Abstract
Spinal muscular atrophy (SMA) is a devastating motor neuron disease that is one of the leading genetic causes of infant mortality. Currently, there is no cure for SMA. Mouse models that genetically and phenotypically resemble SMA have been generated and have the potential to be used for the discovery of novel therapeutics. Oral administration is a commonly used mode of drug delivery in humans as well as in rodents. Unfortunately, there is no method of drug delivery that can accurately and reliably deliver drug compounds orally to neonatal mice. In this report, we describe a novel method to orally administer compounds to neonatal SMA mice. Oral delivery to neonatal mice, usually starting at postnatal day 4 (PND04), is both rapid and safe to the pup. Oral delivery of two different commonly used vehicle formulations, distilled water and 2-hydroxypropyl-β-cyclodextrin, does not affect the survival of SMA mice. After oral delivery for 3 days, 5-bromo-2′-deoxyuridine could be detected in the kidneys, brains and spinal cords of treated non-SMA as well as SMA neonatal pups. In conclusion, we have developed a method by which drugs can be safely and reliably administered orally to neural targets of neonatal mice. This approach offers a simple and rapid means by which potential therapeutics for SMA can be identified.
Keywords: spinal muscular atrophy, mouse, neonatal, drug delivery, oral administration, spinal cord
1. INTRODUCTION
Proximal spinal muscular atrophy (SMA) is an autosomal recessive degenerative disease characterized by selective loss of α motor neurons in the anterior horn of the spinal cord (Crawford and Pardo, 1996). SMA is one of the leading genetic causes of infant death in the world. SMA results from the loss or mutation of the SMN1 (survival motor neuron) gene with retention of SMN2 (Lefebvre et al., 1995). The severity of the SMA phenotype depends on the copy number of SMN2 and the levels of SMN protein (Coovert et al., 1997; McAndrew et al., 1997; Lefebvre et al., 1997). SMN is ubiquitously expressed and its levels are reduced in all tissues of SMA patients especially in type I, severe patients (Coovert et al., 1997; Lefebvre et al., 1997). The mechanism which accounts for the motor neuron specificity of SMA is presently unclear.
We now have mouse models of SMA with varying degrees of phenotypic severity (reviewed in Butchbach and Burghes, 2004). Unlike humans, mice carry only one SMN gene (mSmn) which is equivalent to SMN1 (DiDonato et al., 1997; Viollet et al., 1997). Loss of mSmn results in embryonic lethality in the mouse suggesting that the mSmn gene product is essential for cell function and survival (Schrank et al., 1997). Insertion of SMN2 into mSmn null mice by transgenesis rescues the embryonic lethality phenotype (Monani et al., 2000). However, mice with low copy numbers (i.e. 1–2) of SMN2 develop severe (type I-like) SMA and die at 6–8 days (Monani et al., 2000; Hsieh-Li et al., 2000). Introducing SMN lacking exon 7 (SMNΔ7) into SMN2;mSmn−/− mice partially ameliorates the SMA phenotype and these mice die at 14–15 days (Le et al., 2005). The SMN2;SMNΔ7; mSmn−/− mice show that SMNΔ7 is functional but it does not produce enough functional SMN protein to completely rescue SMA-like motor neuron degeneration. Introduction of a missense mutation (SMN(A2G)) found in type III SMA patients (Parsons et al., 1998) into SMN2;mSmn−/− mice modulates the SMA phenotype such that the transgenic mice can survive for over 1 year (type III SMA) (Monani et al., 2003).
One of the many potential uses for these mouse models of SMA is the testing of potential therapeutic agents for the amelioration of the neurodegenerative phenotype seen in these mice. A common route of administration of drugs to humans as well as to rodents is oral delivery. In rodents, oral administration is usually accomplished by placing the drug of interest into the water supply. For example, treatment of type II/III SMA-like mice (SMN2;mSmn(Δ7)−/−) with sodium butyrate (0.8–8.0 mg/mL) via the drinking water increased their survival by 4–5 days (29%) (Chang et al., 2001). Delivery of drugs via voluntary consumption of water does not account for individual variability in the amount of water—and, hence, drug—consumed in a given day. To further complicate drug delivery to neonatal mice, the drug which is consumed by the dam via the water supply must be able to accumulate in the dam’s milk so that it can delivered to the suckling pups. Administration of drugs ad libitum via the water supply, therefore, does not reliably deliver the drug to individual neonatal mice.
