SUMMARY
Inducible and reversible perturbation of the activity of selected neurons in vivo is critical to understanding the dynamics of brain circuits. Several genetically encoded systems for rapid inducible neuronal silencing have been developed in the past few years offering an arsenal of tools for in vivo experiments. Some systems are based on ion-channels or pumps, others on G-protein coupled receptors and yet others on modified presynaptic proteins. Inducers range from light to small molecules to peptides. This diversity results in differences in the various parameters that may determine the applicability of each tool to a particular biological question. Although further development would be beneficial, the current silencing tool kit already provides the ability to make specific perturbations of circuit function in behaving animals.
INTRODUCTION
Underlying the function and dysfunction of the brain are complex neuronal circuits, consisting of diverse classes of specific neuronal populations. A major goal in systems neuroscience is to relate the activity in specific neuronal circuits to physiological processes, behavioral responses and disease states. This is a challenging task especially in the case of the highly complex mammalian brain and requires tools that allow rapidly inducible and reversible modulation of specific parts of neural circuits in vitro and in vivo; a need that has been widely recognized for some time [1–4]. The past decade has seen an upsurge in the development and optimization of genetically encodable tools for perturbing neuronal activity and several systems have been described [5•–11]. More importantly, initial in vivo applications of these tools have been described in the past two years opening the exciting new field of highly specific perturbation of circuit function in behaving animals [8••, 12••, 13••, 19••]. As these systems differ in the approach to neuronal silencing, mechanism of triggering and kinetics of induction and reversal, they may be best applicable in different experimental contexts. In this review, we discuss the most promising rapidly inducible silencing systems with the focus on how the different properties of each system make it particularly suitable for certain types of biological questions while highlighting the need for further characterization and optimization of their various features.
APPROACHES TO RAPID SILENCING
Allatostatin receptor-allatostatin system
Callaway and colleagues pioneered the use of the Drosophila allatostatin receptor (AlstR) [14] for inducible inactivation of neuronal activity (Figure 1A) [6]. This system is based upon the expression of a G-protein coupled receptor that is selectively activated by the insect peptide hormone allatostatin (AL) but is insensitive to endogenous mammalian peptides [14]. Following application of allatostatin, AlstR indirectly causes opening of the G-protein inward rectifier K+ (GIRK) channels leading to a hyperpolarizing potassium current, which in turn suppresses action potential generation. In dissociated cultures, a decrease in excitability is observed within ten to twenty minutes of allatostatin application. Similarly, in slices of the spinal cord from mice expressing AlstR in a subpopulation of spinal cord inhibitory neurons, reversible suppression of excitability was observed in the presence of allatostatin (10 nM) with a time course of approximately twenty minutes for both induction and reversal [15•]. Tan and colleagues used this system successfully in rat, ferrets and monkeys to selectively suppress neuronal activity in genetically defined visual cortical and thalamic neurons [12••]. Local application of allatostatin (100 nM) to the relevant neuronal tissue (within ~ 1 millimeter) was necessary due to restricted diffusion of the peptide, and its effect on neuronal excitability was inducible and reversible with the similar time course of ten to twenty minutes.
Figure 1.
Schematics of four rapidly inducible and reversible, genetically encoded neuronal inactivation systems described here.
A, Allatostatin receptor (AlstR; green) couples of the binding of allatostatin (red spheres) to the activation of a G-protein (white). This then leads to the opening of a G-protein couples inward rectifier K+ channel (blue).
B, The two subunit GluClα/β receptor (blue, purple) is opened by ivermectin (black circle). The resulting Cl− current suppresses neuronal activity.
C, Chloride current through the five subunit GABAA channel is enhanced by binding of zolpidem (black circle) to the interface between γ2 and α1 subunits leading to suppression of excitability.
D, Two Molecular Systems for the Inactivation of Synaptic Transmission (MISTs). The one component system is based on crosslinking of modified VAMP (light blue). The two component system is based on mislocalization of modified Synaptophysin (Sph; purple) non-specifically along the plasma membrane using the transmembrane domain of Syntaxin (StxTM).
E, Halorhodopsin (NpHR) pumps chloride into the cell when opened by yellow light.
