Abstract
OBJECTIVES
To determine whether counseling and support reduces the burden and depressive symptoms of spouse caregivers of Alzheimer’s patients during the institutionalization transition.
DESIGN
A randomized controlled trial of an enhanced counseling and support program for spouse caregivers of persons with Alzheimer’s disease compared to usual care. Structured interviews were conducted with spouse caregivers at baseline, every 4 months during Year 1 and every 6 months thereafter for up to 16 years.
SETTING
Outpatient research clinic in the New York City metropolitan area.
PARTICIPANTS
Referred volunteer sample of 406 spouse caregivers of community-dwelling Alzheimer’s patients enrolled over a 9.5 year period.
INTERVENTION
Enhanced counseling and support consisting of 6 sessions of individual and family counseling, support group participation and continuous availability of ad hoc telephone counseling.
MEASUREMENTS
Outcome measures included burden (modified Zarit Burden Interview) and depressive symptoms (Geriatric Depression Scale).
RESULTS
Burden and depressive symptoms were significantly lower for caregivers in the treatment group when compared to usual care controls at the time of and after institutionalization. Nursing home admission itself significantly reduced burden and depressive symptoms in the intervention and control groups.
CONCLUSION
Institutionalization alone can reduce caregiver burden and depressive symptoms, but enhanced counseling provides additional long-term benefits. The results offer some of the first clinical evidence for the benefits of enhanced counseling during the transition to institutionalization for Alzheimer’s caregivers.
Keywords: Caregiving, nursing home placement, nursing home admission, informal long-term care, psychosocial intervention
INTRODUCTION
It is estimated that 8.9 million family caregivers provide assistance to someone 50 years of age and over with AD or a related dementia.1 Families provide personal care that is integral to helping disabled older adults remain in their homes.1,2 Family care is not without its costs, particularly in the context of Alzheimer’s disease. As the disabilities and care needs of the person with dementia increase over time, the accumulated financial, social, psychological, and physiological impacts of family caregiving also intensify.3-8
The perspective that tends to dominate much of the literature is that care by family members is solely provided to older adults living at home. When caregivers are followed over longer periods of time it becomes evident that family caregiving responsibilities do not end with institutionalization of the disabled relative. Instead, this key transition appears to impact the type and intensity of help provided.9-11 Unlike earlier studies that treated institutionalization as an ‘endpoint’ in family caregiving, recent research has emphasized the continued involvement of relatives in care and the effects of nursing home admission (NHA) on the stress and mental health of family members.9,12-14
Whether NHA actually leads to reduced depressive symptoms and burden for caregivers is an important clinical question that has yet to be definitively answered. One early descriptive study suggested that NHA provides relief to family caregivers and reduces stressors such as feelings of exhaustion and fatigue related to care provision (“role overload”), at least temporarily.15 More recent studies conclude that NHA results in guilt, anger, anxiety, and depression for dementia caregivers, although others suggest that NHA does little to influence the trajectories of stress or negative mental health prior to and after institutionalization.13,14 16-20 Additional descriptive research focused on the new challenges that institutionalization pose for families, such as the negative interactions between family caregivers and staff as control of care is shifted to the facility and the family member is left with an ambiguous care role.11,16,18
Parallel to descriptive analyses of dementia caregiving and institutionalization, a series of clinical efforts have sought to alleviate the psychological, emotional, and physiological distress that can occur as a function of intensive family care provision. Recent meta-analyses and multi-site, randomized controlled evaluations indicate that psychosocial interventions for caregivers such as skills training, education, therapeutic counseling, and information-based services are generally effective in producing clinically meaningful improvements in psychological well-being.21-26 Nevertheless, there is little research to document the effects of these interventions in easing the transition of NHA for family caregivers. The goal of the current study is to unite these two streams of research to determine whether the availability of long-term counseling and support reduces burden (the emotional, psychological, physical, and emotional “load” of care provision27) and depressive symptomatology across the institutionalization transition.
The NYU Aging and Dementia Research Center (NYU-ADRC) has been conducting a pioneering randomized trial of a psychosocial intervention for spouse-caregivers of people with AD since 1987.28-34 Due to its prospective data collection strategy throughout the provision of dementia caregiving, the NYU Caregiver Intervention (NYUCI) is uniquely positioned to answer the research questions of interest in this study. Moreover, the randomized controlled design of the NYUCI offers clinical insights beyond those offered in descriptive research.
Based on the previously reported effects of the NYUCI in delaying institutionalization29-31 and the equivocal findings of prior research on the ramifications of NHA for family caregivers, we conducted analyses of the NYUCI to answer the following questions:
Does the NYUCI reduce Alzheimer’s caregivers’ burden and depressive symptoms prior to and following NHA? and
What are the long-term, post-NHA changes in burden and depressive symptoms for Alzheimer’s caregivers who do or do not receive the NYUCI?
METHODS
Procedure
Participants in the parent NYUCI included 406 spouses of persons with a clinical diagnosis of AD. Slightly more than 50% of participants were recruited through the NYU-ADRC (n = 214) while the remaining participants were recruited through a number of channels, including local Alzheimer’s Association chapters, physicians, public media announcements, and referring community providers (e.g., adult day centers, social service agencies, lawyers, etc.). Participants recruited via the NYU-ADRC had spouses who received an AD diagnosis according to National Institute of Neurologic and Communicative Diseases and Stroke-AD and Related Disorders Association (NINCDS-ADRDA) criteria.35 The other participants’ spouses received a physician-diagnosis of AD. All spouses were living with the person with AD and were residing at home at the time of study enrollment. Each participant (caregiver or spouse with AD) had to have at least one other family member or relative living in the New York City metropolitan area.
