Abstract
Treatment for schistosomiasis, which is responsible for hundreds of thousands of deaths annually, depends almost exclusively on praziquantel. Hundreds of millions of people are treated annually with praziquantel and drug resistant parasites are likely to evolve. Phosphinic amides and oxadiazole 2-oxides, identified from a quantitative high-throughput screen, were shown to be inhibitors of a parasite enzyme, thioredoxin glutathione reductase (TGR), with activities in the low micromolar to low nanomolar range. Incubation of parasites with these compounds led to rapid inhibition of TGR activity and parasite death. The activity of the oxadiazole 2-oxides was associated with a donation of nitric oxide. Treatment of schistosome-infected mice with 4-phenyl-1,2,5-oxadiazole-3-carbonitrile-2-oxide led to dramatic reductions in worm burdens of 99%, 88% and 94%, from treatments against skin-, liver-, and adult-stage parasites, respectively, and egg-associated pathologies. These protective effects exceed benchmark activity criteria set by the WHO for lead compound development for schistosomiasis.
Schistosomiasis is a chronic disease caused by trematode flatworms of the genus Schistosoma. The disease remains a major neglected, poverty-related health problem in many tropical areas1. It is estimated that more than 200 million people are infected with schistosomiasis, with 779 million at risk of infection, resulting in 280,000 deaths annually while more than 20 million infected individuals experience high morbidity2-4. Chemotherapy is the main schistosomiasis control method and is mediated largely through the use of praziquantel (PZQ). The low cost of the drug (< $0.25/dose) and its efficacy against adult worms of all schistosome species that infect humans has led to its very widespread use; currently tens of millions receive annual treatments of PZQ5. However, because of high re-infection rates drugs must be administered on an annual or semi-annual basis. The widespread reliance on a single drug for schistosomiasis control may hasten the selection of drug-resistant parasites. In fact, PZQ resistant isolates of Schistosoma mansoni and S. haematobium have been identified6-8 and PZQ resistant parasites have been selected for in the laboratory9. Artemether has shown promise as a new drug for schistosomiasis although its use for schistosomiasis may be restricted in areas of malaria transmission so that its use as an antimalarial is not put at risk10. Oxamniquine, a single dose anthelmintic, is effective only against S. mansoni and resistance to oxamniquine has been reported11. The dependence on a single drug for the treatment of schistosomiasis is not sustainable; there is an urgent need to identify new targets and drugs for schistosomiasis treatment.
Schistosome parasites have a complex lifecycle involving snail intermediate and human definitive hosts. Humans become infected when contacting water containing cercariae released by infected snails. After penetration, cercariae remain in the skin for several days, then enter the general circulation and are carried to the lungs, where they reside for several further days before finally entering the liver. Once in the liver, parasites undergo rapid growth, development and sexual differentiation and locate a mate. After pairing, adult parasites migrate to the mesenteric venules (S. mansoni and S. japonicum) or the venules of the urogenital system (S. haematobium) of their human host where they commence egg production. Because schistosome parasites reside in an aerobic environment in their mammalian hosts they must have means to minimize damage caused by reactive oxygen species (superoxide, H2O2, hydroxyl radical) produced by their own aerobic respiration as well as by the host immune assault. Schistosomes completely lack catalase and have minimal glutathione peroxidase activity12, two enzymes involved in the reduction of H2O2. Defense against oxidative stress in schistosomes relies mainly on the activity of peroxiredoxins (Prx), a relatively recently described class of thiol-dependent peroxidases13. Silencing of schistosome Prx expression by RNA interference leads to rapid parasite death, indicating that they are essential parasite proteins14. The activity of schistosome peroxiredoxins is supported by cellular thiol reducing agents, either the tripeptide glutathione (GSH) or the 12 kDa protein thioredoxin15. In vertebrates there are two largely independent systems to detoxify reactive oxygen species, one based on GSH and the other based on thioredoxin. Each of these systems has a dedicated NADPH-dependent flavoenzyme to maintain GSH or thioredoxin in the reduced state, GSH reductase and thioredoxin reductase, respectively16,17. However, based on biochemical and genomic analyses it is clear that these two pathways in S. mansoni are dependent on a single multifunctional selenocysteine-containing flavoenzyme, thioredoxin-glutathione reductase (TGR), which replaces both glutathione reductase and thioredoxin reductase in the parasite18 (DLW unpublished results). This suggests that the parasite's redox system is subject to a bottleneck dependence on TGR. It has been shown that TGR is essential for parasite survival, is biochemically distinct from host enzymes and appears to be a molecular target of potassium antimonyl tartrate19, which was used for schistosomiasis therapy for nearly 70 years.
Because of the unusual organization of the schistosome enzymatic defense against oxygen radicals we hypothesized that the parasite redox pathway would be an effective target for the development of new antischistosomal chemotherapies. As part of the NIH Molecular Libraries Initiative, we have recently completed a quantitative high throughput screen (qHTS)20 of 71,028 compounds comprising the Molecular Libraries Small Molecule Repository and NIH Chemical Genomics Center libraries. The screen, followed by confirmatory and target deconvolution experiments, identified several promising active series, notably phosphinic amides and oxadiazole 2-oxides, active against the S. mansoni antioxidant pathway (AS, A. Jadhav, AAS, Y. Wang, M.E. Nelson, CJT, JI, DLW, CPA, submitted). In the present study, the activity of oxadiazoles 2-oxides and phosphinic amides against TGR, the molecular target of the compounds, live cultured worms and on S. mansoni-infected mice was investigated in detail.
