Abstract
The carotenoids lutein and zeaxanthin (LZ) are found throughout the central nervous system but reach their highest concentration within the macular region of the primate retina where they are commonly referred to as the macular pigments. Although LZ are a major integral feature of the central fovea, no information currently exists regarding the effects of variability in the concentration of these pigments on the developing retina. In particular, the long-term effects of very low levels of macular pigment are not known and potentially meaningful. Macular pigment levels depend upon dietary intake since LZ cannot be synthesized de novo. Infants with low intake of LZ (eg, infants receiving unfortified infant formula or breast milk from mothers with low carotenoid diets) would be expected to have considerably lower macular pigment compared with infants with high LZ intake (eg, breast-fed infants with mothers on carotenoid-rich diets). In this paper we discuss possible implications of this difference and the available evidence suggesting that LZ could influence the developing visual system.
Keywords: lutein and zeaxanthin, carotenoids, macular pigment, infant
Introduction
The human macula is known to contain three yellow carotenoids, 3R,3’R-zeaxanthin, 3R,3’S (meso)-zeaxanthin, and 3R,3’R,6’R)-lutein (Bone et al 1993). These carotenoids are absorbed in the gut and ultimately deposited throughout the tissues of the eye. In the macula, for instance, the average concentration of lutein and zeaxanthin (LZ) is about 1 mM which is about three times higher than the average concentration of carotenoids in other tissues of the body (Landrum et al 1999). This accumulation is conspicuous in terms of amount and selectivity. Of the 30–40 carotenoids typically found in the blood with very similar molecular configurations, only LZ are normally absorbed within ocular tissues. Within the eye, LZ are found in iridial tissue, the retinal pigment epithelium, the epithelium of the crystalline lens, and subretinal fluid (Chan et al 1998; Bernstein et al 2001). This predominance of LZ extends throughout most layers of the visual system. Craft and colleagues (2004) found that LZ are the dominant carotenoids within the brain comprising some 66%–77% of the total carotenoid concentration. The ubiquitous and selective presence of LZ within the visual system has led many researchers to believe that LZ play a special role in visual function and protection against visual disease. Although a number of comprehensive reviews have been written regarding the role of LZ in preventing age-related eye disease (cataracts and macular degeneration), a possible role of LZ in the early development of the visual system has received minimal attention. In this paper, we highlight the available evidence regarding possible functions of LZ early, as opposed to later, in life.
Lutein and zeaxanthin within food
Carotenoids are found in most fruits and vegetables with the exception of some root vegetables (Scott et al 1996). There exists over 500 different types of carotenoids in nature, but only about 30–40 are found within human sera with lutein, lycopene, and betacarotene tending to be the most abundant depending on diet composition. Because humans do not synthesize carotenoids, all carotenoids found within tissue must originate from the diet (or related sources like human milk or supplements). The richest sources of LZ within the diet are dark green leafy vegetables like spinach and kale that can contain 75–150 μg per gram of prepared food. Intermediate sources like broccoli, peas, maize, and lettuce can contain 10–30 μg per gram, and other sources like snap beans, carrots, and oranges tend to have less than 10 μg per gram (Holden et al 1999). Animal products like eggs and chicken skin can contain variable but significant amounts of LZ especially when marigolds are added to chicken feed to improve yolk or skin coloration (NRC 1994).
Lutein and zeaxanthin concentrations in breast milk and infant formula
The LZ content of breastmilk varies widely based on stage of lactation and individual diets (Table 1). The largest survey of breast milk carotenoids included 471 mothers from nine sites around the world (Canfield et al 2003). In this survey, individual LZ milk concentrations ranged from 3–232 μg/L. Mean concentrations at individual sites (Table 1) ranged from approximately 15 μg/L in Australia, Canada, UK, and USA to approximately 43 μg/L in China and Japan. The average LZ concentration across all of these sites was 25 ± 19 μg/L.
Table 1.
Published milk lutein concentrations
| Location | Days postpartum | Lutein concentration (mcg/L)a | Reference |
|---|---|---|---|
| Germany (n = 21) | Day 4 and 19 | 93 ± 48 (day 4) | (Schweigert et al 2004) |
| 50 ± 21 (day 19) | |||
| Ireland (n = 13) | 1 to 41 days | 80 (7–193)b | (Jewell et al 2004) |
| United Kingdom (n = 50) | 1–12 months | 15 ± 1 | (Canfield et al 2003) |
| Brazil (n = 49) | 30 to 120 days | 3 ± 1 | (Meneses and Trugo 2005) |
| United States (n = 12) | < 6 months | 11 ± 1 | (Canfield et al 1997) |
| United States (n = 19) | 4–32 days | 146 ± 99 (day 4) | (Gossage et al 2002) |
| 65 ± 36 (day 16) | |||
| 58 ± 44 (day 31) | |||
| Australia (n = 53) | 1–12 months | 15 ± 1 | (Canfield et al 2003) |
| Canada (n = 55) | 1–12 months | 17 ± 1 | (Canfield et al 2003) |
| Chile (n = 51) | 1–12 months | 32 ± 3 | (Canfield et al 2003) |
| China (n = 52) | 1–12 months | 43 ± 5 | (Canfield et al 2003) |
| Japan (n = 51) | 1–12 months | 44 ± 2 | (Canfield et al 2003) |
| Mexico (n = 50) | 1–12 months | 25 ± 2 | (Canfield et al 2003) |
| Philippines (n = 60) | 1–12 months | 20 ± 2 | (Canfield et al 2003) |
| United States (n = 49) | 1–12 months | 15 ± 1 | (Canfield et al 2003) |
Notes: amean ± SEM unless noted otherwise; converted from published units into μg/L for consistency; shaded rows are European data;
median and range; converted from nmol/g lipid using estimated 34 g lipid/L average from Gossage et al 2002.
In contrast to breast milk, commercial infant formulas generally contain only the LZ innate to the formula components. Using an HPLC method similar to Canfield and colleagues (2003), Jewell and colleagues (2004) measured three samples each of six brands of infant formula (three term and three preterm) and found an average lutein concentration of 0.07 μg/L with a range from 0–0.13 μg/L of lutein. For zeaxanthin, the formulas contained an average concentration of 0.005 μg/L with a range from 0–0.022 μg/L of zeaxanthin. The HPLC method used by Jewell and colleagues (2004) includes a saponification step that is known to reduce the recovery of LZ by approximately 40% (Liu et al 1998) and therefore it is likely that the true LZ content of both breast milk and formula is likely higher.
