Abstract
General anaesthetics act in an agent-specific manner on synaptic transmission in the central nervous system by enhancing inhibitory transmission and reducing excitatory transmission. The synaptic mechanisms of general anaesthetics involve both presynaptic effects on transmitter release and postsynaptic effects on receptor function. The halogenated volatile anaesthetics inhibit neuronal voltage-gated Na+ channels at clinical concentrations. Reductions in neurotransmitter release by volatile anaesthetics involve inhibition of presynaptic action potentials as a result of Na+ channel blockade. Although voltage-gated ion channels have been assumed to be insensitive to general anaesthetics, it is now evident that clinical concentrations of volatile anaesthetics inhibit Na+ channels in isolated rat nerve terminals and neurons, as well as heterologously expressed mammalian Na+ channel α subunits. Voltage-gated Na+ channels have emerged as promising targets for some of the effects of the inhaled anaesthetics. Knowledge of the synaptic mechanisms of general anaesthetics is essential for optimization of anaesthetic techniques for advanced surgical procedures and for the development of improved anaesthetics.
Keywords: anaesthetics volatile; anaesthetics volatile, halogenated hydrocarbons; nerve, neurotransmitters; pharmacology, anaesthetic action; pharmacology, neurotransmission
The pharmacology and toxicology of general anaesthetics are remarkably incomplete for such a widely used and clinically important class of drugs. Despite their widespread clinical use, our understanding of the molecular and cellular mechanisms of general anaesthetic action in the CNS is insufficient to explain how any anaesthetic produces amnesia, unconsciousness, or immobilization (at increasing doses), the cardinal clinical features of general anaesthesia. Early optimism that potentiation of ligand-gated ion channel receptors for inhibitory neurotransmitters like γ-aminobutyric (GABA) acid and/or glycine might underlie the actions of all anaesthetics has given way to current concepts of multiple agent-specific mechanisms underlying the diverse features of anaesthesia.27,81 It is now clear the general anaesthetics act at multiple anatomic sites in the nervous system to produce these distinct behavioural effects involving actions on multiple molecular targets.64,75 For example, there is now convincing evidence that volatile anaesthetics produce immobilization by effects in the spinal cord, while amnesia and unconsiousness involve distinct supraspinal mechanisms.1,15,66
Ion channels have emerged as the most likely molecular targets for general anaesthetics. Neurotransmitter-gated ion channels, in particular GABAA, glycine, and N-methyl-d-aspartate (NMDA)-type glutamate receptors, are leading candidates due to their appropriate central nervous system (CNS) distributions, essential physiological roles in inhibitory and excitatory synaptic transmission, and sensitivities of one or more of these channels to clinically relevant concentrations of all anaesthetics.15,27,102,103 Broadly speaking, general anaesthetic targets vary between the major classes of anaesthetics. Two classes of inhaled anaesthetics can be distinguished based on their distinct pharmacological. properties: (i) the potent inhaled (volatile) anaesthetics exhibit positive modulation of GABAA receptors, and also produce significant anaesthesia-compatible effects on a number of other receptors/channels including enhancement of inhibitory glycine receptors, inhibition of excitatory NMDA-type glutamate and neuronal nicotinic acetylcholine receptors, activation of two-pore domain K2P channels and leak K+ channels,59,79 and inhibition of presynaptic Na+ channels (see in what follows); and (ii) the gaseous inhaled anaesthetics, which include cyclopropane, nitrous oxide, and xenon, are inactive at GABAA receptors, but block NMDA receptors and activate certain K2P channels at clinical concentrations.23 Intravenous anaesthetics like propofol and etomidate represent more potent and specific positive modulators of GABAA receptors, and the i.v. anaesthetic ketamine is a more potent and specific blocker of NMDA receptors.103 Here I review accumulating evidence that suggests that some of the effects of volatile anaesthetics are mediated by inhibition of neuronal voltage-gated Na+ channels, an ion channel family often overlooked as a putative target for general anaesthesia (though widely recognized as the principal target for local anaesthetics).
Synaptic actions of general anaesthetics
General anaesthetics depress fast excitatory and enhance fast inhibitory synaptic transmission mediated primarily by glutamate and GABA (γ-aminobutyric acid), respectively.27,62,81 The relative importance of anaesthetic effects on excitatory vs inhibitory synaptic transmission and the mechanisms involved are less clear. Prolongation of synaptic inhibition by modulation of postsynaptic GABAA receptor function at GABAergic synapses is recognized as an important component of the depressant effects of volatile anaesthetics and of several i.v. anaesthetics at clinical concentrations,21,30,49,108 and significant progress has been made in identifying critical anaesthetic binding sites on GABAA receptors.27 More recent studies have implicated anaesthetic actions on tonic inhibitory currents mediated by extrasynaptic GABAA receptors as well as enhanced release of GABA mediated by a presynaptic increase in miniature inhibitory postsynaptic current (mIPSC) frequency (see in what follows). Evidence also supports depression of excitatory transmission at clinical concentrations of many general anaesthetics.40,53,61,91 The molecular mechanisms of these depressant effects on excitatory transmission are less clear, but could include depressed membrane excitability,64 depressed presynaptic action potential conduction,4,44,54,100 inhibition of transmitter release,43,77 and/or blockade of postsynaptic receptors. The latter is the principal mechanism for ketamine7 and for the gaseous anaesthetics xenon,103 nitrous oxide,42 and cyclopropane.80 Blockade of postsynaptic glutamate receptors has been shown to contribute to the effects of volatile anaesthetics at some synapses.12,25 The roles of enhanced inhibitory transmission vs reduced excitatory transmission to the overall depression of neuronal activity in anaesthesia likely vary between specific networks.64,85
Presynaptic vs postsynaptic anaesthetic effects
The relative contributions of presynaptic vs postsynaptic anaesthetic effects on synaptic transmission have been difficult to resolve.11,19 Electrophysiological evidence supports both presynaptic (release) and postsynaptic (receptor) mechanisms for the synaptic actions of general anaesthetics. Intravenous anaesthetics and volatile anaesthetics decrease excitatory postsynaptic potentials (EPSPs) in spinal33,93 and hippocampal neurons,5,40,61,74 which has been attributed indirectly to a presynaptic mechanism, and decrease cortical neuron sensitivity to applied glutamate, a postsynaptic mechanism.45,73,90,104 General anaesthetics also decrease depolarization-induced glutamate release from brain slices,8,16,35,36 but it is difficult to localize drug effects in such intact polysynaptic neuronal circuits. Volatile anaesthetics have limited effects on cloned α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) or N-methyl-d-aspartic acid (NMDA) glutamate receptors but potentiate kainate receptors,13,45 consistent with a predominantly presynaptic mechanism for inhibition of glutamatergic synapses, although recent evidence suggests that NMDA receptor blockade could contribute to inhaled anaesthetic effects.12,25 Most i.v. anaesthetics are remarkable for their relatively potent and selective potentiation of postsynaptic GABA responses.103 In contrast to glutamatergic synapses, augmentation of GABAergic responses by most general anaesthetics is mediated primarily by potentiation of postsynaptic and extrasynaptic GABAA receptors.27 Anaesthetics also have presynaptic effects at GABA terminals to increase IPSC frequency and GABA release,2,46,52,64 which combine with prolongation of action-potential-evoked IPSCs to increase inhibitory charge transfer and net inhibitory tone.6
Investigations of the molecular targets responsible for these synaptic effects of general anaesthetics have focused on voltage-gated and ligand-gated ion channels, many of which have been cloned and are accessible to direct electrophysiological analysis.19,27 GABAA receptors in particular are potentiated by most general anaesthetics, which accounts for their postsynaptic actions on inhibitory synaptic transmission. Potentiation of GABAA receptors is clearly an important mechanism for i.v. anaesthetics such as propofol, the immobilizing effects of which can be antagonized by intrathecal injection of the GABAA receptor antagonist bicuculline6 or by a β3 receptor N265M knock-in mutation in vivo.31 In contrast, potentiation of GABAA receptors is insufficient to explain the actions of volatile anaesthetics, since bicuculline does not antagonize immobilization by isoflurane.37,107 Thus volatile anaesthetic actions are less specific than those of most i.v. anaesthetics, and other sites of action must be involved. In addition to possible effects on glutamate receptors, convincing evidence exists for volatile anaesthetic effects on voltage-gated Na+ channels,54,58,71,100 two-pore-domain background K+ (K2P) channels,18,22,60 nicotinic cholinergic receptors,17,89 voltage-gated Ca2+ channels,32,50,84 and presynaptic SNARE proteins.47 An important goal is to identify the relevant molecular mechanisms for the presynaptic effects of volatile anaesthetics from among the multiple potential targets.
