Abstract
Multiple object tracking is hypothesized to utilize visual indexes, which may provide rapid, parallel access to a limited number of visual objects, thereby supporting a variety of spatial tasks. We examined whether faulty indexing might play a role in the severe visuospatial deficits found in Williams syndrome. We asked observers to track from one to four targets in a display of eight identical objects. Objects remained stationary (static condition) or moved randomly and independently (moving condition) for 6 s, after which observers pointed to the objects they thought were targets. People with Williams syndrome were impaired in the moving condition, but not the static condition, compared with mental-age-matched control participants. Normal children who were younger than the mental-age-matched control children did not show the same profile as individuals with Williams syndrome, which suggests that the difference between the tasks in WS did not reflect simple developmental immaturity. Error analysis revealed that all groups had “slippery” indexes, falsely identifying target neighbors, and further suggested that people with Williams syndrome deploy fewer indexes than do people without this disorder.
Adults can simultaneously track up to four or five objects as they move, change, or briefly disappear (Pylyshyn & Storm, 1988; Scholl & Pylyshyn, 1999). Similarly, infants can track at least two objects as they move behind and emerge from occluders (e.g., Spelke, 1990; Wynn, 1992). Tracking multiple objects requires complex spatial computations; the fact that this capacity is robust in adulthood and available in infancy suggests that it reflects a core spatial mechanism, separate from memory for static locations (Carey & Xu, 2001; Leslie, Xu, Tremoulet, & Scholl, 1998; Richardson & Kirkham, 2004). Pylyshyn and his colleagues proposed a mechanism, called visual indexing, allows people to track several objects simultaneously, and may play a crucial role in other visuospatial tasks, including block construction and subitizing (Ballard, Hayhoe, Pook, & Rao, 1997; Leslie et al., 1998; Pylyshyn, 2000; Ullman, 1984).
In the study we report here, we examined visual indexing in children and adults with Williams syndrome (WS), a rare genetic disorder characterized by severe visuospatial impairment. We asked whether indexing is damaged in children with WS relative to normally developing children. If visual indexing reflects a core spatial mechanism, it could be impaired in WS even while other spatial functions—such as memory for static location—are spared. Impaired indexing could be partly responsible for the hallmark spatial deficit in WS, difficulty copying spatial models. Furthermore, a unique pattern of performance among people with WS would suggest one possible causal mechanism for their impaired spatial development.
To study visual indexing (originally called FINSTs, or Fingers of INSTantiation), Pylyshyn and Storm (1988) designed the multiple object tracking (MOT) task, which examines people’s ability to keep track of multiple moving objects. Observers in this study viewed 10 identical objects, 1 to 5 of which were cued as targets. All 10 objects then moved along independent trajectories, and at some point, one of the objects flashed. Participants judged whether this object was a target. Adults did surprisingly well on this task, although performance decreased with larger numbers of targets. Pylyshyn and Storm argued that these results are most compatible with a parallel, limited-resource model such as indexing.
Crucially, this task discriminates between the use of indexes and the use of other spatial representations. Tasks that test memory for static object location, for example, can be accomplished using other processes (e.g., remembering spatial coordinates or using spatial language. But tracking multiple moving objects requires continual and simultaneous updating of multiple locations, and therefore seems to entail indexes. Pylyshyn and Storm (1988) proposed that adults possess a maximum of five indexes that act like “sticky fingers,” adhering to objects on the basis of spatiotemporal continuity. Subsequent research confirmed that the tracking mechanism seems to operate on objects (Scholl & Pylyshyn, 1999). For instance, it is difficult to track items that move like substances (e.g., pouring; vanMarle & Scholl, 2003). Imaging research suggests that the neural substrates of object tracking are located in parietal and frontal cortices (Culham et al., 1998).
The case of WS presents a unique opportunity to examine whether this mechanism can help explain severe developmental spatial impairment. WS is a rare genetic deficit (1 in 20,000 live births) resulting from a hemizygous microdeletion on chromosome 7q11.23 (Ewart et al., 1993). WS causes moderate retardation (mean IQ of approximately 60; Howlin, Davies, & Udwin, 1998; Mervis et al., 2000), along with severe visuospatial deficits. These deficits are not well understood, but not all aspects of spatial representation are equally damaged. People with WS are severely impaired in block construction and copying tasks (Bellugi, Lichtenberger, Jones, Lai, & St. George, 2000; Hoffman, Landau, & Pagani, 2003; Mervis et al., 2000). But they perform as well as or better than normal mental-age-matched (MA-matched) observers on tests of biological motion (Jordan, Reiss, Hoffman, & Landau, 2002), motion coherence (J.E. Reiss, Hoffman, & Landau, in press; see also Atkinson et al., 1997), object identification (Landau, Hoffman, & Kurz, in press), and face recognition (Paul, Stiles, Passarotti, Bavar, & Bellugi, 2002; Tager-Flusberg, Plesa-Skwerer, Faja, & Joseph, 2003).
