Abstract
Electron paramagnetic resonance (EPR) spectroscopy was used to measure the free radicals in the particulate matter (PM) emissions from wood and coal combustion. The intensity of radicals in PM dropped linearly within two months of sample storage and stabilized after that. This factor of storage time was adjusted when comparing radical intensities among different PM samples. An inverse relationship between coal rank and free radical intensities in PM emissions was observed, which was in contrast with the pattern of radical intensities in the source coals. The strong correlation between intensities of free radical and elemental carbon in PM emissions suggests that the radical species may be carbon-centered. The increased g-factors, 2.0029−2.0039, over that of purely carbon-centered radicals may indicate the presence of vicinal oxygen heteroatom. The redox and biology activities of these carbon-centered radicals are worthy of evaluation.
Introduction
Although extensive epidemiological evidence associates airborne particulate matter (PM) with increases in human mortality,1,2 the mechanisms of PM-related health effects are still incompletely understood. One mechanistic hypothesis for PM injury is the ability of certain PM properties to induce oxidative stress, which refers to the situation of a serious imbalance between the production of reactive oxygen species (ROS) and antioxidant defense. Oxidative stress occurs in an organism when the concentration of ROS generated exceeds the antioxidant capability of that organism. ROS is a collective term that includes both oxygen-centered radicals (O2•− and OH•) and nonradical derivatives of O2 such as hydrogen peroxide (H2O2). A free radical is a molecular fragment with an unpaired valence electron, conventionally symbolized by a superscript dot “•”. The presence of the unpaired electron causes free radicals to be attracted slightly to a magnetic field; thus, it is called paramagnetic and can be measured by electron paramagnetic resonance (EPR) spectroscopy.
Most previous studies on PM-induced oxidative stress have not made the difficult distinction between endogenous and exogenous sources of free radicals, so the contribution of each source to the PM effect is unknown.(3) To differentiate the contribution of each source to the PM effects, we need to first measure the free radicals that already exist on the particles before inhaled. This is particularly important for combustion-generated carbonaceous particles, which may contain not only transition metals and polycyclic aromatic hydrocarbons (PAHs) that are responsible for in situ production of free radicals in the lung but also free radicals in the PM itself.
The current paper presents measurements of free radicals in PM emissions from wood and coal combustion. The coal samples were taken from Xuan Wei, China, where the extremely high indoor air pollution due to bituminous coal combustion was linked to the highest women lung cancer rate of China.(4) We explored the effects of sample storage time and fuel type on the measurements of free radicals in PM emissions.
Experimental Section
Combustion Experiment and Air Sampling
The experimental methods for combustion and air sampling were reported elsewhere5,6 and are only summarized here. Fifteen types of coal (12 bituminous and 3 anthracite) and two wood samples (one kindling wood Fatwood StarterStix and another oven-dried pine wood sample) were burned under controlled conditions in the laboratory to measure the gaseous and particulate emissions. An open fire was assembled with firebricks to simulate the household fire pit used in Xuan Wei, China, where the extremely high indoor air pollution was linked to the highest female lung cancer rate of China.(4) The combustion experiment involves heating 1.5 kg of water from ambient temperature to boiling, kept simmering for 10 min. Air is drawn from the exhaust duct through 1/4 in. stainless steel sampling probes placed 0.5 m downstream of the duct inlet and 1 m upstream of the damper. Duplicate sets of particulate matter (PM) were collected with various kinds of filter media for different analytic techniques: quartz fiber filters for EC/OC analysis and glass fiber filters (Pallflex, Gelman Sciences Inc.) for free radical analysis. Filter samples were stored in −10 °C freezers before analysis. Over 90% of the particulate mass was attributed to submicrometer particles.(6)
EC/OC Analysis
The thermal/optical reflectance (TOR) method was applied to determine elemental and organic carbon (EC/OC). The TOR method is based on the principle that different types of carbon-containing particles are converted to gases under different temperature and oxidation conditions. Quartz fiber filter samples were analyzed for EC/OC by a DRI Model 2001 thermal/optical carbon analyzer. The protocol involves heating a 0.526 cm2 punch aliquot of a filter stepwise in a nonoxidizing helium (He) atmosphere to measure organic carbon and in an oxidizing atmosphere of 2% oxygen in a balance of helium to measure elemental carbon.(7)
Free Radical Analysis
Electron paramagnetic resonance (EPR) spectroscopy analysis was carried out using the standard continuous wave method in which the microwave frequency, v, is held constant and the magnetic field strength, H, is swept. The EPR spectra were obtained with a Bruker Instruments model EMX EPR spectrometer using filter samples placed in quartz tubes. One quarter of a Pallflex glass fiber filter was cut out, folded to cover the particle collection side, rolled into a small cylinder 2–3 mm in height, and inserted into a quartz EPR tube until reaching 1.5 cm from the bottom of the tube. The EPR parameters were 100 kHz, X-band; microwave frequency, about 9.25 GHz; attenuation, 7 dB; modulation amplitude, 4 G; time constant, 1 s; receiver gain, 2500; scan time, 2 min; scan range, 200 G. All measurements were done at room temperature. The radical intensities were quantified using a standard curve of 2,2-diphenyl-1-picryhydrazyl (DPPH). The g-factors that describe the EPR peak positions were estimated using DPPH (g = 2.0036) as a standard. To determine how the radical intensity changes over time, we measured two duplicate quarters from one same glass fiber filter loaded with PM for 5 times within 6 months. Filter samples placed in quartz tubes were stored in −10 °C freezers between each measurement.