Anaesthetic effects on neurotransmitter release
The basic mechanisms underlying release are conserved among different neurotransmitters.86 However, transmitter- and nerve-terminal-specific specializations exist, such as transmitter-specific involvement of individual Ca2+ channel types in release and the modulation of release by presynaptic receptors.39,41,72,98 Transmitter release is tightly coupled to the concentration of presynaptic cytoplasmic Ca2+. The relationship between extracellular Ca2+ concentration and neurotransmitter release is exponential. Thus, Ca2+ entering through presynaptic voltage-gated Ca2+ channels acts in a highly cooperative manner to evoke exocytosis, with a degree of cooperativity (Hill slope) of ∼3–4 depending on the synapse.14,99 Factors that influence the modulation of neurotransmitter release include the amount of Ca2+ entering the presynaptic terminal, the efficiency with which Ca2+ controls exocytosis (Ca2+-secretion coupling), the cooperativity of Ca2+ action, and the maximal amount of release reflected in the number of docked and primed vesicles (the readily releasable pool).
Depolarization and Ca2+ entry determine the amount of transmitter released, which is regulated by presynaptic ion channels (e.g. Na+, Ca2+, and K+ channels), modulatory presynaptic receptors, and cell signalling mechanisms (e.g. second messengers and protein phosphorylation). Potential presynaptic targets for general anaesthetic action include: nerve terminal excitability/depolarization, Ca2+ influx (by direct effects on Ca2+ channels, indirectly through Ca2+ modulatory pathways, or via other ion channels), synaptic vesicle availability and mobilization (by effects on the cytoskeleton and/or synaptic vesicle-associated proteins), coupling between Ca2+ and exocytosis, fusion/exocytosis mediated by SNARE proteins, and vesicle endocytosis/recycling. The involvement of these and possibly other targets in the presynaptic actions of general anaesthetics on neurotransmitter release is not clearly established.
The presynaptic effects of anaesthetics on transmitter release can be measured directly in isolated nerve terminals (synaptosomes) without interference from intrinsic neuronal networks or somatic effects that limit interpretation of results obtained using brain slices or cultured neurons.48 Volatile anaesthetics inhibit pharmacologically evoked release of endogenous glutamate from cortical nerve terminals, indicating a direct presynaptic site of action.38,43,77 These results are supported by recent studies using advanced techniques to measure presynaptic anaesthetic effects. A high-resolution optical technique for imaging exocytosis showed that isoflurane inhibits action potential-evoked synaptic vesicle exocytosis in cultured rat hippocampal neurons.28 Electrophysiological techniques have also been used to show that isoflurane inhibits glutamatergic transmission in the unusually large rat calyx of Held synapse.63,100 Inhibition of transmitter release by volatile anaesthetics is conserved in the phylogenetically distant organisms Caenorhabditis elegans88 and Drosophila.51,76 Volatile anaesthetics also inhibit evoked GABA release from rat cortical nerve terminals, but with lower potency compared with glutamate release (see in what follows).95,97 The mechanism for this selectivity/differential sensitivity is intriguing but currently unknown. In addition, volatile anaesthetics enhance basal GABA release while inhibiting basal glutamate release, consistent with reports of increased IPSC and decreased excitatory postsynaptic current (EPSC) frequency in hippocampal slices.52,64
Anaesthetic effects on transmitter release from isolated nerve terminals
Isolated CNS nerve terminals (synaptosomes) provide an in vitro model to study the pharmacology of neurotransmitter release that has allowed a detailed pharmacological analysis of presynaptic anaesthetic pharmacology. Synaptosomes contain all the cellular machinery necessary for the generation and maintenance of ion gradients, and for synthesis, storage, uptake, and release of transmitters.48 They are depleted of glial and neuronal cell body elements, and are therefore devoid of cellular and network interactions, and can be prepared from various CNS regions that differ in their transmitter content and modulatory mechanisms. Release from synaptosomes can be evoked chemically by several methods, each of which involves activation of distinct components of the endogenous release mechanisms (Fig. 1). Thus, synaptosomes provide an unexcelled system for analyzing the presynaptic mechanisms of anaesthetic effects on synaptic transmission in isolation of indirect effects present in intact neural networks. Since transmitter release is coupled to changes in the activity of various ion channels, presynaptic receptors, and second messenger pathways, many of which have been implicated as anaesthetic targets, this system provides a functional assay for analyzing the mechanisms of anaesthetic effects at a number of potential target sites.
Fig 1.
