Abstract
Bacterial lipopolysaccharide (LPS) induces monocytes/macrophages to express proinflammatory cytokines and tissue factor (TF), the primary activator of the coagulation cascade. Anti-inflammatory signaling pathways including the phosphatidylinositol-3-kinase (PI3K)-Akt pathway inhibit proinflammatory and TF gene expression in macrophages. We determined the role of Akt, the mammalian target of rapamycin (mTOR) and interleukin-10 in the inhibition of LPS-induced proinflammatory cytokine and TF gene expression in peritoneal macrophages (PMs). We used wild type (WT) peritoneal macrophages (PMs), and PMs from PTENflox/flox/LysMCre mice (PTEN−/− PMs), which have increased Akt activity. Pharmacologic inhibition of mTOR with rapamycin inhibited LPS induction of IL-10 mRNA and protein, and enhanced the expression of TF and the proinflammatory cytokine TNFα in WT PMs. Furthermore, neutralizing IL-10 with anti-IL-10 antibody enhanced LPS induction of TNFα and TF expression in WT PMs. The addition of recombinant IL-10 abolished rapamycin enhancement of LPS-induced TNFα and TF expression in WT PMs. Consistent with enhanced Akt activation, LPS-induced IL-10 expression was increased in PTEN−/− PMs compared to WT PMs. In contrast, LPS-induced TNFα and TF expression was significantly reduced in PTEN−/− PMs compared to WT PMs. However, the neutralizing IL-10 antibody did not completely prevent inhibition of LPS-induced TNFα and TF expression in PTEN−/− PMs. The results indicate that mTOR-dependent IL-10 expression leads to inhibition of LPS induction of TF and the proinflammatory cytokine TNFα in WT macrophages. In contrast, the decrease in LPS-induced TNFα and TF expression in PTEN−/− PMs also requires an IL-10-independent pathway.
Keywords: tissue factor, lipopolysaccharide, interleukin-10, Akt, mammalian target of rapamycin
Introduction
Bacterial lipopolysaccharide (LPS) stimulation of monocytes/macrophages induces expression of the anti-inflammatory cytokine interleukin-10 (IL-10) (Williams et al., 2002). IL-10 exerts both autocrine and paracrine anti-inflammatory effects through the activation of diverse intracellular signaling pathways and the transcription factor signal transducer and activator of transcription 3 (STAT3) (Williams et al., 2004a;Williams et al., 2004b). IL-10 also induces the expression of other anti-inflammatory proteins, including the soluble interleukin-1 receptor antagonist (sIL-1ra) and Bcl-3 (Kuwata et al., 2003;Carl et al., 2004). Neutralization of IL-10 expressed by monocytes enhanced LPS induction of the proinflammatory cytokine tumor necrosis factor-α (TNFα) and tissue factor (TF), the principal activator of the coagulation cascade (Poitevin et al., 2007;Fiorentino et al., 1991). Treatment of a macrophage cell line and peritoneal macrophages with high concentrations of exogenous IL-10 also inhibited LPS induction of TNFα and TF (Kamimura et al., 2005). Moreover, exogenous IL-10 inhibited LPS-induced inflammatory cytokine expression in Kupffer cells and alveolar macrophages (Isler et al., 1999; Yokoyama et al., 2004). Of importance, IL-10 has also been shown to limit LPS-induced inflammation and coagulation in vivo (Howard et al., 1993;Pajkrt et al., 1997).
LPS-induced IL-10 expression in monocytes/macrophages requires activation of the p38 mitogen activated protein kinase (MAPK) and IκB-kinase signaling pathways (Ma et al., 2001;Liu et al., 2006). The phosphatidylinositol-3-phosphate kinase (PI3K)-Akt signaling pathway has also been shown to induce the expression of IL-10 (Pengal et al., 2006;Martin et al., 2003). Interestingly, pharmacologic inhibition of PI3K inhibits IL-10 expression, but enhances LPS activation of p38 (Martin et al., 2003;Guha and Mackman, 2002). This suggests that the PI3K-Akt signaling pathway plays a role in IL-10 expression that cannot be rescued by enhanced MAPK activation. Indeed, other studies have suggested that Akt activation of mammalian target of rapamycin (mTOR) enhances IL-10 expression (Jorgensen et al., 2001;Ohtani et al., 2008). However, the interaction between the PI3K-Akt-mTOR and MAPK signaling pathways in LPS-treated macrophages requires clarification.
LPS-induced inflammation and coagulation is coordinated at multiple steps, including feedback inhibition conferred by activation of anti-inflammatory signaling pathways in monocytes/macrophages. We and others have shown that LPS activation of the phosphatidylinositol-3-kinase-Akt signaling pathway inhibits LPS-induced cytokine and TF expression in vitro and in vivo (Ruse and Knaus, 2006;Luyendyk et al., 2008;Fukao and Koyasu, 2003). Specifically, LPS activation of the p38, JNK1/2 and ERK1/2 MAPK signaling pathways that regulate proinflammatory gene expression in macrophages were significantly reduced in PTEN−/− macrophages, which have enhanced Akt activation (Luyendyk et al., 2008;Cao et al., 2004). Similarly, LPS activation of ERK1/2 and induction of TNFα expression were reduced in SH2 domain-containing inositol 5 -phosphatase (SHIP)-1−/− macrophages, which have enhanced Akt activity (Fang et al., 2004). This suggests that activation of the PI3K-Akt pathway inhibits LPS activation of monocytes/macrophages by limiting MAPK signaling. Indeed, Akt has been shown to inhibit Raf-1 and ASK1, two upstream MAP3Ks critical for LPS signaling (Kim et al., 2001;Rommel et al., 1999). However, the anti-inflammatory effects of PI3K-Akt pathway activation in macrophages likely occur by multiple mechanisms. Indeed, the relative contribution of mTOR and IL-10 to the Akt-dependent inhibition of LPS-induced cytokine and TF expression in macrophages is not completely understood.
