Abstract
Rodent hibernators experience low core body temperature (as low as −2° C) and reduced metabolic rates during hibernation. Concordant with energetic constraints, protein synthesis is negligible during torpor. To maintain pools of key regulatory proteins, proteolysis must be depressed as well. Ubiquitin-dependent proteolysis consists of two major steps: 1) ubiquitylation or tagging of a protein substrate by ubiquitin and 2) the protein substrate’s subsequent degradation by the 26S proteasome. Earlier, we demonstrated that the low temperatures typical of torpor virtually arrest proteolytic processing. Here, we demonstrate that in vitro ubiquitylation still continues at greater than 30% of maximal rates at temperatures as low as 0° C. Continued ubiquitylation in the presence of severely depressed proteolysis may explain the previously observed 2–3 fold increase of ubiquitin conjugates during torpor. We determined if there is a qualitative change in the type of ubiquitylation e.g. monoubiquitylation vs polyubiquitylation that occurs during torpor. We found no bias for monoubiquitylation in any state of the torpor cycle. We further determined that substrate limitation of free ubiquitin is not limiting ubiquitylation during torpor. We conclude that while the cold temperatures of torpor may limit proteolysis in accordance with metabolic demands, continued ubiquitylation may result in increased ubiquitin conjugate concentrations that must be processed upon arousal.
Keywords: torpor, proteolysis, polyubiquitylation, monoubiquitylation, ground squirrels
Introduction
A variety of mammals use hibernation presumably as a means for energy conservation [reviewed in 6, 33]. Hibernation is not static. Hibernators oscillate between 1) torpor where body temperature (Tb) may approach that of ambient temperature to as low as −2° C [3, 11] and metabolic rate may be as low as 1% of the active rate [38] and 2) a short interbout arousal where animals restore body temperature and metabolic rates to euthermic values. A torpor bout may last from 1 to 3 weeks depending on time of year, species, and ambient temperature.
Concordant with a depressed metabolic rate, protein synthesis is depressed to as low as 0.13–0.5% of active levels [39, 34 and references therein]. Continued proteolysis in the absence of significant protein synthesis would lead to a net depletion of protein pools. Therefore, it follows that there should be a coordination of both protein synthetic and degradative processes in order to maintain homeostatic balance.
There are several proteolytic pathways in the mammalian cell. Ubiquitin-mediated proteolysis appears to be responsible for degrading virtually all regulatory proteins; estimates are as high as 80–90% of cytosolic proteins are degraded by ubiquitin-mediated proteolysis [7, 26]. Ubiquitin-mediated proteolysis consist of two major events: 1) ubiquitylation or the tagging of a protein substrate by ubiquitin and 2) the subsequent degradation of the ubiquitylated protein by the 26S proteasome [reviewed in 21, 22].
Typically, conjugation of a polyubiquitin chain with a protein targets that protein for degradation by the 26S proteasome [21, 22, 36]. An examination of ubiquitin conjugate concentrations during torpor revealed a 2–3 fold elevation over values of active squirrels depending on tissue type [31]. This finding would seemingly suggest increased proteolytic activity, as an increase in ubiquitin conjugates usually correlates with increased proteolysis [20, 32]. However, no study to our knowledge has demonstrated wide spread depletion of protein pools during torpor and we found that activity through the 26S proteasome would be reduced to negligible values at the cold temperatures typical of torpor [35]. Even moderate ubiquitylation in the presence of severely limited proteolysis would result in an accumulation of ubiquitin conjugates during torpor. Here, we developed a method to measure the active process of ubiquitylation in liver lysates.
Ubiquitin may be conjugated to a protein through either a monoubiquitylation event or a polyubiquitylation chain. Although polyubiquitylation usually serves as a strong signal for degradation, monoubiquitylation events may be involved in such activities as DNA and ribosomal preservation [37, 16, 17, 18]. One possibility is that the increased levels of ubiquitin conjugates that are found in torpid squirrels represent an increase in monoubiquitylated proteins rather than polyubiquitylated proteins. Here, we determined an index of monoubiquitylated proteins vs polyubiquitylated proteins as a function of torpor state.
The concentrations of ubiquitin conjugates increased 2–3 fold but not more in torpid squirrels [31]. A possible limiting factor to continued ubiquitylation might be substrate limitation e.g. free ubiquitin. We measured the concentrations of free ubiquitin present at different states of the torpor cycle.