This uneven profile suggests that the WS genetic deficit impairs some visuospatial functions and not others. Indexing is a good candidate for impairment. First, it may play a role in tasks such as block construction, which people with WS fail (Ballard et al., 1997; Hoffman et al., 2003). Second, it may provide a foundation for counting or subitizing (Trick & Pylyshyn, 1993), and people with WS may be impaired in related aspects of numerical representation (Ansari et al., 2003; Howlin et al., 1998). Finally, indexing engages the parietal areas (Culham et al., 1998), and these appear to be a major locus of damage in WS (Atkinson et al., 1997; Meyer-Lindenberg et al., 2004; Paul et al., 2002; A.L. Reiss et al., 2000).
We evaluated indexing in people with WS by comparing their performance in the MOT task with their performance on a static location task that was identical to the MOT task except that the objects never moved. Performance on both tasks was examined relative to that of normally developing children. By hypothesis, multiple object tracking and memory for multiple static objects need not rely on the same mechanism and should be empirically separable. Tracking targets in the MOT task should require indexing. However, identifying targets in the static task could be done by indexing or by other mechanisms such as remembering the objects’ coordinates or the overall configuration of objects in the display.
EXPERIMENT 1
Participants
The WS group included 8 males and 7 females (mean age = 18 years 0 months, range = 10 years 5 months through 38 years 11 months) positively diagnosed by a geneticist and the FISH (fluoride in situ hybridization) test (Ewart et al., 1993). Normally developing control participants were 7 males and 8 females (mean age = 5 years 11 months, range = 4 years 6 months through 7 years 3 months). We individually matched normally developing and WS participants on MA, using raw scores on the Kaufman Brief Intelligence Test (KBIT; Kaufman & Kaufman, 1990). The Verbal subtest requires picture naming, a relative strength in WS; mean scores were 38 ± 8.2 for the WS group and 35 ± 7.2 for the control group, t(28) =1.04, p =.3. The Matrices subtest requires picture-based category matching and has few spatial items; therefore, it does not overly penalize people with WS for their spatial deficit; mean scores were 20 ± 4.9 for the WS group and 21 ± 5.1 for the control group, t(28) = −0.18, p = .9. The WS profile was typical, with a mean IQ of 61 ± 16 (KBIT) and a mean block construction score at the 1st percentile for age (Differential Abilities Score; Elliott, 1990). People with WS were recruited through the Williams Syndrome Association, and control subjects through local preschools.
Design, Stimuli, and Procedure
Participants viewed an LCD monitor from a distance of approximately 18 to 25 in. The screen (resolution of 1024 × 768 × 32) subtended approximately 28° × 21° × of visual angle. The moving and static conditions were counterbalanced, and in each, there were 2 practice trials followed by 24 randomly ordered test trials, 6 each with one, two, three, or four targets.
In each condition, eight red squares (2.8° on a side) appeared on a black background (Fig. 1). Before each trial, the objects were assigned random starting positions, with centers at least 5.7° apart and edges 0.8° from the screen boundaries. The red cards then “flipped over” one at a time to reveal from one to four targets (i.e., cats). Participants were told to remember the target locations (static condition) or to track the targets (moving condition). They were given as much time as they wanted to study the display. The experimenter then clicked the computer mouse, and all the cards flipped back over, becoming identical again. The cards then either remained stationary (static condition) or moved (moving condition) for 6 s. Motion paths were computed by assigning each object an initial random starting direction, which changed as a function of the object’s distance from other objects or the sides. If an object was within 3.78° of another object or one of the sides, it was assigned a new random direction to avoid contact. Velocity was constant at 3.6°/s. Participants were encouraged throughout the delay to “look at the screen” and “remember where the cats are hiding” (or “running’’).
Fig. 1.
