Abstract
The reorganization of multicellular populations to produce an elongated tissue structure is a conserved mechanism for shaping the body axis and several organ systems. In the Drosophila germband epithelium, this process is accompanied by the formation of a planar polarized network of junctional and cytoskeletal proteins in response to striped patterns of gene expression. Actomyosin cables and adherens junctions are dynamically remodeled during intercalation, providing the basis for polarized cell behavior. Quantitative analysis of cell behavior in living embryos reveals unexpected cell population dynamics that include the formation of multicellular rosette structures as well as local neighbor exchange.
Introduction
A basic mechanism for generating three-dimensional structure during development is the elongation of multicellular tissues by intercalation, the polarized movements of cells perpendicular to the direction of elongation. Cell intercalation in mesenchymal tissues plays a major role in shaping the body axis in Xenopus and zebrafish [1–4], and intercalation in epithelial tissues drives the elongation of the Drosophila germband [5], the ascidian notochord [6], the chick primitive streak [7] and organ systems such as the gut, lung, spinal cord and inner ear [8–12]. The outcome of intercalation is similar in mesenchymal and epithelial tissues, but the underlying cell behaviors appear to be different. Intercalating mesenchymal cells in zebrafish and Xenopus elongate in the direction of cell movement and extend polarized protrusions that support short- and long-range cell migrations [13–15]. By contrast, epithelial cells in the Drosophila germband are proposed to intercalate through the spatially regulated assembly and disassembly of intercellular adherens junctions [16]. Consistent with this possibility, several proteins required for intercalation are associated with the adherens junctions, including cytoskeletal and junctional components that may directly influence the tenacity or transience of cell contacts within the sheet [16–18]. This review will focus on epithelial intercalation in the Drosophila germband, with an emphasis on the mechanisms that establish polarity within cells and the cell behaviors that translate these polarities into tissue elongation.
Two models for axis elongation in Drosophila: differential adhesion and planar polarity
During body axis formation in the Drosophila embryo, the germband epithelium increases 2.5-fold in length along the head-to-tail (anterior-posterior) axis and narrows in width along the back-to-front (dorsal-ventral) axis to create the basic layout of the body plan [5, 19]. This morphogenetic process is completed in less than two hours, with most of the elongation taking place in the first 30–40 minutes through cell rearrangements that occur largely in the absence of cell division [5]. During intercalation, germband epithelial cells interpose themselves between their dorsal or ventral neighbors, resulting in a decrease in the number of cells along the dorsal-ventral (DV) axis and an increase in the number of cells along the anterior-posterior (AP) axis (Figure 1A,B). These oriented movements –perpendicular to the direction of tissue elongation – allow relatively short-range changes in cell position to produce a major change in tissue shape.
Figure 1. Axis elongation in the Drosophila embryo.
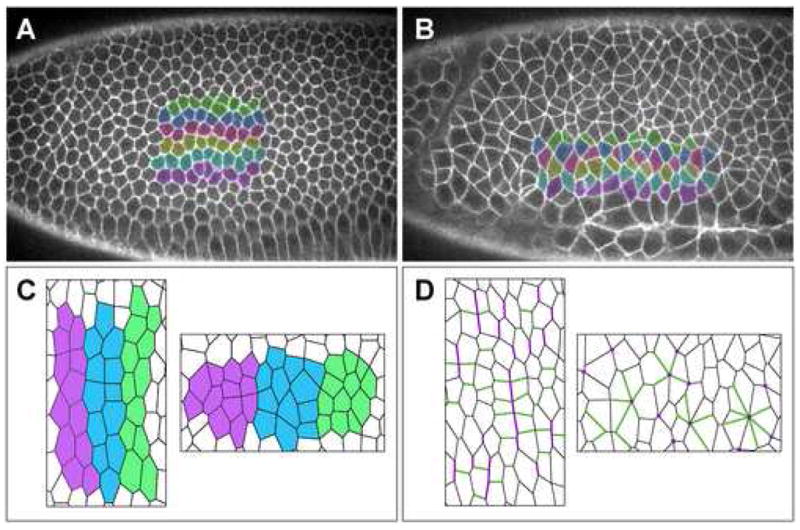
(A,B) Living embryos expressing a cell-surface GFP marker showing six rows of cells before (A) and during (B) axis elongation. Anterior left, dorsal up. Cells become separated from their anterior and posterior neighbors by intervening cells from dorsal and ventral rows. (C) In a differential adhesion model, stripes of cells with similar adhesive preferences rearrange to maximize contact with cells of the same stripe and minimize contact between adjacent stripes. (D) In a planar polarization model, adherens junction proteins (green) accumulate at interfaces between dorsal and ventral cells and actomyosin cables (purple) accumulate at interfaces between anterior and posterior cells. The contraction of these cables is likely to drive neighbor exchange and multicellular rosette formation. Panels A and B provided by Ori Weitz.
