Abstract
We describe here the implementation of a flash-photolysis system for time-resolved cryo-electron microscopy. A previously designed computer-controlled cryo-plunging apparatus (White et al., 2003) was used as a hardware platform, onto which a xenon flash lamp and liquid light pipe were mounted. The irradiation initiates a reaction through cleavage of the photolabile blocking group from a biologically active compound. The timespan between flashing and freezing in cryogen is on the order of milliseconds, and defines the fastest observable reaction. Blotting of excess fluid, which takes on the order of 1 sec, is done before irradiation and thus does not represent a rate-limiting step. A specimen-heating problem, identified by measurements with a thermocouple, was alleviated with the use of thick, aluminum-coated grids.
Introduction
The fundamental goal in any structural biological investigation is to understand the function of a biological complex from the study of the complex’s three-dimensional organization. If a static structure is already informative, then snapshots of the structure undergoing conformational changes should be far more so. Rapid freezing followed by cryo-electron microscopy (cryoEM) is one method by which to trap and visualize transient intermediate states of such a structure undergoing a conformational change.
Several methods exist by which a sample can be allowed to react for a given length of time before cryo-fixation and subsequent examination by cryoEM. Conceptually, the simplest method is similar to a stopped-flow experiment, in which the contents of two syringes are mixed (White et al., 1998; White et al., 2003); in this case there are the additional steps of depositing the mixture on an EM grid and then freezing it by plunging into cryogen. The primary disadvantage of this method is that the grid must be blotted prior to freezing. While freezing takes place on the millisecond timescale, blotting takes on the order of 1 sec, and thereby severely limits the accessible set of biologically interesting chemical reactions.
Another method to trap transient conformational intermediates is to spray droplets, using either an atomizer (Berriman & Unwin, 1994) or an electrospray device (White et al., 2003), of one reactant onto an aqueous film containing a second reactant. The primary advantage here is that the reactant on the grid will have been blotted prior to spraying, so that blotting does not serve as the rate-limiting step as it does in the stopped-flow method. Some disadvantages, however, are the lack of control of the many variables contributing to droplet size (humidity, ionic strength, detergent concentration, air pressure of the atomizer), the risks of physical alteration of the specimen (due to the high electric field associated with electrospray, or surface tension in droplets), and the incomplete and uneven nature of the mixing that occurs when the reactants in the droplet diffuse laterally from the point of contact on the grid.
A third method is to blot a grid with an aqueous solution containing one reactant with filter paper impregnated with a second reactant (Frederik & Sommerdijk, 2005). The question of what biological reactions can be studied using dry reactants has not yet been explored.
A fourth method uses light to trigger a reaction, by irradiating a light-sensitive macromolecule (Subramaniam et al., 1993) or by liberating a caged reactant so that it can react with a macromolecule of interest (Ménétret et al., 1991). As in the spraying methods, the grid can be blotted before the reaction occurs. An additional advantage, in contrast to the spraying methods, is that the two reactants are evenly mixed before the reaction begins. One obvious disadvantage of flash photolysis is that a caged analogue of the reactant of interest must be available. Nevertheless, caged analogues of many small biomolecules are commercially available and caged versions of peptides and proteins have been synthesized (Shigeri et al., 2002). The goal of the present work was to implement and characterize a flash-photolysis system for time-resolved cryoEM.