Upon study intake, participants were administered an intensive baseline assessment protocol consisting of structured questionnaires. Participants were then randomized via lottery to one of two conditions: the experimental, comprehensive counseling condition (n = 203) or the control group (n = 203) which consisted of the usual care provided to patients and their caregivers at the NYU-ADRC. All participants could utilize services outside of the study. The assessment battery was re-administered on a quarterly basis during the first year of participation and on a biannual basis in subsequent years; all follow-up assessments were conducted in-person or over the telephone. Following NHA, caregiver assessments continued at the regularly scheduled follow-up intervals unless the caregiver refused or was lost to follow-up.
Sample
Participants were followed for up to 15.9 years. Twelve percent of the cases (n = 48) have at least 10 years of follow-up data. During the course of the study 210 care recipients were institutionalized; 99 (48.8%) of those in the intervention condition placed their spouse in a nursing home at some point compared to 111 (54.7%) of those in the usual care condition. Previously published Cox proportional hazards models have indicated that there was a significantly longer time to NHA in the intervention group. The median times to NHA were 4.8 and 3.3 years after baseline for intervention and control care recipients, respectively.31
Ten cases (4 intervention, 6 usual care) completed a baseline assessment but were lost to follow-up. In 11 cases (8 intervention, 3 usual care) the care recipients died prior to the first (i.e., 4 month) follow-up interview. As the focus of this analysis is on the effects of comprehensive counseling prior to and after NHA, these 21 individuals are not included in subsequent analyses. This resulted in a longitudinal sample of 385 caregivers. Comparisons between these 385 cases and the 21 cases excluded from the longitudinal analysis indicated that these two groups did not differ significantly on the baseline values of either burden or depressive symptoms (p > .21). Inclusion status also did not differ by treatment group or gender (p > .25). Consequently, no evidence for selection bias was observed as a result of including 385 cases for analysis from the larger pool of 406 randomized cases.
Descriptive data for the sample are presented in Table 1. As detailed in our previous publications from the NYUCI,32 there was a random gender imbalance at baseline, with a higher proportion of husband-caregivers being randomly assigned to the intervention condition. Consequently, gender and baseline scores on the outcome variables were included as covariates in all subsequent analyses.
Table 1. Descriptive Characteristics.
| Variable | Enhanced Treatment | Usual Care |
|---|---|---|
| (N=191) | (N=194) | |
| Female caregiver, n (%) | 103 (53.9) | 126 (65.0) |
| Age of caregiver, mean (±SD) | 71.55 (8.71) | 71.03 (9.46) |
| Age of care recipient, mean (±SD) | 73.67 (8.19) | 74.60 (8,23) |
| Global Deterioration Scale = 4, n (%) | 71 (37.2) | 63 (32.5) |
| Global Deterioration Scale = 5, n (%) | 85 (44.5) | 73 (37.6) |
| Global Deterioration Scale ≥ 6, n (%) | 35 (18.3) | 58 (29.9) |
| Caregiver Geriatric Depression Scale, mean (±SD) | 8.97 (5.73) | 10.33 (7.11) |
| Caregiver Modified Zarit Burden Scale, mean (±SD) | 25.55 (9.46) | 26.87 (10.86) |
NOTE: Sample is the subset of 385 caregivers who provided sufficient follow-up data for the analyses.
Intervention
The NYUCI consisted of three components: individual and family counseling, support group participation and ad hoc counseling. During the four months following the baseline assessment spouse caregivers participated in 6 individual and family sessions with the study counselor (2 with the spouse caregiver only and 4 with the spouse caregiver and at least 1 other family member; the person with AD did not participate in these sessions). The content of these sessions was oriented to the stated needs of the caregiver and included information on AD, skills related to the management of behavioral problems, and strategies to bolster communication among involved and non-involved family members. Caregivers agreed at intake that after the 4-month follow-up they would participate in a support group that met weekly under the auspices of the Alzheimer’s Association. These groups were widely available in the New York City metropolitan area. The third component, provided throughout the duration of the spouse caregiver’s participation in the intervention, was “ad hoc” counseling -- caregivers and participating family members were free to contact the study counselors via telephone to address any issues, crises or other significant changes that occurred. The NYUCI was delivered by counselors with advanced degrees in social work, psychology, counseling or gerontology. The content of NYUCI is described in detail elsewhere.36
Caregivers in the usual care group did not receive the formal counseling sessions provided to those in the intervention group, and their family members did not have contact with the study counselors. For ethical reasons, caregivers in the usual care group were free to identify and utilize services on their own and they were informed upon enrollment that they could contact study counselors for information or referral purposes. For these reasons, caregivers in the “usual” care group were likely to have received more services than are typically provided in clinical care settings.
A prior report has described methods of tracking enactment used in the NYUCI.37 Data on contact with participants were collected to provide counselors with information about utilization of recommended resources. The NYUCI began in 1987, and the treatment fidelity measures in current psychosocial interventions were not routinely utilized two decades ago. We find it noteworthy that our records indicate that 8 of the first 11 participants in the treatment group that institutionalized did so on advice from the counselors, who knew via their work with spouse caregivers and other family members that NHA was in the best interest of all concerned.29 This is particularly relevant when interpreting the findings of the current study, which are detailed below.
