Abstract
The existence of anti-inflammatory circuits centered on melanocortin receptors (MCRs) has been supported by the inhibitory properties displayed by melanocortin peptides in models of inflammation and tissue injury. Here we addressed the pathophysiological effect that one MCR, MCR type 3 (MC3R), might have on vascular inflammation. After occlusion (35 min) and reopening of the superior mesenteric artery, MC3R-null mice displayed a higher degree of plasma extravasation (45 min postreperfusion) and cell adhesion and emigration (90 min postreperfusion). These cellular alterations were complemented by higher expression of mesenteric tissue CCL2 and CXCL1 (mRNA and protein) and myeloperoxydase, as compared with wild-type animals. MC1R and MC3R mRNA and protein were both expressed in the inflamed mesenteric tissue; however, no changes in vascular responses were observed in a mouse colony bearing an inactive MC1R. Pharmacological treatment of animals with a selective MC3R agonist ([d-Trp8]-γ-melanocyte-stimulating hormone; 10 μg i.v.) produced marked attenuation of cell adhesion, emigration, and chemokine generation; such effects were absent in MC3R-null mice. These new data reveal the existence of a tonic inhibitory signal provided by MC3R in the mesenteric microcirculation of the mouse, acting to down-regulate cell trafficking and local mediator generation.—Leoni, G., Patel, H. B., Sampaio, A. L. F., Gavins, F. N. E., Murray, J. F., Grieco, P., Getting, S. J., Perretti, M. Inflamed phenotype of the mesenteric microcirculation of melanocortin type 3 receptor-null mice after ischemia-reperfusion.
Keywords: intravital microscopy, cell trafficking, GPCR
The process of leukocyte trafficking from the microcirculation to the site of inflammation is a hallmark of the response that the host sets up on infection and/or ingestion of xenobiotic products (1). It is therefore imperative that this response occurs promptly; it is equally very important that the trafficking of white blood cells out of the blood circulation is tightly regulated in both a spatial and temporal manner. The latter aspect is becoming an accepted view in the scientific community in parallel to the appreciation that several endogenous mediators could become operative in the microcirculation to produce an inhibitory effect on the process of leukocyte trafficking (2).
Classes of proinflammatory mediators and chemoattractants act in concert with endogenous inhibitory mediators to assure the strict time dependence of the inflammatory reaction. Whereas the first group comprises the widely known cytokine interleukin (IL) -1, IL-8, and tumor necrosis factor (TNF) -α (to quote just a few) and adhesion molecules as well as proteolytic enzymes, the effectors of the latter group of mediators have been discovered in more recent years (3, 4). The portfolio of endogenous inhibitors of inflammation, which often possess proresolving properties, include short-lived lipids such as lipoxins, resolvins, and cyclopentanone prostaglandins; purine bases such as adenosine and peptides; and proteins, such as IL-10, galectin-1, and annexin-1 (5,6,7).
It is likely that activation of the pathways centered on these counter-regulatory circuits is more swift and crucial in the context of an ischemia and reperfusion (I/R) injury, often referred to as “inflammation from within” (8). Indeed, effectors of the endogenous anti-inflammatory arm are particularly active in controlling cell trafficking and the ensuing tissue damage when tested in experimental models of I/R: for instance, administration to mice of annexin-1 and its peptides abrogates cell adhesion in the mesenteric microcirculation after I/R, whereas their inhibition would reach the 40–50% value when tested against classical inflammatory stimuli such as the Toll-like receptor-2 (TLR2) agonist zymosan (9, 10).
ACTH and α-melanocyte-stimulating hormone (MSH) belong to the group of endogenous mediators of anti-inflammation, with several studies reporting, on the one hand, their expression in the context of tissue injury and inflammation and, on the other hand, their ability in suppressing cardinal signs of the inflammatory response of the host. For instance, natural and synthetic melanocortin peptides protect against myocardial infarct (11,12,13); they inhibit rejection after organ transplantation (14) and display inhibitory properties in a variety of models of acute and chronic inflammation (15, 16). In addition, there is also clinical evidence that these hormones are able to control cell activation and cell trafficking in inflammation (17, 18).
The potential benefits of the research on ACTH and α-MSH must come through a better appreciation of receptors responsible for their anti-inflammatory and tissue protective properties (15, 16). Five melanocortin receptors (MCRs) have been cloned and characterized with respect to ligand specificity (19); all five receptors are positively coupled to adenylate cyclase via a Gαs protein, so that their activation leads to rapid intracellular accumulation of cyclic adenosine monophosphate.
We and others (20,21,22) have used predominantly pharmacological approaches to pinpoint either the MC1R or the MC3R as a major transducer for the anti-inflammatory actions of natural and synthetic derivatives of ACTH and α-MSH. Activation of each of the five cloned melanocortin receptors will lead to cellular accumulation of cyclic-adenosime monophosphate (19); by activating protein kinase A and possibly also in an independent fashion, downstream events activated by melanocortin peptides through their receptors involve inhibition of NF-kB activation (23, 24), leading to an inhibition of cytokine synthesis and release and delayed up-regulation of heme oxygenase-1 (25) and IL-10 (26). More recent studies (27), using unbiased screening, have revealed profiles of gene expression on application of tissue-protective and anti-inflammatory melanocortin peptides. In all cases, these experimental pharmacological investigations have not addressed the specific functions that each receptor might play in a given model of pathophysiology.
In the present study, we focused our attention on the impact that the endogenous circuit centered on either MC1R or MC3R might have in the context of vascular inflammation associated with I/R. To do so, we have taken advantage of mice nullified for MC3R (28) or bearing a frameshift mutation such that an inactive MC1R is then expressed (29). The data obtained indicate that, at least when inflammation develops in the mesenteric microcirculation, MC3R produces a tonic inhibitory signal such that in its absence the cellular and soluble response is more pronounced.
MATERIALS AND METHODS
Animals
Male mice (2–3 wk old, ∼20 g body weight) were maintained on a standard chow pellet diet and had free access to water, with a 12-h light-dark cycle. The MC3R-null mouse colony was developed from founders kindly donated by Dr. Howard Y. Chen (Merck; Whitehouse Station, NJ, USA; ref. 28). Founders of the recessive yellow (e/e) mouse colony, bearing a frameshift mutation in the MC1R gene (29), were originally a gift from Dr. Nancy Levin (Trega Bioscience, San Diego, CA, USA). Wild-type (WT) animals (strain C57BL/6J; B&K, Hull, UK) were used 7 days after arrival according to the guidelines laid down by the Ethical Committee for the Use of Animals, Barts and The London School of Medicine. Animal work was performed according to Home Office Regulations (Guidance on the Operation of Animals, Scientific Procedures Act, 1986).
In vivo protocols
Intravital microscopy of the mouse mesentery
Intravital microscopy was performed as previously reported (30). Mice were anesthetized and placed in the supine position on a heating pad (37°C) for maintenance of body temperature. A polyethylene catheter (PE-10 with an internal diameter of 0.28 mm) was placed into the internal jugular vein for administration of drugs and fluorescein isothiocyanate (FITC) -labeled albumin (30). Mesenteric ischemia was induced with a microaneurysm clip (Harvard Apparatus, Kent, UK), clamping the superior mesenteric artery for 35 min. The clip was then removed, and reperfusion was allowed for 45 min (for albumin leakage assessment; see below) or 90 min (for evaluation of white blood cell reactivity). Sham-operated animals underwent the same surgical procedure except for the clamping of the superior mesenteric artery.
