Summary
The establishment of functional neural circuits requires the guidance of axons in response to the actions of secreted and cell-surface molecules such as the semaphorins and their neuropilin and plexin receptors. Semaphorin 3E, acting through its receptor PlexinD1, elicits repulsion in non-neuronal systems. Sema3E and PlexinD1 are expressed in the brain but their functions are unknown. Here, we show that Sema3E/PlexinD1 signaling plays an important role in initial development of descending axon tracts in the forebrain. Early errors in axonal projections are reflected in behavioral deficits in Sema3E null-mutant mice. Two distinct signaling mechanisms can be distinguished downstream of Sema3E. Some corticofugal and striatonigral axons express PlexinD1 but not neuropilin-1, and for these neurons Sema3E acts as a repellent. In contrast, subiculo-mammillary neurons co-express neuropilin-1 and PlexinD1, and for these Sema3E acts as an attractant. The extracellular domain of neuropilin-1 is sufficient to convert repulsive signaling by PlexinD1 to attraction. Our data therefore reveal a novel “gating” function of neuropilins in semaphorin-plexin signaling during the assembly of forebrain neuronal circuits.
Keywords: axon guidance, semaphorin, plexin, neuropilin, brain development
Introduction
The guidance of axons toward their targets is a key early step in the establishment of functional neural circuits. Axonal growth cones respond to long- and short-range signals that can be either repulsive or attractive (Tessier-Lavigne and Goodman, 1996; Chilton, 2006; Wen and Zheng, 2006), as well as to factors that accelerate or inhibit the rate of axonal extension (Filbin, 2006). Understanding how different classes of guidance molecules interact to signal repulsion and attraction remains a major challenge in developmental neuroscience. Most approaches to this question have studied the response of individual growth cones to guidance signals in vitro. In cultured neurons, repulsion by a given guidance cue can be switched to attraction by the modulation of cyclic nucleotide and Ca2+ levels, or by the expression of different combinations of receptor components (Song et al., 1998; Henley and Poo, 2004; Hong et al., 1999). However, it remains to be determined whether such mechanisms play a role in vivo during development of the vertebrate nervous system.
The semaphorins constitute a diverse family of axonal guidance molecules that share a common Sema domain (Yazdani and Terman, 2006). Semaphorins have generally been considered as repellents or inhibitors of axon growth, but there is emerging evidence that they can play attractive or growth-promoting roles (Pasterkamp et al., 2003; Bagnard et al., 1998; Kantor et al., 2004; Gonthier et al., 2006). Bifunctional attractive and repulsive signaling by semaphorins has also been reported in vivo (Dalpe et al., 2004; Wolman et al., 2004; Falk et al., 2005), but in no case has the underlying molecular mechanism been elucidated. Vertebrate class 3 semaphorins (Sema3A to Sema3G) are secreted proteins that play important roles in nervous system development (Kruger et al., 2005, Mann et al., 2007). Their receptor complexes generally comprise neuropilins as ligand-binding components and plexins as signal-transducing components (Bagri and Tessier-Lavigne, 2002; Tamagnone et al., 1999).
Among class 3 semaphorins, Sema3E is unusual in that it does not interact directly with neuropilins but instead binds directly, and with high affinity, to PlexinD1 (Gu et al., 2005). In vivo, Sema3E/PlexinD1 signaling controls blood vessel patterning by triggering a repulsive response (Gu et al., 2005). Both Sema3E (Chedotal et al., 1998; Christensen et al., 1998; Miyazaki et al., 1999b; Miyazaki et al., 1999c; Cohen et al., 2005; Watakabe et al., 2006) and PlexinD1 (Cheng et al., 2001; Gesemann et al. 2001; van der Zwaag et al., 2002; Chen et al., 2005; Vilz et al., 2005; Mauti et al., 2006; Watakabe et al., 2006) are expressed in the nervous system. However, the contribution of either Sema3E or PlexinD1 to brain development remains unknown.
The mammalian forebrain is one tissue in which semaphorin receptor components, including PlexinD1 and neuropilins, show complex expression patterns (Skaliora et al., 1998; Cheng et al., 2001; Worzfeld et al., 2004). In the forebrain, axons in descending pathways follow complex trajectories to reach lower brain centers. These include the corticofugal and striatonigral tracts (Fig. 1A), which grow through the cerebral peduncle to innervate distinct targets at the midbrain, brain stem and spinal cord levels (Joosten et al., 1987; Martin, 2005; Fishell and Van der Kooy, 1987). A second pathway, the subiculo-mammillary tract (Fig. 2A), is the major output structure of the hippocampal formation and projects through the postcommissural fornix to the mammillary bodies in the caudal hypothalamus (Swanson and Cowan, 1977; Kishi et al., 2000; Krout et al., 2002). Although not all milestones in axonal growth along these pathways have been precisely mapped, developmental growth through the brain occurs in the period from E15 (at which axonal growth begins) to early postnatal stages (at which striatal and subicular axons have reached their targets, and corticospinal axons have left the brain to enter the spinal cord). The signals that guide descending axons through the forebrain during embryonic development remain poorly understood.
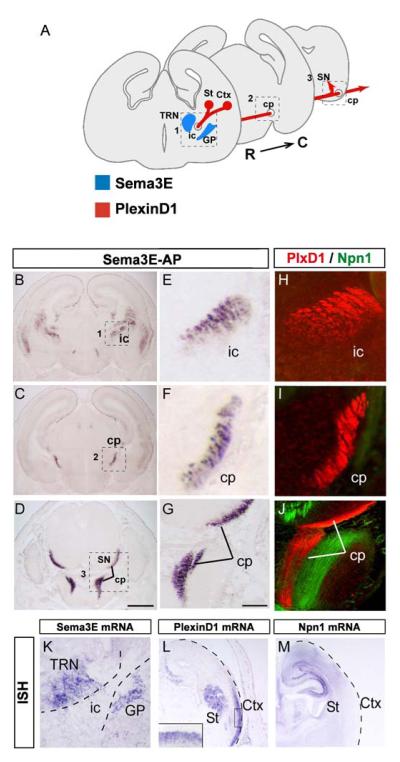
Fig. 1. Binding sites for Sema3E along the corticofugal and striatonigral tracts.
(A) Schematic representation of the path taken by corticofugal and striatonigral axons from the ventrolateral cortex (Ctx) and striatum (St), respectively, through the internal capsule (ic) and the cerebral peduncles (cp). At the midbrain level, striatal axons leave the cerebral peduncles and terminate in the substantia nigra (SN), whereas cortical axons continue into the pons.
(B-G) Localization of Sema3E binding sites along the corticofugal and striatonigral projections on coronal sections of wild-type brain at E17.5. (B-D) Binding of Sema3E-AP is apparent in the internal capsule (ic in B) and cerebral peduncles (cp in C, D). (E-G) Higher magnifications of the areas in boxes 1-3 in B-D.
(H-J) Immunolabeling of the candidate receptor components PlexinD1 (red) and Npn-1 (green) on coronal sections of wild-type brain at E17.5. High-magnification views at different levels of the corticofugal and striatonigral pathways (ic in H, cp in I, J) show that PlexinD1 is expressed throughout the corticofugal and striatonigral projections but that no co-expression of Npn-1 can be detected on these axons. At brainstem level, Npn-1 is expressed in an unidentified fiber tract adjacent to the corticofugal axons (I).Apparent interruption of the cerebral peduncles corresponds to a level at which corticofugal axons are out of the plane of section.
(K-M) Coronal sections of E17.5 wild-type mouse brain were taken through the globus pallidus and thalamic reticular nucleus (K) and through the left hemisphere (L, M). They were hybridized with probes for: Sema3E (K), PlexinD1 (L) and Npn-1 (M). Strong signal for Sema3E can be seen in the globus pallidus (GP) and thalamic reticular nucleus (TRN) between which corticofugal and striatonigral axons must pass (box 1 in panel A). PlexinD1 mRNA is expressed ventrolateral regions of the cortex, whereas Npn-1 mRNA shows complementary expression in dorsomedial regions of the cortex. PlexinD1 mRNA, but not Npn-1 mRNA, is expressed in the striatum. Inset panel in L show high-magnification view of staining in the cortical plate of the ventrolateral cortex.
ISH: in situ hybridization. Scale bars: 650 μm (A-D), 100 μm (E-K), 50 μm (L-M).
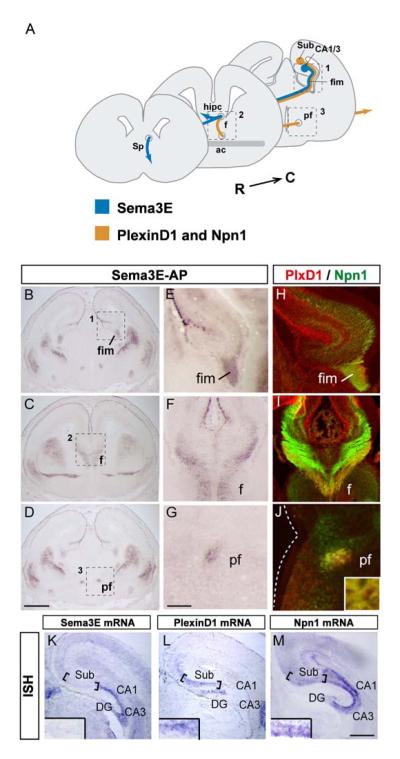
Fig. 2. Binding sites for Sema3E along the subiculo-mammillary tract.
(A) Schematic representation of the pathways taken by subiculo-mammillary projections, from the subiculum (Sub), through the fimbria (fim), fornix (F) and postcommissural fornix (PF). The target areas in the caudal hypothalamus are more caudal and are not shown on the scheme.
(B-G) Localization of Sema3E binding partners along the subiculo-mammillary tract on coronal sections of wild-type brain at E17.5. (B-D) Binding of Sema3E-AP is apparent in the fimbria (fim in B), fornix (F in C) and postcommissural fornix (PF in D). (E-G) Higher magnifications of the areas boxed in B-D.
(H-J) Immunolabeling of the candidate receptor components PlexinD1 (red) and Npn-1 (green) on coronal sections of wild-type brain at E17.5. High-magnification views of immunostaining at different levels of the subiculo-mammillary body tract (fim in H, F in I and PF in J) show that PlexinD1 is co-localized with Npn-1 along the full length of the subiculo-mammillary tract. Inset panel in J shows overlap in individual axonal fascicles.
(K-M) Coronal sections of wild-type brain at E17.5 were taken through the hippocampal formation. Sections were hybridized with probes for: Sema3E (K), PlexinD1 (L) and Npn-1 (M). Note that the pyramidal layers in the subiculum co-express PlexinD1 together with Npn-1. Sema3E is expressed in CA1 and CA3 pyramidal neurons adjacent to the subiculum. Inset panels in K, L, M show high-magnification views of staining in pyramidal layer of the subiculum.
ac: anterior commissure, CA1 and CA3: cornus ammonis 1 and 3, DG: dentate gyrus, fr: fasciculus retroflexus, hipc: hippocampal commissure, ISH: in situ hybridization, Sp: septum. Scale bars: 650 μm (A-D), 250 μm (K-M), 100 μm (E-J).
