Abstract
Ocular myositis represents a subgroup within the idiopathic orbital inflammatory syndrome, formerly termed orbital pseudotumor. Ocular myositis describes a rare inflammatory disorder of single or multiple extraocular eye muscles. Unilateral or sequential bilateral subacute painful diplopia is the leading symptom of eye muscle myositis. There are at least two major forms, a limited oligosymptomatic ocular myositis (LOOM) with additional conjunctival injections only, and a severe exophthalmic ocular myositis (SEOM) with additional ptosis, chemosis, and proptosis. Eye muscle myositis is an idiopathic inflammation of the extraocular muscles in the absence of thyroid disease, ocular myasthenia gravis, and other systemic, particularly autoimmune mediated diseases, resembling CD4+ T cell-mediated dermatomyositis. Contrast-enhanced orbital magnetic resonance imaging most sensitively discloses swelling, signal hyperintensity, and enhancement of isolated eye muscles. Typically, corticosteroid treatment results in prompt improvement and remission within days to weeks in most patients. Compiled data of five patients and a review of the clinical pattern, diagnostic procedures, differential diagnoses, and current treatment options are given.
Keywords: ocular myositis, idiopathic orbital inflammation, painful diplopia, enlarged extraocular muscles
Entity, clinical signs and symptoms, and forms of ocular myositis
Orbital pseudotumor, first described by Gleason in 1903 and later termed by Birch-Hirschfield in 1930, is a benign idiopathic inflammatory disease that may affect any structure in the orbit (Scott and Siatkowski 1997). Today, ocular myositis represents a subgroup within the entity of idiopathic orbital inflammatory syndrome (IOIS), formerly termed orbital pseudotumor. Ocular myositis describes a rare inflammatory disorder of single or multiple extraocular eye muscles. Primary manifestations encompass subacute orbital painful diplopia, exacerbated by eye movement. Diplopia is caused by handicapped contraction and distraction of affected eye muscles, not by neurogenic affection. In most of the patients, beyond orbital discomfort, no additional signs or symptoms are present. There are two major forms, 1) a limited oligosymptomatic ocular myositis (LOOM) with additional conjunctival injections only, and 2) a severe exophthalmic ocular myositis (SEOM) with additional ptosis, chemosis, and proptosis. Furthermore, signs and symptoms of other subgroups of IOIS, such as dacryoadentitis, periscleritis, and perineuritis may be evident (Moorman and Elston 1995; Lacey et al 1999; Jacobs and Galetta 2002; Harris 2006).
Extraocular muscles
Extraocular muscle (EOM) is significantly different from limb skeletal muscle, but it is not precisely known why extraocular muscles are preferentially affected by inflammation in IOIS. EOMs have smaller motor unit sizes, higher motor neuron discharge rates, higher blood flow, and higher mitochondrial volume fractions compared with skeletal muscle. This suggests that the energy requests and susceptibility to mitochondrial dysfunction are higher as compared with skeletal muscle (Yu Wai Man 2005; Schoser and Pongratz 2006). EOMs have a unique myofibrillar protein isoform composition reflecting their structural and functional properties (Kjellgren et al 2006). Furthermore, due to the high blood flow and vascularisation, inflammatory cells circulate more easily within this specialized body compartment (Yu Wai Man 2005; Schoser and Pongratz 2006).
Pathogenetic background of ocular myositis
Idiopathic variants
Very rarely muscle biopsies of extraocular muscle were performed, reporting mixed infiltrates of plasma cells, lymphocytes, macrophages, and polymorphonuclear cells. More chronic forms are associated with fibrosis. Thus, pathogenesis of the so called idiopathic cases are widely not investigated. Recently, Gerald Harris (2006) provided a perspective paper on idiopathic orbital inflammation. The monocyte-macrophage line in combination with B-cells and helper and effector T-cells have multiple functions. Macrophages with major histocompatibility complex molecules are able to process, present, and initiate a cellular immune response. A clonal proliferation of helper cells produces cytokines, causing effector T-cells to multiply and lyse antigen-bearing cells. Cytokines mobilize and activate macrophages in a feedback circle. Beyond lysis of cells bearing foreign antigens, they also cause tissue injury and fibrosis. Simultaneously, a B-cell proliferation is initiated with transformation into antibody-producing plasma cells. Some bacterial or viral antigens and endotoxins can directly activate macrophages or B-cells, leading to neutralizing antibodies, but can also cause tissue damage. Intracellular antigens may not be fully digested, therefore activated macrophages and T-cells proliferate, aggregate, and isolate these antigens in a granulomatous pattern. Injury or structural similarities can alter the antigenicity of tissue specific proteins, which are then recognized as foreign and lead to a secondary, autoimmune wave of inflammation (molecular mimicry) (Dalakas 2006; Harris 2006). In the context of ocular myositis, the precise components and timing of this role model of inflammation has to be analyzed in future. Some of the known findings of ocular myositis are in line with the current model of the pathogenesis of dermatomyositis as a complement-mediated microangiopathy (Dalakas 2006).