Differences in serum lutein and zeaxanthin between breastfed and formula-fed infants
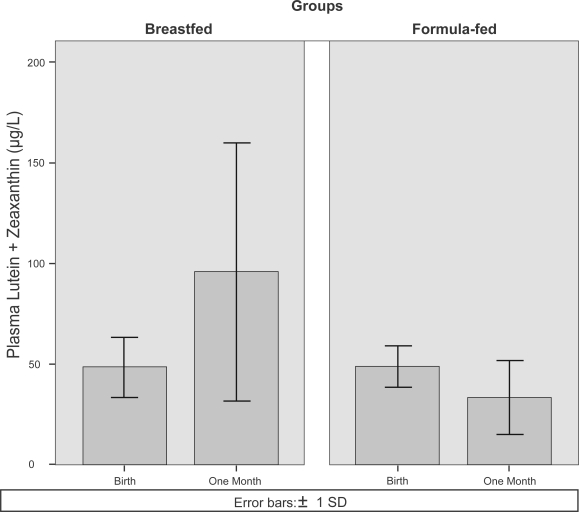
A study of infant plasma carotenoid concentrations (Johnson et al 1995) showed that breastfed and formula-fed infants had similar plasma LZ concentrations at birth (48 ± 15 μg/L and 49 ± 10 μg/L for breast and formula-fed, respectively). After one month, breastfed infant plasma LZ increased to 96 ± 64 μg/L while formula-fed infant plasma LZ decreased below baseline to 33 ± 19 μg/L (Figure 1).
Figure 1.
Plasma lutein and zeaxanthin concentrations at birth and one month of age for breastfed and formula-fed infants. The bar graphs were derived from the original study data presented in Johnson and colleagues (1995).
Similar trends have been observed for other infant plasma carotenoids, with plasma concentrations of betacarotene (Ostrea et al 1986; Sommerburg et al 2000), lycopene and cryptoxanthin (Sommerburg et al 2000) all increasing above newborn levels over time in breastfed infants and dropping below newborn levels in formula-fed infants. Although the amounts of data are limited, it seems clear that if breast milk contains higher average levels of LZ which results in higher serum levels of LZ, then higher retinal levels are sure to result.
Lutein and zeaxanthin intake and serum concentrations in toddlers
The 2005 Dietary Guidelines for Americans recommend that children ages 2–3 consume one cup of vegetables per day at a caloric intake of 1000 kcal/day (USDA 2005). Specifically, this recommended vegetable intake should include one cup per week of dark green vegetables—the richest sources of LZ in the diet. According to the 2002 Feeding Infants and Toddlers Study (FITS), a national sample of US children 12–24 months old, mean vegetable intake in this age group was 0.4 cups per day (Fox et al 2006) with only 3.1% of non-Hispanic children consuming any dark green vegetables between the ages of 6–11 months increasing to 7.5% between the ages of 12–24 months (Mennella et al 2006). This low vegetable dietary intake pattern would seem to present the risk of inadequate LZ consumption among toddlers as well.
Possible roles of lutein and zeaxanthin within the developing visual system
Although LZ are found throughout the tissues of the eye, they are most highly concentrated within the inner layers of the fovea. The area around and including the fovea is called the macula and is of special clinical and scientific interest because it is here that we experience our best acuity and damage to this area leads to legal blindness. The cortical areas corresponding to the fovea are greatly magnified (eg, about half of V1 is devoted to processing information from the macula) reflecting the importance of this area for later information processing by the more anterior portions of the brain. The peripheral retina is essentially mature at birth but the fovea (ie, central 5 degrees) is so poorly developed as to be essentially nonfunctional (Abramov et al 1982). Newborns can generally only discern vague shapes that are at a high contrast relative to their surroundings (Teller 1997).
In adults, the macula has the highest concentration of lutein found in the eye with a predominance of zeaxanthin isomers over lutein at a ratio of 2.4:1 (Bone et al 1988). In contrast, infants (0–2 years) have an incompletely formed and diffuse macula with lutein predominant over zeaxanthin at a ratio of 1.49:1 (Bone et al 1988). At birth, the area that will become the macula contains both rod and cone photoreceptors, and the photoreceptors themselves are short and immature (Hendrickson and Yuodelis 1984; Provis et al 1998). The change in lutein: zeaxanthin ratio appears to be closely related to steps in anatomical development.
Over the first 16 weeks of life, cone cells gradually become predominant in the fovea with a cone cell density similar to that found in adults resulting in improved infant visual acuity (Teller 1997). During this time frame, the outer plexiform layer also becomes discernable within the layers of the retina (Provis et al 1998). The outer plexiform layer (also called the fibers of Henle or receptor axon layer) is structurally relevant in that it contains the highest concentration of LZ in the retina (Snodderly et al 1984). These changes in the anatomical structure of the fovea are consistent with the shift in lutein: zeaxanthin ratio over this time period from a still lutein dominant profile (1.2–1.7:1) to a more adult profile of approximately 0.7:1 (Bone et al 1988). By four years of age, the photoreceptors have lengthened to near-adult lengths and the outer plexiform layer is fully formed (Provis et al 1998). During this same time period, the child’s visual acuity also improves to near adult levels (Teller 1997).
Antioxidant functions of lutein and zeaxanthin
The retina is uniquely susceptible to oxidative damage compared with other tissues. It is exposed to an intense energy source (ie, focused light from the lens) that can generate free radicals, high oxygen tension from the extensive vasculature of the retina, photosensitizing compounds, and a high concentration of an easily oxidized substrate (eg, DHA-rich outer segments). Under these conditions, the singlet oxygen free radical can be readily generated (Hardy et al 2000). Singlet oxygen is electrophilic and preferentially reacts with molecules with a double bond (eg, DHA has six double bonds) in order to extract the hydrogen needed to return to ground state. When DHA loses a hydrogen atom from a double bond, it becomes a hydroperoxide free radical that can create more free radicals and begin a self-perpetuating chain reaction known as lipid peroxidation (Halliwell and Chirico 1993).