We determined the effect of genetically increasing activation of the PI3K-Akt-mTOR pathway on LPS-induced IL-10 expression and whether this impacted LPS-induced proinflammatory cytokine and TF expression. The results indicate that LPS activation of the PI3K-Akt-mTOR pathway promotes LPS induction of IL-10 and the subsequent feedback inhibition of TNFα and TF expression. However, IL-10-independent inhibition of LPS-induced TF and cytokine expression was evident in PTEN−/− peritoneal macrophages (PMs).
Materials and Methods
Materials
Escherichia coli LPS of serotype 0111:B4 and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St Louis, MO). Rapamycin was purchased from LC Laboratories (Woburn, MA). The neutralizing anti-mouse IL-10 antibody JESS-2A5 and recombinant murine IL-10 were purchased from BioLegend (San Diego, CA) and isotype control rat IgG was purchased from BioXCell (West Lebanon, NH).
Mice
All studies were approved by the University of Kansas Animal Care and Use Committee and comply with National Institutes of Health guidelines. Male and female mice used for these studies were between 6 and 8 weeks of age. Wild-type (WT) C57BL/6J mice were obtained from The Jackson Laboratory. PTENflox/flox/LysMCre mice and PTENflox/flox mice backcrossed 6 times onto the C57BL/6J background were generated as described previously (Luyendyk et al., 2008) by breeding male PTENflox/flox/LysMCre mice with female PTENflox/flox mice.
Isolation and culture of PMs
Thioglycollate-elicited peritoneal macrophages (PMs) were isolated from mice by peritoneal lavage 3 days after injection of mice with 2 ml of 3% thioglycollate solution. Adherent cells adjusted to a density of 1 × 106 cells/ml in RPMI 1640 supplemented with 2 mM L-glutamine, 10 mM HEPES, 50 U/ml penicillin 50 μg/ml streptomycin, and 8% heat-inactivated fetal calf serum (Omega Scientific, Tarzana, CA) were cultured overnight at 37°C in 5% CO2. Cells were stimulated with 1 μg/ml LPS, a concentration of LPS shown to require TLR4 for induction of TNFα expression (Lehner et al., 2001). Rapamycin (10 ng/ml) or vehicle (0.1% DMSO) were added to the culture medium 30 minutes prior to LPS treatment. Rat isotype control IgG (0–5 μg/ml) or anti-mouse IL-10 antibody JESS-2A5 (0–5 μg/ml) were diluted in endotoxin-free PBS (Sigma) and added to the culture medium immediately after LPS stimulation. Recombinant murine IL-10 (400 pg/ml) was diluted in endotoxin-free PBS and added to the culture medium 2 hours after LPS stimulation.
Determination of supernatant cytokine concentration
Levels of TNFα and IL-10 in cell culture supernatant were determined using commercially available ELISA kits (R&D Systems).
Procoagulant Activity (PCA)
Cell pellets (1.5 × 106 cells) were solubilized in 15 mM n-Octyl-β-D-glucopyranoside at 37 °C for 15 minutes. The procoagulant activity (PCA) of TF in cell lysates was then measured using a single-stage clotting assay as described previously (Morrissey et al., 1988) with a Start4 coagulation analyzer (Dianostica Stago). Clotting times were converted to PCA by comparison with a standard curve that was established using mouse tissue factor. The PCA was then normalized to total protein concentration, which was determined using a Bio-Rad DC protein assay (Bio-Rad).
Western blotting
Whole cell extracts were prepared by lysis of cell pellets in 2X LDS sample buffer (Invitrogen) containing PhosStop phosphatase inhibitor cocktail (Roche). Proteins were separated by SDS-PAGE on 12% Bis-Tris criterion gels (Bio-Rad) and transferred to Immobilon-P membrane (Millipore). The phosphorylation of Akt (Ser473) and p38 (Thr180/Tyr182) was determined using phospho-specific antibodies (Cell Signaling Technology) and a secondary anti-rabbit IgG-HRP conjugated antibody (Pierce Biotechnology). Membranes were washed and incubated with Supersignal West Pico substrate (Pierce Biotechnology) solution and exposed to Blue lite film (ISC Bioexpress). Blots were stripped and reprobed using antibodies against the nonphosphorylated forms of Akt and p38 (Cell Signaling Technology) to monitor protein loading.
RNA isolation, cDNA synthesis, and Real Time PCR
RNA was isolated from 3 × 106 PMs using TRI Reagent (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. cDNA was synthesized from 1 μg of RNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the manufacturer’s protocol using a MyCycler thermal cycler (Bio-Rad). Levels of TNFα, IL-10, TF, and GAPDH mRNA were determined using Taqman gene expression assays (Applied Biosystems) and Taqman gene expression mix (Applied Biosystems) and an ABI Prism 7300 sequence detection system. The expression of each gene was normalized relative to GAPDH expression levels and relative expression levels were determined using the comparative Ct method.