Materials and Methods
Animals
Adult golden–mantled ground squirrels (Spermophilus lateralis) were captured during the summer from southern Nevada and California. Some animals were sacrificed immediately as a seasonal control (Summer Active). Temperature sensitive radiotelemeters were implanted as described previously in order to allow for precise determination of torpor status [19]. Animals were housed in an environmental chamber at 4° C and allowed to hibernate. Torpid body temperature (Tb) was ~5° C. In addition to summer active squirrels, winter squirrels representing torpid and interbout aroused animals were sampled. Torpid animals were sacrificed when Tb was approximately 5° C. Interbout aroused animals were sacrificed when euthermic between torpor bouts. Rats were used as an additional non-hibernator control where indicated. Squirrels were sacrificed by CO2 asphyxiation except for torpid animals. Torpid animals were killed by decapitation because of their low respiratory rates. Livers were removed and snap frozen in liquid nitrogen and stored at −80° C until use.
Ubiquitylation rates
An assay was developed to measure the rates of ubiquitylation as a function of temperature. Frozen liver was pulverized in liquid N2, and homogenized in 3 volumes of 100 mM HEPES, pH 7.8, 5 mM MgCl2, and 10 mM KCl. Cellular debris was removed by centrifuging the lysates at 20,000 × g for 30 min at 4° C. Lysates were ATP depleted by supplementing them with 20 mM 2-deoxyglucose and incubating at 25° C for 30 min. In order to remove free ATP and reduce levels of endogenous ubiquitin, lysates were passed across two sequential Sephadex G-50 desalting columns [15]. These columns remove smaller molecular weight molecules below 10,000 daltons (ubiquitin is 8,360 daltons). The Sephadex G-50 used in the spin columns was pre-equilibrated to 100 mM Hepes, pH 7.8, 5 mM MgCl2, and 10 mM KCl. The resulting lysates were frozen at −80° C until use. Lysate protein concentrations were determined by a modified Lowry protein assay.
The ubiquitylation reaction volumes totaled 50 μl and contained 75 μg of liver protein; an ATP regenerating system consisting of 45 mM creatine phosphate, 5 mM ATP, 0.1 mM inorganic pyrophosphate, and 8 ng creatine phosphokinase; 0.5 mM dithiothreitol (DTT) and 50 μM MG115 (specific 26S proteasome inhibitor; Biomol International). The reaction was initiated with 5 μg biotinylated ubiquitin (Biomol International) and allowed to proceed for 1 h at the indicated temperatures (0, 5, 10, 15, 20, 25, 30, and 37° C). Reactions were stopped by adding 100 μl of 50 mM Tris-HCl, pH 8.3, 20% glycerol, 2% SDS, and 0.4 M β-mercaptoethanol. Ubiquitylation was determined by following incorporation of the biotinylated ubiquitin with western blotting. SDS-PAGE was performed using a 12% polyacrylamide gel. After transfer to PVDF membrane, blots were incubated in the presence of 10 μg of streptavidin conjugated to horseradish peroxidase and visualized using ECL+ on a Molecular Dynamics Typhoon multivariable imager (GE Life Sciences). Ubiquitylation as a function of temperature was determined for three animals from each state (Summer Active, Torpid, and Interbout Aroused) and for rat as a non-hibernator control. Preliminary western blotting using lysates that were not supplemented with biotinylated ubiquitin revealed interference by endogenous biotinylated proteins as indicated by bands whose intensity did not vary between lysates supplemented with biotinylated ubiquitin and control lysates. As a result, we restricted analyses to portions of the western blot where endogenous biotin was not present e.g. we analyzed ubiquitylated proteins between 20 and 75 kDa molecular weight.