The mesenteric vascular bed was exteriorized, and monitoring of the cellular events in postcapillary venules was conducted exactly as described previously in detail (30). After the microcirculation was positioned under the microscope, a 5 min equilibration period preceded recording for quantitative measurements. Analyses of leukocyte-endothelium interactions were made in 1 to 3 randomly selected postcapillary venules (diameter 20 to 40 μm; visible length ≥100 μm) for each mouse.
Quantification of microcirculatory parameters was performed offline by frame-to-frame analysis of the videotaped images. White blood cell rolling velocity (VWBC) was determined from the time required for a leukocyte to roll a given distance along the length of the venule and is reported in micrometers per second. Rolling leukocyte flux was determined by counting the number of leukocytes passing a reference point in the venule per minute and expressed as cells per minute. Leukocyte adhesion was measured by counting cells clearly visible cells on the vessel wall in a 100 μm stretch. An adherent cell was qualified as a cell that has remained stationary for 30 s or longer. Leukocyte emigration from the microcirculation into the tissue was calculated by counting the number of cells in a 100 × 50 μm2 area on both sides of the 100 μm vessel segment. Red blood cell centerline velocity was measured in venules with an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University, Dallas, TX, USA) and venular wall shear rate was determined based on the Newtonian definition: wall shear rate = 8000 [(red blood cell velocity/1.6)/venular diameter].
In some cases, the selective MC3R agonist [d-Trp8]-γ-MSH (31) was used in both WT and MC3R-null mice. The compound was given at the dose of 10 μg/mouse intravenously before ischemia. Dose and route of administration were chosen from recent studies (32, 33) conducted with models of acute inflammation.
Albumin leakage
A saline solution of FITC-labeled albumin (50 mg/kg) was administered intravenously (Sigma Aldrich, Poole, UK) 10 min before analysis (which occurred at 45 min into reperfusion): a fluorescent light was switched on, and the vessel was recorded for 15 s. Visualization was possible using a block filter (excitation 450–490 nm, emission 525–620 nm). Albumin leakage was quantified by measuring mean fluorescence intensity, with video analysis software, of two 10 × 50 μm2 windows, one placed within the venule (FIin) and the other 10 μm away from the vessel wall (FIout) in relation to background. Albumin leakage was then determined as follows: ([FIout − background]/[FIin − background]) × 100%.
In vitro protocols
ELISA measurements
At the end of the intravital microscopy procedure, mesentery tissues were harvested and stored at −80°C. Mesenteric tissue fragments of sham-operated animals and mice subjected to I/R were homogenized in 1 ml of PBS containing antiproteases (0.1 mM/L phenylmethyl sulfonyl fluoride, 0.1 mM/L benzethonium chloride, 10 mmol/L ethylenediaminetetraacetic acid, and 20 IU aprotinin A) and 0.05% Tween 20. Quantitative ELISA to monitor tissue content for monocyte chemoattractant protein (MCP-1; CCL2 or JE in the mouse), keratinocyte-derived chemokine (KC; CXCL1), IL-1β, or TNF-α was then run, according to the manufacturer’s instructions (R&D Systems Europe, Oxford, UK).
Quantitative polymerase chain reaction (PCR)
Frozen mesenteric tissue was homogenized in TRIzol reagent (Invitrogen, Paisley, UK). RNA was extracted from homogenate, and genomic DNA was eliminated using the Turbo DNA-free kit (Applied Biosystems, Cheshire, UK). After cDNA synthesis by standard methodology, quantitative PCR was performed using the ABI Prism 7900 sequence detector system (Applied Biosystems), whereby 50 ng of cDNA was mixed with Taqman PCR mastermix (Applied Biosystems), QuantiTect primers (Qiagen, Crawley, UK), and SYBR Green I (Invitrogen, Paisley, UK), and the relative quantification was assessed for the KC and MCP-1 genes. The PCR cycle parameters were 50°C for 2 min, 95°C for 15 min, 40 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 30 s. Data were analyzed using SDS 2.1 (Applied Biosystems). The ΔΔCt method was used to calculate relative gene expression, whereby all Ct values were normalized to endogenous GAPDH reference.
Immunocytochemistry
Mesenteric tissues samples from sham-operated mice or mice subjected to the 35–90 I/R protocol were fixed in 4% paraformaldehyde and then embedded in paraffin. Serial sections (3 μm thick) were mounted on gelatin-subbed slides, dewaxed in xylene, and rehydrated in descending concentrations of alcohol. Sections were incubated overnight at 4°C in a humidified chamber with either polyclonal anti-MC1R-Ab (Sigma M 9193) or anti-MC3R-Ab (Sigma M 4937) at a 1:500 final dilution in PBS. Negative control sections were incubated with either PBS alone or a 1:500 dilution of normal rabbit serum in PBS. After being washed, sections were incubated with biotinylated anti-rabbit immunoglobulin G (IgG; 1:300 dilution in PBS for 1 h; Vector Laboratories, Peterborough, UK) followed by an incubation with avidin-biotin complex (ABC Elite; Vector Laboratories). The visualization of the antigen-antibody-ABC complex was achieved by the addition of 0.2 mg/ml diaminobenzidine tetrahydrochloride and 0.06% hydrogen peroxide in 0.1 M Tris (pH 7.6). Sections were counterstained with Gill’s hematoxylin before examination by light microscopy (Leica microscope DM-2000) with images captured using Leica software (Leica Microsystems, Wetzlar, Germany).
Measurement of myeloperoxidase activity
Leukocyte myeloperoxidase (MPO) activity was assessed by measuring the H2O2-dependent oxidation of 3,3′,5,5′-tetramethylbenzidine following a published protocol (34). Mesentery tissue samples from sham- and I/R-treated mice were homogenized in PBS containing 0.5% hexadacyl trimethylammonium bromide detergent. The homogenate was centrifuged at 13,000 g for 5 min before addition of 20 μl supernatant volumes to 160 μl of tetramethylbenzidine and 20 μl of H2O2 in 96-well plates. Plates were incubated for 5 min at room temperature, and optical density was read at 620 nm using GENios (Tecan, Reading, UK). Assays were performed in duplicate and normalized for protein content (BCA protein assay; Pierce, Cramlington, UK).
Analysis in blood and plasma samples
Blood was collected by cardiopuncture under anesthesia into heparinized tubes, diluted 1:10 in Turk’s solution (0.01% crystal violet in 3% acetic acid), before total and differential counting performed by means of a Neubauer chamber using light microscopy (Nikon YS2; ×40 objective; Nikon, Tokyo, Japan). Harvested leukocytes were distinguished in polymorponuclear (PMN) and mononuclear (PBMC) leukocytes.