Statistics
Data are presented as mean ± SEM. Unpaired or paired t-test were used as appropriate for the comparison of two treatment groups. When more than two groups were compared, one way analysis of variance was utilized. Multiple comparisons were made using Student-Neuman-Keuls test. The criterion for significance for all experiments was p < 0.05.
Results
Modulation of LPS-induced cytokine and tissue factor expression by mTOR in macrophages
To determine the role of mTOR in LPS-induced cytokine and tissue factor expression, we utilized the pharmacologic inhibitor rapamycin. Rapamycin pretreatment enhanced the LPS induction of TNFα and TF mRNA expression (Fig. 1A). Rapamycin also increased LPS induction of TNFα protein and cellular procoagulant activity (PCA) in WT macrophages (Fig. 1A). Rapamycin did not affect the LPS-induced phosphorylation of Akt or p38 MAPK (Fig. 1B). Interestingly, rapamycin pretreatment significantly inhibited LPS induction of IL-10 mRNA and protein in WT macrophages (Fig. 2A and B). The data indicate that rapamycin impairs expression of anti-inflammatory IL-10 and enhances expression of TNFα and TF in LPS-treated macrophages.
Figure 1. Effect of rapamycin on LPS activation of Akt and p38 pathways and induction of TF activity and TNFα expression in WT PMs.

WT PMs were pretreated with rapamycin (10 ng/ml) or vehicle (0.1% DMSO) for 30 minutes prior to stimulation with LPS (1 μg/ml). (A) Levels of TNFα and TF mRNA were determined 120 and 240 minutes after LPS stimulation. mRNA levels normalized to GAPDH mRNA are expressed as mean ± SEM relative to WT cells stimulated with LPS for 240 min (defined as 100%). The supernatant level of TNFα and procoagulant activity (PCA) were determined 6 hours after LPS stimulation. (n = 8–22) *, p < 0.05. (B) The activation of Akt and p38 at various times (0–30 min) was determined in whole cell protein extracts by Western blotting using anti-phospho specific Abs. Each blot was stripped and reprobed for the nonphosphorylated form of each protein to assess loading. Representative western blots are shown.
Figure 2. Effect of rapamycin on LPS induction of IL-10 expression in WT PMs.
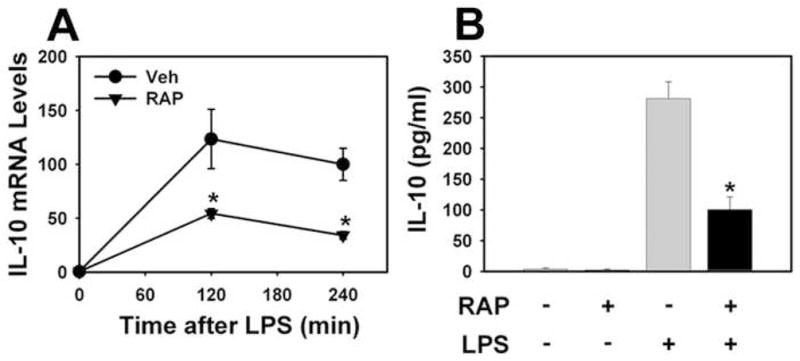
WT PMs were pretreated with rapamycin (10 ng/ml) or vehicle (0.1% DMSO) for 30 minutes prior to stimulation with LPS (1 μg/ml). Levels of IL-10 mRNA (A) were determined 120 and 240 minutes after LPS stimulation and the levels of IL-10 protein (B) in the culture medium were determined 6 hours after LPS stimulation. mRNA levels normalized to GAPDH mRNA are expressed as mean ± SEM relative to Veh-treated cells stimulated with LPS for 240 min (defined as 100%). (n = 8–10) *, p < 0.05.
IL-10 neutralizing antibody enhances LPS induction of TNFα and TF expression in macrophages
Our results suggested that impaired IL-10 synthesis could contribute to the effect of rapamycin on LPS induction of TF and TNFα. To determine whether LPS induction of IL-10 contributed to the expression of TF and proinflammatory cytokines in PMs, we utilized an IL-10 neutralizing antibody (clone JESS-2A5). LPS induction of TNFα protein and PCA was enhanced in macrophages co-treated with anti-IL-10 antibody compared to macrophages co-treated with an isotype control rat IgG antibody (Fig. 3A–B). The results indicate that endogenous IL-10 inhibits the LPS-induced expression of TNFα and TF.
Figure 3. Effect of a neutralizing anti-IL-10 antibody on LPS induction of TF activity and TNFα expression in WT PMs.

WT PMs were stimulated with LPS (1 μg/ml) in the presence of various concentrations (0–5 μg/ml) of anti-IL-10 antibody. Supernatant levels of TNFα (A) and (B) procoagulant activity (PCA) were determined 6 hours after LPS stimulation. (n = 6–8) *, p < 0.05.
Exogenous IL-10 abolishes rapamycin enhancement of LPS-induced TNFα and TF expression in macrophages
Next, we determined whether the effect of rapamycin on LPS-induced TNFα and TF expression could be reversed by the addition of exogenous IL-10. Rapamycin significantly inhibited LPS induction of IL-10 (Fig. 4A). Addition of 400 pg/ml recombinant IL-10 to the culture medium 2 hours after LPS stimulation restored supernatant IL-10 concentration to that observed in PMs stimulated with LPS in the absence of rapamycin (Fig. 4A). Of importance, the restoration of IL-10 levels in the culture medium abolished the rapamycin-mediated enhancement of LPS-induced PCA and TNFα expression (Fig. 4B–C). The data suggest that rapamycin enhances LPS-induced TNFα and TF expression by reducing IL-10 expression.