Mono vs polyubiquitylation
An index of mono vs polyubiquitylation as a function of torpor state was determined using a competitive antibody technique. Frozen livers were pulverized in liquid N2 and homogenized in 3 volumes of 50 mM Tris-HCl, pH 8.3, 20% glycerol, 2% SDS, and 0.4 M β-mercaptoethanol. Lysates were cleared of cellular debris by centrifugation at 20,000 × g for 30 min at 4° C. Protein concentrations of the supernatant were determined by a modified Lowry protein assay. Dot blots were performed using 60 or 90 μg of protein from livers of Summer Active, Torpid, and Interbout Aroused squirrels (n = 3 animals from each state). These concentrations of protein are below the manufacturer’s indicated binding capacity of the membrane (Sequi-Blot PVDF; Bio-Rad; binding capacity is 170–200 μg/cm2). The dot blot membranes were blocked overnight with 3% nonfat dried milk in a solution of 25 mM Tris-HCl, pH 7.5 and 145 mM NaCl (TBS) with the addition of 0.1% Tween 20 (TTBS). The membranes were washed in TBS, washed twice in TTBS and washed once more in TBS. The dot blots were incubated overnight with monoclonal antibodies to conjugated ubiquitin (Biomol international; CosmoBio). Antibo dy clone FK1 belongs to the IgM subclass of immunoglobulins and recogn izes only polyubiquitylated proteins [5, 12–14]. Clone FK2 belongs to the IgG subclass of immunoglobulins and recognizes both mono and polyubiquitylated proteins [5, 13, 14, 27–29]. Simultaneous exposure to both of these antibody clones should reveal relative contributions of mono and polyubiquitylated proteins to the overall ubiquitin conjugate pool. Analogous assays utilizing competitive antibody approaches with both IgG and IgM antibodies were performed previously [e.g. 25]. Both FK1 and FK2 were added simultaneously in a concentration of 1:1000 in a solution of 1% nonfat dried milk in TTBS. Blots were washed in TBS, twice in TTBS, and once more in TBS before incubating the membranes with secondary antibodies. The secondary antibodies were: a) goat anti-mouse IgM conjugated with AlexaFluor 594 (red) and b) goat anti-mouse IgG conjugated with AlexaFluor 488 (green; Invitrogen). Both secondary antibodies were added at a concentration of 1:10,000 in a solution of 1% bovine serum albumin (BSA) in TTBS. Following visualization on a Molecular Dynamics Typhoon multivariable imager, an index of the relative contributions of monoubiquitylation and polyubiquitylation was determined. The index value was determined by dividing the green signal (FK2) by the red signal (FK1). A higher value is indicative of relatively increased monoubiquitylation.
Concentrations of free ubiquitin
Free ubiquitin was measured by western blotting. Frozen liver was pulverized in liquid N2 and homogenized in 3 volumes of 50 mM Tris-HCl, pH 8.3, 20% glycerol, 2% SDS, and 0.4 M β-mercaptoethanol. Lysates were cleared of cellular debris by centrifugation at 20,000 × g for 30 min at 4° C. Protein concentrations of the supernatant were determined by a modified Lowry protein assay. SDS-PAGE was performed using a 20% resolving gel. Lanes were loaded with 70 μg of total protein from Summer Active, Torpid, and Interbout Aroused animals (n = 3 from each state) or varying concentrations of free ubiquitin (0–150 ng; Sigma Chemical). Following transfer to PVDF membrane, the membranes were blocked overnight with 3% nonfat dried milk in a solution of TTBS. The membranes were washed once in TBS, twice in TTBS, and once more in TBS and then incubated with polyclonal antibody that recognizes free ubiquitin (a gift from Dr. Art Haas). The antibody was diluted 1:1000 in a solution of 3% nonfat dried milk in TTBS. Secondary antibody incubation was performed at a 1:3000 dilution in 1% BSA in TTBS for 1 h using a goat anti-rabbit IgG antibody conjugated to horseradish peroxidase (Sigma Chemical Co). Blots were washed as above. ECL+ was used to visualize the samples on a Molecular Dynamics Typhoon multivariable imager. A standard curve was constructed using the varying concentrations of free ubiquitin. The correlation coefficient (r2) of the standard curve was 0.99. The standard curve was used to calculate the concentrations of free ubiquitin in different states of the torpor cycle.