Methods and Results
Hardware setup
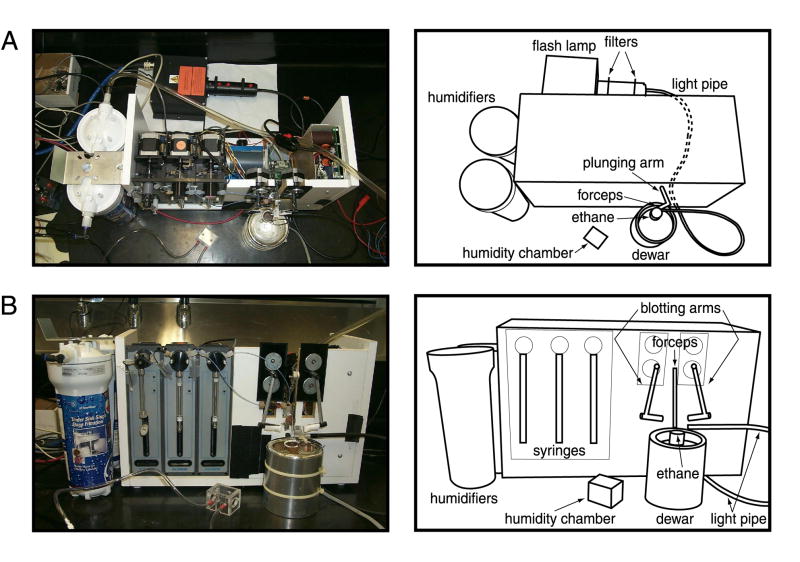
The hardware setup is depicted in Figure 1. The template platform was a computer-controlled time-resolved instrument designed by Howard White (White et al., 2003). This instrument can be operated in a stopped-flow or electrospray mode. The light source for photolysis was a xenon flash lamp (model JML-C1; Rapp OptoElectronic, Hamburg, Germany).
Figure 1. Experimental setup.
(A) Top view of the flash-photolysis apparatus. (B) Side view.
Two short-pass, UV-permissive filters (model 1UV-1H; Rapp OptoElectronic) were placed in series in the optical path, to reduce undesirable infrared wavelengths while passing the optimal wavelengths, 350 to 360 nm, for the caged compounds described below. Light was directed from the flash lamp to the sample via a 2-mm liquid light pipe (Rapp OptoElectronic). Light pulses were generated by a 3000 μF capacitor at 350 V. This configuration resulted in a pulse of approximately 24 mJ and 5 msec in duration, as measured with a JM20 energy meter (Rapp OptoElectronic) read out on a trace oscilloscope. Next to the plunger arm, an optical digital transistor was mounted; this transistor produces the signal that triggers the flash lamp when the grid passes the light pipe. The forceps can be paused for an arbitrary length of time –with the duration of the pause specified on the associated computer – before being plunged into the ethane. In the absence of a pause, the fastest observable reaction time is limited by the travel of the forceps, about 5 msec. For testing of photolysis yields, the flash lamp was triggered manually with the grid stationary, and the grid was not plunged.
Energy deposition
To determine the amount of energy delivered to a grid, we measured the amount of energy exiting the light pipe, both with and without EM grids in the light path. Energy was measured using the JM20 (Rapp OptoElectronic) energy meter. Given that much of the incident light passes through the mesh of a single grid, a stack of six grids with unaligned meshes was mounted on the forceps; inclusion of more grids in the stack did not eclipse more light. Grids were mounted on the forceps on the time-resolved apparatus, with the light pipe mounted 2 mm away. At this distance, approximately 75% of the light from the light pipe illuminated the grid and forceps (Table 1). Light exiting the light pipe is not well collimated, with an exit angle of up to about 35° (data not shown), so minimization of the distance to the grid is vital.
Table 1.
Energy deposition
| Configuration | Energy | Percent energy eclipsed |
|---|---|---|
| no forceps, no grid | 24 mJ | 0% |
| forceps, no grid | 22.5 mJ | 6.25% |
| forceps and grids | 4.5 mJ | 75.00% |
Energy deposition measured using energy meter (model JM20, Rapp OptoElectronic) for various grid configurations. See text for details.
Case study: caged calcium
A caged-calcium compound, DM-nitrophen (Calbiochem, Eugene, OR), was used at 0.8 mM concentration in a buffer of 20 mM MOPS (pH 7.4) and 0.5 M NaCl. Aliquots of caged compounds were enclosed in aluminum foil, but no other special precautions were taken. According to the manufacturer, caged compounds remain unphotolyzed under room light over a timespan of hours.