Results and Discussion
Decay of Free Radicals over Storage Time
The intensity of radicals dropped by 15% in the first two months since first measurement and stabilized after that (Figure 1). The g-factor and peak-to-peak line width ΔHp−p had no significant changes over storage time. The first EPR measurement was not performed until 8 days after the particulate sample was taken. It was likely that an even sharper decrease already occurred before the first EPR measurement at day 9. Decay of thermally generated free radicals in nuts was studied by Yordanov.(8) Free radical intensity in thermally treated flakes of almond decreased over time: a sharp decrease by 50% was observed in the first two days and remains practically unchanged over a period of 15 days. It took only two days for the radical intensity to stabilize in the study of almond nuts, while in the present study of wood and coal emissions it took two months. The contrast here may reflect the effects of different source materials, thermal treatment procedures, and sample storage conditions. One possible reason for the decrease of radical intensities within the first two months of the storage time is the “oxygen effect”,9,10 the rapid decrease of radical intensities when the oxygen diffuse through the carbonized specimen. The decrease in radical intensity may be caused by the physical interaction between the radicals in the specimen and oxygen, a paramagnetic gas.
Figure 1.
Change of radical intensities over storage time. The first EPR measurement was not performed until 8 days after the particulate sample was taken. The intensity of radicals dropped by 15% in the first two months since first measurement and stabilized after that.
There are probably two types of initially formed radicals: one relatively short-lived and the other more persistent. The more persistent radicals are thought to account for the stabilized radical intensities 2−6 months after the samples were taken (Figure 1). These radicals are inactive and chemically stable due to the delocalization of the unpaired electron over many conjugated or aromatic chemical bonds.(11) The lifetime of some unpaired electrons, strongly delocalized over the condensed aromatic rings in charcoal or coal, can be extremely long.(12) Autopsy lung tissue from coal miners exhibited similar free radical activity as that of coal dust.(13) The intensity of unpaired electron EPR signals in charcoal produced today is comparable to that found in the pyramids in Egypt.(9) Some radicals found in cigarette tar have an essentially infinite lifetime.(14) Radicals on PM2.5 collected from urban air are remarkably persistent and can be observed in samples that had been stored for several months.(15)
Influence of Fuel Type on Free Radical Measurement
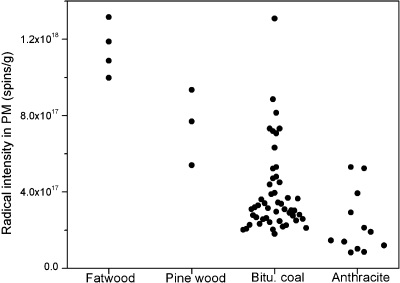
Radical intensities in PM emissions tend to decrease with respect to fuel types from Fatwood, pine wood, and bituminous coal to anthracite (Figure 2). Along with this order of fuel types are decreasing reactivity due to the declining contents of volatiles, aliphatic carbon, and oxygen-containing functional groups. The Fatwood kindling, made of 100% natural wood, burns quickly with a large flame; the oven-dried pine wood burns slower with shorter flames. As compared to bituminous coals, the higher-rank anthracites are less reactive because their structure is approaching that of graphite. A large variability of the radical intensities is seen in PM emissions from the bituminous coals. Carbon aromaticity, as an indicator of coal rank, was calculated for these bituminous coals based on H/C atomic ratio: 1.22−0.58 × H/C.(16) Figure 3 shows an inverse relationship between coal rank and free radical intensities in PM emissions from the 12 bituminous coals. The inverse relationship observed here for the PM emissions is intriguing because it is just opposite to what was reported for the source fuels: coals show a steady increase in radical densities with increasing coalification.(17) This contrast emphasizes the complexity of the free radical creation and recombination processes during combustion of different fuels.
Figure 2.
Effect of fuel type on free radical measurements. Radical intensities in PM emissions tend to decrease with respect to fuel types from Fatwood, pine wood, and bituminous coal to anthracite.
Figure 3.