Results and Discussion
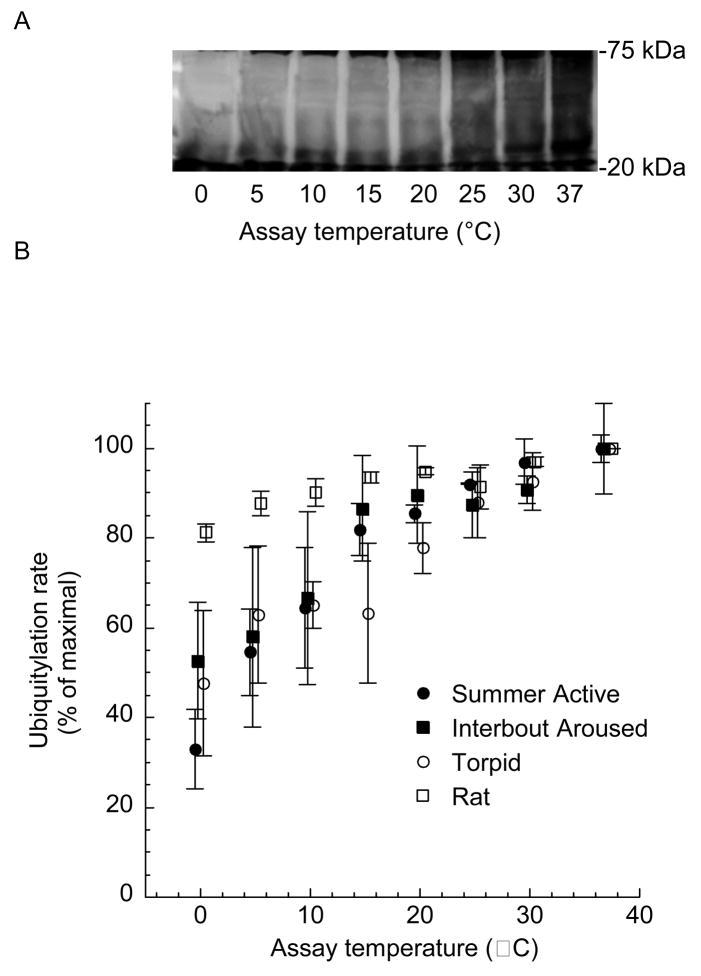
Ubiquitylation continues at a moderate rate even at the lowest temperatures typical of torpor; ubiquitylation at 0° C was greater than 30% of maximal rates (Fig. 1). Previously, we demonstrated that cold temperatures reduce 26S proteasome activity to negligible values [35]. In the presence of negligible 26S proteasome activity, continued ubiquitylation would lead to an accumulation of ubiquitylated proteins. Concordant with that expectation, ubiquitin conjugates increase 2–3 fold during torpor [31].
Fig. 1.
Ubiquitylation in hepatic lysates as a function of temperature. Hepatic lysates from Summer Active, Torpid and Interbout Aroused squirrels and rats were supplemented with biotinylated ubiquitin and incubated for 30 min at indicated assay temperatures. A) Western blot analyses were performed. An area of the blot that did not contain endogenous biotin was used for analyses (20–75 kDa). B) Western blots were quantified. Data represent means ± SE, n = 3 animals for each state. Data were shifted along the axis of abscissa to allow for greater clarity.
An accumulation of 2–3 fold higher concentrations of ubiquitin conjugates may have significant physiological consequences for the hibernating animal. Upon arousal, ubiquitin conjugate concentrations are restored to values typical of active animals [31]. A significant question is how this restoration is accomplished. Proteins that are conjugated to ubiquitin may either enter into the degradation process with the proteasome or they may be deubiquitylated in an editing type fashion [1, 8]. Does the reduction in ubiquitin conjugate concentrations upon arousal represent proteolytic processing or deubiquitylation of proteins?
If the elevated ubiquitin conjugates found during torpor are degraded upon arousal when permissive body temperatures are available, then there may be a significant energetic outlay. Increased proteolysis would necessitate increased de novo protein synthesis to maintain protein pools. Indeed, protein synthesis is 30% higher during the interbout arousal over summer values [39]. These data suggest compensatory protein synthesis when euthermic body temperatures are restored. An interesting observation is that ubiquitylation in ground squirrels appears to be more temperature sensitive than in rats (Fig. 1). Although speculative at best, and based on only two species comparisons, it is possible that the increased thermal sensitivity represents an evolutionary transition toward greater control of ubiquitylation in hibernators. However, additional species comparisons would need to be done before any significant conclusion could be made.
The increase in ubiquitin conjugates may not necessarily represent proteins targeted for degradation. One possible solution to reduce the increased ubiquitin conjugate levels might be the activity of deubiquitylating enzymes (DUBs) [for review, see 1, 8]. These enzymes catalyze the cleavage of the bond between ubiquitin and its target proteins. At least 140 DUBs belonging to several subfamilies have been described based on either genetic similarity (such as ubiquitin-specific processing protein groups, UBP), and/or the mechanism of action (such as ubiquitin carboxy-terminal hydrolases, UCH) [1, 8]. It is tempting to speculate that there may be a role for DUBs during torpor or arousal in recycling proteins that were conjugated to ubiquitin so as to avoid degradation. Our initial efforts to measure DUB activity in crude lysates of hibernators failed (data not shown). Additional efforts to use purified DUBs are underway.