Measures
Nursing Home Admission
Dates of NHA were derived from follow-up interviews, NYU-ADRC records, or ad hoc telephone contacts with spouse caregivers or other family members.
Burden
Caregivers’ burden was measured with a subset of questions from the Zarit Burden Interview (ZBI). The ZBI is one of the most widely-used instruments to assess caregiving burden.27,38 The modified ZBI includes 15 questions that measure areas of potential stress (e.g., perceived time pressure, emotional distress, financial strain, guilt, and overall burden) that could arise both before and following NHA for spouse caregivers.
Depressive Symptoms
The 30-item version of the Geriatric Depression Scale (GDS)39 was administered at baseline and each follow-up interval to measure spouse caregivers’ mood and psychological well-being.
Statistical Analyses
The effects of the intervention and NHA on the 15-item ZBI and the 30-item GDS were estimated and tested using random effects regression growth curve analyses. A multilevel change model was used that estimated longitudinal trajectories for individual participants at one level, with the intercepts and slopes of these person-specific longitudinal trajectories analyzed as the effects of between-subjects predictors at a higher-order second level.40 All analyses were conducted using restricted maximum likelihood estimation as provided by SAS PROC MIXED.41 The models were based on 4,193 observations over time. These included 3,055 observations during the community caregiving phase and 1,148 observations of caregivers while the care recipients were in the nursing home.
Our primary analytic models included four time-invariant variables: caregiver gender, treatment group, placement group, and the baseline (pre-treatment) value on the outcome being examined. Gender was coded as 1 for female caregivers and 0 for male caregivers. Treatment group was coded as 1 for the intervention group and 0 for the usual care group. Placement group was coded as 1 for cases in which a nursing home admission occurred at some point in the follow-up interval and 0 for cases in which no nursing home admission was observed. The baseline value on the outcome was mean-centered such that the overall mean was subtracted from each participant’s score, resulting in a centered score that was a raw deviation from the mean and equal to 0 for someone who scored at the mean.
The models included two predictors for time: one that measured time elapsed since the date of the baseline assessment and one for the amount of time elapsed since NHA. Both predictors were calculated in actual days and then divided by 365.25 to scale these measures in years. The years since baseline measure was further modified by subtracting 1, resulting in a “centered” measure that indicated the time since (or before) the one-year post-baseline point. The purpose of this centering was to scale the model so that the main effect tests for the time-invariant predictors (e.g., treatment group, gender) were comparisons between the groups at the one-year post-baseline point in time. The years since NHA variable was set to 0 for any observations before placement and for those cases who never placed. Using these two measures for time (years since baseline minus 1 and years since NHA), the main effect for years since baseline tested whether the linear slope across time was significantly different from 0, and the main effect for time since NHA tested whether this linear slope across time changed significantly after NHA for those caregivers who placed their spouses.
A time-varying indicator for institutionalization or type of assessment was also included in the model as a predictor. This indicator was set to 0 for all observations before placement and for those cases who never placed, and set to 1 for all observations after placement among those who did institutionalize. This time-varying indicator provided a test of whether NHA led to an abrupt change in the level of the outcome (burden/ZBI or depressive symptoms/GDS) immediately after institutionalization. In addition to these 7 main effects (gender, treatment group, placement group, baseline, years since baseline, years after NHA, and type of assessment), 6 two-way interaction effects were included in our primary analytic models. All 4 time-invariant predictors were specified to have an interaction effect with years since baseline. This included a treatment group*years since baseline effect that tested whether the linear rates of change before placement differed between intervention and usual care participants. A group*years after NHA interaction term tested whether the linear slope of the outcome variable across time differed between intervention and usual care groups after placement. A group*type of assessment interaction effect tested whether the change in outcome observed from before to immediately after placement differed as a function of treatment group.
Prior research has emphasized the various indicators that are associated with caregiver burden, caregiver depressive symptoms, and NHA.2,4,15-17 The NYUCI considered these factors in its design and data collection protocol. These factors include sociodemographic context and severity of cognitive impairment.15 In spite of the random assignment of cases to conditions, some imbalances were found between treatment and control conditions across key caregiving indicators such as gender (see Table 1). For this reason, caregiver gender and the baseline value of the outcome variable in question were included as covariates in all models when predicting trajectories of change in caregiver burden and depressive symptoms across the NHA transition. Many other potential covariates (i.e., Global Deterioration Scale scores, which measured dementia severity) would be expected and were found to be correlated with baseline value of the outcome. By including baseline outcome as a covariate, statistical control was approximated for many of these other potential covariates that were not explicitly included in the analytic model.