Unfortunately, there is no method of minimally invasive drug delivery that can accurately and reliably deliver drug compounds orally to neonatal mice. In this paper, we describe a novel method by which a drug can be safely administered to neonatal mouse pups. This method of oral administration is similar to gavage except that the feeding needle in our method is not delivered to the stomach thereby preventing any potential damage to the esophagus. The effect of various vehicle formulations on the lifespans of SMNΔ7 SMA mice was assessed. Additionally, we determined if a compound—in this case, BrdU—can reach the brain and spinal cord of neonatal pups when administered orally. This method of drug delivery could be used to test the effect of a given drug on the amelioration of the degenerative phenotype observed in SMA mice as well as other mouse models for other early onset neurological disorders.
2. METHODS
2.1. Animals
SMNΔ7 SMA mice were generated from males and females of the genotype SMN2+/+;SmnΔ7+/+;mSmn+/− (FVB.Cg.Tg(SMN*delta7)4299Ahmb Tg(SMN2)89Ahmb Smn1tm1Msd; Le et al., 2005). These mice are on a FVB/N genetic background. Although the mice used in these experiments were derived from our colony, they are available from Jackson Laboratories. Mice were maintained on a 12 h:12 h light:dark cycle (light period 06:00 until 18:00) with ad libitum access to food and water. All breeding dams were provided with nesting material before parturitation and delivered their pups spontaneously. The date of birth was designated as postnatal day (PND) 01. Neonatal offspring were genotyped using a PCR-based assay on genomic DNA from tail biopsies—obtained after death—as described previously (Le et al., 2005). All experiments were conducted in accordance with the protocols described in the National Institutes of Health Guide for the Care and Use of Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2. Oral Delivery of Drugs
Oral delivery of compounds to neonatal pups was accomplished using a modified gavage procedure. A stainless steel, 24-gauge feeding needle (Harvard Apparatus; needle length = 25.4 mm, ball diameter = 1.25 mm) was bent to form a curve as shown in Figure 1. The mouse pup was gently but securely grasped by the skin on the back of its neck and the feeding needle was gently inserted into the oral cavity just until the needle reached the pharynx (Figure 2). The drug compound was slowly injected into the oral cavity after which the feeding needle was slowly removed from the mouth. The Supplemental Video shows in detail the approach used to administer a given drug orally. Mice were sometimes lightly sedated with the gaseous anesthetic isoflurane prior to administration of drug so as to minimize any potential harm to the animal (Murphy et al., 2001). Sedation does not affect the mortality of mice receiving oral administration of a drug. Drugs were administered to mice until the last SMA pup in the litter exhibited prolonged periods (> 10 minutes) of lethargy and >20% loss of body mass over a 24-hour period.
Figure 1. Feeding needles used in oral delivery of drug compounds to neonatal mice.
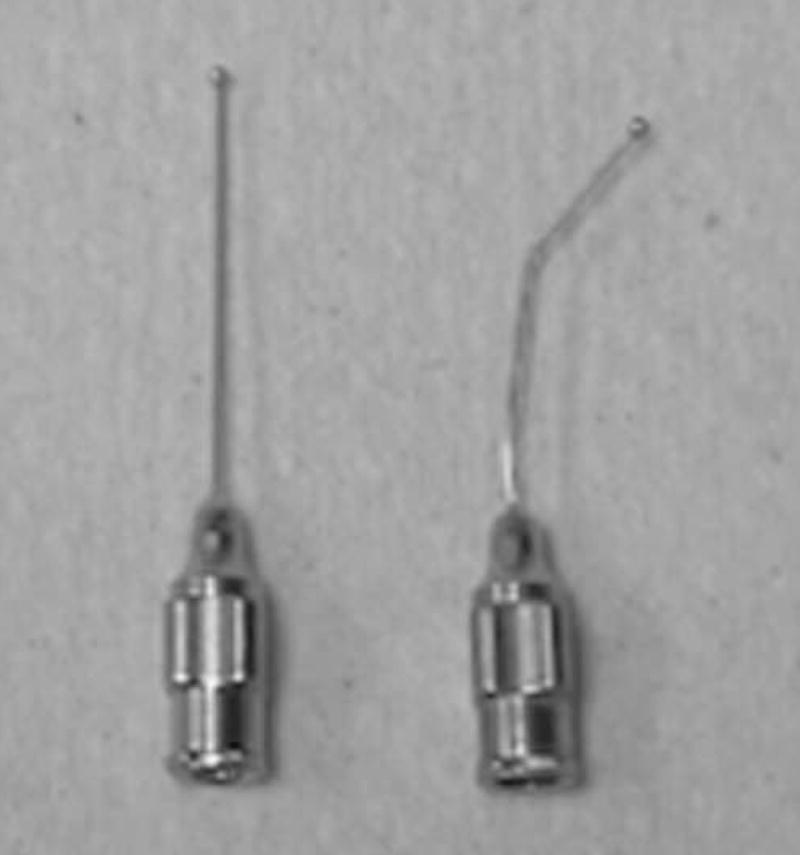
This photograph shows a stainless steel, 24-gauge feeding needle which has been bent ~30° (on right) so as to facilitate insertion into the oral cavity. An unbent feeding needle is shown on the left.