Ubiquitylation during torpor may be qualitatively distinct from the active states i.e. there may be more monoubiquitylation than polyubiquitylation during torpor. Polyubiquitylated proteins are typically destined for degradation [21, 22, 36]. However, monoubiquitylated proteins are generally considered to be poor substrates for the 26S proteasome [23]. Instead, monoubiquitylation is usually associated with stable proteins during stressful periods such as oxidative stress and ischemia [4, 16, 18]. We hypothesized that perhaps, proteins are preferentially monoubiquitylated during the stressful period of torpor. This monoubiquitylation may help maintain protein integrity. However, the data presented here reveal no bias for monoubiquitylation over polyubiquitylation in torpid animals (Table 1).
Table 1.
Index of monoubiquitylation for different states of the torpor cycle
| Summer Active | Interbout Aroused | Torpid | |
|---|---|---|---|
| Trial 1 (60 μg protein) | 1.2794 ± 0.049* | 1.1606 ± 0.029 | 1.2545 ± 0.015 |
| Trial 2 (90 μg protein) | 1.3533 ± 0.062 | 1.1900 ± 0.089 | 1.3088 ± 0.016 |
A higher value represents increased monoubiquitylation. Values represent means ± SE, n = 3 animals. ANOVA revealed no significant difference p ≥ 0.05.
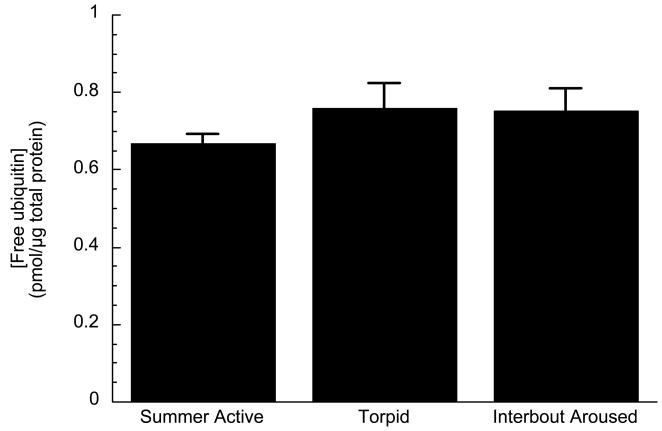
Concentrations of ubiquitin conjugated proteins increase only 2–3 fold but not more during torpor. The total ubiquitin pool in the cell is represented by the sum of both free ubiquitin and ubiquitin conjugate concentrations. If ubiquitylation continued in the cold, one eventuality would be the depletion of free ubiquitin pools. Free ubiquitin concentrations were determined at different states of the torpor cycle. We found no depletion of the free ubiquitin pools (Fig. 2). Rather, free ubiquitin concentrations in the liver of squirrels represent 93–95% of the total ubiquitin pool (Table 2). Thus, a 2–3 fold increase in ubiquitylated proteins represents only a small fraction of the available free ubiquitin. Depletion of free ubiquitin does not seem to be a factor in limiting the increase of ubiquitin conjugate concentrations during torpor. However, the limited accumulation of ubiquitin-conjugated proteins implies that some other factor(s) besides temperature and substrate limitation of ubiquitin must be involved in the regulation of ubiquitylation during torpor.
Fig. 2.
Concentration of free ubiquitin in liver of squirrels from different states of the torpor cycle: Summer Active, Torpid, and Interbout Aroused. Values represent means ± SE, n = 3 animals for each state. ANOVA revealed no significant difference p > 0.05.
Table 2.
Concentrations of ubiquitin conjugates and free ubiquitin from different states of the torpor cycle in livers of ground squirrels
| [Ubiquitin conjugates] pmol/μg of protein * | [Free ubiquitin] pmol/μg of protein | [Ubiquitin conjugates] % of total ubiquitin pool | |
|---|---|---|---|
| Summer Active | 0.0347 ± 0.004 | 0.6676 ± 0.026 | 4.9% |
| Torpid | 0.0606 ± 0.003 | 0.7597 ± 0.066 | 7.4% |
| Interbout Aroused | 0.0430 ± 0.004 | 0.7538 ± 0.058 | 5.4% |
Values represent means ± SE, n = 3 animals for each state.