Specific variants
Some cases are associated with specific myositis either by bacterial or viral infections (eg, Lyme disease, cysticercosis, post-streptococcal, or herpes zoster), or systemic immunomediated disease such as sarcoidosis, systemic lupus erythematosus, Crohn′s disease, giant cell arteriitis, and linear scleroderma (Lacey et al 1999; Harris 2006).
Compilation of five cases
Within the past 10 years, three women and two men, aged 28 to 66 years (mean 46 years) presented with signs and symptoms of ocular myositis in our neuromuscular outpatient clinic (Table 1). All had acute to subacute onset of the disease with painful diplopia exacerbated by eye movement. Two of them presented with the combination of chemosis, ptosis, and proptosis (SEOM form; Table 1; Figure 1). Eye muscle biopsy was performed in none of the patients, but a biopsy of the deltoid muscle was taken without any pathological finding in two of the five patients (data not shown). Axial and coronal magnetic resonance imaging (MRI) of the orbits showed a multilocal edema and enlargement of different eye muscles in all patients (for example see Figure 1, Table 1). In two patients only the medial rectus muscle was affected. Two patients showed a recurrent disease course on the same side after one year, while tapering corticosteroid treatment. In the other three patients, after initiation of immunosuppressive therapy using corticosteroids (1.0 mg/kg/d) and azathioprine (up to 150 mg), within 4 weeks, a complete recovery from all symptoms was evident (Table 1).
Table 1.
Summary of five patients with ocular myositis
| Age of onset (mean, range, years) | 46 (28–66) |
| Sex | 3 female/2 male |
| More than one muscle affected | 3/5 (60%) |
| Bilateral manifestation (sequential) | 2/5 (40%) |
| Medial rectus affection | 5/5 (100%) |
| Superior rectus affection | 3/5 (60%) |
| Lateral rectus affection | 2/5 (40%) |
| Inferior rectus affection | 1/5 (20%) |
| LOOM form (3 of 5 patients) | |
| Subacute onset of retrobulbar/orbital pain | 3/3 (100%) |
| Diplopia | 3/3 (100%) |
| Conjunctival injections | 2/3 (66%) |
| Ptosis | 0/3 (0%) |
| Proptosis | 0/3 (0%) |
| SEOM form (2 of 5 patients) | |
| Subacute onset of retrobulbar/orbital pain | 2/2 (100%) |
| Diplopia | 2/2 (100%) |
| Ptosis | 1/2 (50%) |
| Chemosis | 2/2 (100%) |
| Proptosis | 2/2 (100%) |
Abbreviations: LOOM, limited oligosymptomatic ocular myositis; SEOM, severe exophthalmic ocular myositis.
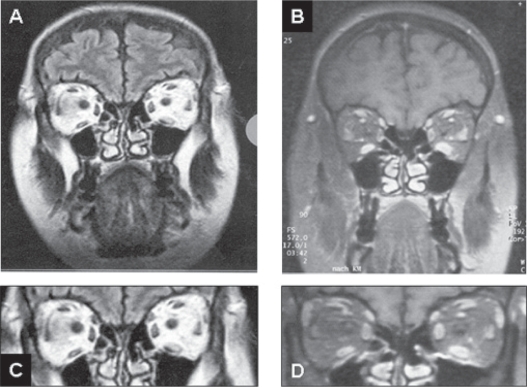
Figure 1.
