Abstract
The biodegradation of collagen and the deposition of new collagen-based extracellular matrices are of central importance in tissue remodeling and function. Similarly, for collagen-based biomaterials used in tissue engineering, the degradation of collagen scaffolds with accompanying cellular infiltration and generation of new extracellular matrix is critical for integration of in vitro grown tissues in vivo. In earlier studies we observed significant impact of collagen structure on primary lung fibroblast behavior in vitro in terms of collagen uptake and matrix remodeling. Therefore, in the present work, the response of human fibroblasts (IMR-90) to the structural state of collagen was studied with respect to phagocytosis in the presence and absence of inhibitors. Protein content and transcript levels for Collagen I (Col-1), Matrix Metalloproteinase 1 (MMP-1), Matrix Metalloproteinase 2 (MMP-2), Tissue Inhibitor of Matrix Metalloproteinase 1 (TIMP-1), Tissue Inhibitor of Matrix Metalloproteinase 2 (TIMP-2), and Heat Shock Protein 70 (HSP-70) were characterized as a function of collagen matrix concentration, structure and cell culture time to assess effects on cellular collagen matrix remodeling processes. Phagocytosis of collagen was assessed quantitatively by uptake of collagen coated fluorescent beads incorporated into the pre-formed collagen matrices. Significantly higher levels of collagen phagocytosis were observed for the cells grown on the denatured collagen vs. native collagen matrices. Significant reduction in collagen phagocytosis was observed by blocking several phagocytosis pathways when the cells were grown on denatured collagen versus non-denatured collagen. Collagen phagocytosis inhibition effects were significantly greater for PDL57 IMR-90 cells versus PDL48 cells, reflecting a reduced number of collagen processing pathways available to the older cells. Transcript levels related to the deposition of new extracellular matrix proteins varied as a function of the structure of the collagen matrix presented to the cells. A four-fold increase in transcript level of Col-1 and a higher level of collagen matrix incorporation were observed for cells grown on denatured collagen versus cells grown on non-denatured collagen. The data suggest that biomaterial matrices incorporating denatured collagen may promote more active remodeling toward new extracellular matrices in comparison to cells grown on non-denatured collagen. A similar effect of cellular action toward denatured (wound-related) collagen in the remodeling of tissues in vivo may have significant impact on tissue regeneration as well as the progression of collagen-related diseases.
Keywords: Collagen, phagocytosis, remodeling, biodegradation, extracellular matrix, denatured
Introduction
The remodeling of collagen is essential to the maintenance of tissue structure and function as well as to the progression of a number of diseases. Wound healing as well as tissue remodeling depend on the degradation and phagocytosis process initiated by neutrophils and macrophages, followed by the deposition of new extracellular matrix (ECM) by fibroblasts [1]. The remodeling of collagen is also integral in cardiomyopathy, spinal injury associated ulcers, scleroderma, and bone structure conditions such as osteoporosis, osteogenesis imperfecta, and osteoarthritis [1–3]. Skeletal metastases are also associated with unregulated breakdown of collagen and the asessment of collagen and its breakdown products is essential for monitoring and managing these patients [4]. The remodeling of collagen as a component of the extracellular matrix is integral to the development and progression of metastases of hepatocellular cancers, melanoma tumors, and colon cancers [5–7]. In all of these diseases, a better understanding of collagen remodeling may provide improved insight into both mechanisms as well as modes of treatment. For example, over 5 million patients in the United States suffer from congestive heart failure and as many as 10,000 cardiomyopathic deaths occur per year [8],5,[9]. Collagen biomaterials are used extensively in tissue engineering scaffolds and also require successful remodeling and integration into surrounding tissues once impanted in vivo [10].
Physiological processes with respect to extracellular matrix degradation and biosynthesis have been studied to characterize the process of collagen remodeling. For example, in the oral gingiva the degradation and remodeling of collagen rich extracellular matrix is essential to maintaining normal oral mucosa [11]. The uptake of collagen by gingival cells has been studied by monitoring the uptake of collagen coated fluorescent beads [12]. Collagen coated beads have also been used to study collagen processing in trophoblastic cells [13–15]. Several small molecules have been identified as inhibitors of steps in phagocytic pathways. Cyclosporin A inhibits collagen phagocytosis by blocking the release of calcium from ER stores [12]. Internally, processing through lysosomic vacuoles can be inhibited by cystine proteinase inhibitors leupeptin, fluromethylketone, and (E-64) trans-epoxysuccinyl-L-leukylamido (4-guanidino) butone [14, 16]. Inhibition of phagocytosis by cytochalasin B prevents the appearance of intracellular collagen fibrils without interfering with the synthesis of new collagen [14].
Some cell responses to collagen are mediated through integrin receptors on cell surfaces. Cells recognize collagen matrices through the integrin family of transmembrane proteins; specifically, α2β1 and α1β1 integrin receptors [17]. The β1 integrins and the α2β1 integrin in particular have been associated with fibroblast collagen binding pathways [18, 19]. Extracellular collagen degradation occurs by the action of enzymes including matrix metalloproteinases (MMPs) and through phagocytosis by macrophages and fibroblasts [20, 21]. Tissue inhibitors of matrix metalloproteinases (TIMP’s) are a set of enzyme inhibitors that work in concert with the MMPs to control matrix remodeling [22]. Although MMP’s represent a major degradation pathway for extracellular matrix components, they are not expressed at detectable levels under routine physiological conditions [23]. Expression of MMP’s increases during reproduction, in wound healing, and in several disease states [23]. Collagen integrin receptors can induce the upregulation of MMP production and also serve to activate the formation of several pro-MMP molecules [24]. Integrin activation by collagen is also responsible for activating signal transduction pathways controlling cell death, migration, and matrix remodeling [25].
Phagocytosis is an important pathway for collagen remodeling but the regulatory mechanisms are not understood. Thus, in the present study we study some of these regulatory systems in the context of the structural nature of the collagen substrate. Collagen remodeling is an important part of the homeostasis of connective tissue as well as in wound repair in such pathological conditions as inflammatory diseases and neoplasia [26]. Interruption of normal collagen phagocytosis may result in the formation of fibrotic lesions [27]. Intracellular collagen processing via phagocytosis occurs in some fibroblasts by cell receptor clustering followed by invagination of the cell membrane [28]. In several diseases total collagen content has been shown to be controlled in vitro by artificially adjusting the cells’ rates of collagen breakdown and deposition to a matched rate in a continual feedback process [29]. A better understanding of the mechanisms that exist in vivo in these diseases may help with elucidating treatments.
During collagen matrix remodeling and new extracellular matrix deposition, the chemical, morphological, and topographic cues that control the process are largely unknown. To further understand the mechanistic basis for the responses between collagen matrix structure, phagocytosis and cell physiology, we hypothesized that cells respond differently to different structural states of collagen. We addressed this issue with IMR-90 human fetal lung fibroblasts on two structural forms of collagen (native and denatured), along with tissue culture plastic as a control. Fibroblasts are active in both the digestion of damaged collagen and production of new collagen [30, 31]. IMR-90 fibroblasts also exhibit age specific responses including senescence around population doubling of 58 (PDL 58) [32]. As IMR-90 cells age they exhibit distinct morphological changes, demonstrate increased production of the β-galactosidase senescence associated enzyme and have reduced potential to proliferate [33]. We have previously reported that these age related responses are influenced significantly by the structural state of the collagen matrix [34]. Insight into the role of collagen structure on rates and extent of phagocytosis, should lead to an improved understanding of collagen biomaterial matrix designs, resulting in more predictable and relevant cell and tissue outcomes in vitro and in vivo. Also, these data should provide fundamental insight into remodeling responses by fibroblasts in normal and wound states, as well as pertaining to many disease states where collagen is critical to the disease pathology.