For flow cytometry analyses, blood aliquots (50 μl) were stained with anti-GR-1 for granulocytes (clone RB6-8C5; BD Pharmingen, San Jose, CA, USA) or anti-F4/80 for monocytes and macrophages (clone Cl:A3-1; Serotec, Oxford, UK) in combination with an anti-CD62L, anti-CD11b, or anti-CD162 (BD Pharmingen) diluted 1:100 in PBS containing 0.1% sodium azide, 10% rat serum, and anti-mouse Fc receptor monoclonal antibody (mAb; anti-CD16/CD32; clone 2.4G2; BD Pharmingen) for 30 min at 4°C. After being stained, red blood cells were lysed using whole blood lysing reagent kit (Beckman Coulter, Galway, Ireland). Data acquisition was performed with a FACScalibur flow cytometer (Becton Dickinson, San Jose, CA, USA) using CellQuest software (Becton Dickinson). Forward and side scatters were set to exclude dead cells. At least 105 cells were analyzed per sample. Positive and negative populations were identified based on dual-color staining performed with a phycoerythrin- and FITC-conjugated irrelevant IgG isotype (eBioscience, San Diego, CA, USA).
Plasma cytokine and chemokine levels were determined using the ELISA methods described above, whereas the soluble form of CD62L was quantified using a specific kit (cat no. MLS00; R&D Systems). In addition, nitrite/nitrate levels were determined with the Griess reaction as described previously (35, 36).
Statistics
All data are reported as means ± se of n observations, using at least 6 mice/group. Statistical evaluation was performed using ANOVA (Prism software; GraphPad, San Diego, CA, USA) with Bonferroni test for post hoc analyses, taking a probability value of P < 0.05 as significant.
RESULTS
Mesenteric microcirculation of MC3R-null mice
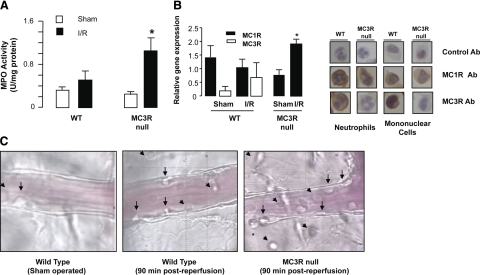
Initial analyses of the tissue reactivity to the I/R protocol (35 min ischemia+90 min reperfusion) revealed augmented MPO activity in tissue samples collected from MC3R-null mice compared to WT animals (∼3-fold increase in MPO activity was measured in MC3R-null mice; n=6; P<0.05; Fig. 1A).
Figure 1.
Indication for a higher inflammatory reactivity in mesenteric tissue of MC3R-null mice after I/R. A) WT and MC3R-null mice were subjected to occlusion of the superior mesenteric artery for 35 min, followed by 90 min reperfusion. For either genotype, a sham-treatment group was also analyzed, where laparotomy was conducted with no occlusion of the artery. MPO was determined on tissue extracts as described in Materials and Methods. Data are means ± se; 6 mice/group. *P < 0.05 vs. respective sham-treatment group. B) Mesenteric tissue was obtained as in A. Left panel: detection by quantitative PCR of MC1R and MC3R mRNA in the mesenteric tissue samples (data from 6 mice); right panel: representative neutrophils and mononuclear cells of WT and MC3R-null mice positive for MC1R and MC3R protein expression. There was no positive immunostaining in leukocytes from tissue sections incubated with either PBS alone or normal rabbit serum diluted in PBS (control). Note that cells from MC3R-null mice were negative for this receptor staining, whereas they expressed MC1R. All sections have been counterstained with Gill’s hematoxylin. Data represent results from 6 distinct tissue preparations. C) Images of inflamed mesenteric microcirculation as taken 90 min postreperfusion. Arrowheads = extravasated leukocytes; arrows = intravascular cells (representative of 6 distinct microvasculatures).
Modest, if any, expression of MC1R and MC3R mRNA could be detected in the mesenteric tissue of sham-operated mice by quantitative PCR (Fig. 1B), with cycle numbers ranging from 30 to 35; the I/R protocol mildly changed the profile of expression in WT mice, whereas a significant increase of MC1R mRNA in MC3R-null mice was evident (Fig. 1B). Immunocytochemistry revealed that white blood cells, such as PMN leukocytes and mononuclear cells, were positive for both MC1R and MC3R protein expression (Fig. 1B); nobably, no MC3R immunoreactivity was detected in tissue cells of MC3R-null mice. Finally, visualization of the mesenteric microcirculation indicated a higher degree of blood leukocyte adhesion and emigration in MC3R-null mice (Fig. 1C). Altogether, these data justified detailed investigations on different cellular processes, and humoral parameters, activated by the I/R protocol.
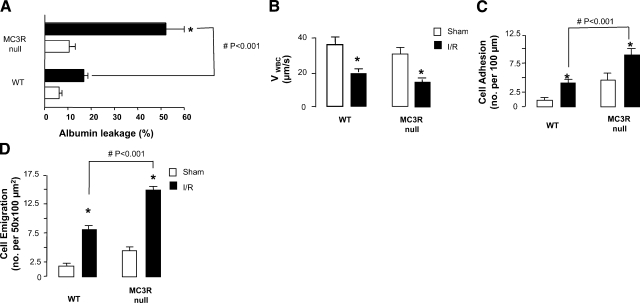
Intravital microscopy analysis indicated a rapid (45 min postreperfusion) albumin leakage, observed in the microcirculation of WT mice (Fig. 2A); MC3R-null mice displayed a marginally higher, not significant, albumin leakage in the sham-treatment group; in contrast, a much more pronounced response was observed after the I/R procedure (Fig. 2A).
Figure 2.
Higher reactivity of microcirculation of MC3R-null mice after I/R. WT and MC3R-null mice were subjected to occlusion of the superior mesenteric artery for 35 min, followed by 90 min reperfusion. For either genotype, a sham-treatment group was also analyzed, where laparotomy was conducted with no occlusion of the artery. A) Plasma protein extravasation was assessed 45 min postreperfusion by monitoring FITC-labeled albumin leakage. B–D) Cellular responses in the inflamed postcapillary venule as determined 90 min postreperfusion, monitoring cell rolling as VWBC (B), adhesion (C), and emigration (D). Data are means ± se; 6 mice/group. *P < 0.05 vs. respective sham-treatment group. #P < 0.01 between genotypes post-I/R.
The cellular events occurring in the mesenteric vessels were more reliably analyzed at the 90 min postreperfusion time point. Figure 2B illustrates the sharp (∼50%) reduction in VWBC on the vessel wall in both WT and MC3R-null mice after I/R. Whereas there was no difference between the two genotypes on this parameter, MC3R-null mice displayed incremented numbers of both adherent and emigrated leukocytes (Fig. 2C, D). This was also evident at other time points, from 45 min to 4 h postreperfusion (data not shown).
Such changes in vascular reactivity were not due to hemodynamic alterations in the vasculature (Table 1).
TABLE 1.
Hemodynamic parameters in mesenteric microcirculation of WT, recessive yellow e/e, and MC3R-null mice
| Treatment and genotype | Venule diameter (μm) | Cell flux (cells/min) | Wall shear rate (s−1) |
|---|---|---|---|
| Sham WT | 26.3 ± 1.7 | 19.4 ± 3.7 | 402.5 ± 12.5 |
| I/R WT | 29.2 ± 0.8 | 17.5 ± 3.3 | 293.7 ± 29.5 |
| Sham MC3R-null | 27.7 ± 1.6 | 13.5 ± 2.3 | 376.1 ± 46.0 |
| I/R MC3R-null | 26.6 ± 3.8 | 11.1 ± 2.8 | 363.1 ± 38.2 |
| Sham recessive yellow e/e | 28.0 ± 1.3 | 16.7 ± 1.0 | 415 ± 18.6 |
| I/R recessive yellow e/e | 25.8 ± 2.0 | 14.4 ± 2.8 | 314.7 ± 19.5 |
Diameters of the mesenteric vessels analyzed in Figs. 1 and 5 are reported. Similarly, values for wall shear rate and cell flux are shown. In all cases, mice were exposed to I/R (35 min of ischemia and 90 min of reperfusion) per the procedure described in Materials and Methods. Data are means ± se; 6 mice/group.