We have examined the possibility that the differential expression and signaling functions of plexins and neuropilins determine the repulsive or attractive response of growing axons to individual semaphorins, focusing on the role of Sema3E/PlexinD1 in development of the descending forebrain pathways. We find that Sema3E can act as a bifunctional ligand in vivo, depending on the composition of the receptor complex. For sub-populations of corticofugal and striatonigral neurons, which express PlexinD1 but not neuropilins, Sema3E acts as a repellent. In contrast, in subiculo-mammillary neurons, the presence of receptor complexes involving Npn-1 in addition to PlexinD1 switches the Sema3E signal from repulsion to attraction and/or stimulation of axonal growth. Strikingly, the exposure of neurons to the extracellular domain of Npn-1 alone is sufficient to trigger the switch from repulsion to attraction in vitro. At adult stages, mice deficient for Sema3E show behavioral defects that are consistent with the persistent perturbation of the subiculo-mammillary tract. Taken together, our data reveal a novel gating function for neuropilins in semaphorin-plexin signaling, and reveal that the differential deployment of plexin and neuropilin receptors has a critical role in vivo in shaping the development of major descending forebrain axonal projections.
Results
PlexinD1 is expressed by growing axons in two major descending pathways
To visualize potential neuronal targets of Sema3E activity, we analyzed the binding of Sema3E-alkaline phosphatase (Sema3E-AP) fusion protein to sections of mouse brain at E17.5, when major descending nerve tracts have begun to form (Figs. 1, 2). Sema3E-AP delineated two axonal pathways. A first pathway — through the internal capsule (ic; Fig. 1B, E) and the cerebral peduncle (cp; Fig. 1C, D, F, G) — marks a trajectory common to corticofugal and striatonigral projections (Fig. 1A). A second Sema3E-AP-delineated pathway - through the fimbria (fim; Fig. 2B, E), the fornix (f; Fig. 2C, F) and the post-commissural fornix (pf; Fig. 2 D, G) — marks the trajectory of the subiculo-mammillary tract (Fig. 2A).
In the vascular system, the principal functional receptor for Sema3E is PlexinD1 (Gu et al., 2005). No Sema3E-AP binding could be detected using sections of PlexinD1-/- embryonic brain (Suppl. Fig. 1U, V), demonstrating that PlexinD1 is also the predominant binding partner for Sema3E in the brain. PlexinD1 mRNA was detected in the ventrolateral regions of the cortex (including the piriform, perirhinal and insular cortices), in the striatum and in the pyramidal layer of the subiculum (Fig. 1L, 2L; Suppl. Fig. 2) during the formation of the descending pathways in the forebrain (at E15.5-E17.5). Moreover, PlexinD1 protein was detected along the length of axons in the cerebral peduncle and post-commissural fornix (Fig. 1H-J, 2H-J; Suppl. Fig. 3). Anterograde DiI tracing from the ventrolateral cortex, striatum and hippocampal formation at E17.5 labeled the same axon tracts as did Sema3E-AP binding (Suppl. Fig. 1) and PlexinD1 antibody (Fig. 1 H-J, Fig. 2 H-J), confirming the identity of the pathways identified.
We therefore focused our analysis on the role of Sema3E in controlling the growth and trajectory of axons along these two descending forebrain pathways.
Correlation between Npn-1 expression and differing growth responses to Sema3E in vivo
To understand how Sema3E influences the growth of these forebrain projections, we first identified potential sources of Sema3E in proximity to growing axons in each pathway (E15.5-P2). At each stage examined, the highest levels of Sema3E expression were detected in the globus pallidus and the thalamic reticular nucleus, identified by their expression of Er81 and Dlx-1 homeogenes, respectively (Fig. 1K; Suppl. Fig. 2A-C; Jones and Rubenstein, 1994; Sussel et al., 1999). These nuclei surround the internal capsule, through which corticofugal and striatonigral fibers pass on their way to the cerebral peduncle, and thus could repel and channel axons at this decision point (Fig. 1A). Along the subiculo-mammillary pathway, Sema3E expression was detected only in the pyramidal cell layers in the CA1 and CA3 fields of the hippocampus, adjacent to the subiculum (Fig. 2K; Suppl. Fig. 2M-O; Chedotal et al., 1998; Miyazaki et al., 1999b). Efferent axons from CA3 and CA1 regions could act as sources of Sema3E, since they project along the same initial pathway as subiculo-mammillary axons (Fig. 2A). No other sites of Sema3E expression were observed along the pathway of hippocampal projections, at any stage examined.
Thus, axons in both sets of descending pathways pass close to cells that express Sema3E. Corticofugal and striatonigral axons avoid potential sources of Sema3E by growing between them (see scheme in Fig. 1A) whereas subiculo-mammillary axons grow alongside the axons of neurons that express Sema3E (see scheme in Fig. 2A). A possible explanation for the different growth behaviors of these classes of neurons in vivo is that neurons in each pathway express different receptor complexes for Sema3E, involving receptor components in addition to PlexinD1. Neuropilin-1 (Npn-1) is required for Sema3E activity in some neurons (Miyazaki et al., 1999b) and so we monitored its expression in each neuronal population (E15.5-P2). In the cortex, no Npn-1 was detected in PlexinD1-expressing ventrolateral regions of the cortex; and expression of Npn-1 was restricted to more dorsal areas of the cortex (Fig. 1M; Suppl. Fig. 2G-I). Npn-1 was also absent from the striatum (Fig.1M; Suppl. Fig. 2G-I). Furthermore, Npn-1 protein was not detected on PlexinD1-positive axons of either the corticofugal or striatonigral tracts (Fig. 1H-J; Suppl. Fig. 3), although it was clearly expressed in unidentified adjacent tracts in the pons (Fig. 1J). In contrast, Npn-1 was observed in the subiculum, overlapping with the domain of PlexinD1 expression (Fig. 2M; Suppl. Fig. 2S-U), and Npn-1 protein was expressed along the full length of the subiculo-mammillary tract (Fig. 2H-J; Suppl. Fig. 3). Moreover, PlexinD1 and Npn-1 were co-expressed by individual subicular axon fascicles within the postcommissural fornix (Fig. 2J).
Together, these findings reveal that forebrain neurons exhibit two contrasting patterns of semaphorin ligand-receptor expression. A sub-population of corticofugal axons and striatonigral axons express PlexinD1 but not Npn-1 and evade sources of Sema3E surrounding the internal capsule. In contrast, subiculo-mammillary axons express both PlexinD1 and Npn-1, and grow together with axons of CA1 and CA3 neurons that express Sema3E (schemes in Figs. 1A, 2A).
Differential axonal responses to loss of Sema3E in vivo
We next examined the role of Sema3E and PlexinD1 in the development of descending forebrain axonal projections by comparing axon trajectories in wild-type, Sema3E-/- and PlexinD1-/- mice. The gross morphology of the internal capsule labeled with Sema3E-AP was very similar in E17.5 wild-type and Sema3E-/- embryos (Fig. 3E, F). However, in all mutant embryos, ectopic axon fascicles labeled by Sema3E-AP projected through the thalamic reticular nucleus (Fig. 3F) and terminated abnormally in the dorsal midbrain (Fig. 3G, H; scheme in Fig. 3B; Suppl. Fig. 9; pattern observed in 5/5 Sema3E-/- and 0/7 wild-type littermate embryos). In the absence of specific markers, it was not possible to distinguish whether the ectopic fibers reflected misguidance of corticofugal or striatonigral axons, or both.
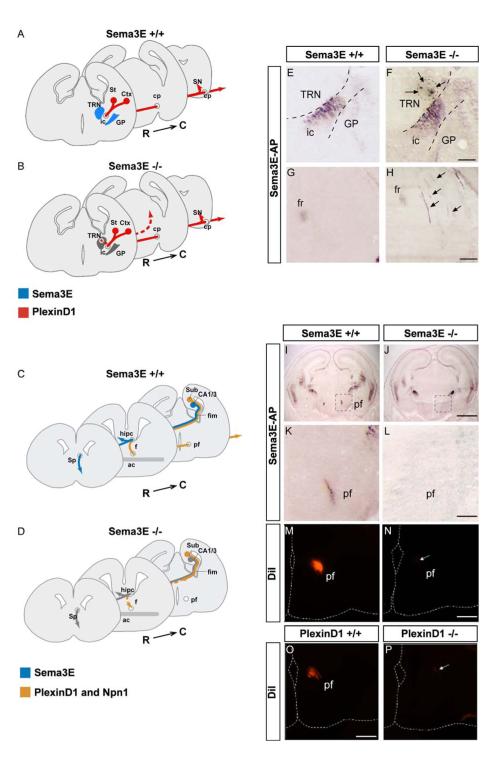
Fig. 3. Opposite effects of Sema3E inactivation on descending pathways correlate with presence or absence of Npn-1.
(A-D) Schemes representing models for Sema3E function in the development of descending forebrain projections. (A, B) Corticofugal and striatonigral projections (red) express PlexinD1 but not Npn-1. Sema3E (blue) is expressed at high levels in the reticular nucleus of the thalamus (TRN), which lies on the dorsal side of the internal capsule (ic) and in the globus pallidus (GP), ventral to the internal capsule. In Sema3E null embryos (gray), some axons depart from their normal trajectory and grow through the reticular nucleus to terminate ectopically in the dorsal midbrain. This suggests that in this system, Sema3E serves as a repulsive signal for growing axons. (C, D) Subiculo-mammillary axons (orange) express both PlexinD1 and Npn-1. Sema3E is expressed by pyramidal neurons of the hippocampal CA1 field (blue) and therefore is likely to be present along the full length of CA1 efferent projections adjacent to subiculo-mammillary axons in the fimbria and fornix. In the absence of Sema3E (gray), the development of subicular projections is massively delayed and only few fibers reach their target in the hypothalamus. This suggests that in this system, Sema3E serves as an attractive and/or growth-promoting signal for growing subicular axons.
(E, F) Sema3E-AP binding to the internal capsule of E17.5 wild-type (E) and Sema3E null (F) embryos. In mutant embryos, a fraction of labeled fibers defasciculates from the internal capsule and grows through the reticular nucleus of the thalamus (arrows). (G, H) Misguided axons terminate ectopically in the dorsal midbrain of Sema3E-/- mutant embryos (arrows). High-magnification views of Sema3E-AP binding in the dorsal midbrain of wild-type (G) and Sema3E-/- (H) embryos.
(I-L) Coronal sections of E17.5 mouse brain showing Sema3E-AP binding to the post-commissural fornix (PF) in wild-type (I, K) but not Sema3E-/- (J, L) embryos. K and L are magnified views of regions of the hypothalamus corresponding to boxed areas in I and J, respectively. (M, N) Coronal sections through the caudal hypothalamus of E17.5 control (M) and Sema3E-/- mutant (N) brains after anterograde DiI tracing of the fornix from the hippocampal formation. Very few labeled axons can be observed in the postcommissural fornix of Sema3E null embryos (arrow).