Effect of the bituminous coal aromaticity on the free radical intensities in PM emissions. There is an inverse relationship between coal rank and free radical intensities in PM emissions.
Table 1 shows a comparison of the free radicals in PM emissions from different fuels. The average radical intensities in PM emissions from Fatwood, pine wood, bituminous coal, and anthracite are 1.2 × 1018, 9.1 × 1017, 4.4 × 1017, and 2.3 × 1017 spins/gram, respectively. For comparison, the radical intensity was reported to be 3 × 1016 spins/gram in cigarette tar(18) and 1.3 × 1016 to 1.5 × 1017 spins/gram in airborne PM2.5.(15)
Table 1. Free Radicals in PM Emissions from Wood and Coal Combustion.
| g-factor | line width (Gauss) | EPR intensity (spins/g) | ||
|---|---|---|---|---|
| fuel | number of PM samples | mean (SE) | mean (SE) | mean (SE) |
| Fatwood | 4 | 2.0029 (8.1 × 10−5) | 5.80 (0.05) | 1.2 × 1018 (6.8 × 1016) |
| pine wood | 3 | 2.0031 (1.2 × 10−4) | 5.65 (0.08) | 9.1 × 1017 (1.1 × 1017) |
| bituminous coal | 49 | 2.0035 (4.0 × 10−5) | 6.21 (0.05) | 4.4 × 1017 (4.0 × 1016) |
| anthracite | 13 | 2.0039 (1.7 × 10−4) | 6.22 (0.17) | 2.3 × 1017 (5.5 × 1016) |
Chemical Speciation of Radicals
The g-factors of EPR spectra can be used to assess whether a radical is carbon-centered or oxygen-centered: whether the unpaired electron is located on a carbon or oxygen atom. Carbon-centered radicals have g-factors that are close to the free electron g-factor 2.0023.10,19 Carbon-centered radicals with an adjacent oxygen atom have higher g-factors in the range of 2.003−2.004, while oxygen-centered radicals have g-factors that are >2.004.(19) The g-factor of 2.0034−2.0039 is characteristic of carbon centered radicals with a nearby oxygen heteroatom that results in increased g-factors over that of purely carbon-centered radicals. The g-factor and line width of the PM emissions from Fatwood, a long flame kindling wood, are similar to those of diesel particulate matter which had a g-factor of 2.0029 and line width of 5.3−6.0 G.(10) Overall, the radicals from wood and coal combustion presented here are similar to the type II radicals (g = 2.0035−2.0040 and ΔHp−p= 8.5 ± 0.2 G) from the oxidative pyrolysis of tobacco, which are surface-associated or bulk polymeric, carbon-centered radicals containing vicinal oxygen.20,21
Figure 4 shows the relation between the intensity of radicals and the percentage of elemental carbon in the PM from wood and coal combustion. The strong correlation between intensities of free radical and elemental carbon (R = 0.87, p < 0.0001) suggests that the predominant radical species is carbon-centered. It has been claimed that free radicals are generated during the thermal breaking down of the carbon−hydrogen bonds in the formation of the condensed carbon rings.(9) The unpaired electron becomes highly stabilized in the π-bond system over the ring structure. The increased g-factors, 2.0029−2.0039, over that of purely carbon-centered radicals may indicate the presence of the vicinal oxygen heteroatom. While oxygen-centered radicals are the most studied and are involved in the pathogenesis of many pulmonary diseases,(22) carbon-centered free radicals may be also reactive to living cells and thus important in biology. The redox and biological activities of these carbon-centered radicals are worthy of evaluation.
Figure 4.
Relation between the radical intensities and percentage of elemental carbon in the PM emissions from wood and coal combustion. The strong correlation between intensities of free radical and elemental carbon suggests that the radical species may be carbon-centered.
Acknowledgments
This work was supported by the Environmental Health Sciences Superfund Basic Research Program (Grant P42ESO47050-01) from the National Institute of Environmental Health Sciences, the Wood Calvert Chair in Engineering (UCB), the National Cancer Institute, and the University of California Toxic Substances Research and Training Program. We also acknowledge NIH Grant GM 55302 (to V.K.Y.) and the Department of Energy, Director, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division, under Contract DE-AC02-05CH11231 (to V.K.Y. and J.Y.).