The ivermectin sensitive chloride channel
A second system that reduces neuronal activity upon ligand application is the ivermectin (IVM)-sensitive chloride channel consisting of Caenorhabditis elegans GluCl α and β subunits (Figure 1B) [7,13••, 16–18]. Expression of GluClα/β using Sindbis virus in dissociated hippocampal neurons allowed inducible and potent inactivation of neuronal activity within seconds of ivermectin (5nM) application. The unbinding of ivermectin from the channel appeared to be very slow as reversal in vitro required eight hours. Although the wild type GluClα/β channel was also sensitive to glutamate, this side-effect was subsequently reduced [18]. Lerchner and colleagues demonstrated that this system can work in vivo by using it to perturb the function of striatal circuits in rats [13••]. By expressing GluClα/β unilaterally in the striatum through co-infection with two adeno-associated viruses encoding the individual subunits, they demonstrated that intraperitoneal administration of 5–10 milligrams of ivermectin per kilogram of body weight led to a perturbation of amphetamine-induced rotational behavior. The observed effect was maximal at 12–24 hours post injection and reversed by 4 days post injection. It is important to emphasize that unlike in the in vivo study with the AlstR/AL system, the readout of the inactivation in this case was not a direct measure of the electrical activity of the targeted neuronal population, but rather behavioral output, making it difficult to compare the time-course of silencing between the two systems. It is not unlikely that the change in behavioral output occurs on a slower time scale than the change in the activity of targeted neurons.
The zolpidem sensitive GABAA channel
The most recently developed system takes advantage of the specificity of the GABAA channel binding to a positive allosteric modulator, zolpidem [19••]. Single amino acid substitution in one of the subunits of the GABAA channel (γ2) leads to abolition of zolpidem sensitivity. Wulff and colleagues used this result to create a strategy for cell type specific enhancement of GABA-ergic currents by first creating zolpidem-insensitive mice using a mutant γ2 subunit and then reintroducing the wild type subunit to genetically defined neuronal populations to restore the sensitivity to the drug to select neuronal populations [19••]. In acute cerebellar slices from mice expressing the zolpidem-sensitive version of the GABAA receptor selectively in Purkinje cells, enhancement of miniature inhibitory post-synaptic currents (mIPSC) in Purkinje cells was observed within minutes of zolpidem (1 μM) delivery. Furthermore, intraperitoneal administration of the inducer in these transgenic mice led to impairment of rotarod performance within 5 minutes. Neither the extent of block of the excitatory input onto Purkinje cells by zolpidem administration nor the time course of reversal was addressed.
MISTs
A completely different approach to silencing is based on blocking neurotransmission by perturbing the function of synaptic proteins; an approach originally exemplified by shibire, a temperature-sensitive mutant of Drosophila dynamin [20]. A different embodiment of this strategy that has proven effective in the mammalian system is Molecular Systems for the Inactivation of Synaptic Transmission (MISTs) [8]. MISTs interfere with the synaptic transmission by inducing the mislocalization or immobilization of modified presynaptic proteins using chemically inducible dimerization (CID) with small cell-permeable chemicals, which are analogs of rapamycin [21]. Two systems have been introduced: VAMP/Syb MIST is based on oligomerization of synaptic vesicle protein VAMP/Synaptobrevin by intravesicular crosslinking, whereas Sph-StxTM MIST is based on inducible crosslinking of synaptic vesicle protein Synaptophysin to the plasma membrane through the transmembrane domain of Syntaxin (Figure 1C). It was demonstrated that in the presence of the relevant dimerizers (100 nM homodimerizer and 250 nM heterodimerizer) the two MISTs induced a reversible block of synaptic transmission in cultured neurons and brain slices within 20–30 minutes. It was further shown the VAMP/Syb MIST system can work in vivo. In transgenic mice expressing VAMP/Syb MIST selectively in Purkinje neurons, intraventricular administration of 0.5 nanomoles of the homodimerizer impaired learning of and performance in the rotarod behavior. A decrease in behavioral performance was observed on the first session following dimerizer administration (~12–18 hours post administration); the performance recovered 2 days later. The time course of the actual silencing of the targeted neurons in vivo was not determined.
Rhodopsin/Halorhodopsin
All three of the abovementioned approaches depend upon the application of an exogenous chemical ligand. An alternative approach is controlling neuronal activity by manipulating neuronal excitability using light-gated membrane channels or pumps (Figure 1D). One example of this is rhodopsin 4 (RO4) that partially suppresses action potential generation by hyperpolarizing neurons in a light dependent manner within milliseconds [9•]. Another potent system is based on the light-activated chloride pump halorhodopsin (NpHR), which, in combination with the cation permeable light-sensitive channelrhodopsin 2 (ChR2), permits bi-directional control of action potential firing with millisecond resolution [10, 11••]. The applicability of halorhodopsin to in vivo manipulation of circuit activity has been verified by demonstration of the control of movement in C. elegans [11••]. The rhodopsin and NpHR systems hold a great promise for rapid in vivo manipulation of neural activity.