Figure 2. Oral delivery of a drug compound to a neonatal mouse.

A neonatal mouse was firmly grasped by the skin between its shoulder blades with the thumb and middle finger. The modified feeding needle was then gently inserted into the oral cavity until the bulbous end of the needle reached the pharynx. The drug was then injected into the oral cavity of the pup and the needle was then gently withdrawn from the oral cavity. The entire procedure can be completed within 10 seconds by a properly trained person. The mouse shown in this photograph is PND04.
2-Hydroxypropyl-β-cyclodextrin (HPCD; Sigma-Aldrich) was dissolved in sterile water to a final concentration of 40% (wt/vol) and filter sterilized into small aliquots. HPCD as well filter sterilized ddH2O were administered to neonatal pups b.i.d. beginning at PND04. For b.i.d. dosing, the drugs were administered at 09.00 and 17.00 daily without fail. The dose volumes for HPCD and ddH2O were 5.0 μL/g. 5-bromo-2-deoxyuridine (BrdU; Sigma-Aldrich) was administered orally to neonatal SMNΔ7 mice at a dose of 50 mg/kg/day (dose volume = 5.0 μl/g) for 3 days beginning at PND04.
2.3. BrdU Detection by Dot-Blot
BrdU incorporation into various tissues was assessed by dot blot analysis as described by Ueda et al. (2005) with modifications. Neonatal mice were treated with BrdU or ddH2O as described above for 3 days after which they were euthanized (at 60 minutes after the last oral administration of BrdU) and their forebrains, spinal cords and kidneys were rapidly dissected and placed in TNES buffer (400 mM NaCl, 100 mM EDTA, 0.6% SDS, 400 μg/mL proteinase K in 10 mM TrisHCl, pH 7.5). The samples were incubated overnight at 55°C followed by a 30- minute incubation at 37°C with 3 μg RNase A. The proteins were precipitated by high salt and the genomic DNAs were further purified by phenol:chloroform:isoamyl alcohol extraction followed by ethanol:sodium acetate (pH 5.2) precipitation. The resultant DNA pellet was resuspended in 10 mM TrisHCl, pH 8.0 and quantified by UV spectrometry. 1 μg of genomic DNA was denatured by incubation with 10 volumes 40 mM NaOH for 30 minutes at room temperature. The denatured DNA was neutralized with 10 volumes 1 M TrisHCl, pH 6.8 and then placed on ice. The single-stranded genomic DNA (100 ng) was then dot blotted onto a Hybond-N+ membrane (GE Healthcare) using a BioDot transfer apparatus (BioRad); the DNA was fixed to the membrane by baking for 2 hours at 70°C. Equal loading of each dot was assessed visually using a UV transluminator (not shown).
The DNA dotted membrane was incubated with mouse anti-BrdU monoclonal antibody (1:1000; G3G4antiBrdU ascites, Developmental Studies Hybridoma Bank) in Antibody Buffer (0.2% Tween-20 and 1% nonfat milk in PBS) for 60 minutes at room temperature. The membrane was thoroughly washed in PBS + 0.2% Tween-20 and then incubated with horseradish peroxidase-conjugated goat anti mouse IgG (1:5000; Rockland Immunochemicals) in Antibody Buffer for 60 minutes at room temperature. After thorough washing with PBS + 0.2% Tween-20, antibody binding was detected using enhanced chemiluminescence (ECL; GE Healthcare). The blot was exposed to autoradiography film and BrdU signal intensity (expressed as relative optical density units) was determined with ImageJ (Scion).