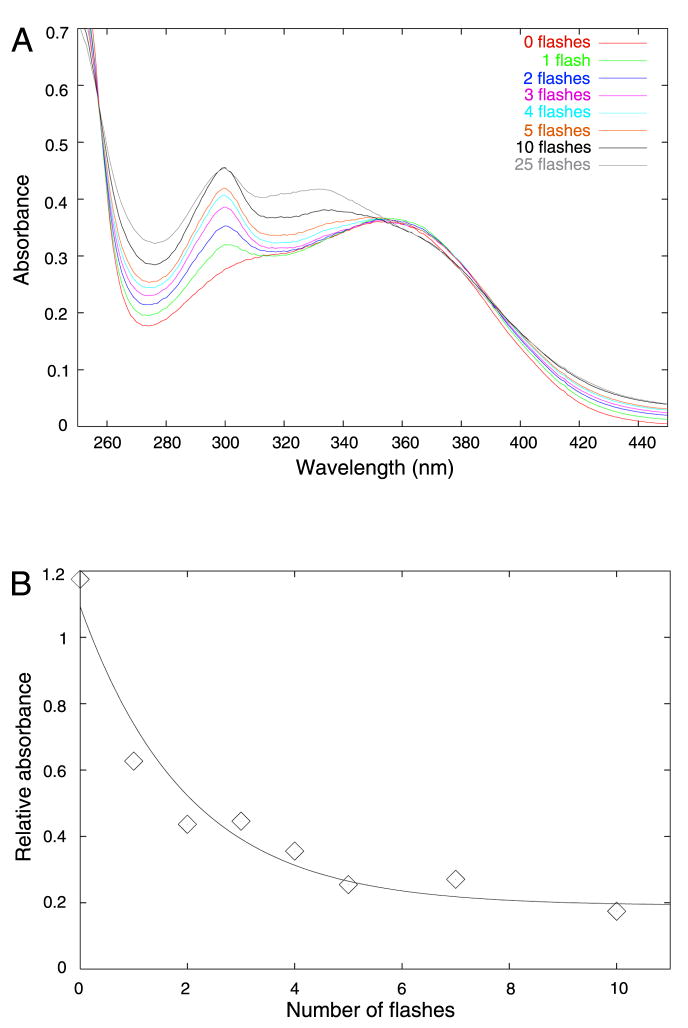
To measure the extent of photolysis, we assessed the effects of photolysis spectrophotometrically. DM-nitrophen was loaded with 10 mM CaCl2. 100-μL samples were placed in a microcuvette. DM-nitrophen undergoes a color change upon photolysis, from yellow to pink. If the contents of the cuvette were not mixed, only the material in the side of the cuvette nearest to the light pipe changed color. Since DM-nitrophen absorbs in the UV range strongly, with an extinction coefficient of 4200 M−1cm−1, light will be strongly attenuated over the 1-cm path length. Thus, when a cuvette was flashed multiple times, the solution was manually mixed in between flashes with a pipet. Spectra recorded after varying numbers of flashes are shown in Figure 2A. With an increasing number of flashes, an increase in absorbance at 300 nm was seen. There was no change at 300 nm from 10 to 25 flashes, indicating maximal photolysis. At greater than 10 flashes, an additional peak was seen at 335 nm, suggesting at least one additional product species. For a volume of 100 μL and path length of 1 cm, the extent of photolysis from a single flash was estimated to be about 10%.
Figure 2. Photolysis of DM-nitrophen.
(A) UV-visible spectra as a function of number of flashes. The concentration of DM-nitrophen used was 0.8 mM, loaded with 10mM CaCl2. Solutions were diluted 10X for measurement on a Perkin-Elmer DU-640 spectrophotometer. (B) Remaining unphotolyzed DM-nitrophen as a function of number of flashes. HPLC was performed using a Delta-Pak C18 column, eluted with a linear gradient, from 100% water to 100% acetonitrile over 15 min. Data were fitted to an exponential function using least-squares minimization.