Data taken from van Breukelen and Carey 2002.
A depleted ATP pool may limit ubiquitylation. During hibernation, there may be a 2-fold reduction of ATP [9, 10]. The hydrolysis of ATP yields a myriad of adenylate metabolites including adenosine, ADP, AMP, hypoxanthine, inosine, and inosine monophosphate [24, 30]. In the embryos of the brine shrimp, Artemia franciscana, active ATP hydrolysis led to the accumulation of an unidentified metabolite(s) that restricted both ubiquitylation and protein degradation [32]. Despite a screening of likely candidates, the precise metabolite was not identified [32]. It should be noted that this inhibition was found when concentrations of ATP were sufficient to support full proteolytic activity in the absence of active ATP hydrolysis. In the hibernator, it is possible that limited ATP hydrolysis could result in the accumulation of this metabolite(s) as well. This hypothesis must be considered in light of our in vitro ubiquitylation data (Fig. 1). In these experiments, ubiquitylation did not cease even at the lowest temperatures typical of torpor. We may have avoided accumulation of this hypothetical metabolite. The same ATP regenerating system was used as in the Artemia experiments and inhibition was only observable when ATP hydrolysis overwhelmed the system.
A successful and prolonged metabolic depression like hibernation requires coordination between anabolic and catabolic processes in order to maintain homeostasis. In the face of limited protein synthesis, hibernators must restrict proteolysis. Indeed, proteolytic processing of the 26S proteasome is virtually arrested at the cold temperatures typical of torpor [35]. However, ubiquitylation of proteins still continues at these temperatures at greater than 30% of maximal rate (Fig. 1). This mismatch between proteolysis and ubiquitylation may result in an accumulation of ubiquitin conjugates as is observed during hibernation [31]. However, we suspect that ubiquitylation does not occur unchecked during hibernation. The increase in ubiquitin conjugated proteins is limited to 2–3 fold despite very high concentrations of free ubiquitin (Fig. 2) i.e. substrate limitation of ubiquitin does not restrict ubiquitylation. Other factors might limit ubiquitylation during torpor such as ATP hydrolysis or, perhaps, the activity of deubiquitylating enzymes. Further experimentation is required to understand the roles of these processes in regulating protein degradation. Concordant with what is required for a successful and prolonged metabolic depression, protein synthesis and degradation are reduced during torpor. However, these processes are not precisely coordinated. Consequently, there is an accumulation of ubiquitylated proteins. Taken together, these data suggest that hibernators exploit cold temperatures, albeit not perfectly, to downregulate critical homeostatic processes. It is possible that the function of the interbout arousal could be to rectify the accumulative effects of physiological mismatches like the one described here.
Acknowledgments
We would like to thank Dr. Art Haas for supplying the antibody for free ubiquitin and technical advice. We also thank members of the laboratory for assistance with animal care.
This work was supported by grants from the National Science Foundation (IOB 0448396) and the National Institutes of Health (2 P20 RR016464).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta. 2004;1695:189–207. doi: 10.1016/j.bbamcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Anchordoguy TJ, Hand SC. Acute blockage of the ubiquitin-mediated proteolytic pathway during invertebrate quiescence. Am J Physiol. 1994;267:R895–900. doi: 10.1152/ajpregu.1994.267.4.R895. [DOI] [PubMed] [Google Scholar]
- 3.Barnes BM. Freeze avoidance in a mammal: body temperature below 0° C in an Arctic hibernator. Science. 1989;244:1593–1595. doi: 10.1126/science.2740905. [DOI] [PubMed] [Google Scholar]