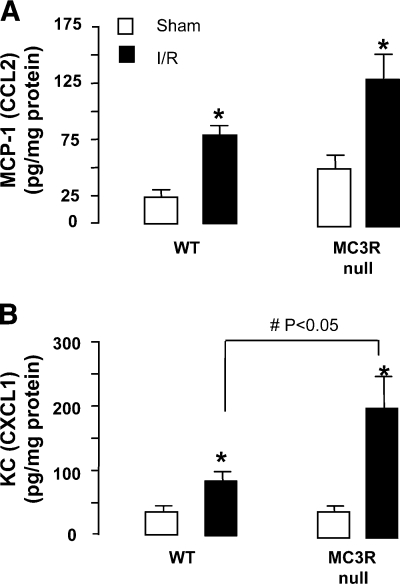
The inflammatory phenotype of MC3R-null mice was reflected, at least in part, by changes in pivotal chemokine levels. The I/R procedure incremented (from doubling to nearly trebling) tissue levels of MCP-1 and KC (Fig. 3). Quantification of their contents in the mesenteric tissue of MC3R-null mice revealed 1) a pronounced response after the I/R procedure as assessed at 90 min postreperfusion with remarkable increases in both chemokines; and 2) a significant increment in the local levels of KC (Fig. 3B) when compared with WT mice, whereas no significant changes could be measured with respect to MCP-1 (Fig. 3A). Tissue levels of IL-1β, IL-6, and TNF-α were below detection in all samples tested (data not shown).
Figure 3.
I/R-induced tissue KC and MCP-1 are augmented in MC3R-null mice. WT and MC3R-null mice were subjected to occlusion of the superior mesenteric artery for 35 min, followed by 90 min reperfusion. For either genotype, a sham-treatment group was also analyzed, where laparotomy was conducted with no occlusion of the artery. Mesenteries were homogenized and protein extracts were used for the ELISA assays to determine tissue content of MCP-1 (A) and KC (B). Data are means ± se; 6 mice/group. *P < 0.05 vs. respective sham-treatment group. #P < 0.05 between genotypes post-I/R.
I/R-induced changes in KC (CXCL1) and MCP-1 (CCL2) protein expression in the mesentery were paralleled by changes in mRNA levels, as detected by real-time PCR. For both genes, WT sham-treated or MC3R-null sham-treated mice were set as the baseline, accordingly. Sham-operated tissue gene levels of either chemokine remained similar between WT and sham MC3R-null mice; in contrast, I/R injured tissue from MC3R-null mice had an ∼19- and 16-fold increase in KC and MCP-1 gene expression, respectively, over WT I/R tissues (data not shown; n=6; P<0.05).
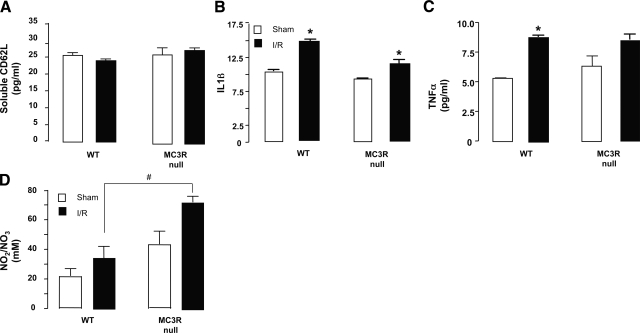
Systemic responses in WT and MC3-null mice: soluble mediators
A selection of analyses was conducted in the plasma of these mice. Whereas MCP-1 and KC could not be detected, soluble CD62L, IL-1, and TNF-α levels were reliably measured, although there were no differences between the two genotypes (Fig. 4A–C). In contrast, the I/R procedure augmented circulating nitrite/nitrate levels, with a more marked response in MC3R-null mice, with values significantly different from WT animals for the I/R groups (P<0.05; Fig. 4D).
Figure 4.
I/R-induced systemic response in WT and MC3R-null mice. WT and MC3R-null mice were subjected to occlusion of the superior mesenteric artery for 35 min, followed by 90 min reperfusion. For either genotype, a sham-treatment group was also analyzed, where laparotomy was conducted with no occlusion of the artery. Plasma samples were analyzed with ELISA or for Griess reactivity as described in Materials and Methods to monitor soluble CD62L (A), IL-1β (B), TNF-α (C), and nitrite/nitrate (D). Data are means ± se; 6 mice/group. *P < 0.05 vs. respective sham-treatment group. #P < 0.05 between genotypes post-I/R.
Systemic responses in WT and MC3-null mice: adhesion molecule expression on blood leukocytes
In resting conditions, MC3R-null and WT mice display a similar percentage of white blood cells; the same held true when analysis was performed 90 min after I/R (Table 2). To investigate the possible effects of MC3R gene ablation on adhesion molecule expression on circulating leukocytes during I/R, we investigated the expression of CD11b and CD62L on granulocytes (GR-1hi) and mononuclear phagocytes (F4/80+).
TABLE 2.
I/R of mesenteric microcirculation and systemic leukocyte differential counts in WT and MC3R-null mice
| Treatment and genotype | PMN (106/ml) | PBMC (106/ml) |
|---|---|---|
| Sham WT | 4.8 ± 0.9 | 32.6 ± 4.6 |
| I/R WT | 16 ± 1.2 | 38.5 ± 6.3 |
| Sham MC3R-null | 7.2 ± 2.1 | 36.6 ± 5.5 |
| I/R MC3R-null | 20 ± 2.2 | 41.6 ± 7.5 |
Peripheral blood was analyzed by light microscopy in sham-treated animals or in mice subjected to 35 min ischemia and 90 min reperfusion of the superior mesenteric artery. Data are means ± se; 6 mice/group.
Within the GR-1hi population, CD11b expression was not modified by the IR injury in either WT or MC3R-null animals; however, granulocytes from IR MC3R-null animals displayed an increased CD11b expression when compared with WT animals. The values of sham-treated animals in both genotypes were not statistically different (Table 3). No statistical changes were observed for CD62L in the GR-1 population even after IR induction (data not shown).
TABLE 3.
I/R of mesenteric microcirculation and profiling of adhesion molecule expression in MC3R-deficient animals
| Treatment and genotype | Gr1hi CD11b expression (MFI) | F4/80
|
|
|---|---|---|---|
| CD11b expression (MFI) | CD62L expression (MFI) | ||
| Sham WT | 12.3 ± 2.6 | 199 ± 69 | 49 ± 11 |
| I/R WT | 13.6 ± 1.0 | 114 ± 28 | 38 ± 7 |
| Sham MC3R-null | 16.0 ± 0.7 | 195 ± 8 | 37 ± 4 |
| I/R MC3R-null | 17.3 ± 1.2 | 131 ± 19# | 23 ± 0.4*,# |
Blood leukocytes of sham-treated or I/R mice were analyzed by flow cytometry for cell adhesion molecule expression. Data are means ± se; 3 mice/group. MFI, mean fluorescence intensity.
