all testing occasions. This prolonged incubation at 4 °C increases the sensitivity of the test: a 1/32 dilution of serum incubated overnight is equivalent to a titer of 1/8–1/16 when incubated for 30–60 min in a conventional MIF test. Slides were washed thoroughly in three changes of PBS solution (pH 7.4), then incubated with fluorescein isothio-cyanate-conjugated rabbit antihuman IgG or IgA (Dakopatts; Glostrup, Denmark) at 37 °C for 30 min. Control sera with specified high and low titres were used on each testing occasion, and tests were accepted only if the titres were within one dilution step of the predetermined mean titres for the respective quality control sera. An experienced microbiologist read all tests with an UV microscope (Zeiss; Jena, Germany) using a 40x oil immersion lens and a 10x ocular lens (total magnification, x400).
Statistical analysis
Statistical analyses were only performed in Study A. Comparisons between the groups were performed by analysis of variance, and in case of statistical significance (p < 0.05), post-hoc analysis was performed with the LSD test. Analyses of difference in serological titres were performed after logarithmic transformation, and negative observations were assigned a number corresponding to one titre step below the detection level.
Results
Study A
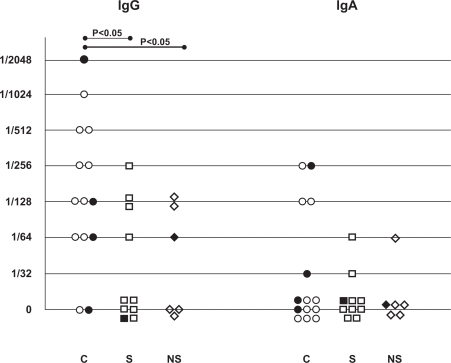
C. pneumoniae species (apple green elementary body like structures) were detected with DFA on cytospin slides from BAL fluid in 29% of the COPD patients, 10% of the healthy smokers and 14% of the never-smokers (Table 1 and Figure 1). No inclusion bodies in the cells were observed on the cytospins. The specific IgG-antibody titres were significantly higher in the COPD patients than in the healthy smokers and non-smokers (Figure 2). The prevalence of specific IgG antibody titre ≥1/128 were 64% in the COPD patients, 30% in the smokers and 33% in the never-smokers. Corresponding figures for specific IgA-antibody titre ≥1/64 were 29%, 10% and 17%. None of the differences between the groups was statistically significant. There was no correlation between high antibody titres and detection of the C. pneumoniae elementary bodies in the cytospin preparations.
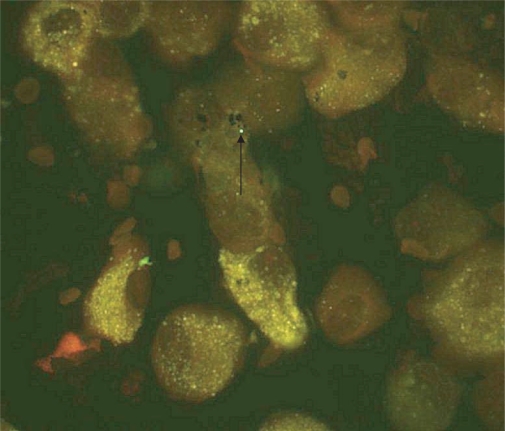
Figure 1.
Round elementary body like structure detected with DFA on cytospin slide from BAL fluid. The apple green color is not visible on this photo.
Figure 2.
Presence of C.pneumoniae in cytospin from bronchoalveolar lavage and antibody titres against C. pneumoniae in patients with COPD (C), healthy smokers (S) and non-smokers (NS) (Study A). Filled figures represent presence and unfilled absence of C.pneumoniae.
Study B
No signs of inflammation were observed in the majority of lung tissue samples from the subjects with advanced emphysema. C. pneumoniae was detected with IHC in lung tissue from 2 of 24 patients with advanced emphysema, one female with α1-antitrypsin deficiency who had never smoked and one male ex-smoker with normal α1-antitrypsin level. Serology for C. pneumoniae antibodies was not available for these two individuals. For those subjects where sera for antibody analyses were available, specific IgG-antibodies titres ≥1/128 were detected in 55% and IgA titres ≥1/64 in 36% of the subjects. In those where sera were available at the time of surgery 57% had IgA titres ≥1/64.
