Abstract
Previous studies suggest that all effective antidepressant (AD) drugs decrease activity of locus coeruleus (LC) neurons. However, little data exist regarding blood levels of drug in these studies, and what data do exist suggest blood levels might have been very high. To assess whether decreased LC activity is produced by drugs that selectively block reuptake for either norepinephrine or serotonin at therapeutically relevant blood levels, effects of chronic administration of desipramine, paroxetine, and escitalopram on LC activity were measured across a range of doses and blood levels of drug. Further, effects of a range of doses of mirtazapine were examined; in that mirtazapine blocks α2 adrenergic receptors, it might be anticipated to increase rather than decrease LC activity. Finally, to begin to assess whether the response of LC to ADs was specific to these drugs, effects of four non-AD drugs (single dose) were measured. Drugs were administered via osmotic minipump for 14 d. Electrophysiological recording of LC activity (assessment of both spontaneous firing rate and sensory-evoked ‘burst’ firing) then took place under isoflurane anaesthesia on the last day of drug treatment. The blood level of drugs present at the end of the recording session was also measured. All AD drugs tested decreased LC spontaneous and sensory-evoked ‘burst’ firing, and this was observed across a wide range of blood levels for the drugs. Non-AD drugs did not decrease LC activity. The findings of this investigation continue to support the possibility that all effective AD drugs decrease LC activity.
Keywords: Electrophysiology, locus coeruleus, mirtazapine, SSRI, tricyclic
Introduction
We previously presented data and reviewed findings, both from experimental animals and humans, suggesting that all effective antidepressant (AD) drugs and electroconvulsive shock cause a decrease in the activity of locus coeruleus (LC) neurons, the major noradrenaline-containing cell body group in the brain (Grant and Weiss, 2001). The results reported in that paper showed that chronic treatment with any one of five different AD drugs — two tricyclics, two selective serotonin reuptake inhibitors (SSRIs), and a monoamine oxidase inhibitor — or a series of electroconvulsive shocks all decreased electrophysiological activity of LC neurons in rat brain, both spontaneous firing rate and sensory-evoked ‘burst’ firing of the LC neurons. Publication of this report elicited a comment from Szabo and Blier (2001a) who pointed out that five other AD drugs could be added to the list of drugs that decreased LC activity (only spontaneous activity was measured in these studies). However, they also pointed out that one AD drug tested in conjunction with a larger study — mirtazapine — produced a small increase in LC activity, and this finding therefore called into question the possibility that all effective AD drugs would have the effect of decreasing LC activity.
Another important concern that arose from our original report related to the blood levels of drugs measured in that study. When blood levels of the two tricyclic drugs used (desipramine, imipramine) as well as the two SSRIs assessed (fluoxetine, sertraline) were measured in some animals, levels were found to be quite high, in most cases well above what have been identified as being therapeutic in humans. Similarly, when we also measured blood levels after intraperitoneal (i.p.) injection of desipramine and sertraline given for 21 d in order to compare these values with those obtained with the minipump delivery used in our study, blood levels were again high. The only other report of AD blood levels in conjunction with electrophysiological recording of LC activity was made by Linnér et al. (1999), who measured the blood level for one drug (imipramine) at one dose, and reported the blood level to be within therapeutic range. These limited data, most of which point to high blood levels of AD drug in the animals, leave open the possibility that decreased LC activity may result from abnormally high blood levels of drug, and raise the question of whether reduced LC activity would occur when blood levels were lower and consistent with levels achieved in therapeutic drug regimens.
The present paper describes further examination of the possibility that effective AD drugs decrease LC activity. First, to address the issue of whether decreases in LC activity might occur only when blood levels are abnormally high, assessment was made of representative ADs across a range of doses, with blood levels measured at the various doses given. The tricyclic that was examined — desipramine — was chosen because of its highly selective action in blocking norepinephrine reuptake. For SSRIs, paroxetine was studied because of its highly effective blocking of serotonin reuptake. We then also studied escitalopram, because it is, of presently used AD drugs, the most specific in blocking serotonin reuptake. Second, we addressed the question of whether mirtazapine indeed represents an exception to the possibility that all effective AD drugs will decrease LC activity. Finally, we examined the effects of four drugs (single dose tested) that have behavioural and psychological effects but are not AD in nature; this was done to begin to assess the specificity of AD drugs with regard to decreasing LC activity.
Methods and materials
Subjects
Male Sprague—Dawley rats (virus/antigen free, bred in our laboratory from stock originally obtained from Charles River) were used; a total of 233 rats were utilized in this study. Because the intent of this study was to assess AD responses in adult subjects, mature males aged 5–7 months, weighing 550–700 g at the time that the minipump for drug delivery was implanted, were used in all studies, except for two additional smaller studies noted directly below. In male rats aged 5–7 months, different doses of drug also can be delivered via minipump relatively accurately insofar as their body weight has stabilized and will not change appreciably across the duration of study (usually 14 d). In examining the effects of mirtazapine, younger rats of two additional ages were also studied — age 60 d (350–400 g) and age 45 d (250–300 g). All animals were group-housed (two per cage) directly on bedding in solid-bottom polycarbonate cages throughout the experiment. The animals housed in any one cage all received the same drug (or vehicle). Food (lab chow) and water were available ad libitum. A 12-h light—dark cycle (lights on 07:00 hours) and temperature of ∼21°C was maintained in the colony room. Procedures and animal use described in this paper were approved by the Emory University Institutional Animal Care and Use Committee.
Drugs and administration
The effects of four ADs were examined : desipramine HCl (Des), a secondary amine dibenzazapine tricyclic norepinephrine reuptake inhibitor (Sigma, St Louis, MO, USA), paroxetine HCl (Par) and escitalopram (Esc), both SSRIs (prepared by Dr M. Owens as described in McConathy et al., 2007, and mirtazapine (Mir), a tetracyclic noradrenergic/specific serotonergic AD (Organon, West Orange, NJ, USA). Different doses of each of the four ADs were administered, the intention being to define a dose—response curve for each drug. Drugs were administered via Alzet Osmotic Minipumps (Durect Corporation, Cupertino, CA, USA) implanted subcutaneously, using Model 2ML2 pumps for 14-d administration. Minipumps, which provide continuous drug delivery beginning approximately 4 h after pump implantation, were used to ensure the presence of drug in the animal throughout the study and at the time of electrophysiological recording. Importantly, use of minipumps also eliminates the need for repeated handling and injection of animals to administer the drug chronically. Studies that have assessed effects of daily injection of vehicle while studying effects of AD drugs have found that repeated injections constitute a stressful procedure. For example, repeated vehicle injections have been observed to produce, in comparisons to no such injections, (a) augmented tyrosine hydroxylase mRNA in LC neurons (Kuteeva et al., 2008) well as elevated norepinephrine release in brain as measured by microdialysis (E. Abercrombie, unpublished data), and (b) increased stress-sensitive tumour growth (Garabal et al., 1991). Consequences of daily handling and injection were therefore avoided in the present study. Each animal’s body weight was determined prior to pump implantation and the flow rate of minipumps was used to compute the drug concentration loaded in the pump to achieve the dose given to that rat. The different doses of these drugs administered (in mg/kg.d) were as follows : Des (0.156, 0.31, 0.62, 1.25, 2.5, 5.0, 7.5, 10.0), Par (0.31, 0.62, 1.25, 2.5, 5.0, 10.0), Esc (2.5, 5.0, 10.0), and Mir (0.31, 0.62, 1.25, 2.5, 5.0, 7.5). In the two studies that employed younger animals (i.e. aged 60 d and 45 d), a single dose of Mir (5.00 mg/kg.d) was used. For rats aged 60 d, the drug was administered for 14 d prior to electrophysiological recording as was done with the 5- to 7-month-old rats. In the study using rats aged 45 d, the intent was to more closely reproduce the conditions used by Haddjeri et al. (1997) in their study of the effects of Mir. Therefore, drug was administered for 21 d before electrophysiological measurement, drug delivery being accomplished by replacing the original 2ML2 pump on day 14 of drug administration with a new 2ML2 pump; also, the amount of drug in the new pump was adjusted for any weight gain over the first 14 d of drug delivery to maintain the 5.0 mg/kg.d dose for each animal. Additionally, as done by Haddjeri et al. (1997), chloral hydrate anaesthesia (400 mg/kg i.p.) was used in the electrophysiological recording of these rats, which was also done for the 60-d-old rats as well. Regarding the vehicle for drug delivery, Des was prepared in distilled water; Par in 25% polyethylene glycol (PEG, MW 400), 50% DMSO, and 25% distilled water; Esc in 25% PEG, 50% DMSO, and 25% distilled water acidified with 1.5 molecular equivalents of HCl, and Mir in 20% DMSO and 80% acidified water (one drop glacial acidic acid added to each ml water). To ensure adequate delivery of Esc in view of its solubility and vehicle-related issues, a further step was taken by delivering the drug into the peritoneal cavity rather than subcutaneously. To accomplish this, the minipump was again implanted subcutaneously as described below. In this case, however, a 15 cm length of silastic tubing (0.04 i.d.×0.085 o.d.) was attached onto the output of the minipump, which was covered at this location by a 10 mm length of tygon tubing (0.02 i.d.×0.06 o.d.) to ensure a tight juncture between the tubing and the minipump output. At 15 mm from the distal end of the length of silastic tubing, a 3 mm piece of the larger silastic tubing (0.062 i.d.×0.095 o.d.) was placed onto it forming a bulb at this location. The distal end of the silastic tubing, including the bulb, was then introduced surgically into the peritoneal cavity and the abdominal wall sutured closed so that the bulb prevented the tubing from being withdrawn back into the subcutaneous space, thereby enabling long-term delivery of drug from the pump into the peritoneal cavity. With use of this technique, a delay of ∼24 h occurred after surgery before drug extruded by the pump filled the 15 cm length of tubing and began entering the peritoneal cavity. For each of the drugs described above, a group of animals treated only with vehicle was included to establish the ‘no drug’ level of LC electrophysiological activity. Pumps were implanted subcutaneously under isoflurane anaesthesia in the dorsal rear flank region and the wound closed with stainless steel clips ; details of this surgery can be found in West and Weiss (1998). Following surgery, animals were returned to the home cage and not disturbed until removed for the electrophysiological recording session.
