Abstract
Background
Adipose tissue consists of mature adipocytes and a mononuclear cell fraction termed adipose tissue-derived cells (ADCs). Within these heterogeneous ADCs exists a mesenchymal stem cell-like cell population, termed adipose tissue-derived stem cells (ASCs). An important clinical advantage of ASCs over other mesenchymal stem cell populations is the fact that they can be isolated in real time in sufficient quantity, such that ex vivo expansion is not necessary to obtain clinically relevant numbers for various therapeutic applications.
Materials and Methods
The aim of this investigation was to evaluate the therapeutic potential of freshly isolated ADCs in treating rats acutely following myocardial infarction (MI). Rats underwent 45 minutes of left anterior descending artery occlusion followed by reperfusion. Fifteen minutes post-MI, saline or 5 × 106 ADCs from GFP-expressing transgenic rats were injected into the chamber of the left ventricle. Left ventricular function and morphometry was followed with 2-D echocardiography for twelve weeks at which point hearts were harvested for histological analysis.
Results
Twelve weeks following cell therapy, left ventricular end-diastolic dimension was less dilated while the ejection fraction and cardiac output of ADC-treated rats were significantly improved compared to control rats (P<0.01). Despite this benefit, absolute engraftment rates were low. This paradox may be partially explained by ADC-induced increases in both capillary and arteriole densities.
Conclusions
These data confirm the therapeutic benefit of freshly isolated ADCs delivered post-MI and suggest a novel beneficial mechanism for ADCs through a potent pro-angiogenic effect.
Keywords: Myocardial infarction, Cell transplantation, Stem cells, Cardiac function
INTRODUCTION
Although advances in the treatment of patients suffering acute myocardial infarction (MI) have improved outcomes significantly; there still remains the need for novel therapies, which will reduce mortality further and prevent the deleterious consequences that follow myocardial damage. In recent years, numerous investigators have evaluated the use of stem/progenitor cells for treating MI both in animal models and recently in human clinical trials [1-6]. The majority of this work has focused on the use of cells isolated from bone marrow, peripheral blood or skeletal muscle, all of which have been shown to improve cardiac function, at least in animal models. However, each of the currently used adult stem cell reservoirs has practical limitations that represent significant obstacles to their widespread use in human disease. These include invasive and painful harvesting techniques, low cell yields, or the requirement for ex vivo manipulation.
Adipose tissue consists of mature adipocytes and a mononuclear cell fraction termed adipose tissue-derived cells (ADCs). ADCs are a diverse mix of cells including endothelial cells (ECs), smooth muscle cells (SMCs), blood cells, and a mesenchymal stem cell population, termed adipose tissue-derived stem cells (ASCs). ASCs have similar phenotypic and functional properties to bone marrow-derived mesenchymal stem cells (MSCs) [7-10]. ASCs express cell surface markers such as CD44, CD90 and CD105 [7, 10], and have multilineage differentiation potential [8, 10]. Of particular relevance, ASCs have been reported to differentiate into cells of the cardiovascular lineage, including cardiomyocytes [11-13], ECs [13-16], and SMCs [13, 16]. Most importantly for their clinical application, ASC-enriched ADCs can be isolated in large quantities by minimally-invasive liposuction with a significantly higher yield of progenitor cells per volume when compared to bone marrow [10].
The ADC fraction of adipose tissue has the potential to improve cardiac function following MI by several mechanisms; delivery of replacement cells (endothelial cells and cardiomyocytes), salvage of host cardiomyocytes through anti-apoptotic mechanism, or stimulation of angiogenesis. Similar to bone marrow-derived MSCs, ASC-enriched ADCs secrete a number of paracrine factors that are angiogenic or anti-apoptotic, which like MSCs may account for at least some of their beneficial effects [6, 17]. Consistent with this idea, conditioned medium from MSCs [18] and ADCs [19] has the ability to improve cardiac function after ischemic injury. We investigated the potential of freshly isolated ADCs to improve left ventricular (LV) function in a rodent model of MI. We demonstrate that ADCs attenuate LV remodeling after MI and are potent inducers of angiogenesis.
MATERIAL AND METHODS
Animal Studies
All animal studies were performed in conformance with the principles described in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of health (NIH Publication No. 85-23, revised 1996) and the Report of the American Veterinary Medical Association (AVMA) Panel on Euthanasia [20] and were approved by the UCLA Institute for Animal Care and Use Committee (IACUC #1999-028). Twenty male Lewis rats (Charles River Laboratories, Wilmington, MA) were randomly divided into two groups at the time of MI induction; group 1: ADC-treated rats (n=11), and group 2: saline controls (n=9).