Materials and Methods
Chemicals
Rat tail collagen type I was purchased from Roche Chemicals (Indianapolis, IN, USA). Tissue culture reagents were purchased from Gibco/Invitrogen (Carlsbad, CA, USA). Molecular Biology reagents were purchased from Qiagen (Valencia, CA, USA) and Promega (Madison, WI, USA). Fluorescent latex beads coated with collagen were purchased from Molecular Probes (Eugene, OR, USA).
Collagen Preparation
Collagen was prepared as we have previously described [35]. Briefly, collagen was dissolved at 2–8°C in sterile filtered 0.1% glacial acetic acid (GAA) at 5–10 mg/mL over at least 3 days for complete dissolution. Once dissolved, collagen was diluted to working concentration just before use for generating non-denatured collagen surfaces. Denaturation was accomplished by a 60-minute treatment in a 50°C water bath and confirmed by Circular Dichroism spectroscopy [36]. Our previous studies [35] showed positive fibroblast growth characteristics on 78 μg/cm2 collagen surfaces. In the present study, we have also explored 156 μg/cm2 for comparison. Plates were typically dried for 12–48 hours until no liquid remained. In cases with collagen coated beads, a first layer of collagen mixed with the beads was completely dried before addition of the second layer of collagen without beads.
Cells
IMR-90 human lung fibroblasts were purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Cells were cultured at 37°C and 5% CO2 in 20% fetal bovine serum, 77% eagle minimum essential medium (MEM), 1% penicillin-streptomycin, liquid, 1% L-glutamine-200 mM, and 1% MEM non-essential amino acids solution 10 mM. Cells were split before confluence approximately 1:10. Cells with fewer than 30 cumulative population doublings (PDL) were designated “young” cells [32]. Cells with more than 48 cumulative population doublings were designated “aged” cells [37]. Metabolism of several proteins has been shown to be two-fold greater in very young cells (PDL=22) versus that in old cells (PDL=48) [37]. Cells used in this study were PDL 47 to PDL 57. Cells were harvested at 70–80% confluence for inoculation of collagen surfaces. Cells were mixed with the media for all wells or plates to allow for homogeneous distribution on the surface.
Collagen Processing
F13083 FluoSpheres® polystyrene microspheres, 1.0 μm diam., red fluorescent (580/605) beads from Molecular Probes, Inc. (Eugene, OR, USA) were used to track cellular uptake of collagen. For “buried bead” experiments, collagen beads were dispersed by sonication, then mixed with collagen just prior to collagen plating. After the bead/collagen layer had dried, a second layer of collagen was added. In the tissue culture plastic (TC) plates, the beads were suspended in the collagen dilution buffer, 0.1% glacial acetic acid (GAA) before addition to the plates. After the second layer of collagen had dried, the wells were equilibrated in media before addition of cells. At appropriate time points, cells were removed by with 0.01% trypsin. Trypsin treatment has previously been found to remove membrane bound beads but not internalized beads from cells [38]. In a separate experiment, native and denatured collagen layers that had been incubated in media were trypsinized up to 60 minutes and no collagen was removed from the plates based on SDS-PAGE analysis (data not shown).
Integrin Blocking
Monoclonal antibodies MAB1998Z Integrin α2β1 [VLA-2] and MAB2253Z Integrin β1 [CD29] were purchased from CHEMICON International, Inc. (Temecula, CA, USA) to block interactions with the collagen/matrix related to cell responses. Blocking methods were based on an adaptation of a previously published ELISA method used in bone marrow stromal cells to identify cell surface integring via Fluorescence-activated Cell Sorting [39, 40]. Antibody saturation levels were defined by growing IMR-90 cells in the presence of increasing concentrations of the MABs. Briefly, after trypsinization, the cells were equilibrated with the MABs for 1 hour before plating to assure that all cell surface receptors could bind to available MABs. Saturation was defined via a modified ELISA procedure. MAB’s were sandwiched with AP124P goat anti-mouse IgG, peroxidase conjugated, followed by ES018 Tetramethylbenzidine (TMB) substrate (Chemicon). The reaction was stopped with 0.5 M sulfuric acid, and the levels of bound MAB were determined by fluorescence at 450 nm. Saturation was defined as a plateau of fluorescence at 450 nm with increasing MAB concentration.
Blocking Phagocytosis
Cyclosporin A, cytochalasin B, (E-64) trans-epoxysuccinyl-L-leukylamido (4-guanidino) butone were purchased from Sigma. Cyclosporin A was used at 125 ng/μL of culture media to inhibit collagen phagocytosis by blocking the release of calcium from ER stores [12]. Internally, processing through lysosomic vacuoles was inhibited by the cystine proteinase inhibitor (E-64) trans-epoxysuccinyl-L-leukylamido (4-guanidino) butone at 2.5 ng/μL culture media [14, 16]. Inhibition of phagocytosis by cytochalasin B at 125 ng/μL culture media was used to prevent the appearance of intracellular collagen fibrils without interfering with the synthesis of new collagen [14].
mRNA Assay
Cells were collected with a single-step acid-phenol guanidinium extraction using Trizol from Invitrogen [41]. mRNA was isolated by chloroform extraction followed by Qiagen kit recovery. The High-Capacity cDNA Archive kit from ABI Biosystems (Perkin Elmer Applied Biosystems, Foster City, CA, USA) was used for synthesis of cDNA. RT-PCR reagents were from Applied Biosystems. Quantitative real time RT-PCR was performed and analyzed using an ABI Prism 7700 Sequence Detection System with settings as described by Martin et. al. [42]. Expression levels for each mRNA transcript were normalized to the housekeeping gene glyceraldehyde phosphate dehydrogenase (GAPDH). Primers and probe sequences were as follows: Col I, forward primer = 5-CAGCCGCTTCACCTACAGC-3, reverse primer = 5-TTTTGTATTCAATCACTGTCTTGCC-3, probe = 5-CCGGTGTGACTCGTGCAGCCATC-3[42]; GAPDH, forward primer = 5-ATGGGGAAGGTGAAGGTCG-3, reverse primer = 5-TAAAAGCCCTGGTGACC-3, probe = 5-CGCCCAATACGACCAAATCCGTTGAC-3[42]; MMP-1 Assays-on Demand (Hs00233958_m1), Celera Gene ID: hCG41471, matrix metalloproteinase 1 (interstitial collagenase); MMP-2, Assays-on Demand (Hs00234422_m1), Celera Gene ID: hCG24148, matrix metalloproteinase 2 (gelatinase A, 72kDa); TIMP-1, Assays-on Demand (Hs00171558_m1), Celera Gene ID: hCG29141, tissue inhibitor of metalloproteinase 1 (erythroid potentiating activity, collagenase inhibitor); TIMP-2, Assays-on Demand (Mm00441825_m1), Celera Gene ID: mCG140800; matrix metalloproteinase 2 (gelatinase A, 72kDa gelatinase, 72kDa type IV collagenase), and HSP-70, Assays-on Demand (Hs00271244_s1), Celera Gene ID hCG43726.