(O, P) PlexinD1 null mutants phenocopy Sema3E-/- embryos. Subiculo-mammillary body projections in E17.5 wild-type (O) and PlexinD1-/- (P) brains were traced by DiI injection in the hippocampal formation. Only a small number of labeled axons was observed in the caudal hypothalamus of PlexinD1 null embryos (arrow).
ac: anterior commissure, cp: cerebral peduncles, Ctx: Cortex; CA1 and CA3: cornus ammonis 1 and 3, f: fornix, fim: fimbriae, fr: fasciculus retroflexus, GP: globus pallidus, hipc: hippocampal commissure, ic: internal capsule, pf: postcommissural fornix, R->C, rostrocaudal axis, SN: substantia nigra, Sp: septum, St: striatum, Sub: subiculum, TRN, thalamic reticular nucleus. Scale bars: 650 μm (I, J), 120 μm (K-P), 100 μm (E-H).
Along the subiculo-mammillary pathway of Sema3E-/- embryos, no Sema3E-AP binding was detected in the region of the postcommissural fornix at E17.5 (Fig. 3I-L; pattern observed in 5/5 Sema3E-/- and 0/7 wild-type littermate embryos), although the number of subicular neurons was unchanged (Suppl. Fig. 4). This observation suggests that subicular axons fail to project along the subiculo-mammillary tract in the absence of Sema3E. To assess this possibility, we performed anterograde DiI tracing from the dorsal hippocampus at E17.5. In Sema3E-/- mutants the postcommissural fornix contained few, if any, labeled axons, whereas it was robustly labeled in wild-type controls (Fig. 3M, N; phenotype observed in 6/6 mutant and 0/4 wild-type embryos). As a control to determine whether the phenotype in Sema3E-/- embryos was specific to subicular axons, we examined the precommissural fornix and hippocampal commissure, through which CA1 and CA3 efferents project (Fig. 3C), and found that labeling was normal (data not shown). The absence of labeled axons in the postcommissural fornix raised the possibility that subicular neurons instead innervated ectopic targets. Along the proximal subiculo-mammillary tract it was not possible to distinguish these fibers from CA1 projections, which are also potentially labeled by DiI injection. However, no ectopic DiI-labeled axons were detected distal to the precommissural fornix in mutant embryos.
To examine whether Sema3E acts through PlexinD1 to control axonal growth, we examined PlexinD1-/- embryos at E17.5. Strikingly, anterograde DiI tracing of fibers projecting from the dorsal hippocampus revealed that, as in PlexinD1-/- mutants, very few axons reached the postcommissural fornix (Fig. 3O, P; phenotype observed in 3/3 PlexinD1-/-, 0/1 wild-type and 0/2 heterozygote embryos). In the absence of specific markers, other than PlexinD1, for cortical and striatal efferents that may be affected in PlexinD1-/- mutants, the characterization of these pathways in the mutant embryos is not currently feasible.
Thus, in the absence of Sema3E signaling, some corticofugal and/or striatonigral axons, which are normally routed through the internal capsule, grow instead into the thalamic reticular nucleus and dorsal midbrain, regions from which they are normally excluded (scheme in Fig. 3B). In contrast, subiculo-mammillary axons in Sema3E/PlexinD1 mutant embryos showed reduced growth towards the postcommissural fornix (scheme in Fig. 3D). One explanation for these findings would be that Sema3E is normally repulsive for corticofugal and/or striatonigral axons but attractive and/or growth-promoting for subiculo-mammillary axons, a possibility we test below.
Adult Sema3E null mice show behavioral defects reflecting decreased anxiety and memory impairment
We also asked whether the perturbations observed in Sema3E-/- embryos persist at later stages. Sections of internal capsule from wild-type and mutant pups at P4 were incubated with Sema3E-AP (Fig. 4A, B; Table 1). The exuberant projections of axons into the thalamic reticular nucleus of Sema3E-/-embryos could no longer be detected at P4, suggesting that the defect in corticofugal and/or striatonigral projections had been corrected by elimination of the ectopic axons. In contrast, neurofilament immunostaining on coronal brain sections of P30 Sema3E-/- mice revealed a subiculo-mammillary tract that, as in mutant embryos, was abnormally thin (Fig. 4C, D; observed in 3/3 mutant and 0/3 wild-type animals). The persistence of this axonal project defect provided an opportunity to determine whether Sema3E inactivation affected adult behavior.
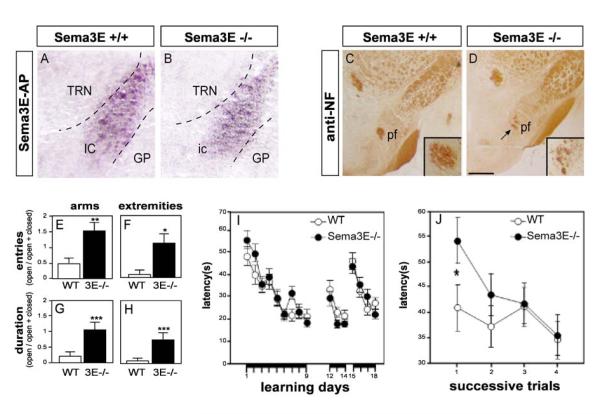
Fig. 4. Adult Sema3E null-mutant mice show behavior consistent with mammillary body denervation.
(A, B) Fibers defasciculating from the internal capsule are no longer detected at postnatal stages. Sema3E-AP binding to the internal capsule of P4 wild-type (A) and Sema3E null (B) mice. (C, D), Reduced size of the postcommissural fornix in adult Sema3E-/- mice (D) as compared to wild-type animals (C). Anti-neurofilament staining of coronal sections of mouse brain at postnatal day 30. (E-H) Anxiety levels in Sema3E-/- mice assessed using the elevated plus maze. Results were quantified using an index which takes into account the number of entries (E, F) or duration (G, H) in each category of arm (E, G) and extremities of arms (F, H) as the ratio open arms/(open + closed arms). Data are presented as mean ± s.e.m. As compared to wild-type mice (open bars), Sema3E-/- mice (black bars) made more frequent visits to open arms than to closed arms (E), and to their extremities (F). They also spent significantly more time in open arms than in closed arms (G) and their extremities (H). (I) Spatial reference memory in Sema3E-/- mice assessed in the hidden platform version of the Morris water maze. Escape latencies (mean ± s.e.m., averaged value from 4 trials) on successive days are shown. Days 1-9: acquisition stage with the hidden platform located in a fixed position; Days 12-14: re-learning after a 13-day rest period; Days 15-18: reversal after relocation of the platform. Wild-type and Sema3E-/- mice learned the three stages of the task equally well. (J) Spatial working memory in Sema3E-/- mice assessed using a version of the Morris water maze in which the platform is changed from day to day. Escape latencies per trial (mean ± s.e.m.; averaged value over 5 days) over successive trials on each day are shown. Sema3E-/- mice showed weaker performance than wild-type mice on the first daily trial (p=0.03) but improved their performance during the four daily trials (p=0.01). Wild-type mice showed no improvement (p=0.26) over the same period.
GP: globus pallidus; ic: internal capsule, pf : postcommissural fornix, TRN : thalamic reticular nucleus. *significantly different with p<0.05, **significantly different with p<0.01, ***significantly different with p<0.005. Scale bars: 100 μm (A, B), 120 μm (C, D).
Experimental lesions of the mammillary bodies lead to defects in emotional behavior and working memory (Beracochea and Krazem, 1991; Santin et al., 1999). To detect potential emotional alterations in Sema3E-/- mice, anxiety was evaluated by testing the mice in an elevated plus maze, consisting of two enclosed and two open arms. When placed in the maze for 8 minutes, Sema3E-/- mice visited the open arms (Fig. 4E) and their extremities (Fig. 4F) >3-fold more frequently than did wild-type controls. They also spent >5-fold longer periods in the open arms (Fig. 4G, H). All effects of genotype were highly significant (n=11 mice per genotype; p<0.016 by ANOVA). Increased exploration did not reflect a general increase in motility since spontaneous activity of mutant mice in isolation was not altered (data not shown). Sema3E-/- mice therefore show behavior suggestive of reduced anxiety levels.
The performance of Sema3E-/- mice was also evaluated in the Morris water maze, which provides a measure of spatial memory. Mutant mice showed normal performance when the platform in the tank was visible, demonstrating that Sema3E-/- mice have no deficit in swimming ability, motivation, or other performance parameters (data not shown). The mutant mice were also normal in the acquisition and retrieval of spatial reference memory tested in the hidden platform version of the task (Fig. 4I).
We therefore used a variant of the Morris water maze in which the position of the platform is changed from day to day (Malleret et al., 1999). Each day, initial placement onto the daily platform (sample stage) was followed by four trials of escape latency. In this paradigm, mice have to solve a new spatial problem each day, while extinguishing their long-term memory of the previously learned platform location. Results for each successive trial were averaged across 5 successive days (Fig. 4J). Wild-type mice learned from their initial placement and performed correctly in the first daily trial. In contrast, Sema3E-/- mice exhibited 30% longer latency than wild-type mice on the first daily trial (p=0.03). Nevertheless, they were able to improve their trial-to-trial performance on a given day, showing a correct short-term processing of spatial information in subsequent trials (Fig. 4J). Thus the null-mutant mice showed intact spatial reference memory but a moderately impaired spatial working memory. The reduced growth of subiculo-mammillary axons in Sema3E-/- embryos therefore leads to an adult behavioral pattern consistent with dysfunction of the mammillary bodies
Both positive and negative effects of Sema3E on axonal growth require PlexinD1
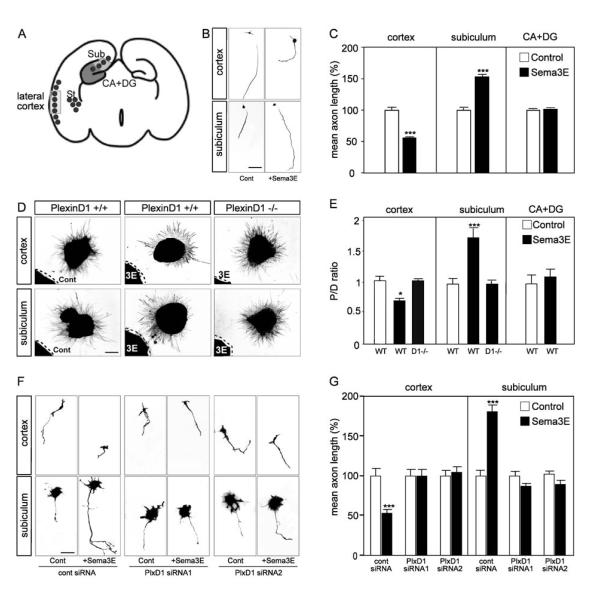
We next addressed the mechanisms underlying Sema3E signaling in the different forebrain neuronal populations. To determine directly how co-expression of Npn-1 with PlexinD1 affects growth responses to Sema3E, we cultured neurons from the ventrolateral region of the cortex expressing PlexinD1 but not Npn-1, or from the subiculum expressing PlexinD1 and Npn-1 (Fig. 5A). The hippocampus proper was taken as a control, since it expresses undetectable levels of PlexinD1 (Fig. 2L). We first determined whether the profile of receptor expression in these cultures faithfully reflected the in vivo pattern. As expected from the high density of PlexinD1 expression in the subdissected regions in vivo (insets in Figs. 1L, 2L), PlexinD1 protein was expressed by 90% of cultured neurons from the ventrolateral cortex and 92% of subicular neurons, whereas Npn-1 could only be detected in subicular cultures, in which it was expressed by 90% of neurons (Suppl. Fig. 5).