Figure 4. Effect of recombinant IL-10 treatment on rapamycin enhanced LPS induction of TF activity and TNFα expression in WT PMs.
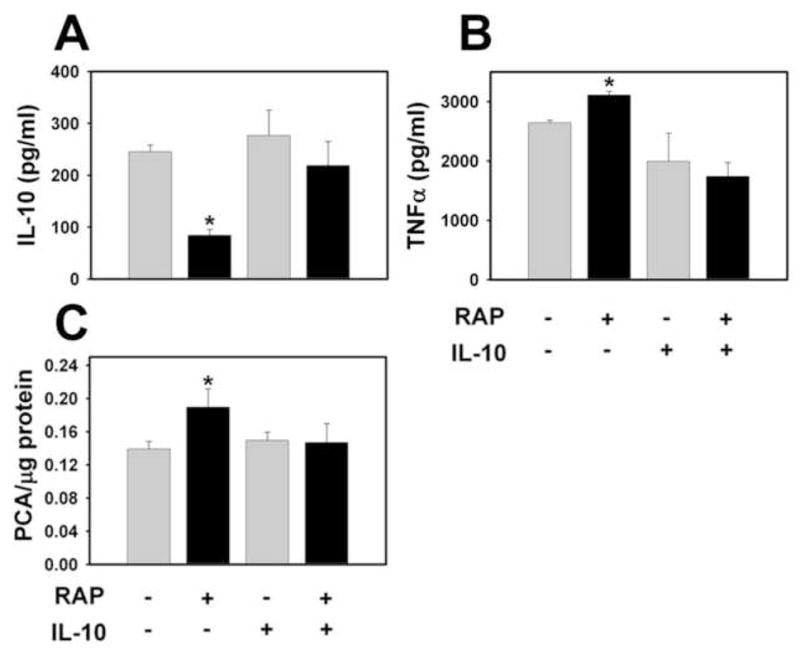
WT PMs were stimulated with LPS (1 μg/ml). Two hours later, recombinant murine IL-10 (400 pg/ml) or sterile PBS was added to the culture medium. Supernatant levels of IL-10 (A), TNFα (B) and procoagulant activity (PCA) (C) were determined 6 hours after LPS stimulation. (n = 6) *, p < 0.05.
Effect of enhanced Akt activation on LPS-induced IL-10 expression in macrophages
Our results indicated that mTOR, which is activated by Akt signaling, contributes to IL-10 expression in macrophages. Accordingly, we determined the effect of genetically increasing Akt activation on LPS induction of IL-10. We have shown that LPS activation of Akt is increased in PTEN−/− PMs compared to WT PMs (Luyendyk et al., 2008). Of importance, LPS induction of IL-10 mRNA was significantly increased in PTEN−/− PMs between 30–120 minutes compared to WT PMs (Fig. 5A). Supernatant IL-10 levels were also significantly increased in LPS-treated PTEN−/− PMs compared to WT PMs (Fig. 5B).
Figure 5. LPS induction of IL-10 mRNA and protein in PTEN−/− PMs.
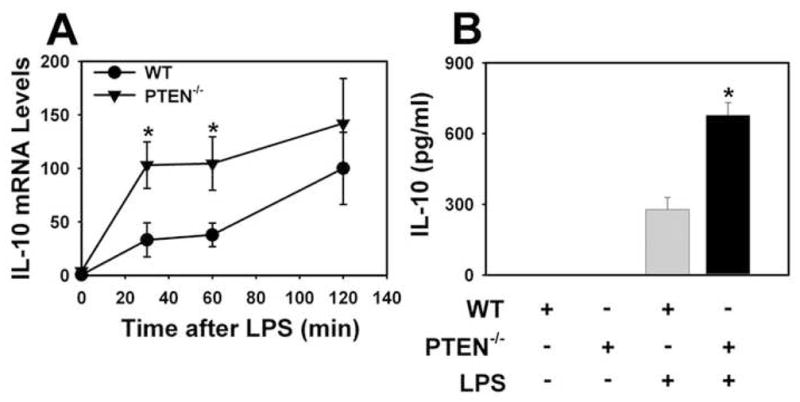
WT and PTEN−/−PMs were stimulated with LPS (1 μg/ml). Levels of IL-10 mRNA (A) were determined 30, 60 and 120 minutes after LPS stimulation. mRNA levels normalized to GAPDH mRNA are expressed as mean ± SEM (n = 6–8) relative to WT cells stimulated with LPS for 120 min (defined as 100%). Levels of IL-10 protein (B) in the culture medium were determined 6 hours after LPS stimulation. Data are expressed as mean ± SEM (n = 8). *, p < 0.05.
