Abstract
Conjunctivitis, or inflammation of the conjunctiva, refers to a diverse group of ocular surface diseases of viral or bacterial origin that primarily affect the conjunctiva. In developed countries, the most common causative bacterial pathogens are Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pneumoniae. Most varieties of conjunctivitis are self-limiting; however, some cases can be extremely contagious or cause serious complications if left unchecked. New ocular antibiotics are needed to keep pace with the increasing incidence of bacterial resistance and provide options that decrease the overall treatment burden and encourage patient compliance. Azithromycin is a well known systemic anti-infective with broad spectrum activity against gram positive-, gram negative-, and atypical bacteria species. Ocular use has been limited because its solubility and stability profiles in aqueous media were not favorable for delivery to the eye. An eyedrop of 1% azithromycin in DuraSite® (AzaSite™, InSite Vision, Alameda, CA, USA), a bioadhesive ocular drug delivery system, was recently developed and evaluated in clinical trials. This formulation is well tolerated, delivers a high concentration of azithromycin to the conjunctiva, has a broader eradication profile than aqueous azithromycin, and can be effectively dosed with 7 drops, a 65% reduction in the amount of drops required by the most popular antibiotics currently used for conjunctivitis.
Keywords: anti-infective, ocular surface, conjunctiva, clinical trial, drug-delivery, azalide
Introduction
The main etiologic agents in bacterial conjunctivitis are Haemophilus influenzae, Staphylococcus aureus, or Steptococcus pnuemoniae. These infections are often self limiting. Antibiotics are used with infectious bacterial conjunctivitis to speed recovery, reduce complications, and prevent reinfection. Physicians need choices to overcome the limitations of frequent dosing, compliance, and bacterial resistance with current antibiotic regimens. This review provides an update on the clinical development of 1% azithromycin formulated with DuraSite® (Insite Vision, Alameda, CA, USA), an innovative ocular delivery system that was used to deliver high bactericidal concentrations of azithromycin to the eye to treat bacterial conjunctivitis.
Unmet patient needs in ocular anti-infective therapy
The choice of antibiotic therapy for bacterial conjunctivitis is usually empirical. New ocular antibiotics are needed to keep pace with the increasing incidence of bacterial resistance and to provide patients with a regimen that decreases their overall treatment burden and improves the chances for adherence to the treatment.
Standard topical vehicles such as eyedrops, ointments, and gels are all highly effective for treating ocular surface infections, although eyedrops may be more practical than ointments for daytime use as ointments may interfere with vision. An ideal topical treatment would have the convenience of a drop but the long-term duration of an ointment.
Antibiotic eyedrops are often used to treat acute infectious diseases and speed recovery, reduce sequelae, and prevent the spread of pathogens through the community. In addition, prophylaxis with topical antibiotics is a prudent and necessary step to reduce the risk of infection and improve outcomes following ophthalmic surgery or ocular trauma (Batequet 2001; De Kaspar 2004). Powerful medications such as 0.5% moxifloxacin (Vigamox®; Alcon, Inc, Fort Worth, TX, USA) and 0.3% gatifloxacin (Zymar®; Allergan, Inc, Irvine, CA, USA) increase the number of potential treatment choices available to clinicians. However, in order to sustain critical bactericidal concentrations of these drops in the eye, dosing requirements range from 3 to 8 drops a day (Table 1) (Allergan Inc. 2004; Alcon Laboratories 2005). For some patients this is a treatment burden that makes compliance difficult.
Table 1.
Topical eyedrop regimens used to treat ocular infection
| Drug class | Eyedrop | Dosing regimena (frequency of administration) |
|---|---|---|
| Fluoroquinolone | 0.5% moxifloxacin | tid, 7 days |
| 0.3% gatifloxacin | q2h days 1 and 2b, d qid days 3–7 | |
| 0.5% levofloxacin | q2h days 1 and 2b, d q4h days 3–7 | |
| 0.3% ofloxacin | q2–4h days 1 and 2b, d q4h days 3–7 | |
| 0.3% ciprofloxacin | q4h 7–14 daysd | |
| Azalide | 1.0% azithromycin in DuraSite | bid days 1 and 2 qd days 3–7 |
| Aminoglycoside | 0.3% tobramycin | qid days 1–7 |
| 0.3% gentamicin | q4h, 7–10 days | |
| Lipopeptide/dihydrofolate reductase inhibitor | 0.1% Trimethoprim sulfate/Polymyxin B sulfate | q3h, 7–10 daysc |
| Polymyxin B/neomycin/gramicidin | q3h, 7–10 days | |
| Translation elongation inhibitors | 0.5% chloramphenicol | q2h days 1 and 2d q4h days 3–5 |
| 1% fusidic acid | bid days 1–7 | |
| Para-aminobenzoic antagonists | 10%–20% sodium sulfacetamide | q2h to qid days 1–7d |
| 4.0% sulfisoxazole diolamine | qid days 1–7 |
All dosing information from full prescribing information.