For Mir, effects of acute drug administration on LC activity were also examined. This was done by injecting either Mir (5.0 mg/kg) or vehicle i.p., and then assessing activity of LC neurons beginning 10 min after the i.p. injection.
Four psychoactive drugs that are not effective AD treatments were also tested. Each of these drugs was also delivered via subcutaneous mimipump (2ML2), and effects tested after 14 d of administration, as with the AD drugs. The drugs were: scopolamine (Scop), an anticholinergic ; chlorpheniramine (Cpa), an antihistamine; chlordiazepoxide (Cdz), a benzodiazepine tranquillizer ; and amphetamine (Amph), a stimulant. For these drugs, it was not deemed necessary to ascertain a dose—response relationship ; rather, the intention was to determine whether an effective dose would produce a change in LC activity similar to what is seen with ADs; therefore, a single dose of these drugs was tested. The doses given were (in mg/kg.d): Scop, 2.0 ; Cpa, 10.0; Cdz, 2.0, and Amph, 2.0. The dose used was selected based on the same criteria we have used previously in studies of drug effects (both of AD and non-AD drugs) in which a single dose has been employed (e.g. Grant and Weiss, 2001; West and Weiss, 1998, 2005); i.e. a clearly effective dose of the drug on acute administration was selected and given via minipump at that dose per day.
Electrophysiological recording
Recording of single-unit electrophysiological activity was performed under isoflurane anaesthesia as described in Borsody and Weiss (1996); technical details of the recording process can be found in that reference. Following anaesthesia and opening of the skull, a glass micropipette was slowly lowered into the brain in the region of the LC until single-unit activity was detected. Recording of LC neurons was verified by criteria described in various studies (Aston-Jones and Bloom, 1981; Borsody and Weiss, 1996; Foote et al., 1980; Graham and Aghajanian, 1971; Korf et al., 1974; Simson and Weiss, 1987); these criteria were: (a) a long-duration action potential with a positive—negative waveform and a notch on the ascending limb (see Figure 1a), (b) a spontaneous firing pattern of 0.1–3.5 Hz, and (c) elicitation of a rapidly occurring succession of spikes (‘burst’ firing) followed by a period of quiescence (post-stimulus inhibition) in response to application of a salient sensory stimulus (in this case, compression of the contralateral hind paw) (see Figure 1b). When a single unit of stable amplitude demonstrating these characteristics was isolated, spontaneous rate of firing was recorded for 3 min, with activity during the last 2 min used to determine the spontaneous firing rate for that unit. The magnitude of the sensory-evoked burst response of the neuron was then measured. For this procedure, the paw was compressed for 1.0 s between the ends of a pair of 13.0 cm surgical forceps (Malony, curved end, Jarit Surgical Instrument Co., Plainsboro, NJ, USA) by applying pressure midway along the forceps such that the opposite sides of the forceps, at this midpoint, came into contact. Paw compression (PC) applied in this manner reliably elicits a burst of action potentials in the anaesthetized rat, as shown Figure 1b. The magnitude of the burst response (i.e. the number of spikes in a ‘burst’) has been shown to be little affected by the intensity of the PC; rather, a burst will occur when the intensity of the PC exceeds the threshold needed to elicit a burst response, with the number of spikes in a burst then determined by factors unrelated to PC intensity such as activation of receptors on the LC neuron, resting potential of the cell, etc. (see Simson and Weiss, 1989). Moreover, application of successive PCs has not been observed to produce sensitization. To determine the amount of sensory-evoked burst firing by a unit, 10 PCs were applied, with each PC spaced at least 10 s apart. Spikes occurring in the first 0.5 s of the PC were counted; this was done because almost all sensory-evoked burst firing for both drug-treated and vehicle-treated animals took place within the first 0.5 s of the PC (see Figure 1). The amount of sensory-evoked burst firing of a unit was established by using the average of the 10 elicited bursts. It should be noted that while drug administration was observed to affect the number of spikes in burst firing, drugs were not observed to affect the rapidity of spikes occurring in a burst. Following the last of the 10 PC applications, spontaneous activity was recorded for 1 min (to verify that the cell returned to its normal baseline firing rate), after which the electrode was slowly moved to isolate another unit. Whenever possible, several units (3–5) were recorded in this manner from an animal, but no more than 5 units from any one animal.
Figure 1.
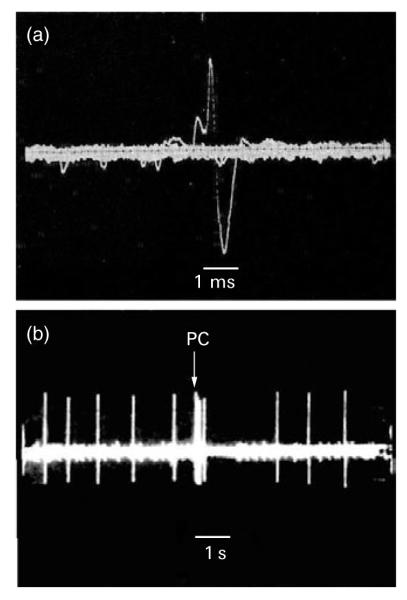
(a) A typical positive—negative action potential waveform, with a notch on the ascending limb, of a locus coeruleus (LC) neuron (bar, 1.0 ms). (b) The typical response of an LC neuron to brief (1 s) paw compression (PC) of the contralateral paw. Application and duration of the PC is marked by the time bar (1.0 s). Note that before PC the cell fires at a slow, regular rate in the isofluane-anaesthetized animal (spontaneous firing), and that a marked increase in firing occurs when the PC is applied (sensory-evoked burst firing) followed by a period of quiescence (post-stimulus inhibition).
Measurement of blood levels of AD drugs
To ascertain the level of the AD drugs in circulation at the time of electrophysiological measurement, blood levels of these drugs were measured. Following the recording session, animals were sacrificed by decapitation and trunk blood collected. Serum from samples was maintained frozen at -80 °C until analysed. The levels of Des were determined by the method of Mazhar and Binder (1989) and the levels of Par, Esc, and Mir determined by the method described in Ritchie and Zhang (1996).
