Abstract
A LCMS method was developed and validated for the simultaneous determination of buprenorphine (BUP), norbuprenorphine (NBUP), buprenorphine glucuronide (BUP-Gluc) and norbuprenorphine glucuronide (NBUP-Gluc) in human umbilical cord. Quantification was achieved by selected ion monitoring of precursor ions m/z 468.4 for BUP; 414.3 for NBUP; 644.4 for BUP-Gluc and 590 for NBUP-Gluc. BUP and NBUP were identified by MS2, with m/z 396, 414 and 426 for BUP, and m/z 340, 364 and 382 for NBUP. Glucuronide conjugates were identified by MS3 with m/z 396 and 414 for BUP-Gluc and m/z 340 and 382 for NBUP-Gluc. The assay was linear 1–50 ng/g. Intra, inter-day and total assay imprecision (%RSD) were <14.5%, and analytical recovery ranged from 94.1% to 112.3% for all analytes. Extraction efficiencies were >66.3%, and process efficiency >73.4%. Matrix effect ranged, in absolute value, from 3.7% to 27.4% (CV<21.8%, n=8). The method was selective with no endogenous or exogenous interferences from 41 compounds evaluated. Sensitivity was high with limits of detection of 0.8 ng/g. In order to prove method applicability, an authentic umbilical cord obtained from an opioid-dependent pregnant woman receiving BUP pharmacotherapy was analyzed. Interestingly, BUP was not detected but concentrations of the other metabolites were NBUP-Gluc 13.4 ng/g, BUP-Gluc 3.5 ng/g and NBUP 1.2 ng/g.
Keywords: umbilical cord, buprenorphine, LCMS, ion trap
INTRODUCTION
In utero drug exposure is associated with negative consequences in fetal development such as adverse mental, physical, and psychological outcomes in newborns [1, 2]. Toxicological analysis of biological matrices from the mother (hair, blood, oral fluid, sweat, urine, breast milk), the newborn (meconium, hair, urine, cord blood, cord tissue), or placenta and amniotic fluid may identify drug-exposed neonates. Recently, Lozano et al. [3] and Gray and Huestis [4] published reviews on specimens for monitoring in utero drug exposure. In newborns, neonatal hair [5, 6] and meconium [7–10] have proven useful, as well as umbilical cord tissue, an interesting alternative matrix for fetal drug exposure detection [11, 12 ]. Umbilical cord offers advantages over hair because many parents reject hair collection for cosmetic or cultural reasons, while umbilical cord is considered a waste product. Compared to meconium, faster results are available with umbilical cord because specimens are collected at delivery rather than several days later for meconium.
In 2002, BUP became available in the United States for office-based treatment of opioid dependence under the Drug Treatment Act of 2000. This law allows physicians to write prescriptions for schedule III, IV, and V narcotic medications specifically approved by the Food and Drug Administration for narcotic addiction treatment [13]. As popularity of BUP increases for opioid dependence treatment, its use has been expanded to high risk populations such as pregnant women in Europe [14, 15] and Australia [16]. In the US, methadone remains the only approved medication for treatment of opioid dependence during gestation, although data are available comparing the safety and efficacy of methadone and buprenorphine in this cohort [17].
BUP is a semi-synthetic opioid derived from thebaine, and a highly lipid soluble base similar in structure to morphine. BUP is well absorbed in the gastrointestinal tract, but has low oral bioavailability due to high first-pass hepatic metabolism [18]. BUP is rapidly metabolized through N-dealkylation mainly by CYP3A4 enzyme in the liver to NBUP [19]. Both compounds, BUP and NBUP, are further metabolized by phase II glucuronidation.
Liquid chromatography mass spectrometry, LCMS, has been applied for BUP and metabolite analyses in a wide variety of matrices including, urine [20–30], plasma [25, 31–37], whole blood [22, 25, 27, 38], hair [6, 22, 25, 27, 39–41], sweat [42], meconium [9], and breast milk [43]. Among these, only four manuscripts describe a method for the simultaneous determination of BUP, NBUP and their glucuronides, one in plasma [36], two in urine [28, 30], and one in both matrices [24].
Umbilical cord specimen data were reported for cocaine [11, 44] and methamphetamine, opiates, cannabinoids and phencyclidine [11]. The concentrations were measured by a meconium method [11, 45], or validation data were not supplied for the umbilical cord matrix [44]. Only Winecker et al. [46] developed a cocaine quantification method in umbilical cord specimens. In order to obtain reliable data, the analytical method employed has to be fully validated in a given biological matrix. This is especially important for LCMS methods because ion suppression or enhancement is highly dependent on the specific matrix.
To our knowledge, this is the first validated assay for the analysis of BUP, NBUP, BUP-Gluc, and NBUP-Gluc in human umbilical cord. The method will be employed to analyze umbilical cords collected in a clinical research study investigating buprenorphine as pharmacotherapy for opioid-dependent pregnant women.
EXPERIMENTAL
Chemicals and materials
BUP, NBUP, BUP-Gluc, NBUP-Gluc, norbuprenorphine-d3 (NBUP-d3) and buprenorphine-d4 (BUP-d4) standards were obtained from Cerilliant (Austin, TX, USA). Stock BUP-Gluc and NBUP-Gluc quality control (QC) solutions were purchased from ElSohly Laboratories (Oxford, MS, USA). BUP and NBUP quality controls (QC) were prepared from different lots of Cerilliant, when possible, or from a different vial, with preparation on different days than for calibrators. Reagent grade formic and perchloric acid were from Sigma Chemicals (St. Louis, MO, USA) and Acros Organics, (Morris Plains, NJ, USA), respectively. All solvents were of HPLC grade. Solid phase extraction (SPE) was performed using Strata-XC columns (100 mg/6 mL) (Phenomenex, Torrance, CA, USA). Anonymized drug-free umbilical cords were confirmed negative for BUP and metabolites prior to use.