2.4. Statistical Analysis
All parametric data were expressed as means ± standard errors. Parametric data were analyzed by one way ANOVA with a Bonferroni post hoc test. Kaplan-Meier curves were generated from the survival data and tested using the Mantel-Cox log rank test. All statistical analyses were performed with SPSS v. 14.0.
3. RESULTS
3.1 Oral administration of drugs to neonatal mice
We describe here a novel method by which solutions containing drugs are administered orally to neonatal mice. Our method is distinct from conventional gavage delivery in that the feeding tube is not inserted into the stomach of mouse pups. Instead, a small feeding needle is inserted into the oral cavity of pups just far enough for the bulbous end of the tube to reach the pharynx. The drug is then delivered via a Hamilton microsyringe attached to the feeding needle and then the needle is gently removed from the mouth of the pup. Shortly after injection of liquid from the syringe into the mouth of the pup, it will usually urinate a small volume. This observation can be used as a indicator for successful oral delivery.
This oral delivery method is quick and minimally invasive to the mouse pup (Figure 2 and Supplemental Video). With proper training, the whole procedure lasts around 10 seconds. The stress experienced by the mouse pup is minimal as evidenced by the normal feeding behavior of treated pups after oral delivery. Furthermore, the handling of the mouse pups and the insertion of the feeding tube into the mouths of these pups have minimal effects on maternal feeding behaviors as the FVB/N dams still nurse and groom the handled pups in the same way and frequency as those unhandled pups.
This oral delivery method is safe to neonatal mice. In fact, we have thus far administered various drug compounds to over 950 neonatal mice. Of those mice, 4.8% of those pups (46/955) died unexpectedly because of improper drug administration. As death of a neonatal mouse due to improper drug administration occurs within 2 hours after oral administration; the mortality observed from improper drug administration is most likely not due to early death resulting from the SMA phenotype (1% of the SMA mice at PND04-PND05) which does not occur as rapidly even in severely affected SMNΔ7 SMA mice (Le et al., 2005; M.E.R.B., J.D.E. and A.H.M.B., manuscript in preparation). This mortality is comparable to or even lower than that observed with intraperitoneal or subcutaneous injection of drugs into neonatal mice. In most cases, oral delivery of drugs began at postnatal day 4 (PND04) as this time point occurs before the onset of motor neuron loss in the SMNΔ7 SMA mice (PND09) (Le et al., 2005). We have been able to successfully administer drugs orally to mice as young as PND02 but the risk of mortality is greater than if the drug treatment had begun at PND04.
3.2 Effect of Various Vehicles on Survival of SMNΔ7 SMA Mice
To determine the effect of oral administration of a given compound on the neonatal SMA mouse, we treated SMNΔ7 SMA mice as well as their non-SMA littermates with one of two commonly used vehicle formulations: 1) distilled water and 2) 40% 2-hydroxypropyl-β-cyclodextrin (HPCD). HPCD is frequently used as a vehicle to deliver hydrophobic compounds in an aqueous format (Hirayama and Uekama, 1999). These vehicles were given to neonatal pups twice at day (b.i.d.) beginning at PND04. It is important to note that the intervals between dosings must be kept constant (i.e. multiple dosings per day should be given at the same times each day) otherwise premature lethality or lack of a drug response will be observed with the SMNΔ7 SMA mice. For comparison, we also measured lifespans of SMNΔ7 SMA mice that were not treated with any compound. As shown in Figure 3, the mean lifespan of SMNΔ7 SMA mice treated with distilled water (red solid line;13.1±0.5 days; n=10) or with 40% HPCD (blue dashed line;12.9±0.5 days; n=10) did not differ significantly from that of untreated pups (black solid line;13.6±0.7 days; χ2 = 1.69, p = 0.19 for water; χ2 = 3.04, p = 0.08 for HPCD). Oral delivery of aqueous formulations does not affect the survival of SMNΔ7 SMA mice.
Figure 3. Effect of oral administration of various vehicle formulations on survival of SMNΔ7 SMA mice.