Statistical analysis
Statistical analysis, which was performed on the activity of the individual units, was conducted primarily by using one-way analyses of variance (ANOVA). If a significant main effect of treatment (p<0.05) was obtained, the significance of the difference between any treatment condition included in the analysis (either dose of drug or different drug) and the control condition in that analysis (i.e. vehicle-treated) was then determined using Dunnett’s test. In instances where only two conditions were included in the experiment (e.g. vehicle vs. drug), statistical significance was determined by t test.
Results
Desipramine
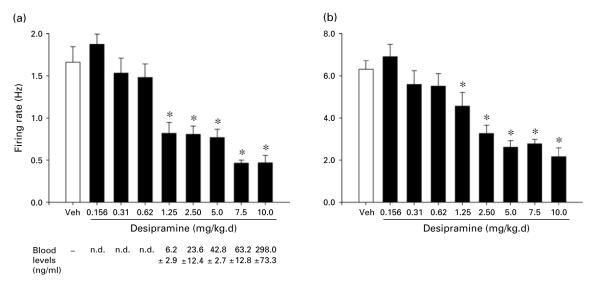
Figure 2 shows the effects on spontaneous firing rate and sensory-evoked burst firing of LC neurons of 14-d treatment with various doses of Des. Effects of eight doses of drug are shown in comparison with the vehicle (Veh, no drug). One-way ANOVAs performed on these data revealed a statistically significant effect of group (i.e. drug dose) for both spontaneous firing rate [F(8, 164)=20.8, p<0.001] and sensory-evoked burst firing [F(8, 164)=15.1, p<0.001]. Subsequent comparisons of each drug to the vehicle condition (by Dunnett’s test) indicated that both spontaneous firing rate and sensory evoked burst firing were significantly decreased by Des at each dose of 1.25 mg/kg.d or higher. Mean blood levels of drug (ng/ml) present in the animals at the end of the recording session are shown below the drug-dose designations in the figure.
Figure 2.
(a) Spontaneous firing rate and (b) sensory-evoked burst firing of LC neurons with administration of vehicle (Veh) or different doses of desipramine (Des) for 14 d via subcutaneous minipump. Des doses ranged from 0.156 to 10.0 mg/kg.d. Means and standard errors are shown. Sensory-evoked burst firing shows the rate based on the number of spikes in the first 0.5 s of a compression of the paw contralateral to the LC from which recording was being made. Numbers of cells comprising the means shown were (for ascending doses): 22, 25, 16, 16, 16, 16, 16, 30, 16; numbers of animals assessed were: 5, 6, 4, 4, 5, 5, 4, 6, 5. * Indicates differs significantly (at least p<0.05) from Veh. Below panel (a) is shown the corresponding mean (±S.E.) blood levels of Des measured at each dose (n.d.=value too low to be reliably detected and quantified). Therapeutic range reported for Des in humans is 75–300 ng/ml plasma (compiled from Gutteck and Rentsch, 2003; Orsulak, 1986; Preskorn, 1989; Van Brunt, 1983).
Paroxetine
Figure 3 shows the effects on LC activity of 14 d treatment with various doses of Par. Effects of six doses of drug are shown in comparison with vehicle. One-way ANOVAs performed on these data revealed a statistically significant effect of group (i.e. drug dose) for both spontaneous firing rate [F(6, 203)=10.5, p<0.001] and sensory-evoked burst firing [F(6, 203)=22.0, p<0.001]. Subsequent comparisons of each drug to the vehicle condition indicated that both spontaneous firing rate and sensory evoked burst firing were significantly decreased by Par at each dose of 0.625 mg/kg.d or higher. Mean blood levels of drug (ng/ml) present at the end of the recording session are shown below the drug-dose designations in the figure.
Figure 3.
(a) Spontaneous firing rate and (b) sensory-evoked burst firing of LC neurons with administration of vehicle (Veh) or different doses of paroxetine (Par) for 14 d via subcutaneous minipump. Par doses ranged from 0.31 to 10.0 mg/kg.d. All details are the same as described in Figure 2 legend. Numbers of cells comprising the means shown were (for Veh and ascending doses): 25, 25, 35, 35, 35, 20, 35; numbers of animals assessed were: 5, 5, 7, 7, 7, 4, 7. * Indicates differs significantly (at least p<0.05) from Veh. Blood levels measured for each dose of Par are shown below panel (a); details as in Figure 2 legend. Therapeutic range reported for Par in humans is 40–120 ng/ml plasma (compiled from Baumann et al., 2004; Devane, 1999; Preskorn, 1997; Rasmussen and Brøsen, 2000).
Escitalopram
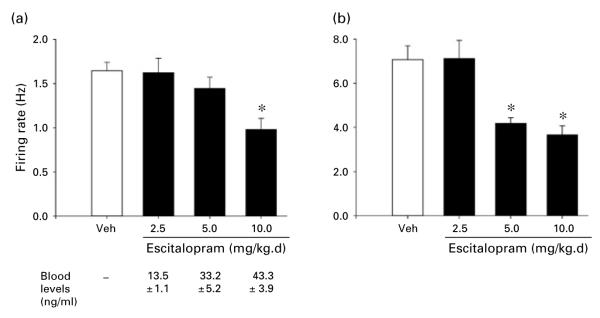
Figure 4 shows the effects on LC activity of 14-d treatment with various doses of Esc. Effects of three doses of drug are shown in comparison with vehicle. One-way ANOVAs performed on these data revealed a statistically significant effect of group (i.e. drug dose) for both spontaneous firing rate [F(3, 116)=5.9, p< 0.002] and sensory-evoked burst firing [F(3, 116)=10.8, p<0.001]. Subsequent comparisons of each drug to the vehicle condition indicated that spontaneous firing rate was significantly decreased by Esc at 10.0 mg/kg.d while sensory-evoked burst firing was decreased at both 5.0 and 10.0 mg/kg.d; neither measure showed a difference from the vehicle condition at 2.5 mg/kg.d. Mean blood levels of drug (ng/ml) present at the end of the recording session are shown below the drug-dose designations in the figure.
Figure 4.
(a) Spontaneous firing rate and (b) sensory-evoked burst firing of LC neurons with administration of vehicle (Veh) or different doses of escitalopram (Esc) for 14 d via subcutaneous minipump. Esc doses ranged from 2.5 to 10.0 mg/kg.d. All details the same as described in Figure 2 legend. Numbers of cells comprising the means were (for Veh and ascending doses): 30 cells in each condition; number of animals assessed in each condition (n=6). * Indicates differs significantly (at least p<0.05) from Veh. Blood levels measured for each dose of Esc are shown panel (a); details as in Figure 2 legend. Therapeutic range reported for Esc in humans is 15–50 ng/ml plasma (compiled from Foglia et al., 1997; Nikisch et al., 2004, 2005; Reis et al., 2003; Sidhu et al., 1997).
Mirtazapine
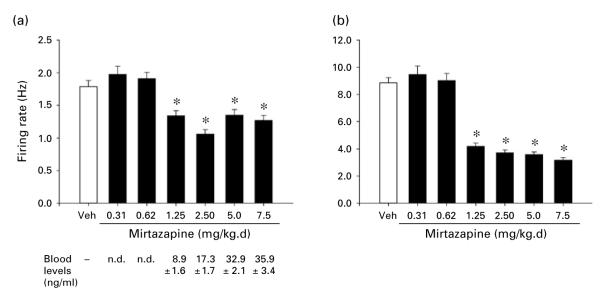
Figure 5 shows the effects on LC activity of 14-d treatment with various doses of Mir. Effects of six doses of drug are shown in comparison with vehicle. One-way ANOVAs performed on these data revealed a statistically significant effect of group (i.e. drug dose) for both spontaneous firing rate [F(6, 309)=16.7, p<0.001] and sensory-evoked burst firing [F(6, 309)=77.8, p<0.001]. Subsequent comparisons of each drug to the vehicle condition indicated that both spontaneous firing rate and sensory-evoked burst firing were significantly decreased by Mir at each dose of 1.25 mg/kg.d or higher. Mean blood levels of drug (ng/ml) present at the end of the recording session are shown below the drug-dose designations in the figure.
Figure 5.