LZ are particularly well suited for protecting the retina from oxidative damage compared with other chain-breaking antioxidants in the eye like alpha-tocopherol (vitamin E). Lutein, for instance, can return singlet oxygen to ground state by temporarily becoming triplet state L and then dissipating the energy as heat—a process that can be repeated over and over again since the lutein molecule remains intact after the energy transfer (Stahl and Sies 2002). Alpha-tocopherol requires a donor antioxidant molecule to return to ground state after neutralizing singlet oxygen (Sies and Stahl 1995). Lutein is also a more effective antioxidant than alpha-tocopherol both in preventing lipid peroxidation (Fukuzawa et al 1998) and oxidation initiated by the retinal photosensitizer A2-PE (composed of two all-trans retinal molecules and a phosphatidyletanolamine molecule) (Kim et al 2006). This latter effect is significant since A2E, the long-wavelength emitting flourophore of lipofuscin, is so cytotoxic to retinal pigment epithelium (RPE) cells and is thought to play an important role in the development of age-related macular degeneration (Shaban and Richter 2002). Appropriately, LZ are found in significant amounts within the DHA-rich photoreceptor outer segments where they can most effectively provide antioxidant protection (Rapp et al 2000). Additional evidence for antioxidant efficacy comes from the fact that when LZ is exposed to more free radical energy than can be dissipated as heat, specific LZ oxidation products are formed. These specific oxidation products have been identified in adult human eyes (Khachik et al 1997) providing indirect evidence that LZ are acting as an antioxidant in vivo.
Antioxidant protection of the eye may be particularly important in infants. Infants cannot down-regulate blood flow in the retinal and choroidal vasculature as well as adults; therefore, these vessels deliver excess oxygen to the retina and favor the generation and proliferation of free radical peroxides (Hardy et al 2000).
The role of lutein and zeaxanthin as an optical filter
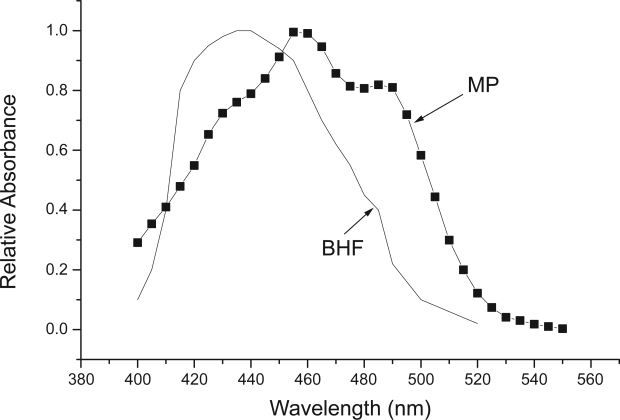
Since light must pass through LZ (ie, macular pigment) before reaching the receptors, it screens the vulnerable, lipid-rich outer retinal layers (eg, the receptoral outer segments and RPE) according to its spectral absorption profile. As shown in Figure 2, the macular pigments selectively absorb the portion of the visible spectrum from about 400–520 nm. Empirical data has shown that light can damage the retina depending on the wavelength of light, intensity and the length of exposure (Ham 1983). Light between the wavelengths of 700 nm (red) and 400 nm (blue) can pass through the cornea and lens and reach the retina efficiently to cause damage while ultraviolet and infrared light do not generally reach the retina (Reme et al 1996). Exposure to high intensity red light for a long period of time can produce thermal lesions on the retina (characterized by raising the temperature of the tissue at least 10 °C). In contrast, exposure to two-fold lower intensity blue light can produce photochemical lesions on the retina in the same amount of time and without raising the temperature of the tissue (Ham et al 1979). Such data have been used to calculate the “blue light hazard function” which is commonly used to define safe exposure limits to short-wave (ie, blue) light (ANSI 2005). These standards are particularly important for occupations that require increased exposure to light between 400–500 nm (such as dentists using blue lasers to quickly cure dental compound). By absorbing actinic short-wave light before it damages the outer retina, macular pigment (MP) would reduce this damage (as shown by the similarity in the photoxic action spectral of the blue light hazard related to the MP absorbance spectra shown in Figure 2). Given equivalent light histories, an individual with high MP, would be expected to suffer less light-initiated damage over many years when compared with an individual with low MP.
Figure 2.
The absorbance spectra of macular pigment (MP) measured ex vivo (derived from tabular data in Hammond et al 2005) shown with the blue light hazard function (BHF) (derived from ANSI 2005).
Quantifying the effects of long-term exposure to visible short-wave light is challenging. The epidemiology, which tends to be cross-sectional, is mixed (Margrain et al 2004). Of course, quantifying light damage over many years is very difficult, especially since, as noted later, so much of the damage could occur very early in life. Moreover, potentially moderating factors are often not considered. For example, there is no data that has considered the long-term effects of individual differences in MP levels. For this reason, laboratory studies using animal models are often used with the assumption that the effects are direct enough that they can be extrapolated to the chronic human situation. Nonhuman primates are the most appropriate models for eye research since they are the only animals that have maculas and accumulate MPs like humans. In a study where a blue light laser (476 nm) was used to induce photochemical lesions in monkey eyes, adult rhesus monkeys fed a LZ-deficient diet throughout life had more damage to their retinas than age-matched controls fed a normal chow diet. When deficient monkeys were then supplemented for 22–28 weeks with lutein or zeaxanthin (3.9 μmol/kg/d or about 10–15 mg per animal per day), the retina damage was significantly reduced compared with unsupplemented, carotenoid-deficient monkeys (Barker et al 2005). Supplemented monkeys also showed some changes in age-related eye damage (drusen particles) compared with unsupplemented, carotenoid-deficient monkeys indicating that LZ may reduce age-related damage to the eyes (Leung et al 2006). In the quail model, zeaxanthin supplementation (36 μg/kg diet) of carotenoid-deficient quail prevented photoreceptor apoptosis induced by intermittent bright white light exposure (Thomson et al 2002). In rats, feeding 1 gram per day of Lycium barbarum L. fruit (wolfberry) to normal Sprague Dawley rats reduced the amount of retina damage induced by bright light exposure when compared with rats fed a normal diet (Na et al 1995). L. barbarum is an extremely rich source of zeaxanthin containing 82 mg zeaxanthin per 100 g weight (Weller and Breithaupt 2003). These experimental models show that dietary LZ can protect the retina from light damage, especially damage due to short-wave light.
If MP does protect the receptors and RPE from actinic damage, it is probably meaningful that individuals vary so widely in the amount of MP they possess. As measured in vivo in adult subjects, individual differences in MP density tend to be large, ranging from a maximum in some individuals of 1.5 optical density units at 460 nm (3% transmission) to a minimum of near zero density (100% transmission) (Hammond et al 1997) (with an average density of about 0.36, SD = 0.22; Mares et al 2006). MP density in infants and children also varies between individuals (Bone et al 1988; Bour et al 2002). As noted, formula-fed infants with lower intake of LZ would be expected to have lower retinal levels compared with infants fed with LZ-rich breast milk.