Results
Analysis of phosphinic amides and oxadiazole 2-oxides
The activities of phosphinic amides and oxadiazole 2-oxides were examined against both the GSH reductase and thioredoxin reductase activities of recombinant schistosome TGR (Table 1). The compounds inhibited both TGR activities with most showing greater inhibition of the thioredoxin reductase activity. The one exception was 9, which had more activity against the GSH reductase activity. The inhibitory activities of 3, 5 and 7 were in the low nanomolar range and near the limit imposed by the concentration of TGR (15 nM) in the assays. Chemical structures of the compounds used in this study are shown in Table 1.
Table 1.
Structure and Potency of Phosphinic Amides and Oxadiazole 2-Oxides Used in this Study. The potency (IC50 in μM) of the compounds against the glutathione reductase (GSSG) and thioredoxin reductase (Trx(S)2) activities of Schistosoma mansoni thioredoxin glutathione reductase are as indicated. Assays used were as described in Methods. IC50 values greater than 50 μM signify lack of fitted curve through the dose-response data, i.e. flat response within the range tested. Notes. NA, not applicable.
| Analogue # | R1 | R2 | GSSG | Trx(S)2 | |
|---|---|---|---|---|---|
Phosphinic Amide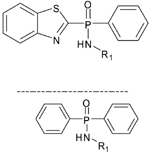
|
1 | 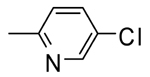 |
NA | 1.54 | 0.789 |
| 2 | 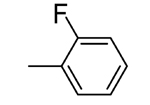 |
NA | 20.3 | 15.1 | |
| 3 | 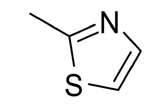 |
NA | 0.121 | 0.021 | |
| 4 | 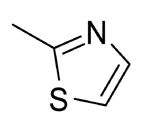 |
NA | >50 | >50 | |
Oxadiazole 2-oxide
|
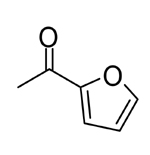
5 | 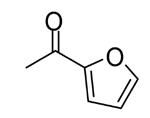 |
 |
0.082 | 0.038 |
| 6 | 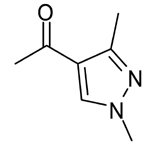 |
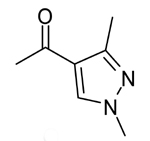 |
4.08 | 1.29 | |
| 7 | 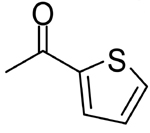 |
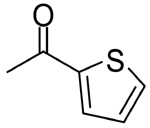 |
0.51 | 0.042 | |
| 8 | 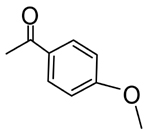 |
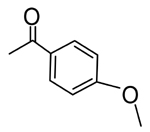 |
2.44 | 0.105 | |
| 9 | -CN | -Ph | 0.32 | 1.67 | |
| 10 | NA | NA | 12.5 | 6.68 |
The action of two compounds, N-(benzothiazol-2-yl-phenyl-phosphoryl)-1,3-thiazol-2-amine, (3), a phosphinic amide, and 4-phenyl-1,2,5-oxadiazole-3-carbonitrile-2-oxide (commonly 4-phenyl-3-furoxancarbonitrile or furoxan) (9), an oxadiazole 2-oxide, were investigated in greater detail. Both compounds exhibited time-dependent inhibition of TGR (not shown). Compound 3 was found to be a reversible inhibitor of TGR while 9 was found to be an irreversible inhibitor (not shown). However, inhibition of TGR by 9 was partially reversible by strong thiol reducing agents (not shown) suggesting that the inhibition of TGR is through the modification of cysteine or selenocysteine residue(s) in the protein.
TGR inhibitors action on cultured worms
We next asked if the TGR inhibitors had any affect on the survival of axenically cultured adult S. mansoni worms. Adult S. mansoni worms were cultured in the presence of different concentrations of the inhibitors and mobility and parasite death were monitored. The oxadiazole 2-oxides showed similar effects on worm survival with activity at concentrations as low as 5 μM (Fig. 1). However, 9 was the most active compound tested: 10 μM 9 resulted in 100% parasite death within 24 hr and 2 μM 9 killed worms in 120 hr. The activities of the phosphinic amides were markedly less than the oxadiazoles: 3 at 50 μM resulted in 100% worm death in 24 hr and at 25 μM in 96 hr, while lower concentrations of 3 and all other phosphinic amides were inactive against worms (Fig. 1).
Fig. 1.