(a) Spontaneous firing rate and (b) sensory-evoked burst firing of LC neurons with administration of vehicle (Veh) or different doses of mirtazapine (Mir) for 14 d via subcutaneous minipump. Mir doses ranged from 0.31 to 7.5 mg/kg.d. All other details the same as described in Figure 2 legend. Numbers of cells comprising the means shown were (for Veh and ascending doses): 50, 35, 35, 47, 50, 47, 50; numbers of animals assessed were: 10, 7, 7,10, 10, 10, 10. * Indicates differs significantly (at least p<0.05) from Veh. Blood levels measured for each dose of Mir are shown below panel (a); details as in Figure 2 legend. Therapeutic range reported for Mir in humans is 15–50 ng/ml plasma (compiled from Bruijn et al., 1996; Grasmäder et al., 2004; Murphy et al., 2003; Timmer et al., 2000).
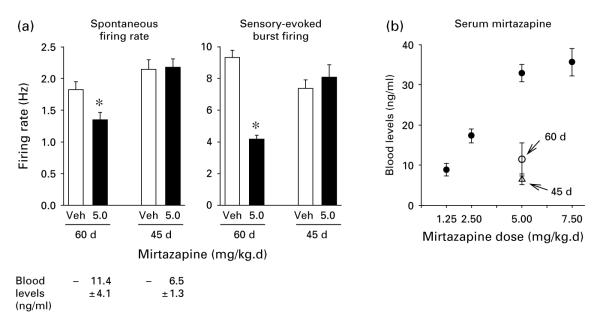
To compare the findings reported directly above with those reported by Haddjeri et al. (1997), effects of treatment with Mir at 5.0 mg/kg.d were also assessed in two groups of younger rats (i.e. aged 60 d, 350–400 g and 45 d, 250–300 g body weight at the time of pump implantation) in addition to the usual rats that were used throughout the present study (i.e. 550–700 g body weight at the time of pump implantation). The results, which are shown in Figure 6a, indicate that 14-d drug treatment given to 60-d-old rats caused a significant decrease in both spontaneous firing rate and sensory-evoked burst firing of LC neurons compared with the vehicle-infused rats (t=2.8, p<0.01 and t=10.0, p<0.001 respectively). Thus, the effects in these rats were similar to what occurred in adult rats aged 5–7 months. This was not the case when 45-d-old rats were treated with Mir for 21 d. In these animals, drug treatment resulted in no significant changes in LC firing rate. Mean spontaneous firing rate was similar in drug- and vehicle-treated animals (t=0.14, n.s.). Sensory-evoked burst firing also was not significantly different (t=0.70, n.s.). Below the bars in the figure denoting firing rates are shown the blood levels of Mir present in these two groups of animals at the conclusion of the recording session. Figure 6b shows these blood levels in relation to the blood levels found in rats aged 5–7 months. Comparing the different age groups that had received 5.0 mg/kg.d, the mean blood level of rats aged 60 d (350–400 g) and 45 d (250–300 g) was marked, and significantly, lower than that of the rats aged 5–7 months (t=5.19, p<0.001, and t=8.93, p<0.001 respectively), while the difference between the two younger groups did not reach statistical significance (t=1.22, n.s.).
Figure 6.
(a) Spontaneous firing rate and sensory-evoked burst firing of LC neurons with administration of vehicle (Veh) or mirtazapine (Mir) (5.0 mg/kg.d) via subcutaneous minipump for rats aged 14–60 d (350–400 g body weight at pump implantation) and for rats aged 21–45 d (250–300 g at pump implantation). Chloral hydrate was used as anaesthetic for these younger rats. All other details the same as described in Figure 2 legend. Number of cells comprising each mean shown was 25; number of animals assessed in each group (n=5). * Indicates differs significantly (at least p<0.05) from Veh. Blood levels of Mir are shown below panel (a). (b) Mean (±S.E.) blood levels for all standard animals (i.e. rats aged 5–7 months, 550–700 g body weight) treated with Mir as a function of the dose given (●), as well as the mean (±S.E.) blood level of Mir for the two groups of younger rats (○, 60 d at 14 d of drug treatment; △, 45 d at 21 d of drug treatment) when given 5.0 mg/kg.d.
Finally with respect to Mir, insofar as this drug blocks α2 adrenergic receptors (de Boer, 1996; de Boer et al., 1988; Nutt, 1997), acute administration of the drug could be anticipated to increase rather than decrease LC activity (Aghajanian and Vandermaelen, 1982; Cedarbaum and Aghajanian, 1976; Simson and Weiss, 1987). We therefore assessed the effect of acute administration (by i.p. injection) of Mir (5.0 mg/kg; rats used were, as usual, aged 5–7 months). The results are shown in Figure 7. When, in animals that had not been given any drug previously, recording of LC activity took place shortly after injection of 5.0 mg/kg Mir, LC activity was significantly increased compared to LC activity after acute injection of vehicle; this was seen for both spontaneous firing rate (t=5.83, p<0.001) and sensory-evoked burst firing (t=4.37, p<0.001).
Figure 7.
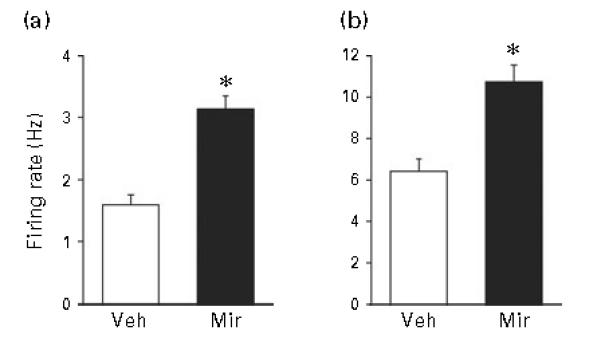
(a) Spontaneous firing rate and (b) sensory-evoked burst firing of LC neurons with administration of vehicle (Veh) or mirtazapine (Mir) (5.0 mg/kg) by intraperitoneal (i.p.) injection followed by electrophysiological recording beginning 10 min later. All details the same as described in Figure 2 legend. Rats in this study had not been treated with any drug prior to i.p. injection. Number of cells comprising means was Veh (n=32), and Mir (n=34); number of animals assessed in each group (n=7). * Indicates differs significantly (at least p<0.05) from Veh.
Non-AD drugs
Figure 8 shows the effects on LC activity of 14-d treatment with Amph, Cdz, Scop, and Cpa. A single dose chosen as described in the Methods section was tested for each of these drugs. The one-way ANOVA analysing spontaneous firing rate failed to show a statistically significant effect of group (i.e. drug type) [F(4, 117)=0.6, n.s.], thus indicating that the drugs tested did not significantly alter spontaneous activity. However, the analysis of sensory-evoked burst firing showed a significant effect of group [F(4, 117)=3.2, p<0.02]. Post-hoc comparison of each of the drug-treated conditions to the vehicle-treated condition revealed that animals given Cdz showed a significant increase in sensory-evoked burst firing, and that animals given Scop also showed an increase that approached statistical significance (p=0.052).
Figure 8.
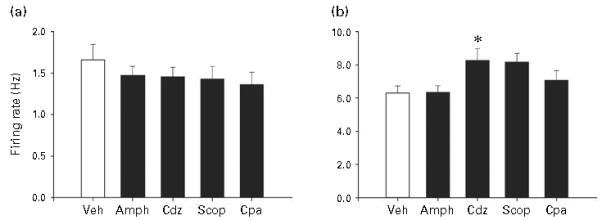
(a) Spontaneous firing rate and (b) sensory-evoked burst firing (right) of LC neurons with administration of four psychoactive drugs that are not effective antidepressant treatments for 14 d via subcutaneous minipump. Drugs and doses given were amphetamine (Amph, 2 mg/kg.d), chlordiazepoxide (Cdz, 2 mg/kg.d), scopolamine (Scop, 2 mg/kg.d) and chlorpheniramine (Cpa, 10 mg/kg.d). All details the same as described in Figure 2 legend. Numbers of cells comprising the means for Vehicle (Veh) and drugs respectively as shown above: 22, 25, 25, 25, 25; numbers of animals assessed in each condition (n=5). * Indicates differs significantly (at least p<0.05) from Veh.