Lower MP levels in children may be particularly meaningful since short wavelength (SW) light may pose a particular hazard to infants and young children. Infants are born with clear lenses that gradually yellow throughout life (due to color changes in proteins and other lens constituents). This yellowing progressively blocks the SW light passing through the lens as a linear function of age (Dillon et al 2004). For example, Werner (1982) obtained lens density data in subjects from birth until 70 years of age using visually evoked cortical potentials as the criterion response. He found that lens density at 400 nm increased (~0.02 O.D. per year) by a factor of 22 when comparing the average one-month old with an average 70-year old. Expressed in relative terms, about 70%–80% of the SW light passes through the lens in 0–2 year olds, 60%–70% in 2–10 year olds but only 20% of SW light is transmitted in 60–90 year olds. Data from various sources is summarized in Reme and colleagues (1996).
Finally, filtering SW light may also have a number of meaningful optical effects as summarized by Nussbaum and colleagues (1981). These include: (1) reducing the effects of chromatic aberration; (2) decreasing glare discomfort and disability; (3) increasing visibility outdoors by absorbing “blue haze”; and (4) enhancing contrast (ie, by differential absorption of a background with shorter wavelengths than a target). Yellow filters like MP have also been shown to improve magnocellular function which could enhance reading performance, motion sensitivity, accommodation, and convergence particularly in reading-impaired children (Ray et al 2005).
Other possible influences of lutein and zeaxathin on neural function within the visual system
All of the major hypotheses of MP function are based on just two fundamental characteristics of the pigments: their filtering and antioxidant properties. In addition to their filtering and antioxidant properties, however, LZ have a number of other known characteristics that might have meaningful influences on the visual system. For example, although most highly accumulated in and around the foveal depression, LZ, unlike the many other carotenoids circulating in the blood, are found throughout the tissues of the eye (Yeum et al 1995; Bernstein et al 2001) and at most layers of the visual system. Craft and colleagues (2004), for instance, found that LZ are the dominant carotenoids within the brain and comprise some 66%–77% of the total carotenoid concentration. There, these pigments would be optimally placed to influence central visual processing (eg, they are concentrated in the gray matter of the occipital region). LZ are also known to associate with the microtubules of axons. During embyogenesis, axons grow over extended distances to reach their targets. Once these targets have been achieved, the microtubules, cylindrical protein lattices, form the cytoskeleton of visual neurons. The walls of microtubules are formed by subunit proteins including tubulin. Whereas traditionally microtubules have been regarded as purely structural in nature, more recent evidence (Glanz 1997; Maniotis 1997a, 1997b) has shown that they may serve mechanical signaling and communicative functions (eg, by influencing second-messenger systems). Bernstein and colleagues (1997) originally identified tubulin as a possible binding protein for the macular carotenoids. Using molecular modeling, Crabtree and colleagues (2001) confirmed tubulin as the most likely binding protein for retinal LZ and suggested that a major function of the macular carotenoids, previously unconsidered, is to modulate the dynamic instability of microtubules. In any event, LZ are found in many locations outside of the inner layers of the fovea, albeit at relatively low concentrations. They are also found in many locations within the cell including the cell membrane and cytoskeleton. It is possible that the presence of the pigments is simply incidental and has no actual function. It is also possible, however, that the pigments influence the function of the neurons that they so closely and structurally associate with.
The idea that the MP carotenoids might have an actual metabolic role is not new. Dartnall and Thomson (1949) originally suggested that LZ facilitate oxygen respiration within the fovea. The xanthophylls do often serve this function in hypoxic situations in other animals and plants (Karnaukhov 1990). The inner layers of the foveal depression, where MP is most densely concentrated, are typically avascular and hypoxic. LZ would, according to this idea, be positively related to oxygen metabolism, particularly in the fovea. For example, MP might be expected to be related to aspects of foveal architecture that might be expected to influence oxygen utilization (eg, the size of the avascular zone). Consistent with such a possibility, Aleman and colleagues (2001) and Liew and colleagues (2006) found that retinal thickness (which would, presumably, cause greater hypoxia in the inner foveal layers due to less oxygen diffusion from the more distant posterior choroid) was positively related to MP density. Dwyer and colleagues (2001) originally suggested that lutein could influence blood flow (most probably including retinal blood flow) by preventing atherosclerotic changes through inhibition of low density lipoprotein-induced migration of plaque-forming monocytes to artery walls. As noted, Hardy and colleagues (2000) argued that it was the absence of autoregulation of choroidal blood flow in the infant that leads to increased oxidative stress in the newborn retina.
Another possibly relevant feature of carotenoids is their ability to enhance gap junctional communication (Stahl and Sies 2001). Gap junctions increase intracellular communication between glial and neuronal cells. Acting as portals, they influence propagation of action potentials, second-messenger systems and the movement of metabolites and electrolytes. Within the retina, gap junctions are crucial to light processing via lateral connections (Cook and Becker 1995; Vaney et al 1998). Gap junctions may therefore be vital to the developing neural circuitry within the visual system (Roerig and Feller 2000).
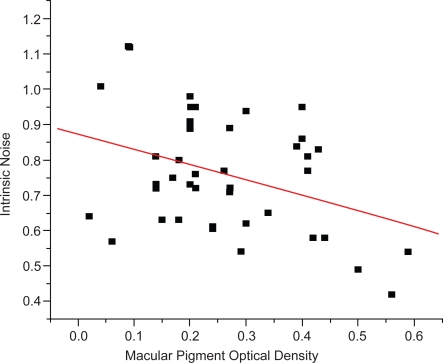
Evidence from such diverse sources suggests that, in addition to the classic hypotheses of MP function, LZ might play a facilitative role in neural processing. Gutherie and colleagues (2005) originally evaluated this possibility by measuring scotopic noise. It is generally thought that visual thresholds that depend primarily on receptoral processes are determined early in life. For example, most absolute thresholds are thought to reach adult levels between about 2–4 months (Brown 1990). Although intrinsic noise can arise at numerous levels of the visual system, noise associated with scotopic thresholds is thought to originate at the input stage (ie, at the level of the retina) (van Rossum and Smith 1998). When in a fully dark-adapted state (ie, scotopic), the visual system is maximally sensitive, making the effects of noise easily measurable. LZ are found within rod outer-segments and therefore a relation to rod function is feasible. As shown in Figure 3, intrinsic noise was significantly related to MP optical density (MPOD). Such a relation is biologically plausible. As noted, LZ are found throughout the retina including within rod outer segments (Rapp et al 2000). It is likely that higher levels of LZ in the macula (ie, the MP values that were measured) correlate with LZ in the outer segments. Such a relation suggests that higher LZ levels in rod outer segments might help improve the efficiency of rod functioning leading to less variability in threshold response (their measure of noise).