Coronal MRI (T2 left (A, C); contrast-enhanced T2 right (B, D, C, and D detail from A, B respectively) view of a patient with ocular myositis. Most prominent is the edema and swelling of the left inferior rectus muscle, although other eye muscles are also involved (A–D). This 48-year-old women developed horizontal diplopia over a 5-day period, which was associated with dull retroorbital pain. She showed 5 mm of axial proptosis, chemosis, tenderness, and restricted ductions on the left side (SEOM patient). Treatment with corticosteroids was initiated, and after 10 weeks azathioprine was added, leading to complete remission of the symptoms within 7 month.
Abbreviations: MRI, magnetic resonance imaging; SEOM, severe exophthalmic ocular myositis.
Summary of data given in the literature
The frequency of major symptoms are acute to subacute orbital and/or retroorbital pain (~95%), diplopia (~85%), conjunctival injections closely related to the affected eye muscle (~70%), and proptosis (~60%) (Weinstein et al 1983; Moorman and Elston 1995; Berkhoff et al 1997; Lacey et al 1999). Recurrence can involve different muscles, and sporadically alternate the eye (~30%) (Moorman and Elston 1995; Berkhoff et al 1997; Lacey et al 1999). Overall, there seems to be a slightly predominance of the disease in females (2:1) (Moorman and Elston 1995; Berkhoff et al 1997; Lacey et al 1999). The mean age at onset is around age 40 (range 7–80 years). Imaging studies corroborate that no particular pattern of muscle involvement is found. Unilateral single muscle inflammation with tendon involvement is the most common presentation. Nevertheless, superior, lateral, and medial rectus muscles are more often affected than inferior rectus muscle (Lacey et al 1999). Multiple involvements at initial presentation seemed to be a risk factor for recurrence, particularly if bilateral. During repeated attacks, additional muscles are often involved (Lacey et al 1999; see Table 2 for summary).
Table 2.
Summary of the frequency of symptoms in ocular myositis
| General aspects | |
|---|---|
| Age of onset (years, range) | 30–40 (7–80) |
| Sex (ratio) | female > male (2:1) |
| More than one muscle affected | 50% |
| Bilateral manifestation (sequential) | 30% |
| Medial rectus affection | 70% |
| LOOM form (75% of all patients) | |
| Subacute onset of retrobulbar/orbital pain | 95% |
| Exacerbation of retrobulbar pain by eye movement | 90% |
| Diplopia | 85% |
| Conjunctival injections | 75% |
| SEOM form (25% of all patients) | |
| Ptosis | 90% |
| Chemosis | 80% |
| Proptosis | 80% |
Abbreviations: LOOM, limited oligosymptomatic ocular myositis; SEOM, severe exophthalmic ocular myositis.
Diagnostic assessment
High-resolution orbital computed tomography is not as sensitive as MRI. When available, contrast-enhanced MRI with multiple coronal views and fat saturation should be the initial diagnostic study performed (Jacobs and Galetta 2002). Eye muscle biopsy or limb muscle biopsy is usually not indicated, and response to therapy frequently confirms the diagnosis. Other additional blood analyses are recommended regarding to optional differential diagnoses.
Differential diagnoses of ocular myositis
Thyroid associated orbitopathy
Thyroid orbitopathy is thought to be an organ-specific autoimmune process and is associated with thyroid disease in 80%–90% of cases. Thyroid orbitopathy is the most common cause of orbital disease (Jacobs and Galetta 2002; Garrity and Bahn 2006). Characteristically, the onset is insidious rather than acute. Lid lag and lid retraction in down-gaze are two characteristic findings of this disorder (Jacobs and Galetta 2002; Garrity and Bahn 2006). Bilateral muscle involvement including inflammation, edema, and secondary fibrosis is very common. Imaging reveals fusifom posterior enlargement of extraocular muscles with relative sparing of the tendon (Lacey et al 1999; Jacobs and Galetta 2002; Garrity and Bahn 2006). Optic neuropathy is a serious complication of thyroid eye disease, but is relatively uncommon, with visual loss occurring in up to 5% of Graves’ orbitopathy patients. Impairment of colour vision, optic disc swelling, and radiological evidence of apical optic nerve compression are the major features of this severe complication of thyroid eye disease (McKeag et al 2007) (Table 3).