Not to exceed 8 doses per day.
Not to exceed 6 doses per day.
During waking hours.
Abbreviations: qd, daily; bid, twice a day; tid, 3 times a day; q2h, every 2 hours; q3h, every 3 hours; q4h, every 4 hours; qid, 4 times a day.
Bacterial coverage is an additional concern and unmet need in ophthalmology. Depending on the patient’s history, ophthalmologists choose from an array of old and new antibiotics for the management of ocular surface infection. Newer broad-spectrum antibiotics may be more desirable than some of the older, narrower-spectrum drugs. However, even with the improved activity of newer generation fluoroquinolones, surveillance studies and regulatory agencies have reported changing trends in the susceptibility of infecting organisms to fluoroquinolones (Goldstein et al 1999; Venezia et al 2001; Ambrose et al 2004). Documentation of methicillin resistance has also shown an increase in frequency (Shanmuganathan et al 2005). Indiscriminate and incorrect ophthalmic usage of these powerful broad-spectrum anti-infectives can promote the development of resistance. There is still a great need in ophthalmology for broad-spectrum antibiotics that can fill the coverage gap created by fluoroquinolone-resistant bacteria.
Properties of azithromycin
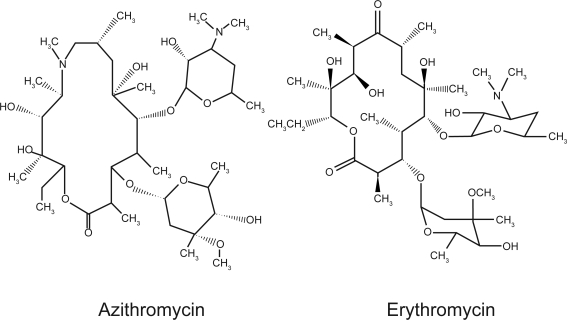
Azithromycin is a semi-synthetic antibiotic that has been successfully used in oral and intravenous dosage forms to treat gram positive-, gram negative-, and atypical infections of the skin and upper respiratory tract (Figure 1). Azithromycin is derived from erythromycin and has been available under the trade names Zithromax® in the United States, Western Europe, and Japan (Pfizer, Inc., New York, NY, USA) and Sumamed® in Eastern Europe (Pliva, Sarajevo, Bosnia and Herzegovina). Compared with the narrower-spectrum macrolide erythromycin, azithromycin, an azalide, is characterized by improved oral bioavailability, increased tissue penetration and persistence, and a longer elimination half-life (Piscitelli et al 1992; Girard et al 1993). The pharmacokinetic properties of azithromycin enable short and simple daily dosage regimens that can contribute to greater patient compliance (Pfizer, Inc. 2004, 2005).
Figure 1.
Azithromycin, an azalide, is derived from erythromycin but differs chemically in that a methyl substituted nitrogen atom is incorporated into the lactone ring, which improves the stability of azithromycin relative to erythromycin.
In vitro studies indicate that azithromycin is highly concentrated within polymorphonuclear leucocytes, which gravitate by chemotactic mechanisms to sites of infection. The high intracellular concentration of azithromycin in inflammatory fluids of abscesses supports the pharmacodynamic properties, which include concentration-dependent killing of bacteria and prolonged persistent effects in tissues (Amsden 2001; Pfizer, Inc. 2004).