Steps in the process of synaptic transmission. The action potential invades the presynaptic bouton (1) leading to depolarization mediated by voltage-gated Na+ channels. This can be mimicked in isolated nerve terminals by the chemical secretogogue 4-aminopyridine. The depolarization activates voltage-gated Ca2+ channels closely coupled to docked and releasable synaptic vesicles. (2) Increased extracellular K+ can depolarize the membrane potential independent of Na+ channel involvement to an extent sufficient to activate Ca2+ channels and transmitter release. The local elevations in intracellular Ca2+ concentration bind to the SNARE vesicle fusion complex, leading to exocytosis of transmitter. (3) The transmitter enters the synaptic cleft where it diffuses to the postsynaptic cell, activates synaptic and extrasynaptic receptors, and thereby modifies the excitability of the postsynaptic cell. (4) Anaesthetics can potentially disrupt this process at multiple points. Considerable evidence implicates inhibition of presynaptic voltage-gated Na+ channels as a probable site of inhaled anaesthetic action, particularly at excitatory glutamatergic synapses.
The presynaptic actions of volatile anaesthetics likely vary between transmitters due to differences in presynaptic physiology and release mechanisms, and between volatile anaesthetics due to their distinct pharmacological profiles. Recent studies on glutamate and GABA release from cortical nerve terminals provide a paradigm for studying the mechanisms of these effects and those of anaesthetics on other transmitters and in other CNS regions. Previous studies with rat cortical nerve terminals demonstrated that volatile anaesthetics (at clinically relevant concentrations) and propofol (at supraclinical concentrations) inhibit Ca2+-dependent glutamate release evoked by secretogogues that require Na+ channel activation (veratridine or 4-aminopyridine) with greater potency than Na+ channel-independent release (evoked by depolarization with elevated extracellular KCl which bypasses Na+ channels) (Fig. 2).38,68,77,95 Taken together with independent neurochemical67,69 and electrophysiological58,70,71 evidence of anaesthetic blockade of neuronal Na+ channels, these findings suggest that volatile anaesthetics inhibit glutamate release by blocking presynaptic Na+ channels, thereby inhibiting nerve terminal depolarization. Additional evidence for Na+ channel involvement is provided by observations that the model anaesthetic 1-chloro-1,2,2-trifluoro-cyclobutane (F3) inhibits Na+ channel-dependent glutamate release and blocks neuronal Na+ channels, while the structurally similar non-immobilizer 1,2-dichlorohexafluorocyclobutane (F6) is ineffective at predicted anaesthetic concentrations.70 This indicates a greater anaesthetic sensitivity of presynaptic Na+ channels than of the Ca2+ channels coupled to glutamate release, and is consistent with the observation that the predominant Ca2+ channel coupled to neurotransmitter release at hippocampal glutamatergic synapses (P/Q-type) is relatively insensitive to isoflurane.24 However, these findings do not preclude involvement of additional presynaptic targets in depression of transmitter release by volatile anaesthetics and do not explain the greater sensitivity of glutamate vs GABA release or the differential effects on basal release.95–97 Other presynaptic mechanisms that have been proposed include enhanced glutamate uptake35 or actions on the vesicle fusion process.88 However, enhanced glutamate uptake is insufficient to explain inhibition of glutamate release,94 and isoflurane effects on exocytosis in hippocampal neurons occur primarily upstream of vesicle fusion.20,28,77 A contribution of anaesthetic effects on the vesicle fusion process is possible,47 but has yet to be directly demonstrated in a vertebrate synapse.
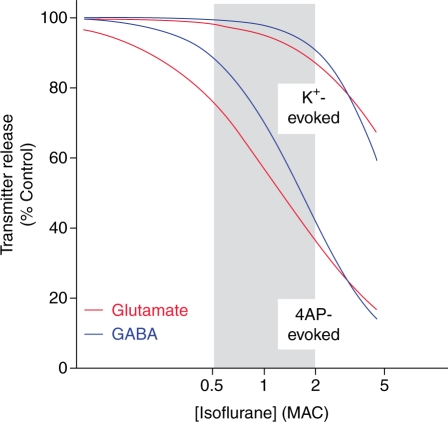
Fig 2.
Selective inhibition of Na+ channel-dependent glutamate release by isoflurane. Nerve terminals isolated from rat cerebral cortex were pre-labelled with radio-labelled glutamate and GABA, and release was measured following stimulation with either 4-aminopyridine (4AP) or elevated extracellular K+ as described in Figure 1.95 Na+ channel-dependent release of both glutamate (IC50=0.44 mM) and GABA (IC50=0.58 mM) evoked by 4AP was more sensitive to inhibition by isoflurane than release of glutamate (IC50=2.6 mM) or GABA (IC50=1.9 mM) evoked by elevated K+, consistent with a greater sensitivity of presynaptic Na+ channels than Ca2+ channels or other downstream processes. Moreover, 4AP-evoked release of the excitatory transmitter glutamate was inhibited at significantly lower concentrations than release of the inhibitory transmitter GABA within the clinical concentration range (0.5–2 MAC, or minimum alveolar concentration, which corresponds to the mean effective dose) indicated by gray shading. IC50, concentration for 50% inhibition. Unpublished data from H.C.H. and R.I. Westphalen.
The complexity of the presynaptic terminal is evident in the modulation of nerve terminal excitability by numerous ion channels and presynaptic receptors, and the multiple molecular interactions involved in vesicle mobilization, docking, fusion, and recycling.86 The small size of most nerve terminals in the CNS (<1 µm diameter) precludes direct electrophysiological analysis of presynaptic events. Isolated neurohypophysial nerve terminals92 have served as a useful model for studying the electrophysiological effects of general anaesthetics on nerve terminal ion currents by whole-terminal patch-clamp recording.54,58 Isoflurane inhibits nerve terminal Na+ currents and action potential amplitude through Na+ channel blockade in isolated rat neurohypophysial terminals. A similar approach used on the rat calyx of Held giant synapse also showed that isoflurane inhibits glutamatergic transmission presynaptically by a mechanism involving a reduction in Na+ channel-mediated action potential amplitude rather than Ca2+ channel blockade. Isoflurane significantly depressed action potential-evoked synaptic vesicle exocytosis and EPSC amplitude with only a small reduction in presynaptic action potential amplitude and no direct effect on Ca2+ current in the rat calyx of Held synapse.100 Simulated reductions in action potential amplitude reproduced this highly nonlinear relationship between peak Na+ current inhibition and exocytosis. These findings identify presynaptic Na+ channels as important anaesthetic targets for volatile anaesthetics. These approaches yielded the first direct recordings of anaesthetic actions on presynaptic ion channels, and helped bridge the gap between neurochemical studies of anaesthetic effects on transmitter release, studies of the molecular actions of anaesthetics on isolated targets, and the effects of anaesthetics on synaptic physiology.