Figure 3.
The relation between macular pigment optical density (measured at 460 nm, 1-degree test) and intrinsic noise within the scotopic system (Y = 0.87 + −0.43X). r = −0.38; p < 0.01; and n = 39 (mean age = 40; SD = 8 years). Macular pigment was measured at 460 nm using a one-degree test stimulus and a psychophysical technique based on heterochromatic flicker photometry. For details regarding the procedure and apparatus see Wooten et al 1999. Scotopic thresholds were assessed in Maxwellian view using a 1.85-deg, 510-nm circular test stimulus located at 10° eccentricity in the left visual field using a two-alternative forced-choice paradigm (an average of 200 trials per participant was obtained). Individual trials were transformed into binomial data (the inverse of the normal probability integral) and then fit with a weighted linear regression. We then defined “intrinsic noise” as the average deviation from this line (see Gutherie et al 2005).
Gutherie and Hammond’s data (2005) suggest that MP might be related to dynamic receptoral function. There is evidence also that MP might be related to post-receptoral processing. Hammond and Wooten (2005) found a moderately strong relation between MP and critical flicker fusion thresholds (CFF) that was independent of age. CFF is largely regarded as a measure of temporal processing speed. Evidence suggests that the CFF (the average of descending flicker and ascending fusion thresholds) is probably determined post-receptorally (Powell 1983; Curran 1990). This possibility is based on the observation that the retina (as measured by electroretinography) responds to flicker even after a subject has reported fusion (Brown 1965) and correlates with measures of cortical arousal like electroencephalogram (Gortelmeyer and Wieman 1982; Grunberger et al 1982). Thus, diseases and drugs that influence central nervous system function often influence CFF thresholds in the same direction (Grunberger et al 1982; MacNab et al 1985; Curran 1990). For example, barbituates decrease CFF thresholds and amphetamines increase CFF thresholds. In most situations, a lower threshold implies increased sensitivity (eg, a lower energy threshold for detection means a higher sensitivity). In the case of flicker, however, a higher threshold for fusion implies a higher flicker sensitivity. Like the MP–noise relation, the MP–CFF relation reported by Hammond and colleagues (2005) was not age-dependent (ie, it is also evident for the younger subjects). CFF thresholds (reviewed by Teller 1997) reach adult levels in early infancy as opposed to some other spatial vision functions, like vernier acuity, that develop much later (~5–6 years of age) and appear not to be associated with MP (Engles et al in press).
Conclusion
The question of how LZ influence the developing visual system is open. There is, however, convincing evidence that a lack of LZ from birth can produce distinct anatomical changes within the retina and RPE. For example, Leung and colleagues (2004) studied the effects of raising Rhesus monkeys on diets containing no LZ. Compared with monkeys raised on normal diets, these xanthophyll-free monkeys displayed changes in the anatomy of their RPE (eg, in the density and distribution of RPE cells) that could be largely reversed by supplementing LZ (especially in conjunction with n-3 fatty acids, a precursor of DHA) later in life. Their results suggested that LZ might be important to the maturation of the fovea based on metabolic effects not associated with protection. With respect to the latter, it is becoming increasingly clear that protection very early in life is critical to preventing degenerative disease later in life. Carotenoid-deficient monkeys also develop signs of premature aging and damage (drusen and transmission defects) in their maculas years earlier than these signs develop in normal chow-fed monkey (Neuringer et al 2003).
One might argue that humans evolved in an environment where dietary intake of carotenoid-rich fruits and vegetables was high (ie, most food is gathered in hunter and gathering societies). Humans also were exclusively breast-fed until relatively recently in our evolutionary history. Although the effects of deviation from this “natural state” are not known, they could be meaningful. Of course, empirical study of this question in humans is difficult. Randomized, placebo-controlled trials of infant formula with and without lutein could require many years of follow-up before changes in the retina and RPE are detectable. Until such data are available, there is reason to believe lutein may have long term benefits based both on animal data and by examining the mechanisms by which damage accumulates in the retina throughout life. For example, one mechanism through which early intake of LZ could have long-term health benefits is through reduction of cumulative eye damage. Over the course of life, a cellular waste product called lipofuscin accumulates in the RPE of the eye. Lipofuscin is a mass of oxidized proteins, lipids, and other compounds created through the incomplete digestion of photoreceptor outer segments in the lysosomes of RPE (Sparrow and Boulton 2005). Lipofuscin cannot be transported out of the cell, and the accumulation of lipofuscin in RPE is accelerated by increased oxygen (Wihlmark et al 1996), light exposure (Ben-Shabat et al 2002) or the absence of macular pigment (Leung et al 2006). In vitro, LZ inhibit the formation of lipofuscin in cultured RPE (Sundelin and Nilsson 2001). The results of failing to forestall lipofuscin accumulation can be seen in vivo in that primates subjected to a life-long carotenoid deficiency develop lipofuscin-related damage (drusen) much earlier than primates fed normal diets (Neuringer et al 2003).
Among the various components that make up lipofuscin is a retinal-derived compound called A2-PE (Lamb and Simon 2004). A2-PE is a photosensitizing fluorophore (a molecule that absorbs light energy and releases it as fluorescence at a different wavelength) that creates oxygen free radicals when exposed to blue light. LZ are efficient quenchers of the photochemical oxidation caused by A2-PE—more so than alpha-tocopherol, another biological antioxidant (Kim et al 2006). Through this mechanism, LZ may help reduce the retina damage caused by lipofuscin in the RPE. As much as half of the lifetime accumulation of lipofuscin in RPE may occur in the first five years of life (Wing et al 1978; Feeney-Burns et al 1984). Increasing MP earlier in life could theoretically slow the accumulation of lipofuscin as well as reduce the free radical oxidants that it produces. Given the low risk and clear potential benefit, it would be prudent to increase lutein consumption earlier in life while further evidence of its benefits emerge.
References
- Abramov I, Gordon J, Hendrickson A, et al. The retina of the newborn human infant. Science. 1982;217:265–7. doi: 10.1126/science.6178160. [DOI] [PubMed] [Google Scholar]
- Aleman TS, Duncan JL, Bieber ML, et al. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Invest Ophthalmol Vis Sci. 2001;42:1873–81. [PubMed] [Google Scholar]
- [ANSI] American National Standards Institute 2005Recommended practice for photobiological safety for lamps and lamp systems: General requirementsANSI/IESNA RP-27.1–05.