WHAT TO CONSIDER WHEN CHOOSING THE SILENCING APPROACH
The relevant biological question and experimental paradigm will define several criteria that will guide the choice of the silencing methodology. A non-exhaustive list of the important issues to consider includes the relevant time frame for the induction and reversal of silencing; the nature of the neuronal population to be targeted for inactivation; the desired expression strategy and the constraints on inducer administration.
Time-course of inactivation and its reversal
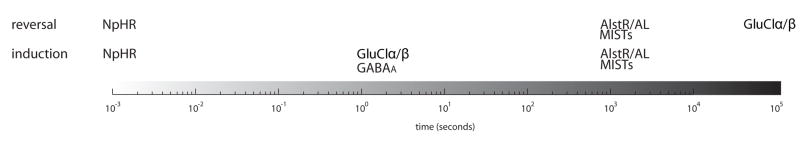
Different experimental paradigms will impose distinct constraints on the time course of induction, maintenance and reversal of silencing (Figure 2). For instance, some experiments in sensory physiology rely on in vivo whole-cell recording of subthreshold membrane activity [22,23]. The limited recording time available in such experiments requires that the circuit perturbation be induced and reversed within minutes. Thus, of the existing methods halorhodopsin might be best suited in this context if silencing needs to be reversible, whereas GABAA or GluClα/β can also be used when reversal is not important. Experiments examining the functional structure and dynamics of cortical circuits might employ imaging of neuronal activity using functional indicators [24,25]. Although a number of genetically-encodable indicators have been described [26,27], their inability to follow single action potentials has limited their applicability up to now [28]. Thus, indicator dyes that are bulk-loaded into the tissue are still employed by most studies [25,29,30]. The time course of such experiments is limited by the retention half-life for these synthetic dyes and usually ends up being on the orders of several hours. In such cases, circuit perturbation needs to be achieved within tens of minutes for longitudinal experiments; thus, all of the abovementioned systems should be applicable. In contrast to network dynamics questions, studies exploring contribution of particular circuit components to animal behavior will often tolerate a longer induction time course for activity perturbation. All of the systems described in this review should be useful in such studies. In cases when long-term silencing is required, however, the exogenous conductances in channel-based approaches may perturb the energy budget of neurons possibly making MISTs a better choice.
Figure 2.
Time course of the induction and reversal of inactivation for the four neuronal silencing systems
The nature of circuit components to be silenced
It is also important to consider which circuit components are to be targeted. One issue is whether the neuronal population is widely distributed or localized to a small region of the brain. As the allatostatin receptor and halorhodopsin require localized delivery of the inducer (allatostatin or light), they may be best suited for the latter case. In contrast, the GABAA, GluClα/β and the MIST systems should be applicable in both. In some cases the limited spread of the induction by AlstR and light will be a great advantage if the required degree of cell-type specificity cannot be achieved by genetic means. Another issue is how large the cells are physically and/or electrotonically. When a high level of expression for exogenous channels is difficult to achieve genetically, channel density might decrease with the size of the targeted cell. Thus, small cells, e.g interneurons, could be easily silenced by channel-based methods whereas large cells, e.g. Purkinje cells, might prove difficult to hyperpolarize and may be better targeted for inactivation using MISTs. On the other hand, if neurons in the circuit are electrically coupled, channel-based methods would be advantageous as MISTs selectively perturb communication through the chemical synapses. Suppression of excitability through the zolpidem/GABAA system depends on the interaction of the drug with the α-γ subunit interface of the channel. Zolpidem is most effective at the α1-γ2 subunit containing complexes with a significantly lower affinity for the α2 and α3 containing channels and no affinity for the α4-α6 subunits [31][32]. As the zolpidem/GABAA systems relies on the endogenous alpha subunit expression, silencing of neurons in regions with little or no α1 subunit expression (like amygdala, ventral striatum and others [33]) may prove challenging. Finally, at present, MISTs represent the only approach for perturbing individual projections within a circuit. Since MIST-dependent inactivation of neuronal activity relies on the perturbation of synapses, localized delivery of the dimerizer should allow targeting of individual projections. In contrast, channel-based interference with action-potential generation will lead to inactivation of the entire set of projections from the targeted neuronal population. It is important to mention, however, that if good axonal targeting of channel-based systems can be achieved, localized inhibition of action potential propagation leading to silencing of specific projections may become possible for these systems as well.