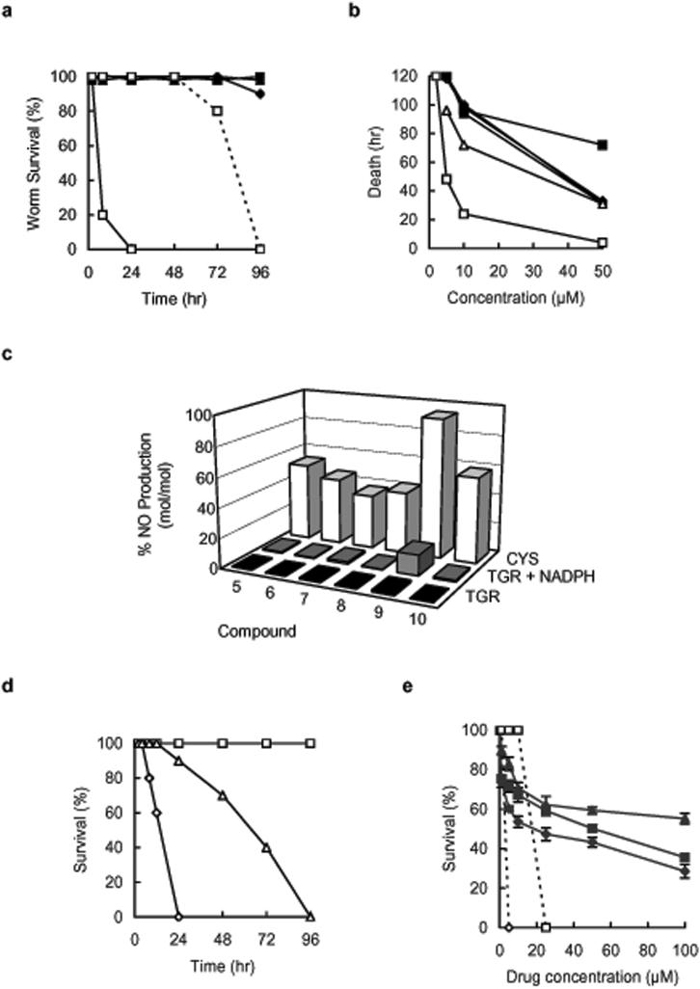
Activity of phosphinic amides and oxadiazole 2-oxides against cultured Schistosoma mansoni worms. (a) Survival of cultured adult S. mansoni worms treated with phosphinic amides. Cultured worms treated with 50 μM 1 (◆), 50 μM 2 (■), 50 μM 3 (□), 25 μM 3 (□, dotted line) and 50 μM 4 (▲). (b) Survival of cultured adult S. mansoni worms treated with oxadiazole 2-oxides. Cultured worms were treated with 50 μM 5 (◆), 6 (■), 7 (▲), 8 (◇), or 10 (△), or 10 μM 9 (□). (c) NO release by oxadiazole 2-oxides (10 μM) after incubation with 15 nM S. mansoni TGR (black bars), with 15 nM S. mansoni TGR plus 100 μM NADPH (gray bars), or with 5 mM cysteine alone (open bars) was determined using ABTS method. (d) Survival of adult worms in 10 μM 9 (◇), 10 μM 9 plus 100 μM carboxy-PTIO (△), and drug carrier plus 100 μM carboxy-PTIO treated control (□). (e) Cytotoxicity after five days culture at the indicated concentrations of compounds 3 (■) and 9 (◆) and praziquantel (▲) against mouse myeloma line SP2/0 (solid lines) and adult Schistosoma mansoni worms (dashed lines). Error bars represent the ± standard deviation form three independent experiments.
Nitric oxide production and activity of oxadiazoles
Because oxadiazoles are known to be nitric oxide (NO) donors in the presence of physiological levels of thiols21 we examined if NO release played a part in their mechanism of action against worms. First, it was determined that all oxadiazoles produced NO at approximately the same efficiency when incubated in 5 mM cysteine (Fig. 1). The NO releasing capability of oxadiazoles was next tested with recombinant S. mansoni TGR with and without NADPH. Compound 9 showed ten times greater NO release in the presence of TGR plus NADPH than in its absence and than the other oxadiazoles (Fig. 1).
Since 9 was both the most efficient NO donor in the presence of TGR and was more active against cultured worms than the other oxadiazoles, we hypothesized that NO production may be involved in the action of 9 against cultured worms. To investigate this we cultured adult S. mansoni worms with 100 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO), a specific tool for NO purging and scavenging22, in the presence or absence of 10 μM 9. Worm viability in the presence of 9 plus the NO scavenger was extended and similar to those seen for worms cultured with the other oxadiazoles (Fig. 1). Survival of worms in exogenously generated NO (sodium nitroprusside + 5 mM cysteine) was not affected by concentrations up to 1 mM sodium nitroprusside (not shown). This corresponds to a concentration of 0.72 mM NO (under these conditions sodium nitroprusside + cysteine generates 72.52 % NO mol/mol, not shown). The lethal effect of sodium nitroprusside on worm survival was only seen at concentrations of 2 mM sodium nitroprusside or higher or about 1,000 times higher concentration than an effective dose of 9.
Drug cytotoxicity screening
To examine if 3 and 9 are tolerated by mammalian cells, we incubated the murine myeloma cell line SP2/0 with the drugs and monitored cell viability after 120 hr. All worms were killed by concentrations of 9 as low as 5 μM and 3 at 25 μM (Fig. 1). At 25 μM 3, 9, or PZQ the viability of the myeloma cells was 59%, 54%, and 62%, respectively (Fig. 1). Although 3 and 9 both appear to be more toxic to mammalian cells than PZQ, concentrations as high as 100 μM were tolerated (Fig. 1). Differential cytotoxicity of both 3 and 9 on the worm survival and myeloma cells was also seen after 24 hrs of drug application (not shown).