- 4.Bergink S, Salomons FA, Hoogstraten D, Groothius TAM, de Waard H, Wu J, Citterio E, Houtsmüller AB, Neefjes J, Hoeijmakers JHJ, Vermeulen W, Dantuma NT. DNA damage triggers nucleotide excision repair-dependent monoubiquitylation of histone H2A. Genes Develop. 2006;20:1343–1352. doi: 10.1101/gad.373706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bishop N, Norman A, Woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J Cell Biol. 2002:91–101. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperatures. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- 7.Ciechanover A, Finley D, Varshavsky A. Ubiquitin dependence of selective protein degradation demonstrated in the mammalian cell cycle mutant ts85. Cell. 1984;37:57–66. doi: 10.1016/0092-8674(84)90300-3. [DOI] [PubMed] [Google Scholar]
- 8.D’Andrea AD, Pellman D. Deubiquitinating enzymes: a new class of biological regulators. Crit Rev Biochem Mol Biol. 1998;33:337–352. doi: 10.1080/10409239891204251. [DOI] [PubMed] [Google Scholar]
- 9.Doherty JC, Kronon MT, Rotermund AJ., Jr The effects of short term cold storage upon ATP and 2,3-BPG levels in the blood of euthermic and hibernating thirteen-lined ground squirrels Spermophilus tridecemlineatus. Comp Biochem Physiol. 1992;104:87–91. doi: 10.1016/0300-9629(93)90013-t. [DOI] [PubMed] [Google Scholar]
- 10.English TE, Storey KB. Enzymes of adenylate metabolism and their role in hibernation of the white-tailed prairie dog, Cynomys leucurus. Arch Biochem Biophys. 2000;376:91–100. doi: 10.1006/abbi.1999.1686. [DOI] [PubMed] [Google Scholar]
- 11.Frank CL. The influence of dietary fatty acids on hibernation by golden-mantled ground squirrels (Spermophilus lateralis) Physiol Zool. 1992;65:906–920. [Google Scholar]
- 12.Fujimuro M, Sawada H, Yokosawa H. Dynamics of ubiquitin conjugation during heat-shock response revealed by using a monoclonal antibody specific to multi-ubiquitin chains. Eur J Biochem. 1997;249:173–180. doi: 10.1111/j.1432-1033.1997.00427.x. [DOI] [PubMed] [Google Scholar]
- 13.Fujimuro M, Sawada H, Yokosawa H. Production and characterization of monoclonal antibodies specific to multiubiquitin chains of polyubiquitinated proteins. FEBS Letts. 1994;349:173–180. doi: 10.1016/0014-5793(94)00647-4. [DOI] [PubMed] [Google Scholar]
- 14.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nature Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 15.Helmerhost E, Stokes GB. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980;104:130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- 16.Hicke L. Protein regulation by monoubiquitin. Nat Rev Mol Cell Biol. 2001;2:195–201A. doi: 10.1038/35056583. [DOI] [PubMed] [Google Scholar]
- 17.Huang TT, D’Andrea AD. Regulation of DNA repair by ubiquitylation. Nat Rev Mol Cell Biol. 2006;7:323–34. doi: 10.1038/nrm1908. [DOI] [PubMed] [Google Scholar]
- 18.Lee DY, Teyssier C, Strahl BD, Stallcup MR. Role of protein methylation in regulation of transcription. Endoc Rev. 2005;26:147–170. doi: 10.1210/er.2004-0008. [DOI] [PubMed] [Google Scholar]
- 19.Martin SL, Maniero GD, Carey C, Hand SC. Reversible depression of oxygen consumption in isolated liver mitochondria during hibernation. Physiol Biochem Zool. 1999;72:255–264. doi: 10.1086/316667. [DOI] [PubMed] [Google Scholar]
- 20.Munro S, Pelham H. What turns on heat shock genes? Nature. 1985;317:477–478. doi: 10.1038/317477a0. [DOI] [PubMed] [Google Scholar]
- 21.Pickart CM. Back to the future with ubiquitin. Cell. 2004;116:181–190. doi: 10.1016/s0092-8674(03)01074-2. [DOI] [PubMed] [Google Scholar]
- 22.Pickart CM, Cohen RE. Proteasomes and their kin: proteasomes in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–187. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- 23.Pickart CM, Raasi S. Controlled synthesis of polyubiquitin chains. Methods Enzymol. 2005;399:21–36. doi: 10.1016/S0076-6879(05)99002-2. [DOI] [PubMed] [Google Scholar]