- Barker F, Neuringer M, Johnson E, et al. Dietary zeaxanthin or lutein improves foveal photo-protection from blue light in xanthophyll-free monkeys [abstract] Invest Ophthalmol Vis Sci. 2005;46:1770. [Google Scholar]
- Ben-Shabat S, Parish C, Vollmer H, et al. Biosynthetic studies of A2E, a major fluorophore of retinal epithelial lipofuscin. J Biol Chem. 2002;277:7183–90. doi: 10.1074/jbc.M108981200. [DOI] [PubMed] [Google Scholar]
- Bernstein PS, Balashov NA, Tsong ED, et al. Retinal tubulin binds macular carotenoids. Invest Ophthalmol Vis Sci. 1997;38:167–75. [PubMed] [Google Scholar]
- Bernstein P, Khachik F, Carvalho L, et al. Identification and quantification of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–23. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- Bone R, Landrum J, Fernandez L, et al. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–9. [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Hime GW, et al. Stereochemistry of the human macular carotenoids. Invest Ophthalmol Vis Sci. 1993;34:2033–40. [PubMed] [Google Scholar]
- Bour LJ, Koo L, Delori FC, et al. Fundus photography for measurement of macular pigment density distribution in children. Invest Ophthalmol Vis Sci. 2002;43:1450–5. [PubMed] [Google Scholar]
- Brown AM. Development of visual sensitivity to light and color vision in human infants: A critical review. Vis Res. 1990;30:1159–88. doi: 10.1016/0042-6989(90)90173-i. [DOI] [PubMed] [Google Scholar]
- Brown JL. Flicker and intermittent stimulation. In: Graham CH, editor. Vision and Visual Perception. Vol 1. New York: John Wiley and Sons; 1965. pp. 251–320. [Google Scholar]
- Canfield L, Clandinin M, Davies D, et al. Multinational study of major breast milk carotenoids of healthy mothers. Eur J Nutr. 2003;42:133–41. doi: 10.1007/s00394-003-0403-9. [DOI] [PubMed] [Google Scholar]
- Canfield L, Giuliano A, Neilson E, et al. Beta-carotene in breast milk and serum is increased after a single beta-carotene dose. Am J Clin Nutr. 1997;66:52–61. doi: 10.1093/ajcn/66.1.52. [DOI] [PubMed] [Google Scholar]
- Chan C, Leung IYF, Lam KW, et al. The occurrence of retinol and carotenoids in human subretinal fluid. Curr Eye Res. 1998;17:890–5. doi: 10.1076/ceyr.17.9.890.5141. [DOI] [PubMed] [Google Scholar]
- Cook JE, Becker DL. Gap junctions in the vertebrate retina. Microsc Res Tech. 1995;31:408–19. doi: 10.1002/jemt.1070310510. [DOI] [PubMed] [Google Scholar]
- Crabtree DV, Ojima I, Geng X, et al. Tubulins in the primate retina: evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site. Bioorg Med Chem. 2001;9:1967–76. doi: 10.1016/s0968-0896(01)00103-1. [DOI] [PubMed] [Google Scholar]
- Craft NE, Haitema HB, Garnett KM, et al. Carotenoid, tocopherol and retinol concentrations in the elderly human brain. J Nutr Health Aging. 2004;8:156–62. [PubMed] [Google Scholar]
- Curran S. Critical flicker techniques in psychopharmacology. In: Hindmarch I, Stonier PD, editors. Human Psychopharmacology: Methods and Measures. Vol 3. Chichester: John Wiley & Sons Ltd; 1990. pp. 21–38. [Google Scholar]
- Dartnall HJA, Thomson LC. Retinal oxygen supply and macular pigmentation. Nature. 1949;164:876. doi: 10.1038/164876a0. [DOI] [PubMed] [Google Scholar]
- Dillon J, Zheng L, Merriam J, et al. Transmission of light to the aging human retina: possible implications for age related macular degeneration. Exp Eye Res. 2004;79:753–9. doi: 10.1016/j.exer.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Dwyer JH, Navab M, Dwyer KM, et al. Oxygenated carotenoid lutein and progression of early atherosclerosis. The Los Angeles atherosclerosis study. Circulation. 2001;103:2922–7. doi: 10.1161/01.cir.103.24.2922. [DOI] [PubMed] [Google Scholar]
- Engles M, Wooten BR, Hammond BR.2007Macular pigment: The acuity hypothesis IOVS, in press. [DOI] [PubMed] [Google Scholar]
- Feeney-Burns L, Hilderbrand E, Eldridge S. Aging human RPE: Morphometric analysis of macular, equitorial and peripheral cells. Invest Ophthalmol Vis Sci. 1984;25:195–200. [PubMed] [Google Scholar]
- Fox MK, Reidy K, Karwe V, et al. Average portions of food commonly eaten by infants and toddlers in the United States. J Am Diet Assoc. 2006;106:S66–S76. doi: 10.1016/j.jada.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Fukuzawa K, Inokami Y, Tokumura A, et al. Rate constants for quenching singlet oxygen and activities for inhibiting lipid peroxidation of carotenoids and alpha-tocopherol in liposomes. Lipids. 1998;33:751–6. doi: 10.1007/s11745-998-0266-y. [DOI] [PubMed] [Google Scholar]
- Glanz J. Force-carrying web pervades living cell. Science. 1997;276:678–9. doi: 10.1126/science.276.5313.678. [DOI] [PubMed] [Google Scholar]
- Gossage C, Deyhim M, Yamini S, et al. Carotenoid composition of human milk during the first month postpartum and the response to β-carotene supplementation. Am J Clin Nutr. 2002;76:193–7. doi: 10.1093/ajcn/76.1.193. [DOI] [PubMed] [Google Scholar]
- Gortelmeyer R, Wieman H. Retest reliability and construct validity of critical flicker fusion frequency. Pharmacopsychiatry. 1982;15(Supp. 1):24–8. [Google Scholar]
- Grunberger J, Saletu B, Berner P, et al. CFF and assessment of pharmacodynamics: Role and relationship to psychometric, EEG, and pharmacokinetic variables. Pharmacopsychiatry. 1982;15(Supp. 1):29–35. [Google Scholar]
- Gutherie AH, Hammond BR. Macular pigment and scotopic noise. ARVO Abstracts [abstract] Invest Ophthalmol Vis Sci. 2005:E1784. [Google Scholar]
- Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57(suppl):715S–725S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- Ham W. Ocular hazards of light sources: Review of current knowledge. J Occup Med. 1983;25:101–3. [PubMed] [Google Scholar]