Table 3.
Differential diagnoses of ocular myositis
| OM | TED | Ocular MG | OPMD | DM1 | PEO | |
|---|---|---|---|---|---|---|
| Ptosis | + | + | +++ | +++ | ++ | ++ |
| Proptosis | + | +++ | − | − | − | − |
| Diplopia | ++ | + | +++ | −/+ | −/+ | −/+ |
| Laterality | ||||||
| - unilateral | ++ | + | ++ | + | + | + |
| - bilateral | + | ++ | + | ++ | ++ | ++ |
| Orbital pain | ++ | + | − | − | − | − |
| Chemosis | + | + | + | − | − | − |
| Conjunctival Injection | + | + | + | − | − | − |
| Systemic symptoms | −/+ | + | − | + | + | + |
| Vision impaired | − | −/+ | − | − | − | − |
| CK elevation | − | − | − | + | + | −/+ |
| Antibodies | − | + | + | − | − | − |
Notes: Semiquantitative rating of signs and symptoms: − = absent; −/+ = minimal/rare; + = mild/infrequent; ++ = moderate/frequent; +++ = severe/most frequent.
Abbreviations: CK, creatine kinase; DM1, myotonic dystrophy type 1; Ocular MG, ocular myasthenia gravis; OM, ocular myositis; OPMD, oculopharygeal muscular dystrophy; PEO, progressive external ophthalmoplegia; TED, thyroid eye disease.
Ocular myasthenia gravis
The term ocular myasthenia contrasting generalized myasthenia is to define the clinical subtype of myasthenia gravis with isolated eye muscle weakness with blepharoptosis or ophthalmoparesis, resulting in diplopia. Ocular dysfunction accounts for 50%–80% of the initial manifestation of myasthenia gravis. Asymmetric ptosis is most frequently related to acquired autoimmune myasthenia gravis. Moreover, inherited congenital myasthenic syndromes are frequently associated with ptosis and ophthalmoplegia. The eyelid ice test, edrophonium test, and nerve conduction studies with repetitive nerve stimulation of the facial nerves are supportive in making the diagnosis. Additionally, acetylcholine receptor antibodies are present in 40%–60% of the patients (Elrod and Weinberg 2004; Beeson et al 2005; Romi et al 2005) (Table 3).
Tolosa-Hunt syndrome
This syndrome of painful ophthalmoplegia consists of periorbital or hemicranial pain, combined with ipsilateral ocular motor nerve palsies, oculosympathetic paralysis, and sensory loss in the distribution of the ophthalmic and occasionally the maxillary division of the trigeminal nerve. Multiple combinations of cranial nerve palsies may occur, localizing the pathological process to the region of the cavernous sinus/superior orbital fissure. The Tolosa-Hunt syndrome is caused by an inflammatory process with granulomatous inflammation, epithelioid cells, and occasional giant cells (Kline and Hoyt 2001). Spontaneous remissions may occur; corticosteroids markedly reduce the pain and syndrome.
Oculopharyngeal muscular dystrophy
The combination of ptosis and pharyngeal weakness combined with ophthalmoparesis, dysphagia, and weakness and wasting of face, neck, proximal, and distal limb muscles characterizes the autosomal dominant, late-onset oculopharyngeal muscular dystrophy (OPMD). OPMD is caused by expansions in a 6 GCG trinucleotide repeat tract located in the first exon of the polyadenylate binding protein nuclear 1 gene on chromosome 14q (Brais 2003; Ruegg et al 2005). Genetic testing is available (Table 3).
Myotonic dystrophy type 1 (DM1)
Among the well known multisystemic features of the classic from of myotonic dystrophy (DM1) eg, muscle weakness and myotonia, the eye is affected in multiple ways. Not only the myotonic cataracts, but also retinal abnormalities, ptosis, and blepharospasm are common features of the disease. DM1 is caused by expansions in CTG trinuclotide repeat tract located in the myotonic dystrophy protein kinase gene on chromosome 19q. To exclude this disease, genetic testing is available (Harper 2001; Schara and Schoser 2006) (Table 3).