Action of inhibitors on cultured worms
Because both 3 and 9 inhibit recombinant parasite TGR and cause rapid death of cultured parasites, we examined if the mechanism of action of these compounds in living worms was through, at least in part, inhibition of TGR activity. Freshly perfused adult S. mansoni worms were cultured with 10 μM 9 or 50 μM 3. Because parasite TGR is a multifunctional enzyme and possesses both thioredoxin reductase and GSH reductase activities both activities were determined in worm homogenates. The activity of lactate dehydrogenase was monitored as a control enzyme. A progressive decrease in TGR activity was seen after treatment with either 3 or 9 and after 18 hrs thioredoxin reductase and GSH reductase activities were reduced by 83% and 93% for 9 and 69% and 59% for 3, respectively, compared to carrier treated-control worms (Fig. 2). The activity of parasite lactate dehydrogenase in homogenates from 3- and 9-treated worm showed no significant change from carrier only treated worms (Fig. 2).
Fig. 2.
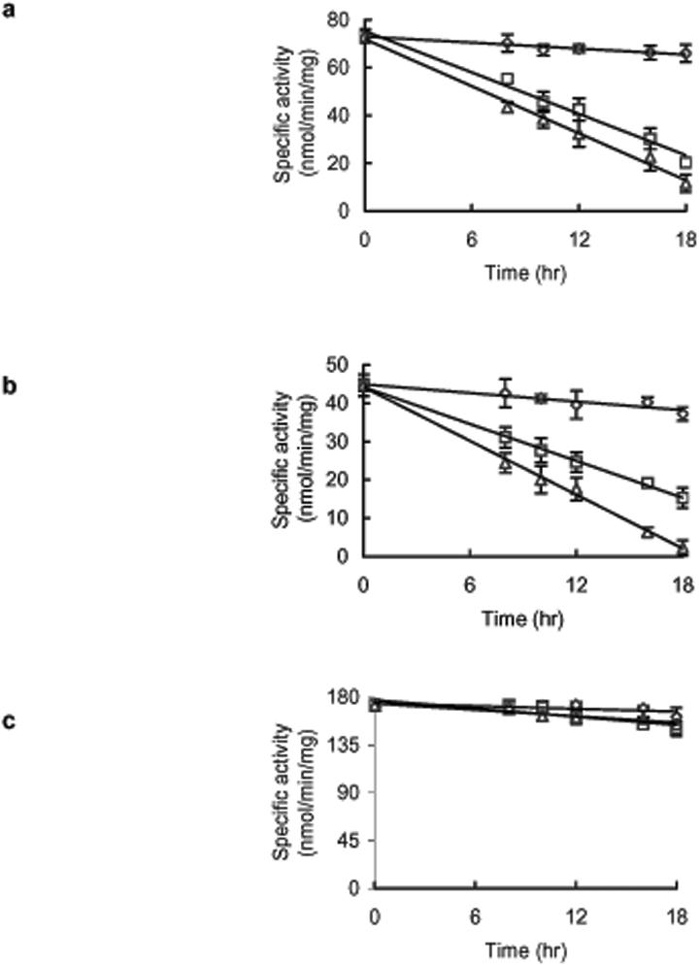
The action of compounds 3 and 9 on TGR activities in cultured Schistosoma mansoni worms. (a) The specific thioredoxin reductase activity of TGR in worm homogenates from control worms (◇), 50 μM 3 treated worms (□), and 10 μM 9 treated worms (△). (b) The specific glutathione reductase activity of TGR in worm homogenate from control worms (◇), 50 μM 3 treated worms (□), and 10 μM 9 treated worms (△). (c) The specific activity of lactate dehydrogenase in worm homogenates from control worms (◇), 50 μM 3 treated worms (□), and 10 μM 9 treated worms (△).The error bars represent ± standard deviation from the average of three independent experiments.
In vivo action of 4-phenyl-1,2,5-oxadiazole-3-carbonitrile-2-oxide against schistosome infections
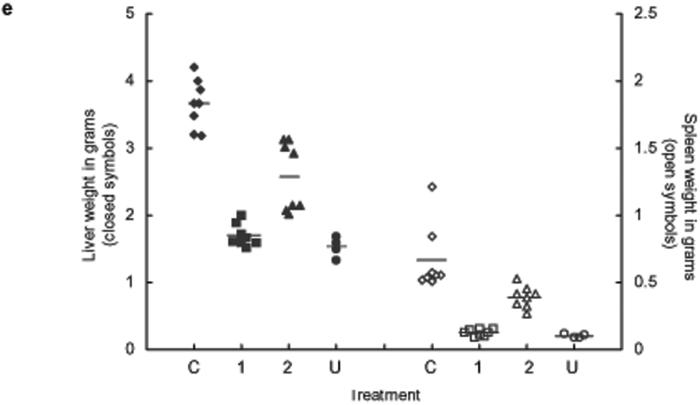
The efficacy of 9 against different parasite life stages in an experimental mammalian host was determined. In all treatments 9 was administrated once daily for 5 consecutive days by intraperitoneal injection at 10 mg/kg to S. mansoni infected mice; treatment 1 started one day after infection (skin stage parasites), treatment 2 started 23 days after infection (juvenile, liver-stage parasites), and treatment 3 started 37 days after infection (adult, egg laying parasites in the mesenteric system) (Fig. 3). In all treatments adult worms were quantified after perfusion of mice 49 days post infection. In all experimental treatments large and highly significant reductions in worm burdens were observed. Treatment 1 resulted in decrease in worm burden of >99% (P < 0.0001) compared to the control S. mansoni infected mice (Fig. 3). The protective effect of 9 was also seen in a significant reduction of hepato- and splenomegaly (P < 0.0001, Fig. 3) compared to control infected mice; indeed the weights of the spleens and livers in this treatment were not statistically different from livers from uninfected age-matched mice (P > 0.1, Fig. 3) and showed no signs of egg-induced liver granulomas. Treatment 2 resulted in a decrease of 89 % (P < 0.0001) in worm burdens (Fig. 3) and dramatic reductions in hepato- and splenomegaly relative to control infected mice (P < 0.0001, Fig. 3). The number of liver granulomas was also considerably reduced compared to the control infections (Fig. 3). Moreover, the worms recovered were distinctly smaller after this treatment, especially male worms (Fig. 3). An equal number of male and female worms were recovered from this treatment indicating that 9 was equally active against both worm sexes. Treatment 3 resulted in a decrease of 94 % (P < 0.0001) in worm burdens relative to control infected mice (Fig. 3). The livers collected from this treatment also exhibited a greatly reduced numbers of egg-induced granulomas compared to the control group (Fig. 3) and dramatic reductions in hepatomegaly (P < 0.0001, not shown).