- Ham W, Mueller H, Ruffolo J, et al. Sensitivity of the retina to radiation damage as a function of wavelength. Photochem Photobiol. 1979;29:735–43. doi: 10.1111/j.1751-1097.1979.tb07759.x. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Johnson EJ, Russell RM, et al. Individual variations in the spatial profile of macular pigment. J Opt Soc Am A Opt Image Sci Vis. 1997;14:1187–96. doi: 10.1364/josaa.14.001187. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Wooten BR, Smollon B. Assessment of the validity of in vivo methods of measuring human macular pigment optical density. Optom Vis Sci. 2005;82:387–404. doi: 10.1097/01.opx.0000162652.85875.d2. [DOI] [PubMed] [Google Scholar]
- Hammond BR, Wooten BR. CFF Thresholds: Relation to macular pigment optical density. Ophthalmic and Physiological Optics. 2005;25:315–19. doi: 10.1111/j.1475-1313.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Hardy P, Dumont I, Bhattacharya M, et al. Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: a basis for ischemic retinopathy. Cardiovasc Res. 2000;47:489–509. doi: 10.1016/s0008-6363(00)00084-5. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91:603–12. doi: 10.1016/s0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]
- Holden J, Eldridge A, Beecher G, et al. Carotenoid content of U.S. foods: An update of the database. J Food Comp Anal. 1999;12:169–96. [Google Scholar]
- Jewell VC, Mayes CBD, Tubman TRJ, et al. A comparison of lutein and zeaxanthin concentrations in formula and human milk samples from Northern Ireland mothers. Eur J Clin Nutr. 2004;58:90–7. doi: 10.1038/sj.ejcn.1601753. [DOI] [PubMed] [Google Scholar]
- Johnson L, Norkus E, Abbasi S, et al. 1995Contribution of beta-carotene(BC) from BC enriched formulae to individual and total serum carotenoids in term infants [abstract] FASEB J 9(4 Pt 3)1869 [Google Scholar]
- Karnaukhov VN. Carotenoids: recent progress, problems and prospects. Comp Biochem Physiol B. 1990;95:1–20. doi: 10.1016/0305-0491(90)90241-k. [DOI] [PubMed] [Google Scholar]
- Khachik F, Bernstein P, Garland D. Identification of lutein and zeaxanthin oxidation products in human and monkey retinas. Invest Ophthalmol Vis Sci. 1997;38:1802–11. [PubMed] [Google Scholar]
- Kim S, Nakanishi K, Itagaki Y, et al. Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res. 2006;82:828–39. doi: 10.1016/j.exer.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Lamb L, Simon J. A2E: a component of ocular lipofuscin. Photochem Photobiol. 2004;79:127–36. doi: 10.1562/0031-8655(2004)079<0127:aacool>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Landrum JT, Bone RA, Chen Y, et al. Carotenoids in the human retina. Pure Appl Chem. 1999;71:2237–44. [Google Scholar]
- Leung IY, Sandstrom MM, Zucker CL, et al. Nutritional manipulation of primate retinas, II: effects of age, n-3 fatty acids, lutein, and zeaxanthin on retinal pigment epithelium. IOVS. 2004;45:3244–56. doi: 10.1167/iovs.02-1233. [DOI] [PubMed] [Google Scholar]
- Leung IY, Snodderly DM, Neuringer M, et al. Nutritional effects of n-3 fatty acids, lutein and zeaxanthin on the lipofuscin accumulation in the foveal retinal pigment epithelium of rhesus monkeys [abstract] IOVS. 2006;47:2822. [Google Scholar]
- Liew SHM, Gilbert CE, Spector TD, et al. Central retinal thickness is positively correlated with macular pigment optical density. Exp Eye Res. 2006;82:915–20. doi: 10.1016/j.exer.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Liu Y, Xu M, Canfield L. Enzymatic hydrolysis, extraction, and quantitation of retinol and major carotenoids in mature human milk. J Nutr Biochem. 1998;9:178–83. [Google Scholar]
- MacNab MW, Foltz EL, Sweitzer J. Evaluation of signal detection theory on the effects of psychotropic drugs on critical flicker-fusion frequency in normal subjects. Psychopharmacology. 1985;85:431–5. doi: 10.1007/BF00429659. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Bojanowski K, Ingber DE. Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J Cellular Biochem. 1997a;65:114–30. [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DI. Demonstration of mechanical connections between integrins, cytoskeletal ®laments and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997b;94:849–54. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mares JA, LaRowe TL, Snodderly DM, et al. CAREDS Macular Pigment Study Group and Investigators. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women’s Health Initiative. Am J Clin Nutr. 2006;84:1107–22. doi: 10.1093/ajcn/84.5.1107. [DOI] [PubMed] [Google Scholar]
- Margrain TH, Boulton M, Marshall J, et al. Do blue light filters confer protection against age-related macular degeneration. Prog Ret Eye Res. 2004;23:523–31. doi: 10.1016/j.preteyeres.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Ziegler P, Briefel R, et al. Feeding Infants and Toddlers Study: The types of foods fed to Hispanic Infants and Toddlers. J Am Diet Assoc. 2006;106:S96–S106. doi: 10.1016/j.jada.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Meneses F, Trugo NMF. Retinol, beta-carotene, and lutein + zeaxanthin in the milk of Brazilian nursing women: associations with plasma concentrations and influences of maternal characteristics. Nutr Res. 2005;25:443–51. [Google Scholar]
- Na L, Ziliang L, Mom T. A study of the action of Lycium barbarum L. in rescuing the retina from photic injury in rats. Zhonghua Yandibing Zazhi. 1995;11:31–3. [Google Scholar]
- [NRC] National Research Council . 9th ed. Washington, DC: Natl Acad Pr; 1994. Nutrient requirements of poultry. [Google Scholar]
- Neuringer M, Jeffrey B. Visual development: neural basis and new assessment methods. J Pediatr. 2003;143:S87–S95. doi: 10.1067/s0022-3476(03)00406-2. [DOI] [PubMed] [Google Scholar]