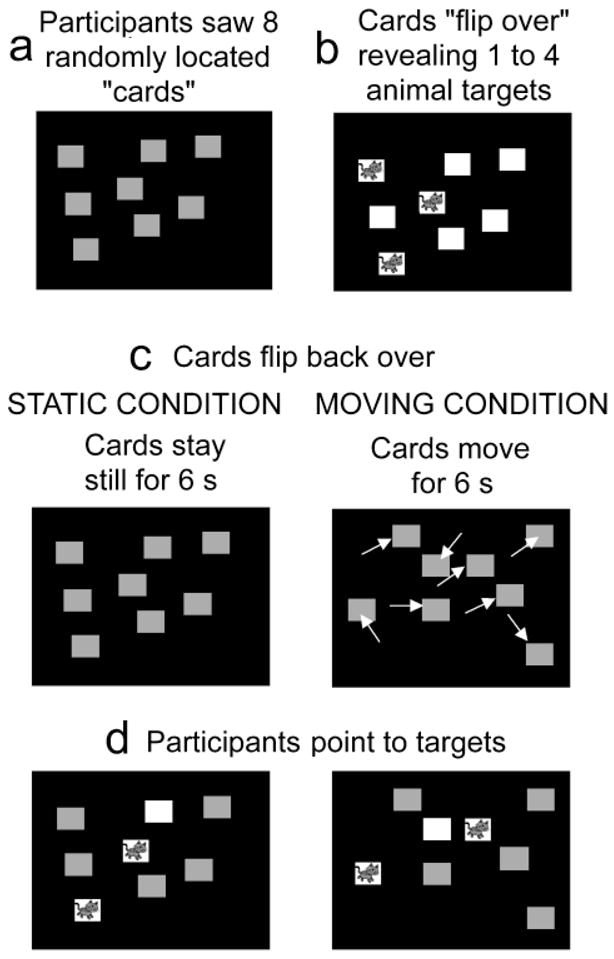
Illustration of the task. Participants saw eight cards (a), which then flipped over to reveal the targets (b). After the cards turned back over so that the targets were hidden, the cards stayed still (static condition) or moved (moving condition) for 6 s. Finally, the subjects indicated which cards were the targets.
After the 6 s, a tone sounded, and the mouse pointer appeared on the screen; in the moving condition, the cards stopped moving. Finally, participants used the mouse pointer to indicate the cards they thought were targets, and the experimenter recorded the choices by clicking the computer mouse. With each click, the selected card flipped over, revealing either a plain white side (nontarget) or a cat that meowed (target).
Results
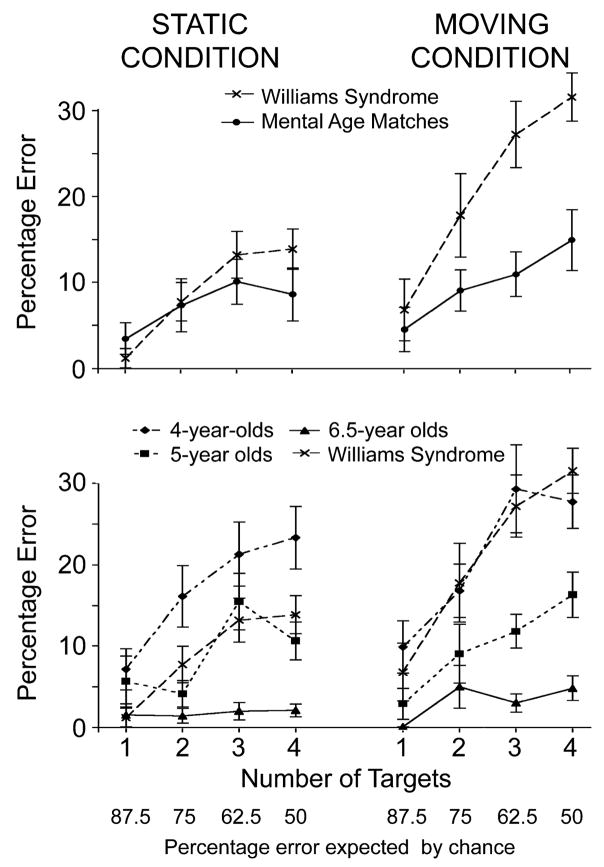
The WS group performed more poorly than the MA-matched control group in the moving condition, but not the static condition, and this impairment was most evident with three and four targets (Fig. 2, top panel).1 A 2 (group) × 2 (condition) × 4 (target number) repeated measures analysis of variance on percentage error revealed main effects of group, F(1, 28) =5.08, p = .03; condition, F(1, 28) = 20.41, p = .001; and target number, F(3, 84) = 29.76, p = .001. These effects were modulated by two-way interactions between group and condition, F(1, 28) = 8.65, p = .006; group and target number, F(3, 84) = 5.38, p = .002; and condition and target number, F(3, 84) = 2.87, p = .05. The three-way interaction was not significant, F(3, 84) = 0.77, p = .5. Planned comparisons showed that the WS group performed reliably worse than the control group on three- and four-target trials in the moving condition—one target: t(28) = −0.52, p = .61, Cohen’s d = 0.19; two targets: t(28) = −1.61, p =.12, Cohen’s d =0.59; three targets: t(28) =−3.51, p = .002, d = 1.28; four targets: t(28) = −3.69, p = .001, d = 1.35. The groups did not perform significantly differently for any number of targets in the static condition; although the graph in the top panel of Figure 2 hints that the WS group may have performed worse on three- or four-target trials, this difference was not reliable—three targets: t(28) = −0.83, p = .41, d = 0.30; four targets: t(28) = −1.36, p = .19; d = 0.50. Our non-parametric analyses (discussed next) also showed no significant differences in the static condition.
Fig. 2.