ELISA
MMP-1, MMP-2, MMP-9, MMP-13, TIMP-1, and TIMP-2 ELISA kits were obtained from Merck Biosciences AG (Laeufelfingen, Switzerland) and run according to the manufacturer’s directions. The samples were lysed cells that had been collected after 2 days growth on 156 μg/cm2 collagen substrates.
Statistics
All samples were run minimally in triplicate. Outliers were identified using a z-test. Post hoc comparison of the means was accomplished with a student t test. A p value less than 0.05 was defined as significant. All data are represented as mean ± standard error of the mean.
Results
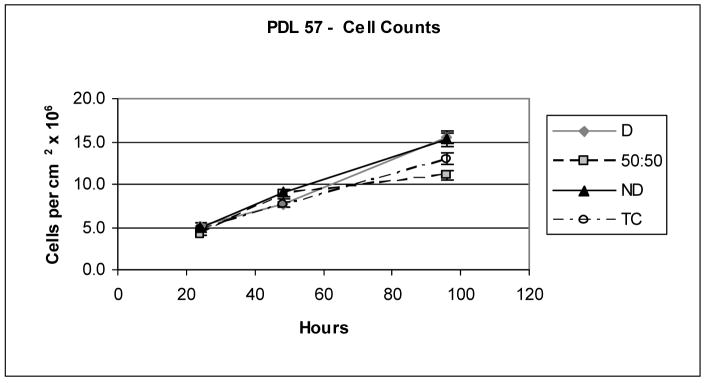
Similar cell numbers and growth rates were found on the various samples (Figure 1) in this study, including the denatured (D) and non-denatured collagen (ND), as well as the tissue culture plastic (TC). However, the rates of substrate phagocytosis have been found previously [35] and in this study to be significantly greater for cells grown on denatured collagen than for cells grown on non-denatured collagen (e.g., 7% on denatured and 1.8% on native collagen when phagocytosis was not inhibited). In addition to the bead uptake measure of phagocytosis, this process has also been quantified and confirmed using radiolabeled collagen [43]. An illustration of the various paths and inhibitors studied is included in Figure 2.
Figure 1.
Cell counts showing similar growth rates for PDL 57 cells grown seeded at approximately 20% to 30% confluence on denatured and non-denatured collagen substrates.
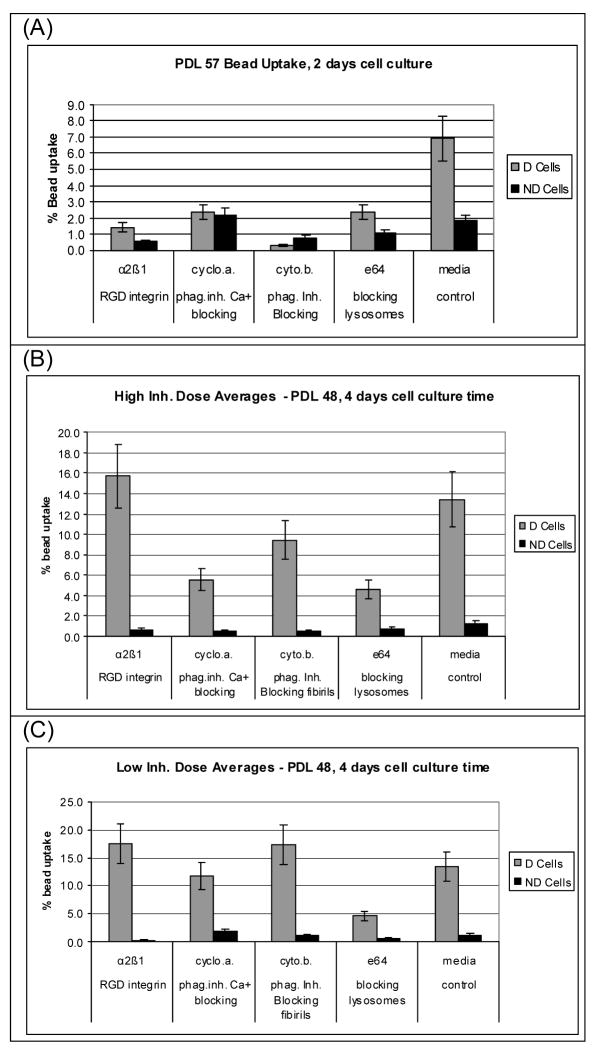
Figure 2.
Phagocytosis as a function of phagocytosis inhibitors. (A) inhibited phagocytosis of collagen substrates for PDL 57 cells, (B) inhibited phagocytosis for PDL 48 cells at “high” doses of inhibitors, (D) inhibited phagocytosis for PDL 48 cells at “low” doses of inhibitors.
To assess the involvement of the α2β1 and β1 integrins in the responses of fibroblasts to the different collagen substrate structures (native vs. denatured), the goal was to block α2β1 and β1 integrins and examine differences in cell responses. Saturation levels for the α2β1 and β1 integrins were determined (data not shown). The cell receptors for the α2β1 integrin MAB reached saturation at 0.150 μg MAB per μL cell culture media. The cell receptors for β1 integrin MAB were not saturated for the MAB concentrations studied.
In addition to the α2β1 integrin pathway, a goal was to compare the responses of cells to collagen substrates as a function of possible collagen processing pathways. Therefore, collagen phagocytosis was assayed in the presence of various pathway inhibitors (Figures 3A–C). Two cell passage stages, PDL57 and PDL48 were studied at two time points, 2 or 4 days. PDL57 cells were selected as near senescence. Following evaluation of the PDL57 results where limited phagocytosis was seen in the control samples, PDL48 was selected to represent an aged cell passage stage, but expected to have better cell growth than the older PDL57 cells. For the PDL57 cells grown in the absence of inhibitors on denatured collagen (156 μg/cm2), 6.9% of the beads in the collagen were incorporated into the cells. For the non-denatured collagen and all of the samples with phagocytosis inhibitors present, the uptake of the beads was negligible. In order to examine the effect of less stringent phagocytosis inhibition, experiments were repeated with PDL48 cells grown for 4 days both in the presence of standard levels of phagocytosis inhibitors as well as ¼ the standard concentrations for cyclosporin A (125 ng/μL and 31.25 ng/μL), cytochalasin B (125 ng/μL and 31.25 ng/μL), (E-64) and trans-epoxysuccinyl-L-leukylamido (4-guanidino) butone (2.5 ng/μL and 0.5 ng/μL). The α2β1 integrin MAB was used at the standard saturation concentration of 167 ng/μL in all experiments.