P < 0.05 vs. I/R WT group;
P < 0.05 vs. sham-treated MC3R-null group.
Within the F4/80 population, I/R triggered a nonsignificant decrease in CD11b expression in WT animals; however, in MC3R-null animals, a significant decrease was observed (Table 3). I/R leads to a nonsignificant decrease in CD62L expression in WT F4/80 cells, whereas a significant decrease in CD62L expression was observed in MC3R-null animals (Table 3), suggesting that the mononuclear phagocyte compartment is highly activated in the MC3R-null animals after I/R.
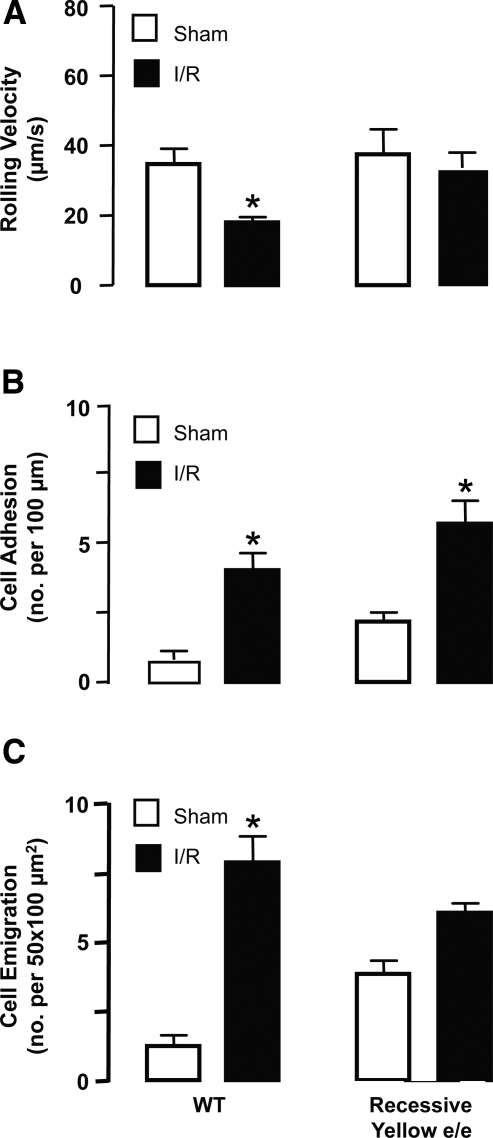
Analyses in recessive yellow e/e mice
The fact that MC1R mRNA was present in mesenteric tissue samples prompted us to determine what function it might have in these experimental conditions; to do so, the intravascular response was monitored in mice bearing an inactive MC1R, the recessive yellow e/e mouse. Application of the I/R protocol to this genotype, conducting analyses at 90 min postreperfusion, revealed a cellular response not dissimilar to that promoted in WT mice; this was evident both for VWBC values and cell adhesion and emigration (Fig. 5). Table 1 reports the hemodynamic parameters measured in this set of experiments.
Figure 5.
Analyses of the microcirculation of recessive yellow e/e mice. WT and recessive yellow e/e (bearing an inactive MC1R mutant) mice were subjected to occlusion of the superior mesenteric artery for 35 min, followed by reperfusion. For either genotype, a sham-treatment group was also analyzed, where laparotomy was conducted with no occlusion of the artery. Cellular responses in the inflamed postcapillary venule were determined 90 min postreperfusion, monitoring cell rolling as VWBC (A), adhesion (B), and emigration (C). Data are means ± se; 6 mice/group. *P < 0.05 vs. respective sham group.
This lack of difference in the inflammatory cellular responses activated in the microcirculation between WT and MC1R mutants (recessive yellow e/e) was mirrored by a lack of difference in tissue levels in chemokines. Calculated values of 79 ± 9 and 83 ± 4 pg/mg tissue protein were measured for MCP-1 (CCL2) in WT and MC1R mutant mice. Similarly, tissue levels of KC (CXCL1) were 85 ± 15 vs. 80 ± 12 pg/mg in WT and MC1R mutants, respectively (values are means ± se of 5 mice/group; not significant).
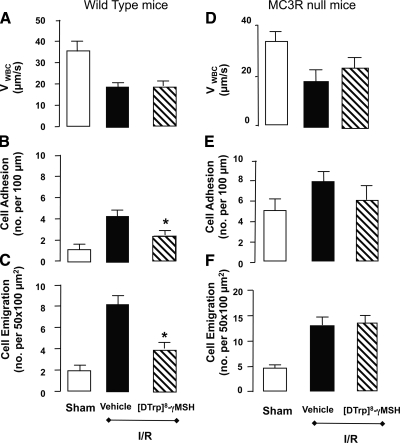
Inhibitory properties of a selective MC3R agonist
Finally, the pharmacological effects of a highly selective MC3R agonist, [d-Trp8]-γ-MSH (31), known to possess anti-inflammatory properties (32), were assessed on the inflammatory response induced by the mesenteric microcirculation of WT and MC3R-null mice. When administered to WT mice at the dose of 10 μg i.v., [d-Trp8]-γ-MSH produced selective inhibitory effects in the inflamed microcirculation: the compound did not affect VWBC (Fig. 6A) or cell flux (not shown), whereas it did inhibit the extent of cell adhesion (∼50% attenuation of the I/R response, that is, the increment of cell adhesion induced by I/R above the sham-treatment values; Fig. 6B) and, more effectively, that of cell emigration (>70% reduction, when considering the I/R response; Fig. 6C).
Figure 6.
Anti-inflammatory properties of [d-Trp8]-γ-MSH selectively in the microcirculation of WT, but not MC3R-null, mice. WT (A–C) and MC3R-null (D–F) mice were subjected to occlusion of the superior mesenteric artery for 35 min, followed by 90 min reperfuction. For either genotype, a sham-treatment group was also analyzed, where laparotomy was conducted with no occlusion of the artery. In the case of the I/R groups, some mice received vehicle or 10 μg i.v. [d-Trp8]-γ-MSH 1 h before inducing ischemia. Cellular responses in the inflamed postcapillary venule were determined 90 min postreperfusion, monitoring cell rolling as VWBC (A, D), adhesion (B, E), and emigration (C, F). Data are means ± se; 6 mice/group. *P < 0.05 vs. respective vehicle I/R group.
These inhibitory effects were mirrored by a marked inhibition in the mesenteric tissue protein expression of both MCP-1 and KC, as well as of MPO activity; calculated inhibitions of 65, 80, and 90% were obtained for each marker of inflammation, respectively, when compared with WT mice treated with vehicle and subjected to I/R procedure (data not shown; n=6; P<0.05).
In contrast to this panel of inhibitory readouts observed in WT mice, treatment of MC3R-null mice with 10 μg i.v. [d-Trp8]-γ-MSH failed to significantly modify VWBC (Fig. 6D) and the extent of cell adhesion (Fig. 6E) and cell emigration (Fig. 6F). Similarly, no modification of mesenteric tissue markers of inflammation was produced by treatment with the anti-inflammatory compound [d-Trp8]-γ-MSH when it was administered to MC3R-null mice (data not shown).