The primary antibacterial effect of azithromycin differs from that of fluoroquinolones and involves inhibition of protein synthesis and binding of the 50S ribosomal subunit (Champney and Miller 2002; Mabe et al 2004). Compared with older antibiotics, both azithromycin and the fluoroquinolones gatifloxacin and moxifloxacin are effective against gram negative-, gram positive-, and atypical bacteria (Allergan Inc. 2004; Pfizer, Inc. 2004; Alcon Laboratories 2005). Both drug classes have a well known efficacy profile, but systemic use of fluoroquinolones is not recommended as a first-line anti-infective in young children (Committee on Infectious Diseases 2006).
A stable, sustained-release ophthalmic delivery system for azithromycin
Until recently, ophthalmic uses of azithromycin were limited to oral dosing for the treatment of Chlamydia trachomatis infections. The safety of three-dose (once per week for 3 weeks) and single-dose regimens has been demonstrated in clinical trials (West 1999). In comparison trials, a single 1-g dose of azithromycin was just as effective as a standard 10-day treatment with doxycycline (100 mg twice daily) in the treatment of adult inclusion conjunctivitis (Katusic et al 2003).
Comparative corneal penetration studies in animals indicate that aqueous formulations of azithromycin created by reconstituting the intravenous dry form of the drug achieved higher tissue penetration than the related azalide clarithromycin (Kuehne et al 2004). Reconstituted azithromycin, although effective and capable of penetrating the cornea, is not suitable as a commercial eyedrop because it lacks long-term chemical stability and its sterility is not preserved in the bottle (Pfizer, Inc. 2003). These hurdles have recently been overcome.
DuraSite® is a drug delivery system that solubilizes azithromycin in a sterile aqueous eyedrop. It is a lightly cross-linked bioadhesive polymer of polyacrylic acid suitable for medical applications that require topical delivery and sustained release. The molecular weight of the polymer exceeds 1 × 106 Da. The topical formulation of 1% azithromycin in DuraSite (AzaSite™; InSite Vision) is a gel-forming solution that entraps water and the active molecules in a bioadhesive matrix. Azithromycin is then held within the matrix by continued physical entrapment and reversible ionic interaction. In eyedrop formulations with DuraSite, drug delivery can be sustained, with release from the matrix taking place over a period of up to 6 hours (Keller et al 1993; Harper et al 1995).
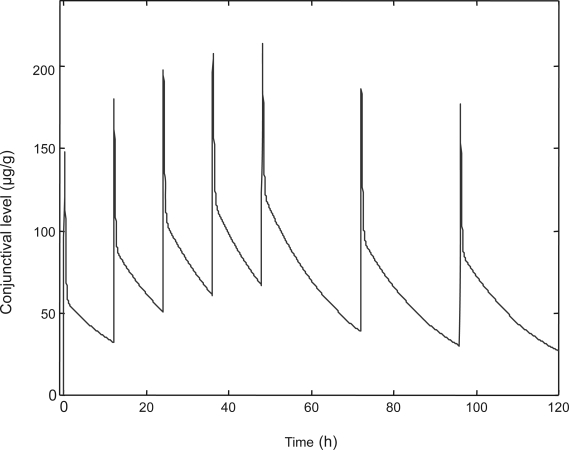
Azithromycin in DuraSite is preserved with benzalkonium chloride. The formulation is sterile and stable for 9–12 months at room temperature. Initial pharmacokinetic modeling studies of the conjunctival concentrations achieved with a regimen of once-a-day dosing for 5 days indicated that peak concentrations of 150–200 μg/g and trough concentrations of 40 μg/g could be sustained during a 24-hour period (Figure 2). These concentrations obtained from once-a-day dosing are several fold higher than the minimum inhibitory concentrations (MICs) needed to fight most ocular surface infections. This is of clinical value for patients who are poorly compliant with multi-dose eyedrop regimens. Even with these trough concentrations, a therapeutic level of azithromycin can be maintained throughout the night with once-a-day dosing.
Figure 2.
Modeled bioavailability of azithromycin from 1% azithromycin in DuraSite.