Discussion
In this study we demonstrated that C. pneumoniae elementary body like structures could be detected in cytospin preparations of BAL fluid with a higher, although not statistically significant, prevalence in subjects with COPD than in healthy smokers and non-smokers. Further, the specific IgG and IgA antibody titres were higher in subjects with COPD than in healthy smokers and non-smokers. We have also shown that C. pneumoniae could be detected in lung tissue from 2 of 24 subjects undergoing surgery for severe emphysema.
Sero-epidemiological studies have demonstrated that C. pneumoniae is a common and widespread pathogen with 50% of adults in the general population having detectable IgG antibody titres (Grayston 1989). This was confirmed in the present study, which demonstrated a high prevalence (71%) of IgG titres ≥1/64. Several studies have shown an association between chronic C. pneumoniae infection defined by serological criteria and COPD (von Hertzen et al 1996; von Hertzen et al 1997; Smieja et al 2002; Brandén et al 2005; Kurashima et al 2005). This study confirms such an association, since subjects with COPD had significantly higher specific IgG titres than healthy individuals with a similar smoking history. The persistence of elevated titres of short-lived IgA has been proposed as a marker for chronic, recurrent or active carrier state (Saikku et al 1992). Chronic C. pneumoniae infection is thus strongly indicated in case of elevated specific IgA levels in paired serum samples taken with an extended interval. Since our study did not include repeated serological investigations, the results must be interpreted with caution, since a single elevated value can represent an acute infection. However, none of the subjects showed any signs of respiratory infection at the time of the investigation. In this study a larger proportion of subjects with COPD than healthy smokers and non-smokers had IgA titres ≥1/64, but the difference was not statistically significant.
Although the association between serological signs of chronic C. pneumoniae infection and COPD is well established, knowledge about the host cells and tissues for the infection is limited. It has been reported that COPD patients are more likely to have C. pneumoniae DNA in peripheral blood mononuclear cells than patients undergoing coronary angiography (Smieja et al 2002), and there are several publications demonstrating an association between C. pneumoniae and atherosclerosis (Saikku et al 1988; Muhlestein et al 1996; Campbell et al 1998). To strengthen a causal relationship with COPD, presence of the micro-organism should ideally be demonstrated in lung tissue. C. pneumoniae can be detected by a number of methods such as immunohistochemistry, PCR (polymerase chain reaction) on lung tissue and culture of fresh tissues. We only analyzed the specimens with immunohistochemistry, and due to lack of good specimens we could unfortunately not verify the findings with other methods.
C. pneumoniae has previously been identified in emphysematous tissue (Wu et al 2000; Rupp et al 2004; Theegarten et al 2004), and we could confirm the presence of C. pneumoniae in lung tissue from two out of 24 subjects who had undergone lung volume reduction surgery due to severe emphysema. The detection rate corresponds with the findings in two of the previously reported studies (Rupp et al 2004; Theegarten et al 2004), but was considerably lower than in another study (Wu et al 2000). However, in Wu’s study archival tissue from subjects who had undergone lobectomy for bronchial carcinoma was analyzed, and this could have influenced the results. Several studies have reported an association between chronic C. pneumoniae infection and lung cancer (Laurila et al 1997; Jackson et al 2000; Koyi et al 2001; Kocazeybek 2003), and the organism has been detected in macrophages close to the lung cancer using the same staining techniques as in the present study (Koyi et al 2001).
The low rate of detection of C. pneumoniae in emphysematous tissue in this and other studies may be explained by the patchy localization of the infection (Kuo 2000), and that the investigated tissue samples only represent a very limited part of the lungs. BAL covers a larger compartment including small airways, which are affected in COPD. The retained BAL fluid can either be used for cultivation or for identification of micro-organisms with immunological methods. Cultivation of C. pneumoniae is difficult, and tissue specimens should undergo primary isolation procedures plus 4–6 additional passages (Dowell et al 2001). Since this was a retrospective study, freshly procured samples were not available for cultivation, and we used immunological techniques with monoclonal antibodies for detection of the micro-organism on fixed cytospin preparations.