Inhibition of LPS-induced TNFα and TF expression caused by PTEN-deficiency does not require enhanced IL-10 expression
Our previous studies indicated that enhanced Akt activation in PTEN−/− PMs inhibited LPS activation of the MAPK signaling pathways (Luyendyk et al., 2008). Insofar as LPS induction of IL-10 was increased in PTEN−/− PMs, we hypothesized that the enhanced IL-10 expression could also contribute to the inhibition of LPS-induced TNFα and TF expression in these cells. To this end, we determined the effect of IL-10 neutralization on the inhibition of LPS-induced TNFα and procoagulant activity caused by PTEN-deficiency in PMs. Addition of neutralizing anti-IL-10 antibody (5 μg/ml) enhanced the LPS induction of TNFα and procoagulant activity in WT PMs and PTEN−/− PMs (Fig. 6A–B). This suggests that IL-10 has similar anti-inflammatory effects regardless of PTEN-deficiency in LPS-treated PMs. In agreement with our previous findings (Luyendyk et al., 2008), LPS induction of TNFα and TF expression was significantly reduced (53% and 35%, respectively) in PTEN−/− PMs compared to WT PMs (Fig. 6A–B). Interestingly, LPS-induced TNFα and TF expression was also significantly reduced (30% and 23%, respectively) in PTEN−/− PMs compared to WT PMs in the presence of the neutralizing anti-IL-10 antibody (Fig. 6A–B). Taken together, the data suggest that PTEN-deficiency inhibits LPS-induced TNFα and TF expression in PMs in part by an IL-10 independent mechanism
Figure 6. Effect of neutralizing anti-IL-10 antibody on LPS induction of TNFα expression and TF activity in PTEN−/− PMs.
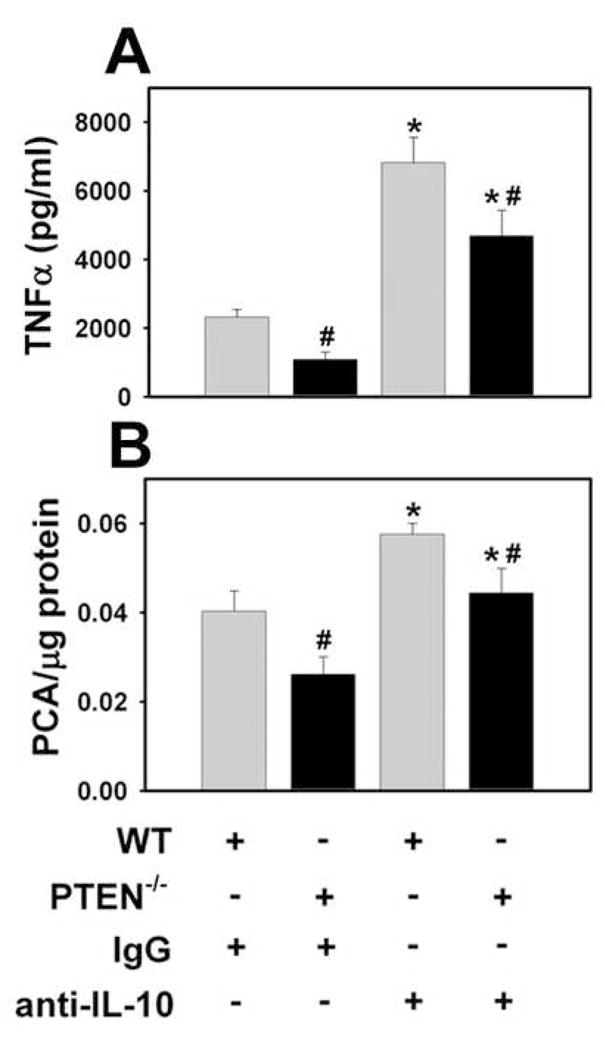
WT and PTEN−/− PMs were treated with LPS (1 μg/ml) in the presence of neutralizing anti-IL-10 antibody (5 μg/ml) or control IgG (5 μg/ml). Supernatant levels of TNFα (A) and procoagulant activity (PCA) (B) were determined 6 hours after LPS stimulation. (n = 5–6) *, p < 0.05.
Discussion
Using a genetic approach we show that the PI3K-Akt pathway supports LPS induction of the anti-inflammatory cytokine IL-10. Our results are in agreement with previous studies showing that pharmacologic inhibitors of PI3K reduce LPS induction of IL-10 in monocytes/macrophages (Martin et al., 2003). Indeed, we also found that IL-10 expression was decreased in Pik3r1−/− macrophages, which have decreased Akt activation after treatment with LPS (not shown). In contrast, IL-10 mRNA and protein expression were significantly increased in PTEN−/− PMs, which have enhanced basal and LPS activation of Akt (Luyendyk et al., 2008). This is consistent with the enhanced LPS induction of IL-10 protein in macrophages expressing a constitutively activated Akt (Pengal et al., 2006). Taken together, the data indicate that enhancing Akt activation supports LPS-induced IL-10 transcription in macrophages.
The MAPK signaling pathways are required for LPS induction of IL-10 expression in macrophages (Ma et al., 2001;Liu et al., 2006). Indeed, we found that a pharmacologic inhibitor (SB203580) of the p38 MAPK signaling pathway reduced LPS induction of IL-10 in PMs (not shown). Interestingly, enhanced Akt activation inhibited p38 activation in LPS-treated PTEN−/− PMs (Luyendyk et al., 2008). However, our current findings indicate that LPS-induced IL-10 expression was enhanced in PTEN−/−PMs. This suggests that LPS activation of the MAPKs in PTEN−/− PMs is retained above the threshold required to elicit IL-10 expression, and that a second, Akt-dependent parallel pathway further increases IL-10 expression. LPS activation of the Akt-mTOR signaling pathway has been implicated in the induction of IL-10 in dendritic cells and whole blood (Ohtani et al., 2008;Jorgensen et al., 2001). We found that rapamycin did not inhibit LPS activation of the p38 MAPK signaling pathway, but significantly reduced LPS induction of IL-10 mRNA and protein in LPS-treated PMs. This suggests that mTOR modifies LPS-induced IL-10 expression independently of MAPK activation (Fig. 7).
Figure 7. Proposed regulation of LPS-induced cytokine and TF expression by Akt, mTOR, and the MAPKs in macrophages.