Clinical trials of 1% azithromycin in DuraSite
Evaluation of the safety and efficacy of 1% azithromycin in DuraSite was recently completed in a controlled phase 3 clinical trial for bacterial conjunctivitis. Patients aged 1–96 years were enrolled in the trial. Clinical and bacteriologic confirmation of infection was obtained for the worst affected eye at study entry and exit. In most cases, only one eye was treated, but the fellow eye could be treated if the infection spread. Two masked trials were run simultaneously in which the efficacy and safety of a 5-day regimen of 1% azithromycin in DuraSite was compared with a 5-day regimen of 0.3% tobramycin eyedrops or vehicle, dosed 4 times per day.
Subjects in both treatment groups received 20 drops of masked study medication. Those who completed the azithromycin arm of the trial received a combination of active drug and vehicle-only drops. A loading dose of the active azithromycin eyedrop was administered twice a day on days 1 and 2 and once a day on days 3 through 5. Clinical resolution was defined as the absence of clinical signs and symptoms of bacterial conjunctivitis, specifically conjunctival discharge, bulbar conjunctival injection, and palpebral conjunctival injection at study exit (visit 3, day 6). At the test-of-cure visit on day 6, the efficacy data demonstrated that the clinical resolution rates of 1% azithromycin in DuraSite were superior to vehicle (63.1% vs 49.7%; p = 0.030 by Fisher exact test) and equivalent to 0.3% tobramycin (79.9% vs 78.3%; p = 0.783). The most frequently observed ocular adverse events seen in 1% to 3% of the overall study population were eye irritation, conjunctival hyperemia, and worsening conjunctivitis usually seen as transfer of infection to the fellow eye during treatment (Table 2) (Abelson et al 2006a, b). Upon analysis of tolerability, azithromycin in DuraSite was found to be just as comfortable as tobramycin or vehicle.
Table 2.
Ocular adverse events in phase 3 clinical trials
| Active controlled | Vehicle controlled | |||
|---|---|---|---|---|
| 1% azithromycin in DuraSite (n = 365) | 0.3% tobramycin (n = 378) | 1% azithromycin in DuraSite (n = 333) | Vehicle (n = 350) | |
| Eye irritation | 7 (1.9%) | 4 (1.1%) | 5 (1.5%) | 1 (0.3%) |
| Conjunctival hyperemia | 4 (1.1%) | 4 (1.1%) | 1 (0.3%) | 0 (0%) |
| Worsening bacterial conjunctivitis | 4 (1.1%) | 8 (2.1%) | 5 (1.5%) | 3 (0.9%) |
Bacterial eradication was demonstrated as the absence of suprathreshold levels of new pathogens in cultures taken at study exit. Suprathreshold levels of bacteria were defined as levels that could be detected in cultures. The results of the exit visit cultures were compared with those obtained at the baseline visit. Bacterial eradication with 1% azithromycin in DuraSite was just as robust as with 0.3% tobramycin (88.1% vs 94.3%, p = 0.073). The most frequently observed bacteria upon study entry were S. aureus, S. pneumoniae, and H. influenzae. The minimum inhibitory concentrations against these bacteria ranged from 0.12 to >1024 μg/mL (Table 3). Overall, the clinical resolution rate for these three most common bacteria was 82%.
Table 3.
Clinical and microbiological outcomes
| Species | MIC μg/uL (range) | N(total) | Bacterial eradication | Clinical resolution |
|---|---|---|---|---|
| Gram (+) strains | ||||
| Aerococcus viridans | 4 | 1 | 100% | 100% |
| CDC coryneform group G | 0.008 – >1024 | 3 | 100% | 100% |
| Enterococcus faecalis | 8 | 1 | 100% | 100% |
| Staphylococcus aureus | 1 – >1024 | 17 | 82.4% | 70.6% |
| Staphylococcus capitis | 1 | 1 | 100% | 100% |
| Staphylococcus epidermidis | 0.5–256 | 4 | 75% | 100% |
| Staphylococcus simulans | 1 | 1 | 100% | 100% |
| Streptococcus mitis | 0.06 | 4 | 100% | 100% |
| Streptococcus mitis group | 0.06–4 | 3 | 100% | 100% |
| Streptococcus oralis | 0.12–8 | 3 | 100% | 100% |
| Streptococcus pnuemoniae | 0.12 – >1024 | 55 | 87.3% | 85.5% |
| Streptococcus salivarius | 8 | 1 | 100% | 100% |
| Viridans streptococcus | 8 | 1 | 100% | 100% |
| Gram (–) strains | ||||
| Enterobacter cloacae | 64 | 1 | 100% | 0% |
| Haemophilus influenzae | 0.5–4 | 57 | 93% | 89.5% |
| Klebsiella pneumoniae | 16 | 1 | 100% | 0 |
| Moraxella catarrhalis | 0.06 | 1 | 100% | 100% |
Notes: Minimum inhibitory concentration (MIC) values were evaluated at the baseline visit on day 1. Bacterial eradication and clinical resolution were evaluated in the per protocol population at the point of cure visit on day 6.