C. pneumoniae has previously been detected by DFA on buffy coat cell pellets from BAL of children with asthma and other lung diseases (Webley et al 2005), but to our knowledge, no previous study has aimed to detect C. pneumoniae from BAL fluid cytospin preparations. Using DFA, we found elementary body like structures on cytospin slides in four subjects with COPD, in one healthy smoker and in one non-smoker. As the specimens were archival we had no possibility to confirm the identity of these structures. It is known that some bacteria, like Staphylococcus aureus containing Protein A can unspecifically bind the FITC-labeled antibody and cause false positive results, but this bacterium should not be present in the lung and the organisms are usually larger than the elementary body of C. pneumoniae. Further analysis with other techniques such as PCR are needed to confirm the identification of C. pneumoniae. However, our finding of elementary body like structures in cytospin preparations from BAL is interesting, and if confirmed, could add information on an association between C. pneumoniae infection and COPD. Appropriately designed and powered studies are needed to confirm this finding.
A disadvantage of studying BAL in COPD is that the recovery of fluid often is low (Linden et al 1993; Soler et al 1999), which also was seen in this study. This might influence the reliability of the sampling, since in case of low recovery the BAL fluid may not ideally represent the distal bronchial tree but rather reflect conditions in large airways.
Long term studies using C. pneumoniae serology are needed to reveal whether chronic infections initiate the development of COPD or occur as a consequence of reduced immune response in a compromised lung. To further strengthen a causal relationship between chronic infections and COPD, the bacteria should ideally be identified in the lungs. Although this study was not powered to confirm an association between C. pneumoniae infection and COPD, we have demonstrated that the assays used for detection of the organism in cytospin from BAL and resected lung tissue are feasible, and thus could be adapted in adequately sized studies.
Acknowledgments
The study was supported by Karolinska Institutet, Stockholm Sweden and the Swedish Heart and Lung Foundation.
Good assistance was provided from Annika Lundbäck, microbiologist at the Department of Clinical Microbiology, Gävle County Hospital, Gävle, Sweden.
References
- Branden E, Koyi H, Gnarpe J, et al. Chronic Chlamydia pneumoniae infection is a risk factor for the development of COPD. Respir Med. 2005;99:20–6. doi: 10.1016/j.rmed.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Campbell LA, Kuo CC, Grayston JT. Chlamydia pneumoniae and cardiovascular disease. Emerg Infect Dis. 1998;4:571–9. doi: 10.3201/eid0404.980407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell SF, Peeling RW, Boman J, et al. Standardizing Chlamydia pneumoniae assays: recommendations from the Centers for Disease Control and Prevention (USA) and the Laboratory Centre for Disease Control (Canada) Clin Infect Dis. 2001;33:492–503. doi: 10.1086/322632. [DOI] [PubMed] [Google Scholar]
- Gnarpe J, Naas J, Lundback A. Comparison of a new commercial EIA kit and the microimmunofluorescence technique for the determination of IgG and IgA antibodies to Chlamydia pneumoniae. APMIS. 2000;108:819–24. doi: 10.1111/j.1600-0463.2000.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Gnarpe J, Sparr A, Naas J, et al. Serological analysis of specific IgA to Chlamydia pneumoniae: increased sensitivity of IgA antibody detection using prolonged incubation and high antigen concentration. APMIS. 2000;108:357–62. doi: 10.1034/j.1600-0463.2000.d01-68.x. [DOI] [PubMed] [Google Scholar]
- Grayston JT. Chlamydia pneumoniae, strain TWAR. Chest. 1989;95:664–9. doi: 10.1378/chest.95.3.664. [DOI] [PubMed] [Google Scholar]
- Haslam P, Baughman R, editors. Report of European Respiratory Society (ERS) Task Force: guidelines for measurement of acellular components and recommendations for standardization of bronchoalveolar lavage (BAL) Eur Respir Rev. 1999;9:25–157. doi: 10.1034/j.1399-3003.1999.14b01.x. [DOI] [PubMed] [Google Scholar]
- Jackson LA, Wang SP, Nazar-Stewart V, et al. Association of Chlamydia pneumoniae immunoglobulin A seropositivity and risk of lung cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:1263–6. [PubMed] [Google Scholar]
- Kocazeybek B. Chronic Chlamydophila pneumoniae infection in lung cancer, a risk factor: a case-control study. J Med Microbiol. 2003;52:721–6. doi: 10.1099/jmm.0.04845-0. [DOI] [PubMed] [Google Scholar]