Fig. 5. PlexinD1 is required for both positive and negative effects of Sema3E.
(A) Schematic diagram of a coronal section through E17.5 brain illustrating the cortical and hippocampal regions dissected for in vitro assays. Black dots indicate regions of PlexinD1 expression. (B) Typical images of dissociated neurons cultured in the presence or absence of 5 nM Sema3E. Adding Sema3E inhibits the extension of cortical axons but stimulates the growth of subicular axons. (C) Histograms Sema3E effects in cultures of subicular, cortical and control hippocampal (CA+DG) dissociated cells. Data are presented as mean axonal length ± s.e.m. (n=3) and are normalized to 100% for values obtained in control conditions. Sema3E significantly inhibits cortical growth and stimulates growth of subicular axons, but has no effect on hippocampal axons. (D) Typical patterns of axonal outgrowth from explants co-cultured with Sema3E-expressing HEK293T cells, and stained with SMI-31 neurofilament antibody. Cortical explants show chemorepulsion whereas subicular explants show chemoattraction. Neither attraction nor repulsion was seen in explants from PlexinD1-/- embryos. (E) Quantification of the axonal guidance response of cortical, subicular and hippocampal neurons to control and Sema3E-expressing HEK293T cells. Data are expressed as P/D ratio, where P and D are the mean lengths of axons in the quadrant proximal and distal to the cell aggregate (Cheng et al., 2001). A P/D ratio close to 1 indicates radial outgrowth. Cortical axons are repelled by a source of diffusible Sema3E, whereas subicular axons are significantly attracted and hippocampal axons show no response. All growth responses to Sema3E are PlexinD1-dependent (columns labeled D1-/- performed using PlexinD1-/- explants). (F) Dissociated cortical and subicular neurons electroporated with GFP-expressing vector together with either control siRNA or PlexinD1 siRNAs 1 and 2 (see Experimental Procedures) were cultured in the presence or absence of 5 nM Sema3E. (G) Quantification of the effects of siRNAs on axon length. Knock-down of PlexinD1 abolishes both the growth-inhibitory and the growth-promoting effects of Sema3E. Mean axonal lengths are calculated as a percentage of mean values obtained in control conditions for each experiment.
CA: cornus ammonis, DG: dentate gyrus, Sub: subiculum. *significantly different with p<0.05; ***significantly different with p<0.001. Scale bars: 30 μm (B, F), 150 μm (D).
To measure the effects of Sema3E on axonal elongation, dissociated neurons were cultured in the presence or absence of recombinant Sema3E (5 nM) and axon length was quantified after 2-3 days. The number of living neurons was not affected by Sema3E (data not shown), but in the presence of Sema3E, cortical axon length was reduced by 45% (p<0.001; Fig. 5B, C). In contrast, the mean length of subicular axons was increased by 55% in the presence of Sema3E (p<0.001; Fig. 5B, C). The length of hippocampal axons was unaffected by the addition of Sema3E (Fig. 5C).
To determine whether the opposite effects of Sema3E on axonal length of cortical and subicular axons are also reflected in its effects on axonal guidance, explants of ventrolateral cortex, subiculum or hippocampus from PlexinD1-/- or control embryos were co-cultured with aggregates of HEK293T cells expressing Sema3E or control cells (Fig. 5D, E). After 2 days in vitro, axons from cortical explants co-cultured with control HEK293T cells extended in a radially symmetric manner. When confronted with Sema3E-expressing cells, axonal length in the quadrant closest to the explant was reduced by 40% compared to that in the opposite quadrant (p<0.05; Fig. 5D, E). Thus, Sema3E repels the axons of cortical neurons. In contrast, subicular axons were attracted to Sema3E. Axonal length was ∼70% greater in the quadrant proximal to Sema3E-secreting cells as compared to the distal quadrant (p<0.001; Fig. 5D, E).
We next asked whether PlexinD1 was required for both repulsive and attractive effects of Sema3E on axons. Explants of either ventrolateral cortex or subiculum taken from PlexinD1-/- mice showed radial growth despite the presence of Sema3E-expressing cells (Fig. 5D, E), demonstrating that PlexinD1 is required for both the repulsive and attractive effect. Neurons from each region therefore use PlexinD1 to signal contrasted axonal responses to Sema3E - repulsion/growth inhibition for ventrolateral cortex, and attraction/growth stimulation for subiculum - consistent with the in vivo phenotypes of Sema3E-/- and PlexinD1-/- embryos.
To examine whether the altered behavior of neurons isolated from PlexinD1-/- mice directly reflected the absence of PlexinD1 signaling, rather than an earlier developmental defect, we studied the effects of knock-down of PlexinD1 in dissociated neurons, using two different siRNAs that lowered PlexinD1 expression in COS-7 cells by >95%, without affecting Npn-1 levels (Suppl. Fig. 6A-C). The siRNAs were co-electroporated with a GFP reporter vector, and only cells that had been transduced (as indicated by GFP fluorescence) and had a clear neurite >1 cell diameter in length were included in the analysis. As with PlexinD1-/- neurons, knockdown of PlexinD1 to low levels abrogated both positive and negative effects of Sema3E on axonal length (Fig. 5F, G). As a control, we grew cultures of neurons isolated from the dorsal part of the cortex, which exhibits minimal expression of PlexinD1 in mouse embryos (see Suppl. Fig. 2E). The same siRNAs had no effects on inhibition of axon growth by Sema3A, indicating that effects of PlexinD1 knockdown were specific to Sema3E (Suppl. Fig. 7; Bagnard et al., 1998).
These findings indicate that cortical and subicular neurons show intrinsic differences in their PlexinD1-dependent responses to Sema3E in vitro. Sema3E acts as a repulsive/inhibitory signal for cortical axons but as an attractive/promoting cue for subicular axons. Since both axonal populations express PlexinD1, but only subiculo-mammillary processes express Npn-1, our data suggest a model in which “gating” by Npn-1 of the response of PlexinD1 to Sema3E transforms the repulsive signal mediated by PlexinD1 into an attractive effect.
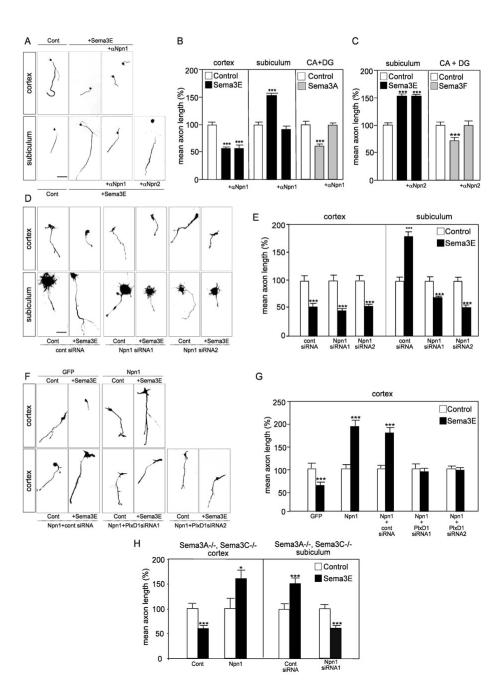
Npn-1 is required for stimulatory, but not inhibitory, effects of Sema3E on axonal growth
To analyze the role of Npn-1 in each forebrain neuronal population, we used neutralizing antibodies to the extracellular domain of Npn-1. These antibodies completely block the inhibitory response of hippocampal axons to Sema3A, which is known to act through Npn-1 (Fig. 6B; Chedotal et al., 1998). Npn-1 blocking antibodies had no effect on the inhibitory growth response to Sema3E of cortical axons. In contrast, the antibodies completely inhibited the axonal growth-promoting effects of Sema3E on subicular neurons (Fig. 6A, B). Since it has been suggested that neuropilin-2 may also form a complex with PlexinD1 (Gitler et al., 2004), antibodies to Npn-2 were used as a control. As expected, Npn-2 antibodies completely blocked the response of hippocampal neurons to Sema3F (Fig. 6C; Bielenberg et al., 2004). However, they did not interfere with the response of subicular neurons to Sema3E (Fig. 6A, C), despite the fact that Npn-2 is expressed by this population (data not shown). These results suggest that Npn-1, but not Npn-2, is a necessary component of the Sema3E signaling mechanism involved in increased axonal growth, but not growth inhibition.
Fig. 6. Levels of Npn-1 gate the responses of cortical and subicular axons to Sema3E.
(A) Typical images of dissociated neurons cultured in the presence or absence of 10 μg/ml polyclonal anti-Npn-1 or anti-Npn-2, and of Sema3E. Blockade of Npn-1 prevents stimulation of subicular axon growth by Sema3E, but not inhibition of cortical axon growth by Sema3E. Anti-Npn-2 antibodies do not affect the growth-promoting effect of Sema3E on subicular axons. (B, C) Quantification of the results illustrated in A. Data are presented as mean axonal length ± s.e.m. (n=3) and are normalized to 100% for values obtained in control conditions. The anti-Npn-1 and anti-Npn-2 antibodies prevent inhibition of hippocampal axon growth by Sema3A (B) and Sema3F (C), respectively, confirming their neutralization efficiency. (D) Typical images of dissociated neurons cultured in the presence or absence of 5 nM Sema3E after electroporation of the indicated siRNAs. Knock-down of Npn-1 causes subicular neurons to switch their response to Sema3E from promotion to inhibition but does not affect inhibition of cortical axon growth by Sema3E. (E) Quantification of the results illustrated in D. Data are presented as mean axonal length ± s.e.m. (n= 3) and are normalized to 100% for values obtained in control conditions. (F) Typical images of dissociated neurons cultured in the presence or absence of 5 nM Sema3E after electroporation of expression vectors encoding GFP or Npn-1 together with the indicated siRNAs. Misexpression of Npn-1 confers on cortical neurons the ability to respond positively to Sema3E in a PlexinD1-dependent manner. (G) Quantification of the results illustrated in F. Data are presented as mean axonal length ± s.e.m. (n= 3) and are normalized to 100% for values obtained in control conditions. (H) To exclude the possibility that Npn-1 was acting as a receptor for other semaphorins produced in an autocrine manner (Bachelder et al., 2003; Serini et al., 2003; De Wit et al; 2005), responses of neurons from double mutant Sema3A-/-; Sema3C-/- embryos to Sema3E were quantified. Data are presented as mean axonal length ± s.e.m. (n= 3) and are normalized to 100% for values obtained in control conditions. Even in these mutant cells, misexpression of Npn-1 in cortical neurons and knock-down of Npn-1 in subicular neurons induce switches in axonal responses to Sema3E that are similar to those observed using wild-type neurons.
CA: cornus ammonis, DG: dentate gyrus. *significantly different with p<0.05; *** significantly different with p<0.001. Scale bar: 30 μm (A, D, F).