Percentage of errors as a function of number of targets in the moving condition (right) and static condition (left). The average percentage of errors expected at chance performance, which decreases as target number increases, is shown along the bottom. The top graph presents the data from the Williams syndrome (WS) group and the mental-age-matched control group in Experiment 1. The bottom graph presents the data from normally developing 4, 5, and 6.5-year-olds in Experiment 2, along with the results for the WS group in Experiment 1.
Participants performed above chance for all numbers of targets, but this does not mean that they necessarily tracked all targets. For example, participants might obtain the same score in the four-target condition by sometimes tracking all four targets or by always tracking just two or three of the four targets. Therefore, we tallied the number of participants who accurately located all targets on at least half of the six trials for each target number. In the static condition, the number of participants reaching this criterion did not differ reliably between the groups (all ps > .7).2 In the moving condition, all participants reached this criterion for one-target trials, but more MA-matched control subjects than WS subjects reached criterion for two-, three-, and four-target trials—two targets: 15 of 15 MA subjects versus 12 of 15 WS subjects, χ2(29, N = 30) = 3.33, p = .07; three targets: 14 of 15 MA subjects versus 8 of 15 WS subjects, χ2(29, N =30) = 6.14, p = .01; four target: 8 of 15 MA subjects versus 4 of 15 WS subjects, χ2(29, N = 30) = 2.22, p = .14.
We also analyzed the errors participants made, specifically, the distance of chosen items from actual targets, to examine whether the errors in the two groups were similar. Errors in this task could be produced by guessing, which would lead to random choice among objects. Alternatively, errors could occur if indexes “slip off’’ targets onto nearby distractors during tracking; this would lead to errors associated with distractors that were close to targets at some point during their movement. According to this latter hypothesis, distractors that are incorrectly chosen as targets (false alarms, FAs) should be associated with closer approaches to a missed target during the animation than distractors that are correct rejections (CRs). We therefore measured the closest approach between each missed target and each distractor, determining the average distance separately for FAs and CRs for each subject. We removed from this analysis subjects who had fewer than 3 FAs, which left 15 WS subjects and 11 control subjects.
For both groups, FAs had a closer approach, on average, to a missed target than did CRs (see Fig. 3a). This result is consistent with a role for index slippage in errors on this task. This difference between FAs and CRs was larger for the MA-matched control group than the WS group, which suggests a more prominent role for other sources of error—perhaps guessing—among WS participants. An analysis of variance conducted on these data revealed a significant effect of response type (CR vs. FA), F(1, 24) =36.65, p <.001, and an interaction of group and response type, F(1, 24) = 4.33, p < .05.
Fig. 3.
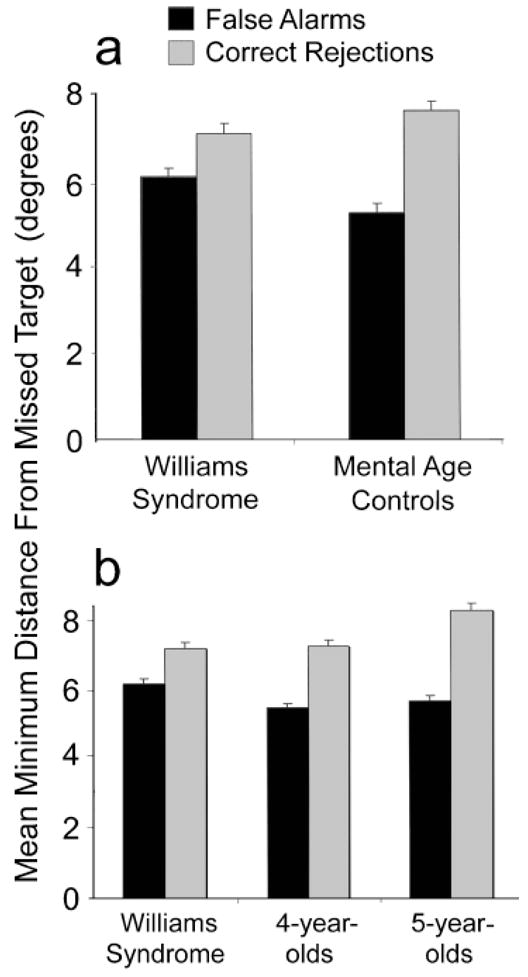
Average closest approach between the target and false alarms (nontargets that were incorrectly chosen) and between the target and correct rejections (nontargets that were correctly not chosen) in the moving condition. A smaller distance from the target for false alarms than for correct rejections suggests a bias toward making errors on distractors that pass close to the target. The data for the Williams syndrome (WS) group in Experiment 1 are presented along with the data for mental-age-matched control subjects in Experiment 1 (a) and the data for typically developing 4- and 5-year-olds in Experiment 2 (b).