RESULTS
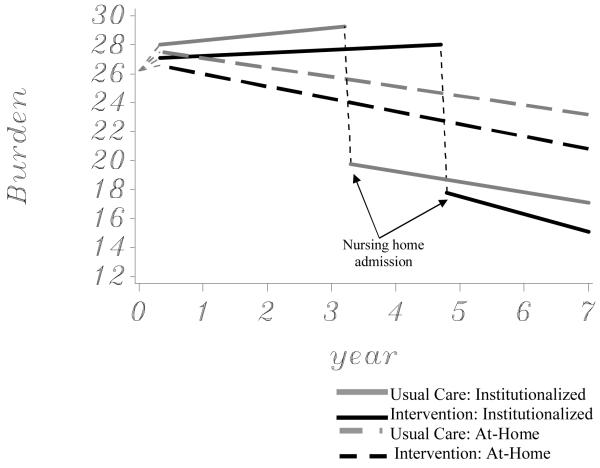
Model-predicted trajectories of the modified burden/ZBI score for intervention and usual care participants are illustrated in Figure 1. Solid lines are used to illustrate predicted values for caregivers where placements were observed, and dashed lines are used to illustrate predicted values for caregivers where no placements were ever observed. The NHA transition points were the median times to placement established in prior evaluations of the NYUCI’s effect on institutionalization.31 The statistical results of the random effects growth curve model for burden are presented in Table 2. The estimates in Tables 2 and 3 represent the amount of change in the outcome variable that is predicted for a one unit change in that predictor variable. In the analysis for the modified burden score in Table 2, a strong immediate effect for NHA was found. The estimate of -9.52 for type of assessment indicates that, after accounting for the other predictors as covariates, the post-NHA measurements of the modified ZBI were 9.52 units lower on average than the measurements obtained immediately prior to NHA for the usual care participants and 10.26 units lower for intervention group participants.
Figure 1.
Effects of the enhanced counseling intervention on burden trajectories (N = 385)
Table 2. Effects of Nursing Home Placement and Intervention on Burden.
| Effect | Estimate | SE | t | df | p |
|---|---|---|---|---|---|
| Intercept | 27.00 | 0.75 | 35.96 | 380 | <.0001 |
| Years after baseline (centered at 1 year) | -0.87 | 0.24 | -3.59 | 355 | 0.0004 |
| Years after NHA | -1.15 | 0.30 | -3.90 | 3069 | <.0001 |
| Type of Assessment (NHA = 1, Community = 0) | -9.52 | 0.54 | -17.61 | 3069 | <.0001 |
| Burden at baseline (mean centered) | 0.57 | 0.03 | 16.85 | 3069 | <.0001 |
| Baseline burden*Years after baseline | -0.10 | 0.01 | -9.94 | 3069 | <.0001 |
| Caregiver gender (Female = 1, Male = 0) | 0.69 | 0.70 | 0.99 | 3069 | 0.3221 |
| Gender*Years after baseline | 0.32 | 0.20 | 1.56 | 3069 | 0.1187 |
| Placement Group (Yes = 1, No = 0) | 1.23 | 0.67 | 1.84 | 3069 | 0.0652 |
| Placement Group*Years after baseline | 1.08 | 0.24 | 4.54 | 3069 | <.0001 |
| Treatment Group (Intervention = 1, Usual Care = 0) | -1.08 | 0.67 | -1.63 | 3069 | 0.1042 |
| Treatment Group*Years after baseline | -0.22 | 0.24 | -0.90 | 3069 | 0.3668 |
| Treatment Group*Years after NHA | -0.31 | 0.36 | -0.85 | 3069 | 0.3980 |
| Treatment Group*Type of Assessment | -0.74 | 0.72 | -1.02 | 3069 | 0.3060 |
NOTE: NHA = nursing home admission; Estimate represents the amount of change in the outcome variable that is predicted for a one unit change in that predictor variable
Table 3. Effects of Nursing Home Placement and Intervention on Symptoms of Depression.
| Effect | Estimate | SE | t | df | p |
|---|---|---|---|---|---|
| Intercept | 10.04 | 0.48 | 20.84 | 380 | <.0001 |
| Years after baseline (centered at 1 year) | -0.11 | 0.16 | -0.69 | 355 | 0.4909 |
| Years after NHA | -0.49 | 0.18 | -2.63 | 3069 | 0.0087 |
| Type of Assessment (NHA = 1, Community = 0) | -1.73 | 0.34 | -5.12 | 3069 | <.0001 |
| Depression at baseline (mean centered) | 0.69 | 0.03 | 20.26 | 3069 | <.0001 |
| Baseline depression*Years after baseline | -0.06 | 0.01 | -5.55 | 3069 | <.0001 |
| Caregiver gender (Female = 1, Male = 0) | 0.22 | 0.45 | 0.48 | 3069 | 0.6316 |
| Gender*Years after baseline | -0.11 | 0.13 | -0.81 | 3069 | 0.4169 |
| Placement Group (Yes = 1, No = 0) | 0.34 | 0.43 | 0.78 | 3069 | 0.4338 |
| Placement Group*Years after baseline | 0.16 | 0.15 | 1.07 | 3069 | 0.2841 |
| Treatment Group (Intervention = 1, Usual Care = 0) | -1.35 | 0.43 | -3.12 | 3069 | 0.0018 |
| Treatment Group*Years after baseline | -0.13 | 0.15 | -0.82 | 3069 | 0.4105 |
| Treatment Group*Years after NHA | 0.60 | 0.23 | 2.62 | 3069 | 0.0089 |
| Treatment Group*Type of Assessment | 0.24 | 0.45 | 0.54 | 3069 | 0.5879 |
NOTE: NHA = nursing home admission; Estimate represents the amount of change in the outcome variable that is predicted for a one unit change in that predictor variable
Although the estimates in Table 2 did not demonstrate a significant treatment effect at one-year after baseline, pairwise comparisons of the treatment and usual care groups at other time points revealed significant differences. Direct pairwise comparisons using the LSMEANS statement in SAS PROC MIXED indicated that the burden of the intervention group was significantly lower than the burden of the usual care group at each point after NHA (p < .03), and the difference at 3.3 years before placement closely approached statistical significance (difference in estimates = -1.56, p = .063) .