Congenital cranial dysinnervation disorders and congenital fibrosis of extraocular muscles
The congenital cranial dysinnervation disorders (CCDDs) encompass congenital, nonprogessive, sporadic, or familial abnormalities of cranial musculature that result from developmental abnormalities of, or complete absence of, one or more cranial nerves with primary or secondary muscle dysinnervation. Among CCDDs, the congenital fibrosis of the extraocular muscles (CFEOM) are rare inherited strabismus syndromes presenting with congenital, nonprogressive bilateral ophthalmoplegias with active and passive restriction of globe movement, and fibrosis of the extraocular muscles innervated by the oculomotor and/or trochlear nerves. At least three distinct syndromes are recognized: CEFOM1 is an autosomal dominant form and caused by mutations in the KIF21a gene on chromosome 12p. CFEOM2 is an autosomal recessive form and homozygous mutations in the ARIX gene on chromosome 11q are found. CFEOM3 is mapped in separate families to different loci on chromosomes 12q, 13q, and 16q (Engle 2002; Bau and Zierz 2005; Schoser and Pongratz 2006).
Mitochondrial myopathies
The most common form is late onset bilateral progressive external ophthalmoplegia (PEO). PEO onset ranges between age 11 and 82 years. PEO is characterized by ptosis and weakness of extraocular muscles leading to limitation of extraocular movements with relative sparing of downgaze, and occasionally dysconjugate ocular movements. Although transient diplopia may occur, the majority of patients rarely complain diplopia and are mostly unaware of their restrictions. Ptosis and ophthalmoplegia may occur jointly, but each can occur alone. The ptosis is often asymmetric. Up to 90% of PEO patients have additional weakness of the facial, bulbar, or limb muscles. Thus, many patients might be classified as “PEO plus” by presenting additional multisystemic symptoms such as other neurological symptoms, hearing disturbances, or diabetes. In about 15% of PEO autosomal dominant or recessive inheritance is noticed. Autosomal dominant PEO (adPEO) is characterized by accumulation of multiple deletions of mtDNA in patient’s tissue. Autosomal recessive PEO is rarely found. Clinically, no differences between hereditary and sporadic PEO can be elaborated, thus further information may be only be found in family history (DiMauro and Hirano 2005; Schoser and Pongratz 2006). The Kearns-Sayre Syndrome (KSS)/PEO Plus is characterized by onset before age 20 years and encompasses PEO, atypical pigmentary retinopathy (salt- and pepper-like appearance), and often myopathic weakness, heart block, cerebellar ataxia, and high cerebrospinal fluid (CSF) protein levels. KSS might include concomitant strabismus, mental deterioration, pyramidal signs, short stature, diabetes, or delayed sexual maturation (DiMauro and Hirano 2005; Schoser and Pongratz 2006). The mitochondrial myopathy and encephalopathy, with lactate acidosis, and stroke-like episodes (MELAS) was introduced in 1984 to designate one of the most common maternally-inherited mitochondrial syndromes. At an age of onset between 3 to 40 years, early symptoms include muscle weakness (87%), easy fatigability (15%–18%), recurrent headaches and seizures (28%). The typical clinical manifestations of MELAS include stroke-like episodes (99%–100%), seizures (85%–96%), short stature (55%–100%), muscle weakness (87%–89%), headache/vomiting (77%–92%), hearing loss (27%–75%), encephalopathy (20%–95%), optic atrophy (20%), pigmentary retinopathy (16%), and PEO (13%) (Dimauro and Hirano 2005; Schoser and Pongratz 2006). In patients with the rare sensory ataxic neuropathy with dysarthria and ophthalmoparesis (SANDO) compound heterozygosity for 2 mutations and homozygous mutations in the POLG gene were found. Lately, a heterozygous mutation in the C10ORF2 gene was found. The finding indicated that SANDO is a variant of autosomal recessive PEO (Hudson et al 2005; Schoser and Pongratz 2006). Finally, external ophthalmoplegia may also occur as a symptom in mitochondrial syndromes such as, myoclonic epilepsy, myopathy with ragged red fibers (MERRF), mitochondrial neurogastrointestinal encephalopathy (MNGIE), and neuropathy, ataxia, retinopathia pigmentosa (NARP) (Schoser and Pongratz 2006). A muscle biopsy is still needed for making the diagnosis in patients with PEO and KSS/PEO Plus. Taking a biopsy of a proximal limb muscle instead of an extraocular muscle is recommended. Southern blot analysis of muscle DNA as a first step is useful to test single or multiple deletions. In some cases with multiple mtDNA deletions and autosomal inheritance molecular analysis of POLG1, Twinkle and ANT1 are suggested (Table 3).