- Koyi H, Branden E, Gnarpe J, et al. An association between chronic infection with Chlamydia pneumoniae and lung cancer A prospective 2-year study. APMIS. 2001;109:572–80. doi: 10.1034/j.1600-0463.2001.d01-177.x. [DOI] [PubMed] [Google Scholar]
- Kuo C, Campbell LA. Detection of Chlamydia pneumoniae in arterial tissues. J Infect Dis. 2000;181(Suppl 3):S432–6. doi: 10.1086/315615. [DOI] [PubMed] [Google Scholar]
- Kurashima K, Kanauchi T, Takayanagi N, et al. Serum IgG and IgA antibodies to Chlamydia pneumoniae and severity of emphysema. Respirology. 2005;10:572–8. doi: 10.1111/j.1440-1843.2005.00752.x. [DOI] [PubMed] [Google Scholar]
- Laurila AL, Anttila T, Laara E, et al. Serological evidence of an association between Chlamydia pneumoniae infection and lung cancer. Int J Cancer. 1997;74:31–4. doi: 10.1002/(sici)1097-0215(19970220)74:1<31::aid-ijc6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Linden M, Rasmussen JB, Piitulainen E, et al. Airway inflammation in smokers with nonobstructive and obstructive chronic bronchitis. Am Rev Respir Dis. 1993;148:1226–32. doi: 10.1164/ajrccm/148.5.1226. [DOI] [PubMed] [Google Scholar]
- Lofdahl JM, Cederlund K, Nathell L, et al. Bronchoalveolar lavage in COPD: fluid recovery correlates with the degree of emphysema. Eur Respir J. 2005;25:275–81. doi: 10.1183/09031936.05.00033504. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Murray CC. The global burden of disease 1990–2020. Nat Med. 1998;4:1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- Michaud CM, Murray CJ, Bloom BR. Burden of disease – implications for future research. JAMA. 2001;285:535–9. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- Muhlestein JB, Hammond EH, Carlquist JF, et al. Increased incidence of Chlamydia species within the coronary arteries of patients with symptomatic atherosclerotic versus other forms of cardiovascular disease. J Am Coll Cardiol. 1996;27:1555–61. doi: 10.1016/0735-1097(96)00055-1. [DOI] [PubMed] [Google Scholar]
- Quanjer PH. Standardized lung function testing. Report working party standardization lung function tests. European Community for Coal and Steel. Bull Europ Physiopath Respir. 1983;19:1–95. [PubMed] [Google Scholar]
- Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J. 1993;6(Suppl 16):5–40. [PubMed] [Google Scholar]
- Rupp J, Droeman D, Goldmann T, et al. Alveolar epithelial cells type II are major target cells for C. pneumoniae in chronic but not in acute respiratory infection. FEMS Immunol Med Microbiol. 2004;41:197–203. doi: 10.1016/j.femsim.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Saikku P, Leinonen M, Mattila KJ, et al. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;2:983–6. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- Saikku P, Leinonen M, Tenkanen L, et al. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann Intern Med. 1992;116:273–8. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- Smieja M, Leigh R, Petrich A, et al. Smoking, season and detection of chlamydia pneumoniae DNA in clinically stable COPD patients. BMC Infectious Dis. 2002;2:12. doi: 10.1186/1471-2334-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler N, Ewig S, Torres A, et al. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14:1015–22. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- Theegarten D, Anhem O, Hotzel H, et al. A comparative ultrastructural and molecular biological study on Chlamydia psittaci infection and alpha-I antitrypsin deficiency and non alpha-I antitrypsin deficiency emphysema versus lung tissue of patients with hamartochondroma. BMC Infectious Dis. 2004;4:38. doi: 10.1186/1471-2334-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hertzen L, Alakarppa H, Koskinen R, et al. Chlamydia pneumoniae infection in patients with chronic obstructive pulmonary disease. Epidemiol Infect. 1997;118:155–64. doi: 10.1017/s095026889600725x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hertzen L, Isoaho R, Leinonen M, et al. Chlamydia pneumoniae antibodies in chronic obstructive pulmonary disease. Int J Epidemiol. 1996;25:658–64. doi: 10.1093/ije/25.3.658. [DOI] [PubMed] [Google Scholar]
- Webley WC, Salva PS, Andrzejewski C, et al. The bronchial lavage of pediatric patients with asthma contains infectious Chlamydia. Am J Respir Crit Care Med. 2005;171:1083–8. doi: 10.1164/rccm.200407-917OC. [DOI] [PubMed] [Google Scholar]
- Wu L, Skinner SJ, Lambie N, et al. Immunohistochemical staining for Chlamydia pneumoniae is increased in lung tissue from subjects with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:1148–51. doi: 10.1164/ajrccm.162.3.9912134. [DOI] [PubMed] [Google Scholar]