For the induction of MI, rats were anesthetized, intubated, ventilated, and a left thoracotomy was created. A length of 7-O Prolene® suture was placed around the left anterior descending artery (LAD) and tightened to occlude the vessel. Blanching of the myocardium and ECG ST-segment elevation were indicative of successful occlusion. After 45 minutes of LAD occlusion, the ligature was loosened and subsequently removed. All animals were allowed to stabilize for at least 15 minutes before 0.2 ml of ADCs at 25 × 106 cells/ml in saline or saline control were injected into the LV using a 26G needle as a slow bolus. The thoracic cavity was then closed and the animals recovered.
All rats underwent morphometric and functional assessment prior to the MI and again 6 and 12 weeks after MI using echocardiography (echo) with a Siemens Acuson Sequoia C256 instrument (Siemens Medical Solutions, Mountain View, CA). Ventricular dimensions were obtained using methods identical to those previously described for mice using M-mode echo [21, 22]. Since an MI generally elicits abnormal wall motion, one-dimensional M-Mode measures of ventricular function can be misleading if they do not include infarcted areas. Therefore, we outlined the interiors of the ventricular chambers from sequences of two-dimensional (2-D) images to obtain better estimates of ventricular areas and volumes at the peak of systole and diastole using AccessPoint software (Freeland Systems LLC, Santa Fe, NM). From these ventricular volumes and the heart rate (HR), we calculated the ejection fraction (EF), stroke volumes (SV) and cardiac outputs (CO) from each rat at each time point of the study.
Isolation and Characterization of ADCs
ADCs were isolated from the green fluorescent protein (GFP) transgenic Lewis rat strain [Lewis-Tg (EGFP) F458.7], which was obtained from the NIH-funded Rat Resources and Research Center (RRRC), University of Missouri, Columbia, MO. Briefly, inguinal subcutaneous adipose tissue was removed from donor GFP-positive rats and minced into pieces weighting < 10 mg each. Adipose tissue was enzymatically digested with Celase™ 1 (Cytori Therapeutics, San Diego, CA) for 45-60 minutes at 37°C, under shaking conditions. The ASC-rich ADC fraction was then separated from the buoyant adipocytes by centrifugation at 600g for 5 minutes as previously described [16]. ADCs were further washed in sterile phosphate buffered saline (PBS; Invitrogen, Carlsbad, CA), counted and resuspended at 25 × 106 cells/ml in saline for infusion into post MI rats, prepared for fluorescence activated cell sorter (FACS) analysis, or placed into low density culture for colony forming unit fibroblast (CFU-F) analysis as previously described [23].
FACS analysis
For further characterization of the rat ADC fraction, cell surface antigen phenotyping was performed using freshly isolated, non-fixed cells. The following cell surface epitopes were marked with anti-rat antibodies: phycoerythrin (PE)-conjugated CD31 (555027; BD Pharmingen, San Diego, CA); PE-conjugated CD34 and fluorescein isothiocyanate (FITC)-conjugated CD34 (ICO115; Santa Cruz Biotechnology Inc., Santa Cruz, CA); as well as Flk1 (EIC), c-kit (CD117; C-19), CD133 (M-20) and CD105 (endoglin; 2Q1707) (all purchased from Santa Cruz). All nonspecific isotype- and species-matched unconjugated or PE- and FITC-conjugated IgG controls were purchased from Santa Cruz. Secondary detection was done using appropriate Alexa Fluor 488-, and Alexa Fluor 647-conjugated antibodies (Molecular Probes, Eugene, OR). Staining with 7-amino-actinomycin (7-AAD; 559925; BD Pharmingen) was performed to exclude dead cells according to the manufacturer’s instructions. All analyses were performed using a BD LSR2 flow cytometer (BD Bioscience, San Jose, CA). FCS files were exported and analyzed using the FlowJo 8.3.3 software (Tree Star Inc., Ashland, OR).