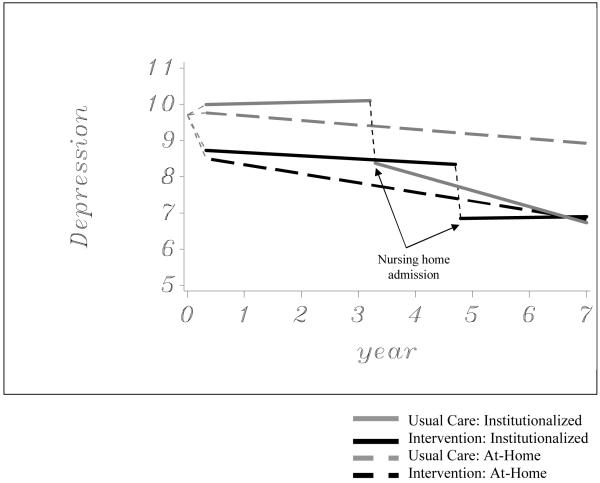
The results of the random effects growth curve model for symptoms of depressive symptoms are presented in Table 3. There was a significant effect for depressive symptom reduction after NHA (p < .0001) and for the accelerated decrease of depressive symptoms after NHA (p = .0087). Treatment group was also found to have a significant main effect, with a covariate-adjusted difference of 1.33 at the 1-year post-baseline assessment. In addition, a statistically significant group*years since placement effect was found. These effects are evident in the trajectories for depressive symptoms displayed in Figure 2. The linear slope for a decrease in GDS scores over time was statistically significant only for usual care participants after NHA. Covariate-adjusted means were compared between intervention and usual care participants at each time point. Intervention GDS scores were significantly lower than usual care GDS scores at all points prior to NHA with the exception of baseline (p < .01). These significant differences were maintained following NHA for the treatment group for approximately four months (128 days). By that point, GDS scores for the usual care group were statistically similar to GDS scores in the treatment group for the remainder of study follow-up. The depressive symptoms of the treatment and usual care groups were statistically similar because of the more rapid decrease in depressive symptoms observed after NHA for the usual care group.
Figure 2.
Effects of the enhanced counseling intervention on depressive symptom trajectories (N = 385)
DISCUSSION
The current analyses present a dynamic picture of how comprehensive counseling and support can benefit spouse caregivers throughout the progression of AD and the transition to institutionalization. Spouse caregivers in the usual care group appear to have experienced a year and a half of reduced burden compared to those in the intervention group (see Figure 1) as a result of institutionalizing earlier. This trend is temporary. Following the period of delayed NHA, caregivers in the intervention group once again indicated significantly lower perceptions of burden than those receiving usual care.
We did not observe a similar trend in depressive symptoms across the NHA transition. Caregivers in the intervention group had fewer depressive symptoms before and at the time of NHA than those in the control group, even though caregivers in the control group placed earlier and experienced some immediate reduction in symptoms at the point of NHA. Following institutionalization, only the control group reported a continuing decrease in depressive symptoms, reaching roughly the same level as caregivers in the intervention group 128 days after the latter placed. These findings are important from a clinical standpoint, as the depressive symptoms of informal caregivers is of perhaps greater public health concern than burden given the strong empirical associations between depression and other key outcomes such as health service utilization and mortality.42
Our previous work has shown that counseling and support had a clinically significant and long-lasting effect on depressive symptoms for caregivers.32 Institutionalization did not have as strong an impact on the depressive symptoms of people in the treatment group, perhaps because the counseling intervention exerted maximal effect in reducing depressive symptoms long before NHA. On the other hand, caregivers in the control group had more depressive symptoms prior to NHA which were only relieved after placement. As they did not have access to the formal support provided in the intervention condition, controls may have had to resort to institutionalization of the spouse with AD in order to achieve some sort of relief from depressive symptoms.
When examining the trajectories in the figures illustrating change in both burden and depressive symptoms (see Figures 2 and 3), the effects of the NHA transition itself is apparent. Regardless of treatment or control assignment, spouse caregivers indicated considerable reductions in burden and depressive symptoms shortly after institutionalization. These results are in contrast to prior research, which has indicated that NHA has either modest effects in reducing caregiver distress or no effect at all.13,15-17,20 One reason for this finding is that the NYUCI collected follow-up data from caregivers at relatively frequent intervals (every 4 months in the first year and every 6 months thereafter). This allowed for an analysis of caregiver burden and depressive symptoms at a time point more proximal to NHA than earlier studies. In addition, the multi-level, mixed modeling analyses were more sensitive to capturing changes in burden or depressive symptom trajectories prior to and following NHA than traditional analytic approaches (e.g., repeated measures analyses of variance). The results emphasize that the NHA transition may be necessary to alleviate emotional or psychological distress for some caregivers after providing many years of at-home care. These findings also suggest that instead of attempting to avoid NHA entirely (or suggesting it early in the disease process), good clinical care demands that health care providers recognize the point at which NHA is necessary for the well-being of caregiving families.