Expression strategy
Two strategies for introducing silencing systems into animals have been pursued: viral expression with stereotactic delivery [12••, 13••], and transgenesis [8••]. For both strategies the choice between one and two-component systems is important. Whereas two-component systems (GluClα/β and Sph-StxTM MIST) will give a greater combinatorial control over the population of neurons to be silenced, the accompanying requirement for co-expression of both components can be technically difficult in viral and genetic systems. One component systems are easier to express and allow for much simpler breeding schemes when used in transgenic mice, which is an important issue when many animals are needed for experiments.
Delivery of the inducer
Another factor to consider is the possible restriction on the way, in which the inducer is delivered. For AlstR and halorhodopsin, the delivery has to be rather localized. Allatostatin needs to be applied within ~1millimeter of the targeted neurons. If cells in the cortex are being silenced, the peptide can be applied to the surface of the brain, however it needs to be injected with a glass pipette if deeper brain areas are targeted [12••]. The light source for the activation of halorhodopsin may need to be close to the targeted neurons as well given the power required for stimulation and the light-scattering and absorption properties of the brain tissue and is often done with an optical fiber for stimulating deep in the brain. Although both glass pipettes for the AL delivery and optical fibers can be quite small in diameter (~30 micrometers for the pipette [12••] and ~100 micrometers for the fiber [34]) and thus may not cause much damage, care may need to be taken in some cases to ensure that an important fiber tract has not been disrupted. Dimerizers that are used for the induction of MIST-dependent silencing do not impose such strict restrictions on delivery. Karpova et al. administered the homodimerizer used with VAMP/Syb MIST into the lateral ventricle, far removed from the terminals of the Purkinje cells [8••]. Further studies will be needed to determine if this homodimerizer penetrates the blood brain barrier (BBB) sufficiently well that it can be administered peripherally. Ivermectin and zolpidem are so far the only inducers discussed here that have been shown to work in the brain following peripheral delivery [13••]. The heterodimerizer used with the Sph-StxTM MIST is known to cross the blood brain barrier (Dr. Victor Rivera, personal communication), and thus should be able to work when administered systemically as well.
For many experiments, the stress to animals following surgical delivery of the inducer may prove to be a non-issue. In others, a single surgery implanting the optical fiber or a miniature osmotic pump days in advance of the actual behavioral testing will circumvent the problem with stress. Yet in some cases, the behavioral studies may be exquisitely sensitive to any stress the animal experiences. Under those circumstances, the less invasive the route of the inducer delivery is, the more advantageous the silencing system. In the ideal case, the chemical inducer would be delivered with food or water and the inducer would not only need to cross the blood brain barrier but also be sufficiently water-soluble. This can be accomplished for the tetracycline-inducible gene expression, which has been useful for the slower induction of neuronal silencing using tetanus toxin light chain [35] and could be used to make the elegant system based on tethered toxins [5•] inducible as well. However, none of the inducers discussed here are sufficiently water soluble for this route of delivery. Short of that, intraperitoneal injection would be the best drug delivery method when stress is a factor.
CURRENT CHALLENGES
Although great progress has been made developing genetically encoded systems for rapidly inducible and reversible silencing, none of the systems described here is ideal. The limitations of the existing systems can be partly overcome by a careful choice of the silencing system (or systems) given the biological question and experimental context. However, further characterization of 1) the pharmacokinetics of the inducers, 2) the time course of the actual neuronal silencing rather than of behavioral perturbation and 3) the dynamic range of the effect at different expression levels of each system would help in making a proper choice. In addition, it is already clear that some optimization of the existing systems would be beneficial. For instance, it is known that ivermectin can cause toxicity at high concentrations [36]. Although no toxicity was observed in the study by Lerchner et al., the concentration of ivermectin necessary to achieve behavioral perturbation was only 2–4 fold smaller than what has been reported to cause side-effects [6]. Furthermore, ivermectin affects other endogenous murine channels including GABA channels [37] and P2X4 purinergic receptors [38]. It will be important to address these potentially negative side-effects in the future. Similarly, the heterodimerizer used with the Sph-StxTM MIST system binds to the endogenous FKBP protein and is thus likely to have side-effects at some concentrations. Finding ways to modify these chemicals to alleviate these non-specific effects while retaining their functionality would be a major advance. Improving their blood-brain barrier permeability may be helpful as well.