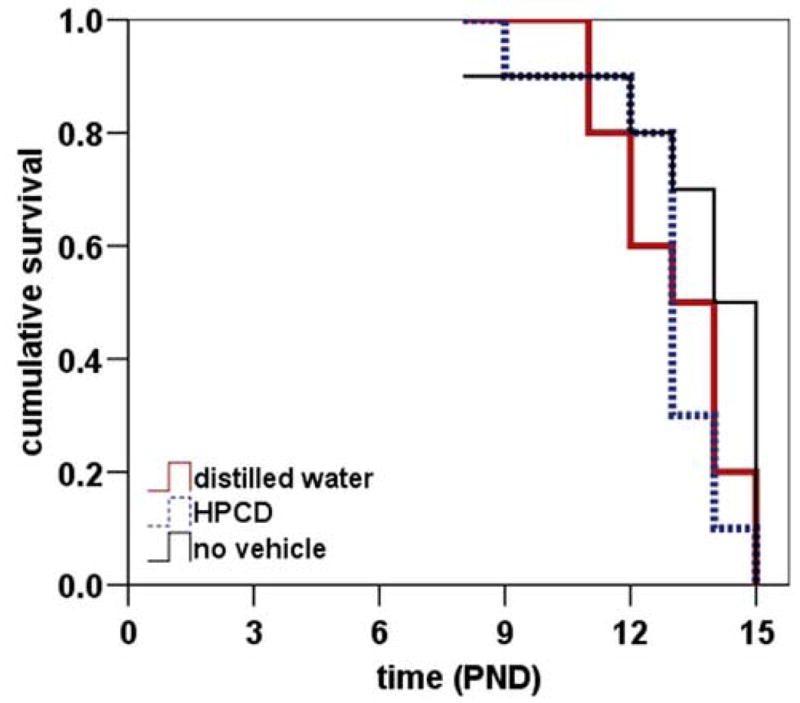
The effect of oral administration of two commonly used vehicle formulations—water and 40% hydroxypropyl-β-cyclodextrin (HPCD)—on the survival of SMNΔ7 SMA mice was determined. The vehicle formulations were administered b.i.d. beginning at PND04 until the last SMA pup in a given litter died. For comparison, the average lifespan of SMNΔ7 SMA mice that were not treated with any compound was also determined. The mean lifespans of SMNΔ7 SMA mice treated either with distilled water (red solid line; 13.1±0.5 days; n=10) or with 40% HPCD (blue dashed line;12.9±0.5 days; n=10) were not significantly different from that of untreated pups (black solid line;13.6±0.7 days; χ2 = 1.69, p = 0.19 for water; χ2 = 3.04, p = 0.08 for HPCD).
The dose volume used in the aforementioned experiment was 5.0 μL/g and each mouse received this dose volume twice a day. We have been able to administer solutions at a dose volume as high as 10 μL/g and a dose frequency as high as 3 times a day without increasing the mortality of the neonatal mice.
3.3 CNS Levels of Drug Following Oral Administration to Neonatal Mice
Since SMA is a disease which primarily affects motor neurons in the spinal cord, we must determine if a given drug can reach its target, in this case, the spinal cord, when delivered via oral administration. 5-bromo-2′-deoxyuridine (BrdU) was selected as a proof of concept compound since BrdU can reach the CNS when administered via the water supply, e.g. (Santosa et al., 2006), and can be rapidly detected by dot blot analysis (Ueda et al., 2005). Neonatal SMNΔ7 SMA (SMN2+/+;SMNΔ7+/+;mSmn−/−) as well as non-SMA mice (either SMN2+/+;SMNΔ7+/+;mSmn+/− or SMN2+/+;SMNΔ7+/+;mSmn+/+) received BrdU (50 mg/kg/day) via oral administration for 3 days beginning at PND04. Oral administration of BrdU had no effect on the survival of SMNΔ7 SMA mice when administered over this window. CNS (forebrain and spinal cord) as well as peripheral (kidney) tissues were harvested from treated mice at 1 hour after the last dosing. Genomic DNA extracted from these tissues was analyzed for BrdU content by dot blot analysis. As shown in Figure 4A, BrdU incorporation could be detected in the kidneys, brains and spinal cords of neonatal mice when administered orally. In fact, the relative levels of BrdU are similar between SMA and non-SMA mice in the three tissues tested (Figure 4B) although BrdU levels were higher in the forebrains of SMA mice than in non-SMA mice (p=0.009). The relevance of this observation is not known. Importantly, a drug—in this case, BrdU—can reach CNS targets when administered orally to neonatal mouse pups.
Figure 4. Distribution of BrdU following oral administration to neonatal pups.