Fig. 3.
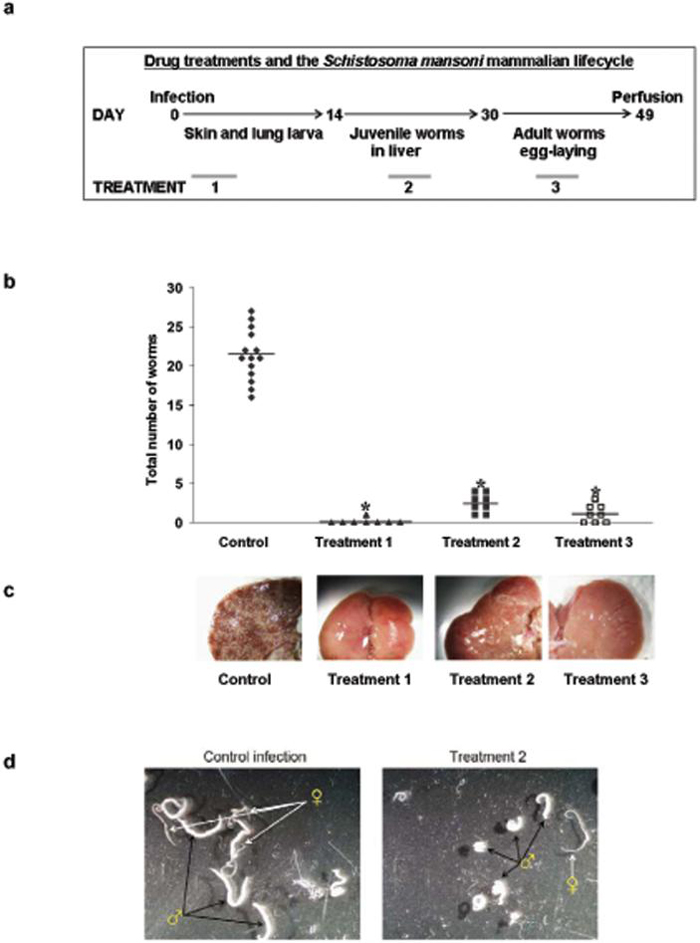
In vivo drug treatment with compound 9. (a) Administration of compound 9 at 10 mg/kg for 5 days at different points during the development of Schistosoma mansoni in the mouse. (b) The effect on adult worm burdens after treatment of S. mansoni infected mice with compound 9 in treatment 1 (▲, skin-stage parasites, n= 8), treatment 2 (■, juvenile liver-stage parasites, n = 8), treatment 3 (□, Adult egg-laying parasites, n= 8); drug carrier treated control infected mice (◆, n = 16). Points represent data from individual mice; * P < 0.0001. The horizontal bars represent the average point of each treatment. (c) Pictures of the livers collected from the different treatments, the white dots are liver granulomas resulting from host immunological reaction against trapped parasite eggs. (d) Worms recovered from treatment 2 and control infected mice. Worms recovered from treatment 2 are small and stunted, especially males, relative to worms recovered from untreated mice. (e) Anti-pathology effects of treatment with compound 9. Liver (closed symbols) and spleen (open symbols) weights from infected control mice (I, ◆), treatment 1 (1, ■), treatment 2 (2, ▲) and uninfected age matched control mice (U, •); * P < 0.0001.
Discussion
In this study we have identified oxadiazole 2-oxides as new lead compounds for schistosomiasis chemotherapy. Compound 9, the most active in the series, provides parasitological cures of infected animals when given at any point during the infection. Furthermore, we have shown that the primary target of 9 in cultured worms is the parasite redox protein TGR, which is rapidly inhibited after exposure to the compound, and that the anti-schistosome activity of 9 is associated with a donation of NO through the action of the target enzyme.
The nearly complete reliance on PZQ for schistosomiasis treatment jeopardizes control efforts should resistance arise. Therefore, it is important to identify drugs to replace PZQ or, preferably, to be used in combination with PZQ to prevent resistance from developing. The development of drugs for Neglected Tropical Diseases (NTDs) is hampered by the general lack of interest by the pharmaceutical industry in this class of diseases and fewer than 1% of all new drugs target NTDs23. Partnerships to develop drugs for NTDs (as well as rare and orphan diseases) include the NIH Molecular Libraries Initiative (MLI), which was started in 2004 to provide academic investigators with the infrastructure and technologies necessary to discover both chemical probes for investigating fundamental biological phenomena and starting points for development of novel therapeutics for human disease24. The work described here is the product of the first assay officially accepted for screening by the MLI.