Discussion
The results described above revealed that chronic administration of the ADs tested — Des, a tricyclic, Par and Esc, two SSRIs, and Mir, an atypical AD — decreased both spontaneous firing rate and sensory-evoked burst firing in a dose-dependent manner. For each of these drugs, testing included a low dose that did not affect LC activity as well as progressively higher doses that did have this effect. The tricyclic and SSRI drugs used were chosen for being highly potent and/or selective in blocking either norepinephrine (NE) or serotonin (5-HT) reuptake sites and thus were selected to determine whether chronic blockade of either NE or 5-HT transporters would result in a decrease in LC activity. Des has been found to be highly specific in blocking NE reuptake, with little or no direct effect on 5-HT or dopamine (DA) reuptake sites (Bolden-Watson and Richelson, 1993; Richelson and Pfenning, 1984). Par is amongst the most potent ADs in blocking 5-HT reuptake (Bolden-Watson and Richelson, 1993); however, Par at therapeutically effective doses has been reported also to block NE reuptake sites (reviewed in Owens and Nemeroff, 2003). Consequently, in addition we tested Esc which blocks 5-HT reuptake while having little capacity to bind to NE transporters and thus is highly selective for blocking 5-HT reuptake (Owens et al., 2001; Sánchez et al., 2003). The results described here therefore show that AD drugs that are highly selective for blocking either NE or 5-HT reuptake will produce a decrease in LC activity, both for spontaneous firing rate and sensory-evoked burst firing, when administered chronically as is done in a therapeutically effective regimen. We do not here reiterate discussion of the mechanisms by which blockade of NE and 5-HT reuptake produces this effect on LC activity ; this has been discussed previously by us (Grant and Weiss, 2001) as well as others (Szabo and Blier, 2001b).
In addition to drugs that produce potent and selective blockade of noradrenergic and serotonergic reuptake, the effect of chronic administration of Mir was also tested across a range of doses. Perhaps the most significant challenge to the possibility that all effective AD drugs will decrease LC activity is posed by Mir and mianserin. These two drugs have been found to block α2 receptors (de Boer, 1996; de Boer et al., 1988; Nickolson et al., 1982; Nutt, 1997; Sambunaris et al., 1997). Because an apparently tonic inhibitory influence on LC activity is exerted via stimulation of α2 receptors located on the cell bodies of LC neurons, blockade of α2 receptors increases LC activity. Acute administration of drugs that possess the capability to block α2 receptors consistently has been found to increase LC electrophysiological activity, especially increasing sensory-evoked burst firing but also increasing spontaneous firing rate at higher doses (Cedarbaum and Aghajanian, 1976; Freedman and Aghajanian, 1984; Simson and Weiss, 1987, 1989). However, effects on LC activity from chronic administration of drugs that block α2 receptors, and particularly the effects of such drugs that are ADs (i.e. Mir and mianserin), are less clear. In the course of testing effects of chronic (21-d) administration of Mir on the serotonergic function, Haddjeri et al. (1997) reported a small but statistically significant increase in spontaneous firing rate of LC neurons. On the other hand, Valentino and colleagues reported that chronic administration of mianserin resulted in no change in LC activity (Curtis and Valentino, 1991); moreover, inspection of their data reveals that animals that received drug showed a reduction in spontaneous and sensory-evoked burst LC firing in comparison to the control animals that received a similar schedule of vehicle injections. Finally, in a human study of direct relevance, Mendlewicz et al. (1982) reported that therapeutic administration of mianserin in treatment of depression produced a significant decrease in the noradrenergic metabolite 3-methoxy-6-hydroxyphenylglycol (MHPG) in cerebrospinal fluid, suggesting that NE release in brain, 70% of which originates from LC terminals, was reduced in patients given the drug; these data point to reduced LC activity in human patients taking mianserin.
We therefore examined the effect of chronic (14-d) administration of Mir. We focused on Mir because it is in widespread use in the USA whereas mianserin is not licensed for use here. The chemical structure of Mir and mianserin are the same except for a difference at one position on the molecule, mianserin having a methine group at this location while Mir has a nitrogen (Kelder et al., 1997). Both drugs possess essentially the same affnity to bind to α2 receptors, but do show a number of other distinct functional differences (de Boer, 1996; de Boer et al., 1988; Kelder et al., 1997). The results, shown in Figure 5, clearly indicated that 14-d administration of Mir produced a reduction in LC activity, both in spontaneous firing rate and sensory-evoked burst firing. The effect of this drug seemed sufficiently important to define that we attempted to repeat the procedure used by Haddjeri and colleagues who had seen a small increase in LC spontaneous firing rate when they administered Mir chronically. In contrast to the particular conditions we had used for the study reported here, Haddjeri and colleagues employed considerably smaller rats (250–300 g) and also administered the drug (5.0 mg/kg.d, by minipump) for a slightly longer period (21 d). They also used chloral hydrate as the anaesthetic in measuring LC electrophysiological activity rather than isofluorane which we used for the data reported in the present study. Therefore, we first tested rats that were considerably smaller than those used throughout the research reported here (i.e. average body weight 367.5±8.7 g at pump implantation vs. 550–700 g normally used in present studies) and performed the electrophysiological measurements under choral hydrate anaesthesia. For this initial study, measurement was made after 14 d of drug administration (5.0 mg/kg.d) as in the main part of the present study. When this was done, we still found that Mir produced a notable reduction in LC activity. However, the blood levels of Mir were markedly lower in these smaller (younger) rats than had been found in our normally used, larger rats. We then approximated the conditions employed by Haddjeri et al., testing rats of still smaller size (280.8±5.5 g at pump implantation), administering drug (via minipump) for 21 d (5.0 mg/kg.d), and carrying out electrophysiological measurement under chloral hydrate anaesthesia. When this was done, Mir treatment was found not to produce a decrease in LC activity, and this lack of effect was accompanied by blood levels of Mir that were even lower than in the previous study (see Figure 6). As an additional step, we tested effects of Mir administered acutely to determine whether we would obtain an increase in LC activity when such an effect clearly should occur. Acute administration produced a clear increase in LC activity, so it was apparent that our measurement conditions are not biased against seeing increases in LC activity. Our conclusion is as follows: although we did not see an actual increase in LC activity as did Haddjeri and colleagues, our results indicate that young (and small) rats, as they used, metabolize Mir with considerably greater efficiency than do older (and larger) rats, and blood levels of the drug can be appreciably lower in young/small rats than in older/larger rats given the same initial dose of drug. Coincident with this, effects of chronic administration of Mir on LC activity were very much diminished in young/small rats (i.e. 250–300 g) — administration of 5.0 mg/kg.d of Mir for 21 d failed to cause a decrease in LC activity of such animals. We speculate that the small increase seen by Haddjeri et al. occurred in conjunction with a similar low blood level but in that case chronic Mir administration was even less effective than we observed in small/young rats and therefore failed to completely blunt the increase in LC activity normally observed from acute administration of Mir. As a final note, this aspect of our study indicates that the use of young/small rats in examining drug action may well yield results that are quite different from what will be seen if mature older and larger rats are used.
Table 1 shows a summary of the findings of the different studies that have examined the influence of chronic administration of effective AD drugs or electroconvulsive shock on the activity of LC neurons recorded in vivo, including the findings described in this report.
Table 1.
Effects of chronic treatment (≥1 wk) with antidepressant drugs or a series of electroconvulsive shocks on spontaneous and sensory-evoked firing rate of locus coeruleus neurons
NRI, Norepinephrine reuptake inhibitors; MAOI, monamine oxidase inhibitors; SSRI, selective serotonin reuptake inhibitors; ECS, Electroconvulsive shock.
The firing rate shown by animals receiving drug or electroconvulsive shock in comparison with animals not receiving such treatment was: ↓, decreased; ↑, increased; —, unchanged.
Evoked response assessed, but results not reported for this measure. Report presents only the ratio of evoked to spontaneous rate; because spontaneous rate was decreased by phenelzine, effect on evoked rate could not be determined from data presented.
Spontaneous firing rate of sertraline-treated rats was not different from untreated rats but was significantly lower than that of rats that received a similar schedule of injections of vehicle.