To exclude the possibility of indirect steric hindrance by Npn-1 antibodies, we modulated Npn-1 levels in cultured neurons from ventrolateral cortex and subiculum using RNA interference. Two different siRNA sequences reduced Npn-1 levels to <5% of normal values (Suppl. Fig. 6D-F). Strikingly, in dissociated cultures from E17.5 subiculum, knockdown of Npn-1 transformed the positive growth response to Sema3E into an inhibitory one (p<0.001; Fig. 6D, E). The same siRNAs had no effect on the inhibition of cortical axonal growth by Sema3E (Fig. 6D, E). Thus, Npn-1 is necessary for the growth-promoting effects of Sema3E on subicular neurons.
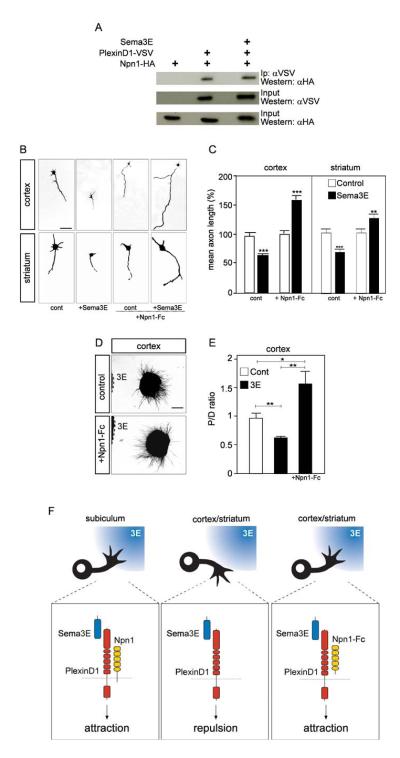
To determine whether Npn-1 is alone sufficient to confer on cortical axons a positive growth response to Sema3E, we electroporated cortical neurons with an expression vector encoding Npn-1, co-transfected with a GFP reporter vector. Cortical neurons over-expressing Npn-1 showed an 80% increase in axonal length when cultured in the presence of Sema3E, whereas neurons transfected with the GFP vector alone showed a 40% decrease in length in response to Sema3E (p<0.001; Fig. 6F, G). This Npn-1-dependent growth-promoting effect required PlexinD1 function, since it could be inhibited using siRNAs specific to PlexinD1 (Fig. 6F, G). Thus both Npn-1 and PlexinD1 are required to initiate a growth-promoting response to Sema3E. Indeed, Npn-1 could be co-precipitated with PlexinD1 after heterologous expression of epitope-tagged proteins in COS-7 cells (Fig. 7A), supporting the idea that Npn-1 and PlexinD1 form part of a receptor complex (Gitler et al., 2004). In contrast, although it could be co-immunoprecipitated with PlexinD1 in the COS-7 system (Suppl. Fig. 10), Npn-2 did not convert the growth inhibitory response of cortical neurons to Sema3E into growth promotion (not shown).
Fig. 7. The extracellular domains of Npn-1 and PlexinD1 interact to signal growth.
(A) Co-immunoprecipitation experiments showing that PlexinD1 can form a complex with Npn-1 in both the presence and absence of Sema3E. COS-7 cells were transfected with the indicated combinations of HA-tagged Npn-1 and VSV-tagged PlexinD1, and treated or not with Sema3E (5 nM). (B) Typical images of dissociated cortical and striatal neurons cultured in the presence or absence of 5 nM Sema3E and 2 μg/ml Npn1-Fc. Like cortical neurons, striatal neurons show a growth inhibitory response to Sema3E. Soluble Npn1-Fc switches the growth response of cortical and striatal neurons to Sema3E from inhibition to stimulation. (C) Quantification of the results illustrated in B. Data are presented as mean axonal length ± s.e.m. (n= 3) and are normalized to 100% for values obtained in control conditions. (D) Typical patterns of axonal growth from cortical explants, stained with SMI-31 antibody, in co-cultures with Sema3E-expressing HEK293T cells. Cortical axons, normally repelled by secreted Sema3E, show chemoattraction toward Sema3E in the presence of 2 μg/ml Npn1-Fc. (E) Quantification of the guidance response of cortical neurons to control and Sema3E-expressing HEK293T cells in presence of 2 μg/ml Npn1-Fc. Data are expressed as P/D ratio, where P and D are the length of axons in the quadrants proximal and distal to the cell aggregate (Cheng et al., 2001). A P/D ratio close to 1 indicates radially symmetric outgrowth. (F) Molecular model for bifunctional signaling by Sema3E. Axons of subicular neurons express both PlexinD1 and Npn-1 and show a chemoattractive (or growth-promoting) response to Sema3E (left panel). In contrast, Sema3E chemorepels (or inhibits growth from) axons of cortical and striatal neurons, which express PlexinD1 but not Npn-1 (middle panel). The extracellular domain of Npn-1 is sufficient to “gate” PlexinD1 signaling and cause cortical and striatal neurons to grow towards a source of Sema3E (right panel).
*significantly different with p<0.05; **significantly different with p<0.01; ***significantly different with p<0.001. Scale bar: 30 μm (B), 150 μm (D).
Mechanism of gating of PlexinD1 signaling by Npn-1
Npn1-PlexinD1 interactions have previously been shown to require the Sema domain in the extracellular portion of PlexinD1 (Gitler et al., 2004). We therefore asked whether interactions between the extracellular domains of PlexinD1 and Npn-1 were sufficient to convert axonal responses to Sema3E from inhibition to growth enhancement. We used Npn1-Fc - the Npn-1 extracellular domain fused to a human Fc immunoglobulin domain - which functions as an agonist, since it rescues the vascular phenotype of Npn1-/- embryos in vivo (Yamada et al., 2001). As in earlier experiments, addition of Sema3E to control cortical neurons led to a 40% decrease in mean axonal length (Fig. 7B, C). In contrast, addition of Npn1-Fc to the culture medium of cortical neurons led to a 60% increase in mean axon length in response to Sema3E (p<0.001; Fig. 7B, C), just as observed following forced expression of full-length Npn-1 (Fig. 6F, G). A strikingly similar switch in growth responses to Sema3E from inhibition to stimulation was observed following addition of Npn1-Fc to striatal neurons (p<0.01; Fig. 7B, C), which express PlexinD1 but not Npn-1 (Fig. 1N, O). Moreover, in co-culture experiments using cortical explants, Npn1-Fc caused growing axons to be attracted towards a source of Sema3E (p<0.05; Fig. 7D, E). Thus, exposure of neurons to the extracellular domain of Npn-1 is sufficient to switch their response to PlexinD1 signaling.
We investigated the possibility that Npn-1 might affect Sema3E signaling by modulating levels of PlexinD1. However, co-expression of Npn-1 in COS-7 cells did not alter surface levels of PlexinD1 (Suppl. Fig. 8B). Moreover, the Kd of Sema3E binding to PlexinD1 is unchanged in the presence of full length Npn-1, whereas the number of binding sites is modified (Suppl. Fig. 8C). This result showed that changes in Kd are not necessary for switching to occur but left open the possibility that the gating might involve changes in Bmax. However, addition of Npn1-Fc to COS-7 cells expressing PlexinD1 induced no significant change in the number of binding sites for Sema3E (Suppl. Fig. 8E). Thus the switch in PlexinD1 signaling triggered by Npn-1 likely reflects changes in intracellular signaling events, which are currently unknown.
Together, our findings demonstrate that Npn-1 is necessary and sufficient to “gate” the normally inhibitory/repulsive signal generated by binding of Sema3E to PlexinD1. Strikingly, the extracellular domain of Npn-1 is sufficient to determine whether PlexinD1 signals repulsion or attraction (Fig. 7F). The expression of Npn-1 in subicular neurons but not in cortical or striatal neurons therefore appears to explain the differential response of these neuronal populations to Sema3E in vitro, and their contrasting responses to Sema3E inactivation in vivo.
DISCUSSION
Our aim in this study was to determine whether differential receptor usage permits a single guidance cue to exert both attractive and repulsive actions in vivo. We describe a gating mechanism for signal modulation by the semaphorin receptor component Npn-1, whose extracellular domain is sufficient to convert signaling by Sema3E/PlexinD1 from repulsion to attraction. We show that Sema3E and PlexinD1 are key signals for axon guidance during forebrain development, and demonstrate a novel attractive and growth-promoting role for Sema3E, that appears to be required for establishment of normal behaviors in adult mice. Lastly, we demonstrate that the effects of individual guidance molecules vary as a function of cellular context even between different classes of forebrain projection neurons, providing a refined level of guidance control during the assembly of neural circuits.
Central role of PlexinD1 in Sema3E signal transduction
The composition of the neuronal receptor complex for Sema3E has long remained obscure. Using biochemical and functional approaches, we first identified PlexinD1 as one key player. The only binding partner for Sema3E in the developing brain appears to be PlexinD1, as in the vascular system (Gu et al., 2005). Second, deletion of PlexinD1 by gene targeting or by RNAi demonstrates that PlexinD1 is required for both the stimulatory/attractive and inhibitory/repulsive activities of Sema3E on axon growth in vitro. Third, PlexinD1-/- and Sema3E-/- mutants show the same defects in subiculo-mammillary projections in vivo. Together, these findings indicate that PlexinD1 is a major component of the Sema3E receptor in brain.
Our findings do not exclude the possibility that PlexinD1 also participates in receptor complexes for other semaphorin ligands. This is indeed suggested by the more severe perinatal lethality of the PlexinD1-/- mutation as compared to Sema3E-/- mice, and by the ability of complexes of PlexinD1 with Npn-1 or Npn-2 to bind Sema3A, Sema3C and/or Sema4A in vitro (Gitler et al., 2004; Toyofuku et al., 2007). Thus, in addition to its role in Sema3E signaling in axons, PlexinD1 may have other receptor functions during brain development.
Npn-1 cooperates with PlexinD1 to specify Sema3E-induced attraction
Although PlexinD1 is required for all the known effects of Sema3E on growing axons, it is not sufficient to explain the ability of Sema3E to trigger both attraction and repulsion. Our data demonstrate a novel role for Npn-1 in diversifying neuronal responses to Sema3E. In particular, Npn1 cooperates with PlexinD1 to specify axonal attraction/growth. In support of this view, we find that Npn-1 is expressed on subicular axons, which show a positive growth response to Sema3E in vitro and in vivo, but not on cortical and striatal projections, which are repelled by Sema3E. Moreover, knock-down of Npn-1 causes subicular axons to be inhibited by Sema3E, whereas misexpression of Npn-1 in cortical neurons converts Sema3E-induced repulsion into PlexinD1-dependent attraction. A switch from repulsion to attraction can also be elicited using solely the extracellular domain of Npn-1. This striking finding supports a model in which the PlexinD1 response is “gated” by the presence of Npn-1 in the receptor complex. Our findings argue against the possibility that Npn-1 plays a role in an independent attractive mechanism which competes with PlexinD1-mediated repulsion. In that case, inactivation of PlexinD1 would be expected to enhance attraction in subicular but not cortical neurons. Instead, inactivation of PlexinD1 it leads to loss of response to Sema3E in both populations.