The most potent compound investigated in this study was an oxadiazole 2-oxide, which have been of pharmacological interest because of their ability to donate NO and have been investigated for vasodilating, platelet anti-aggregating, anti-infective and other properties25. The death of treated cultured worms is preceded by the inhibition of the glutathione reductase and thioredoxin reductase activities of TGR providing evidence that the antihelminthic effects of the 3 and 9 are due to the inhibition of TGR. No inhibition of an abundant and essential enzyme lactate dehydrogenase was seen. It is likely that the action on worms of 9 is intricately associated with NO donation and TGR inhibition. Nitric oxide produced by human white cells has been shown to kill larval schistosome parasites26. The release of NO by 9 has been demonstrated to be contingent upon a ligation event with free thiols27. The combined role of TGR inhibition by covalent modification of a cysteine or selenocysteine and the subsequent release of NO are undoubtedly associated with worm killing. Here it was determined that NO scavenging decreased the activity of 9. However, we found that NO was well tolerated by adult worms when generated exogenously. Production of NO was found to be enhanced by addition of NADPH, which indicated an enzymatic NO release by TGR. Inhibition of TGR activity would lead directly to the inactivation of both the thioredoxin- and glutathione-based defenses and the accumulation of reactive oxygen and nitrogen species. With the concomitant production of NO by TGR, there would be increased nitrosative stress. Furthermore, it has been shown that inhibition of the related mammalian enzyme thioredoxin reductase leads to an increase in the NADPH oxidase activity of that enzyme resulting in the production of superoxide28. It is likely that inhibition of TGR will increase its NADPH oxidase activity.
The activity of compound 9 surpasses criteria established by the World Health Organization for potential lead compounds for schistosomiasis: active `hits' should result in 100% inhibition of motility of adult parasites at 5 μg/ml and highly active lead compounds are defined as those with 80% reduction in worm burdens when administered in 10% DMSO in 5 injections at 100 mg/kg29. At 1.87 μg/ml 9 produces 100% inhibition of motility of adult parasites in 24 hr and concentrations as low as 0.38 μg/ml are active at longer times. All trials in mice resulted in at least an 88% reduction in worm burdens when 9 was administered in five doses at 10 mg/kg. Furthermore, because schistosome TGR is not inhibited by PZQ19, 9 and PZQ have different mechanisms of action and selection of cross-resistant parasites is highly unlikely.
It is significant to note that 9 is highly active against all intra-mammalian lifecycle stages of S. mansoni, with at least an 88% and as high as 99% reductions in worm burdens depending on the lifecycle stage present during the treatment, which is equal to or better than PZQ and artemether. In schistosomiasis worm burden reductions are associated with reduced pathology and there is no concern about relapse as schistosome parasites do not multiply in the mammalian host. Compound 9 appears to be particularly active against early stages of the infection as only one adult parasite was recovered from the eight mice infected. Previous studies indicated that neither PZQ nor artemether display such broad activity. PZQ is much less effective against juvenile liver parasites than against adult parasites, with only a 25-30% reduction in worm burdens30. Although artemether affords ~80% reduction if juvenile parasites are targeted, it is less effective against adult schistosome parasites, resulting in less than 50% reduction in worm burden31. Therefore, 9, or its derivatives, could be useful as a prophylactic treatment (it is effective against skin and migrating parasites) and would kill adult parasites, which trigger disease by egg production and are typically the diagnostic stage.
In this study we have identified oxadiazole 2-oxides as new lead compounds for schistosomiasis chemotherapy. The level of activity of 9 was equal to or better than the currently used drug and surpasses all landmarks for new lead compounds for schistosomiasis control. The low cytotoxicity and high bioavailability, bioactivity and tolerance by mice of 9 support the potential of this compound as a highly effective lead compound for human schistosomiasis. Compound 9 is amenable to derivatization and efforts are underway to identify selective and active derivatives and uncover the mechanism of inhibition of TGR.
Methods
Active Compounds
The phosphinic amides and oxadiazole 2-oxides were identified from a quantitative high-throughput screen against the TGR/Prx2 redox cascade of S. mansoni (AS, A. Jadhav, AAS, Y. Wang, M.E. Nelson, CJT, JI, DLW, CPA, submitted), in which screening library members were assayed in a concentration-response format in 1536-well plate format20. After data analysis, actives identified from the screen, as well as untested analogues, were purchased as powders of greater than 90% purity from the respective primary vendors. Confirmatory, target deconvolution, and the present studies were performed using these separately-sourced samples.
Parasite preparation
Mice (NIH-Swiss) were infected by percutaneous tail exposure for 2 hr to 60 S. mansoni cercariae (NMRI strain) for in vivo studies or 180 cercariae to obtain adult parasites for in vitro studies32. Adult S. mansoni worms were obtained from 7-week infected mice by perfusion with RPMI1640 media32. The worms were collected and washed at least twice with fresh media and incubated for 1 hr at 37 °C in 5% CO2 incubator before any in vitro drug treatments were conducted. This study was approved by the Institutional Animal Care and Use Committee of Illinois State University (05-2006; DHHS animal welfare assurance number A3762-01).