DISCUSSION
In this study, we reveal the existence of a tonic inhibitory signal provided by MC3R in the mesenteric microcirculation of the mouse. The absence of this receptor is associated with an exacerbated inflammatory reaction of the microvasculature, characterized by distinct and selected increments in the extent of leukocyte adhesion emigration and local generation of proinflammatory mediators. Treatment of WT mice with a selected agonist to MC3R produced remarkable inhibitory effects on intravascular cellular reactivity and markers of inflammation. Collectively, these data highlight the pathophysiological role of an endogenous inhibitory circuit centered on this specific receptor during vascular inflammation.
In the past two decades, a large number of studies (11, 12, 15,16,17, 27, 37,38,39) have reported the efficacy of melanocortin peptides (the natural agonists ACTH or α-MSH, or their synthetic derivatives) in several models of acute and chronic inflammation, spanning from tissues and organs as diverse as the liver, kidney, lung, and joint. However, this wealth of data begs two major questions, by and large still unanswered: 1) what is the main receptor target responsible for these protective effects and 2) what is the pathophysiological rather pharmacological effect of these peptides in experimental and possibly also human inflammation.
The first question has been partly addressed by us and others, such that of the five receptors cloned so far for melanocortin peptides (19), two subtypes, MC1R and MC3R, seem to play a central role in transducing the pharmacological properties of these agents. The model that emerged from our own work indicates the existence of MC3R in the tissue resident macrophage; its activation by exogenously administered agonists would down-regulate macrophage activation, such as to obtain, on the one hand, inhibition of the production of proinflammatory mediators and, on the other hand, induction of proresolving and tissue protective proteins (16, 25, 26). Other studies have highlighted a role for MC1R, specifically in the skin compartment, whereas again activation of this receptor would lead to inhibition of cytokine and chemokine processing, thereby producing a net inhibitory effect on the induction of the inflammatory reaction (37, 40). It was important that we could detect mRNA for both receptors in the mesenteric tissue, even in the absence of injury.
With respect to pathophysiological relevance of these receptors and the circuits that ligand-receptor pairs might produce, a great help to elucidate functions derives from the use of mice nullified for specific receptor subtypes. A natural MC1R mutant (the recessive yellow e/e mouse; ref. 29) was described a few years ago, but, so far, it has been scarcely investigated in the context of inflammation. Using these mice, we were able to demonstrate a redundant role for MC1R in the context of peritoneal inflammation elicited by urate crystals (41). More recent studies (42) performed with a chronic model of inflammation (7-day colitis) demonstrated a proinflammatory phenotype in the recessive yellow e/e mouse, indicating that at least in these experimental conditions activation of MC1R would elicit anti-inflammatory signals with a major outcome on the inflammatory phenotypes of the mouse.
MC3R-null mice were generated quite a few years ago (28) and so far have been predominantly studied in the context of energy metabolism, whereby subtle differences in adipocyte functions have been reported (28). Using this colony, we have been able to validate the central role that MC3R possesses in bringing about the anti-inflammatory actions of melanocortin peptides in crystal-induced inflammation (32). Here we used this mouse colony to investigate potential changes in the vascular inflammatory response activated by an I/R protocol. This protocol was chosen because it was previously validated and found to be sensitive to the action of other endogenous effectors of endogenous anti-inflammation, such as annexin-1 and lipoxin A4 (30).
Gene deficiency in MC3R produced remarkable augmentation of the cellular responses in the mesenteric microcirculation after I/R; this was particularly consistent at the 90 min time point as shown here and as evident at other time points as well (we have tested up to 4 h postreperfusion; unpublished results). Therefore, activation of MC3R is essential to exert a tonic inhibitory action during the inflammatory response that occurs in this experimental model. Such an inflammatory profile is more likely to be consequent to augmented local inflammatory signals and processes, as we could not detect increases in markers of white blood cell activation. Indeed, whereas no changes of CD11b and CD62L expression could be measured in circulating PMN, a lower degree of expression was measured in circulating F4/80+ cells selectively after I/R. Therefore, such a reduction would not underlie the observed higher extent of cell adhesion and emigration (with no modification of cell rolling) detected in mesenteric postcapillary venules.
However, what would be the source of MC3R agonists? Both ACTH and α-MSH could reach the inflamed mesentery as part of the plasma protein externalization response that is observed in this model; as expected, this response was optimal at earlier time points compared with the cellular response (45 min vs. ≥60 min). However, we cannot exclude the local generation of melanocortin peptides by mast cells (43), macrophages (44), or other tissue-dwelling cells. This hypothesis would require new experiments that would form the basis of future studies; in any case, the expression of both MC3R and MC1R mRNA (although at a low level, as positivity by quantitative PCR was not confirmed by the less sensitive RT-PCR method) in the mesentery favors the possible existence of local counter-regulatory circuits that could be rapidly activated after an I/R event. This time-dependent activation hypothesis is plausible, since migrated neutrophils and mononuclear cells seemed positive for both MC1R and MC3R, instead of stromal and other cells resident in the mesenteric microvascular bed.
These data obtained with markers of inflammation deserve full analysis. Within this scenario of induction and maintenance of the vascular inflammatory response after I/R, pluri-potent cytokines seem to sustain the systemic (and possibly second-organ injury) response, whereas chemokines might regulate white blood cell trafficking and activation in the affected tissue. Such a dichotomy of properties may justify the partially surprising effects we have observed, although we cannot exclude that kinetic assessment over time might have yielded different results. What is more relevant for the present study, though, is that gene deficiency in MC3R did not affect the systemic response to any large extent, the only exception possibly being the generation of nitrate/nitrite. In contrast, potent regulation of mesenteric MCP-1 (CCL2) and KC (CXCL1) levels by the MC3R pathway was observed, with augmented protein and mRNA expression. Such results are in line with the pharmacological properties of melanocortin receptor agonists that inhibit generation of cytokines and chemokines in inflamed tissues or from activated cells, including KC from macrophages (21, 22, 39, 45). In our experimental conditions, we could not detected mesenteric expression of IL-1β, TNF, or IL-6.
The novel findings that we report are in apparent contrast to a recent study that reported a reduced inflammatory reaction in MC3R-null mice (46). The transgenic mouse colony used (47) is different from the one we used here; in any case, we do not have a clear explanation for this discrepancy; the large majority of publications in the field indicate, in a clear manner, the inhibitory role that melanocortin peptides and their receptors play in inflammation (15); it is possible that the high-fat diet might have somehow subverted the pathophysiological properties of this receptor.
It is worth noting that the application of our experimental protocols to recessive yellow e/e mice did not produce cellular responses different from those observed in WT animals; on the same vein, no differences in local and systemic mediator generation were observed between the animals bearing an inactive MC1R mutant and WT mice. Such a lack of involvement is striking when compared with the major alterations observed and quantified in the microcirculation of MC3R-null mice.
There were also indications that the microvasculature of MC3R animals might be more prone to reactivity even when very mild stimuli are applied, such as observed in sham animals, although it rarely reached statistical significance. Such a mild hyper-reactivity was unlikely due to inherent alterations in white blood cell phenotype, as we did not detect different basal expression of PMN or monocyte markers in WT and MC3R-null circulating cells. The fact that higher markers and readouts of inflammation were often detected in sham-operated MC3R-null mice was also a constant feature of the present study. Together with more pronounced differences seen after I/R and discussed above, we wish to propose that MC3R is pivotal to an inhibitory circuit that possesses physiological as well as pathological inhibitory roles within the mesenteric microcirculation.