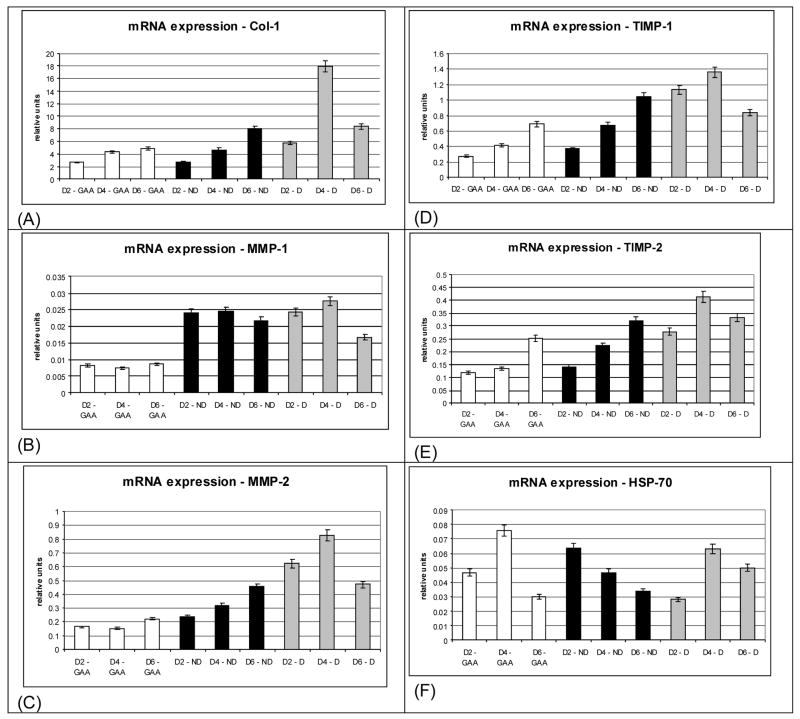
Figure 3.
Transcript levels for cells grown on collagen substrates as a function of time. (A) Collagen (Col-1), (B) matrix metalloproteinase-1 (MMP-1), (C) matrix metalloproteinase-2 (MMP-2), (D) tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), (E) tissue Inhibitor of matrix metalloproteinase-2 (TIMP-2), (F) heat shock protein-70 (HSP-70).
Inhibition of the pathways involved in uptake of denatured collagen was significant for phagocytosis blocking via cyclosporin A, cytochalasin B, and (E-64) trans-epoxysuccinyl-L-leukylamido (4-guanidino) butone. There was no significant change in collagen uptake for cells grown on denatured collagen in the presence of the α2β1 integrin MAB blocking, indicating that the enhanced collagen uptake observed for cells grown on denatured collagen is not likely a α2β1 integrin mediated event. For the cells grown on native collagen phagocytosis of collagen measured by bead uptake was minimal for all cases.
In addition to the direct measurement of phagocytosis of the substrate via tracking imbedded beads, collagen remodeling by the cells was assessed at the molecular level based on transcript levels. The time course for upregulation of Col-1, MMP-1, MMP-2, TIMP-1, TIMP-2, and HSP-70 was determined (Figures 4A–F). Two, 4, and 6 days were selected to correspond to ~30%, ~60%, and ~90% cell confluence, respectively. At both 2 day and 4 days transcript levels for Col-1, MMP-2, TIMP-1 and TIMP-2 were significantly higher for cells grown on denatured collagen versus native collagen or on the collagen solubilizing buffer (0.1% glacial acetic acid) treated tissue culture plastic (GAA/TCP). At 6 days cells grown on both denatured and native (non-denatured) collagen produced significantly higher transcript levels of Col-1, MMP-2, TIMP-1, and TIMP-2 versus glacial acetic acid on tissue culture plastic grown cells. Transcript levels for MMP-1 were significantly higher for cells grown on both denatured and non-denatured collagen versus those for cells grown on glacial acetic acid on tissue culture plastic. The non-heat induced levels of HSP-70 transcript were low in all samples.
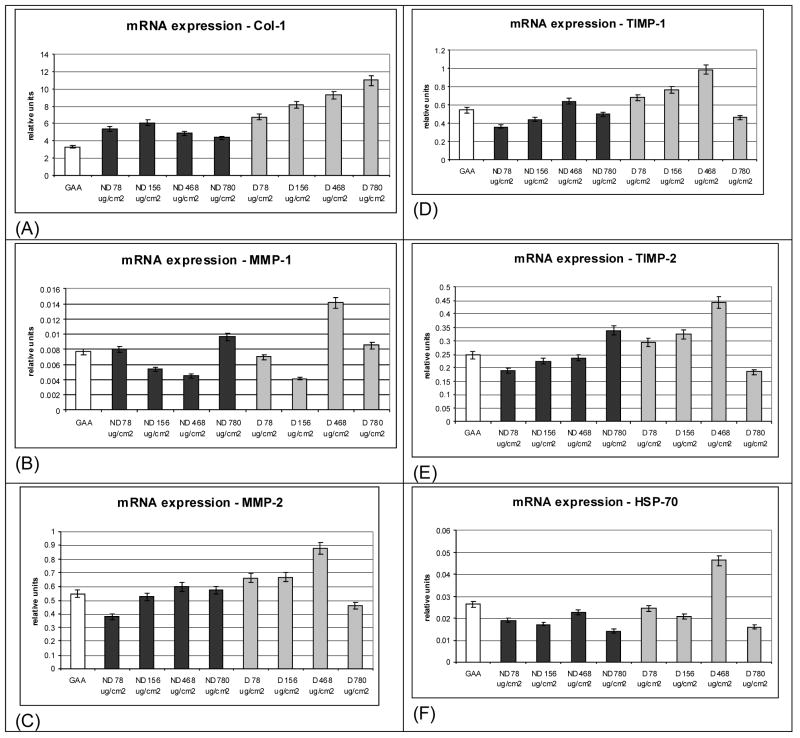
Figure 4.
Transcript levels for cells grown on various collagen substrates as a function of substrate concentration. (A) Collagen (Col-1), (B) matrix metalloproteinase-1 (MMP-1), (C) matrix metalloproteinase-2 (MMP-2), (D) tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), (E) tissue Inhibitor of matrix metalloproteinase-2 (TIMP-2), (F) heat shock protein-70 (HSP-70).
In addition to culture time, collagen concentration was of interest in relation to collagen remodeling responses. Earlier work suggested that cell health varied as a function of the structure of the collagen matrix and one measure of cell health was based on HSP-70 transcript levels [44]. Transcript levels for Col-1, MMP-1, MMP-2, TIMP-1, TIMP-2, and HSP-70 as a function of matrix collagen concentration were determined (Figures 5A–F). At each collagen concentration (78, 156, 468, and 780 μg/cm2) transcript levels for Col-1 were significantly higher for cells grown on denatured collagen versus those for cells grown on native collagen. Transcript levels of MMP-1 were low at all concentrations for cells grown on both denatured and native collagen matrices. At collagen concentrations 78, 156, 468 μg/cm2 transcript levels for TIMP-1, TIMP-2, and MMP-2 were significantly greater for cells grown on denatured collagen versus the cells grown on the same concentration of native collagen. The non-heat induced levels of HSP-70 transcript were low in all samples, including cells on collagen substrates and those on GAA/TCP controls.
Figure 5.