Enzyme assays
Enzyme preparation and assays were as described as described19 with 15 nM TGR at 25°C in 0.1 M potassium phosphate, pH 7.4, 10 mM EDTA. Thioredoxin reductase activity of TGR was determined using either 3 mM 5,5' dithiobis(2-nitrobenzoic acid) (DTNB, Ellman's reagent) or 10 μM recombinant 6-histidine tagged S. mansoni thioredoxin-2 (Lisa Beers and DLW, unpublished). One enzyme unit was defined as the NADPH-dependent production of 2 μmol of 2-nitro-5-thiobenzoic acid per minute using ε412 nm = 13.6 mM-1 cm-1 or the consumption of 1 μmol of NADPH (ε340 nm = 6.22 mM-1 cm-1) during the first three minutes. Glutathione reductase activity was determined with 100 μM GSH disulfide and 100 μM NADPH by measuring the decrease in A340 nm due to consumption of NADPH (ε340 nm = 6.22 mM-1 cm-1) during the first three minutes. Activity of the control enzyme lactate dehydrogenase (LDH) was determined with 10 μM sodium pyruvate and 100 μM NADPH. Each assay was done in triplicate and each experiment was repeated three times.
Inhibitor Studies on Cultured Worms
Compounds were dissolved in dimethylsulfoxide (DMSO) and added at concentrations indicated to freshly perfused worms in RPMI1640 containing 25 mM Hepes, pH 7, 150 units/ml penicillin, 125 μg/ml streptomycin, and 10% fetal calf serum (Cell Grow, Fisher). Media were replaced every two days with fresh media with addition of the compounds at the designated concentrations. Control worms were treated with equal amounts of DMSO alone. Worms were subsequently observed for motility and mortality and collected at the indicated times for analysis. Worms were homogenized by sonication in PBS and homogenates were assayed for enzyme activities as described. To asses the importance of NO production the potassium salt of the compound 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO, Invitrogen), a NO scavenger, was dissolved in water and incubated with freshly perfused S. mansoni worms at 100 μM in the presence or absence of 10 μM 9 as described.
In vivo drug treatments
Compound 9 was dissolved in DMSO and administrated by intraperitoneal injection to S. mansoni infected mice (NIH-Swiss, National Cancer Institute) at 10mg/kg once a day for 5 consecutive days following the schedule in Figure 3. Compound 9 at this dosage has been shown to be well tolerated by mice33. Control S. mansoni infected mice were administrated a corresponding amount of the drug carrier on the same timetable. Age-matched uninfected mice were used as reference group.
Cytotoxicity Assay
Cytotoxicity assays were performed using sulforhodamine B to determine cellular protein content as described34. Murine myeloma cell line SP2/0 was cultured in 96-well microtiter plates containing 0.2 ml of RPMI-1640 per well at a cell density of 900 per well at 37 °C in 5% CO2. Cells were treated with drug concentrations (or drug carrier alone) for times as indicated. After treatment, cells were fixed with 10% TCA at 4 °C for 1 hr. Fixed cells were rinsed to remove fixative and then stained in 0.4% (wt/vol) sulforhodamine B (Sigma) in 1% acetic acid for 35 min. After washing with 1% acetic acid and dye extraction in 10 mM Tris (pH 10.5), plates were read at A564nm. The A564nm of drug-treated cells was compared to carrier-only-treated cells.
Determination of NO release
NO release by different oxadiazoles was determined by 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS, Sigma) oxidation method35. Different oxadiazoles at 10 μM were incubated at RT for 50 minutes with either 5 mM cysteine or 15 nM recombinant TGR in the presence or absence of 100 μM NADPH. The reaction mixture was then added to aerobic PBS, pH 7.4, containing 5 mM ABTS. The absorbance of the resulting green ABTS+ were measured at A660. The conversion from 1 ml reaction to 96-well plate format used was performed by plotting the oxidized form of ABTS (ABTS+) from the reduced form ABTS (ABTS+-ABTS) versus different concentration of tested ABTS. The slope of the regression line was calculated and used to recalculate the data at A660 in a 200 μl reaction. The concentrations of the released NO were calculated as a percentage of the added oxadiazoles. Each reaction was done in triplicate and the data is the average of three independent experiments.
Statistical analysis
The statistical significance of worm burden, hepatomegaly, and splenomegaly reduction between experimental groups compared to the controls was determined by two-tailed Student's T test.
Supplementary Material
Acknowledgements
Funding. This work was supported in part by NIH/NIMH grant R03MH076449 (DLW) and by NIH/NIAID grant R01AI065622 (DLW). Schistosome life stages used in this research were supplied in part by the National Institute of Allergy and Infectious Diseases Schistosomiasis Resource Center at the Biomedical Research Institute (Rockville, Maryland, United States) through NIAID Contract N01-AI-30026.
Footnotes
Competing interests. The authors have declared that no competing interests exist.