- 24.Pillwein K, Jayaram HN, Weber G. Effect of ischemia on nucleosides and bases in rat liver and hepatoma 3924A. Cancer Res. 1987;47:3092–3096. [PubMed] [Google Scholar]
- 25.Robinson WH, DiGennaro C, Hueber W, Haab BB, Kamachi M, Dean EJ, Fournel S, Fong D, Genovese MC, Neuman de Vegvar HE, Skriner K, Hirschberg DL, Morris RI, Muller S, Pruijn GJ, van Venrooij WJ, Smolen JS, Brown PO, Steinman L, Utz PJ. Autoantigen microarrays for multiple chracterization of antibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 26.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteosome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 27.Takada K, Hirakawa T, Yokosawa H, Okawa Y, Taguchi H, Ohkawa K. Isolation of ubiquitin-E2 (ubiquitin conjugating enzyme complexes from erythroleukaemia cells using immunoaffinity techniques. Biochem J. 2001;356:199–206. doi: 10.1042/0264-6021:3560199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takada K, Nasu H, Hibi N, Tsukada Y, Ohkawa K, Fujimuro M, Sawada H, Yokosawa H. Immunoassay for the quantification of intracellular multiubiquitin chains. Eur J Biochem. 1995;233:42–47. doi: 10.1111/j.1432-1033.1995.042_1.x. [DOI] [PubMed] [Google Scholar]
- 29.Takada K, Nasu H, Hibi N, Tsukada Y, Shibasaki T, Fujise K, Fujimuro M, Sawada H, Yokosawa H, Ohkawa K. Serum concentrations of free ubiquitin and multiubiquitin chains. Clin Chem. 1997;43:1188–1195. [PubMed] [Google Scholar]
- 30.Tongaonkar P, Beck K, Shinde UP, Madura K. Characterization of a temperature-sensitive mutant of a ubiquitin-conjugating enzyme and its use as a heat-inducible degradation signal. Anal Biochem. 1999;272:263–269. doi: 10.1006/abio.1999.4190. [DOI] [PubMed] [Google Scholar]
- 31.van Bilsen M, van der Vussse GJ, Coumans WA, de Groot MJM, Willemsen PHM, Reneman RS. Degradation of adenine nucleotides in ischemic and reperfused rat heart. Am J Physiol. 1989;257:H47–H54. doi: 10.1152/ajpheart.1989.257.1.H47. [DOI] [PubMed] [Google Scholar]
- 32.van Breukelen F, Carey H. Ubiquitin conjugate dynamics in the gut and liver of hibernating ground squirrels. J Comp Physiol B. 2002;172:269–273. doi: 10.1007/s00360-002-0252-5. [DOI] [PubMed] [Google Scholar]
- 33.van Breukelen F, Hand SC. Characterization of ATP-dependent Proteolysis in Embryos of the Brine Shrimp, Artemia franciscana. J Comp Physiol B. 2000;170:125–133. doi: 10.1007/s003600050267. [DOI] [PubMed] [Google Scholar]
- 34.van Breukelen F, Martin SL. Invited Review: Molecular adaptations in mammalian hibernators: unique adaptations or generalized responses? J Appl Physiol. 2002;92:2640–2647. doi: 10.1152/japplphysiol.01007.2001. [DOI] [PubMed] [Google Scholar]
- 35.van Breukelen F, Martin SL. Translational inititiation is uncoupled from elongation at 18 C during mammalian hibernation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1374–R1379. doi: 10.1152/ajpregu.2001.281.5.R1374. [DOI] [PubMed] [Google Scholar]
- 36.Velickovska V, Lloyd BP, Qureshi S, van Breukelen F. Proteolysis is depressed during torpor in hibernators at the level of the 20S core protease. J Comp Physiol B. 2005;175:329–335. doi: 10.1007/s00360-005-0489-x. [DOI] [PubMed] [Google Scholar]
- 37.Volk S, Wang M, Pickart CM. Chemical and genetic strategies for manipulating polyubiquitin chain structure. Methods Enzymol. 2005;399:3–20. doi: 10.1016/S0076-6879(05)99001-0. [DOI] [PubMed] [Google Scholar]
- 38.von Mickezc A. The nuclear ubiquitin-proteasome system. J Cell Sci. 2006;119:1977–1984. doi: 10.1242/jcs.03008. [DOI] [PubMed] [Google Scholar]
- 39.Wang LCH, Lee TF. Perspectives on metabolic suppression during mammalian hibernation and daily torpor. In: Heldmaier G, Klingenspor M, editors. Life in the Cold. Springer-Verlag; Berlin: 2000. pp. 152–158. [Google Scholar]
- 40.Zhegunov GF, Mikulinsky YE, Kudokotseva EV. Hyperactivation of protein synthesis in tissues of hibernating animals on arousal. Cryo Lett. 1988;9:236–245. [Google Scholar]