Na+ channel pharmacology
Voltage-gated Na+ channels are responsible for the rapid depolarization phase of the action potential in electrically excitable cells such as nerve, muscle and heart.9 Voltage-gated channels respond to changes in the plasma membrane potential by opening to allow the passive flux of ions down their electrochemical gradient into or out of the cell. Their modular architecture allows interactions between multiple regions of the channel to orchestrate gating, rapid channel opening and closure. A dynamic model of receptor gating has been developed to explain the pharmacological response of this channel and its ion-selective conductance. A variety of drugs and toxins, including local anaesthetics, class I anti-arrhythmic drugs, and class I anti-epileptic drugs, exhibit voltage-dependent and frequency-dependent block of Na+ channels as described by the modulated receptor hypothesis.65 According to this model, these properties are conferred by different drug affinities for the various functional states of the Na+ channel: resting, open, and inactivated. Voltage-dependent inhibition is explained by drug binding to the inactivated state of the channel. This impedes the voltage-dependent transition of the channel from its inactivated state back to its resting state, which effectively reduces the number of resting channels available for activation in response to depolarization. Frequency-dependent inhibition is explained by selective drug binding to the open state of the channel. Na+ channels stimulated with increased frequency are statistically more likely to be in the open state. This allows increased drug binding, but does not prevent subsequent channel inactivation. Local anaesthetics, which show frequency-dependent inhibition, enter from the intracellular side and bind to the inner pore of the Na+ channel with high affinity.
The Na+ channel family consists of nine homologous pore-forming α subunits with distinct cellular and subcellular distributions (Table 1).9 The principle pore forming component of Na+ channels in mammalian brain is the 260 kilodalton glycoprotein α-subunit. It is a transmembrane protein with large intracellular N- and C-termini. The subunit contains four internally homologous repeated domains (I–IV) with over 50% sequence identity. Each domain consists of six segments (S1–S6) that form transmembrane α-helices. Four additional integral membrane glycoprotein subunits have been identified. The β1 and β3 (36 kDa) subunits interact non-covalently with the α-subunit, while the β2 and β4 subunits (33 kDa) are attached via a disulfide bond. There are at least nine isoforms of the α-subunit which vary in species and tissue expression. The α-subunit is sufficient to carry out the basic function of the channel. Coexpression of β-subunits accelerates inactivation and shifts voltage dependence toward more negative membrane potentials.
Table 1.
Voltage-gated Na+ channel family members. DRG, dorsal root ganglion; CNS, central nervous system; PNS, peripheral nervous system
| Channel α-subunits | Tissue expression | Modulators |
|---|---|---|
| NaV1.1 | CNS, PNS | Antagonists: Tetrodotoxin, Saxitoxin, µ-Conotoxin, Sea-anemone toxin, Local anaesthetics |
| NaV1.2 | CNS | |
| NaV1.3 | CNS | Activators: Veratridine, Batrachotoxin, α/β-Scorpion toxins |
| NaV1.4 | Skeletal muscle | |
| NaV1.5 | Heart, skeletal muscle | |
| NaV1.6 | CNS, PNS | |
| NaV1.7 | PNS, Schwann cells | |
| NaV1.8 | PNS (DRG) | |
| NaV1.9 | PNS | |
| Nax | Heart, uterus, skeletal muscle, astrocytes, DRG |
Several potent toxins have been used to classify, purify, and define functional domains of Na+ channels.10 The puffer fish poison tetrodotoxin (TTX) and the dinoflagellate toxin saxitoxin bind to an extracellular site (site 1) of the α-subunit. These toxins block Na+ permeability with high potency (KI=1–10 nM) to TTX-sensitive Na+ channels, and have enabled the identification of outer pore structures and the selectivity filter. Tissue selectivity is evident in the 200-fold lower affinity of TTX for TTX-insenstive Na+ channels (Table 1). Lipid soluble steroids such as the frog-skin toxin batrachotoxin and the plant alkaloids aconitine and veratridine bind to site 2. These toxins have a high affinity for the open state of the channel and lead to channel activation by slowing inactivation.
Direct evidence for Na+ channel block by volatile anaesthetics
Voltage-gated Na+ channels are critical to axonal conduction, synaptic integration, and neuronal excitability. Axonal action potentials were initially reported to be relatively resistant to clinical concentrations of volatile anaesthetics,34 which was consistent with the relative insensitivity of Na+ currents in squid26 and crayfish3 giant axons to volatile anaesthetics. This established the widespread notion ‘that clinical concentrations of general anaesthetics almost certainly do not act by blocking Na+ channels’ or any other voltage-gated ion channel.19 However, axonal conduction in small (0.1–0.2 µm) unmyelinated hippocampal axons is significantly depressed by inhaled anaesthetics,4,44 and other small diameter structures such as nerve terminals might also be sensitive. Patch clamp recordings of accessible nerve terminals have shown that isoflurane inhibits action potential amplitude,54 and that reductions in nerve terminal action potential amplitude have significant effects on transmitter release and hence on postsynaptic responses.100
Evidence that mammalian voltage-gated Na+ channels are sensitive to clinically relevant concentrations of general anaesthetics has come from careful analysis of anaesthetic effects on heterologously expressed channels. Analysis of volatile anaesthetic effects on heterologously expressed channels indicates that mammalian voltage-gated Na+ channels are sensitive to clinically relevant concentrations of general anaesthetics. One neuronal isoform (Nav1.2) is inhibited by multiple potent inhaled anaesthetics through a voltage-independent block of peak current and a hyperpolarizing shift in the voltage dependence of steady-state inactivation.71 Isoflurane and other inhaled anaesthetics inhibit multiple mammalian Na+ channel isoforms55 including Nav1.2,71 Nav1.4 and Nav1.6,56,78 Nav1.5,82 and Nav1.8. Initial reports suggested that the peripheral tetrodotoxin-resistant isoform Nav1.8 expressed in amphibian oocytes was resistant to inhaled anaesthetics,78 but more focused studies in neuronal cells indicates that Nav1.8 is inhibited by isoflurane at concentrations similar to those that inhibit other Nav isoforms.29
Potent inhaled anaesthetics also inhibit native Na+ channels in isolated nerve terminals58,69 and dorsal root ganglion neurons, while the non-immobilizer F6 is ineffective.70 In contrast, xenon has no detectable effect on Na+, Ca2+, or K+ channels in isolated cardiomyocytes.83 Recent studies indicate that xenon can in fact block neuronal Na+ channels at clinically relevant concentrations (H.C.H. and K.F. Herold unpublished data). Two principal mechanisms contribute to Na+ channel inhibition by isoflurane: voltage-independent block of peak currents and enhanced inactivation due to a hyperpolarizing shift in the voltage dependence of steady-state fast inactivation, with significant differences between isoform in the contributions of each mechanism to overall inhibition.55,71 Volatile anaesthetics, but not non-immobilizers, also inhibit native neuronal and nerve terminal Na+ channels, lending support to the notion that depression of synaptic neurotransmitter release occurs by Na+ channel blockade.55,70 The recent demonstration that NaChBac, a prokaryotic homologue of voltage-gated Na+ channels, is also inhibited by volatile anaesthetics opens the way for structure-function studies of these channels.57 Anaesthetic interactions with NaChBac might ultimately allow co-crystallization with anaesthetic for three-dimensional structure determinations by X-ray crystallography, as achieved for voltage-gated K+ channels, to determine the site(s) of interaction of anaesthetics with a voltage-gated ion channel. It is also intriguing that the binding sites for anaesthetics on ion channels exist in prokaryotic homologues, indicating a remarkable evolutionary conservation.