References
- 1.Hotez PJ, et al. Control of neglected tropical diseases. New Eng. J. Med. 2007;357:1018–1027. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 2.van der Werf MJ, et al. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 3.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 4.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 5.Fenwick A, Webster JP. Schistosomiasis: challenges for control, treatment and drug resistance. Curr. Opin. Infect. Dis. 2006;19:577–582. doi: 10.1097/01.qco.0000247591.13671.6a. [DOI] [PubMed] [Google Scholar]
- 6.Herwaldt BL, Tao LF, van Pelt W, Tsang VC, Bruce JI. Persistence of Schistosoma haematobium infection despite multiple courses of therapy with praziquantel. Clin. Infect. Dis. 1995;20:309–315. doi: 10.1093/clinids/20.2.309. [DOI] [PubMed] [Google Scholar]
- 7.Murray-Smith SQ, Scott BJ, Barton DP, Weinstein P. A case of refractory schistosomiasis. Med. J. Aust. 1996;165:458. doi: 10.5694/j.1326-5377.1996.tb138598.x. [DOI] [PubMed] [Google Scholar]
- 8.Ismail M, et al. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg. 1999;60:932–935. doi: 10.4269/ajtmh.1999.60.932. [DOI] [PubMed] [Google Scholar]
- 9.Fallon PG, Doenhoff MJ. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am. J. Trop. Med. Hyg. 1994;51:83–88. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- 10.Utzinger J, Xiao SH, Tanner M, Keiser J. Artemisinins for schistosomiasis and beyond. Curr. Opin. Investig. Drugs. 2007;8:105–116. [PubMed] [Google Scholar]
- 11.Cioli D, Pica-Mattoccia L, Archer S. Drug resistance in schistosomes. Parasitol. Today. 1993;9:162–166. doi: 10.1016/0169-4758(93)90138-6. [DOI] [PubMed] [Google Scholar]
- 12.LoVerde PT. Do antioxidants play a role in schistosome host-parasite interactions? Parasitol. Today. 1998;14:284–289. doi: 10.1016/s0169-4758(98)01261-7. [DOI] [PubMed] [Google Scholar]
- 13.Rhee SG, Chae HZ, Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Sayed AA, Cook SK, Williams DL. Redox balance mechanisms in Schistosoma mansoni rely on peroxiredoxins and albumin and implicate peroxiredoxins as novel drug targets. J. Biol. Chem. 2006;281:17001–17010. doi: 10.1074/jbc.M512601200. [DOI] [PubMed] [Google Scholar]
- 15.Sayed AA, Williams DL. Biochemical characterization of 2-Cys peroxiredoxins from Schistosoma mansoni. J. Biol. Chem. 2004;279:26159–26166. doi: 10.1074/jbc.M401748200. [DOI] [PubMed] [Google Scholar]
- 16.Townsend DM, Tew KD, Tapiero H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lillig CH, Holmgren A. Thioredoxin and related molecules--from biology to health and disease. Antioxid. Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 18.Alger HM, Williams DL. The disulfide redox system of Schistosoma mansoni and the importance of a multifunctional enzyme, thioredoxin glutathione reductase. Mol. Biochem. Parasitol. 2002;121:129–139. doi: 10.1016/s0166-6851(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 19.Kuntz AN, et al. Thioredoxin Glutathione Reductase from Schistosoma mansoni: An Essential Parasite Enzyme and a Key Drug Target. PLoS Med. 2007;4:e206. doi: 10.1371/journal.pmed.0040206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inglese J, et al. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feelisch M, Schönafinger K, Noack E. Thiol-mediated generation of nitric oxide accounts for the vasodilator action of furoxans. Biochem. Pharmacol. 1992;44:1149–1157. doi: 10.1016/0006-2952(92)90379-w. [DOI] [PubMed] [Google Scholar]
- 22.Akaike T, et al. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing facto/•NO through a radical reaction. Biochemistry. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- 23.Trouiller P, et al. Drug development for neglected diseases: a deficient market and a public-health policy failure. Lancet. 2002;359:2188–2194. doi: 10.1016/S0140-6736(02)09096-7. [DOI] [PubMed] [Google Scholar]
- 24.Austin CP, Brady LS, Insel TR, Collins FS. NIH Molecular Libraries Initiative. Science. 2004;306:1138–1139. doi: 10.1126/science.1105511. [DOI] [PubMed] [Google Scholar]
- 25.Cerecetto H, Porcal W. Pharmacological properties of furoxans and benzofuroxans: recent developments. Mini Rev. Med. Chem. 2005;5:57–71. doi: 10.2174/1389557053402864. [DOI] [PubMed] [Google Scholar]
- 26.James SL, Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J. Immunol. 1989;143:4208–4212. [PubMed] [Google Scholar]
- 27.Medana C, et al. Furoxans as nitric oxide donors. 4-Phenyl-3-furoxancarbonitrile: thiol-mediated nitric oxide release and biological evaluation. J. Med. Chem. 1994;37:4412–4416. doi: 10.1021/jm00051a020. [DOI] [PubMed] [Google Scholar]
- 28.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 29.Nwaka S, Hudson A. Innovative lead discovery strategies for tropical diseases. Nat. Rev. Drug Discov. 2006;5:941–955. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- 30.Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Exp. Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- 31.Xiao SH, Chollet J, Weiss NA, Bergquist RN, Tanner M. Preventive effect of artemether in experimental animals infected with Schistosoma mansoni. Parasitol. Int. 2000;49:19–24. doi: 10.1016/s1383-5769(00)00028-3. [DOI] [PubMed] [Google Scholar]
- 32.Lewis F. Schistosomiasis. Suppl. 28: 19.1.1-19.1.28. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons; New York: 1998. [Google Scholar]
- 33.Aguirre G, et al. Furoxan derivatives as cytotoxic agents: preliminary in vivo antitumoral activity studies. Pharmazie. 2006;61:54–59. [PubMed] [Google Scholar]
- 34.Skehan P, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990;82:107–112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 35.Nims RW, et al. Colorimetric methods for the determination of nitric oxide concentration in neutral aqueous solutions. Methods. 1995;7:48–54. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.