The spatial information that guides cell behavior during axis elongation in Drosophila is provided locally by interactions between neighboring cells, rather than by a distant polarizing signal. The local nature of these cues was first suggested by experiments demonstrating that interfering with tissue specification dorsal, ventral, or posterior to the germband does not disrupt intercalation [5]. Instead, patterning information within the germband epithelium itself is required for its elongation. The Even-skipped (Eve) and Runt transcription factors are initially expressed in seven stripes along the AP axis through the action of a global patterning system that subdivides the embryo into segments [20, 21]. In addition to their intrinsic role in cell fate, the Eve and Runt stripes also influence interactions between cells that bias the direction of cell movement. Disruption of these stripes in embryos in which Eve or Runt are absent or uniformly misexpressed causes a substantial reduction in elongation [5, 17], and an ectopic source of either protein is sufficient to reorient the axis of cell polarity [17]. A related mechanism operates in Xenopus, in which cells from the developing notochord intercalate when explants from anterior and posterior regions are combined in culture, but not when the explants are from the same region [22]. These results suggest that intercalary behavior is guided locally by signaling events at boundaries between cells with different identities. The molecular nature of these signals is unknown.
How do striped patterns of spatial information determine the direction of cell movement? In one model, Eve and Runt expression could confer differential adhesion between adjacent stripes of cells [5, 23]. Quantitative or qualitative differences in adhesion can account for the tissue-specific aggregation of dissociated cells [24] and patterns of cell sorting in culture [25]. In addition, in vivo evidence supports a role for differential adhesion in a range of developmental processes including oocyte positioning, tissue separation, and compartmental boundary formation [26–28]. In the Drosophila germband, adhesive differences present in a striped configuration could trigger tissue elongation if cells rearrange to maximize contact with cells of the same stripe and minimize contact between stripes (Figure 1C) [5, 23]. While blocks of similarly adhesive cells the width of a typical Eve or Runt stripe are in principle sufficient for elongation [5], a finer pattern of adhesive differences created by the combinatorial action of multiple striped genes is more compatible with the observation that intercalary behavior occurs throughout the germband, not just near stripe boundaries [23].
An alternative model postulates that different domains within a single cell – rather than absolute differences between cells – provides the driving force for intercalation. In this planar polarization model, each cell could acquire a polarized architecture that is asymmetric with respect to the AP and DV axes of the embryo (Figure 1D). Intercalating cells in this model rely on striped spatial cues not to determine their adhesive properties, but to generate and orient intrinsic cellular asymmetries. For example, specific proteins may be targeted to interfaces between neighboring cells along the AP axis (AP interfaces) that encounter recognizable differences in Eve and Runt expression. Several epithelial tissues display molecular planar polarity, including the Drosophila wing, eye, and notum and the mammalian cochlear epithelium. In these cases, polarized proteins are components of a conserved planar cell polarity (PCP) signaling pathway mediated by the Frizzled receptor [29–31]. Some components of this pathway are dispensable for germband extension [17], but a distinct set of cytoskeletal and adherens junction proteins is planar polarized in the Drosophila embryo [16–18]. These findings support a role for a planar polarization mechanism in elongation of the Drosophila body axis.
Establishing cellular asymmetry along planar axes
To distinguish between different mechanisms for intercalary behavior, it is necessary to identify proteins essential for this process that have spatially specific patterns of expression or subcellular localization. In particular, intercalating cells in the Drosophila germband display a planar polarized distribution of cytoskeletal and adherens junction proteins (Figure 2). The homophilic adhesion molecule E-cadherin, its associated cytoplasmic protein Armadillo/β-catenin, and the Bazooka/PAR-3 PDZ-domain protein are concentrated in interfaces between dorsal and ventral neighbors (DV interfaces) [17, 18]. Conversely, filamentous actin (F-actin) and the actin-based myosin II motor protein are enriched in the reciprocal AP interfaces [16–18]. These polarities are specific to intercalating cells, appear just before the onset of cell movement, and require the AP patterning system that is essential for axis elongation [17, 18]. Consistent with a functional role for these planar polarized proteins, axis elongation is reduced in embryos that are defective for the myosin II heavy or regulatory light chain subunits and embryos injected with an inhibitor of the Rho kinase myosin activator [16], as well as embryos that are defective for Bazooka or its binding partners, the Par6 PDZ-domain protein or atypical protein kinase C (aPKC) [17, 18]. A related system of planar polarity may extend to other organisms, as Xenopus homologs of Par6 and aPKC are enriched in a planar polarized fashion in intercalating notochord cells [32].
Figure 2. Establishment of planar polarity in intercalating cells.
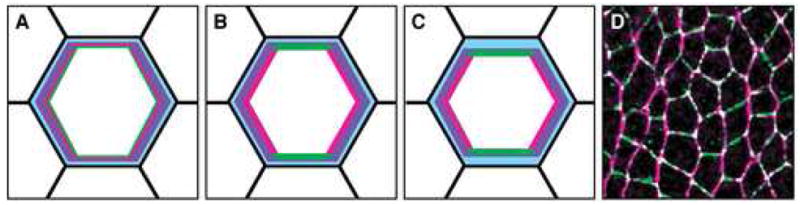
(A–C) Schematics of planar polarity in the Drosophila germband at early (A), middle (B), and late (C) stages of polarization. Anterior left, dorsal up. Planar polarity is indicated for the central cell in an apical plane at the level of the adherens junctions. The transition from a symmetric to a planar polarized subcellular organization occurs in a 15-minute time window. (A) The earliest known planar symmetry-breaking event is an accumulation of F-actin (purple) at AP interfaces. (B) Bazooka (green) and myosin II (red) are initially uniform at the apical cortex and subsequently become restricted to complementary domains. (C) E-cadherin and Armadillo/β-catenin (blue) become enriched at DV interfaces by the onset of intercalation. Bazooka and myosin II are largely mutually exclusive. Adherens junctions and F-actin are present throughout the apical cortex. (D) Confocal image of the Drosophila germband showing myosin II (magenta) enrichment at AP interfaces and Bazooka (green) enrichement at DV interfaces.