Since the light of the flash is attenuated strongly over distance in a 1-cm cuvette, it is likely that the photolysis yield in a large volume underestimates the yield on an EM grid, where the aqueous film is much thinner. To test photolysis on small samples, we placed 5 μL of 1 mM DM-nitrophen solution, loaded with excess CaCl2, on an EM grid coated with a continuous carbon layer (0.03 inches in thickness). The humidity chamber was not used for these experiments. Enough molecules of DM-nitrophen in 5 μL (4 nmol) were present to permit spectrophotometric detection of a signal. However, since more than one photoproduct was evident, we used reversed-phase high-performance liquid chromatography (HPLC) to discriminate among the products. After flashing a grid with a 5-μL droplet a given number of times, we submerged the grid in 20 μL of buffer to increase the volume needed for an HPLC injection. We then injected 10 μL of the sample onto a Delta-Pak C18, 3.9×100 mm column. The solvent gradient was varied linearly from water to acetonitrile over 15 min. For use as an internal concentration standard, we included 5 mM AMP in DM-nitrophen solutions since such a component would not be photolyzed. To quantify remaining unphotolyzed DM-nitrophen, we normalized the absorbance at 352 nm, corresponding to the peak assigned to the starting product, to the absorbance at 280 nm assigned to AMP (Fig. 2B). After five flashes, nearly maximal photolysis was achieved. After manual fitting to an exponential curve, the proportion of DM-nitrophen photolyzed after a single flash was estimated to be 40% or higher. This yield is comparable to those achieved in physiological applications that use caged calcium (reviewed in Ellis-Davies, 2003).
Case study: caged GTP
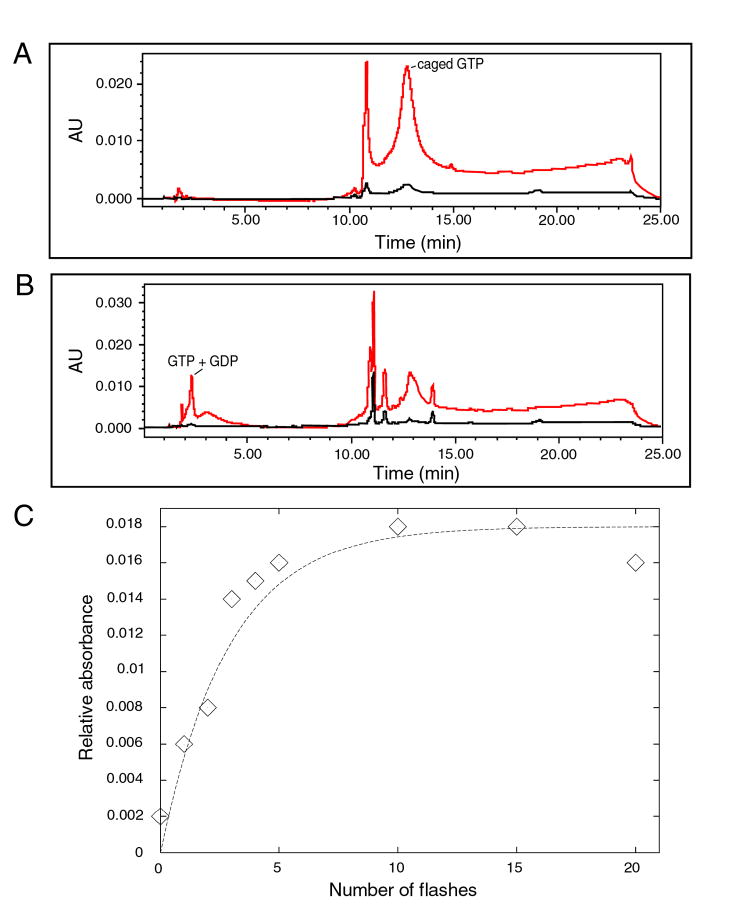
A second caged molecule that we tested was caged GTP (Calbiochem, Eugene, OR). We explored assays for the uncaging using a Delta-Pak C18 HPLC column after flashing 100 μL of solution 25 times in a cuvette. Caged GTP at 1 mM concentration was buffered in 20 mM Tris (pH 7.6), 100 mM NH4Cl, 10.5 mM magnesium acetate, and 0.5 mM EDTA. Photoproducts resulting from the flashes are indicated by peaks at short retention times (Figs. 3A–B), corresponding to liberated GTP and/or GDP (Schlichting et al., 1989). In addition to the GTP and GDP peaks, there was a peak at 11-min retention time representing absorption at longer wavelengths, with a maximum at 309 nm. This peak corresponded to nitrosoacetophenone, the protecting group of caged GTP. The OD309 of this peak was used to quantify photolysis (Fig. 3C). Upon least-squares fitting to an exponential function, photolysis was estimated to be 30% per flash, similar to the value found for another structural biological application (Schlichting et al., 1989).