A similar analysis of the static condition (14 subjects in the WS group and 7 in the control group) also showed a main effect of response type, F(1, 19) =4.62, p < .05. For both groups, FAs were closer to targets, on average, than CRs were. There was no main effect of group, F(1, 19) = 2.94, p = .10, nor a group-by-response-type interaction, F < 1.
Only in the moving condition was the performance of people with WS impaired relative to the performance of MA-matched control subjects. This result suggests that WS involves deficits in multiple object tracking, but not in retention of static location. Is this pattern caused by simple developmental immaturity? To find out, we examined the normal developmental profile of performance on both tasks in children who ranged in age from 4 to 7 years. This range included children younger than the MA-matched control subjects.
EXPERIMENT 2
Method
Participants were 6 typically developing boys and 6 typically developing girls in each of three age groups: 4-year-olds (M =4 years 6 months, range = 3 years 11 months through 4 years 10 months), 5-year-olds (M = 5 years 4 months, range = 5 years 0 months through 5 years 10 months), and 6.5-year-olds (M = 7 years 0 months, range = 6 years 6 months to 7 years 3 months). The 4-year-olds were the youngest children able to reliably sit through the task. The design, stimuli, and procedure were the same as in Experiment 1.
Results
The bottom panel in Figure 2 shows the percentage of errors as a function of target number across the three normal groups, as well as the WS group from Experiment 1. A repeated measures analysis of variance on the normal children’s data showed main effects of all three factors: age, F(2, 33) = 22.93, p = .001; condition, F(1, 33) =4.91, p =.03; and target number, F(3, 99) = 23.43, p = .001.3 Performance improved with age and was better in the static than the moving condition. Tukey’s post hoc analyses showed reliably different performance between all pairs of age groups (4 vs. 5, p = .001; 4 vs. 6.5, p < .001; 5 vs. 6.5, p = .02). There was also an Age × Target Number interaction, F(6, 99) = 5.15, p = .001, with 4- and 5-year-olds, but not 6.5-year-olds, performing more poorly with larger numbers of targets: F(3, 33) = 13.58, p = .001, for 4-year-olds; F(3, 33) =9.99, p =.001, for 5-year-olds; F(3, 33) =1.6, p =.2, for 6.5-year-olds. This pattern held for both the static and the moving conditions. Unlike the WS group, normal children showed no special difficulty in the moving condition as the number of targets increased.
Additional analyses examined the number of children able to correctly identify all targets on at least half of the trials with each target number. Eleven of the twelve 6.5-year-olds and the majority of 5-year-olds4 met this criterion for all target numbers in both the static and the moving conditions. However, 4-year-olds differed from older children. In the static condition, fewer 4 year-olds than 5 year-olds reached criterion on four-target trials (3 of 12 vs. 11 of 12), χ2(23, N = 24) = 10.97, p = .001. In the moving condition, fewer 4-year-olds than 5-year-olds reached criterion on both three-target trials (5 of 12 vs. 12 of 12 ), χ2(23, N = 24) = 9.88, p = .002, and four-target trials (3 of 12 vs. 8 of 12), χ2(23, N = 24) = 4.20, p = .04. Overall, these results suggest that the capacity to track multiple objects—and to remember four static locations—develops rapidly during the 4th year of life.
WS Versus Normal Children
The bottom graph in Figure 2 suggests that the WS group performed like 5-year-olds in the static condition, but like 4-year-olds in the moving condition. We confirmed these impressions using independent-sample t tests on percentage of error. In the static condition, the WS group performed better than the 4-year-olds for all target numbers, though for two and three targets, the result was marginally significant—one target: t(25) =2.30, p = .05, d =0.86; two targets: t(25) =2.01, p =.06, d =0.76; three targets: t(25) = 1.75, p = .09, d = 0.67; four targets: t(25) = 2.19, p =.04, d =0.83. In contrast, the WS group performed no differently from the 5 year-olds for any target numbers (ps > .2). In the moving condition, the WS group performed no differently from the 4 year-olds for any target numbers (ps > .4), but performed worse than the 5-year-olds on the three- and four-target trials—three targets: t(25) = −3.28, p = .003, d = 1.32; four targets: t(25) = −3.82, p = .001, d = 1.49.
Error Analysis
Figure 3b shows distances from the closest missed targets for FAs and CRs (4-year-olds, n = 12; 5-year-olds, n = 10; WS group, n =15).5 In the moving condition, there was a main effect of response type, F(1, 34) = 54.9, p < .001, with FAs closer to the missed targets than CRs were. When making errors, all groups tended to choose an object that passed close to a missed target. The Response Type × Group interaction was not reliable, F(2, 34) = 2.41, p = .11. In the static condition (4-year-olds, n = 11; 5-year-olds, n = 9; WS group, n = 14), there were no significant effects or interactions, though for all groups, FAs tended to be closer to the target than CRs were, F(1, 31) =2.52, p = .12.