More importantly, the data showed that the antibacterial spectrum of azithromycin in DuraSite included several species that were resistant to azithromycin. Resistance was defined by the systemic breakpoints as assessed by the Clinical Laboratory and Standards Institute (CLSI). Table 4 lists clinical isolates that were resistant when grown in cultures to 1% azithromycin as determined by the MIC but susceptible to 1% azithromycin in DuraSite as evaluated by the absence of ocular pathogens at the end of the study. The bacteria were isolated from study participants and evaluated for susceptibility to azithromycin at visit 1 (day 1). At the point-of-cure visit (day 6), the data showed that 1% azithromycin in DuraSite eradicated 21 of 29 resistant bacterial species (72.4%). Both gram positive and gram negative species were eradicated. This result suggests that the CLSI systemic breakpoints may not be a fair representation of antibiotic activity with topical dosing and that the antibiotic effect of azithromycin may have been enhanced by the formulation of the azalide in DuraSite.
Table 4.
Eradication of azithromycin-resistant organisms by 1% azithromycin in DuraSite
| Azithromycin-resistant organisms killed, % (n/N) | |
|---|---|
| Resistant organism | |
| Staphylococcus aureus | 50% (2/4) |
| Staphylococcus epidermidis | 100% (2/2) |
| Staphylococcus simulans | NAa |
| Streptococcus mitis | 100% (3/3) |
| Streptococcus mitis group | 100% (1/1) |
| Streptococcus oralis | 100% (2/2) |
| Streptococcus pneumoniae | 60% (9/15) |
| Streptococcus salivarius | 100% (1/1) |
| Streptococcus viridans | 100% (1/1) |
| Total | 72.4% (21/29) |
Organisms without minimum inhibitory concentration result interpretation or resistant organism not available.
Infection in children: special issues
In children, the most common cause of conjunctivitis is bacterial infection. A 1993 review of cultured pathogenic organisms in children found that 80% of conjunctivitis cases were bacterial infections, 13% were viral, 2% were allergic, and 5% had unidentifiable causes (Gigliotti et al 1981; Weiss et al 1993). According to a review by Lichtenstein and colleagues the most common pathogens that cause bacterial conjunctivitis in children are H. influenzae, S. pneumoniae, and Moraxella catarrhalis (Lichtenstein 2003).
In the US, and less often in the UK, children with bacterial conjunctivitis may be excluded from the school setting until adequate intervention has been used to prevent contagion (American Academy of Pediatrics 2003; Health Protection Agency 2006). Sometimes adequate intervention is problematic with currently available prescription therapies that call for 3–8 doses per day because doses may be skipped or taken improperly. Currently in the US, fluoroquinolone, aminoglycoside, or the combination of polymyxin B sulfate and trimethoprim sulfate in an ophthalmic solution are the most widely prescribed ocular anti-infectives for bacterial conjunctivitis in children (Lichtenstein 2003; Schuman 2003). Although similar data on the use of ocular solutions in Canada are not currently available, the most frequently dispensed antimicrobials by retail pharmacies in Canada are extended-spectrum penicillins (25%), macrolides (20%), tetracyclines (14%), fluoroquinolones (12%), and second-generation cephalosporins (5%) (Public Health Agency of Canada 2004). In the United Kingdom, a 5-day regimen of chloramphenicol ointment or drops (1 drop every 2 hours on days 1 and 2, and 1 drop every 4 hours on days 3 through 5) is available to treat ocular surface infections. An over-the-counter formulation of chloramphenicol was recently made available for the treatment of bacterial conjunctivitis. However, nursing studies have challenged its effectiveness and safety in children (Harkless 2006). Additionally, results from a controlled clinical trial in the UK indicated that chloramphenicol was no more effective than placebo (Everitt 2006).