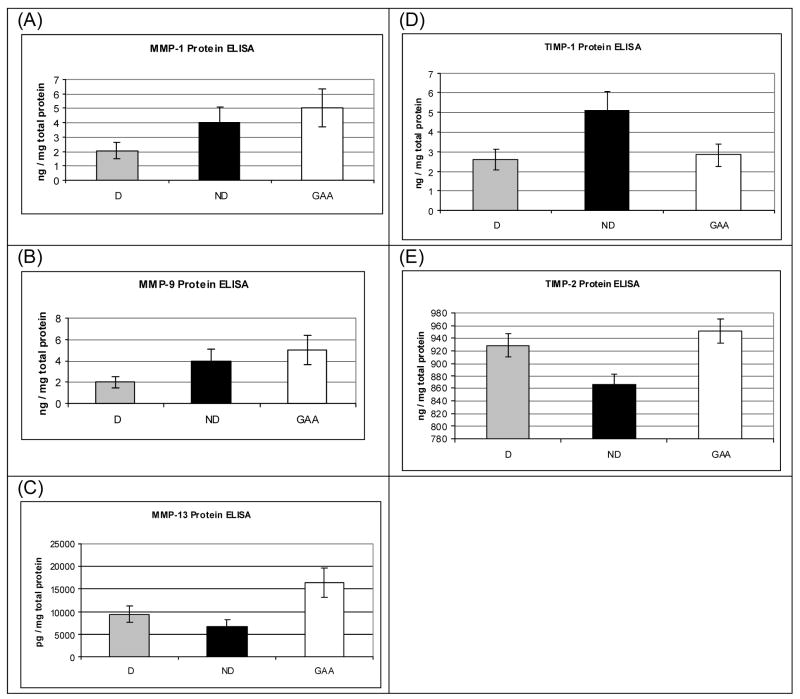
ELISA results for cells grown on collagen substrates. (A) MMP-1, (B) MMP-9, (C) MMP-13, (D) TIMP-1, (D) TIMP-2.
In order to determine if the levels of mRNA’s observed via quantitative RT-PCR were similar to the levels of expressed proteins, ELISA was used to directly measure protein expression for several collagen remodeling related proteins (Figures 6, A–E). The intracellular levels of all of the proteins were higher for the tissue culture plastic samples than expected based on the mRNA results. MMP-1 and TIMP-1 protein levels were higher intracellularly for cells grown on native collagen versus those for cells grown on denatured collagen or control cells on the tissue culture plastic.
The similar cell counts for each substrate demonstrated that all treatments allowed cells to proliferate on a rougly equivalent basis. Therefore, the differences reported in mRNA levels and bead uptake [35] were not related to differences in cell amounts or growth rates on the different matrices.
Discussion
To determine the role of α2β1 and β1 integrins in cellular responses to the different collagen matrices, cellular saturation levels with MABs for these integrins was determined. Variation in substrate phagocytosis levels in the presence and absence of integrin inhibition provided information about the involvement of these integrins in the phagocytosis responses seen on denatured versus native collagen. The saturation level for RGD integrin α2β1 was 150 ng/μL and this value, as expected would be higher for the less specific β1 integrin due to the many possible ‘α’ with β1 combinations [45]. The saturation level for α2β1 were approximately 20 ng/μL for early passage bone marrow stromal cells using the same MAB [39]. The cells used in this study were high passage number fibroblasts. Due to the large amount of collagen processing that is required of fibroblasts, it would be expected that the fibroblasts would have higher levels of α2β1 integrin expression than the bone marrow stromal cells.
The data from the control samples (media only) agrees with previous results that also showed greater phagocytosis levels for cells grown on denatured collagen versus cells grown on native collagen [35]. This data set validates the bead model for monitoring phagocytosis in that the results show the expected reduction in bead uptake with the collagen phagocytosis pathways blocked. For the PDL48 cells at 4 days culture, each of the blocking pathways except the α2β1 integrin were involved in the cellular phagocytosis response of cells grown on denatured collagen.
The results indicate that the cells grown on denatured collagen were more active in phagocytosis than cells grown on the native collagen. In previous studies, collagen coated beads have been used to study the phagocytosis of individual beads [46–48]. In gingival cells, the presence of cyclosporin A was shown to inhibit collagen phagocytosis in vitro [48, 49]. In the present study, the cells grown in the presence of cyclosporin A also displayed reduced production of new collagen. It is likely that the enhanced level of collagen production observed on denatured collagen involved these phagocytosis pathways. Similarly, E-64 and cytochalasin B have been shown to interfere with fibroblastic digestion of intracytoplasmic collagen fibrils and the phagocytosis of collagen, respectively [16]. The complete reduction of enhanced collagen uptake for the cells grown on the denatured collagen in the presence of E-64 provides strong evidence that digestion of intracytoplasmic collagen fibrils is facilitated for fibroblasts grown on denatured collagen matrices when compared to the native collagen. Cytochalasin B is known to allow collagen synthesis while inhibiting phagocytosis via interfering with the appearance of intracellular collagen fibrils [14]. Specifically, it has been shown that in the formation of hydroxyproline from proline in collagen was not inhibited by Cytochalasin B [50]. Cytochalasin B impairs microfilament function but had no effect on collagen secretion [50]. Here, the presence of Cytochalasin B for the PDL48 cells grown on denatured collagen substates reduced the cell’s collagen substrate uptake somewhat, but not to the levels of the control cells. This suggests that ingestion of collagen fibrils via phagocytosis may be accompanied by additional collagen uptake mechanisms for cells grown on denatured collagen substrates.
The results observed for collagen trafficking suggest that a portion of the collagen uptake by the PDL48 fibroblasts grown on denatured collagen is the result of partial degradation of the collagen external to the cell, followed by pinocytosis and or phagocytosis of degraded collagen [44]. The significant reduction of collagen uptake observed with the same inhibition with cytochalasin B for PDL57 cells indicates that the older cells were less capable of utilizing the external collagen degradation pathways than cells just three passages younger. In addition, the quantities of collagen undergoing synthesis in organelles including lysosomes was around 1–2% of the total intracellular collagen [44].
The E64 mediated blocking of lysosome formation significantly reduced the levels of phagocytosis for cells grown on denatured collagen substrates. Taken together these data suggest that although the total quantity of collagen in the lysosomes is small, the kinetics of this processing may be rapid, accounting for the large amount of collagen processed when lysosome formation is not inhibited.
MMP-1, MMP-8, MMP-13, and MMP-14 (MT1-MMP) are known to target collagen type 1[23, 51, 52]. MMP-2 and MMP-9 as well as the membrane bound MMP-26 target denatured collagen (gelatin) [23, 51]. Using fragments of MMP-1, it has been shown that MMP-1(Δ243–450) cleaves type 1 collagen when incubated with the fragment responsible for unwinding the collagen - MMP-1(Glu200-Ala) [52]. There is some disagreement in the literature on the specificity of TIMP-1-4 for inhibiting MMP’s. Wong et al note that all the TIMP’s inhibit all MMP’s [51]. Sternlicht and Werb note that the TIMP’s have specific MMP targets [53]. Laliberté et al note that TIMP-1 inhibits all MMP’s and TIMP-2 has specificity for MMP-1, MMP-2, and MMP-9 [47]. Since the matrix substrates used in the present study were collagens, MMP-1, MMP-2, TIMP-1, and TIMP-2 were selected along with Col-1 as transcripts of interest. With the goal of observing remodeling characteristics that might be applicable for tissue engineering, it was necessary to monitor the expression levels of transcripts related to matrix generation, maintenance, and degradation. Specifically the generation of new collagen was followed using Col-1 transcript levels. Breakdown of denatured and non-denatured collagen was followed using, respectively, MMP-2 and MMP-1. TIMP-1 and TIMP-2 are reported to have more wide-ranging activity in inhibiting a range of collagen degradation reactions. However, these markers still provide an indication of cellular efforts to reduce rates of collagen and denatured collagen matrix breakdown.