Figure 3. Photolysis of caged GTP.
A Delta-Pak C18 column was eluted with a linear gradient from 100% water to 100% acetonitrile over 15 min. Absorbance at 280 nm is shown in red, and that at 309 nm is shown in black. (A) Unflashed. Caged GTP was detected at an elution time of 13 min at 280 nm. (B) Flashed three times. Liberated GTP was detected at an elution time of about 2 minutes at 280 nm. The protecting group, nitrosoacetophenone, eluted at 11 min (arrow) and was detected at 309 nm. (C) Absorbance at 309 nm as a function of number of flashes. Data were fitted to an exponential function using least-squares minimization.
Temperature jumps
The possibility must be considered that the heat from a flash of light can damage the biological molecules of interest, or can complicate their chemical behaviors. To determine the peak temperature rise upon flashing, we spot-welded a copper-constantan thermocouple to a copper EM grid (Siegel et al., 1994) and read out the values with a trace oscilloscope. The temperature rise upon flash illumination of a bare copper grid was 41°C. In order to promote reflectivity of UV light instead of absorption, we evaporated a layer of aluminum onto the grid. This layer reduced the subsequent temperature rise to 15°C. Evaporative cooling reduced the temperature rise by 1 to 2 °C, although the variation in temperature of a wet grid was erratic on the timescale of seconds.
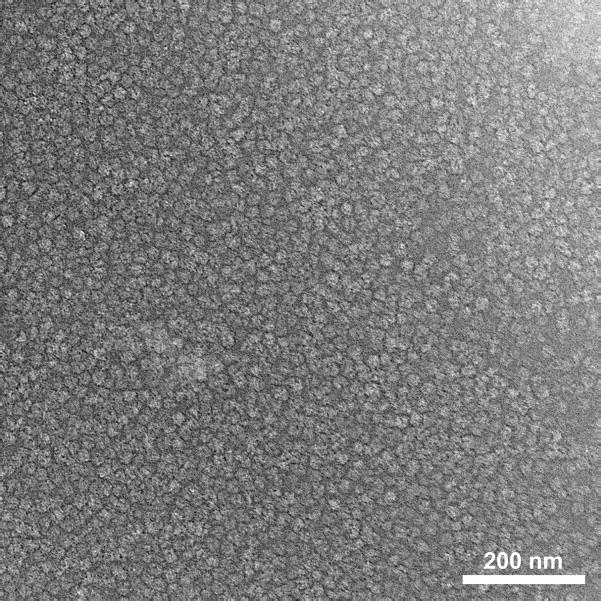
To further buffer the temperature rise, we increased the thermal mass of the grid by use of 50-μm thick, single-hole grids. (For comparison, a typical grid used for single-particle data collection is 20 μm thick.) In the absence of an aluminum coating, the temperature rise was 8°C. With a coating of aluminum, the temperature rise was reduced to 5°C. Since single-hole grids are not ideal for single-particle analysis, we ordered custom, 45-μm thick 300-mesh grids from Ladd Research (Williston, VT). These grids, after coating with aluminum, yielded a temperature rise of 7.7°C. Results are summarized in Table 2. Use of these thick grids permitted otherwise normal cryoEM imaging (Fig. 4).
Table 2.
Temperature rise upon photoflashing grids
| Grid thickness | No coating | Aluminum coated |
|---|---|---|
| 20 μm, 300 mesh | 43.6°C ± 5.0°C (n=24) | 6.9°C ± 0.5°C (n=18) |
| 50 μm, single hole | 15.6°C ± 1.3°C (n=27) | 4.8°C ± 0.3°C (n=18) |
| 45 μm, 300 mesh | NA | 7.7°C ± 0.4°C (n=6) |
Voltage readings from thermocouple were converted to degrees assuming 0.04mV/degree (CRC Handbook of Chemistry and Physics, 2007). Temperature rises listed are of the form average ± one standard deviation (n = number of measurements).
Figure 4. CryoEM of a flashed grid.