The models also emphasize sustained benefit of comprehensive counseling and support for participants continuing to provide at-home care to spouses. As demonstrated in Figures 1 and 2, those who never institutionalize their spouses and received the intervention indicate significantly lower burden and fewer depressive symptoms when compared to caregivers who were in the usual care control group. The findings of the current analysis confirm prior results of the NYUCI which demonstrate that the benefits of individual and family counseling, support groups, and ad hoc support are sustained over a long time period.31-34 These accumulated results point to the unique benefits of the NYUCI for caregiving families, and suggest the need to adopt similar long-term treatment strategies when implementing psychosocial interventions to manage the clinical, multi-year course of Alzheimer’s disease.
There are several limitations to this study. The setting (NYU-ADRC), focus on spouse caregivers, and the lack of ethnic/racial diversity of the sample hinder the generalizability of the results to the larger population of AD caregivers. This study was not originally designed to determine the effect of the NHA transition. In future studies, more specific measures of distress or subjective experiences during or after the transition could provide greater insight into the effects of comprehensive counseling on spouse caregivers’ adaptation to institutionalization. An additional limitation of the NYUCI is that it does not provide detailed information on the process by which the intervention improved adaptation to NHA.
CONCLUSION
The results have several important clinical applications. The research approach and models emphasize the need to consider NHA as not only an endpoint, but a key clinical transition as well. In both models and in the entire sample, institutionalization had the most powerful effect in reducing burden and depression for spouse caregivers. These results suggest the need to balance the policy and quality of life benefits of keeping chronically-disabled older adults at home (i.e., “aging in place”) with the needs of family caregivers who must eventually relinquish such responsibilities to a 24-hour residential setting in order to experience significant emotional or psychological relief. Clinical intervention approaches that can effectively delay placement, but at the same time recognize when such a decision is appropriate for the family that has done all that it can to provide at-home assistance, can successfully meet the needs of key stakeholders in long-term care (e.g., the person with AD, the family caregiver, and the public that shoulders the costs of residential long-term care through Medicaid). As demonstrated in the results of this study, the NYUCI is one such clinical approach that can balance these two needs.
Dementia caregiving is in many ways a “career,” and a key aspect of this conceptualization is recognition of the various transitions that may occur for family caregivers during the course of dementia care.15 By moving beyond NHA as an endpoint and instead considering this event as a transition, the current study demonstrates the essential benefits of providing continuity of care for families. A comprehensive counseling model such as the NYUCI can effectively meet the goal of delaying institutionalization29-31 and offer benefits during and following this transition as well. Caregivers in the intervention group received only 6 individual and family sessions shortly after enrolling in the study, although they had continual access to counselors on an ad hoc basis. Our previous results demonstrate that the intervention group showed increased participation in support groups as well as perceptions of social support.43,44 We believe that the long-term benefits that occurred after NHA—and long after formal in-person counseling sessions had ended—are likely to be the result of the enhanced social support that the intervention produced. As the results of this study suggest, guidance and support during NHA may exert positive effects for dementia caregivers if offered as part of a long-term counseling program. Intervention approaches that provide support prior to, during, and after major transitions (e.g., from community to residential care)45 may offer similar benefits to families caring for elderly relatives with other chronic diseases.
As the findings from the NYUCI and other randomized controlled evaluations make clear, the state-of-the art in AD caregiving intervention research is entering a transformative phase. Specifically, the accumulation of high quality evidence has made it possible to shift from demonstrations of efficacy to identification of strategies that facilitate translation. Physicians should make it a practice to refer caregivers not only to support groups, but also to individual and family counseling where available. Replication in community settings of comprehensive counseling and support interventions has the potential to help caregivers weather the strains of transitions such as NHA. To facilitate NYUCI’s implementation in clinical settings, Mittelman and colleagues have developed a detailed handbook to support and train potential counselors in the field.36 Clinicians or practitioners interested in implementing a long-term counseling intervention approach are encouraged to review this handbook. The NYUCI is included in the National Registry of Evidence-based Programs and Practices (NREPP), a service of the Substance Abuse and Mental Health Services Administration (SAMHSA).46 A recent request for applications from the Administration on Aging47 to support states in implementing the NYUCI and other evidence-based caregiving interventions (such as the Resources for Enhancing Alzheimer’s Caregivers’ Health protocol) also demonstrates broader interest in this new phase of research.25,26 With the emergence of high quality evidence from randomized controlled evaluations such as the NYUCI, subsequent research efforts can focus on translating these approaches into viable AD treatment options.
ACKNOWLEDGMENT
| Elements of Financial/Personal Conflicts | JEG | DLR | WEH | MSM | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | √ | √ | √ | √ | ||||
| Grants/Funds | √ | √ | √ | √ | ||||
| Honoraria | √ | √ | √ | √ | ||||
| Speaker Forum | √ | √ | √ | √ | ||||
| Consultant | √ | √ | √ | √ | ||||
| Stocks | √ | √ | √ | √ | ||||
| Royalties | √ | √ | √ | √ | ||||
| Expert Testimony | √ | √ | √ | √ | ||||
| Board Member | √ | √ | √ | √ | ||||
| Patents | √ | √ | √ | √ | ||||
| Personal Relationship | √ | √ | √ | √ | ||||
Grants/Funds: The authors of this study were supported by grant R01 MH 42216 (PI: Mary S. Mittelman).
Funding sources and related paper presentations:
Funded by NIMH (R01 MH 42216) and NIA (R01 AG14634). Additional funding was provided through the NYU Alzheimer’s Disease Center (P30-AG08051). Dr. Haley was supported by the Florida AD Research Center (P50-AG025711). A version of this paper was presented at the 2006 Gerontological Society of America annual meeting in Dallas, Texas.