Current treatment options of ocular myositis
Typically, oral corticosteroid treatment (1.0 to 1.5 mg/kg/d for 1 to 2 weeks and then taper dosage to zero over 6 to 12 weeks) results in prompt improvement and remission within days to weeks in most patients. Beyond corticosteroids, other immunosuppressives are helpful in tapering corticosteroid doses in selected patients. Antimetabolites, such as azathioprine, methotrexat, mycophenolate mofetil are frequently used immunosuppressives in this condition, but T-cell inhibitors (eg, cyclosporine, tacrolimus) have also been used in a few cases (Lacey et al 1999; Franczo et al 2006; Harris 2006). Additionally, the use of tumor necrosis factor alpha blocker and other new monoclonal antibodies are anecdotally reported (Franczo et al 2006; Harris 2006). Besides, in selected cases, particularly with recurred and severe disease manifestation, high dose of intravenous immunoglobulin or rituximab (CD-20 antibody) infusion may be helpful (Lacey et al 1999; Dalakas 2006; Franczo et al 2006; Harris 2006).
Conclusions
Although ocular myositis is a rare disease, prompt recognition, diagnosis, and treatment is important. The most sensitive diagnostic tool is the axial and coronal MRI of the orbit. In addition assessment of thyroid hormones and antibodies, and acetylcholine receptor antibody is suggested. First-line treatment option is corticosteroid therapy. Without prompt relief within the next 4 weeks, addition of other immunosuppressive agents is recommended. Nevertheless, the underlying pathogenetic mechanism of idiopathic eye muscle myositis remains to be elucidated.
Acknowledgments
I thank Hanns Lochmüller for continuous excellent cooperation and helpful comments.
References
- Bau V, Zierz S. Update on chronic progressive external ophthalmoplegia. Strabismus. 2005;13:133–42. doi: 10.1080/09273970500216432. [DOI] [PubMed] [Google Scholar]
- Beeson D, Hantai D, Lochmuller H, et al. 126th International Workshop: congenital myasthenic syndromes, 24–26 September 2004, Naarden, the Netherlands. Neuromuscul Disord. 2005;15:498–512. doi: 10.1016/j.nmd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Berkhoff M, Sturzenegger M, Schroth G, et al. Ocular myositis. Nervenarzt. 1997;68:792–800. doi: 10.1007/s001150050196. [DOI] [PubMed] [Google Scholar]
- Birch-Hirschfeld A. [Handbuch der Gesamten Augenheilkunde] Vol. 9. Berlin: Julius Springer; 1930. p. 251. [Google Scholar]
- Brais B. Oculopharyngeal muscular dystrophy: a late-onset polyalanine disease. Cytogenet Genome Res. 2003;100:252–60. doi: 10.1159/000072861. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Therapeutic targets in patients with inflammatory myopathies: present approaches and a look to the future. Neuromuscl Disord. 2006;16:223–36. doi: 10.1016/j.nmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Hirano M. Mitochondrial encephalomyopathies: an update. Neuromuscl Disord. 2005;15:276–86. doi: 10.1016/j.nmd.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Engle EC. Applications of molecular genetics to the understanding of congenital ocular motility disorders. Ann NY Acad Sci. 2002;956:55–63. doi: 10.1111/j.1749-6632.2002.tb02808.x. [DOI] [PubMed] [Google Scholar]
- Gleason J. Idiopathic myositis involving the intraocular muscles. Ophthalmol Rec. 1903;12:471–8. [Google Scholar]
- Harper PS. Myotonic Dystrophy. 3rd Ed. London: WB Saunders; 2001. [Google Scholar]