A 4 μL sample of volume of 70S ribosomes at 1.9 μM was applied to a 45 μm thick, 300-mesh grid, blotted for 3 sec, flashed, and plunged into liquid ethane without a programmed delay. CryoEM was performed on an FEI Tecnai F20 operating at 200kV, at 50,000X magnification, under low-dose conditions. Contrast is inverted, i.e., ribosomes are light on a dark background. Scale bar represents 200 nm.
Discussion
A flash-photolysis system to trap transient intermediate states of macromolecules circumvents some of the drawbacks of other methods for time-resolved cryoEM, such as stopped-flow analogs or microdroplet spraying. The stopped-flow method, as currently practiced, requires that the grid be blotted between initiation of the reaction and plunge-freezing. Conventional blotting takes ≥1 sec, thereby precluding the study of many interesting structural transitions that occur on a more rapid timescale in macromolecular complexes. In the microdroplet spraying method, the grid containing one reactant can be blotted before the spraying of a second reactant, so that the blotting does not represent the rate-limiting step. However, the microdroplet spraying method has certain limitations. For aerosol spraying (Berriman & Unwin, 1994), large amounts of the reactant to be sprayed are required, and the droplets are not evenly dispersed. Droplets from an electrospray device are potentially smaller and smaller quantities of material are required (White et al., 2003), but control of droplet size requires optimization of many parameters, including ionic strength, detergent concentration, and accelerating voltage. The spraying of one reactant onto another in general requires that the sprayed reactant diffuse laterally from the point of contact on the aqueous film. This spreading is fast but finite, and the concentration of reactant is nonuniform (Berriman & Unwin, 1994); methods to improve mixing two components and then spraying are currently under investigation. With the use of caged compounds, the two reactants can be present on the grid simultaneously before the reaction is initiated, thus avoiding the concentration gradients arising from the diffusion from droplets. Also, the solution containing the two reactants can be blotted before initiation of the reaction.
However, the use of caged compounds and flash photolysis has its own drawbacks. A pulse of light, even after filtration of long-wavelength radiation, will heat an EM grid. Under the conditions used here – 3000 μF capacitance and 350 V for the flash lamp, 2 mm distance between the light pipe and the grid – a bare copper grid showed a peak temperature rise of over 40°C upon flashing, similar to values measured previously (Siegel et al., 1994). By means of the combined use of a reflective aluminum coating and thicker grids, we were able to reduce the temperature rise to under 8°C. The extra thermal mass of the thick grids should not buffer the temperature reduction during plunge-freezing to the extent that that non-vitreous ice is produced (Fig. 4). In single-particle cryoEM preparations, a surface layer of only 100 nm needs to be vitrified. This vitrification depth should be easily attained, given that 10 to 20 μm of macroscopic, hydrated material can be vitrified in the course of sample-preparation for electron tomography (Handley et al., 1980), even at atmospheric pressure. As noted earlier, the thick, single-hole grids used for testing are not ideal for single-particle microscopy, so custom-made, 300-mesh grids were designed for use in actual, biological experiments.
In addition to thermal issues, there are practical concerns such as the availability and optimization of the geometric configuration and suitability of particular caged compounds (described below). For the optimization step, the distance between the light pipe and the grid proved crucial. Since light exiting the light pipe is not well collimated, intensity falls off quickly with distance. Indeed, while testing photolysis of DM-nitrophen, we found that, for a distance of 4 mm between the light pipe and the grid, only 10% was photolyzed in one flash (data not shown), under conditions where, at 2 mm distance, at least 40% had been photolyzed.
Among light sources alternative to the xenon flash tube used here are: UV lasers (reviewed in Rapp, 1998); a shuttered, continuous xenon lamp (Zucker, 1993); and UV light-emitting diodes (LEDs) (Bernardinelli et al., 2005). Lasers permit shorter pulses than does a xenon flash tube, and therefore their time resolution is superior. However, longer pulses can lead to multiple excitation events, with consequently higher photolysis yield (Ellis-Davies, 2003). Also, UV lasers are the most costly of the light sources listed here. For higher-intensity light sources, another issue is that the degree of grid heating increases. Continuous xenon sources, in contrast, offer longer pulses, but at lower power. UV-LEDs represent a relatively new technology, and cheaper than flash lamps or lasers, but the photolysis yield is low: Bernardinelli et al. (2005) found it to be no higher than 23% among four compounds tested. The choice of light source will thus involve a trade-off among the factors of power output, photolysis yield, grid heating, and monetary cost.