The gating effect of Npn-1 on PlexinD1 signaling differs significantly from other roles reported for this type I transmembrane protein. In receptor complexes with PlexinA family members, Npn-1 is a required binding component for Sema3A (He and Tessier-Lavigne, 1997; Takahashi et al., 1999). Npn-1 also interacts with specific isoforms of VEGF and enhances their affinity for the VEGFR2 receptor (Soker et al., 1998), and can mediate heterophilic cell adhesion (Fujisawa et al., 1997; Shimizu et al., 2000). Moreover, Npn-1 may directly transduce signals during angiogenesis (Wang et al., 2006). Thus, in its interactions with these other signaling molecules, Npn-1 initiates or potentiates a signal, but does not switch the sign of the response, as with PlexinD1.
Several mechanisms might underlie the “gating” of PlexinD1 function by Npn-1. Most simply, Npn-1 binding to PlexinD1 could result in the recruitment of an additional component of receptor complex that initiates an attractive intracellular signalling pathway. Alternatively, Npn-1 may cause conformational changes in the cytoplasmic tail of PlexinD1 protein, therefore controlling the selective recruitment of adaptors molecules to the activated PlexinD1 receptor. Such a model has received recent support in the finding that the association of FARP2, a downstream molecule of the Sema3A receptor complex, to PlexinA1 is dependent on the interaction between the extracellular domains of Npn-1 and PlexinA1 (Toyofuku et al., 2005).
“Gating” of plexin responses by neuropilins: a novel mechanism for modulation of semaphorin signaling
Gating of the PlexinD1 response by Npn-1 provides a novel receptor-based mechanism through which a single semaphorin can play both attractive and repulsive roles during axon guidance. Our findings bear comparison with the modulation of the response of UNC-40/DCC to UNC-6/netrin-1 by UNC-5 in the nematode worm C. elegans (Chan et al., 1996). Invertebrate axons are attracted to UNC-6/netrin-1 when they express the UNC-40/DCC receptor alone but repelled when they express UNC-40/DCC together with UNC-5 (Chan et al., 1996; Keleman and Dickson, 2001). Similar changes in axonal responses to netrin-1 are observed in cultured Xenopus neurons expressing UNC-40/DCC in the presence or absence of UNC-5, allowing the signaling interactions between UNC-40/DCC and UNC-5 to be analyzed (Hong et al., 1999). Comparison of these results with ours reveals three striking mechanistic differences between the sign-switch systems underlying the responses of vertebrate neurons to netrin-1 and Sema3E. First, complex formation involving PlexinD1 and Npn-1 can be detected in the absence of Sema3E, whereas DCC/UNC-5 interactions appear to be ligand-dependent (Hong et al., 1999). Second, Npn-1 does not bind Sema3E, whereas both DCC and UNC-5 bind netrin. Lastly, DCC and UNC-5 interact through their intracellular domains whereas only the extracellular domain of Npn-1 is required to effect the switch from repulsive to attractive signaling by PlexinD1. Moreover, modulation of DCC signaling by UNC-5 has yet to be demonstrated in vivo during vertebrate development (Fazeli et al., 1997; Braisted et al., 2000; Finger et al., 2002). Thus, by both its mechanistic basis and in vivo relevance, the gating of PlexinD1 by Npn-1 provides insight into the principles that direct the diversity of axonal responses during pathfinding decisions.
Other semaphorins have been shown to exhibit both attractive and repulsive activities in vitro and in vivo (Song et al., 1998; Castellani et al., 2000, 2002, 2004; Dalpe et al., 2004; Wolman et al., 2004). However, the mechanisms underlying these differential responses in vivo have not been elucidated. The clearest example to date of both attractive and repulsive signaling by a single semaphorin in vivo is in the context of Sema3B activity during the development of the anterior commissure (Falk et al., 2005). A dorsal source of Sema3B attracts anterior commissural axons (ACa) dorsally, while a ventral source repels posterior commissural axons (ACp) so that they too turn dorsally, but through a different mechanism. However, it remains unclear why ACa axons are attracted and ACp repelled. Since all commissural axons express the receptor components Npn-2 and NrCAM (Falk et al., 2005), the basis of attraction and repulsion is apparently not directed by differences in receptor composition.
Repulsion by Sema3E patterns descending projections at the internal capsule
Our findings demonstrate a role for Sema3E in axonal guidance along the cerebral peduncle, the shared trajectory of corticofugal and striatonigral projections. We show that subsets of axons in these tracts express PlexinD1. In the absence of Sema3E, which is normally expressed on either side of the internal capsule, some PlexinD1-expressing fibers grow exuberantly through the thalamic reticular nucleus (TRN) and terminate in the dorsal midbrain. The origin of the ectopic bundles in Sema3E null-mutant mice could be either cortical and/or striatal. Our in vitro experiments suggest that both tracts may be involved, since Sema3E constitutes a repulsive signal for the growth of both cortical and striatal axons.
TRN cells send pioneer axons to the dorsal thalamus and may ensure the guidance of corticothalamic projections (Mitrofanis and Guillery, 1993). Our findings indicate that the TRN seems also involved in directing corticofugal and/or striatonigral projections towards the developing cerebral peduncle as they course through the internal capsule. It is likely that other cues are also required to ensure axonal guidance along this pathway. For example, Slits appear to play important role in preventing corticofugal axons from entering ventral regions of the forebrain (Bagri et al., 2002; Lopez-Bendito et al., 2007).
Sema3E is an attractive/growth-promoting factor essential for establishment of a functional subiculo-mammillary pathway
Analysis of the phenotype of the subiculo-mammillary tract in Sema3E mutant mice has permitted us to probe the link between developmental defects in axonal projections and later behavioral changes. Subicular projections are routed through the postcommissural fornix to the caudal hypothalamus, a major output of the hippocampus (Nauta, 1958; Swanson and Cowan, 1977; Stanfield et al., 1987), but the molecules that establish this trajectory have not been defined. We find that in Sema3E null-mutant mice, very few subicular axons reach the caudal hypothalamus, even at adult stages. The most likely source of Sema3E’s influence on subicular neurons in vivo is the axons of hippocampal pyramidal neurons. These neurons express Sema3E mRNA and their axons grow alongside the subiculo-mammillary fibers until they leave the fornix. Axons are a plausible source since in vitro, another semaphorin, Sema3A, is released from the axons of cortical neurons (De Wit et al., 2005, 2006) and Sema3E can be detected on axonal surfaces (S.C., O.G., F.M., unpublished observations). Since Sema3E attracts axons of subicular neurons and stimulates their growth in vitro, we conclude that Sema3E expression by CA1/CA3 projections is required to guide subicular efferents out of the hippocampus towards the fimbria and/or to promote their growth along the fornix. Axo-axonal interactions have also been proposed to regulate axonal growth patterns in dense white matter tracts such as the optic nerve or limb plexi, suggesting that the interaction we observe in the subicular system may have general relevance in other brain regions (Landmesser, 2001; Oster et al., 2004).
Since Sema3E mutants are viable, and the defects in the subiculo-mammillary projection persist in adults, we have been able to explore the behavioral consequences of Sema3E inactivation. Prior lesion studies in rats demonstrated an involvement of the mammillary bodies in spatial working memory but not reference memory (Santin et al., 1999; Santin et al., 2003; Conejo et al., 2004). Moreover, lesions of the mammillary bodies lead to altered behavior in the elevated plus maze, with mice entering more often and spending more time in the open arms compared with controls (Beracochea and Krazem, 1991; Beracochea, 2005). Our findings show that Sema3E mutant mice also spend more time in the open arms of the elevated plus maze, suggesting reduced anxiety levels, and in addition they exhibit deficits consistent with impaired working memory. We emphasize that the disruption of other axonal tracts in Sema3E mutants could contribute to the phenotype. Nevertheless, the striking similarities between the behavioral phenotypes of Sema3E-null and lesioned mice support the idea that the embryonic defect in axonal growth along the subiculo-mammillary pathway underlies, at least in part, the behavioral deficits.
Receptor-based mechanisms for modulation of semaphorin signaling
The gating properties of neuropilin-1 demonstrated here have clear significance in forebrain circuit assembly. They may also provide more general insights into semaphorin actions in other tissues. Sema3E repels blood vessels during embryogenesis (Gu et al., 2005), but in tumors it appears to promote the growth of blood vessels, which express PlexinD1 (Christensen et al., 2005; Roodink et al., 2005). It will be important to determine whether the differential effects of Sema3E on endothelial cells are also gated by Npn-1. The potential involvement of Npn-1 in activities reported for Sema3E in other neuronal systems (Chedotal et al., 1998; Miyazaki et al., 1999a; Miyazaki et al., 1999b; Sakai et al., 1999; Castellani et al., 2000; Steinbach et al., 2002; Steffensky et al., 2006) also remains to be explored. Since Npn-1 can interact with Plexins-A and -B, it may potentially modulate responses to the transmembrane semaphorins (classes 4-6) with which these plexins interact (Tamagnone et al., 1999; Artigiani et al., 2004; Toyofuku et al., 2004; Yoshida et al., 2006; Suto et al., 2007). The conditions for the occurrence of “gating” are likely to be more stringent than simple co-expression of Npn1 and Plexins. Npn1, in spite of its widespread expression in developing heart, does not interact with PlexinA1 in ventricular cells (Toyofuku et al., 2004). Since plexin-neuropilin complex formation precedes ligand binding (Fig. 7A; Tamagnone et al., 1999; Gitler et al., 2004), regulation of this assembly may also have a determinant effect on the outcome of semaphorin signaling. More generally, the controlled local assembly of receptor complexes for guidance molecules may drive diversity and provide specificity that helps accommodate the inordinately large number of axonal guidance decisions taken during development of the vertebrate brain.
Experimental procedures
In situ Hybridization
In situ hybridization was performed on 20-μm cryostat sections from E15, E17.5 and P2 wild-type and E17.5 Sema3E null mice, using DIG-labeled probes as described (Carroll et al., 2001). PlexinD1 and Sema3E probes were generously provided by M. Tessier-Lavigne and C. Christensen, respectively. Npn-1 probe was from A. Chedotal and Dlx1 probe from J.L. Rubenstein and S. Garel.
Production of Sema3E-AP and binding assays
Mouse Sema3E-AP was obtained by cloning cDNA encoding mouse Sema3E in pAPtag-5 vector (GenHunter Corporation), which contains a sequence coding for secreted alkaline phosphatase. To produce AP-tagged proteins, HEK293T cells were transfected with the Sema3E-AP vector, Sema3A-AP vector (gift from A. Chedotal) or empty pAPtag-5 vector as control, using Lipofectamine plus (Invitrogen). After 3 days of culture in Opti-MEM serum-free medium, the supernatant was collected and concentrated using Centricon filters (Millipore). AP activity was assessed as described (Gu et al., 2005). Sema3E-AP binding experiments on tissue sections were performed as described previously (Feiner et al., 1997).