LPS activation of the mitogen activated protein kinases (MAPKs) induces the expression of tissue factor (TF) as well as the proinflammatory cytokine TNFα. LPS activation of the MAPKs is also required for the expression of the anti-inflammatory cytokine, IL-10. LPS activation of the phosphatidylinositol-3-kinase (PI3K)-Akt signaling pathway inhibits the expression of TF and TNFα by 1) direct Akt-dependent inhibition of the MAPK signaling cascades and 2) supporting IL-10 production through the activation of mTOR. The relative contribution of each anti-inflammatory mechanism likely depends on the degree to which Akt is activated. Our studies in PTEN−/− PMs suggest that Akt-dependent MAPK inhibition is a critical anti-inflammatory pathway under conditions of exaggerated Akt activation.
Previous studies have shown that genetic or pharmacologic inhibition of PI3K in macrophages enhanced MAPK activation and the expression of proinflammatory cytokine and TF mRNAs after 2 hours of LPS stimulation (Luyendyk et al., 2008;Cao et al., 2004). In contrast, we found that rapamycin did not impact the LPS-induced expression of TNFα and TF mRNAs in PMs at this time. However, rapamycin inhibited IL-10 expression as early as 2 hours and this was associated with prolonged expression of TNFα and TF mRNA, increased TNFα protein supernatant concentration, and cellular procoagulant activity. In agreement with other studies (Poitevin et al., 2007;Fiorentino et al., 1991), we found that neutralizing endogenous IL-10 enhanced LPS induction of TF and TNFα. The observation that direct neutralization of IL-10 in the culture medium enhanced LPS-induced TF and TNFα expression to a greater degree than rapamycin likely relates to our finding that rapamycin does not completely block LPS-induced IL-10 expression. Of importance, restoration of IL-10 concentration in the culture supernatant blocked the rapamycin enhancement of LPS-induced TNFα and TF expression. Taken together, the results indicate that rapamycin enhances LPS induction of proinflammatory cytokines and TF in WT macrophages by inhibiting IL-10 expression (Fig. 7).
The requirement of mTOR for LPS-induced IL-10 expression in WT PMs is further evidence of the anti-inflammatory role of the PI3K-Akt signaling pathway in LPS-treated macrophages. We and others have shown that enhanced activation of Akt in PTEN-deficient PMs inhibits LPS activation of the MAPKs (Luyendyk et al., 2008;Ruse and Knaus, 2006). Indeed, Akt negatively regulates Raf-1 and ASK-1, both of which are upstream activators of the MAPK signaling cascades (Kim et al., 2001;Rommel et al., 1999). Neutralization of IL-10 secreted by the LPS-treated PMs did not completely prevent the decrease in LPS-induced TNFα and PCA in PTEN−/− PMs, despite being added in excess (5 μg/ml) to neutralize the higher levels of IL-10 produced by the PTEN−/− PMs. Interestingly, neutralization of IL-10 enhanced, regardless of the degree of Akt activation (WT or PTEN−/−), the LPS induction of TNFα and TF expression. The results imply that the anti-inflammatory effects of IL-10 are similar in WT and PTEN−/− PMs, but that a distinct Akt-dependent, IL-10 independent anti-inflammatory pathway contributes to inhibition of LPS-induced cytokine and TF expression in PMs. This finding is consistent with the direct inhibition of MAPK signaling by the PI3K-Akt signaling pathway under conditions where Akt activation is dramatically increased (Fig. 7).
IL-10 has been shown to inhibit inflammation and coagulation in models of endotoxemia and sepsis (Van Der et al., 1995;Howard et al., 1993;Pajkrt et al., 1997). However, to determine whether PI3K-Akt-dependent IL-10 expression inhibits inflammation and coagulation in vivo is complex. For example, it has been reported that plasma IL-10 levels are increased in endotoxemic mice expressing a constitutively active Akt in myeloid cells (Pengal et al., 2006). However, PTEN-deficiency in vivo also increased the number of circulating myeloid cells, complicating the interpretation of these results (Zhang et al., 2006;Luyendyk et al., 2008). Interestingly, administration of the PI3K-Akt pathway activator insulin significantly inhibited LPS-induced inflammation and coagulation in mice and rats (Kidd et al., 2008;Jeschke et al., 2004). Moreover, insulin significantly increased plasma IL-10 levels in endotoxemic rats (Jeschke et al., 2004). These studies suggest that activation of the PI3K-Akt pathway in vivo may rebalance LPS-induced gene expression toward resolution of inflammation (i.e., decreased TNFα and increased IL-10) and inhibition of coagulation (decreased TF).
This shift in pro- and anti-inflammatory cytokine expression may be important for paradigms of treatment for inflammatory diseases associated with endotoxemia, such as Gram-negative bacterial sepsis. Unlike targeted pharmacologic inhibition of the MAPK signaling pathways, which limits both proinflammatory (e.g., TNFα) and anti-inflammatory (e.g., IL-10) cytokine expression (Branger et al., 2002), activation of the PI3K-Akt signaling pathway reduces inflammation and coagulation by inhibiting the MAPKs (Luyendyk et al., 2008). Moreover, our data indicate that distinct from MAPK inhibition, the PI3K-Akt pathway exaggerates IL-10 expression by activation of mTOR. Overall, this suggests that exploiting the endogenous anti-inflammatory effects of the PI3K-Akt signaling pathway may afford a more balanced anti-inflammatory treatment strategy in endotoxemia and sepsis. Interestingly, the shift in cytokine expression by the PTEN−/− PMs is consistent with a role for the PI3K-Akt pathway in generating alternatively activated macrophages, which produce higher levels of IL-10. For example, a previous study showed that macrophages from SHIP−/− mice had features of M2 (i.e., alternatively activated macrophages), including enhanced IL-10 expression (Rauh et al., 2005). Moreover, a mixed M1/M2 macrophage response was associated with increased survival in septic baboons (Mehta et al., 2004). Further studies are required to elucidate the complex anti-inflammatory effects of PI3K-Akt activation in macrophages in vivo.