Testing the relevance of Na+ channel block as an anaesthetic target
Voltage-gated Na+ channels have received short shrift as anaesthetic targets, largely because early studies failed to demonstrate significant effects on action-potential conduction in myelinated axons. However smaller diameter unmyelinated fibres and bare nerve terminals are more sensitive to Na+ channel block and do not possess the considerable reserve in conduction seen in myelinated nerves. Numerous studies summarized earlier demonstrate that inhaled anaesthetics partially impair Na+ channel function at MAC (minimum alveolar concentration). Moreover, a variety of evidence supports a role for Na+ channels in general anaesthesia in vivo. An increase in cerebrospinal fluid Na+ concentration increases MAC (equivalent to ED50), while a reduction decreases MAC, in rats.87 Intravenous administration of the Na+ channel blocker lidocaine reduces MAC for several volatile anaesthetics in rats,105 and i.v. or intrathecal infusions of riluzole, a potent inhibitor of Na+ channels and glutamate release, decrease isoflurane MAC in rats.101 Finally, intrathecal but not intraventricular administration of veratridine, a toxin that maintains Na+ channels in their open state, increases the MAC for isoflurane in rats.106 Collectively, these results point to anaesthetic inhibition of Na+ channels as a plausible mechanism for the mediation of immobility produced by inhaled anaesthetics.
Funding
Supported by a grant from the U.S. National Institutes of Health (GM 58055).
Acknowledgement
The invaluable contribution of R.I. Westphalen to the preparation of Figure 2 is acknowledged.
References
- 1.Antognini JF, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993;79:1244–9. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Banks MI, Pearce RA. Dual actions of volatile anesthetics on GABA(A) IPSCs: dissociation of blocking and prolonging effects. Anesthesiology. 1999;90:120–34. doi: 10.1097/00000542-199901000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Bean BP, Shrager P, Goldstein DA. Modification of sodium and potassium channel gating kinetics by ether and halothane. J Gen Physiol. 1981;77:233–53. doi: 10.1085/jgp.77.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg-Johnsen J, Langmoen IA. The effect of isoflurane on unmyelinated and myelinated fibres in the rat brain. Acta Physiology Scand. 1986;127:87–93. doi: 10.1111/j.1748-1716.1986.tb07879.x. [DOI] [PubMed] [Google Scholar]
- 5.Berg-Johnsen J, Langmoen IA. The effects of isoflurane on excitatory synaptic transmission in the rat hippocampus. Acta Anaesthesiol Scand. 1992;36:350–4. doi: 10.1111/j.1399-6576.1992.tb03480.x. [DOI] [PubMed] [Google Scholar]
- 6.Bieda MC, Maclver MB. A major role for tonic GABAA conductances in anesthetic suppression of intrinsic neuronal excitability. J Neurophysiol. 2004;92:1658–67. doi: 10.1152/jn.00223.2004. [DOI] [PubMed] [Google Scholar]
- 7.Brockmeyer DM, Kendig JJ. Selective effects of ketamine on amino acid-mediated pathways in neonatal rat spinal cord. Br J Anaesth. 1995;74:79–84. doi: 10.1093/bja/74.1.79. [DOI] [PubMed] [Google Scholar]
- 8.Buggy DJ, Nicol B, Rowbotham DJ, Lambert DG. Effects of intravenous anesthetic agents on glutamate release: a role for GABAA receptor-mediated inhibition. Anesthesiology. 2000;92:1067–73. doi: 10.1097/00000542-200004000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 10.Cestele S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–92. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- 11.de Sousa SL, Dickinson R, Lieb WR, Franks NP. Contrasting synaptic actions of the inhalational general anesthetics isoflurane and xenon. Anesthesiology. 2000;92:1055–66. doi: 10.1097/00000542-200004000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson R, Peterson BK, Banks P, et al. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–67. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- 13.Dildy-Mayfield JE, Eger E, III, Harris RA. Anesthetics produce subunit-selective actions on glutamate receptors. J Pharmacol Exp Ther. 1996;276:1058–65. [PubMed] [Google Scholar]
- 14.Dodge FAJ, Rahamimoff R. On the relationship between calcium concentration and the amplitude of the end-plate potential. J Physiol. 1967;189:90P–92P. [PubMed] [Google Scholar]
- 15.Eger EI, II, Raines DE, Shafer SL, Hemmings HC, Jr, Sonner JM. Is a new paradigm needed to explain how inhaled anesthetics produce immobility? Anesth Analg. 2008;107:832–48. doi: 10.1213/ane.0b013e318182aedb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eilers H, Kindler CH, Bickler PE. Different effects of volatile anesthetics and polyhalogenated alkanes on depolarization-evoked glutamate release in rat cortical brain slices. Anesth Analg. 1999;88:1168–74. doi: 10.1097/00000539-199905000-00037. [DOI] [PubMed] [Google Scholar]
- 17.Flood P, Ramirez-Latorre J, Role L. Alpha 4 beta 2 neuronal nicotinic acetylcholine receptors in the central nervous system are inhibited by isoflurane and propofol, but alpha 7-type nicotinic acetylcholine receptors are unaffected. Anesthesiology. 1997;86:859–65. doi: 10.1097/00000542-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Franks NP, Honoré E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci. 2004;25:601–8. doi: 10.1016/j.tips.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–13. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 20.Fu D, Vissavajjhala P, Hemmings HC., Jr Volatile anaesthetic effects on phospholipid binding to synaptotagmin 1, a presynaptic Ca2+ sensor. Br J Anaesth. 2005;95:216–21. doi: 10.1093/bja/aei163. [DOI] [PubMed] [Google Scholar]
- 21.Gage PW, Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985;85:675–81. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray AT, Zhao BB, Kindler CH, et al. Volatile anesthetics activate the human tandem pore domain baseline K+ channel KCNK5. Anesthesiology. 2000;92:1722–30. doi: 10.1097/00000542-200006000-00032. [DOI] [PubMed] [Google Scholar]