Footnotes
Sponsor’s Role: The sponsor had no role in the design, methods, subject recruitment, data collection, analysis or preparation of this paper.
REFERENCES
- 1.Family Caregiver Alliance [Accessed June 12, 2007];Selected caregiver statistics [On-line] www.caregiver.org/caregiver/jsp/content_node.jsp?nodeid=439.
- 2.Gaugler JE, Duval S, Anderson KA, et al. Predicting nursing home admission in the U.S.: A meta-analysis. BMC Geriatr. 2007;7:12. doi: 10.1186/1471-2318-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore MJ, Zhu CW, Clipp EC. Informal costs of dementia care: Estimates from the national longitudinal caregiver study. J Gerontol B Psychol Sci Soc Sci. 2001;56:S219–S228. doi: 10.1093/geronb/56.4.s219. [DOI] [PubMed] [Google Scholar]
- 4.Pinquart M, Sorensen S. Associations of stressors and uplifts of caregiving with caregiver burden and depressive mood: A meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2003;58B(2):P112–P128. doi: 10.1093/geronb/58.2.p112. [DOI] [PubMed] [Google Scholar]
- 5.Pinquart M, Sorensen S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychol Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- 6.Schulz R, Beach SR. Caregiving as a risk factor for mortality: The caregiver health effects study. JAMA. 1999;282:2215–2260. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- 7.Schulz R, O’Brien AT, Bookwala MS, et al. Psychiatric and physical morbidity effects of dementia caregiving: Prevalence, correlates, and causes. Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- 8.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 9.Gaugler JE. Family involvement in residential long-term care: A synthesis and critical review. Aging Ment Health. 2005;9:105–118. doi: 10.1080/13607860412331310245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Port CL, Gruber-Baldini AL, Burton L, et al. Resident contact with family and friends following nursing home admission. Gerontologist. 2001;41:589–596. doi: 10.1093/geront/41.5.589. [DOI] [PubMed] [Google Scholar]
- 11.Port CL. Identifying changeable barriers to family involvement in the nursing home for cognitively impaired residents. Gerontologist. 2004;44:770–778. doi: 10.1093/geront/44.6.770. [DOI] [PubMed] [Google Scholar]
- 12.Gaugler JE, Anderson KA, Zarit SH, et al. Family involvement in the nursing home: Effects on stress and well-being. Aging Ment Health. 2004;8:65–75. doi: 10.1080/13607860310001613356. [DOI] [PubMed] [Google Scholar]
- 13.Schulz R, Belle SH, Czaja SJ, et al. Long-term care placement of dementia patients and caregiver health and well-being. JAMA. 2004;292:961–967. doi: 10.1001/jama.292.8.961. [DOI] [PubMed] [Google Scholar]
- 14.Tornatore JB, Grant LA. Burden among family caregivers of persons with Alzheimer’s disease in nursing homes. Gerontologist. 2002;42:497–506. doi: 10.1093/geront/42.4.497. [DOI] [PubMed] [Google Scholar]
- 15.Aneshensel CS, Pearlin LI, Mullan JT, et al. Profiles in Caregiving: The Unexpected Career. Academic Press; San Diego, CA: 1995. [Google Scholar]
- 16.Gaugler JE, Leitsch SA, Zarit SH, et al. Caregiver involvement following institutionalization: Effects of preplacement stress. Res Aging. 2000;22:337–359. [Google Scholar]
- 17.Whitlatch CJ, Feinberg LF, Stevens EJ. Predictors of institutionalization for persons with Alzheimer’s disease and the impact on family caregivers. Journal of Mental Health and Aging. 1999;5:275–288. [Google Scholar]
- 18.Whitlatch CJ, Schur D, Noelker LS, et al. The stress process of family caregiving in institutional settings. Gerontologist. 2001;41:462–473. doi: 10.1093/geront/41.4.462. [DOI] [PubMed] [Google Scholar]
- 19.Grant I, Adler KA, Patterson TL, et al. Health consequences of Alzheimer’s caregiving transitions: Effects of placement and bereavement. Psychosom Med. 2002;64:477–486. doi: 10.1097/00006842-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Gaugler JE, Pot AM, Zarit SH. Long-term adaptation to institutionalization in dementia caregivers. Gerontologist. doi: 10.1093/geront/47.6.730. in press. [DOI] [PubMed] [Google Scholar]
- 21.Bourgeois MS, Beach S, Schulz R, et al. When primary and secondary caregivers disagree: Predictors and psychosocial consequences. Psychol Aging. 1996;11:527–537. doi: 10.1037//0882-7974.11.3.527. [DOI] [PubMed] [Google Scholar]
- 22.Schulz R, O’Brien A, Czaja S, et al. Dementia caregiver intervention research: In search of clinical significance. Gerontologist. 2002;42:589–602. doi: 10.1093/geront/42.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sörensen S, Pinquart M, Habil X, et al. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42:356–372. doi: 10.1093/geront/42.3.356. [DOI] [PubMed] [Google Scholar]
- 24.Sörensen S, Duberstein P, Gill D, et al. Dementia care: Mental health effects, intervention strategies, and clinical implications. Lancet Neurol. 2006;5:961–973. doi: 10.1016/S1474-4422(06)70599-3. [DOI] [PubMed] [Google Scholar]