Alternative narrow-spectrum approaches involving preparations of fusidic acid that can be dosed twice daily for 5–10 days are also being investigated for bacterial conjunctivitis. Bacteriologic clearance rates of 80% have been reported in children of age 2–9 years. The formulations are viscous so the drug can stay in contact with the conjunctiva longer than traditional drops (Jackson et al 2002; Doughty and Dutton 2006). When all options have been considered, the dosing regimen for 1% azithromycin in DuraSite appears more favorable than the currently available choices in the UK and US. In the clinical study, a regimen of 7 drops of 1% azithromycin in DuraSite (2 drops on days 1 and 2 and 1 drop on days 3 through 5) was found to be equally effective as 20 drops of tobramycin (65% more drops). Upon approval of 1% azithromycin in DuraSite by the Food and Drug Administration, the duration of dosing will most likely be extended to 7 days as it has been with all recently approved ocular anti-infectives. In either case, this dosing regimen lowers the treatment burden on parents and caregivers and increases the probability of real-world compliance and successful treatment outcomes. Azithromycin in DuraSite fills an unmet need in ophthalmology.
Usefulness in ophthalmic practice
Azithromycin provides excellent coverage of the pathogens that are the most common cause of bacterial conjunctivitis in adults, S. aureus, H. influenzae, and S. pneumoniae (Morrow and Abbott 1998), and its combination with the bioadhesive properties of DuraSite may enhance its antibiotic effect against microbes that were commonly considered resistant to azithromycin.
For example, although other bacteria may be involved, Staphylococcus species are prevalent in many patients with chronic bacterial conjunctivitis. This type of conjunctivitis often develops in association with blepharitis, a common but often unrecognized inflammatory condition related to bacterial colonization of the eyelid margins. In adults, blepharitis often is associated with systemic diseases such as rosacea.
The work-up of patients with chronic conjunctivitis and blepharoconjunctivitis involves culturing the conjunctiva and the eyelid margins to identify the predominant bacterial pathogen. Treatment includes the establishment of good eyelid hygiene, using warm compresses and eyelid margin scrubs and the application of appropriate topical antimicrobials (eg, erythromycin or sulfacetamide ointment) for 1–2 weeks (Morrow and Abbott 1998; American Academy of Ophthalmology 2003).
Recurrence of blepharitis in adults is high (American Academy of Ophthalmology 2003). Twenty-four-hour coverage with ointments may be difficult to achieve since their use can cause blurriness, thereby making daytime application inconvenient for patients.
The gel-forming eyedrop of azithromycin in DuraSite drop provides the desired long-term residence time in the eye and in clinical trials. In the trial, 1% azithromycin in DuraSite eliminated 82% of S. aureus, including some species that are considered resistant to azithromycin. Formulations of sustained-acting drugs in DuraSite have been evaluated before. In these cases as well as with azithromycin, blurriness from the formulation was not a major safety finding (Harper et al 1995). Patients with blepharoconjunctivitis could benefit from the antibiotic coverage of ocular azithromycin in DuraSite.
Conclusions
There are several compelling reasons why 1% azithromycin in DuraSite will be a welcome addition to the class of ophthalmic antibiotics; it offers a simplified dosing regimen that is convenient and effective, the potential for increased compliance and successful therapeutic intervention because therapy can be completed with 7 drops administered over 5 days, and high bactericidal levels that can be sustained in the eye overnight, and the potential for reduction in the development of resistance by effectively killing sensitive organisms.