Spontaneous and evoked firing rates of mianserin-treated rats were not different from untreated rats but were less than that of rats that received a similar schedule of injections of vehicle; however, the statistical significance of the comparison of these rates in mianserin-treated rats with vehicle-treated rats was not presented (see Curtis and Valentino, 1991, fig. 4, p. 334).
Dose—response defined. Doses tested included a low dose that produced no decrease.
In addition to testing ADs as described above, the effect on LC activity of four drugs that are not ADs was also examined. Effects of chronic administration (14-d) of Scop, an anticholinergic (muscarinic receptor antagonist), Cpa, an antihistamine, Cdz, a benzodiazepine tranquillizer, and Amph, a stimulant, were examined, using a dose that is effective on acute administration. None of these drugs decreased either spontaneous firing rate or sensory-evoked burst firing of LC neurons; in one case, a significant increase in sensory-evoked burst firing was observed (Cdz). These results indicate that the decrease in LC activity brought about by AD drugs is at least to some extent specific to the action of ADs. Additionally, insofar as the anticholinergic and antihistamine tested did not decrease LC activity, the findings also indicate that these concomitant actions of various AD drugs (e.g. see Frazer, 1997) are not responsible for the decrease in LC activity produced by ADs.
The electrophysiological findings reported previously and described here are not the only results supporting the idea that effective AD drugs decrease LC activity. In a comprehensive study in rats, Nestler and colleagues assessed the effect of a variety of AD drugs and electroconvulsive shock on tyrosine hydroxylase (TH) in LC neurons, and found that all of these AD treatments they tested decreased the amount of TH in LC neurons (Nestler et al., 1990). Insofar as synthesis of TH is activity dependent, decreased TH in LC cells indicates that the AD treatments reduced activity of these neurons. Additionally, a significant body of research exists consisting of studies in which MHPG has been measured in CSF of human depressed patients treated with various AD drugs. In our previous paper, we listed 16 studies (see table 4 in Grant and Weiss, 2001) that reported findings for this measure. The results are remarkably consistent (i.e. no exceptions) in showing that MHPG in CSF is reduced in patients taking AD medications. Insofar as most of the norepinephrine released in brain derives from terminals on LC neurons, this measure also indicates that LC activity is reduced by AD treatments. Thus, assessment of a measure that reflects LC activity in human patients undergoing AD drug treatment points to the same changes in LC activity as are seen in rats given AD drugs.
In concluding the discussion of how LC activity is affected by treatments for affective disorders, it is interesting to note that treatments for reducing mania appear to have an effect opposite to what is described above, i.e. results suggest these treatments increase LC activity. Drugs that are effective in treating bipolar disorder, such as valproate, have been reported to increase TH in LC neurons (Olpe et al., 1983; Sands et al., 2000), and carbamazapine was found to increase LC firing measured electrophysiologically (Olpe and Jones, 1983). Olanzapine and the combination of olanzapine and fluoxetine, approved for the treatment of bipolar disorder, increase TH in LC (Ordway and Szebeni, 2004) and also LC activity (Seager et al., 2005). Thus, it is interesting to note that whereas effective treatments to reduce depression decrease LC activity, treatments to reduce mania may have the opposite effect on the LC activity.
A major reason for examining the response of LC neurons to different doses of the ADs studied here was the high blood levels of drug found in our previous report (Grant and Weiss, 2001). In that report, blood levels for both the tricylic ADs tested [Des, imipramine (10 mg/kg.d)] as well as the SSRIs [fluoxetine (10 mg/kg.d) and sertraline (10 and 25 mg/kg.d)] were quite high and generally above the accepted therapeutic range; consequently, it was unclear whether decreased LC activity indeed characterized the response to effective ADs or whether this might be seen only with blood levels of drug above the therapeutic range. The results reported here make clear that decreased LC firing as a consequence of chronic administration of AD drugs is not the result of abnormally high blood levels of drug. Decreased LC activity was seen with all of the drugs tested at blood levels of drug that are considered to be therapeutic in humans for the various drugs used. However, it also should be noted that decreased LC activity was in fact seen at blood levels that were lower than levels indicated to be therapeutic in humans, suggesting that decreased LC activity can occur at low and possibly non-therapeutic levels of these drugs studied. In evaluating this last statement, two points need to be taken into consideration. First, it is not known whether therapeutic blood levels for the rat (i.e. levels that will produce behavioural and physiological effects relevant to AD action) are the same as for the human, and thus it cannot be said with certainty that the low blood levels at which decreased LC was observed in the rat represent ‘non-therapeutic’ levels for this species. Second, the studies conducted here were performed in normal animals, whereas ‘depressed’ animals would be expected to have higher LC activity and therefore could well require higher blood levels of drug to reduce their LC firing rates. However, with these caveats having been noted, it is nevertheless evident that the findings presented in this report, while demonstrating that all drugs tested decreased LC activity, do not indicate that the presence of a statistically significant decrease in LC activity is correlated with a blood level of drug that in humans is required to produce an AD effect.
To summarize, the findings reported here continue to indicate that chronic administration of all AD drugs examined to date, as well as electroconvulsive shock, results in a reduction in the activity of LC neurons. Results reported here show that this occurs with drugs that are highly specific for blocking either NE or 5-HT reuptake, and, importantly, with a drug that blocks α2 adrenergic receptors (Mir) and therefore might have been conjectured to produce an increase rather than a decrease in LC activity. Also of importance, decreased LC activity is seen at blood levels of drug where, in humans, therapeutic AD effects are observed. However, reduction of LC activity was also seen at blood levels of drug below the human therapeutic range. But insofar as extrapolation of the present data to effective blood levels in human patients is of course complicated by the use of normal (i.e. non-depressed) rats in the studies reported here, it therefore remains to be determined whether the reduction in LC activity produced by AD drugs is or is not correlated with blood levels of drug that produce a therapeutic response in human patients.
Acknowledgements
Mirtazapine (Remeron) was generously provided by Organon, West Orange, NJ, USA. We thank Dr Michael Owens of Emory University for his help in preparing the paroxetine and escitalopram used in these studies. This research was supported by Public Health Service grants MH65737 and MH79794.
Footnotes
Statement of Interest: None.