It is intriguing that in addition to LPS-stimulated PMs, rapamycin has been shown to enhance TNFα- and thrombin-induced TF expression by other cell types including endothelial cells and smooth muscle cells (Steffel et al., 2005). This observation is postulated to link the induction of TF expression to the risk of thrombosis associated with sirolimus-eluting stents (Steffel et al., 2005;McFadden et al., 2004;Iakovou et al., 2005). Analogous to our findings with LPS-stimulated PMs, rapamycin did not impact activation of the MAPKs, but substantially enhanced TF expression in endothelial cells stimulated with thrombin (Steffel et al., 2005). This suggests that rapamycin inhibition of IL-10 expression could be a common path to enhanced TF expression in different cell types. Such a connection would imply that modulation of the cytokine environment by drug-eluting stents could contribute to TF expression and thrombosis. However, further studies are required to determine whether impaired IL-10 expression contributes to TF induction and thrombosis in these other models.
In summary, the data indicate that the PI3K-Akt signaling pathway supports LPS induction of IL-10 mRNA and protein in macrophages in an mTOR dependent manner and that rapamycin enhances LPS induction of TF and TNFα expression in WT macrophages by reducing the expression of IL-10. In contrast, under conditions where Akt activity is enhanced (PTEN−/− macrophages), the decrease in LPS-induced TF and TNFα expression is partly independent of enhanced IL-10 expression.
Acknowledgments
This publication was made possible by grant number P20 RR016475 from the National Center for Research Resources (NCRR) and NIH grant P20 RR021940. The authors wish to thank Huina Cai for outstanding technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Branger J, van den Blink B, Weijer S, Madwed J, Bos CL, Gupta A, Yong CL, Polmar SH, Olszyna DP, Hack CE, van Deventer SJ, Peppelenbosch MP, van der Poll T. Anti-inflammatory effects of a p38 mitogen-activated protein kinase inhibitor during human endotoxemia. J Immunol. 2002;168:4070–7. doi: 10.4049/jimmunol.168.8.4070. [DOI] [PubMed] [Google Scholar]
- Cao X, Wei G, Fang H, Guo J, Weinstein M, Marsh CB, Ostrowski MC, Tridandapani S. The inositol 3-phosphatase PTEN negatively regulates Fc gamma receptor signaling, but supports Toll-like receptor 4 signaling in murine peritoneal macrophages. J Immunol. 2004;172:4851–4857. doi: 10.4049/jimmunol.172.8.4851. [DOI] [PubMed] [Google Scholar]
- Carl VS, Gautam JK, Comeau LD, Smith MF., Jr Role of endogenous IL-10 in LPS-induced STAT3 activation and IL-1 receptor antagonist gene expression. J Leukoc Biol. 2004;76:735–742. doi: 10.1189/jlb.1003526. [DOI] [PubMed] [Google Scholar]
- Fang H, Pengal RA, Cao X, Ganesan LP, Wewers MD, Marsh CB, Tridandapani S. Lipopolysaccharide-induced macrophage inflammatory response is regulated by SHIP. J Immunol. 2004;173:360–366. doi: 10.4049/jimmunol.173.1.360. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- Guha M, Mackman N. The phosphatidylinositol 3-kinase-akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Howard M, Muchamuel T, Andrade S, Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- Isler P, de Rochemonteix BG, Songeon F, Boehringer N, Nicod LP. Interleukin-12 production by human alveolar macrophages is controlled by the autocrine production of interleukin-10. Am J Respir Cell Mol Biol. 1999;20:270–8. doi: 10.1165/ajrcmb.20.2.3313. [DOI] [PubMed] [Google Scholar]
- Jeschke MG, Klein D, Bolder U, Einspanier R. Insulin attenuates the systemic inflammatory response in endotoxemic rats. Endocinology. 2004;145:4084–4093. doi: 10.1210/en.2004-0592. [DOI] [PubMed] [Google Scholar]
- Jorgensen PF, Wang JE, Almlof M, Solberg R, Okkenhaug C, Scholz T, Thiemermann C, Foster SJ, Aasen AO. Sirolimus interferes with the innate response to bacterial products in human whole blood by attenuation of IL-10 production. Scand J Immunol. 2001;53:184–191. doi: 10.1046/j.1365-3083.2001.00862.x. [DOI] [PubMed] [Google Scholar]
- Kamimura M, Viedt C, Dalpke A, Rosenfeld ME, Mackman N, Cohen DM, Blessing E, Preusch M, Weber CM, Kreuzer J, Katus HA, Bea F. Interleukin-10 suppresses tissue factor expression in lipopolysaccharide-stimulated macrophages via inhibition of Egr-1 and a serum response element/MEK-ERK1/2 pathway. Circ Res. 2005;97:305–313. doi: 10.1161/01.RES.0000177893.24574.13. [DOI] [PubMed] [Google Scholar]