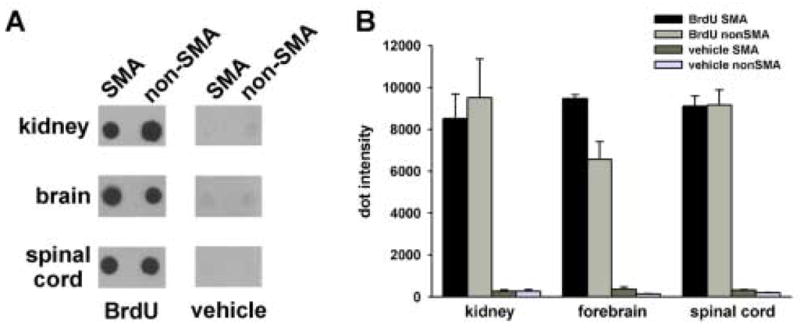
BrdU was administered orally to neonatal SMNΔ7 SMA as well as non-SMA littermates for 3 days beginning at PND04 (n=3/genotype/drug). Genomic DNA from the kidney, forebrain and spinal cord (100 ng) was dot blotted onto a Nytran membrane and probed with a mouse monoclonal antibody directed against BrdU. As shown in (A), BrdU can be detected in the DNA of all three tissues of treated SMA and non-SMA mice. Relative quantitation of the dots (expressed as relative optical density units in B) shows that BrdU incorporation is similar between SMNΔ7 SMA (SMN2+/+;SMNΔ7+/+;mSmn−/−) and non-SMA (either SMN2+/+;SMNΔ7+/+;mSmn+/− or SMN2+/+;SMNΔ7+/+;mSmn+/+) mice in all tissues tested except that BrdU levels were higher in the forebrains of SMA mice than in non-SMA mice (p=0.009).
4. DISCUSSION
We describe here a novel method by which drugs can be orally delivered to neonatal SMNΔ7 SMA mice. This approach permits rapid (~10 seconds per pup) and safe (4.8% mortality) administration of a potential therapeutic agent to SMA mice as young as PND04, well before the onset of loss of motor neurons in this model of SMA (Le et al., 2005). Oral administration does not affect the survival of SMNΔ7 SMA mice as the mean lifespans of SMA mice receiving either one of two vehicle formulations (distilled water and 40% HPCD) were not different from that of unmanipulated SMA mice. Additionally, we have demonstrated using BrdU that drugs can reach the brain and spinal cord—the primary target for SMA—when administered orally to neonatal mice.
A commonly used mode of oral delivery of a drug is through the water supply. Ad libitum delivery in the water supply assumes that the water (drug) consumption rates are the same between all mice in a given cage and that this consumption rate is the same over time. As an example, Chang et al. (2001) administered sodium butyrate to type II/III SMA-like mice (SMN2;mSmn(Δ7)−/−) via the drinking water which resulted in a ~30% increase in survival. The authors mention that there was variability in the amount of water consumed by the mice each day such as their mice received 0.8–8.0 mg/mL butyrate in a given day. This variability in consumption makes these observations difficult to reproduce. One way to ensure that consistent dosing of mice occurs using water supply delivery would involve the use of transponder-tagged mice (Santosa et al., 2006). While this method of controlled delivery would work in adult mice, it would be difficult to apply to neonatal mice as these pups rely on maternal milk for water and food. In order for a drug that is consumed by dam via the water supply to reach the neonatal pups, it must be able to accumulate in the dam’s milk so that it can delivered to the suckling pups. Again, one must assume that the feeding rates of the individual pups in that litter are the same. It would, therefore, appear that oral administration of compounds ad libitum through the water supply may not be the most reliable way to deliver compounds to neonatal mice.
Oral gavage, or insertion of a feeding tube down the esophagus to the stomach, can be a reliable means to delivery a drug to healthy mice. It has been used as a means to deliver compounds such as 3′-azido-3′-deoxythymidine (ADT), dideoxyinosine (ddI) and clofibrate to neonatal mice as young as PND04-06 (Bishop et al., 2004; Nesfield et al., 2005). However, SMNΔ7 SMA mice are smaller than their non-SMA littermates at PND04 (Le et al., 2005; M.E.R.B., J.D.E. and A.H.M.B., manuscript in preparation) which makes administration of a drug via oral gavage difficult. Recently, two groups have reported novel methods of oral administration of compounds to neonatal (PND15) (Wheeler et al., 2006) and adult (Schleimer et al., 2005) rats whereby drugs were delivered to the mouths with a syringe or a pipette tip for consumption. Additionally, Duysen et al. (2002) reported that hand feeding acetylcholinesterase (AChE) nullizygous mice, which normally live to PND14, with a pipette tip significantly increased the lifespans of these mice. Our method is an extension of this approach to neonatal SMA mice in that we use a feeding needle with a bulbous end to deliver drugs into the oral cavities of young pups.