Histological Analysis
Following the 12 week post MI echo, the rats were euthanized using isoflurane. Subsequently, the hearts were excised, trimmed free of the atria, vena cava and pulmonary vessels, weighted and prepared for histology. In detail, the aorta was attached to a Langendorff apparatus and the heart was perfused in retrograde fashion with saline to remove the residual blood. After the saline buffer ran clear, the hearts were perfused with Tissue-Tek OCT (Sakura Finetek Inc., Torrance, CA) until the LV was fully infused. The hearts were then placed in Tissue-Tek OCT filled embedding chambers, flash frozen and stored at -80°C until cr yo-sectioning was performed. All hearts were cryosectioned at 6 μm in a cross-sectional direction. Evaluation of engraftment and assessment of differentiation was performed by immunostaining using anti-GFP (ab290; Abcam Inc., Cambridge, MA), Troponin T (clone CT-3; Lab Vision Corporation, Fremont, CA), and anti-CD31 (550300; BD PharMingen) antibodies. Blood vessels were quantified using histomorphometry (Simple PCI). Capillaries were distinguished from larger arterioles and venules by deriving lumen diameters from area assessments of each vessel. Only vessels with diameters <10 μm and cut in an orthogonal fashion were considered capillaries and included in the quantification. Arteriole density was compared by quantifying those vessels >10 μm, which also co-stained positive for smooth muscle myosin (BT-562; Biomedical Technologies Inc., Stoughton, MA) surrounding the vessel. For vessel density quantification, twelve high power fields were acquired for each region (infarct, border zone and healthy myocardium) and were analyzed for each heart using PCI image analysis software (PCI Geomatics Inc., Arlington, VA). In addition to the immunohistochemical stains, Masson’s Trichrome stain was employed to detect areas of fibrous scar and healthy myocardium, which was then quantified to derive a fibrosis index/scar size.
Statistical Analyses
Statistical analyses were performed with SPSS for Windows, Version 11.0 (SPSS Inc., Chicago, IL) or with InStat V.3.05 (GraphPad Software Inc., San Diego, CA). Two group comparisons were analyzed using either the student’s t-test or ANOVA and deemed significant if P<0.05. Data are expressed as mean ± standard error of measure (SEM).
RESULTS
Freshly isolated rat ADCs contain several populations of potentially therapeutic cells
The rat ADC isolation procedure averaged in 1.18 ± 0.15 × 106 cells per gram of adipose tissue. Mean ADC viability was 91.33% ± 2.41%; this population contained CFU-F at a frequency of 7.7 ± 1.67 CFU/100 cells after 14 days in culture. To better characterize the heterogeneous population of freshly isolated ADCs we performed flow cytometry using a panel of cell surface markers. Consistent with the CFU-F assay, multicolor FACS analysis revealed the presence of a CD105+/CD117+ mesenchymal progenitor population (3.27% ± 0.002%). The combination of these markers has been previously shown to determine a population of bone marrow-derived cells that have MSC-like properties [24]. ADCs further contained populations of CD34+/CD133+/Flk1+/CD117+ (2.70% ± 0.01%), CD34-/CD133+ (2.69% ± 0.004%) and CD31+/CD34+/CD105+/CD117- cells (0.45% ± 0.002%), cell phenotypic profiles that had been previously reported to identify endothelial progenitor and hematopoietic stem cells respectively [25-27]. 11.3% ± 0.01% of the ADCs were CD31-positive consistent with ECs (results summarized in Table 1).
Table 1.
Cell surface markers in freshly isolated rat ADCs.
| Cell surface markers | Mean ± SEM |
|---|---|
| CD31+/CD34-/CD105- | 11.30% ± 0.009% |
| CD31+/CD34-/CD105+ | 1.89% ± 0.004% |
| CD31+/CD34+/CD105+/CD117- | 0.45% ± 0.002% |
| CD34+/CD133+/Flk1+/CD117+ | 2.70% ± 0.007% |
| CD34-/CD133+ | 2.69% ± 0.004% |
| CD105+/CD117+ | 3.27% ± 0.002% |
| Flk1-/CD34-/CD105-/CD117+ | 5.89% ± 0.018% |
| Flk1+/CD34-/CD117+ | 1.25% ± 0.004% |
| Flk1-/CD34+/CD117+ | 0.58% ± 0.003% |
ADCs engraft poorly but improve LV function post MI
Masson’s trichrome staining revealed infarct scars in all animals by 12 weeks; however, there were no significant differences in scar size between control or ADC-treated hearts. To estimate the amount of the ADC engraftment we examined myocardial tissues sections for the presence of GFP-positive cells. Donor GFP-positive cells were identified in only 2 of 11 rats at 12 weeks. GFP-positive cells were observed primarily within the infarct border zone regions (Figure 1A). Interestingly, the presence of engrafted ADCs was not correlated with LV function at 12-weeks. To determine if ADCs could differentiate into cardiac myocytes in vivo as it had been reported in vitro, we co-immunostained sections with a cardiac-specific marker. GFP-positive donor cells co-expressing Troponin T were very rare and whether this represented de novo differentiation versus fusion could not be distinguished in this study (Figure 1B, C).