Our previous data had shown Col 1 expression was two fold higher and expression for MMP-1, MMP-2, TIMP-1, and TIMP-2 increased for cells grown on 156 μg/cm2 denatured collagen17. In this same study with 156 μg/cm2, with native collagen, Col-1 expression decreased by half and MMP-2 transcript levels increased by 2.5 fold in cell responses to denatured versus native collagen substrates at 4 day time points[35]. In the present study, the higher levels of Col-1 mRNA for cells grown on denatured collagen at the 2 and 4 day time points, as well as the 78 μg/cm2, 156 μg/cm2, and 468 μg/cm2 collagen levels, corresponded to an increased level of matrix remodeling in those samples. Transcript levels for cells grown on native collagen showed little change from those for control cells grown on tissue culture plastic. These results indicate that the cells grown on native collagen remain in a collagen processing and synthesis mode similar to control cells, while the cells grown on denatured collagen show a higher level of collagen remodeling. These results may also help with an improved understanding of the impact of collagen-related diseases. For example, decreased production of MMP-1 has been observed for fibroblasts of patients suffering from diabetes mellitus [54]. These results may further the understanding of fibroblast remodeling behavior as a function of the helical nature of the local collagen matrix in normal vs. abnormal states of collagen, such as related to disease specific mutations.
The finding of differences in intracellular mRNA levels than proteins levels in some cases may be due to secretion of MMP’s into the media, as has been reported for MMP-2 and MMP-9 from fibroblasts [55]. Secretion of MMP’s and TIMP’s has also been correlated with levels of collagen synthesis [47].
The observed changes in the expression of extracellular matrix component encoding genes indicate significantly different cell responses on the denatured versus native collagen. The Col-1 increase with accompanying increase in mRNA level associated with collagen digestion in the cells grown on denatured collagen results from a shift to a cell behavior that includes a greater rate of extracellular matrix remodeling. The opposite effect was observed for the cells grown on native collagen. Therefore less remodeling is required in the presence of native collagen, as would be expected. For tissue engineering where rapid remodeling of the implanted tissue engineered matrix is often desired, denatured collagen may be a more useful matrix to stimulate more rapid remodeling into new extracellular matrix. Perhaps more importantly for the longer term, these results provide clues into relationships between the structural state of matrices such as collagen utilized in vitro, to regulate the rate of tissue remodeling in vivo.
In contrast to coating a small amount of collagen on the surface of a bead, the dual layer presentation of collagen and beads embedded in collagen allowed the study of cell responses in a 3D-like environment that better mimics native extracellular matrix systems. This approach allowed observations of cellular responses to the collagens as well as the quantitative monitoring of collagen matrices phagocytosed by the cells as a function of inhibitors. Higher levels of substrate consumption by cells on denatured collagen were observed, as well as a reduction of these effects in the presence of the phagocytosis inhibitors. Also in the denatured collagen samples as a function of time and substrate concentration, mRNA transcript shift toward a higher level of matrix remodeling was observed. This study demonstrated that fibroblasts can be induced to increase levels of collagen remodeling by providing a denatured (non-helical, gelatin-like) collagen substrate. Increased rates of extracellular matrix recycling could be advantageous in many tissue engineering applications. As cells migrate into a tissue engineering scaffold both in vitro and in vivo, increased remodeling rates may help cells regenerate normal extracellular matrix and normal tissue more rapidly. At the same time, this correlation must be balanced against needs for maintenance of structural integrity of the matrix until new extracecullar matrix formation can provide the appropriate supportive functions for the regenerated tissue.
A better understanding of the pathways involved in cellular remodeling of collagen matrices could have significant benefits both in understanding of collagen diseases as well in the design of tissue engineering matrices to match desired integration rates in vivo. It has been postulated that integrin binding of RGD sites that may become exposed on denatured or damaged collagen molecules may be part of the healing process [56]. Recent studies on cardiac remodeling have indicated that β1 integrin binding by fibroblasts may be involved in the cellular remodeling [57]. The binding of α2β1 integrin, particularly in interaction with the binding of other integrins has been found to affect the tumorigenicity of breast carcinoma cells [58]. With fibroblast cellular remodeling having such critical effects on healing and cancer applications, gaining a better understanding of the fibroblastic remodeling response is important.
α1β1 and α2β1 integrins are involved in binding to native collagen type I while αvβ3 is activated in response to denatured collagen type I [56,59–62]. The α2β1 integrin binds to the GFOGER sequence in native collagen type I and does not bind to denatured collagen type I [60,62,63–65]. The αvβ3 integrin binds to denatured collagen type I almost exclusively via matricryptic RGD sequences that are exposed upon denaturation and unwinding of the triple helix [59,61,66–68]. In our continuing studies to elucidate a mechanistic basis for the results observed, α1β1 and α2β1 in response to native collagen, and αvβ3 in response to denatured collagen and RGD-dependent events, initiate the processes involved in the observations reported (unpublished data). We continue to map the activation of MAP kinase and subsequent intracellular pathways to correlate these events both to matrix remodeling as repored here, as well as to differentiation pathways when stem cells are cultivated on similar matrix variants (unpublished data).
Acknowledgments
We thank the NIH (EB002520) for support of this study. We also thank Joshua Mauney for valulable advice on integrin blocking experiments, and Kelly Gillen and Claudia Clark for technical assistance on mRNA and ELISA assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–9. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 2.Dunkin MA. Handout on Health: Scleroderma. National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), a part of the National Institutes of Health (NIH); 2001. [Google Scholar]
- 3.Prockop DJ. What holds us together? Why do some of us fall apart? What can we do about it? Matrix Biol. 1998;16:519–28. doi: 10.1016/s0945-053x(98)90064-6. [DOI] [PubMed] [Google Scholar]