References
- Aghajanian GK, Vandermaelen CP. Alpha-2-adrenoreceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982;215:1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. Journal of Neuroscience. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P, Hiemke C, Ulrich S, Eckermann G, Gaertner I, Gerlach M, Kuss H-J, Laux G, Müller-Oerlinghausen B, Rao ML, Riederer P, Zernig G. The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry [Review] Pharmacopsychiatry. 2004;37:243–265. doi: 10.1055/s-2004-832687. [DOI] [PubMed] [Google Scholar]
- Béïque J, de Montigny C, Blier P, Debonnel G. Effects of sustained administration of the serotonin and norepinephrine reuptake inhibitor venlafaxine: I. in vivo electrophysiological studies in the rat. Neuropharmacology. 2000;39:1800–1812. doi: 10.1016/s0028-3908(00)00017-4. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Serotonergic but not noradrenergic neurons in rat central nervous system adapt to long-term treatment with monoamine oxidase inhibitors. Neuroscience. 1985;14:949–955. doi: 10.1016/0306-4522(85)90107-1. [DOI] [PubMed] [Google Scholar]
- Bolden-Watson C, Richelson E. Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sciences. 1993;52:1023–1029. doi: 10.1016/0024-3205(93)90194-8. [DOI] [PubMed] [Google Scholar]
- Borsody MK, Weiss JM. Influence of corticotropin-releasing hormone on electrophysiological activity of locus coeruleus neurons. Brain Research. 1996;724:149–168. doi: 10.1016/0006-8993(96)00199-0. [DOI] [PubMed] [Google Scholar]
- Bruijn JA, Moleman P, Mulder PGH, van den Broek WW, van Hulst AM, van der Mast RC, van de Wetering BJM. A double-blind, fixed blood-level study comparing mirtazapine with imipramine in depressed in-patients. Psychopharmacology. 1996;127:231–237. [PubMed] [Google Scholar]
- Cedarbaum JM, Aghajanian GK. Noradrenergic neurons of the locus coeruleus: inhibition by epinephrine and activation by the alpha-antagonist piperoxane. Brain Research. 1976;112:413–419. doi: 10.1016/0006-8993(76)90297-3. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Valentino RJ. Acute and chronic effects of the atypical antidepressant, mianserin on brain noradrenergic neurons. Psychopharmacology. 1991;103:330–338. doi: 10.1007/BF02244286. [DOI] [PubMed] [Google Scholar]
- de Boer Th. The pharmacologic profile of mirtazapine. Journal of Clinical Psychiatry. 1996;57:19–25. [PubMed] [Google Scholar]
- de Boer Th, Maura G, Raiteri M, de Vos CJ, Wieringa JH, Pinder RM. Neurochemical and autonomic pharmacological profiles of the 6-aza-analogue of mianserin, Org 3770 and its enantiomers. Neuropharmacology. 1988;27:399–408. doi: 10.1016/0028-3908(88)90149-9. [DOI] [PubMed] [Google Scholar]
- Devane CL. Metabolism and pharmacokinetics of selective serotonin reuptake inhibitors. Cellular and Molecular Neurobiology. 1999;19:443–466. doi: 10.1023/A:1006934807375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foglia JP, Pollock BG, Kirshner MA, Rosen J, Sweet R, Mulsant B. Plasma levels of citalopram enantiomers and metabolites in elderly patients. Psychopharmacology Bulletin. 1997;33:109–112. [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proceedings of the National Academy of Sciences USA. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. Pharmacology of antidepressants. Journal of Clinical Psychopharmacology. 1997;17:2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- Freedman JE, Aghajanian GK. Idazoxan (RX 781094) selectively antagonizes alpha-2 adrenoceptors on rat central neurons. European Journal of Pharmacology. 1984;105:265–272. doi: 10.1016/0014-2999(84)90618-6. [DOI] [PubMed] [Google Scholar]
- Garabal MF, Nunez MJ, Balboa JL, Suarez JA, Belmonte A. Effects of alpraxolam on the development of MTV-induced mammary tumours in female mice under stress. Cancer Letters. 1991;62:185–189. doi: 10.1016/0304-3835(92)90094-c. [DOI] [PubMed] [Google Scholar]
- Graham AW, Aghajanian GK. Effects of amphetamine on single-cell activity in a catecholamine nucleus, the locus coeruleus. Nature. 1971;234:100–102. doi: 10.1038/234100b0. [DOI] [PubMed] [Google Scholar]
- Grant MM, Weiss JM. Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophysiologic activity. Biological Psychiatry. 2001;49:117–129. doi: 10.1016/s0006-3223(00)00936-7. [DOI] [PubMed] [Google Scholar]
- Grasmäder K, Verwohlt PL, Rietschel M, Dragicevic A, Müller M, Hiemke C, Freymann N, Zobel A, Maier W, Rao ML. Impact of polymorphisms of cytochrome-P450 isoenzymes 2C9, 2C19 and 2D6 on plasma concentrations and clinical effects of antidepressants in a naturalistic clinical setting. European Journal of Clinical Pharmacology. 2004;60:329–336. doi: 10.1007/s00228-004-0766-8. [DOI] [PubMed] [Google Scholar]
- Gutteck U, Rentsch KM. Therapeutic drug monitoring of 13 antidepressant and five neuroleptic drugs in serum with liquid chromatography-electrospray ionization mass spectrometry. Clinical Chemistry and Laboratory Medicine. 2003;41:1571–1579. doi: 10.1515/CCLM.2003.240. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C. Effects of long-term treatment with the α2-adrenoceptor antagonist mirtazapine on 5-HT neurotransmission. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1997;355:20–29. doi: 10.1007/pl00004913. [DOI] [PubMed] [Google Scholar]
- Huang YH, Maas JW, Hu GH. The time course of noradrenergic pre- and postsynaptic activity during chronic desipramine treatment. European Journal of Pharmacology. 1980;68:41–47. doi: 10.1016/0014-2999(80)90058-8. [DOI] [PubMed] [Google Scholar]
- Kelder J, Funke C, De Boer T, Delbressine L, Leysen D, Nickolson V. A comparison of the physicochemical and biological properties of mirtazapine and mianserin. Journal of Pharmacy and Pharmacology. 1997;49:403–411. doi: 10.1111/j.2042-7158.1997.tb06814.x. [DOI] [PubMed] [Google Scholar]
- Korf J, Bunney BS, Aghajanian GK. Noradrenergic neurons: morphine inhibition of spontaneous activity. European Journal of Pharmacology. 1974;25:165–169. doi: 10.1016/0014-2999(74)90045-4. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Wardi T, Lundström L, Sollenberg U, Langel Ü, Hökfelt T, Ögren SO. Differential role of galanin receptors in the regulation of depression-like behaviour and monoamine/stress-related genes at the cell body level. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301660. Published online: 2 January 2008. doi:10.1038/sj.npp.1301660. [DOI] [PubMed] [Google Scholar]
- Linnér L, Arborelius L, Nomikos GG, Bertilsson L, Svensson TH. Locus coeruleus neuronal activity and noradrenaline availability in the frontal cortex of rats chronically treated with imipramine: Effect of α2-adrenoceptor blockade. Biological Psychiatry. 1999;46:766–774. doi: 10.1016/s0006-3223(99)00126-2. [DOI] [PubMed] [Google Scholar]
- Mazhar M, Binder SR. Analysis of benzodiazepines and tricyclic antidepressants in serum using a common solid-phase clean-up and a common mobile phase. Journal of Chromatography. 1989;497:201–212. doi: 10.1016/0378-4347(89)80019-2. [DOI] [PubMed] [Google Scholar]
- McConathy J, Capello C, Jarkas N, Stowe ZN, Owens MJ. Preparation of antidepressants for use in preclinical research. International Journal of Neuropsychopharmacology. 2007;10:759–763. doi: 10.1017/S1461145706007474. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Warnack W, German DC, Shore PA. Effects of chronic desipramine treatment on rat brain noradrenergic responses to α-adrenergic drugs. European Journal of Pharmacology. 1980;61:239–246. doi: 10.1016/0014-2999(80)90126-0. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Pinder RM, Stulemeijer SM, Van Dorth R. Monoamine metabolites in cerebrospinal fluid of depressed patients during treatment with mianserin or amitritriptyline. Journal of Affective Disorders. 1982;4:219–226. doi: 10.1016/0165-0327(82)90006-4. [DOI] [PubMed] [Google Scholar]
- Mongeau R, Weiss M, de Montigny C, Blier P. Effect of acute, short- and long-term milnacipran administration on rat locus coeruleus noradrenergic and dorsal raphe serotonergic neurons. Neuropharmacology. 1998;37:905–918. doi: 10.1016/s0028-3908(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Murphy GM, Jr., Kremer C, Rodrigues HE, Schatzberg AF. Pharmacogenetics of antidepressant medication intolerance. American Journal of Psychiatry. 2003;160:1830–1835. doi: 10.1176/appi.ajp.160.10.1830. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, McMahon A, Sabban EL, Tallman JF, Duman RS. Chronic antidepressant administration decreases the expression of tyrosine hydroxylase in the rat locus coeruleus. Proceedings of the National Academy of Sciences USA. 1990;87:7522–7526. doi: 10.1073/pnas.87.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickolson VJ, Wieringa JH, van Delft AML. Comparative pharmacology of mianserin, its main metabolites and 6-azamianserin. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1982;319:48–53. doi: 10.1007/BF00491478. [DOI] [PubMed] [Google Scholar]