DISCUSSION AND CONCLUSIONS
In Experiment 1, we found that people with WS were no different from MA-matched control subjects at remembering static locations, but were impaired at tracking three or four moving targets. Experiment 2 showed that people with WS performed like 5-year-olds in the static condition, but like 4-year-olds in the moving condition. The profile for people with WS was unique in that the moving condition caused disproportionate impairment. The relatively intact performance of people with WS in the static condition suggests that their impairment in the moving condition was not due to a general lack of attention or to other factors that the two tasks had in common. Nor was it due to deficient motion perception in WS: Participants were very accurate at tracking one object. In addition, their perception of biological motion and motion coherence is spared (Jordan et al., 2002; J.E. Reiss et al., in press). Because both conditions required remembering the locations of up to four objects, the deficit in WS does not seem to result from a general limit in visuospatial memory. Instead, it seems to reflect a limit on the mechanism underlying object tracking.
The profile of normally developing children revealed comparable limits in the number of items that they could track (moving condition) and remember (static condition). The non-parametric analyses suggest that most of the 5–to 6.5-year-olds could track up to four moving objects, whereas most of the 4-year-olds reliably tracked only two objects. The skill of even the youngest children in multiple object tracking is consistent with proposals that the same core spatial mechanism underlies performance in MOT tasks and in the paradigms examining object tracking in infancy (Leslie et al., 1998; vanMarle & Scholl, 2003). However, the improvement between 4 and 7 years of age also suggests there is development in the number of items that can be tracked simultaneously. The developmental change in both conditions could have been due to improved vigilance or increasing use of adultlike strategies (e.g., Yantis, 1992).
Further analyses examined two possible explanations of errors, both reflecting possible characteristics of indexes (i.e., stickiness and capacity limitations). First, more errors might reflect less sticky indexes—indexes that are more likely to slip off targets. Second, more errors could indicate fewer indexes. If indexes are slippery, distractors mistakenly chosen as targets should have passed close to the targets during the animation. In contrast, if errors are due to a limited number of indexes, errors should reflect random choice among the available objects. All groups tended to choose distractors that had passed close to targets, which suggests that some errors reflected a tendency for indexes to slip off targets onto nearby distractors (see also Pylyshyn, 2004). This source of error seems to have affected all the groups in the experiments reported here and may be continuous throughout development. However, the distance effects for FAs and CRs were weaker for WS participants than MA-matched control subjects in Experiment 1. This suggests that people with WS are more likely than normal observers to produce errors that are random guesses. The pattern of the distance effects, along with the poor performance of people with WS on three- and four-target trials in the moving condition, leads us to speculate that they have fewer indexes than typically developing children matched on MA.
This interpretation is consistent with ongoing studies in our lab suggesting that people with WS have a smaller subitizing range than MA-matched control subjects (O’Hearn, Landau, & Hoffman, 2005). Like the MOT task, subitizing tasks do not depend on storing multiple static locations, but rather, might depend on the indexing mechanism (Trick & Pylyshyn, 1993). In most subitizing tasks, the objects’ spatial locations change over trials, ruling out the possibility that number is estimated on the basis of stored locations. Interestingly, the subitizing range might also be smaller than expected for normal individuals in people with two other genetic disorders that have a profile of visuospatial impairment: Turner syndrome (Bruandet, Molko, Cohen, & Dehaene, 2004) and 22q11.2 deletion, a deficit that has a cognitive and neuroanatomical profile similar to that of WS (Bearden, Wang, & Simon, 2002; Simon, Bearden, Mc-Ginn, & Zackai, 2005). The indexing deficit found in WS might also be evident in other genetic disorders characterized by visuospatial impairment.
The MOT task was originally designed to distinguish two alternative means of relocating an object. One way is to use its spatial location (e.g., spatial coordinates). Another method requires applying an index and using that to follow the object through space. Our evidence suggests that these two spatial abilities are indeed distinct. People with WS are particularly impaired at multiple object tracking, but not at remembering the static locations of objects. Nonetheless, people with WS did track several objects in Experiment 1, which suggests that indexing exists in these individuals but is limited. Interestingly, our WS participants performed at the level of 4-year-olds in the MOT task—the same level of performance they achieve in the block construction task (Hoffman et al., 2003). This developmental parallel is consistent with theories postulating that both object tracking and block construction rely on visual indexing (Pylyshyn, 2000). Both tasks rely on parietal and possibly frontal areas, as would be expected if the tasks utilized similar brain functions. People with WS are thought to have parietal lobe damage, which could result in impaired performance on these tasks and others (Atkinson et al., 1997; Meyer-Lindenberg et al., 2004; A.L. Reiss et al., 2000). If people with WS are impaired in the number of items they can index, or “mark,’’ this basic spatial deficit could cascade over development (Karmiloff-Smith, 1998; Hoffman et al., 2003), leading to the complex, unexpected patterns of visuospatial deficits found in WS.