- 25.Schulz R, Burgio L, Burns R, et al. Resources for enhancing Alzheimer’s caregiver health (REACH): Overview, site-specific outcomes, and future directions. Gerontologist. 2003;43:514–520. doi: 10.1093/geront/43.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belle SH, Burgio L, Burns R, et al. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: A randomized, controlled trial. Ann Intern Med. 2006;145:727–738. doi: 10.7326/0003-4819-145-10-200611210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: Correlates of feelings of burden. Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- 28.Mittelman MS, Ferris SH, Shulman E, et al. A comprehensive support program: Effect on depression in spouse-caregivers of AD patients. Gerontologist. 1995;35:792–802. doi: 10.1093/geront/35.6.792. [DOI] [PubMed] [Google Scholar]
- 29.Mittelman MS, Ferris SH, Steinberg G, et al. An intervention that delays institutionalization of Alzheimer’s disease patients: Treatment of spouse caregivers. Gerontologist. 1993;33:730–740. doi: 10.1093/geront/33.6.730. [DOI] [PubMed] [Google Scholar]
- 30.Mittelman MS, Ferris SH, Shulman E, et al. A family intervention to delay nursing home placement of patients with Alzheimer’s disease: A randomized controlled trial. JAMA. 1996;276:1725–1731. [PubMed] [Google Scholar]
- 31.Mittelman MS, Haley WE, Clay OJ, et al. Improving caregiver well-being delays nursing home placement of patients with Alzheimer’s disease. Neurology. 2006;67:1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- 32.Mittelman MS, Roth DL, Coon DW, et al. Sustained benefit of supportive intervention for depressive symptoms in Alzheimer’s caregivers. Am J Psychiatry. 2004;161:850–856. doi: 10.1176/appi.ajp.161.5.850. [DOI] [PubMed] [Google Scholar]
- 33.Mittelman MS, Roth DL, Haley WE, et al. Effects of a caregiver intervention on negative caregiver appraisals of behavior problems in patients with Alzheimer’s disease: Results of a randomized trial. J Gerontol B Psychol Sci Soc. 2004;59B:P27–P34. doi: 10.1093/geronb/59.1.p27. [DOI] [PubMed] [Google Scholar]
- 34.Jang Y, Clay OJ, Roth DL, et al. Neuroticism and longitudinal change in caregiver depression: Impact of a spouse-caregiver intervention program. Gerontologist. 2004;44:311–317. doi: 10.1093/geront/44.3.311. [DOI] [PubMed] [Google Scholar]
- 35.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of Department of Health & Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 36.Mittelman MS, Epstein C, Pierzchala A. Counseling the Alzheimer’s Caregiver: A Resource for Health Care Professionals. AMA Press; Chicago: 2003. [Google Scholar]
- 37.Mittelman MS. Community caregiving. Alzheimer’s Care Quarterly. 2003;4:273–285. [Google Scholar]
- 38.Gaugler JE, Kane RA, Langlois J. Assessment of family caregivers of older adults. In: Kane RL, Kane RA, editors. Assessing the Well-being of Older People: Measures, Meaning, and Practical Applications. Oxford University Press; New York, NY: 2000. pp. 320–359. [Google Scholar]
- 39.Brink TL, Yesavage JA, Lum O, et al. Screening tests for geriatric depression: Geriatric Depression Scale. Clin Gerontol. 1982;1:37–43. [Google Scholar]
- 40.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; New York, NY: 2003. [Google Scholar]
- 41.Littell RC, Milliken GA, Stroup WW, et al. SAS System for Mixed Models. SAS Institute; Cary, NC: 1996. [Google Scholar]
- 42.Aldwin CM, Gilmer DF. Health, Illness, and Optimal Aging: Biological and Psychosocial Perspectives. Sage Publications; Thousand Oaks, CA: 2003. [Google Scholar]
- 43.Drentea P, Clay OJ, Roth DL, et al. Predictors of improvement in social support: Five-year effects of a structured intervention for caregivers of spouses with Alzheimer’s disease. Soc Sci Med. 2006;63:957–967. doi: 10.1016/j.socscimed.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 44.Roth DL, Mittelman MS, Clay OJ, et al. Changes in social support as mediators of the impact of a psychosocial intervention for spouse caregivers of persons with Alzheimer’s disease. Psychol Aging. 2005;20:634–644. doi: 10.1037/0882-7974.20.4.634. [DOI] [PubMed] [Google Scholar]
- 45.Wilson RS, McCann JJ, Li Y, et al. Nursing home placement, day care use, and cognitive decline in Alzheimer’s disease. Am J Psychiatry. 2007;164:910–915. doi: 10.1176/ajp.2007.164.6.910. [DOI] [PubMed] [Google Scholar]
- 46.National Registry of Evidence-Based Programs-Substance Abuse and Mental Health Services Administration [Accessed September 19, 2007];Intervention summary: New York University Caregiver Intervention (NYUCI) [On-line] http://www.nrepp.samhsa.gov/programfulldetails.asp?PROGRAM_ID=122.
- 47.U.S. Administration on Aging [Accessed September 18, 2007];Alzheimer’s Disease Demonstration Grants to States (ADDGS) program: Translating evidence-based Alzheimer’s disease and related dementia direct services research into practice [On-line] http://www.aoa.gov/doingbus/fundopp/announcements/2007/ADDGS_Program_Announcement _JULY_2007.doc.