Planar polarized junctional and cytoskeletal structures in the Drosophila embryo precedes intercalation and are likely to drive the separation of anterior and posterior cells and the formation of contacts between dorsal and ventral cells (Figure 3A–C) [16–18]. The functional relationships between different aspects of polarity are not clear, although F-actin is the first protein to localize asymmetrically in the plane of the tissue and adherens junction polarity is not detected until a later stage (Figure 2C) [18]. Bazooka is required for the apical localization of adherens junction proteins [33, 34] and can bind to Armadillo/β-catenin [35], suggesting that Bazooka may directly stabilize junctions at DV interfaces. Actomyosin contractility can also influence adhesion, although evidence from the literature is contradictory on this point. In some cell types myosin II promotes the disassembly of adherens junctions [36, 37], consistent with the reciprocal distributions of myosin II and E-cadherin in the Drosophila germband [18]. However, in other cell types myosin II enhances adhesion through the lateral clustering of E-cadherin molecules [38] or spatially localized contractility at the periphery of growing contacts [39]. Cells from myosin heavy chain II-A knockout mice display reduced adhesion and less surface E-cadherin [40], indicating that some myosin II activity is necessary for adhesion. However, higher levels of myosin II or the spatial distribution of contractile forces in the cell may determine whether myosin II supports or opposes the stabilization of junctional contacts.
Figure 3. Polarized cell behaviors during intercalation.
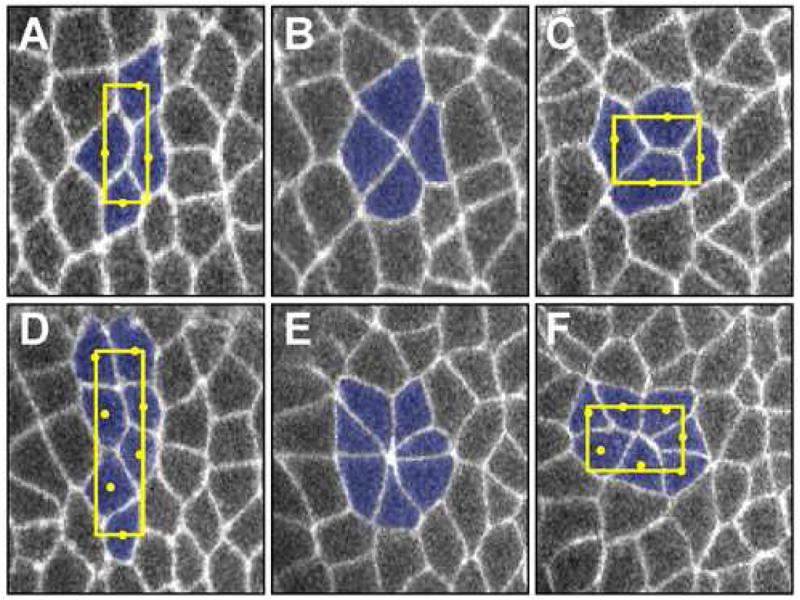
(A–F) Living embryos expressing a cell-surface GFP marker at sequential time points during neighbor exchange (A–C) or rosette formation (D–F). Anterior left, dorsal up. (A–C) In neighbor exchange, a single interface between anterior and posterior cells contracts to produce a 4-cell vertex (B) that resolves directionally (C), creating contact between the dorsal and ventral cells. This is described as a T1 process [67] or type 1 (A), type 2 (B), and type 3 (C) junctions [16]. The geometric center of each cell is indicated by a yellow circle; a box circumscribing the cellular geometric centers was used to calculate the aspect ratio (the ratio of the length of the cellular assembly along the AP axis relative to its height along the DV axis). (D–F) In rosette formation, several AP interfaces contract together to bring two columns of cells into contact at a high-order vertex (E) that resolves directionally (F), producing elongation along the AP axis.
Cell polarization through extracellular signals and intracellular self-organization
The polarized architecture of intercalating cells could be generated by the self-organization of distinct cellular domains in response to a single polarizing cue or landmark. Alternatively, different regions of the cell could be specified independently in response to multiple extracellular inputs. The establishment of planar polarity in the Drosophila embryo is not yet understood, but other systems provide instructive examples of intrinsic and extrinsic cellular mechanisms that could contribute to this process.