It has been shown that adult rats receiving large volumes of oil-based vehicles via gavage show a marked stress response as evidenced by elevated corticosterone levels (Brown et al., 2000). Water-based vehicle formulations nor smaller dosing volumes did not elevate corticosterone levels in these rats. It would be reasonable to suggest that oral gavage may have also elicit a stress response in neonatal mice. We did not observe any significant signs of stress, such as precocious loss of body mass or rejection by the dam, in those neonatal mice which underwent oral administration of a vehicle or a drug. Likewise, Wheeler et al. (2006) and Schleimer et al. (2005) reported that their oral delivery methods were minimally stressful on their rats.
In summary, we have a developed a novel approach to orally deliver drugs to neonatal (PND04) SMNΔ7 SMA mice that is rapid, safe, reliable and minimally stressful. This mode of drug delivery can be used to test various drugs for amelioration of the motor neuron degenerative phenotype observed in SMA mice. Although this route of administration has been optimized for neonatal SMNΔ7 SMA mice, oral delivery can also be used for other mouse models of neonatal neurological disorders.
Supplementary Material
Video 1. Oral delivery of drug compounds to neonatal mice. This video clip shows the oral administration of a drug solution to two neonatal mice (PND04).
Acknowledgments
We would like to thank Dr. Thanh T. Le for providing the initial SMNΔ7 carrier mice used to establish our colony and Dr. Daniel D. Coovert for his assistance during the early phase of this study. This project was supported in part by grants from the NINDS (#NS3860; AHMB) and the Families of SMA (AHMB). MERB was supported by the Families of SMA. The G3G4antiBrdU monoclonal antibody developed by Dr. S. J. Kaufman was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bishop JB, Witt KL, Tice RR, Wolfe GW. Genetic damage detected in CD-1 mouse pups exposed perinatally to 3′-azido-3′-deoxythymidine and dideoxyinosine via maternal dosing, nursing and direct gavage. Environ Mol Mutagen. 2004;43:3–9. doi: 10.1002/em.10210. [DOI] [PubMed] [Google Scholar]
- Brown AP, Dinger N, Levine BS. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci. 2000;39:17–21. [PubMed] [Google Scholar]
- Butchbach MER, Burghes AHM. Perspectives on models of spinal muscular atrophy for drug discovery. Drug Discov Today Dis Model. 2004;1:151–156. [Google Scholar]
- Chang JG, Hsieh-Li HM, Jong YJ, Wang NM, Tsai CH, Li H. Treatment of spinal muscular atrophy by sodium butyrate. Proc Natl Acad Sci USA. 2001;98:9808–9813. doi: 10.1073/pnas.171105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coovert DD, Le TT, McAndrew PE, Strasswimmer J, Crawford TO, Mendell JR, Coulson SE, Androphy EJ, Prior TW, Burghes AHM. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet. 1997;6:1205–1214. doi: 10.1093/hmg/6.8.1205. [DOI] [PubMed] [Google Scholar]
- Crawford TO, Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol Dis. 1996;3:97–110. doi: 10.1006/nbdi.1996.0010. [DOI] [PubMed] [Google Scholar]
- DiDonato CJ, Chen XN, Noya D, Korenberg JR, Nadeau JH, Simard LR. Cloning, characterization and copy number of the murine survival motor neuron gene: homolog of the spinal muscular atrophy-determining gene. Genome Res. 1997;7:339–352. doi: 10.1101/gr.7.4.339. [DOI] [PubMed] [Google Scholar]
- Duysen EG, Stribley JA, Fry DL, Hinrichs SH, Lockridge O. Rescue of the acetylcholinesterase knockout mouse by feeding a liquid diet; phenotype of the adult acetylcholinesterase deficient mouse. Dev Brain Res. 2002;137:43–54. doi: 10.1016/s0165-3806(02)00367-x. [DOI] [PubMed] [Google Scholar]
- Hirayama F, Uekama K. Cyclodextrin-based controlled drug release system. Adv Drug Deliv Rev. 1999;36:125–141. doi: 10.1016/s0169-409x(98)00058-1. [DOI] [PubMed] [Google Scholar]
- Hsieh-Li HM, Chang JG, Jong YJ, Wu MH, Wang NM, Tsai CH, Li H. A mouse model for spinal muscular atrophy. Nat Genet. 2000;24:66–70. doi: 10.1038/71709. [DOI] [PubMed] [Google Scholar]