- 23.Gruss M, Bushell TJ, Bright DP, et al. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65:443–52. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- 24.Hall AC, Lieb WR, Franks NP. Insensitivity of P-type calcium channels to inhalational and intravenous general anesthetics. Anesthesiology. 1994;81:117–23. doi: 10.1097/00000542-199407000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Haseneder R, Kratzer S, Kochs E, et al. Xenon reduces N-methyl-D-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated synaptic transmission in the amygdala. Anesthesiology. 2008;109:998–1006. doi: 10.1097/ALN.0b013e31818d6aee. [DOI] [PubMed] [Google Scholar]
- 26.Haydon DA, Urban BW. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983;341:429–39. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Hemmings HC, Jr, Yan W, Westphalen RI, Ryan TA. The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol. 2005;67:1591–9. doi: 10.1124/mol.104.003210. [DOI] [PubMed] [Google Scholar]
- 29.Herold KF, Nau C, Ouyang W, Hemmings HC., Jr Isoflurane inhibits the tetrodotoxin-resistant voltage-gated sodium channel Nav1.8. Anesthesiology. 2009 doi: 10.1097/ALN.0b013e3181af64d4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones MV, Harrison NL. Effects of volatile anesthetics on the kinetics of inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurophysiol. 1993;70:1339–49. doi: 10.1152/jn.1993.70.4.1339. [DOI] [PubMed] [Google Scholar]
- 31.Jurd R, Arras M, Lambert S, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 32.Kamatchi GL, Chan CK, Snutch T, Durieux ME, Lynch C., III Volatile anesthetic inhibition of neuronal Ca channel currents expressed in Xenopus oocytes. Brain Res. 1999;831:85–96. doi: 10.1016/s0006-8993(99)01401-8. [DOI] [PubMed] [Google Scholar]
- 33.Kullman DM, Martin RL, Redman SJ. Reduction by general anesthetics of group Ia excitatory postsynaptic potentials and currents in the cat spinal cord. J Physiol (London) 1989;412:277–96. doi: 10.1113/jphysiol.1989.sp017615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larrabee MG, Posternak JM. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol. 1952;15:91–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- 35.Larsen M, Grondahl TO, Haugstad TS, Langmoen IA. The effect of the volatile anesthetic isoflurane on Ca2+-dependent glutamate release from rat cerebral cortex. Brain Res. 1994;663:335–7. doi: 10.1016/0006-8993(94)91282-3. [DOI] [PubMed] [Google Scholar]
- 36.Liachenko S, Tang P, Somogyi GT, Xu Y. Concentration-dependent isoflurane effects on depolarization-evoked glutamate and GABA outflows from mouse brain slices. Br J Pharmacol. 1999;127:131–8. doi: 10.1038/sj.bjp.0702543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao M, Sonner JM, Jurd R, et al. Beta3-containing gamma-aminobutyric acidA receptors are not major targets for the amnesic and immobilizing actions of isoflurane. Anesth Analg. 2005;101:412–8. doi: 10.1213/01.ANE.0000154196.86587.35. [DOI] [PubMed] [Google Scholar]
- 38.Lingamaneni R, Birch ML, Hemmings HC., Jr Widespread inhibition of sodium channel-dependent glutamate release from isolated nerve terminals by isoflurane and propofol. Anesthesiology. 2001;95:1460–6. doi: 10.1097/00000542-200112000-00027. [DOI] [PubMed] [Google Scholar]
- 39.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–85. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 40.MacIver MB, Mikulec AA, Amagasu SM, Monroe FA. Volatile anesthetics depress glutamate transmission via presynaptic actions. Anesthesiology. 1996;85:823–34. doi: 10.1097/00000542-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Meir A, Ginsburg S, Butkevich A, et al. Ion channels in presynaptic nerve terminals and control of transmitter release. Physiol Rev. 1999;79:1019–88. doi: 10.1152/physrev.1999.79.3.1019. [DOI] [PubMed] [Google Scholar]
- 42.Mennerick S, Jevtovic-Todorovic V, Todorovic SM, Shen W, Olney JW, Zorumski CF. Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 1998;18:9716–26. doi: 10.1523/JNEUROSCI.18-23-09716.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miao N, Frazer MJ, Lynch C., III Volatile anesthetics depress Ca2+ transients and glutamate release in isolated cerebral synaptosomes. Anesthesiology. 1995;83:593–603. doi: 10.1097/00000542-199509000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Mikulec AA, Pittson S, Amagasu SM, Monroe FA, Maclver MB. Halothane depresses action potential conduction in hippocampal axons. Brain Res. 1998;796:231–8. doi: 10.1016/s0006-8993(98)00348-5. [DOI] [PubMed] [Google Scholar]
- 45.Minami K, Wick MJ, Stern-Bach Y, et al. Sites of volatile anesthetic action on kainate (glutamate receptor 6) receptors. J Biol Chem. 1998;273:8248–55. doi: 10.1074/jbc.273.14.8248. [DOI] [PubMed] [Google Scholar]
- 46.Murugaiah KD, Hemmings HC., Jr Effects of intravenous anesthetics on [3H]GABA release from rat cortical synaptosomes. Anesthesiology. 1998;89:919–28. doi: 10.1097/00000542-199810000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Nagele P, Mendel JB, Placzek WJ, Scott BA, d'Avignon DA, Crowder CM. Volatile anesthetics bind rat synaptic SNARE proteins. Anesthesiology. 2005;103:768–78. doi: 10.1097/00000542-200510000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Nicholls DG. The glutamatergic nerve terminal. Eur J Biochem. 1993;212:613–31. doi: 10.1111/j.1432-1033.1993.tb17700.x. [DOI] [PubMed] [Google Scholar]
- 49.Nicoll RA, Eccles JC, Oshima T, Rubia F. Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature. 1975;258:625–6. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- 50.Nikonorov IM, Blanck TJ, Recio-Pinto E. The effects of halothane on single human neuronal L-type calcium channels. Anesth Analg. 1998;86:885–95. doi: 10.1097/00000539-199804000-00038. [DOI] [PubMed] [Google Scholar]
- 51.Nishikawa K-I, Kidokoro Y. Halothane presynaptically depresses synaptic transmission in wild-type Drosophila larvae but not halothane-resistant (har) mutants. Anesthesiology. 1999;90:1691–7. doi: 10.1097/00000542-199906000-00026. [DOI] [PubMed] [Google Scholar]
- 52.Nishikawa K-I, MacIver MB. Excitatory synaptic transmission mediated by NMDA receptors is more sensitive to isoflurane than are non-NMDA receptor-mediated responses. Anesthesiology. 2000;92:228–36. doi: 10.1097/00000542-200001000-00035. [DOI] [PubMed] [Google Scholar]