Cellular self-organization can be achieved through a variety of molecular mechanisms, including inhibitory phosphorylation events displacing kinase from substrate [41–43], competitive interactions between mutually exclusive protein complexes [29–31], and actomyosin-dependent transport of proteins within the cell [44–46]. A well-characterized example of cellular self-organization is anterior-posterior axis formation in the C. elegans embryo, which occurs at the one-cell stage. A single polarizing event mediated by sperm entry determines the posterior pole of the cell [47] and leads to the anterior accumulation of PAR-3 (Bazooka), PAR-6, and PKC-3 (aPKC) and the posterior accumulation of the PAR-1 serine/threonine kinase and the PAR-2 RING domain protein [44–46]. These molecularly distinct cortical domains are generated by an actomyosin-dependent flow that initiates at the site of sperm entry and reorganizes the entire cell cortex and cytoplasm [48]. The signals that trigger this directional flow emanate from the sperm centrosome [49] as well as the localized deposition of CYK-4 RhoGAP and downregulation of ECT-2 RhoGEF at the posterior pole of the embryo [50–52]. These events lead to inactivation of the RHO-1 GTPase at the posterior pole and a displacement of the cortical actomyosin network toward the anterior of the embryo. Actomyosin-dependent cortical flow colocalizes with and is required for the delivery of PAR-3, PAR-6, and PKC-3 to the anterior cortex [48, 53–56]. Conversely, feedback from the anterior PAR-3 protein is required to sustain this cortical flow [48, 57, 58]. Therefore, a network of molecular interactions leads to the autonomous organization of both anterior and posterior cellular domains in the C. elegans embryo.
An alternative mechanism for polarization is available to cells in multicellular tissues, which are exposed to a range of environmental inputs that could specify distinct aspects of planar polarity. Consistent with this possibility, intercalating cells in the Xenopus embryo integrate signals from multiple sources. Polarized protrusions in the developing notochord are restricted to a two-dimensional plane by the extracellular matrix [59] and these protrusions are locally inhibited at the notochord boundary [60]. Moreover, Xenopus neural plate cells convert from bipolar to a directional unipolar motility in response to contact with the underlying mesoderm [15] and exposure to a diffusible signal from the midline [61]. In support of a multi-signal polarization mechanism in Drosophila, some properties of planar polarity appear to be separable in the germband epithelium. An actomyosin network is still enriched at AP interfaces in Par6 and aPKC mutants, despite the fact that large areas of DV interfaces are vacated by Bazooka [18]. Mislocalized Bazooka aggregates also retain their affinity for DV interfaces in basolateral regions, whereas myosin II remains concentrated apically [18, 62]. These findings suggest that different properties of planar polarity can be regulated independently in Par6 and aPKC mutant backgrounds.
The independent modulation of distinct aspects of cell polarity by extracellular signals could provide a flexible mechanism for maintaining planar polarity in a dynamic and disordered cellular environment. Germband cells display a wide range of topologies [18, 63], and the organization of a four-sided cell is likely to be very different from that of a six- or seven-sided cell, presenting a challenge to self-organizing strategies in which polarity is organized around a single landmark. Moreover, intercalating cells have to continually remodel their polarity through multiple rounds of cell rearrangement. An externally-driven cell polarization could provide a mechanism to regenerate asymmetry in moving cells that transition between different structural states.
Cytoskeletal and junctional dynamics during intercalation
The planar polarized distribution of junctional and cytoskeletal proteins in the Drosophila embryo is consistent with a model in which increased contractility and decreased adhesion at AP interfaces leads to the disassembly of contact between anterior and posterior cells during intercalation [16, 18]. Little is known, however, about how these lost contacts are replaced by new contacts between dorsal and ventral cells to produce directional cell rearrangement. One possibility is that a rapid reloading of myosin II could cause AP interfaces to shrink whenever they attempt to reform [16]. Alternatively, increased adhesion at DV interfaces could facilitate cell rearrangement by favoring new contacts between dorsal and ventral cells [17, 18]. However, the association of Bazooka, which is thought to stabilize adherens junctions, is not detected until several minutes after the establishment of contact between cells [18], suggesting that Bazooka may not affect the formation of cell contacts but may instead act to stabilize them once they form.
The actin cytoskeleton can act as a positive or negative regulator of junctional stability and could therefore influence the formation and elimination of cell contacts during intercalation. Actin filaments in the Drosophila germband not only assemble a contractile actomyosin network at shrinking interfaces, but are also present as a transient burst at growing interfaces [18]. The establishment of contact between epithelial cells in culture is also accompanied by cytoskeletal reorganization, including the formation of actin-rich lamellipodia and the localized recruitment of the Rac1 GTPase, the actin-nucleating formin-1 and Arp2/3 complex proteins, the Arp2/3 activators N-WASP and cortactin, and VASP [64–66]. Sites of actin polymerization dynamically redistribute to the expanding periphery of growing interfaces and are replaced by a central zone of adherens junctions [39]. By analogy, a noncontractile, polymerizing actin structure could drive the formation and growth of new contacts between cells in the Drosophila germband. Mutually exclusive contractile and polymerization-driven cytoskeletal structures could provide a mechanism to ensure the directionality of cell rearrangement. For example, the disassembly of actomyosin cables could render adjacent anterior and posterior cells temporarily refractory to actin polymerization, favoring the actin-dependent stabilization new junctions between dorsal and ventral cells.