- Le TT, Pham LT, Butchbach MER, Zhang HL, Monani UR, Coovert DD, Gavrilina TO, Xing L, Bassell GJ, Burghes AHM. SMNΔ7, the major product of the centromeric survival motor neuron gene (SMN2), extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum Mol Genet. 2005;14 :845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, Dreyfuss G, Melki J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16:265–269. doi: 10.1038/ng0797-265. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, Le Paslier D, Frézal J, Cohen D, Weissenbach J, Munnich A, Melki J. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- McAndrew PE, Parsons DW, Simard LR, Rochette C, Ray PN, Mendell JR, Prior TW, Burghes AHM. Identification of proximal spinal muscular atrophy carriers and patients by analysis of SMNT and SMNC gene copy number. Am J Hum Genet. 1997;60:1411–1422. doi: 10.1086/515465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monani UR, Pastore MT, Gavrilina TO, Jablonka S, Le TT, Andreassi C, DiCocco JM, Lorson C, Androphy EJ, Sendtner M, Podell M, Burghes AHM. A transgene carrying an A2G missense mutation in the SMN gene modulates phenotypic severity in mice with severe (type I) spinal muscular atrophy. J Cell Biol. 2003;160:41–52. doi: 10.1083/jcb.200208079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monani UR, Sendtner M, Coovert DD, Parsons DW, Andreassi C, Le TT, Jablonka S, Schrank B, Rossol W, Prior TW, Morris GE, Burghes AHM. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn −/− mice and results in a mouse with spinal muscular atrophy. Hum Mol Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, Smith P, Shaivitz AB, Rossberg MI, Hurn PD. The effect of brief halothane anesthesia during daily gavage on complications and body weights in rats. Contemp Top Lab Anim Sci. 2001;40:9–12. [PubMed] [Google Scholar]
- Nesfield SR, Williams TC, Hoivik DJ, Miller RT, Allen JS, Selinger K, Rickert D, Santostefano MJ. Evaluation of the carcinogenic potential of clofibrate in the neonatal mouse. Int J Toxicol. 2005;24:341–348. doi: 10.1080/10915810500210401. [DOI] [PubMed] [Google Scholar]
- Parsons DW, McAndrew PE, Iannaccone ST, Mendell JR, Burghes AHM, Prior TW. Intragenic telSMN mutations: frequency, distribution, evidence of a founder effect and modification of the spinal muscular atrophy phenotype by cenSMN copy number. Am J Hum Genet. 1998;63:1712–1723. doi: 10.1086/302160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa A, Kaiser A, Winter Y. Individually dosed oral administration to socially-living transponder-tagged mice by a water dispenser under RFID control. J Neurosci Meth. 2006;153:208–213. doi: 10.1016/j.jneumeth.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Schleimer SB, Johnston GAR, Henderson JM. Novel oral administration in an animal model of neuroleptic therapy. J Neurosci Meth. 2005;146:159–164. doi: 10.1016/j.jneumeth.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Schrank B, Götz R, Gunnersen JM, Ure JM, Toyka KV, Smith AG, Sendtner M. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc Natl Acad Sci USA. 1997;94:9920–9925. doi: 10.1073/pnas.94.18.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda J, Saito H, Watanabe H, Evers BM. Novel and quantitative DNA dot-blotting methods for assessment of in vivo proliferation. Am J Physiol. 2005;288:G842–G847. doi: 10.1152/ajpgi.00463.2004. [DOI] [PubMed] [Google Scholar]
- Viollet L, Bertrandy S, Beuno Brunialti AL, Lefebvre S, Burlet P, Clermont O, Cruaud C, Guénet JL, Munnich A, Melki J. cDNA isolation, expression and chromosomal localization of the mouse survival motor neuron gene (Smn) Genomics. 1997;40:185–188. doi: 10.1006/geno.1996.4551. [DOI] [PubMed] [Google Scholar]
- Wheeler TL, Eppolito AK, Smith LN, Huff TB, Smith RF. A novel method for oral stimulant administration in the neonate rat and similar species. J Neurosci Meth. 2006 doi: 10.1016/j.jneumeth.2006.07.019. in press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Oral delivery of drug compounds to neonatal mice. This video clip shows the oral administration of a drug solution to two neonatal mice (PND04).