- 4.Demers LM, Costa L, Lipton A. Biochemical markers and skeletal metastases. Cancer. 2000;88:2919–26. doi: 10.1002/1097-0142(20000615)88:12+<2919::aid-cncr7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Theret N, Musso O, Turlin B, Lotrian D, Bioulac-Sage P, Campion JP, Boudjema K, Clement B. Increased extracellular matrix remodeling is associated with tumor progression in human hepatocellular carcinomas. Hepatology. 2001;34:82–8. doi: 10.1053/jhep.2001.25758. [DOI] [PubMed] [Google Scholar]
- 6.Hendrix MJ, Seftor EA, Kirschmann DA, Quaranta V, Seftor RE. Remodeling of the microenvironment by aggressive melanoma tumor cells. Ann N Y Acad Sci. 2003;995:151–61. doi: 10.1111/j.1749-6632.2003.tb03218.x. [DOI] [PubMed] [Google Scholar]
- 7.Mook OR, Van Overbeek C, Ackema EG, Van Maldegem F, Frederiks WM. In situ localization of gelatinolytic activity in the extracellular matrix of metastases of colon cancer in rat liver using quenched fluorogenic DQ-gelatin. J Histochem Cytochem. 2003;51:821–9. doi: 10.1177/002215540305100613. [DOI] [PubMed] [Google Scholar]
- 8.Chen K, Li D, Zhang X, Hermonat PL, Mehta JL. Anoxia-reoxygenation stimulates collagen type-I and MMP-1 expression in cardiac fibroblasts: modulation by the PPAR-gamma ligand pioglitazone. J Cardiovasc Pharmacol. 2004;44:682–7. doi: 10.1097/00005344-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Thohan V, Torre-Amione G, Koerner MM. Aldosterone antagonism and congestive heart failure: a new look at an old therapy. Curr Opin Cardiol. 2004;19:301–8. doi: 10.1097/01.hco.0000129667.34622.14. [DOI] [PubMed] [Google Scholar]
- 10.Friess W. Collagen--biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45:113–36. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 11.McCulloch CA. Drug-induced fibrosis: interference with the intracellular collagen degradation pathway. Curr Opin Drug Discov Devel. 2004;7:720–4. [PubMed] [Google Scholar]
- 12.Arora PD, Silvestri L, Ganss B, Sodek J, McCulloch CA. Mechanism of cyclosporin-induced inhibition of intracellular collagen degradation. J Biol Chem. 2001;276:14100–9. doi: 10.1074/jbc.M010298200. [DOI] [PubMed] [Google Scholar]
- 13.Fisher SJ, Leitch MS, Kantor MS, Basbaum CB, Kramer RH. Degradation of extracellular matrix by the trophoblastic cells of first-trimester human placentas. J Cell Biochem. 1985;27:31–41. doi: 10.1002/jcb.240270105. [DOI] [PubMed] [Google Scholar]
- 14.Manyonda IT, Choy MY. Collagen phagocytosis by human extravillous trophoblast: potential role in trophoblastic invasion. J Soc Gynecol Investig. 1999;6:158–66. doi: 10.1016/s1071-5576(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 15.Bischof P, Meisser A, Campana A. Biochemistry and molecular biology of trophoblast invasion. Ann N Y Acad Sci. 2001;943:157–62. doi: 10.1111/j.1749-6632.2001.tb03799.x. [DOI] [PubMed] [Google Scholar]
- 16.Everts V, Beertsen W, Tigchelaar-Gutter W. The digestion of phagocytosed collagen is inhibited by the proteinase inhibitors leupeptin and E-64. Coll Relat Res. 1985;5:315–36. doi: 10.1016/s0174-173x(85)80021-2. [DOI] [PubMed] [Google Scholar]
- 17.Riikonen T, Westermarck J, Koivisto L, Broberg A, Kahari VM, Heino J. Integrin alpha 2 beta 1 is a positive regulator of collagenase (MMP-1) and collagen alpha 1(I) gene expression. J Biol Chem. 1995;270:13548–52. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- 18.Chen M, O’Toole EA, Li YY, Woodley DT. Alpha 2 beta 1 integrin mediates dermal fibroblast attachment to type VII collagen via a. Exp Cell Res. 1999;249:231–9. doi: 10.1006/excr.1999.4473. [DOI] [PubMed] [Google Scholar]
- 19.Durr J, Goodman S, Potocnik A, von der Mark H, von der Mark K. Localization of beta 1-integrins in human cartilage and their role in chondrocyte adhesion to collagen and fibronectin. Exp Cell Res. 1993;207:235–44. doi: 10.1006/excr.1993.1189. [DOI] [PubMed] [Google Scholar]
- 20.Lauer-Fields JL, Juska D, Fields GB. Matrix metalloproteinases and collagen catabolism. Biopolymers. 2002;66:19–32. doi: 10.1002/bip.10201. [DOI] [PubMed] [Google Scholar]
- 21.Cate AR, Syrbu S. A relationship between alkaline phosphatase activity and the phagocytosis and degradation of collagen by the fibroblast. J Anat. 1974;117:351–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Huppertz B, Kertschanska S, Demir AY, Frank HG, Kaufmann P. Immunohistochemistry of matrix metalloproteinases (MMP), their substrates, and their inhibitors (TIMP) during trophoblast invasion in the human placenta. Cell Tissue Res. 1998;291:133–48. doi: 10.1007/s004410050987. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro SD. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr Opin Cell Biol. 1998;10:602–8. doi: 10.1016/s0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 24.Vogel WF. Collagen-receptor signaling in health and disease. Eur J Dermatol. 2001;11:506–14. [PubMed] [Google Scholar]
- 25.Tian B, Lessan K, Kahm J, Kleidon J, Henke C. beta 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem. 2002;277:24667–75. doi: 10.1074/jbc.M203565200. [DOI] [PubMed] [Google Scholar]
- 26.Knowles GC, McKeown M, Sodek J, McCulloch CA. Mechanism of collagen phagocytosis by human gingival fibroblasts: importance of collagen structure in cell recognition and internalization. J Cell Sci. 1991;98:551–8. doi: 10.1242/jcs.98.4.551. [DOI] [PubMed] [Google Scholar]
- 27.McCulloch CA, Knowles GC. Deficiencies in collagen phagocytosis by human fibroblasts in vitro: a mechanism for fibrosis? J Cell Physiol. 1993;155:461–71. doi: 10.1002/jcp.1041550305. [DOI] [PubMed] [Google Scholar]
- 28.Lee W, McCulloch CA. Deregulation of collagen phagocytosis in aging human fibroblasts: effects of integrin expression and cell cycle. Exp Cell Res. 1997;237:383–93. doi: 10.1006/excr.1997.3802. [DOI] [PubMed] [Google Scholar]
- 29.Kivirikko KI. Collagens and their abnormalities in a wide spectrum of diseases. Ann Med. 1993;25:113–26. doi: 10.3109/07853899309164153. [DOI] [PubMed] [Google Scholar]
- 30.Johnson G, Jenkins M, McLean KM, Griesser HJ, Kwak J, Goodman M, Steele JG. Peptoid-containing collagen mimetics with cell binding activity. J Biomed Mater Res. 2000;51:612–24. doi: 10.1002/1097-4636(20000915)51:4<612::aid-jbm9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 31.Mauch C, Kreig T. Fibroblast-matrix interactions and their role in the pathogenesis of fibrosis. Rheum Dis Clin North Am. 1990;16:93–107. [PubMed] [Google Scholar]
- 32.Garfinkel S, Wessendorf JH, Hu X, Maciag T. The human diploid fibroblast senescence pathway is independent of interleukin-1 alpha mRNA levels and tyrosine phosphorylation of FGFR-1 substrates. Biochim Biophys Acta. 1996;1314:109–19. doi: 10.1016/s0167-4889(96)00105-x. [DOI] [PubMed] [Google Scholar]
- 33.Severino J, Allen RG, Balin S, Balin A, Cristofalo VJ. Is beta-galactosidase staining a marker of senescence in vitro and in vivo? Exp Cell Res. 2000;257:162–71. doi: 10.1006/excr.2000.4875. [DOI] [PubMed] [Google Scholar]
- 34.Volloch V, Kaplan D. Matrix-mediated cellular rejuvenation. Matrix Biol. 2002;21:533–43. doi: 10.1016/s0945-053x(02)00038-0. [DOI] [PubMed] [Google Scholar]