Local interactions and global cell population dynamics during intercalation
An understanding of the global patterns of behavior that arise from local interactions between cells requires the quantitative analysis of cell population dynamics. The number of neighbors that a cell is in contact with, via a shared interface, is a useful measure of cell topology [63, 67–69]. The distribution of polygon classes provides a statistical measurement of cell interactions. Epithelial sheets are often presumed to consist predominantly of hexagonal cells, but it is becoming apparent that many tissues diverge significantly from this pattern [18, 63, 68, 69]. The germband epithelium contains a substantial fraction of nonhexagonal cells before intercalation (~35%), and this fraction nearly doubles during intercalation [18, 63]. Topological diversity (or disorder) is a signature of intercalating populations that increases as the germband elongates [18, 63]. Therefore, rather than describing intercalating populations in terms of uniform behaviors, may be more appropriate to view them as a collection of cells with similar tendencies that behave differently under different topological and geometric constraints.
The germband epithelium maintains its integrity throughout axis elongation and the disassembly of contacts between some neighboring cells is balanced by the acquisition of contacts between new neighbors. Elementary neighbor exchange events are overwhelmingly directional: adjacent cells along the AP axis tend to separate while dorsal and ventral cells come together (Figure 3A–C). A requisite intermediate during neighbor exchange, also known as a T1 process, is a transient structure in which four cells come into contact at a single point or vertex [67]. T1 processes are a topological necessity during cell rearrangement in two dimensions and have been reported in several instances of intercalation [6, 16, 18, 63, 70]. The existence of these structures does not, by itself, yield information about the underlying molecular mechanisms. An important insight into the mechanism of intercalation in Drosophila came from the finding that myosin II localizes to the interfaces that disappear during intercalation [16]. Therefore, in contrast to intercalation in mesenchymal tissues in which motile protrusions allow two cells to “push” their way into contact [1, 71], intercalation in Drosophila instead appears to be driven by a contractile “pulling” force in the cells that become separated [16–18].
In addition to elementary neighbor exchange, axis elongation in the Drosophila embryo involves the formation of higher-order structures that are topologically distinct from T1 processes and may reflect coordination within the sheet. Live imaging studies reveal that intercalating populations form multicellular rosette structures in which 5 or more cells meet at a single point in clusters containing up to 11 cells [18]. During rosette formation, two columns of cells constrict their shared interfaces to bring a group of cells into contact at a common vertex (Figure 3D–F). These multicellular assemblies resolve in a directional fashion to produce two rows of cells, elongating the cellular array along the AP axis. Rosette formation amplifies the effects of neighbor exchange, producing a 5.5-fold average change in the AP:DV aspect ratio, compared to a 2.5-fold average change for T1 processes (Figure 3). Rosettes occur at a consistent frequency during axis elongation, but with a variable spatial distribution, and expand the range of cell interactions beyond what is possible in a system dominated by local neighbor exchange. Neighbor exchange and rosette formation represent local and higher-order behaviors, respectively, that link the dynamics of individual cell interfaces to the global restructuring of a multicellular tissue.
Rosette formation can account for the increase in disorder in the germband, but rosettes are not a necessary consequence of disorder. This is evident from a comparison with two-dimensional soap films, which are highly disordered but contain only three-fold vertices due to the constraints of surface tension [67]. Instead, rosettes occur when shrinking edges are interconnected within the epithelial sheet. It is not known if these linkages are actively generated by chemical or mechanical signals between cells, or if they can occur randomly through the stochastic collision of T1 processes. During rosette formation, actomyosin structures align across multiple cells to form cables of linked interfaces that contract together to form a rosette [18]. These actomyosin structures may be functionally associated among multiple pairs of cells, analogous to the supracellular contractile networks that drive epithelial advance and embryonic wound healing [72–74]. Higher-order actomyosin structures in line, arc and ring-like configurations have been implicated in compartmental boundary formation, tracheal placode invagination and ommatidial precluster morphogenesis downstream of Notch, EGF receptor and Hedgehog signaling [75–78].
Open questions: axis elongation through regulated adhesion and polarity
Whether differences between cells or differences across the surface of a single cell direct polarized cell behavior during intercalation remains to be determined. These mechanisms are not mutually exclusive. Differences in adhesion between cells could provide the spatial cue that creates planar polarity, and a planar polarization mechanism could influence multiple processes including adhesion. A differential adhesion mechanism is appealing in that it can successfully predict the outcome of various striped configurations in the Drosophila embryo [5]. Moreover, cell sorting events triggered by differences in adhesion resemble the sorting patterns of intercalating Xenopus cells when randomly mixed in culture [22, 25]. However, some aspects of intercalary behavior in the Drosophila embryo appear to be inconsistent with a differential adhesion model. First, AP interfaces decrease in length during intercalation, but this is not accompanied by a systematic increase in the length of DV interfaces, arguing against a tendency to maximize certain cell contacts. Second, the differential adhesion model predicts that cell movements should be random, followed by the stabilization of favored interfaces, whereas cell movement in the germband is highly directional [5, 16, 18]. Finally, computer simulations of cell sorting by differential adhesion are not able to reproduce higher-order rosette behaviors [79], whereas rosette structures are in principle compatible with the formation of actomyosin cables in a planar polarization model [18]. Ultimately, identification of the signals provided by striped patterns of gene expression will be necessary to understand how cells send and receive spatial information and translate this information into polarized cell behavior.