CONCLUSIONS
With the recent developments in the field of molecular tools for perturbing neural activity, highly specific manipulations of circuit function in vivo are becoming possible. Although some challenges remain, we are finally well under way to go beyond classical lesion studies in understanding the neural basis of behavior.
Acknowledgments
The authors thank Drs. Winfried Denk, Thomas Südhof and Karel Svoboda for helpful comments on the manuscript. D.G.R.T was supported, in part, by the Howard Hughes Medical Institute predoctoral fellowship and George A. and Majorie H. Anderson Fellowship. A.Y.K. was supported, in part, by the Burroughs Wellcome Foundation and Howard Hughes Medical Institute (HHMI). Development of the MIST silencing system was supported by HHMI and NIH (R01MH070052 to Karel Svoboda).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dougal Gowanlock Robinson Tervo, Watson School of Biological Sciences, Cold Spring Harbor Laboratory, 1 Bungtown Rd, Cold Spring Harbor, NY 11724, (516)606-2697, gowantervo@gmail.com.
Alla Y Karpova, Howard Hughes Medical Institute, Janelia Farm Research Campus, 19700 Helix Drive, Ashburn, VA 20147, (571) 209-4135, karpovaa@janelia.hhmi.org.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Shah EM, Jay DG. Methods for ablating neurons. Curr Opin Neurobiol. 1993;3:738–742. doi: 10.1016/0959-4388(93)90146-p. [DOI] [PubMed] [Google Scholar]
- 2.Marek KW, Davis GW. Controlling the active properties of excitable cells. Curr Opin Neurobiol. 2003;13:607–611. doi: 10.1016/j.conb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Miesenbock G. Genetic methods for illuminating the function of neural circuits. Curr Opin Neurobiol. 2004;14:395–402. doi: 10.1016/j.conb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Callaway EM. A molecular and genetic arsenal for systems neuroscience. Trends Neurosci. 2005;28:196–201. doi: 10.1016/j.tins.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 5•.Ibanez-Tallon I, Wen H, Miwa JM, Xing J, Tekinay AB, Ono F, Brehm P, Heintz N. Tethering naturally occurring peptide toxins for cell-autonomous modulation of ion channels and receptors in vivo. Neuron. 2004;43:305–311. doi: 10.1016/j.neuron.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of Mammalian neurons. J Neurosci. 2002;22:5287–5290. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slimko EM, McKinney S, Anderson DJ, Davidson N, Lester HA. Selective electrical silencing of mammalian neurons in vitro by the use of invertebrate ligand-gated chloride channels. J Neurosci. 2002;22:7373–7379. doi: 10.1523/JNEUROSCI.22-17-07373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Karpova AY, Tervo DG, Gray NW, Svoboda K. Rapid and Reversible Chemical Inactivation of Synaptic Transmission in Genetically Targeted Neurons. Neuron. 2005;48:727–735. doi: 10.1016/j.neuron.2005.11.015. Provides in vitro and in vivo characterization of MISTs. [DOI] [PubMed] [Google Scholar]
- 9•.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. Characterizes rhodopsin 4-dependent silencing in vitro and in chick embryos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han X, Boyden ES. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11••.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. Introduces the use of the chloride pump halorhodopsin for rapid supression of neuronal activity and demonstrates its use in C elegans. [DOI] [PubMed] [Google Scholar]
- 12••.Tan EM, Yamaguchi Y, Horwitz GD, Gosgnach S, Lein ES, Goulding M, Albright TD, Callaway EM. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. Extends the use of allatostatin receptor to in vivo applications in the thalamus and cortex of rats, ferrets and monkeys. [DOI] [PubMed] [Google Scholar]
- 13••.Lerchner W, Xiao C, Nashmi R, Slimko EM, van Trigt L, Lester HA, Anderson DJ. Reversible silencing of neuronal excitability in behaving mice by a genetically targeted, ivermectin-gated cl(−) channel. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. In vivo application of the GluCl system to silencing of the striatal circuits. [DOI] [PubMed] [Google Scholar]
- 14.Birgül N, Weise C, Kreienkamp HJ, Richter D. Reverse physiology in drosophila: identification of a novel allatostatin-like neuropeptide and its cognate receptor structurally related to the mammalian somatostatin/galanin/opioid receptor family. 1999;18:5892–5900. doi: 10.1093/emboj/18.21.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Gosgnach S, Lanuza GM, Butt SJB, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. 2006;440:215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 16.Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. Cloning of an ivermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Slimko EM, Lester HA. Selective elimination of glutamate activation and introduction of fluorescent proteins into a Caenorhabditis elegans chloride channel. FEBS Lett. 2002;528:77–82. doi: 10.1016/s0014-5793(02)03245-3. [DOI] [PubMed] [Google Scholar]