Figure 1.
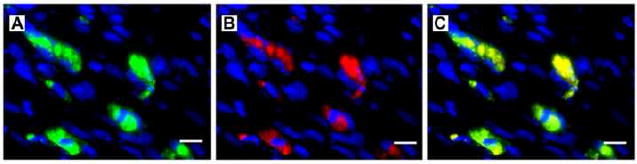
(A) GFP-positive donor cells were found within infarct regions 12 weeks post MI (green = GFP, blue = DAPI). (B) Troponin T-positive cells in the infarct region correlated to the location of the GFP-positive ADCs at 12 weeks post MI (red = Troponin T, blue = DAPI). (C) represents a merged image of (A) and (B). Scale bars equal 10 μm; 40× magnification.
All echo data were obtained under light isoflurane anesthesia with heart rates in the physiological range. As expected, LV function measured by EF and SV was reduced by 19.7% (P<0.001) and 17.8% (P<0.01) respectively in the control (saline-injected) rats after MI (Fig. 2, Table 2). EFs were reduced by an average of 8.9% in the ADC-treated group after MI; this reduction was significantly less than the control group (P<0.01). Likewise, both SV and CO were higher in the ADC-treated group compared to control rats 12 weeks post MI (P<0.01). These results suggest that ventricular contractile function was improved by ADC treatment. Echo assessments at 6 weeks post MI were either similar to the 12 week data or had intermediate values.
Figure 2.
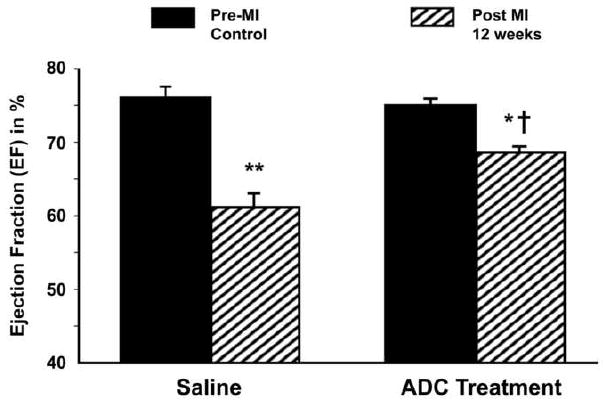
ADC-treated rats demonstrated significantly improved ejection fractions when compared with saline-treated rats at 12 weeks post MI. * = P<0.01 to baseline of the same group; ** = P<0.001 to baseline of the same group; † = P<0.01 to saline group at 12 weeks post MI.
Table 2.
Echocardiographic Analysis of LV Function After MI.
| Control Rats (n=9) | ADC-treated Rats (n=11) | |||
|---|---|---|---|---|
| Baseline | 12 weeks post MI | Baseline | 12 weeks post MI | |
| LVEDD (mm) | 8.16± 0.25 | 9.14± 0.20* | 8.25± 0.10 | 8.92± 0.21 |
| LVESD (mm) | 4.73± 0.20 | 5.91± 0.19* | 5.15± 0.14 | 5.65± 0.23 |
| PWT (mm) | 1.36± 0.05 | 1.40± 0.08 | 1.24± 0.05 | 1.40± 0.08 |
| VST (mm) | 1.39± 0.06 | 1.42± 0.06 | 1.49± 0.04 | 1.39± 0.04 |
| SV (μl) | 306± 15 | 252± 15* | 291± 10 | 328± 14 † |
| HR (bpm) | 363± 9 | 365± 6 | 361± 6 | 369± 5 |
| CO (ml/min) | 111± 5 | 92± 6 | 105± 3 | 121± 6† |
P < 0.01 to baseline
P < 0.001 to baseline
P < 0.01 to control under the same condition
LVEDD = Left ventricular end-diastolic dimension; LVESD = Left ventricular end-systolic dimension; PWT = Posterior wall thickness; VST = Ventricular septal thickness; SV = Stroke volume; HR = Heart rate; CO = Cardiac output
ADC treatment prevents LV remodeling post MI
Cellular therapy often results in an improvement in LV function with little effect on preventing LV remodeling. To determine if ADCs also attenuated the expected LV remodeling that occurs after MI we examined morphometric parameters from the echocardiographic analysis before and 12 weeks post MI in both the control and ADC-treated rats (Table 2). There were no significant differences in wall thickness (posterior wall thickness (PWT) or ventricular septal thickness (VST)) from the non infarcted regions between the groups. The LV end-diastolic dimension (LVEDD) was significantly greater in the control rats at 12 weeks post MI compared to baseline (P<0.01). In contrast ADC-treated rats did not demonstrate statistically significant LV dilation. Similar results were seen when LV end-systolic dimensions (LVESD) were examined. These morphometric data suggest that the significant remodeling seen in the control rats at 12 weeks post MI is blunted in the ADC-treated rats.