In the future, realistic models of epithelial intercalation must account not only for the outcome of elongation, but also for the dynamic trajectories of cells that engage in a range of local and higher-order interactions. Of particular interest are features of cell populations that do not arise through mathematical necessity but may reflect active biological regulation. The ability to distinguish these properties through the judicious application of mathematical and physical approaches to biological systems was articulated by British botanist K. J. Dormer in 1980:
So long as a system is mathematically determinate there is no room for any biological phenomenon to show itself. Biology begins specifically at the point where there are two or more mathematically admissible results, between which the organism must choose upon some basis other than that of geometrical necessity. We have: (a) to recognize, and as soon as possible to eliminate from further discussion, aspects of tissue structure deriving from pure mathematical necessity; (b) to estimate, once the available principles of mathematical causation have been exhausted, just how much remains undecided, and how much freedom of choice is consequently left to the cells; and (c) within the limits fixed by (b), to devise practical schemes of laboratory observation for extracting information about biological decisions taken by cells in specified plant and animal tissues [80].
Thanks to modern imaging techniques for time-lapse microscopy and quantitative analysis of cell behavior in living embryos, Dormer’s practical schemes are now within reach.
Acknowledgments
We thank Richard Zallen, Eric Wieschaus, Mimi Shirasu-Hiza and the members of the Zallen lab for helpful comments on the manuscript. JAZ is supported by a Burroughs Wellcome Fund Career Award in the Biomedical Sciences, a March of Dimes Basil O’Connor Starter Scholar Award, a Searle Scholar Award, a W. M. Keck Foundation Distinguished Young Scholar in Medical Research Award, and NIH/NIGMS R01 grant GM079340.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P. Mechanisms of convergence and extension by cell intercalation. Philos Trans R Soc Lond B Biol Sci. 2000;355:897–922. doi: 10.1098/rstb.2000.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tada M, Concha ML, Heisenberg CP. Non-canonical Wnt signalling and regulation of gastrulation movements. Semin Cell Dev Biol. 2002;13:251–260. doi: 10.1016/s1084-9521(02)00052-6. [DOI] [PubMed] [Google Scholar]
- 3.Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 4.Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr Biol. 2005;15:R213–228. doi: 10.1016/j.cub.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Irvine KD, Wieschaus E. Cell intercalation during Drosophila germband extension and its regulation by pair-rule segmentation genes. Development. 1994;120:827–41. doi: 10.1242/dev.120.4.827. [DOI] [PubMed] [Google Scholar]
- 6.Munro EM, Odell GM. Polarized basolateral cell motility underlies invagination and convergent extension of the ascidian notochord. Development. 2002;129:13–24. doi: 10.1242/dev.129.1.13. [DOI] [PubMed] [Google Scholar]
- 7.Voiculescu O, Bertocchini F, Wolpert L, Keller RE, Stern CD. The amniote primitive streak is defined by epithelial cell intercalation before gastrulation. Nature. 2007;449:1049–1052. doi: 10.1038/nature06211. [DOI] [PubMed] [Google Scholar]
- 8.Hardin J. Local shifts in position and polarized motility drive cell rearrangement during sea urchin gastrulation. Dev Biol. 1989;136:430–445. doi: 10.1016/0012-1606(89)90268-6. [DOI] [PubMed] [Google Scholar]
- 9.Iwaki DD, Johansen KA, Singer JB, Lengyel JA. drumstick, bowl, and lines are required for patterning and cell rearrangement in the Drosophila embryonic hindgut. Dev Biol. 2001;240:611–626. doi: 10.1006/dbio.2001.0483. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro C, Neumann M, Affolter M. Genetic control of cell intercalation during tracheal morphogenesis in Drosophila. Curr Biol. 2004;14:2197–2207. doi: 10.1016/j.cub.2004.11.056. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, Chen P. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shih J, Keller R. Cell motility driving mediolateral intercalation in explants of Xenopus laevis. Development. 1992;116:901–914. doi: 10.1242/dev.116.4.901. [DOI] [PubMed] [Google Scholar]
- 14.Concha ML, Adams RJ. Oriented cell divisions and cellular morphogenesis in the zebrafish gastrula and neurula: a time-lapse analysis. Development. 1998;125:983–994. doi: 10.1242/dev.125.6.983. [DOI] [PubMed] [Google Scholar]
- 15.Elul T, Keller R. Monopolar protrusive activity: a new morphogenic cell behavior in the neural plate dependent on vertical interactions with the mesoderm in Xenopus. Dev Biol. 2000;224:3–19. doi: 10.1006/dbio.2000.9746. [DOI] [PubMed] [Google Scholar]
- 16.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 17.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 18.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Hartenstein V, Campos-Ortega JA. The spatio-temporal pattern of embryonic cell divisions. Roux’s Arch Dev Biol. 1985;195:181–195. 20. [Google Scholar]