In the final part of the study, we determined the potentially inhibitory actions of a selective MC3R agonist ([d-Trp8]-γ-MSH; ref. 31). We (32) recently reported that this compound produces anti-inflammatory activities in models of peritonitis as well joint inflammation, and these actions are due to MC3R activation, since absent in mice nullified for this receptor. In addition, and in line with its receptor binding profile (31), [d-Trp8]-γ-MSH fully retained its pharmacological properties in the animals bearing the MC1R mutant (33). Here we have assessed the role of this compound on leukocyte interaction with inflamed postcapillary venules. Treatment of mice with [d-Trp8]-γ-MSH produced remarkable inhibition, between 50–70% of both cell adhesion and cell emigration, whereas it did not affect the responses observed in MC3R-null mice. In line with the data produced by comparing inflammatory responses in MC3R-null vs. WT mice, administration of the MC3R selective agonist to the latter mouse strain did inhibit also chemokine generation in the mesenteric tissue. This effect complements the reported inhibition on KC and IL-1β released into the supernatant of cultured mouse peritoneal macrophages or detected in inflammatory exudates (32). Together with the studies (11, 12, 38, 45) by other groups where the protective actions of cellular melanocortins on I/R injury of the heart, kidneys, and other organs have been observed, our new data indicate that [d-Trp8]-γ-MSH or other selective MC3R agonists would be suitable for development as novel therapeutics to control I/R pathological conditions.
In summary, we have taken advantage of a mouse colony nullified for MC3R to reveal novel and crucial properties for this receptor in controlling the vascular reactivity of the mesenteric microcirculation, observing tonic inhibitory properties on the white blood cell trafficking process as well local generation of inflammatory mediators. The pathophysiological relevance that we reveal here for MC3R might bear therapeutic implications, since a selective agonist at this receptor produced remarkable inhibitory effects in WT mice subjected to the I/R reperfusion procedure. In fact, irrespective of whether MC1R or MC3R is the major receptor target responsible for bringing about the anti-inflammatory properties of melanocortin peptides (it is plausible that tissue and/or species specificity might exist), it is clear that studying the biology of melanocortin peptides and their receptor might help in the design and development of new anti-inflammatory therapeutics.
Acknowledgments
This study was funded by the British Heart Foundation (studentship FS/05/078/19406). A.L.F.S. is a Fellow of CNPq Brasil (201172/2004–06).
References
- Ley K, Laudanna C, Cybulsky M I, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Gilroy D W, Lawrence T, Perretti M, Rossi A G. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Newby A C. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- Wainwright C L. Matrix metalloproteinases, oxidative stress and the acute response to acute myocardial ischemia and reperfusion. Curr Opin Pharmacol. 2004;4:132–138. doi: 10.1016/j.coph.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Perretti M. Endogenous mediators that inhibit the leukocyte-endothelium interaction. Trends Pharmacol Sci. 1997;18:418–425. doi: 10.1016/s0165-6147(97)01116-4. [DOI] [PubMed] [Google Scholar]
- Serhan C N, Brain S D, Buckley C D, Gilroy D W, Haslett C, O'Neill L A, Perretti M, Rossi A G, Wallace J L. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21:325–332. doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- Serhan C N, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- Lim L H, Solito E, Russo-Marie F, Flower R J, Perretti M. Promoting detachment of neutrophils adherent to murine postcapillary venules to control inflammation: effect of lipocortin 1. Proc Natl Acad Sci U S A. 1998;95:14535–14539. doi: 10.1073/pnas.95.24.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damazo A S, Yona S, Flower R J, Perretti M, Oliani S M. Spatial and temporal profiles for anti-inflammatory gene expression in leukocytes during a resolving model of peritonitis. J Immunol. 2006;176:4410–4418. doi: 10.4049/jimmunol.176.7.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzani C, Guarini S, Botticelli A R, Zaffe D, Tomasi A, Bini A, Cainazzo M M, Ferrazza G, Mioni C, Bertolini A. Protective effect of melanocortin peptides in rat myocardial ischemia. J Pharmacol Exp Ther. 2001;297:1082–1087. [PubMed] [Google Scholar]
- Mioni C, Giuliani D, Cainazzo M M, Leone S, Iannone C, Bazzani C, Grieco P, Novellino E, Tomasi A, Bertolini A, Guarini S. Further evidence that melanocortins prevent myocardial reperfusion injury by activating melanocortin MC(3) receptors. Eur J Pharmacol. 2003;477:227–234. doi: 10.1016/s0014-2999(03)02184-8. [DOI] [PubMed] [Google Scholar]
- Getting S J, Di Filippo C, Christian H C, Lam C W, Rossi F, D'Amico M, Perretti M. MC-3 receptor and the inflammatory mechanisms activated in acute myocardial infarct. J Leukoc Biol. 2004;76:845–853. doi: 10.1189/jlb.0306175. [DOI] [PubMed] [Google Scholar]
- Gatti S, Colombo G, Buffa R, Turcatti F, Garofalo L, Carboni N, Ferla L, Fassati L R, Lipton J M, Catania A. alpha-Melanocyte-stimulating hormone protects the allograft in experimental heart transplantation. Transplantation. 2002;74:1678–1684. doi: 10.1097/00007890-200212270-00005. [DOI] [PubMed] [Google Scholar]
- Catania A, Gatti S, Colombo G, Lipton J M. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- Getting S J. Targeting melanocortin receptors as potential novel therapeutics. Pharmacol Ther. 2006;111:1–15. doi: 10.1016/j.pharmthera.2005.06.022. [DOI] [PubMed] [Google Scholar]
- Ritter J, Kerr L D, Valeriano-Marcet J, Spiera H. ACTH revisited: effective treatment for acute crystal induced synovitis in patients with multiple medical problems. J Rheumatol. 1994;21:696–699. [PubMed] [Google Scholar]
- Catania A, Airaghi L, Colombo G, Lipton J M. Alpha-melanocyte-stimulating hormone in normal human physiology and disease states. Trends Endocrinol Metab. 2000;11:304–308. doi: 10.1016/s1043-2760(00)00296-4. [DOI] [PubMed] [Google Scholar]
- Gantz I, Fong T M. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284:E468–E474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- Guarini S, Schioth H B, Mioni C, Cainazzo M, Ferrazza G, Giuliani D, Wikberg J E, Bertolini A, Bazzani C. MC(3) receptors are involved in the protective effect of melanocortins in myocardial ischemia/reperfusion-induced arrhythmias. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:177–182. doi: 10.1007/s00210-002-0572-8. [DOI] [PubMed] [Google Scholar]