References
- Abelson M, Protzko EE, Shapiro AM, et al. 2006aA randomized trial assessing microbial eradication and clinical efficacy of 1.0% azithromycin ophthalmic solution vs. tobramycin in adult subjects with bacterial conjunctivitis [abstract] Invest Ophthalmol Vis Sci 47E-Abstract 3589 [PMC free article] [PubMed] [Google Scholar]
- Abelson MB, Shapiro AM, Heller W, et al. 2006bEfficacy of azithromycin 1% eye drops vs vehicle as first-line therapy for bacterial conjunctivitis [abstract] Presented at annual meeting of the American Academy of OphthalmologyNovember 10–14, 2006Las Vegas, NVAbstract PO074 [Google Scholar]
- Alcon Laboratories . Fort Worth, TX: Alcon Laboratories; 2005. Vigamox (moxifloxacin hydrochloride ophthalmic solution) 0.5% as base [prescribing information] [Google Scholar]
- Allergan Inc . Irvine, CA: Allergan Inc; 2004. Zymar (gatifloxacin ophthalmic solution) 0.3% [prescribing information] [Google Scholar]
- Ambrose PG, Blast D, Doern GV, et al. Fluoroquinolone-resistant Streptococcus pnuemoniae, an emerging but unrecognized public health concern: Is it time to resight the goal posts. Clin Infect Dis. 2004;39:1554–6. doi: 10.1086/425508. [DOI] [PubMed] [Google Scholar]
- American Academy of Ophthalmology 2003Blepharitis. Preferred Practice Pattern [online]Accessed January 3, 2007. URL: http://www.aao.org/aao/education/library/ppp/upload/Blepharitis.pdf
- American Academy of Pediatrics . School Health. In: Pickering LK, editor. Book: 2003 Report of the Committee on Infectious Diseases. 26th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2003. p. 142. [Google Scholar]
- Amsden GW. Advanced-generation macrolides: tissue directed antibiotics. Int J Antimicrob Agents. 2001;18(Suppl 1):S11–S5. doi: 10.1016/s0924-8579(01)00410-1. [DOI] [PubMed] [Google Scholar]
- Batequet IS, Jabbur NS, Barron Y, et al. Perioperative microbiologic profile of the conjunctiva in photorefractive keratectomy. J Refract Surg. 2001;1:55–62. doi: 10.3928/1081-597X-20010101-07. [DOI] [PubMed] [Google Scholar]
- Champney WS, Miller M. Inhibition of 50S ribosomal subunit assembly in Haemophilus influenzae cells by azithromycin and erythromycin. Curr Microbiol. 2002;44:418–24. doi: 10.1007/s00284-001-0016-6. [DOI] [PubMed] [Google Scholar]
- Committee on Infectious Diseases The use of systemic fluoroquinolones. Pediatrics. 2006;118:1287–92. doi: 10.1542/peds.2006-1722. [DOI] [PubMed] [Google Scholar]
- De Kaspar HM, Chang RT, Shriver EM, et al. Three-day application of topical ofloxacin reduces the contamination rate of microsurgical knives in cataract surgery: a prospective randomized study. Ophthalmology. 2004;111:1352–5. doi: 10.1016/j.ophtha.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Doughty MJ, Dutton GN. Fusidic acid viscous eyedrops – an evaluation of pharmacodynamics, pharmacokinetics, and clinical use for UK optometrists. Ophthalmic Physiol Opt. 2006;26:343–61. doi: 10.1111/j.1475-1313.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- Everitt HA, Little PS, Smith PW. A randomized controlled trial of management strategies for acute infective conjunctivitis in general practice. BMJ. 2006;333:321. doi: 10.1136/bmj.38891.551088.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliotti F, Williams WT, Hauden FG, et al. Aetiology of acute conjunctivitis in children. J Pediatr. 1981;98:531–6. doi: 10.1016/s0022-3476(81)80754-8. [DOI] [PubMed] [Google Scholar]
- Girard D, Bergeron JM, Milisen WB, et al. Comparison of azithromycin, roxithromycin, and cephalexin penetration kinetics in early and mature abscesses. J Antimicrob Chemother. 1993;31(Suppl E):17–28. doi: 10.1093/jac/31.suppl_e.17. [DOI] [PubMed] [Google Scholar]
- Goldstein MH, Kowlaski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis. Ophthalmology. 1999;106:1313–8. [PubMed] [Google Scholar]