- 20.Ingham PW, Martinez Arias A. Boundaries and fields in early embryos. Cell. 1992;68:221–235. doi: 10.1016/0092-8674(92)90467-q. [DOI] [PubMed] [Google Scholar]
- 21.St Johnston D, Nusslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992;68:201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- 22.Ninomiya H, Elinson RP, Winklbauer R. Antero-posterior tissue polarity links mesoderm convergent extension to axial patterning. Nature. 2004;430:364–367. doi: 10.1038/nature02620. [DOI] [PubMed] [Google Scholar]
- 23.Wieschaus E, Sweeton D, Costa M. Convergence and extension during germband elongation in Drosophila embryos. In: Keller R, editor. Gastrulation. New York: Plenum Press; 1991. pp. 213–223. [Google Scholar]
- 24.Steinberg MS. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science. 1963;141:401–408. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- 25.Steinberg MS, Takeichi M. Experimental specification of cell sorting, tissue spreading, and specific spatial patterning by quantitative differences in cadherin expression. Proc Natl Acad Sci USA. 1994;91:206–209. doi: 10.1073/pnas.91.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 27.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 28.Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 31.Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 32.Hyodo-Miura J, Yamamoto TS, Hyodo AC, Iemura S, Kusakabe M, Nishida E, Natsume T, Ueno N. XGAP, an ArfGAP, is required for polarized localization of PAR proteins and cell polarity in Xenopus gastrulation. Dev Cell. 2006;11:69–79. doi: 10.1016/j.devcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Muller HA, Wieschaus E. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol. 1996;134:149–163. doi: 10.1083/jcb.134.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris TJ, Peifer M. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J Cell Biol. 2004;167:135–147. doi: 10.1083/jcb.200406024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei SY, Escudero LM, Yu F, Chang LH, Chen LY, Ho YH, Lin CM, Chou CS, Chia W, Modolell J, Hsu JC. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev Cell. 2005;8:493–504. doi: 10.1016/j.devcel.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Sahai E, Marshall CJ. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol. 2002;4:408–415. doi: 10.1038/ncb796. [DOI] [PubMed] [Google Scholar]
- 37.Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. Rho/Rho-associated Kinase-II Signaling Mediates Disassembly of Epithelial Apical Junctions. Mol Biol Cell. 2007;18:3429–3439. doi: 10.1091/mbc.E07-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279:41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- 41.Betschinger J, Knoblich JA. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol. 2004;14:R674–685. doi: 10.1016/j.cub.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 42.Wodarz A. Molecular control of cell polarity and asymmetric cell division in Drosophila neuroblasts. Curr Opin Cell Biol. 2005;17:475–481. doi: 10.1016/j.ceb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 44.Nance J. PAR proteins and the establishment of cell polarity during C.elegans development. Bioessays. 2005;27:126–135. doi: 10.1002/bies.20175. [DOI] [PubMed] [Google Scholar]
- 45.Munro EM. PAR proteins and the cytoskeleton: a marriage of equals. Curr Opin Cell Biol. 2006;18:86–94. doi: 10.1016/j.ceb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Cowan CR, Hyman AA. Acto-myosin reorganization and PAR polarity in C. elegans. Development. 2007;134:1035–1043. doi: 10.1242/dev.000513. [DOI] [PubMed] [Google Scholar]
- 47.Goldstein B, Hird SN. Specification of the anteroposterior axis in Caenorhabditis elegans. Development. 1996;122:1467–1474. doi: 10.1242/dev.122.5.1467. [DOI] [PubMed] [Google Scholar]
- 48.Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev Cell. 2004;7:413–424. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Cowan CR, Hyman AA. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature. 2004;431:92–96. doi: 10.1038/nature02825. [DOI] [PubMed] [Google Scholar]
- 50.Jenkins N, Saam JR, Mango SE. CYK-4/GAP provides a localized cue to initiate anteroposterior polarity upon fertilization. Science. 2006;313:1298–1301. doi: 10.1126/science.1130291. [DOI] [PubMed] [Google Scholar]
- 51.Motegi F, Sugimoto A. Sequential functioning of the ECT-2 RhoGEF, RHO-1 and CDC-42 establishes cell polarity in Caenorhabditis elegans embryos. Nat Cell Biol. 2006;8:978–985. doi: 10.1038/ncb1459. [DOI] [PubMed] [Google Scholar]
- 52.Schonegg S, Hyman AA. CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development. 2006;133:3507–3516. doi: 10.1242/dev.02527. [DOI] [PubMed] [Google Scholar]
- 53.Guo S, Kemphues KJ. A non-muscle myosin required for embryonic polarity in Caenorhabditis elegans. Nature. 1996;382:455–458. doi: 10.1038/382455a0. [DOI] [PubMed] [Google Scholar]