- 53.Ouanonou A, Zhang Y, Zhang L. Changes in the calcium dependence of glutamate transmission in the hippocampal CA1 region after brief hypoxia-hypoglycemia. J Neurophysiol. 1999;82:1147–55. doi: 10.1152/jn.1999.82.3.1147. [DOI] [PubMed] [Google Scholar]
- 54.OuYang W, Hemmings HC., Jr Depression by isoflurane of the action potential and underlying voltage-gated ion currents in isolated rat neurohypophysial nerve terminals. J Pharmacol Exp Ther. 2005;312:801–8. doi: 10.1124/jpet.104.074609. [DOI] [PubMed] [Google Scholar]
- 55.Ouyang W, Hemmings HC., Jr Isoform-selective effects of isoflurane on voltage-gated Na+ channels. Anesthesiology. 2007;107:91–8. doi: 10.1097/01.anes.0000268390.28362.4a. [DOI] [PubMed] [Google Scholar]
- 56.Ouyang W, Herold KF, Hemmings HC., Jr Comparative effects of halogenated inhaled anesthetics on voltage-gated Na+ channel function. Anesthesiology. 2009;110:582–90. doi: 10.1097/ALN.0b013e318197941e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.OuYang W, Jih T-y, Zhang T-t, Correa AM, Hemmings HC., Jr Isoflurane inhibits NaChBac, a prokaryotic voltage-gated sodium channel. J Pharmacol Exp Ther. 2007;322:1076–83. doi: 10.1124/jpet.107.122929. [DOI] [PubMed] [Google Scholar]
- 58.OuYang W, Wang G, Hemmings HC., Jr Isoflurane and propofol inhibit presynaptic Na+ channels in isolated rat neurohypophysial nerve terminals. Mol Pharmacol. 2003;64:373–81. doi: 10.1124/mol.64.2.373. [DOI] [PubMed] [Google Scholar]
- 59.Patel AJ, Honore E. Anesthetic-sensitive 2P domain K+ channels. Anesthesiology. 2001;95:1013–21. doi: 10.1097/00000542-200110000-00034. [DOI] [PubMed] [Google Scholar]
- 60.Patel AJ, Honoŕe E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nature Neurosci. 1999;2:422–6. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- 61.Perouansky M, Baranov D, Salamn M, Yaari Y. Effects of halothane on glutamate receptor-mediated excitatory postsynaptic currents: A patch-clamp study in adult mouse hippocampal slices. Anesthesiology. 1995;83:109–19. doi: 10.1097/00000542-199507000-00014. [DOI] [PubMed] [Google Scholar]
- 62.Perouansky M, Hemmings HC Jr. Presynaptic actions of general anesthetics. In: Antognini JF, Carlens E, Raines D, editors. Neural Mechanisms of Anesthesia. Totowa, NJ: Humana Press; 2003. pp. 345–70. [Google Scholar]
- 63.Perouansky M, Hemmings HC, Jr, Pearce RA. Anesthetic effects on glutamatergic transmission: Lessons learned from a large synapse. Anesthesiology. 2004;100:470–2. doi: 10.1097/00000542-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Pittson S, Himmel AM, MacIver MB. Multiple synaptic and membrane sites of anesthetic action in the CA1 region of rat hippocampal slices. BMC Neurosci. 2004;5:52. doi: 10.1186/1471-2202-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci USA. 1996;93:9270–5. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rampil IJ, Mason P, Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology. 1993;78:707–12. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- 67.Ratnakumari L, Hemmings HC., Jr Inhibition by propofol of [3H]batrachotoxinin-A 20-α-benzoate binding to voltage-dependent sodium channels in rat cortical synaptosomes. Br J Pharmacol. 1996;119:1498–504. doi: 10.1111/j.1476-5381.1996.tb16064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ratnakumari L, Hemmings HC., Jr Effects of propofol on sodium channel-dependent sodium influx and glutamate release in rat cerebrocortical synaptosomes. Anesthesiology. 1997;86:428–39. doi: 10.1097/00000542-199702000-00018. [DOI] [PubMed] [Google Scholar]
- 69.Ratnakumari L, Hemmings HC., Jr Inhibition by presynaptic sodium channels by halothane. Anesthesiology. 1998;88:1043–54. doi: 10.1097/00000542-199804000-00025. [DOI] [PubMed] [Google Scholar]
- 70.Ratnakumari L, Vysotskaya TN, Duch DS, Hemmings HC., Jr Differential effects of anesthetic and nonanesthetic cyclobutanes on neuronal voltage-gated sodium channels. Anesthesiology. 2000;92:529–41. doi: 10.1097/00000542-200002000-00037. [DOI] [PubMed] [Google Scholar]
- 71.Rehberg B, Xiao Y-H, Duch DS. Central nervous system sodium channels are significantly suppressed at clinical concentrations of volatile anesthetics. Anesthesiology. 1996;84:1223–33. doi: 10.1097/00000542-199605000-00025. [DOI] [PubMed] [Google Scholar]
- 72.Reid CA, Clements JD, Bekkers JM. Nonuniform distribution of Ca2+ channel subtypes on presynaptic terminals of excitatory synapses in hippocampal cultures. J Neurosci. 1997;17:2738–45. doi: 10.1523/JNEUROSCI.17-08-02738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richards CD, Smaje JC. Anaesthetics depress the sensitivity of cortical neurones to L-glutamate. Br J Pharmacol. 1976;58:347–57. [PMC free article] [PubMed] [Google Scholar]
- 74.Richards CD, White AE. The actions of volatile anaesthetics on synaptic transmission in the dentate gyrus. J Physiol. 1975;252:241–57. doi: 10.1113/jphysiol.1975.sp011142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 76.Sandstrom DJ. Isoflurane depresses glutamate release by reducing neuronal excitability at the Drosophila neuromuscular junction. J Physiol. 2004;558:489–502. doi: 10.1113/jphysiol.2004.065748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schlame M, Hemmings HC., Jr Inhibition by volatile anesthetics of endogenous glutamate release from synaptosomes by a presynaptic mechanism. Anesthesiology. 1995;82:1406–16. doi: 10.1097/00000542-199506000-00012. [DOI] [PubMed] [Google Scholar]
- 78.Shiraishi M, Harris RA. Effects of alcohols and anesthetics on recombinant voltage-gated Na+ channels. J Pharmacol Exp Ther. 2004;309:987–94. doi: 10.1124/jpet.103.064063. [DOI] [PubMed] [Google Scholar]
- 79.Sirois JE, Lynch C, III, Bayliss DA. Convergent and reciprocal modulation of a leak K+ current and I(h) by an inhalational anaesthetic and neurotransmitters in rat brainstem motoneurones. J Physiol. 2002;541:717–29. doi: 10.1113/jphysiol.2002.018119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Solt K, Eger EI, II, Raines DE. Differential modulation of human N-methyl-D-aspartate receptors by structurally diverse general anesthetics. Anesth Analg. 2006;102:1407–11. doi: 10.1213/01.ane.0000204252.07406.9f. [DOI] [PubMed] [Google Scholar]