ADCs induce new capillary and arteriole formation
We and others have suggested that transplanted cells may secrete autocrine or paracrine factors that could have beneficial effects independent of myocardial regeneration, for example, by promoting angiogenesis. Therefore, to quantitate new vessel formation, myocardial sections were immunostained for the endothelial specific marker, CD31. Capillary density was evaluated in the infarct region, border zone and the healthy myocardium. As shown in Figure 3, ADC-treated animals showed significantly more capillaries per mm2 in the infarct border zone (288.9 ± 17.8 versus 206.5 ± 15.9; P<0.01). There was a similar trend in the infarct region that did not reach statistical significance (32.2 ± 17.4 versus 19.7 ± 3.5; P=ns). Interestingly, we also found significantly more capillaries in the healthy myocardium of ADC-treated rats versus controls (499.7 ± 13.4 versus 439.7 ± 13.6; P<0.01). In addition, we examined arteriole density in the healthy, infarct and border zones. ADC-treated animals showed significantly more arterioles per mm2 within the border zones (19.9 ± 1.8 versus 14.9 ± 1.2, Fig. 4; P<0.05) and infarcted myocardium (15.8 ± 2.0 versus 9.6 ± 2.1, P<0.05) than the control rats.
Figure 3.
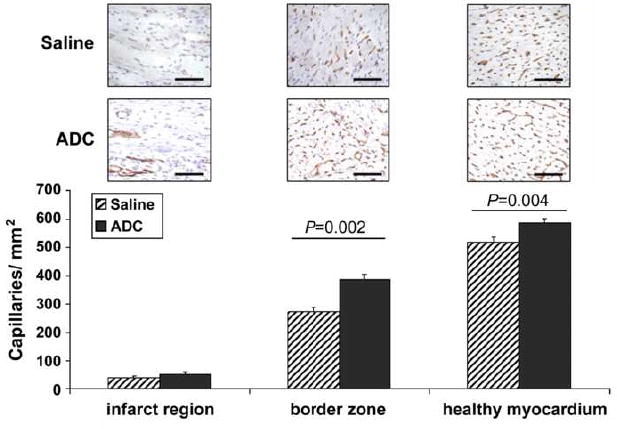
Myocardial capillary density within the infarcted, border and healthy regions of hearts 12 weeks post MI demonstrated significantly more capillaries in the ADC-treated rats when compared with the saline controls. Representative images of CD31 immunostaining (red) of each zone for both ADC-treated and control rats are shown directly above the graph. Scale bars equal 100 μm; 20× magnification.
Figure 4.
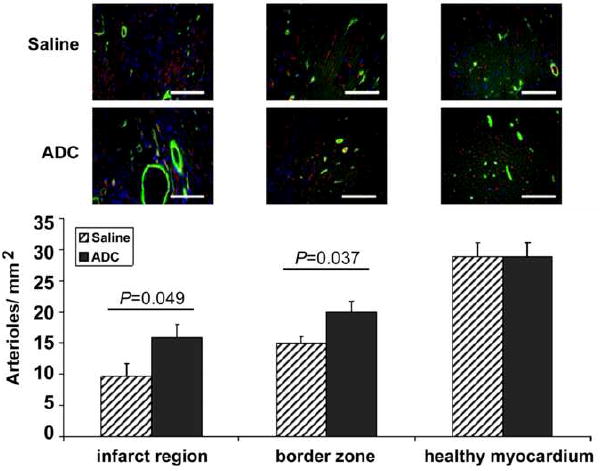
Myocardial arteriole density was significantly greater in the infarct and border regions of ADC-treated versus control rats 12 weeks post MI. Green = Smooth Muscle Myosin, Red = CD31, Blue = DAPI. Scale bars equal 100 μm; 20× magnification.