- Neuringer M, Wallace P, Billingslea A, et al. Incidence of drusen-like changes in a rhesus monkey colony: Effects of age, gender and dietary carotenoids [abstract] Invest Ophthalmol Vis Sci. 2003;44:4949. [Google Scholar]
- Nussbaum JJ, Pruett RC, Delori FC. Historic perspectives. Macular yellow pigment. The first 200 years. Retina. 1981;1:296–310. [PubMed] [Google Scholar]
- Ostrea E, Balun J, Winkler R, et al. Influence of breast-feeding on the restoration of the low serum concentration of vitamin E and beta-carotene in the newborn infant. Am J Obstet Gynecol. 1986;154:1014–17. doi: 10.1016/0002-9378(86)90740-4. [DOI] [PubMed] [Google Scholar]
- Powell RR. Flicker fusion as a typological index of nervous system ‘reactivity’. Percept Mot Skills. 1983;57(3, part 1):701–2. doi: 10.2466/pms.1983.57.3.701. [DOI] [PubMed] [Google Scholar]
- Provis J, Diaz C, Dreher B. Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol. 1998;54:549–81. doi: 10.1016/s0301-0082(97)00079-8. [DOI] [PubMed] [Google Scholar]
- Rapp L, Maple S, Choi J. Lutein and zeaxanthin concentrations in rod outer segment membranes from perifoveal and peripheral human retina. Invest Ophthalmol Vis Sci. 2000;41:1200–9. [PubMed] [Google Scholar]
- Ray NJ, Fowler S, Stein JF. Yellow filters can improve magnocellular function: motion sensitivity, convergence, accommodation, and reading. Ann N Y Acad Sci. 2005;1039:283–93. doi: 10.1196/annals.1325.027. [DOI] [PubMed] [Google Scholar]
- Reme C, Reinboth J, Clausen M, et al. Light damage revisited: converging evidence, diverging views. Graefes Arch Clin Exp Opthalmol. 1996;234:2–11. doi: 10.1007/BF00186512. [DOI] [PubMed] [Google Scholar]
- Roerig B, Feller MB. Neurotransmitters and gap junctions in developing neural circuits. Brain Res Brain Res Rev. 2000;32:86–114. doi: 10.1016/s0165-0173(99)00069-7. [DOI] [PubMed] [Google Scholar]
- Scott KJ, Thurnham DI, Hart DJ, et al. The correlation between the intake of lutein, lycopene and beta carotene from vegetables and fruits, and blood plasma concentrations in a group of women aged 50–65 years in the UK. Br J Nutr. 1996;75:409–18. doi: 10.1079/bjn19960143. [DOI] [PubMed] [Google Scholar]
- Schweigert F, Bathe K, Chen F, Buscher U, Dudenhausen J. Effect of the stage of lactation in humans on carotenoid levels in milk, blood plasma and plasma lipoprotein fractions. Eur J Nutr. 2004;43:39–44. doi: 10.1007/s00394-004-0439-5. [DOI] [PubMed] [Google Scholar]
- Shaban H, Richter C. A2E and blue light in the retina: the paradigm of age-related macular degeneration. Biol Chem. 2002;383:537–45. doi: 10.1515/BC.2002.054. [DOI] [PubMed] [Google Scholar]
- Sies H, Stahl W. Vitamins E and C, beta-carotene, and other carotenoids as antioxidants. Am J Clin Nutr. 1995;62(suppl):1315S–21S. doi: 10.1093/ajcn/62.6.1315S. [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Auran J, Delori F. The macular pigment. II. Spatial distribution in primate retinas. Invest Ophthalmol Vis Sci. 1984;25:674–85. [PubMed] [Google Scholar]
- Sommerburg O, Meissner K, Nelle M, et al. Carotenoid supply in breast-fed and formula-fed neonates. Eur J Pediatr. 2000;159:86–90. doi: 10.1007/pl00013811. [DOI] [PubMed] [Google Scholar]
- Sparrow J, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H. Effects of carotenoids and retinoids on gap junctional communication. Biofactors. 2001;15:95–8. doi: 10.1002/biof.5520150209. [DOI] [PubMed] [Google Scholar]
- Stahl W, Sies H. Antioxidant effects of carotenoids: implication in photoprotection in humans. In: Cadenas E, Packer L, editors. Handbook of antioxidants. 2nd ed. New York, NY: Marcel Dekker; 2002. pp. 223–33. [Google Scholar]
- Sundelin S, Nilsson S. Lipofuscin-formation in retinal pigment epithelial cells is reduced by antioxidants. Free Radic Biol Med. 2001;31:217–25. doi: 10.1016/s0891-5849(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Teller D. First glances: The vision of infants. Invest Ophthalmol Vis Sci. 1997;38:2183–203. [PubMed] [Google Scholar]
- Thomson L, Toyoda Y, Langner A, et al. Elevated retinal zeaxanthin and prevention of light-induced photoreceptor cell death in quail. Invest Ophthalmol Vis Sci. 2002;43:3538–49. [PubMed] [Google Scholar]
- [USDA] United States Department of Agriculture 2005Dietary guidelines for Americans [online]Accessed on March 6, 2007. URL: http://www.healthierus.gov/dietaryguidelines
- Vaney DI, Nelson JC, Pow DV. Neurotransmitter coupling through gap junctions in the retina. J Neurosci. 1998;18:10594–602. doi: 10.1523/JNEUROSCI.18-24-10594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rossum MC, Smith RG. Noise removal at the rod synapse of mammalian retina. Vis Neurosci. 1998;15:809–21. doi: 10.1017/s0952523898155037. [DOI] [PubMed] [Google Scholar]
- Weller P, Breithaupt D. Identification and quantification of zeaxanthin esters in plants using liquid chromatography-mass spectrometry. J Agric Food Chem. 2003;51:7044–9. doi: 10.1021/jf034803s. [DOI] [PubMed] [Google Scholar]
- Werner JS. Development of scotopic sensitivity and the absorption spectrum of the human ocular media. J Opt Soc Am A. 1982;72:247–57. doi: 10.1364/josa.72.000247. [DOI] [PubMed] [Google Scholar]
- Wihlmark U, Wrigstad A, Roberg K, et al. Lipofuscin formation in cultured retinal pigment epithelial cells exposed to photoreceptor outer segment material under different oxygen concentrations. APMIS. 1996;104:265–71. doi: 10.1111/j.1699-0463.1996.tb00716.x. [DOI] [PubMed] [Google Scholar]
- Wing G, Blanchard G, Weiter J. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978;17:601–7. [PubMed] [Google Scholar]
- Yeum K-J, Taylor A, Tang G, et al. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Invest Ophthalmol Vis Sci. 1995;36:2756–61. [PubMed] [Google Scholar]