Funding Statement
National Institutes of Health, United States
References
- Samet J. A.; Schwartz J.; Suh H. H. Fine particulate air pollution and mortality in 20 US cities. New Engl. J. Med. 2001, 344 (16), 1253–1254. [DOI] [PubMed] [Google Scholar]
- Pope C. A.; Burnett R. T.; Thun M. J.; Calle E. E.; Krewski D.; Ito K.; Thurston G. D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA (Journal of the American Medical Association) 2002, 287 (9), 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F.; Gonzalez-Flecha B.; Kobzik L. Reactive oxygen species in pulmonary inflammation by ambient particulates. Free Radical Biol. Med. 2003, 35 (4), 327–40. [DOI] [PubMed] [Google Scholar]
- Mumford J. L.; He X. Z.; Chapman R. S.; Cao S. R.; Harris D. B.; Li X. M.; Xian Y. L.; Jiang W. Z.; Xu C. W.; Chuang J. C.; Wilson W. E.; Cooke M. Lung cancer and indoor air pollution in Xuan Wei, China. Science 1987, 235 (4785), 217–220. [DOI] [PubMed] [Google Scholar]
- Tian L. W.Coal Combustion Emissions and Lung Cancer in Xuan Wei, China. Ph.D. dissertation, University of California, Berkeley, California, 2005, pp 1−221.
- Tian L. W.; Lucas D.; Fischer S. L.; Lee S. C.; Koshland C. P. Particle and gas emissions from a simulated fire pit. Environ. Sci. Technol. 2008, 42 (7), 2503–2508. [DOI] [PubMed] [Google Scholar]
- Cao J. J.; Lee S. C.; Ho K. F.; Zhang X. Y.; Zou S. C.; Fung K.; Chow J. C.; Watson J. G. Characteristics of carbonaceous aerosol in Pearl River Delta Region, China during 2001 winter period. Atmos. Environ. 2003, 37 (11), 1451–1460. [Google Scholar]
- Yordanov N. D.; Mladenova R. EPR study of thermally generated free radicals in nuts. Int. J. Food Sci. Technol. 2007, 42 (12), 1384–1389. [Google Scholar]
- Ingram D. J. E.Biological and biochemical applications of electron spin resonance; Plenum Press: New York, 1969; p x, 311. [Google Scholar]
- Ross M.; Chedekel M.; Risby T.; Lestz S.; Yasbin R. Electron paramagnetic resonance spectrometry of diesel particulate matter. Environ. Int. 1982, 7, 325–329. [Google Scholar]
- Borg D. C.Oxygen free radicals and tissue injury: a reference outline. In Oxygen free radicals in tissue damage; Tarr M., Samson F., Eds.; Birkhauser: Boston, 1993; pp 12−53. [Google Scholar]
- Yordanov N. D.Introduction to the theory of Electron Paramagnetic Resonance and its application to the study of aerosols. In Analytical chemistry of aerosols, Spurny K. R., Ed. Lewis: Boca Raton, 1999; pp 197−213. [Google Scholar]
- Dalal N. S.; Suryan M. M.; Vallyathan V.; Green F. H. Y.; Jafari B.; Wheeler R. Detection of Reactive Free-Radicals in Fresh Coal-Mine Dust and Their Implication for Pulmonary Injury. Ann. Occup. Hyg. 1989, 33 (1), 79–84. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. Biological Effects of Cigarette-Smoke, Wood Smoke, and the Smoke From Plastics - the Use of Electron-Spin-Resonance. Free Radical Biol. Med. 1992, 13 (6), 659–676. [DOI] [PubMed] [Google Scholar]
- Squadrito G. L.; Cueto R.; Dellinger B.; Pryor W. A. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radical Biol. Med. 2001, 31 (9), 1132–1138. [DOI] [PubMed] [Google Scholar]
- Maroto-Valer M. M.; Andersen J. M.; Snape C. E. Verification of the linear relationship between carbon aromaticities and H/C ratios for bituminous coals. Fuel 1998, 77 (7), 783–785. [Google Scholar]
- Czechowski F.; Jezierski A. EPR studies on petrographic constituents of bituminous coals, chars of brown coals group components, and humic acids 600 degrees C char upon oxygen and solvent action. Energy Fuels 1997, 11 (5), 951–964. [Google Scholar]
- Pryor W. A.; Hales B. J.; Premovic P. I.; Church D. F. The Radicals in Cigarette Tar - Their Nature and Suggested Physiological Implications. Science 1983, 220 (4595), 425–427. [DOI] [PubMed] [Google Scholar]
- Dellinger B.; Loninicki S.; Khachatryan L.; Maskos Z.; Hall R. W.; Adounkpe J.; McFerrin C.; Truong H. Formation and stabilization of persistent free radicals. Proc. Combust. Inst. 2007, 31, 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos Z.; Khachatryan L.; Dellinger B. Formation of the persistent primary radicals from the pyrolysis of tobacco. Energy Fuels 2008, 22 (2), 1027–1033. [Google Scholar]
- Maskos Z.; Dellinger B. Radicals from the oxidative pyrolysis of tobacco. Energy Fuels 2008, 22 (3), 1675–1679. [Google Scholar]
- Vallyathan V.; Shi X. The role of oxygen free radicals in occupational and environmental lung diseases. Environ. Health Perspect. 1997, 105 (Suppl 1), 165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]