DISCUSSION
Current treatment of MI still leaves a significant number of patients with impaired cardiac function that leads to more severe LV dysfunction as the ventricle remodels. Thus, cell therapy has generated much excitement as a novel therapy that might provide additive benefits over conventional treatment to restore or prevent further LV dysfunction after MI. Stem or progenitor cells from peripheral blood, bone marrow and skeletal muscle, autologous or allogeneic, have now all been tested in humans and have shown modest improvements in cardiac function [3-5]. While these early clinical results are promising, the practical utility of using cells from each of these sources is limited. Depending on the source, low cell yields, extended culture periods between tissue collection, and potential immune rejection associated problems have all been identified as barriers for mainstream clinical adoption. Adipose tissue provides an attractive source of adult stem cells due to the minimal harvest morbidity associated with liposuction and the high cell yield per unit tissue, which obviate the need for cell expansion, thus, allowing for autologous cell treatment to be given acutely following injury.
Although there have been several reports that ASCs or ADCs improve cardiac function post-MI [28-31], no clear mechanism has been proposed to explain this beneficial effect. This study demonstrates the ability of a freshly isolated ASC-rich ADC fraction, delivered acutely following LAD occlusion/reperfusion, to improve cardiac function independent of engraftment. In contrast to many previous studies on adult stem cell therapy [2, 13, 29-32], this study demonstrated very poor long-term cell engraftment and significant cardiac myocyte differentiation was not seen. However, this paradox is consistent with the findings of Limbourg et al. who also showed that hematopoietic stem cells can improve cardiac function post MI, even in the absence of sustained engraftment [33]. The results of the current study suggest an alternate explanation for the functional improvements seen in infarcted hearts following ADC-treatment. ADCs accelerated angiogenesis in the infarcted areas, namely, increased capillary and arteriole density within the infarct border zones. Given the absence of significant numbers of donor cells retained within this region (or incorporated into the vessel wall) at 12 weeks, and the known fact that ADCs are able to secrete numerous angiogenic, arteriogenic, chemotactic and anti-apoptotic growth factors [6, 17, 19, 34], it is much likely that a paracrine signaling mechanism may be responsible for the observed improvements rather than cell differentiation and engraftment.
This mechanism has been suggested by other investigators examining adult stem cells from other sources administered acutely post MI [6, 35]. Furthermore, angiogenic and cytoprotective growth factors and cytokines such as vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), as well as hepatocyte growth factor (HGF) have been shown to significantly improve cardiac function through increased angiogenesis and decreases in infarct size, when either directly administered acutely post-MI [34] or delivered as a gene construct [36] in a rat MI model. Similar data were reported by Li et al. who showed increased capillary densities along with significantly higher VEGF protein levels and VEGF mRNA expression after adipose stromal cell treatment in a rat MI model [31]. While myocardial hypoxia during infarction has been demonstrated to induce expression of angiogenic growth factors including VEGF as well [37], it is likely that ADCs further augment secretion of potent factors enhancing this mechanism. Interestingly, Shyu et al. recently investigated the effects of individual angiogenic genes (Ang-1 and VEGF) in comparison to adult stem cells in a murine model of MI [38]. The authors noted that while both Ang-1 and VEGF significantly improved cardiac performance, adult stem cells were superior to the individual genes in terms of diastolic dysfunction and capillary density. This may be due to the ability of cells to function as a source of sustainable and controllable production of multiple growth factors in response to microenvironmental cues. In contrast, growth factor gene therapy, to date, has consisted of single growth factors, thereby providing individual stimuli lacking the ability to respond to environmental signals for tissue regeneration.
While many investigators have focused on homogeneous populations of adult stem cells, it may be advantageous to utilize a heterogeneous population of cells such as ADCs in this study that can benefit LV function by multiple mechanisms. In this study, we confirmed the presence of ECs and several subpopulations of stem/progenitor cells within the ADC fraction used for post MI injections. These cell populations are also seen in the ASC-rich ADC fraction obtained from humans [39, 40]. Freshly isolated ASC-rich ADCs also contain CD34+/CD133+/Flk1+/CD117+ cells; most likely a population of EPCs that can also participate in the angiogenic response post ischemic injury [40]. Thus, the heterogeneous ADC population has the potential to restore cardiac function by multiple mechanisms, including therapeutic angiogenesis, anti-inflammation, and cardiomyocytic regeneration.