- Getting S J, Christian H C, Flower R J, Perretti M. Activation of melanocortin type 3 receptor as a molecular mechanism for adrenocorticotropic hormone efficacy in gouty arthritis. Arthritis Rheum. 2002;46:2765–2775. doi: 10.1002/art.10526. [DOI] [PubMed] [Google Scholar]
- Getting S J, Gibbs L, Clark A J, Flower R J, Perretti M. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. J Immunol. 1999;162:7446–7453. [PubMed] [Google Scholar]
- Kalden D H, Scholzen T, Brzoska T, Luger T A. Mechanisms of the anti-inflammatory effects of alpha-MSH. Role of transcription factor NF-kappa B and adhesion molecule expression. Ann N Y Acad Sci. 1999;885:254–261. doi: 10.1111/j.1749-6632.1999.tb08682.x. [DOI] [PubMed] [Google Scholar]
- Manna S K, Aggarwal B B. Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents. J Immunol. 1998;161:2873–2880. [PubMed] [Google Scholar]
- Lam C W, Getting S J, Perretti M. In vitro and in vivo induction of heme oxygenase 1 in mouse macrophages following melanocortin receptor activation. J Immunol. 2005;174:2297–2304. doi: 10.4049/jimmunol.174.4.2297. [DOI] [PubMed] [Google Scholar]
- Lam C W, Perretti M, Getting S J. Melanocortin receptor signaling in RAW264.7 macrophage cell line. Peptides. 2006;27:404–412. doi: 10.1016/j.peptides.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Colombo G, Gatti S, Turcatti F, Sordi A, Fassati L R, Bonino F, Lipton J M, Catania A. Gene expression profiling reveals multiple protective influences of the peptide alpha-melanocyte-stimulating hormone in experimental heart transplantation. J Immunol. 2005;175:3391–3401. doi: 10.4049/jimmunol.175.5.3391. [DOI] [PubMed] [Google Scholar]
- Chen A S, Marsh D J, Trumbauer M E, Frazier E G, Guan X M, Yu H, Rosenblum C I, Vongs A, Feng Y, Cao L, Metzger J M, Strack A M, Camacho R E, Mellin T N, Nunes C N, Min W, Fisher J, Gopal-Truter S, MacIntyre D E, Chen H Y, Van der Ploeg L H. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- Robbins L S, Nadeau J H, Johnson K R, Kelly M A, Roselli-Rehfuss L, Baack E, Mountjoy K G, Cone R D. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Gavins F N, Yona S, Kamal A M, Flower R J, Perretti M. Leukocyte antiadhesive actions of annexin 1: ALXR- and FPR-related anti-inflammatory mechanisms. Blood. 2003;101:4140–4147. doi: 10.1182/blood-2002-11-3411. [DOI] [PubMed] [Google Scholar]
- Grieco P, Balse P M, Weinberg D, MacNeil T, Hruby V J. D-Amino acid scan of gamma-melanocyte-stimulating hormone: importance of Trp(8) on human MC3 receptor selectivity. J Med Chem. 2000;43:4998–5002. doi: 10.1021/jm000211e. [DOI] [PubMed] [Google Scholar]
- Getting S J, Lam C W, Chen A S, Grieco P, Perretti M. Melanocortin 3 receptors control crystal-induced inflammation. FASEB J. 2006;20:2234–2241. doi: 10.1096/fj.06-6339com. [DOI] [PubMed] [Google Scholar]
- Getting S J, Lam C W, Leoni G, Gavins F N, Grieco P, Perretti M. [d-Trp8]-gamma-melanocyte-stimulating hormone exhibits anti-inflammatory efficacy in mice bearing a nonfunctional MC1R (recessive yellow e/e mouse) Mol Pharmacol. 2006;70:1850–1855. doi: 10.1124/mol.106.028878. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Tailor A, Zingarelli B, Salzman A L, Flower R J, Szabø C, Perretti M. Lipocortin 1 protects against splanchnic artery occlusion and reperfusion injury by affecting neutrophil migration. J Immunol. 1997;159:5089–5097. [PubMed] [Google Scholar]
- Di Filippo C, Rossi F, Ongini E, Del Soldato P, Perretti M, D'Amico M. The distinct alterations produced in cardiovascular functions by prednisolone and nitro-prednisolone (NCX-1015) in the rat highlight a causal role for endothelin-1. J Pharmacol Exp Ther. 2004;310:1133–1141. doi: 10.1124/jpet.104.068726. [DOI] [PubMed] [Google Scholar]
- Paul-Clark M, Del Soldato P, Fiorucci S, Flower R J, Perretti M. 21-NO-prednisolone is a novel nitric oxide-releasing derivative of prednisolone with enhanced anti-inflammatory properties. Br J Pharmacol. 2000;131:1345–1354. doi: 10.1038/sj.bjp.0703704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger T A, Brzoska T. alpha-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugs. Ann Rheum Dis. 2007;66:iii52–55. doi: 10.1136/ard.2007.079780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao H, Kohda Y, McLeroy P, Craig L, Housini I, Star R A. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J Clin Invest. 1997;99:1165–1172. doi: 10.1172/JCI119272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao H, Foster S, Thomas R, Lipton J, Star R A. Alpha-melanocyte-stimulating hormone reduces endotoxin-induced liver inflammation. J Clin Invest. 1996;97:2038–2044. doi: 10.1172/JCI118639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger T A, Scholzen T E, Brzoska T, Bohm M. New insights into the functions of alpha-MSH and related peptides in the immune system. Ann N Y Acad Sci. 2003;994:133–140. doi: 10.1111/j.1749-6632.2003.tb03172.x. [DOI] [PubMed] [Google Scholar]
- Getting S J, Christian H C, Lam C W, Gavins F N, Flower R J, Schioth H B, Perretti M. Redundancy of a functional melanocortin 1 receptor in the anti-inflammatory actions of melanocortin peptides: studies in the recessive yellow (e/e) mouse suggest an important role for melanocortin 3 receptor. J Immunol. 2003;170:3323–3330. doi: 10.4049/jimmunol.170.6.3323. [DOI] [PubMed] [Google Scholar]
- Maaser C, Kannengiesser K, Specht C, Lugering A, Brzoska T, Luger T A, Domschke W, Kucharzik T. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut. 2006;55:1415–1422. doi: 10.1136/gut.2005.083634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artuc M, Bohm M, Grutzkau A, Smorodchenko A, Zuberbier T, Luger T, Henz B M. Human mast cells in the neurohormonal network: expression of POMC, detection of precursor proteases, and evidence for IgE-dependent secretion of alpha-MSH. J Invest Dermatol. 2006;126:1976–1981. doi: 10.1038/sj.jid.5700318. [DOI] [PubMed] [Google Scholar]
- Lyons P D, Blalock J E. Pro-opiomelanocortin gene expression and protein processing in rat mononuclear leukocytes. J Neuroimmunol. 1997;78:47–56. doi: 10.1016/s0165-5728(97)00081-7. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Guarini S, Altavilla D, Squadrito G, Campo G M, Arlotta M, Quartarone C, Saitta A, Cucinotta D, Bazzani C, Cainazzo M M, Mioni C, Bertolini A, Caputi A P. Adrenocorticotropin reverses vascular dysfunction and protects against splanchnic artery occlusion shock. Br J Pharmacol. 1999;128:816–822. doi: 10.1038/sj.bjp.0702848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellacott K L, Murphy J G, Marks D L, Cone R D. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology. 2007;148:6186–6194. doi: 10.1210/en.2007-0699. [DOI] [PubMed] [Google Scholar]
- Butler A A, Cone R D. The melanocortin receptors: lessons from knockout models. Neuropeptides. 2002;36:77–84. doi: 10.1054/npep.2002.0890. [DOI] [PubMed] [Google Scholar]