DiI tracing
Adult heterozygous mice were mated to obtain Sema3E-/- and PlexinD1-/- embryos. The genotype of the offspring was determined by PCR as described (Gu et al., 2005). Brains from E17.5 mutant embryos and wild-type littermates were dissected, fixed in 4% PFA in PBS, rinsed with PBS and used for DiI injections. Small crystals of lipophilic tracer DiI were placed in the dorsal hippocampus, in the striatum or in the ventrolateral cortex. After 3-4 weeks in the fixative, brains were sectioned at 100 μm.
Immunohistochemistry
Immunohistochemistry was carried out as described (Carroll et al., 2001). To detect PlexinD1 and Npn-1 protein, we used rabbit polyclonal antiserum raised against a PlexinD1 peptide (CELVEPKKSHRQSHRKK, 1:100) and a goat anti-Npn1 antibody (1:300, R&D System Research). Secondary antibodies were Cy3-conjugated donkey anti-rabbit IgG (1:500, Jackson Immuno Research) and Alexa Fluor 488-conjugated donkey anti-goat IgG antibodies (1:500, Molecular Probes). Neurofilament staining of the postcommissural fornix was performed using monoclonal anti-neurofilament 160 antibody (1:300, clone NN18, Sigma) and biotin-conjugated donkey anti-mouse IgG antibody (1:500, Jackson Immuno Research).
Co-culture experiments and dissociated neuronal cultures
Brains from wild-type or PlexinD1-/- mutants (E17.5-18.5) were dissected to extract small tissue pieces from the ventrolateral or dorsal cortex, striatum, subiculum and hippocampus. Co-cultures were performed as described (Castellani et al., 2000). Explants were co-cultured with HEK293T cell aggregates secreting Sema3E-AP or control AP. For dissociated cell cultures, neurons were dissociated and plated onto polylysine/laminin-coated 4-well plates (Nunc) in Neurobasal medium supplemented with 1 mM glutamine, 1:50 B27 (Gibco) and control or Sema3E-AP supernatants (see above). In some experiments, neurons were electroporated with a GFP-pCAGGS vector alone or together with HA-Npn1 or Npn2-myc expression vectors (gifts from A. Chedotal) or different siRNAs (100 pmol) as described (Junghans et al., 2004). siGENOME PlexinD1 or siGENOME Npn1 Smart Pool siRNAs from Dharmacon RNA Technologies were used. Efficient knock-down of PlexinD1 was obtained using the Smart Pool sequences 5′-GCAUCCAGCUGCAUUCAUGUU-3′ (PlexinD1 siRNA1) and 5′-AAUGAUGGCUGUCGUCUAAUU-3′ (PlexinD1 siRNA2). The following sequences were efficacious for knock-down of Npn-1: 5′-AAUCAGAGUUCCCGACAUAUU-3′ (Npn1 siRNA1) and 5′-UGUCAAGACUUACAGAGUAUU-3′ (Npn1 siRNA2). In some experiments, neurons were cultured in the presence of anti-Npn1 (R&D Systems), anti-Npn2 (kind gift from A. Kolodkin) or Npn1-Fc (R&D Systems).
Quantification of axonal growth and guidance
After 2-3 days in vitro, cultures were fixed and immunostained with mouse SMI31 antibodies (1:1000, Sternberger) or rabbit anti-GFP (1:500, Torrey Pines). Secondary antibodies were goat Cy3-conjugated anti-mouse (1:500 Jackson Immuno Research) and goat Alexa Fluor 488-conjugated anti-rabbit (1:400, Molecular Probes), respectively. Pictures were taken with a Leica DM IRB camera and axonal length was determined using the NeuronJ software. The guidance effect was quantified as described (Cheng et al., 2001) by calculating the P/D Ratio, where P and D represent the length of axons extending in the quadrants proximal and distal to the HEK 293T cell aggregate, respectively. Axonal growth in dissociated cell cultures was analyzed by measuring for each SMI31- or GFP-positive neuron the length of the longest neurite. Statistical differences were determined with a two-tailed Student’s t test. Results for each experimental condition are from 3 independent preparations.
Immunoprecipitation and Western blotting
COS-7 cells were transfected with HA-Npn1 expression vector and/or equal amounts of VSV-PlexinD1 expression vector using Lipofectamine Plus (Invitrogen). Two days after transfection, cells were lysed, immunoprecipitated with a mouse anti-VSV antibody (gift from A. Lebivic). Complexes were separated by SDS-PAGE and electrotransferred onto a membrane (Immobilon-P, Millipore). Membrane were incubated with mouse anti-VSV antibodies or rat anti-HA antibodies (Roche) and secondary antibodies. Signals were detected with enhanced chemiluminescence system (Amersham, Biosciences).
Behavioral tests
The animals used to perform the behavioral study were adult male wild type (n = 11) and Sema3E null (n = 11) mice with equivalent genetic background and the same fetal and neonatal experience. They were aged 5-6 months at the beginning of the experiments. Behavioral testing was conducted as previously described in Malleret et al. (1999). All experimental procedures were conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Supplementary Material
Acknowledgements
We thank J. Raper for the Sema3A/Sema3C mutant mice and B. Molyneaux for helpful discussion. This work was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Université de la Méditerranée, a grant from Agence Nationale de la Recherche (ANR, to F.M.), a grant from Fédération pour la Recherche sur le Cerveau (FRC, to F.M.) and a doctoral fellowship from Association pour la Recherche sur le Cancer (ARC, to S. Cohen). C.E.H. was supported by NYSTAR, Project ALS, and SMA Foundation. T.M.J. is an Investigator of the Howard Hughes Medical Institute, and is supported by grants from NCI, The Wellcome Trust and NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004;5:710–714. doi: 10.1038/sj.embor.7400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230–5233. [PubMed] [Google Scholar]
- Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. doi: 10.1242/dev.125.24.5043. [DOI] [PubMed] [Google Scholar]
- Bagri A, Tessier-Lavigne M. Neuropilins as Semaphorin receptors: in vivo functions in neuronal cell migration and axon guidance. Adv Exp Med Biol. 2002;515:13–31. [PubMed] [Google Scholar]
- Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Beracochea D. Interaction between emotion and memory: importance of mammillary bodies damage in a mouse model of the alcoholic Korsakoff syndrome. Neural Plast. 2005;12:275–287. doi: 10.1155/NP.2005.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beracochea DJ, Krazem A. Effects of mammillary body and mediodorsal thalamic lesions on elevated plus maze exploration. Neuroreport. 1991;2:793–796. doi: 10.1097/00001756-199112000-00016. [DOI] [PubMed] [Google Scholar]
- Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 2004;114:1260–1271. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braisted JE, Catalano SM, Stimac R, Kennedy TE, Tessier-Lavigne M, Shatz CJ, O’Leary DD. Netrin-1 promotes thalamic axon growth and is required for proper development of the thalamocortical projection. J Neurosci. 2000;20:5792–5801. doi: 10.1523/JNEUROSCI.20-15-05792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P, Renoncourt Y, Gayet O, De Bovis B, Alonso S. Sorting nexin-14, a gene expressed in motoneurons trapped by an in vitro preselection method. Dev Dyn. 2001;221:431–442. doi: 10.1002/dvdy.1163. [DOI] [PubMed] [Google Scholar]
- Castellani V. The function of neuropilin/L1 complex. Adv Exp Med Biol. 2002;515:91–102. doi: 10.1007/978-1-4615-0119-0_8. [DOI] [PubMed] [Google Scholar]
- Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Castellani V, Falk J, Rougon G. Semaphorin3A-induced receptor endocytosis during axon guidance responses is mediated by L1 CAM. Mol Cell Neurosci. 2004;26:89–100. doi: 10.1016/j.mcn.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Chan SS, Zheng H, Su MW, Wilk R, Killeen MT, Hedgecock EM, Culotti JG. UNC-40, a C. elegans homolog of DCC (Deleted in Colorectal Cancer), is required in motile cells responding to UNC-6 netrin cues. Cell. 1996;87:187–195. doi: 10.1016/s0092-8674(00)81337-9. [DOI] [PubMed] [Google Scholar]
- Chedotal A, Del Rio JA, Ruiz M, He Z, Borrell V, de Castro F, Ezan F, Goodman CS, Tessier-Lavigne M, Sotelo C, Soriano E. Semaphorins III and IV repel hippocampal axons via two distinct receptors. Development. 1998;125:4313–4323. doi: 10.1242/dev.125.21.4313. [DOI] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HJ, Bagri A, Yaron A, Stein E, Pleasure SJ, Tessier-Lavigne M. Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron. 2001;32:249–263. doi: 10.1016/s0896-6273(01)00478-0. [DOI] [PubMed] [Google Scholar]
- Chilton JK. Molecular mechanisms of axon guidance. Dev Biol. 2006;292:13–24. doi: 10.1016/j.ydbio.2005.12.048. [DOI] [PubMed] [Google Scholar]
- Christensen C, Ambartsumian N, Gilestro G, Thomsen B, Comoglio P, Tamagnone L, Guldberg P, Lukanidin E. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer Res. 2005;65:6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- Christensen CR, Klingelhofer J, Tarabykina S, Hulgaard EF, Kramerov D, Lukanidin E. Transcription of a novel mouse semaphorin gene, M-semaH, correlates with the metastatic ability of mouse tumor cell lines. Cancer Res. 1998;58:1238–44. [PubMed] [Google Scholar]
- Cohen S, Funkelstein L, Livet J, Rougon G, Henderson CE, Castellani V, Mann F. A semaphorin code defines subpopulations of spinal motor neurons during mouse development. Eur J Neurosci. 2005;21:1767–1776. doi: 10.1111/j.1460-9568.2005.04021.x. [DOI] [PubMed] [Google Scholar]
- Conejo NM, Gonzalez-Pardo H, Vallejo G, Arias JL. Involvement of the mammillary bodies in spatial working memory revealed by cytochrome oxidase activity. Brain Res. 2004;1011:107–114. doi: 10.1016/j.brainres.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Coonan JR, Greferath U, Messenger J, Hartley L, Murphy M, Boyd AW, Dottori M, Galea MP, Bartlett PF. Development and reorganization of corticospinal projections in EphA4 deficient mice. J Comp Neurol. 2001;436:248–262. [PubMed] [Google Scholar]
- Dalpe G, Zhang LW, Zheng H, Culotti JG. Conversion of cell movement responses to Semaphorin-1 and Plexin-1 from attraction to repulsion by lowered levels of specific RAC GTPases in C. elegans. Development. 2004;131:2073–2088. doi: 10.1242/dev.01063. [DOI] [PubMed] [Google Scholar]