While this study supports ADC-induced angiogenesis as an important mechanism of action, it does not rule out the other mechanisms outlined above, which also play potentially important roles in the improvement of cardiac function, similar to what we have observed. Further studies will be necessary to determine the relative contribution of each potential mechanism and the critical cell population within ADCs that mediates these benefits. Studies to isolate and characterize the specific ASC population, within ADCs are currently under way. In conclusion, this study suggest that freshly isolated mononuclear cells from adipose tissue are a clinically important source of cells that can be used in real time, without expansion, to improve cardiac function following acute myocardial infarction.
Acknowledgments
We would like to thank K. Hayakawa, P. Zhao, J. Yang, J. Kim, E. Daniels and R. Schreiber for technical assistance. This work was supported by gifts from the Laubisch Fund (WRM and KPR) as well as grants AHA EIA 0340087N, P01 HL080111 and R01 HL62448 to WRM, and the Deutsche Forschungsgemeinschaft (DFG; Sche701/2-1, 3-1 (KSL)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102(32):11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagaya N, Fujii T, Iwase T, Ohgushi H, Itoh T, Uematsu M, Yamagishi M, Mori H, Kangawa K, Kitamura S. Intravenous administration of mesenchymal stem cells improves cardiac function in rats with acute myocardial infarction through angiogenesis and myogenesis. Am J Physiol Heart Circ Physiol. 2004;287(6):H2670–H2676. doi: 10.1152/ajpheart.01071.2003. [DOI] [PubMed] [Google Scholar]
- 3.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 4.Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ, Aaronson KD. Autologous skeletal myoblasts transplanted to ischemia-damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol. 2003;41(5):879–888. doi: 10.1016/s0735-1097(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 5.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 6.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112(8):1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 7.De Ugarte DA, Morizono K, Elbarbary A, Alfonso Z, Zuk PA, Zhu M, Dragoo JL, Ashjian P, Thomas B, Benhaim P, Chen I, Fraser J, Hedrick MH. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174(3):101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 8.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui JH, Li L, Teo YH, Ouyang HW, Lee EH. Comparative study of the ability of mesenchymal stem cells derived from bone marrow, periosteum, and adipose tissue in treatment of partial growth arrest in rabbit. Tissue Eng. 2005;11(56):904–912. doi: 10.1089/ten.2005.11.904. [DOI] [PubMed] [Google Scholar]
- 10.Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, Fraser JK, Hedrick MH. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54(3):132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 11.Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, Penicaud L, Castailla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94(2):223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 12.Rangappa S, Fen C, Lee EH, Bongso A, Sim EK. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75(3):775–779. doi: 10.1016/s0003-4975(02)04568-x. [DOI] [PubMed] [Google Scholar]
- 13.Yamada Y, Wang XD, Yokoyama S, Fukuda N, Takakura N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem Biophys Res Commun. 2006;342(2):662–670. doi: 10.1016/j.bbrc.2006.01.181. [DOI] [PubMed] [Google Scholar]
- 14.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109(5):656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332(2):370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 16.Heydarkhan-Hagvall S, Schenke-Layland K, Yang YQ, Heydarkhan S, Xu Y, Zuk PA, MacLellan WR, Beygui RE. Human Adipose Stem Cells: A Potential Cell Source for Cardiovascular Tissue Engineering. Cells Tissue Organs. 2008 doi: 10.1159/000113407. in press. [DOI] [PubMed] [Google Scholar]
- 17.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm_grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 18.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94(5):678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 19.Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25(12):2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 20.2000 Report of the AVMA Panel on Euthanasia. J Am Vet Med Assoc. 2001;218:669–696. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- 21.Chu DK, Jordan MC, Kim JK, Couto MA, Roos KP. Comparing isoflurane with tribromoethanol anesthesia for echocardiographic phenotyping of transgenic mice. J Am Assoc Lab Anim Sci. 2006;45(4):8–13. [PubMed] [Google Scholar]