- 18.Slimko EM, Lester HA. Codon optimization of Caenorhabditis elegans GluCl ion channel genes for mammalian cells dramatically improves expression levels. J Neurosci Methods. 2003;124:75–81. doi: 10.1016/s0165-0270(02)00362-x. [DOI] [PubMed] [Google Scholar]
- 19••.Wulff P, Goetz T, Leppa E, Linden AM, Renzi M, Swinny JD, Vekovischeva OY, Sieghart W, Somogyi P, Korpi ER, et al. From synapse to behavior: rapid modulation of defined neuronal types with engineered GABA(A) receptors. Nat Neurosci. 2007;10:923–929. doi: 10.1038/nn1927. Describes the use of zolpidem-sensitive GABAA channel to enhance IPSCs onto taregted cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kitamoto T. Targeted expression of temperature-sensitive dynamin to study neural mechanisms of complex behavior in Drosophila. J Neurogenet. 2002;16:205–228. doi: 10.1080/01677060216295. [DOI] [PubMed] [Google Scholar]
- 21.Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- 22.Margrie TW, Brecht M, Sakmann B. In vivo, low-resistance, whole-cell recordings from neurons in the anaesthetized and awake mammalian brain. Pflugers Arch. 2002;444:491–498. doi: 10.1007/s00424-002-0831-z. [DOI] [PubMed] [Google Scholar]
- 23.Lee AK, Manns ID, Sakmann B, Brecht M. Whole-cell recordings in freely moving rats. Neuron. 2006;51:399–407. doi: 10.1016/j.neuron.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 25.Ohki K, Chung S, Kara P, Hubener M, Bonhoeffer T, Reid RC. Highly ordered arrangement of single neurons in orientation pinwheels. Nature. 2006;442:925–928. doi: 10.1038/nature05019. [DOI] [PubMed] [Google Scholar]
- 26.Mank M, Reiff DF, Heim N, Friedrich MW, Borst A, Griesbeck O. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys J. 2005 doi: 10.1529/biophysj.105.073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyawaki A, Griesbeck O, Heim R, Tsien RY. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc Natl Acad Sci U S A. 1999;96:2135–2140. doi: 10.1073/pnas.96.5.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pologruto TA, Yasuda R, Svoboda K. Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J Neurosci. 2004;24:9572–9579. doi: 10.1523/JNEUROSCI.2854-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diez-Garcia J, Akemann W, Knopfel T. In vivo calcium imaging from genetically specified target cells in mouse cerebellum. Neuroimage. 2007;34:859–869. doi: 10.1016/j.neuroimage.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan MR, Nimmerjahn A, Sarkisov DV, Helmchen F, Wang SS. In vivo calcium imaging of circuit activity in cerebellar cortex. J Neurophysiol. 2005;94:1636–1644. doi: 10.1152/jn.01013.2004. [DOI] [PubMed] [Google Scholar]
- 31.Pritchett DB, Seeburg PH. Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 32.Wafford KA, Thompson SA, Thomas D, Sikela J, Wilcox AS, Whiting PJ. Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit. Mol Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- 33.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta AD, Jung JC, Flusberg BA, Schnitzer MJ. Fiber optic in vivo imaging in the mammalian nervous system. 2004;14:617–628. doi: 10.1016/j.conb.2004.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamamoto M, Wada N, Kitabatake Y, Watanabe D, Anzai M, Yokoyama M, Teranishi Y, Nakanishi S. Reversible suppression of glutamatergic neurotransmission of cerebellar granule cells in vivo by genetically manipulated expression of tetanus neurotoxin light chain. J Neurosci. 2003;23:6759–6767. doi: 10.1523/JNEUROSCI.23-17-06759.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dadarkar SS, Deore MD, Gatne MM. Comparative evaluation of acute toxicity of ivermectin by two methods after single subcutaneous administration in rats. Regul Toxicol Pharmacol. 2007 doi: 10.1016/j.yrtph.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Adelsberger H, Lepier A, Dudel J. Activation of rat recombinant alpha(1)beta(2)gamma(2S) GABA(A) receptor by the insecticide ivermectin. Eur J Pharmacol. 2000;394:163–170. doi: 10.1016/s0014-2999(00)00164-3. [DOI] [PubMed] [Google Scholar]
- 38.Sim JA, Chaumont Sv, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J Neurosci. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]