- Nikisch G, Mathé AA, Czernik A, Eap CB, Jiménez-Vasquez P, Brawand-Amey M, Baumann P. Stereoselective metabolism of citalopram in plasma and cerebrospinal fluid of depressive patients: relationship with 5-HIAA in CSF and clinical response. Journal of Clinical Psychopharmacology. 2004;24:283–290. doi: 10.1097/01.jcp.0000125680.89843.a6. [DOI] [PubMed] [Google Scholar]
- Nikisch G, Mathé AA, Czernik A, Thiele J, Bohner J, Eap CB, Ågren H, Baumann P. Long-term citalopram administration reduces responsiveness of HPA axis in patients with major depression: relationship with S-citalopram concentrations in plasma and cerebrospinal fluid (CSF) and clinical response. Psychopharmacology (Berlin) 2005;181:751–760. doi: 10.1007/s00213-005-0034-3. [DOI] [PubMed] [Google Scholar]
- Nutt D. Mirtazapine: pharmacology in relation to adverse effects. Acta Psychiatrica Scandinavica. 1997;96:31–37. doi: 10.1111/j.1600-0447.1997.tb05956.x. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Jones RS. The action of anticonvulsant drugs on the firing of locus coeruleus neurons: selective, activating effect of carbamazepine. European Journal of Pharmacology. 1983;91:107–110. doi: 10.1016/0014-2999(83)90369-2. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Laszlo J, Dooley DJ, Heid J, Steinmann MW. Decreased activity of locus coeruleus neurons in the rat after DSP-4 treatment. Neuroscience Letters. 1983;40:81–84. doi: 10.1016/0304-3940(83)90096-4. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Szebeni K. Effect of repeated treatment with olanzapine or olanzapine plus fluoxetine on tyrosine hydroxylase in the rat locus coeruleus. International Journal of Neuropsychopharmacology. 2004;7:321–327. doi: 10.1017/S1461145704004468. [DOI] [PubMed] [Google Scholar]
- Orsulak PJ. Therapeutic monitoring of antidepressant drugs: Current methodology and applications. Journal of Clinical Psychiatry. 1986;47:39–50. [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biological Psychiatry. 2001;50:345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Neuropharmacology of paroxetine. Psychopharmacology Bulletin. 2003;37:8–18. [PubMed] [Google Scholar]
- Preskorn SH. Tricyclic antidepressants: the whys and hows of therapeutic drug monitoring. Journal of Clinical Psychiatry. 1989;50:34–42. [PubMed] [Google Scholar]
- Preskorn SH. Clinically relevant pharmacology of selective serotonin reuptake inhibitors: an overview with emphasis on pharmacokinetics and effects on oxdative drug metabolism. Clinical Pharmacokinetics. 1997;32:1–21. doi: 10.2165/00003088-199700321-00003. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Brøsen K. Is therapeutic drug monitoring a case for optimizing clinical outcome and avoiding interactions of the selective serotonin reuptake inhibitors? [Review] Therapeutic Drug Monitoring. 2000;22:143–154. doi: 10.1097/00007691-200004000-00001. [DOI] [PubMed] [Google Scholar]
- Reis M, Lundmark J, Bengtsson F. Therapeutic drug monitoring of racemic citalopram: a 5-year experience in Sweden, 1992–1997. Therapeutic Drug Monitoring. 2003;25:183–191. doi: 10.1097/00007691-200304000-00007. [DOI] [PubMed] [Google Scholar]
- Richelson E, Pfenning M. Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: most antidepressants selectively block norepinephrine uptake. European Journal of Pharmacology. 1984;104:277–286. doi: 10.1016/0014-2999(84)90403-5. [DOI] [PubMed] [Google Scholar]
- Ritchie JC, Zhang W. Modification of the Bio Rad tricyclic antidepressant methodology to measure the serotonin reuptake inhibitors. Clinical Chemistry. 1996;42:S222. [Google Scholar]
- Sambunaris A, Hesselink JK, Pinder R, Panagides J, Stahl SM. Development of new antidepressants. Journal of Clinical Psychiatry. 1997;58:40–53. [PubMed] [Google Scholar]
- Sánchez C, Bergqvist PBF, Brennum LT, Gupta S, Hogg S, Larsen A, Wiborg O. Escitalopram, the S(+)-enantiomer of citalopram, is an extremely selective serotonin reuptake inhibitor with potent antidepresant and anxiolytic activities. Psychopharmacology. 2003;167:353–362. doi: 10.1007/s00213-002-1364-z. [DOI] [PubMed] [Google Scholar]
- Sands SA, Guerra V, Morilak DA. Changes in tyrosine hydroxylase mRNA expression in the rat locus coeruleus following acute or chronic treatment with valproic acid. Neuropsychopharmacology. 2000;22:27–35. doi: 10.1016/S0893-133X(99)00072-X. [DOI] [PubMed] [Google Scholar]
- Seager MA, Barth VN, Phebus LA, Rasmussen K. Chronic coadministration of olanzapine and fluoxetine activates locus coeruleus neurons in rats: implications for bipolar disorder. Psychopharmacology (Berlin) 2005;181:126–133. doi: 10.1007/s00213-005-2198-2. [DOI] [PubMed] [Google Scholar]
- Seager MA, Huff KD, Barth VN, Phebus LA, Rasmussen K. Fluoxetine administration potentiates the effect of olanzapine on locus coeruleus neuronal activity. Biological Psychiatry. 2004;55:1103–1109. doi: 10.1016/j.biopsych.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Sidhu J, Priskorn M, Poulsen M, Segonzac A, Grollier G, Larsen F. Steady-state pharmacokinetics of the enantiomers of citalopram and its metabolites in humans. Chirality. 1997;9:686–692. doi: 10.1002/(SICI)1520-636X(1997)9:7<686::AID-CHIR9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Simson PE, Weiss JM. Alpha-2 receptor blockade increases responsiveness of locus coeruleus neurons to excitatory stimulation. Journal of Neuroscience. 1987;7:1732–1740. doi: 10.1523/JNEUROSCI.07-06-01732.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simson PE, Weiss JM. Blockade of α2-adrenergic receptors, but not blockade of γ-aminobutyric acidA, serotonin, or opiate receptors, augments responsiveness of locus coeruleus neurons to excitatory stimulation. Neuropharmacology. 1989;28:651–660. doi: 10.1016/0028-3908(89)90147-0. [DOI] [PubMed] [Google Scholar]
- Svensson TH, Usdin T. Feedback inhibition of brain noradrenaline neurons by tricyclic antidepressants: alpha-receptor mediation. Science. 1978;202:1089–1091. doi: 10.1126/science.213833. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Effects of chronic antidepressant drug administration and electroconvulsive shock on locus coeruleus electrophysiological activity [Correspondence] Biological Psychiatry. 2001a;50:644. doi: 10.1016/s0006-3223(01)01260-4. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Functional and pharmacological characterization of the modulatory role of serotonin on the firing activity of locus coeruleus norepinephrine neurons. Brain Research. 2001b;922:9–20. doi: 10.1016/s0006-8993(01)03121-3. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Effect of the selective noradrenergic reuptake inhibitor reboxetine on the firing activity of noradrenaline and serotonin neurons. European Journal of Neuroscience. 2001c;13:2077–2087. doi: 10.1046/j.0953-816x.2001.01583.x. [DOI] [PubMed] [Google Scholar]
- Szabo ST, de Montigny C, Blier P. Modulation of noradrenergic neuronal firing by selective serotonin reuptake blockers. British Journal of Pharmacology. 1999;126:568–571. doi: 10.1038/sj.bjp.0702343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer CJ, Sitsen JM, Delbressine LP. Clinical pharmacokinetics of mirtazapine [Review] Clinical Pharmacokinetics. 2000;38:461–474. doi: 10.2165/00003088-200038060-00001. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Curtis AL. Antidepressant interactions with corticotropin-releasing factor in the noradrenergic nucleus locus coeruleus. Psychopharmacological Bulletin. 1991;27:263–269. [PubMed] [Google Scholar]
- Valentino RJ, Curtis AL, Parris DG, Wehby RG. Antidepressant actions on brain noradrenergic neurons. Journal of Pharmacology and Experimental Therapeutics. 1990;253:833–840. [PubMed] [Google Scholar]
- Van Brunt N. The clinical utility of tricyclic antidepressant blood levels: a review of the literature. Therapeutic Drug Monitoring. 1983;5:1–10. doi: 10.1097/00007691-198303000-00001. [DOI] [PubMed] [Google Scholar]
- West CHK, Weiss JM. Effects of antidepressant drugs on rats bred for low activity in the swim test. Pharmacology, Biochemistry and Behavior. 1998;61:67–79. doi: 10.1016/s0091-3057(98)00076-8. [DOI] [PubMed] [Google Scholar]
- West CHK, Weiss JM. A selective test for antidepressant treatments using rats bred for stress-induced reduction of motor activity in the swim test. Psychopharmacology. 2005;182:9–23. doi: 10.1007/s00213-005-0048-x. [DOI] [PubMed] [Google Scholar]