- 22.Roos KP, Jordan MC, Fishbein MC, Ritter MR, Friedlander M, Chang HC, Rahgozar P, Han T, Garcia AJ, MacLellan WR, Ross RS, Philipson KD. Hypertrophy and heart failure in mice overexpressing the sodium-calcium exchanger. J Card Fail. 2007;13(4):318–329. doi: 10.1016/j.cardfail.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 24.Sun S, Guo Z, Xiao X, Liu B, Liu X, Tang PH, Mao N. Isolation of Mouse Marrow Mesenchymal Progenitors by a Novel and Reliable Method. Stem Cells. 2003;21:527–535. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- 25.Kähler CM, Wechselberger J, Hilbe W, Gschwendtner A, Colleselli D, Niederegger H, Boneberg EM, Spizzo G, Wendel A, Gunsilius E, Patsch JR, Hamacher J. Peripheral infusion of rat bone marrow derived endothelial progenitor cells leads to homing in acute lung injury. Respir Res. 2007;9:8–50. doi: 10.1186/1465-9921-8-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W, Zhang G, Jin H, Hu R. Characteristics of bone marrow-derived endothelial progenitor cells in aged mice. Biochem Biophys Res Commun. 2006;348(3):1018–1023. doi: 10.1016/j.bbrc.2006.07.161. [DOI] [PubMed] [Google Scholar]
- 27.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105(3):369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 28.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28(21):2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 29.Strem BM, Zhu M, Alfonso Z, Daniels EJ, Schreiber R, Beygui R, MacLellan WR, Hedrick MH, Fraser JK. Expression of cardiomyocytic markers on adipose tissue-derived cells in a murine model of acute myocardial injury. Cytotherapy. 2005;7(3):282–291. doi: 10.1080/14653240510027226. [DOI] [PubMed] [Google Scholar]
- 30.Zhang DZ, Gai LY, Liu HW, Jin QH, Huang JH, Zhu XY. Transplantation of autologous adipose-derived stem cells ameliorates cardiac function in rabbits with myocardial infarction. Chin Med J (Engl) 2007;120(4):300–307. [PubMed] [Google Scholar]
- 31.Li B, Zeng Q, Wang H, Shao S, Mao X, Zhang F, Li S, Guo Z. Adipose tissue stromal cells transplantation in rats of acute myocardial infarction. Coron Artery Dis. 2007;18(3):221–227. doi: 10.1097/MCA.0b013e32801235da. [DOI] [PubMed] [Google Scholar]
- 32.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 33.Limbourg FP, Ringes-Lichtenberg S, Schaefer A, Jacoby C, Mehraein Y, Jäger MD, Limbourg A, Fuchs M, Klein G, Ballmaier M, Schlitt HJ, Schrader J, Hilfiker-Kleiner D, Drexler H. Haematopoietic stem cells improve cardiac function after infarction without permanent cardiac engraftment. Eur J Heart Fail. 2005;7(5):722–729. doi: 10.1016/j.ejheart.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Anderson CD, Heydarkhan-Hagvall S, Schenke-Layland K, Yang JQ, Jordan MC, Kim JK, Brown DA, Zuk PA, Laks H, Roos KP, MacLellan WR, Beygui RE. The Role of Cytoprotective Cytokines in Cardiac Ischemia/Reperfusion Injury. J Surg Res. 2007 doi: 10.1016/j.jss.2007.08.005. in press. [DOI] [PubMed] [Google Scholar]
- 35.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98(11):1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi K, Ito Y, Morikawa M, Kobune M, Huang J, Tsukamoto M, Sasaki K, Nakamura K, Dehari H, Ikeda K, Uchida H, Hirai S, Abe T, Hamada H. Adenoviral-delivered angiopoietin-1 reduces the infarction and attenuates the progression of cardiac dysfunction in the rat model of acute myocardial infarction. Mol Ther. 2003;8(4):584–592. doi: 10.1016/s1525-0016(03)00230-2. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Brown LF, Hibberd MG, Grossman JD, Morgan JP, Simons M. VEGF, flk-1, and flt-1 expression in a rat myocardial infarction model of angiogenesis. Am J Physiol. 1996;270:H1803–H1811. doi: 10.1152/ajpheart.1996.270.5.H1803. [DOI] [PubMed] [Google Scholar]
- 38.Shyu KG, Wang BW, Hung HF, Chang CC, Shih DT. Mesenchymal stem cells are superior to angiogenic growth factor genes for improving myocardial performance in the mouse model of acute myocardial infarction. J Biomed Sci. 2006;13(1):47–58. doi: 10.1007/s11373-005-9038-6. [DOI] [PubMed] [Google Scholar]
- 39.Boquest AC, Shahdadfar A, Fronsdal K, Siqurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16(3):1131–1141. doi: 10.1091/mbc.E04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Estrada OM, Munoz-Santos Y, Julve J, Reina M, Vilaro S. Human adipose tissue as a source of Flk-1+ cells: new method of differentiation and expansion. Cardiovasc Res. 2005;65(2):328–333. doi: 10.1016/j.cardiores.2004.11.015. [DOI] [PubMed] [Google Scholar]