- 54.Shelton CA, Carter JC, Ellis GC, Bowerman B. The nonmuscle myosin regulatory light chain gene mlc-4 is required for cytokinesis, anterior-posterior polarity, and body morphology during Caenorhabditis elegans embryogenesis. J Cell Biol. 1999;146:439–451. doi: 10.1083/jcb.146.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cuenca AA, Schetter A, Aceto D, Kemphues K, Seydoux G. Polarization of the C. elegans zygote proceeds via distinct establishment and maintenance phases. Development. 2003;130:1255–1265. doi: 10.1242/dev.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Severson AF, Bowerman B. Myosin and the PAR proteins polarize microfilament-dependent forces that shape and position mitotic spindles in Caenorhabditis elegans. J Cell Biol. 2003;161:21–26. doi: 10.1083/jcb.200210171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirby C, Kusch M, Kemphues K. Mutations in the par genes of Caenorhabditis elegans affect cytoplasmic reorganization during the first cell cycle. Dev Biol. 1990;142:203–215. doi: 10.1016/0012-1606(90)90164-e. [DOI] [PubMed] [Google Scholar]
- 58.Cheeks RJ, Canman JC, Gabriel WN, Meyer N, Strome S, Goldstein B. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr Biol. 2004;14:851–862. doi: 10.1016/j.cub.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 59.Davidson LA, Marsden M, Keller R, Desimone DW. Integrin alpha5beta1 and fibronectin regulate polarized cell protrusions required for Xenopus convergence and extension. Curr Biol. 2006;16:833–844. doi: 10.1016/j.cub.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 60.Keller R, Cooper MS, Danilchik M, Tibbetts P, Wilson PA. Cell intercalation during notochord development in Xenopus laevis. J Exp Zool. 1989;251:134–154. doi: 10.1002/jez.1402510204. [DOI] [PubMed] [Google Scholar]
- 61.Ezin AM, Skoglund P, Keller R. The midline (notochord and notoplate) patterns the cell motility underlying convergence and extension of the Xenopus neural plate. Dev Biol. 2003;256:100–114. doi: 10.1016/s0012-1606(02)00130-6. [DOI] [PubMed] [Google Scholar]
- 62.Harris TJ, Peifer M. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev Cell. 2007;12:727–738. doi: 10.1016/j.devcel.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zallen JA, Zallen R. Cell-pattern disordering during convergent extension in Drosophila. J Phys: Condensed Matter. 2004;16:S5073–S5080. doi: 10.1088/0953-8984/16/44/005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 65.Yap AS, Kovacs EM. Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol. 2003;160:11–16. doi: 10.1083/jcb.200208156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mege RM, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 67.Weaire D, Rivier N. Contemp Phys. 1984;25:59–99. [Google Scholar]
- 68.Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 69.Gibson MC, Patel AB, Nagpal R, Perrimon N. The emergence of geometric order in proliferating metazoan epithelia. Nature. 2006;442:1038–1041. doi: 10.1038/nature05014. [DOI] [PubMed] [Google Scholar]
- 70.Glickman NS, Kimmel CB, Jones MA, Adams RJ. Shaping the zebrafish notochord. Development. 2003;130:873–887. doi: 10.1242/dev.00314. [DOI] [PubMed] [Google Scholar]
- 71.Green JB, Davidson LA. Convergent extension and the hexahedral cell. Nat Cell Biol. 2007;9:1010–1015. doi: 10.1038/ncb438. [DOI] [PubMed] [Google Scholar]
- 72.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 73.Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol. 2005;15:2208–2221. doi: 10.1016/j.cub.2005.11.064. [DOI] [PubMed] [Google Scholar]
- 74.Koppen M, Fernandez BG, Carvalho L, Jacinto A, Heisenberg CP. Coordinated cell-shape changes control epithelial movement in zebrafish and Drosophila. Development. 2006;133:2671–2681. doi: 10.1242/dev.02439. [DOI] [PubMed] [Google Scholar]
- 75.Major RJ, Irvine KD. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development. 2005;132:3823–3833. doi: 10.1242/dev.01957. [DOI] [PubMed] [Google Scholar]
- 76.Major RJ, Irvine KD. Localization and requirement for Myosin II at the dorsal-ventral compartmental boundary of the Drosophila wing. Dev Dyn. 2006;235:3051–3058. doi: 10.1002/dvdy.20966. [DOI] [PubMed] [Google Scholar]
- 77.Nishimura M, Inoue Y, Hayashi S. A wave of EGFR signaling determines cell alignment and intercalation in the Drosophila tracheal placode. Development. 2007;134:4273–4282. doi: 10.1242/dev.010397. [DOI] [PubMed] [Google Scholar]
- 78.Escudero LM, Bischoff M, Freeman M. Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev Cell. 2007;13:717–729. doi: 10.1016/j.devcel.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 79.Zajac M, Jones GL, Glazier JA. Simulating convergent extension by way of anisotropic differential adhesion. J Theor Biol. 2003;222:247–259. doi: 10.1016/s0022-5193(03)00033-x. [DOI] [PubMed] [Google Scholar]
- 80.Dormer KJ. Fundamental Tissue Geometry for Biologists. Cambridge: Cambridge University Press; 1980. pp. 5–6. [Google Scholar]