- Harkless GE. Topical chloramphenicol was not effective for acute infective conjunctivitis in children. Evid Based Nurs. 2006;9:12. doi: 10.1136/ebn.9.1.12. [DOI] [PubMed] [Google Scholar]
- Harper DG, Chen CE, Friedlaender MH. Controlled comparison of two fluorometholone formulations in the antigen challenge model of allergic conjunctivitis. CLAO J. 1995;21:256–60. [PubMed] [Google Scholar]
- Health Protection Agency, Essex Health Protection Unit 2006Factsheet on Conjunctivitis [online]Accessed December 28, 2006. URL: http://www.hpa.org.uk/essex/factsheets/CJCtitis.pdf
- Jackson WB, Low DE, Dattani D, et al. Treatment of bacterial conjunctivitis: 1% fusidic acid viscous drops vs. 0.3% tobramycin. Can J Ophthalmol. 2002;37:228–37. doi: 10.1016/s0008-4182(02)80114-4. [DOI] [PubMed] [Google Scholar]
- Katusic D, Petricek I, Mandic Z, et al. Azithromycin vs. doxycycline in the treatment of inclusion conjunctivitis. Am J Ophthalmol. 2003;135:447–51. doi: 10.1016/s0002-9394(02)02094-9. [DOI] [PubMed] [Google Scholar]
- Keller N, Tsao S-W, Chandrasakaran K, et al. Ocular kinetic of extended duration fluorometholone (0.1%) formulations vs. ointment and aqueous suspension. Presented at Association for Research in Vision and Ophthalmology; May 2–7, 1993; Sarasota, Fla. 1993. [Google Scholar]
- Kuehne JJ, Yu AL, Holland GN, et al. Corneal pharmacokinetics of topically applied azithromycin and clarithromycin. Am J Ophthalmol. 2004;138:547–53. doi: 10.1016/j.ajo.2004.04.071. [DOI] [PubMed] [Google Scholar]
- Lichtenstein SJ.2003Treatment options for pediatric conjunctivitis. In Ocular Infections in Children: New Developments in Fluoroquinolones Infectious Diseases in Children[online]. Accessed December 31, 2006. URL: http://idinchildren.com/monograph/0303/frameset.asp?article=treatment.asp
- Mabe S, Eller J, Champney WS. Structure-activity relationships for three macrolide antibiotics in Haemophilus influenzae. Curr Microbiol. 2004;49:248–54. doi: 10.1007/s00284-004-4312-9. [DOI] [PubMed] [Google Scholar]
- Morrow GL, Abbott RL. Conjunctivitis. Am Fam Physician. 1998;57:735–46. [PubMed] [Google Scholar]
- Piscitelli SC, Danzinger LH, Rodvold KA. Clarithromycin and azithromycin: new macrolide antibiotics. Clin Pharm. 1992;11:137–52. [PubMed] [Google Scholar]
- Pfizer, Inc . New York, NY: Pfizer, Inc; 2003. Zithromax [package insert 70-5179-00-8] (azithromycin for injection) [Google Scholar]
- Pfizer, Inc . New York, NY: Pfizer, Inc; 2004. Zithromax [package insert 70-5179-00-4] (azithromycin tablets) and (azithromycin for oral suspension) [Google Scholar]
- Pfizer, Inc . New York, NY: Pfizer Inc; 2005. Zmax (azithromycin extended release) for oral suspension [prescribing information] [Google Scholar]
- Public Heath Agency of Canada Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2004 full report [online]Accessed January 5, 2007. URL: http://www.phac-aspc.gc.ca/cipars-picra/2004_e.html
- Schuman AJ. A concise history of antimicrobial therapy (serendipity and all) Contemp Pediatr. 20:65. [Google Scholar]
- Shanmuganathan VA, Armstrong M, Butler A, et al. External ocular infections due to methicillin-resistant Staphylococcus aureus (MRSA) Eye. 2005;19:284–91. doi: 10.1038/sj.eye.6701465. [DOI] [PubMed] [Google Scholar]
- Venezia RA, Domaracki BE, Evans AM, et al. Selection of high-level oxacillin resistance in heteroresistant Staphylococcus aureus by fluoroquinolone exposure. J Antimicrob Chemother. 2001;48:375–81. doi: 10.1093/jac/48.3.375. [DOI] [PubMed] [Google Scholar]
- Weiss AH, Brinser JH, Nazar-Stewart V. Acute conjunctivitis in childhood. J Pediatr. 1993;122:10–4. doi: 10.1016/s0022-3476(05)83479-1. [DOI] [PubMed] [Google Scholar]
- West S. Azithromycin for control of trachoma. J Comm Eye Health. 1999;12:55–6. [PMC free article] [PubMed] [Google Scholar]