- 35.Abraham LC, Vorrasi J, Kaplan DL. Impact of collagen structure on matrix trafficking by human fibroblasts. J Biomed Mater Res. 2004;70A:39–48. doi: 10.1002/jbm.a.30057. [DOI] [PubMed] [Google Scholar]
- 36.Abraham LC, Zuena EE, Perez B, Kaplan DL. A review of structure and morphology of collagen tissue engineering matrices. 2005 in review. [Google Scholar]
- 37.Chen KY, Chang ZF. Age dependency of the metabolic conversion of polyamines into amino acids in IMR-90 human embryonic lung diploid fibroblasts. J Cell Physiol. 1986;128:27–32. doi: 10.1002/jcp.1041280106. [DOI] [PubMed] [Google Scholar]
- 38.McKeown M, Knowles G, McCulloch CA. Role of the cellular attachment domain of fibronectin in the phagocytosis of beads by human gingival fibroblasts in vitro. Cell Tissue Res. 1990;262:523–30. doi: 10.1007/BF00305249. [DOI] [PubMed] [Google Scholar]
- 39.Mauney J, Kaplan D. 2004 unpublished. [Google Scholar]
- 40.Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001;28:174–81. doi: 10.1016/s8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]
- 41.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 42.Martin I, Jakob M, Schafer D, Dick W, Spagnoli G, Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage. 2001;9:112–8. doi: 10.1053/joca.2000.0366. [DOI] [PubMed] [Google Scholar]
- 43.Abraham LC, Dice JF, Finn PF, Mesires NT, Lee K, Kaplan DL. Extracellular matrix remodeling-Methods to quantify cell-matrix interactions. Biomaterials. 2007;28:151–61. doi: 10.1016/j.biomaterials.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Abraham LC, Dice JF, Finn PF, Mesires NT, Lee K, Kaplan DL. A quantitative assessment of collagen matrix remodeling by fibroblasts. 2005 in review. [Google Scholar]
- 45.Hay ED. Cell biology of extracellular matrix. 2. Plenum Press; New York: 1991. [Google Scholar]
- 46.Lee W, Sodek J, McCulloch CA. Role of integrins in regulation of collagen phagocytosis by human fibroblasts. J Cell Physiol. 1996;168:695–704. doi: 10.1002/(SICI)1097-4652(199609)168:3<695::AID-JCP22>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 47.Laliberte R, Rouabhia M, Bosse M, Chakir J. Decreased capacity of asthmatic bronchial fibroblasts to degrade collagen. Matrix Biol. 2001;19:743–53. doi: 10.1016/s0945-053x(00)00120-7. [DOI] [PubMed] [Google Scholar]
- 48.Arora PD, Fan L, Sodek J, Kapus A, McCulloch CA. Differential binding to dorsal and ventral cell surfaces of fibroblasts: effect on collagen phagocytosis. Exp Cell Res. 2003;286:366–80. doi: 10.1016/s0014-4827(03)00096-x. [DOI] [PubMed] [Google Scholar]
- 49.Paik JW, Kim CS, Cho KS, Chai JK, Kim CK, Choi SH. Inhibition of cyclosporin A-induced gingival overgrowth by azithromycin through phagocytosis: an in vivo and in vitro study. J Periodontol. 2004;75:380–7. doi: 10.1902/jop.2004.75.3.380. [DOI] [PubMed] [Google Scholar]
- 50.Diegelmann RF, Peterkofsky B. Inhibition of collagen secretion from bone and cultured fibroblasts by microtubular disruptive drugs. Proc Natl Acad Sci U S A. 1972;69:892–6. doi: 10.1073/pnas.69.4.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong TT, Sethi C, Daniels JT, Limb GA, Murphy G, Khaw PT. Matrix metalloproteinases in disease and repair processes in the anterior segment. Surv Ophthalmol. 2002;47:239–56. doi: 10.1016/s0039-6257(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 52.Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, Nagase H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. Embo J. 2004;23:3020–30. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rittie L, Berton A, Monboisse JC, Hornebeck W, Gillery P. Decreased contraction of glycated collagen lattices coincides with impaired matrix metalloproteinase production. Biochem Biophys Res Commun. 1999;264:488–92. doi: 10.1006/bbrc.1999.1519. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi T, Hattori S, Shinkai H. Matrix metalloproteinases-2 and -9 are secreted from human fibroblasts. Acta Derm Venereol. 2003;83:105–7. doi: 10.1080/00015550310007436. [DOI] [PubMed] [Google Scholar]
- 56.Davis GE. Affinity of integrins for damaged extracellular matrix: alpha v beta 3 binds to denatured collagen type I through RGD sites. Biochem Biophys Res Commun. 1992;182:1025–31. doi: 10.1016/0006-291x(92)91834-d. [DOI] [PubMed] [Google Scholar]
- 57.Goldsmith EC, Carver W, McFadden A, Goldsmith JG, Price RL, Sussman M, Lorell BH, Cooper G, Borg TK. Integrin shedding as a mechanism of cellular adaptation during cardiac growth. Am J Physiol Heart Circ Physiol. 2003;284:H2227–34. doi: 10.1152/ajpheart.00920.2002. [DOI] [PubMed] [Google Scholar]
- 58.Baciu PC, Suleiman EA, Deryugina EI, Strongin AY. Membrane type-1 matrix metalloproteinase (MT1-MMP) processing of pro-alpha(v) integrin regulates cross-talk between alpha(v)beta(3) and alpha(2)beta(1) integrinsin breast carcinoma cells. Exp Cell Res. 2003;291:167–75. doi: 10.1016/s0014-4827(03)00387-2. [DOI] [PubMed] [Google Scholar]
- 59.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW, Barnes MJ. The collagen-binding A-domains of integrins α1β1 and α2β1 recognize the same specific amino acid sequence, GFOGER, in native (triple helical) collagens. J Biol Chem. 2000;275:35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 61.Stupack DG, Cheresh DA. Get a ligand, get a life: integrins, signaling and cell Survival. J Cell Sci. 2002;115:3729–3738. doi: 10.1242/jcs.00071. [DOI] [PubMed] [Google Scholar]
- 62.Xu Y, Gurusiddappa S, Rich RL, Owens RT, Keene DR, Mayne R, Hook M. Multiple binding sites in collagen type I for the integrins α1β1 and α2β1. J Biol Chem. 2000;275:38981–38989. doi: 10.1074/jbc.M007668200. [DOI] [PubMed] [Google Scholar]
- 63.Eble JA, Golbik R, Kuhn K. The α1β1 integrin recognition site of the basement membrane collagen molecule [α 1(IV)]2 α 2(IV) EMBO J. 1993;12:4795–4802. doi: 10.1002/j.1460-2075.1993.tb06168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Underwood PA, Bennett FA, Kirkpatrick A, Bean PA, Moss BA. Evidence for the location of a binding sequence for the α2β1 integrin of endothelial cells, in the β1 subunit of laminin. Biochem J. 1995;309:765–771. doi: 10.1042/bj3090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vandenberg P, Kern A, Ries A, Luckenbill-Edds L, Mann K, Kiilm K. Characterization of a Type IV Collagen Major Cell Binding Site with Affinity to the α1β1 and the α2β1 Integrins. J Cell Biol. 1991;113:1475–1483. doi: 10.1083/jcb.113.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP, Jr, et al. New Functions for Non-collagenous Domains of Human Collagen Type IV. J Biol Chem. 2000;275:8051–8061. doi: 10.1074/jbc.275.11.8051. [DOI] [PubMed] [Google Scholar]
- 67.Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–155. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 68.Xiong JP, Stehle T, Goodman SL, Arnaout MA. New insights into the structural basis of integrin activation. Blood. 2003;102:1155–1159. doi: 10.1182/blood-2003-01-0334. [DOI] [PubMed] [Google Scholar]