- De Wit J, De Winter F, Klooster J, Verhaagen J. Semaphorin 3A displays a punctate distribution on the surface of neuronal cells and interacts with proteoglycans in the extracellular matrix. Mol Cell Neurosci. 2005;29:40–55. doi: 10.1016/j.mcn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- De Wit J, Toonen RF, Verhaagen J, Verhage M. Vesicular trafficking of semaphorin 3A is activity-dependent and differs between axons and dendrites. Traffic. 2006;7:1060–1077. doi: 10.1111/j.1600-0854.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Puschel AW, et al. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48:63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Feiner L, Koppel AM, Kobayashi H, Raper JA. Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron. 1997;19:539–545. doi: 10.1016/s0896-6273(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990;63:185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006;361:1565–1574. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger JH, Bronson RT, Harris B, Johnson K, Przyborski SA, Ackerman SL. The netrin 1 receptors Unc5h3 and Dcc are necessary at multiple choice points for the guidance of corticospinal tract axons. J Neurosci. 2002;22:10346–10356. doi: 10.1523/JNEUROSCI.22-23-10346.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, van der Kooy D. Pattern formation in the striatum: developmental changes in the distribution of striatonigral neurons. J Neurosci. 1987;7:1969–1978. doi: 10.1523/JNEUROSCI.07-07-01969.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa H, Kitsukawa T, Kawakami A, Takagi S, Shimizu M, Hirata T. Roles of a neuronal cell-surface molecule, neuropilin, in nerve fiber fasciculation and guidance. Cell Tissue Res. 1997;290:465–470. doi: 10.1007/s004410050954. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Litwack ED, Yee KT, Christen U, O’Leary DD. Identification of candidate genes for controlling development of the basilar pons by differential display PCR. Mol Cell Neurosci. 2001;18:1–12. doi: 10.1006/mcne.2001.0996. [DOI] [PubMed] [Google Scholar]
- Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gonthier B, Nasarre C, Roth L, Perraut M, Thomasset N, Roussel G, Aunis D, Bagnard D. Functional Interaction between Matrix Metalloproteinase-3 and Semaphorin-3C during Cortical Axonal Growth and Guidance. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl082. [DOI] [PubMed] [Google Scholar]
- Gu C, Yoshida Y, Livet J, Reimert DV, Mann F, Merte J, Henderson CE, Jessell TM, Kolodkin AL, Ginty DD. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307:265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Hinck L, Nishiyama M, Poo MM, Tessier-Lavigne M, Stein E. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Jones EG, Rubenstein JL. Expression of regulatory genes during differentiation of thalamic nuclei in mouse and monkey. J Comp Neurol. 2004;477:55–80. doi: 10.1002/cne.20234. [DOI] [PubMed] [Google Scholar]
- Joosten EA, Gribnau AA, Dederen PJ. An anterograde tracer study of the developing corticospinal tract in the rat: three components. Brain Res. 1987;433:121–130. doi: 10.1016/0165-3806(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Junghans D, Chauvet S, Buhler E, Dudley K, Sykes T, Henderson CE. The CES-2-related transcription factor E4BP4 is an intrinsic regulator of motoneuron growth and survival. Development. 2004;131:4425–4434. doi: 10.1242/dev.01313. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, Kolodkin AL. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron. 2001;32:605–617. doi: 10.1016/s0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- Kishi T, Tsumori T, Ono K, Yokota S, Ishino H, Yasui Y. Topographical organization of projections from the subiculum to the hypothalamus in the rat. J Comp Neurol. 2000;419:205–222. doi: 10.1002/(sici)1096-9861(20000403)419:2<205::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- Landmesser LT. The acquisition of motoneuron subtype identity and motor circuit formation. Int J Dev Neurosci. 2001;19:175–182. doi: 10.1016/s0736-5748(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, Tessier-Lavigne M, Marin O. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci. 2007;27:3395–407. doi: 10.1523/JNEUROSCI.4605-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malleret G, Hen R, Guillou JL, Segu L, Buhot MC. 5-HT1B receptor knock-out mice exhibit increased exploratory activity and enhanced spatial memory performance in the Morris water maze. J Neurosci. 1999;19:6157–6168. doi: 10.1523/JNEUROSCI.19-14-06157.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann F, Chauvet S, Rougon G. Semaphorins in development and adult brain: implication for neurological diseases. Prog Neurobiol. 2007;82:57–79. doi: 10.1016/j.pneurobio.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- Mauti O, Sadhu R, Gemayel J, Gesemann M, Stoeckli ET. Expression patterns of plexins and neuropilins are consistent with cooperative and separate functions during neural development. BMC Dev Biol. 2006;6:32. doi: 10.1186/1471-213X-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrofanis J, Guillery RW. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci. 1993;16:240–245. doi: 10.1016/0166-2236(93)90163-g. [DOI] [PubMed] [Google Scholar]
- Miyazaki N, Furuyama T, Amasaki M, Sugimoto H, Sakai T, Takeda N, Kubo T, Inagaki S. Mouse semaphorin H inhibits neurite outgrowth from sensory neurons. Neurosci Res. 1999a;33:269–274. doi: 10.1016/s0168-0102(99)00015-2. [DOI] [PubMed] [Google Scholar]
- Miyazaki N, Furuyama T, Sakai T, Fujioka S, Mori T, Ohoka Y, Takeda N, Kubo T, Inagaki S. Developmental localization of semaphorin H messenger RNA acting as a collapsing factor on sensory axons in the mouse brain. Neuroscience. 1999b;93:401–408. doi: 10.1016/s0306-4522(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Miyazaki N, Furuyama T, Takeda N, Inoue T, Kubo T, Inagaki S. Expression of mouse semaphorin H mRNA in the inner ear of mouse fetuses. Neurosci Lett. 1999c;261:127–129. doi: 10.1016/s0304-3940(98)00988-4. [DOI] [PubMed] [Google Scholar]
- Nauta WJ. Hippocampal projections and related neural pathways to the midbrain in the cat. Brain. 1958;81:319–340. doi: 10.1093/brain/81.3.319. [DOI] [PubMed] [Google Scholar]
- Oster SF, Deiner M, Birgbauer E, Stretavan DW. Ganglion cell axon pathfinding in the retina and optic nerve. Semin Cell Dev Biol. 2004;15:125–136. doi: 10.1016/j.semcdb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2003;424:398–405. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- Roodink I, Raats J, van der Zwaag B, Verrijp K, Kusters B, van Bokhoven H, Linkels M, de Waal RM, Leenders WP. Plexin D1 expression is induced on tumor vasculature and tumor cells: a novel target for diagnosis and therapy? Cancer Res. 2005;65:8317–8323. doi: 10.1158/0008-5472.CAN-04-4366. [DOI] [PubMed] [Google Scholar]
- Sakai T, Furuyama T, Ohoka Y, Miyazaki N, Fujioka S, Sugimoto H, Amasaki M, Hattori S, Matsuya T, Inagaki S. Mouse semaphorin H induces PC12 cell neurite outgrowth activating Ras-mitogen-activated protein kinase signaling pathway via Ca(2+) influx. J Biol Chem. 1999;274:29666–29671. doi: 10.1074/jbc.274.42.29666. [DOI] [PubMed] [Google Scholar]
- Santin LJ, Aguirre JA, Rubio S, Begega A, Miranda R, Arias JL. c-Fos expression in supramammillary and medial mammillary nuclei following spatial reference and working memory tasks. Physiol Behav. 2003;78:733–739. doi: 10.1016/s0031-9384(03)00060-x. [DOI] [PubMed] [Google Scholar]
- Santin LJ, Rubio S, Begega A, Arias JL. Effects of mammillary body lesions on spatial reference and working memory tasks. Behav Brain Res. 1999;102:137–150. doi: 10.1016/s0166-4328(99)00011-x. [DOI] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Murakami Y, Suto F, Fujisawa H. Determination of cell adhesion sites of neuropilin-1. J Cell Biol. 2000;148:1283–1293. doi: 10.1083/jcb.148.6.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaliora I, Singer W, Betz H, Puschel AW. Differential patterns of semaphorin expression in the developing rat brain. Eur J Neurosci. 1998;10:1215–29. doi: 10.1046/j.1460-9568.1998.00128.x. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, Nahin BR, O’Leary DD. A transient postmamillary component of the rat fornix during development: implications for interspecific differences in mature axonal projections. J Neurosci. 1987;7:3350–3361. doi: 10.1523/JNEUROSCI.07-10-03350.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensky M, Steinbach K, Schwarz U, Schlosshauer B. Differential impact of semaphorin 3E and 3A on CNS axons. Int J Dev Neurosci. 2006;24:65–72. doi: 10.1016/j.ijdevneu.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Steinbach K, Volkmer H, Schlosshauer B. Semaphorin 3E/collapsin-5 inhibits growing retinal axons. Exp Cell Res. 2002;279:52–61. doi: 10.1006/excr.2002.5595. [DOI] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Suto F, Tsuboi M, Kamiya H, Mizuno H, Kiyama Y, Komai S, Shimizu M, Sanbo M, Yagi T, Hiromi Y, et al. Interactions between Plexin-A2, Plexin-A4, and Semaphorin 6A Control Lamina-Restricted Projection of Hippocampal Mossy Fibers. Neuron. 2007;53:535–547. doi: 10.1016/j.neuron.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, Hori M. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. Embo J. 2007 doi: 10.1038/sj.emboj.7601589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci. 2005;8:1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Zhang H, Kumanogoh A, Takegahara N, Suto F, Kamei J, Aoki K, Yabuki M, Hori M, Fujisawa H, Kikutani H. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18:435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Zwaag B, Hellemons AJ, Leenders WP, Burbach JP, Brunner HG, Padberg GW, Van Bokhoven H. PLEXIN-D1, a novel plexin family member, is expressed in vascular endothelium and the central nervous system during mouse embryogenesis. Dev Dyn. 2002;225:336–343. doi: 10.1002/dvdy.10159. [DOI] [PubMed] [Google Scholar]
- Vilz TO, Moepps B, Engele J, Molly S, Littman DR, Schilling K. The SDF-1/CXCR4 pathway and the development of the cerebellar system. Eur J Neurosci. 2005;22:1831–1839. doi: 10.1111/j.1460-9568.2005.04378.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Mukhopadhyay D, Xu X. C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J. 2006;20:1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- Watakabe A, Ohsawa S, Hashikawa T, Yamamori T. Binding and complementary expression patterns of semaphorin 3E and plexin D1 in the mature neocortices of mice and monkeys. J Comp Neurol. 2006;499:258–273. doi: 10.1002/cne.21106. [DOI] [PubMed] [Google Scholar]
- Wen Z, Zheng JQ. Directional guidance of nerve growth cones. Curr Opin Neurobiol. 2006;16:52–58. doi: 10.1016/j.conb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Wolman MA, Liu Y, Tawarayama H, Shoji W, Halloran MC. Repulsion and attraction of axons by semaphorin3D are mediated by different neuropilins in vivo. J Neurosci. 2004;24:8428–8435. doi: 10.1523/JNEUROSCI.2349-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worzfeld T, Puschel AW, Offermanns S, Kuner R. Plexin-B family members demonstrate non-redundant expression patterns in the developing mouse nervous system: an anatomical basis for morphogenetic effects of Sema4D during development. Eur J Neurosci. 2004;19:2622–32. doi: 10.1111/j.0953-816X.2004.03401.x. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Takakura N, Yasue H, Ogawa H, Fujisawa H, Suda T. Exogenous clustered neuropilin 1 enhances vasculogenesis and angiogenesis. Blood. 2001;97:1671–1678. doi: 10.1182/blood.v97.6.1671. [DOI] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Han B, Mendelsohn M, Jessell TM. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron. 2006;52:775–788. doi: 10.1016/j.neuron.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.