While a wide range of caged compounds – in particular, small molecules – is available (reviewed in Shigeri et al., 2001), certain limitations remain. For example, it is possible to cage proteins (Shigeri et al., 2001; Ellis-Davies, 2007), although this process may not be sufficient to block large macromolecules from reacting. Thus, caged compounds provide a powerful, but not necessarily widely applicable, exploratory tool. Macromolecules that undergo a conformational change in response to photolysis of a caged compound, and thus should be amenable to study by our method, include: the ryanodine receptor with caged calcium (Sharma et al., 2000; Györke and Fill, 1993); myosin, reacting with caged ATP (Ménétret et al., 1991); GroEL, reacting with caged ATP (von Germar et al., 1999); and the ribosome bound with elongation factors, reacting with caged GTP (Katunin et al., 2002; Limmer et al., 1992)
Ideally a caged compound will not interact with other reactants before photolysis. In practice however, this is not always the case. For example, GTP is required for certain reactions carried out by ribosomes; an obvious experiment would involve the use of caged GTP to study such reactions. We can anticipate that that some reactions will be complicated by the participation of factors such as with elongation-factor G, to which caged GTP binds even before hydrolysis (Katunin et al., 2002). Since photolysis efficiencies never attain 100%, there will be a subset of ribosomes that will be blocked from reacting with the free GTP that is produced upon flashing. A photolysis efficiency of 30% was determined from spectrophotometry of a 5-μL droplet, which has a macroscopic thickness. While we found that UV penetration was attenuated much more strongly across a 1-cm path length in a cuvette than in a 5-μL droplet, it is unlikely that a grid blotted to an aqueous film thickness of 100 to 200 nm will show superior photolysis to 5-μL droplet, given that previous flash-photolysis experiments using pooled, blotted grids showed a similar yield (Ménétret et al., 1991). This incomplete photolysis will contribute heterogeneity to the sample, and will add yet more complexity to subsequent image-processing and classification steps.
Conclusion
We describe here an implementation of a flash-photolysis system for cryoelectron microscopy, with the purpose of trapping transient intermediate states of macromolecules undergoing conformational change, with fine time resolution. While the application of the method may be relatively restricted, we can readily identify a number of biological macromolecules for which this method should yield valuable new information on structural transitions.
Acknowledgments
The authors would like to thank Howard White for assistance regarding the time-resolved instrument, Gert Rapp for advice about the configuration of the flash lamp, David Siegel for helpful discussion about grid heating, Jon Berkowitz for providing lacy carbon films, Ingrid Hahn for providing 70S ribosomes, Michael Watters for help with illustrations, and the Wadsworth Center Biochemistry Core for assistance with spectrophotometry and HPLC. Supported by NIH Biotechnological Resource grant RR01219.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernardinelli Y, Haeberli C, Chatton JY. Flash photolysis using a light emitting diode: an efficient, compact, and affordable solution. Cell Calcium. 2005;37:565–72. doi: 10.1016/j.ceca.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Berriman J, Unwin N. Analysis of transient structures by cryo-microscopy combined with rapid mixing of spray droplets. Ultramicroscopy. 1994;56:241–252. doi: 10.1016/0304-3991(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Lide DR, editor. CRC Handbook of Chemistry and Physics. CRC Handbook of Chemistry and Physics, 2007. CRC Press; 2007. p. 88. [Google Scholar]