Acknowledgments
This work was supported by grants from the National Institute of Child Health and Human Development (F32 HD42346, to K.O.), National Science Foundation (BCS 0117744 and 9808585, to B.L. and J.E.H.), and March of Dimes (12-01-0087, to B.L.). We thank Gitana Chunyo, Elizabeth Crowe, Eric Hsiao, Leslie Huang, and Jason Reiss for their assistance. We gratefully acknowledge our participants and the Williams Syndrome Association. Preliminary results were presented at the 44th Annual Meeting of the Psychonomic Society, Vancouver, British Columbia, Canada, November 2003.
Footnotes
Our WS group included a wide range of ages and abilities. A repeated measures analysis of variance revealed no effects of age (under 18 vs. 18 and over) and no interaction of age with condition or number or targets. A separate analysis revealed no effects of performance on the Matrices subtest of the KBIT (scores at or above 20 vs. under 20), nor interactions of Matrices score with the other factors (condition or number of targets).
All participants reached this criterion with one and two targets, 14 from each group did so with three targets, and 10 WS participants and 11 control participants reached the criterion with four targets.
In a few comparisons, the variance was not equal across groups. Therefore, we also did analyses using statistics appropriate for unequal variances. These always revealed the same pattern of results as reported here.
In the static condition, twelve 5-year-olds reached this criterion with one and two targets, and eleven 5-year-olds reached it with three and four targets. In the moving condition, twelve 5-year-olds reached the criterion with one and three targets, 10 with two targets, and 8 with four targets.
There were not enough 6.5-year-olds reaching the criterion of at least 3 FAs to include this age group in the analysis.
References
- Ansari D, Donlan C, Thomas M, Ewing SA, Peen T, Karmiloff-Smith A. What makes counting count? Verbal and visuospatial contributions to typical and atypical number development. Journal of Experimental Child Psychology. 2003;85:50–62. doi: 10.1016/s0022-0965(03)00026-2. [DOI] [PubMed] [Google Scholar]
- Atkinson J, King J, Braddick O, Nokes L, Anker S, Braddick F. A specific deficit of dorsal stream function in Williams syndrome. NeuroReport. 1997;8:1919–1922. doi: 10.1097/00001756-199705260-00025. [DOI] [PubMed] [Google Scholar]
- Ballard D, Hayhoe M, Pook P, Rao R. Deictic codes for the embodiment of cognition. Behavioral and Brain Sciences. 1997;20:723–767. doi: 10.1017/s0140525x97001611. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Wang PP, Simon TJ. Williams Syndrome cognitive profile also characterizes Velocardiofacial/DiGeorge Syndrome. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2002;114:689–692. doi: 10.1002/ajmg.10539. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Lichtenberger L, Jones W, Lai Z, St George M. The neurocognitive profile of Williams syndrome: A complex pattern of strengths and weaknesses. Journal of Cognitive Neuroscience. 2000;12(Suppl 1):7–29. doi: 10.1162/089892900561959. [DOI] [PubMed] [Google Scholar]
- Bruandet M, Molko N, Cohen L, Dehaene S. A cognitive characterization of dyscalculia in Turner syndrome. Neuropsychologia. 2004;42:288–298. doi: 10.1016/j.neuropsychologia.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Carey S, Xu F. Infants’ knowledge of objects: Beyond object files and object tracking. Cognition. 2001;80:179–213. doi: 10.1016/s0010-0277(00)00154-2. [DOI] [PubMed] [Google Scholar]
- Culham J, Brandt S, Cavanagh P, Kanwisher N, Dale A, Tootell R. Cortical fMRI activation produced by attentive tracking of moving targets. Journal of Neurophysiology. 1998;80:2657–2670. doi: 10.1152/jn.1998.80.5.2657. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential Abilities Scales. San Diego, CA: Harcourt Brace Jovanovich; 1990. [Google Scholar]
- Ewart AK, Morris CA, Atkinson D, Weishan J, Sternes K, Spallone P, Stock AD, Leppert M, Keating MT. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nature Genetics. 1993;5:11–16. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Landau B, Pagani B. Spatial breakdown in spatial construction: Evidence from eye fixations in children with Williams syndrome. Cognitive Psychology. 2003;46:260–301. doi: 10.1016/s0010-0285(02)00518-2. [DOI] [PubMed] [Google Scholar]
- Howlin P, Davies M, Udwin O. Cognitive functioning in adults with Williams syndrome. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1998;39:183–189. [PubMed] [Google Scholar]