- Elrod RD, Weinberg DA. Ocular myasthenia gravis. Ophthalmol Clin North Am. 2004;17:275–309. doi: 10.1016/j.ohc.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Franzco LL, Suhler EB, Smith JR. Biologic therapies for inflammatory eye disease. Clin Experiment Ophthalmol. 2006;34:365–74. doi: 10.1111/j.1442-9071.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- Garrity JA, Bahn RS. Pathogenesis of Graves ophthalmopathy: implications for prediction, prevention, and treatment. Am J Ophthalmol. 2006;142:147–53. doi: 10.1016/j.ajo.2006.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GJ. Idiopathic orbital inflammation: a pathogenetic construct and treatment strategy. Ophthal Plast Reconstr Surg. 2006;22:79–86. doi: 10.1097/01.iop.0000203734.52333.93. [DOI] [PubMed] [Google Scholar]
- Hudson G, Deschauer M, Busse K, et al. Sensory ataxic neuropathy due to a novel C10ORF2 mutation with probable germline mosaicism. Neurology. 2005;64:371–3. doi: 10.1212/01.WNL.0000149767.51152.83. [DOI] [PubMed] [Google Scholar]
- Jacobs D, Galetta S. Diagnosis and management of orbital pseudotumor. Curr Opin Ophthalmol. 2002;12:347–51. doi: 10.1097/00055735-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Kjellgren D, Stal P, Larsson L, et al. Uncoordinated expression of myosin heavy chains and myosin-binding protein C isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci. 2006;47:4188–93. doi: 10.1167/iovs.05-1496. [DOI] [PubMed] [Google Scholar]
- Kline LB, Hoyt WF. The Tolosa-Hunt syndrome. J Neurol Neurosury Psychiatry. 2001;71:577–82. doi: 10.1136/jnnp.71.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey B, Chang W, Rootman J. Nonthyroid causes of extraocular muscle disease. Surv Ophthalmol. 1999;44:187–213. doi: 10.1016/s0039-6257(99)00101-0. [DOI] [PubMed] [Google Scholar]
- McKeag D, Lane CM, Lazarus JH, et al. Clinical features of dysthyroid optic neuropathy: a European group on Graves' Orbitopathy (EUGOGO) survey. Br J Ophthalmol. 2007;91:455–8. doi: 10.1136/bjo.2006.094607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman CM, Elston JS. Acute orbital myositis. Eye. 1995;9:96–101. doi: 10.1038/eye.1995.15. [DOI] [PubMed] [Google Scholar]
- Romi F, Gilhus NE, Aarli JA. Myasthenia gravis: clinical, immunological, and therapeutic advances. Acta Neurol Scand. 2005;111:134–41. doi: 10.1111/j.1600-0404.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- Ruegg S, Hagen ML, Hohl U, et al. Oculopharyngeal muscular dystrophy – an under-diagnosed disorder? Swiss Med Wkly. 2005;135:574–86. doi: 10.4414/smw.2005.11221. [DOI] [PubMed] [Google Scholar]
- Schara U, Schoser BGH. Myotonic dystrophies type 1 and -2: a summary on current aspects. Semi Pediat Neurol. 2006;13:71–79. doi: 10.1016/j.spen.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Schoser BGH, Pongratz D. Extraocular mitochondrial myopathies and their differential diagnoses. Strabismus. 2006;14:107–13. doi: 10.1080/09273970600701218. [DOI] [PubMed] [Google Scholar]
- Scott IU, Siatkowski RM. Idiopathic orbital myositis. Curr Opin Rheumatol. 1997;9:504–12. doi: 10.1097/00002281-199711000-00005. [DOI] [PubMed] [Google Scholar]
- Weinstein GS, Dresner SC, Slamovits TL, Kennerdell JS. Acute and subacute orbital myositis. Am J Ophthalmol. 1983;96:214–17. doi: 10.1016/s0002-9394(14)77789-x. [DOI] [PubMed] [Google Scholar]
- Yu Wai Man CY, Chinnery PF, Griffiths PG. Extraocular muscles have fundamentally distinct properties that make them selectively vulnerable to certain disorders. Neuromuscul Disord. 2005;15:17–23. doi: 10.1016/j.nmd.2004.10.002. [DOI] [PubMed] [Google Scholar]