- Ellis-Davies GC. Development and application of caged calcium. Methods Enzymol. 2003;360:226–238. doi: 10.1016/s0076-6879(03)60112-6. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies GC. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederik PM, Sommerdijk N. Spatial and temporal resolution in cryo-electron microscopy — A scope for nano-chemistry. Curr Opinion in Colloid & Interface Sci. 2005;10:245–249. [Google Scholar]
- Györke S Fill M. Ryanodine receptor adaptation: control mechanism of Ca2+-induced Ca2+ release in heart. Science. 1993;260:807–9. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Handley DA, Alexander JT, Chien S. The design and use of a simple device for rapid quench-freezing of biological samples. J Microsc. 1980;121:273–282. doi: 10.1111/j.1365-2818.1981.tb01224.x. [DOI] [PubMed] [Google Scholar]
- Kaplan JH, Ellis-Davies GC. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988;85:6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunin VI, Savelsbergh A, Rodnina MV, Wintermeyer W. Coupling of GTP hydrolysis by elongation factor G to translocation and factor recycling on the ribosome. Biochemistry. 2002;41:12806–12812. doi: 10.1021/bi0264871. [DOI] [PubMed] [Google Scholar]
- Limmer S, Reiser CO, Schirmer NK, Grillenbeck NW, Sprinzl M. Nucleotide binding and GTP hydrolysis by elongation factor Tu from Thermus thermophilus as monitored by proton NMR. Biochemistry. 1992;31:2970–7. doi: 10.1021/bi00126a018. [DOI] [PubMed] [Google Scholar]
- Ménétret JF, Hofmann W, Schroder RR, Rapp G, Goody RS. Time-resolved cryo-electron microscopic study of the dissociation of actomyosin induced by photolysis of photolabile nucleotides. J Mol Biol. 1991;219:139–144. doi: 10.1016/0022-2836(91)90554-j. [DOI] [PubMed] [Google Scholar]
- Rapp G. Flash lamp-based irradiation of caged compounds. Methods Enzymol. 1998;291:202–22. doi: 10.1016/s0076-6879(98)91014-x. [DOI] [PubMed] [Google Scholar]
- Schlichting I, Rapp G, John J, Wittinghofer A, Pai EF, Goody RS. Biochemical and crystallographic characterization of a complex of c-Ha-ras p21 and caged GTP with flash photolysis. Proc Natl Acad Sci U S A. 1989;86:7687–7690. doi: 10.1073/pnas.86.20.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MR, Jeyakumar LH, Fleischer S, Wagenknecht T. Three-dimensional structure of ryanodine receptor isoform three in two conformational states as visualized by cryo-electron microscopy. J Biol Chem. 2000;275:9485–91. doi: 10.1074/jbc.275.13.9485. [DOI] [PubMed] [Google Scholar]
- Shigeri Y, Tatsu Y, Yumoto N. Synthesis and application of caged peptides and proteins. Pharmacol Ther. 2001;91:85–92. doi: 10.1016/s0163-7258(01)00148-6. [DOI] [PubMed] [Google Scholar]
- Siegel DP, Green WJ, Talmon Y. The mechanism of lamellar-to-inverted hexagonal phase transitions: A study using temperature-jump cryo-electron microscopy. Biophys J. 1994;66:402–414. doi: 10.1016/s0006-3495(94)80790-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Gerstein M, Oesterhelt D, Henderson R. Electron diffraction analysis of structural changes in the photocycle of bacteriorhodopsin. EMBO J. 1993;12:1–8. doi: 10.1002/j.1460-2075.1993.tb05625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Germar F, Galán A, Llorca O, Carrascosa JL, Valpuesta JM, Mäntele W, Muga A. Conformational changes generated in GroEL during ATP hydrolysis as seen by time-resolved infrared spectroscopy. J Biol Chem. 1999;274:5508–13. doi: 10.1074/jbc.274.9.5508. [DOI] [PubMed] [Google Scholar]
- White HD, Walker ML, Trinick J. A computer-controlled spraying-freezing apparatus for millisecond time-resolution electron cryomicroscopy. J Struct Biol. 1998;121:306–313. doi: 10.1006/jsbi.1998.3968. [DOI] [PubMed] [Google Scholar]
- White HD, Thirumurugan K, Walker ML, Trinick J. A second generation apparatus for time-resolved electron cryo-microscopy using stepper motors and electrospray. J Struct Biol. 2003;144:246–252. doi: 10.1016/j.jsb.2003.09.027. [DOI] [PubMed] [Google Scholar]
- Zucker RS. The calcium concentration clamp: spikes and reversible pulses using the photolabile chelator DM-nitrophen. Cell Calcium. 1993;14:87–100. doi: 10.1016/0143-4160(93)90079-l. [DOI] [PubMed] [Google Scholar]