- 81.Sonner JM, Antognini JF, Dutton RC, et al. Inhaled anesthetics and immobility: mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesth Analg. 2003;97:718–40. doi: 10.1213/01.ANE.0000081063.76651.33. [DOI] [PubMed] [Google Scholar]
- 82.Stadnicka A, Kwok WM, Hartmann HA, Bosnjak ZJ. Effects of halothane and isoflurane on fast and slow inactivation of human heart hH1a sodium channels. Anesthesiology. 1999;90:1671–83. doi: 10.1097/00000542-199906000-00024. [DOI] [PubMed] [Google Scholar]
- 83.Stowe DF, Rehmert GC, Kwok WM, et al. Xenon does not alter cardiac function or major cation currents in isolated guinea pig hearts or myocytes. Anesthesiology. 2000;92:516–22. doi: 10.1097/00000542-200002000-00035. [DOI] [PubMed] [Google Scholar]
- 84.Study RE. Isoflurane inhibits multiple voltage-gated Ca2+ currents in hippocampal pyramidal neurons. Anesthesiology. 1994;81:104–16. doi: 10.1097/00000542-199407000-00016. [DOI] [PubMed] [Google Scholar]
- 85.Stuth EA, Krolo M, Tonkovic-Capin M, Hopp FA, Kampine JP, Zuperku EJ. Effects of halothane on synaptic neurotransmission to medullary expiratory neurons in the ventral respiratory group of dogs. Anesthesiology. 1999;91:804–14. doi: 10.1097/00000542-199909000-00033. [DOI] [PubMed] [Google Scholar]
- 86.Südhof TC. The synaptic vesicle cycle. Ann Rev Neurosci. 2004;27:509–47. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 87.Tanifuji Y, Eger EI., II Brain sodium, potassium, and osmolality: Effects on anesthetic requirement. Anesth Analg. 1978;57:404–10. doi: 10.1213/00000539-197807000-00007. [DOI] [PubMed] [Google Scholar]
- 88.van Swinderen B, Saifee O, Shebester L, Roberson R, Nonet ML, Crowder CM. A neomorphic syntaxin mutation blocks volatile-anesthetic action in Caenorhabditis elegans. Proc Natl Acad Sci USA. 1999;96:2479–84. doi: 10.1073/pnas.96.5.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Violet JM, Downie DL, Nakisa RC, Lieb WR, Franks NP. Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology. 1997;86:866–74. doi: 10.1097/00000542-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 90.Wakamori M, Ikemoto Y, Akaike N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. J Neurophysiol. 1991;66:2014–21. doi: 10.1152/jn.1991.66.6.2014. [DOI] [PubMed] [Google Scholar]
- 91.Wakasugi M, Hirota K, Roth S, Ito Y. The effects of general anesthetics agents on excitatory and inhibitor synaptic transmission in area CA1 of the rat hippocampus in vitro. Anesth Analg. 1999;88:676–80. doi: 10.1097/00000539-199903000-00039. [DOI] [PubMed] [Google Scholar]
- 92.Wang G, Dayanithi G, Newcomb R, Lemos JR. An R-Type Ca2+ current in neurohypophysial terminals preferentially regulates oxytocin secretion. J Neurosci. 1999;19:9235–41. doi: 10.1523/JNEUROSCI.19-21-09235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weakly JN. Effect of barbiturate on quantal synaptic transmission in spinal motoneurones. J Physiol (London) 1969;204:63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Westphalen RI, Hemmings HC., Jr Effects of isoflurane and propofol on glutamate and GABA transporters in isolated cortical nerve terminals. Anesthesiology. 2003;98:364–72. doi: 10.1097/00000542-200302000-00016. [DOI] [PubMed] [Google Scholar]
- 95.Westphalen RI, Hemmings HC., Jr Selective depression by general anesthetics of glutamate vs GABA release from isolated nerve terminals. J Pharmacol Exp Ther. 2003;304:1188–96. doi: 10.1124/jpet.102.044685. [DOI] [PubMed] [Google Scholar]
- 96.Westphalen RI, Hemmings HC., Jr Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: Basal release. J Pharmacol Exp Ther. 2006;316:208–15. doi: 10.1124/jpet.105.090647. [DOI] [PubMed] [Google Scholar]
- 97.Westphalen RI, Hemmings HC., Jr Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: 4-Aminopyridine evoked release. J Pharmacol Exp Ther. 2006;316:216–23. doi: 10.1124/jpet.105.090662. [DOI] [PubMed] [Google Scholar]
- 98.Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–12. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 99.Wu LG, Westenbroek RE, Borst JGG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J Neurosci. 1999;19:726–36. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu XS, Sun JY, Evers AS, Crowder M, Wu LG. Isoflurane inhibits transmitter release and the presynaptic action potential. Anesthesiology. 2004;100:663–70. doi: 10.1097/00000542-200403000-00029. [DOI] [PubMed] [Google Scholar]
- 101.Xing Y, Zhang Y, Stabernack CR, Eger EI II, Gray AT. The use of the potassium channel activator riluzole to test whether potassium channels mediate the capacity of isoflurane to produce immobility. Anesth Analg. 2003;97:1020–4. doi: 10.1213/01.ANE.0000077073.92108.E7. [DOI] [PubMed] [Google Scholar]
- 102.Yamakura T, Bertaccini E, Trudell JR, Harris RA. Anesthetics and ion channels: molecular models and sites of action. Annu Rev Pharmacol Toxicol. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]
- 103.Yamakura T, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology. 2000;93:1095–101. doi: 10.1097/00000542-200010000-00034. [DOI] [PubMed] [Google Scholar]
- 104.Yang J, Zorumski CF. Effects of isoflurane on N-methyl-D-aspartate gated ion channels in cultured rat hippocampal neurons. Ann NY Acad Sci. 1991;625:287–9. doi: 10.1111/j.1749-6632.1991.tb33851.x. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y, Laster MJ, Eger EI II, Sharma M, Sonner JM. Lidocaine, MK-801, and MAC. Anesth Analg. 2007;104:1098–102. doi: 10.1213/01.ane.0000260318.60504.a9. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Sharma M, Eger EI, II, Laster MJ, Hemmings HC, Jr, Harris RA. Intrathecal veratridine administration increases MAC in rats. Anesth Analg. 2008;107:875–8. doi: 10.1213/ane.0b013e3181815fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y, Sonner JM, Eger EI II, et al. Gamma-aminobutyric acidA receptors do not mediate the immobility produced by isoflurane. Anesth Analg. 2004;99:85–90. doi: 10.1213/01.ANE.0000118108.64886.42. [DOI] [PubMed] [Google Scholar]
- 108.Zimmerman SA, Jones MV, Harrison NL. Potentiation of gamma-aminobutyric acid A receptor Cl-current correlates with in vivo anesthetic potency. J Pharmacol Exp Ther. 1994;270:987–91. [PubMed] [Google Scholar]