- Kidd L, Schabbauer GA, Luyendyk JP, Holscher TD, Tilley RE, Tencati M, Mackman N. Insulin Activation of the PI3K/Akt Pathway Reduces LPS-induced Inflammation in Mice. J Pharmacol Exp Ther. 2008;326:348–53. doi: 10.1124/jpet.108.138891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AH, Khursigara G, Sun X, Franke TF, Chao MV. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol Cell Biol. 2001;21:893–901. doi: 10.1128/MCB.21.3.893-901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwata H, Watanabe Y, Miyoshi H, Yamamoto M, Kaisho T, Takeda K, Akira S. IL-10-inducible Bcl-3 negatively regulates LPS-induced TNF-alpha production in macrophages. Blood. 2003;102:4123–4129. doi: 10.1182/blood-2003-04-1228. [DOI] [PubMed] [Google Scholar]
- Lehner MD, Morath S, Michelsen KS, Schumann RR, Hartung T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J Immunol. 2001;166:5161–5167. doi: 10.4049/jimmunol.166.8.5161. [DOI] [PubMed] [Google Scholar]
- Liu YW, Chen CC, Tseng HP, Chang WC. Lipopolysaccharide-induced transcriptional activation of interleukin-10 is mediated by MAPK- and NF-kappaB-induced CCAAT/enhancer-binding protein delta in mouse macrophages. Cell Signal. 2006;18:1492–1500. doi: 10.1016/j.cellsig.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Schabbauer GA, Tencati M, Holscher T, Pawlinski R, Mackman N. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. J Immunol. 2008;180:4218–4226. doi: 10.4049/jimmunol.180.6.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Lim W, Gee K, Aucoin S, Nandan D, Kozlowski M, az-Mitoma F, Kumar A. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J Biol Chem. 2001;276:13664–13674. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, Suddath WO, Weissman NJ, Torguson R, Kent KM, Pichard AD, Satler LF, Waksman R, Serruys PW. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- Mehta A, Brewington R, Chatterji M, Zoubine M, Kinasewitz GT, Peer GT, Chang AC, Taylor FB, Jr, Shnyra A. Infection-induced modulation of m1 and m2 phenotypes in circulating monocytes: role in immune monitoring and early prognosis of sepsis. Shock. 2004;22:423–30. doi: 10.1097/01.shk.0000142184.49976.0c. [DOI] [PubMed] [Google Scholar]
- Morrissey JH, Fair DS, Edgington TS. Monoclonal antibody analysis of purified and cell-associated tissue factor. Thromb Res. 1988;52:247–261. doi: 10.1016/0049-3848(88)90084-9. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajkrt D, van der Poll T, Levi M, Cutler DL, Affrime MB, van den Ende A, Wouter ten Cate J, van Deventer SJH. Interleukin-10 inhibits activation of coagulation and fibrinolysis during human endotoxemia. Blood. 1997;89:2701–2705. [PubMed] [Google Scholar]
- Pengal RA, Ganesan LP, Wei G, Fang H, Ostrowski MC, Tridandapani S. Lipopolysaccharide-induced production of interleukin-10 is promoted by the serine/threonine kinase Akt. Mol Immunol. 2006;43:1557–1564. doi: 10.1016/j.molimm.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Poitevin S, Cochery-Nouvellon E, Dupont A, Nguyen P. Monocyte IL-10 produced in response to lipopolysaccharide modulates thrombin generation by inhibiting tissue factor expression and release of active tissue factor-bound microparticles. Thromb Haemost. 2007;97:598–607. [PubMed] [Google Scholar]
- Rauh MJ, Ho V, Pereira C, Sham A, Sly LM, Lam V, Huxham L, Minchinton AI, Mui A, Krystal G. SHIP represses the generation of alternatively activated macrophages. Immunity. 2005;23:361–74. doi: 10.1016/j.immuni.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- Ruse M, Knaus UG. New players in TLR-mediated innate immunity: PI3K and small Rho GTPases. Immunol Res. 2006;34:33–48. doi: 10.1385/IR:34:1:33. [DOI] [PubMed] [Google Scholar]
- Steffel J, Latini RA, Akhmedov A, Zimmermann D, Zimmerling P, Luscher TF, Tanner FC. Rapamycin, but not FK-506, increases endothelial tissue factor expression: implications for drug-eluting stent design. Circulation. 2005;112:2002–2011. doi: 10.1161/CIRCULATIONAHA.105.569129. [DOI] [PubMed] [Google Scholar]
- Van Der PT, Marchant A, Buurman WA, Berman L, Keogh CV, Lazarus DD, Nguyen L, Goldman M, Moldawer LL, Lowry SF. Endogenous IL-10 protects mice from death during septic peritonitis. J Immunol. 1995;155:5397–5401. [PubMed] [Google Scholar]
- Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004a;172:567–576. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- Williams L, Jarai G, Smith A, Finan P. IL-10 expression profiling in human monocytes. J Leukoc Biol. 2002;72:800–809. [PubMed] [Google Scholar]
- Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation--a continuing puzzle. Immunology. 2004b;113:281–292. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama Y, Kitchens WC, Toth B, Schwacha MG, Rue LW, 3rd, Bland KI, Chaudry IH. Role of IL-10 in regulating proinflammatory cytokine release by Kupffer cells following trauma-hemorrhage. Am J Physiol Gastrointest Liver Physiol. 2004;286:G942–6. doi: 10.1152/ajpgi.00502.2003. [DOI] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, Wu H, Li L. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]