- Jordan H, Reiss JE, Hoffman JE, Landau B. Intact perception of biological motion in the face of profound spatial deficits: Williams syndrome. Psychological Science. 2002;13:162–167. doi: 10.1111/1467-9280.00429. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Development itself is the key to understanding developmental disorders. Trends in Cognitive Sciences. 1998;2:389–398. doi: 10.1016/s1364-6613(98)01230-3. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Landau B, Hoffman J, Kurz N. Object recognition with severe spatial deficits in Williams Syndrome: Sparing and breakdown. Cognition. doi: 10.1016/j.cognition.2005.06.005. (in press) [DOI] [PubMed] [Google Scholar]
- Leslie A, Xu F, Tremoulet P, Scholl B. Indexing and the object concept: Developing ‘what’ and ‘where’ systems. Trends in Cognitive Sciences. 1998;2:10–18. doi: 10.1016/s1364-6613(97)01113-3. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Bertrand J, Morris CA, Klein-Tasman BP, Armstrong S. The Williams syndrome cognitive profile. Brain and Cognition. 2000;44:604–628. doi: 10.1006/brcg.2000.1232. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, Kippenhan JS, Olsen R, Morris CA, Berman KF. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43:623–631. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- O’Hearn K, Landau B, Hoffman JE. Subitizing in people with Williams syndrome and normally developing children; Poster presented at the biennial meeting of the Society for Research in Child Development; Atlanta GA. 2005. Apr, [Google Scholar]
- Paul B, Stiles J, Passarotti A, Bavar N, Bellugi U. Face and place processing in Williams syndrome: Evidence for a dorsal-ventral dissociation. NeuroReport. 2002;13:1115–1119. doi: 10.1097/00001756-200207020-00009. [DOI] [PubMed] [Google Scholar]
- Pylyshyn Z. Situating the world in vision. Trends in Cognitive Sciences. 2000;4:197–207. doi: 10.1016/s1364-6613(00)01477-7. [DOI] [PubMed] [Google Scholar]
- Pylyshyn Z. Some puzzling findings in multiple object tracking (MOT): I. Tracking without keeping track of object identities. Visual Cognition. 2004;11:801–822. [Google Scholar]
- Pylyshyn Z, Storm R. Tracking multiple independent objects: Evidence for a parallel tracking mechanism. Spatial Vision. 1988;3:179–197. doi: 10.1163/156856888x00122. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, Straus E, Lai Z, Jones W, Bellugi U. Neuroanatomy of Williams syndrome: A high-resolution MRI study. Journal of Cognitive Neuroscience. 2000;12(Suppl 1):65–73. doi: 10.1162/089892900561986. [DOI] [PubMed] [Google Scholar]
- Reiss JE, Hoffman JE, Landau B. Motion processing in Williams syndrome: Evidence against a general dorsal stream deficit. Vision Research (in press) [Google Scholar]
- Richardson DC, Kirkham NZ. Multi-modal events and moving locations: Eye movements of adults and 6-month-olds reveal dynamic spatial indexing. Journal of Experimental Psychology: General. 2004;133:46–62. doi: 10.1037/0096-3445.133.1.46. [DOI] [PubMed] [Google Scholar]
- Scholl B, Pylyshyn Z. Tracking multiple objects through occlusion: Clues to visual objecthood. Cognitive Psychology. 1999;38:259–290. doi: 10.1006/cogp.1998.0698. [DOI] [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, Mc-Ginn D, Zackai E. Visuospatial and numerical cognitive deficits in children with Chromosome 22q11.2 Deletion Syndrome. Cortex. 2005;41:131–141. doi: 10.1016/s0010-9452(08)70889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelke E. Principles of object perception. Cognitive Science. 1990;14:29–56. [Google Scholar]
- Tager-Flusberg H, Plesa-Skwerer D, Faja S, Joseph RM. People with Williams syndrome process faces holistically. Cognition. 2003;89:11–24. doi: 10.1016/s0010-0277(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Trick L, Pylyshyn Z. What enumeration studies can show us about visual attention: Evidence for limited capacity preattentive processing. Journal of Experimental Psychology: Human Perception and Performance. 1993;19:331–351. doi: 10.1037//0096-1523.19.2.331. [DOI] [PubMed] [Google Scholar]
- Ullman S. Visual routines. Cognition. 1984;18:97–159. doi: 10.1016/0010-0277(84)90023-4. [DOI] [PubMed] [Google Scholar]
- vanMarle K, Scholl B. Attentive tracking of objects versus substances. Psychological Science. 2003;14:498–504. doi: 10.1111/1467-9280.03451. [DOI] [PubMed] [Google Scholar]
- Wynn K. Addition and subtraction by human infants. Nature. 1992;358:749–750. doi: 10.1038/358749a0. [DOI] [PubMed] [Google Scholar]
- Yantis S. Multielement visual tracking: Attention and perceptual organization. Cognitive Psychology. 1992;24:295–340. doi: 10.1016/0010-0285(92)90010-y. [DOI] [PubMed] [Google Scholar]