Instrumentation
LCMS analyses were performed on a Thermo Finnegan LCQ Deca XP Plus ion trap mass spectrometer, with an electrospray ionization (ESI) source interfaced with a Surveyor autosampler and LC pump (Thermo Electron, San Jose, CA, USA). Specimen homogenization was achieved with a Tissue Tearor (Biospec Products, INC., Bartlesville, OK, USA). Solvent evaporation was carried out on a TurboVap LV evaporator from Zymark (Hopkinton, MA, USA).
Preparation of standard solutions
Solutions containing 10 and 1 μg/mL of BUP, NBUP, BUP-Gluc and NBUP-Gluc were prepared separately in methanol from 100 μg/mL stock calibrators. Different working solutions of the four analytes were prepared by appropriate dilution in methanol. The internal standard solution (IStd) at 1 μg/mL of BUP-d4 and NBUP-d3 was prepared by dilution of 100 μg/mL stock solutions in methanol. QC solutions containing BUP, NBUP, BUP-Gluc and NBUP-Gluc were prepared in methanol at three different working concentrations across the linear range of the assay.
Calibrators and quality control sample preparation
A seven-point calibration curve (1, 2, 5, 10, 20, 30 and 50 ng/g) was prepared by addition of 50 μL working calibrator and 50 μL IS solution to 2±0.05 g of blank umbilical cord. In the same manner, low, medium and high QC samples containing 3, 15 and 40 ng/g were prepared by adding 50 μL working QC solution and 50 μL IStd solution to 2±0.05 g of blank umbilical cord. After adding 8 mL of 0.1% perchloric acid in water, homogenization was performed for 0.5–1 min at 30,000 rpm followed by centrifugation at 7,500 rpm for 15 min. The supernatant was separated into a clean test tube and subjected to SPE.
Specimen preparation
2±0.05 g umbilical cord was weighed and cut into small pieces. After adding 8 mL 0.1% perchloric acid in water and 50 μL IStd solution, homogenization was performed for 0.5–1 min at 30,000 rpm. After centrifugation at 7,500 rpm for 15 min, the supernatant was separated into a clean test tube and subjected to SPE.
SPE
SPE columns were preconditioned with 2 mL methanol and 2 mL 0.1% v/v perchloric acid in water. Specimen homogenate was applied followed by wash steps of 2 mL 2% v/v formic acid in water and 2 mL methanol. Cartridges were dried for 15 min under vacuum, before eluting with 3 mL methylenechloride:isopropanol:concentrated ammonium hydroxide (60:35:5, v/v/v). Eluates were completely dried under nitrogen at 45°C and reconstituted with 100 μL mixture of 85% A (0.1% formic acid in water) and 15% B (0.1% formic acid in acetonitrile). After centrifugation at 1,500 rpm for 2 min, the supernatant was transferred to a clean vial, and 20 μL injected into the LCMS.
Liquid Chromatography
Chromatographic separation was achieved with a Synergi Polar-RP 80A (75 × 2 mm, 4 μm) column with a 4×2 mm, identically packed, guard column (Phenomenex, Torrence, CA, USA) and gradient elution with mobile phase A and B. Initial flow conditions were 15% B at 200 μL/min for 2.5 min, then flow was increased to 300 μL/min and B increased to 65% over 7.5 min. From 10 to 12 min, B was increased again to 95%. Flow and mobile phase ratios were returned to initial conditions over 1 min, and the column re-equilibrated for additional 7 min, yielding a total run time of 20 min.
Mass Spectrometry
Mass spectrometric data were acquired in ESI positive ion mode with the following parameters: sheath gas flow rate 50; auxiliary gas flow rate 10; spray voltage 5 kV; and transfer capillary temperature 275°C. MSn optimization was established by directly infusing 1 μg/mL solutions of single analytes in methanol. Two scan events were performed for each analyte for quantification and identification purposes. The quantification scan event was selected ion monitoring (SIM) of the precursor ion without fragmentation. Glucuronide conjugates were identified by performing MS3, and BUP and NBUP were identified via MS2. MS3 fragmentation was monitored in consecutive reaction monitoring (CRM) mode and MS2 in selected reaction monitoring (SRM) mode. Each free analyte was identified by the presence of 4 characteristic ions from MH+ fragmentation: BUP and NBUP were identified by the precursor ion and 3 MS2 fragment ions; in the case of glucuronidated analytes, MH+ was fragmented cleaving the glucuronide moiety, the surviving molecule was further fragmented, and two characteristic MS3 ions monitored. Table 1 displays monitored ions and collision energies employed.
Table 1.
LCMS parameters, retention times and corresponding internal standards for buprenorphine (BUP) and metabolites norbuprenorphine (NBUP); buprenorphine glucuronide (BUP-Gluc) and norbuprenorphine glucuronide (NBUP-Gluc).
| Analyte | SIM m/z | SRM Transition | CRM Transition | MS2 Collision Energy (%) | MS3 Collision Energy (%) | Retention Time (min) | Internal Standard |
|---|---|---|---|---|---|---|---|
| NBUP-Gluc | 590 | - | 590>414.2>340 590>414.2>382 |
29 | 32 | 2.6 | NBUP-d3 |
| BUP-Gluc | 644.4 | - | 644.4>468.3>396 644.4>468.3>414 |
31 | 36 | 8.6 | NBUP-d3 |
| NBUP | 414.4 | 414.4>340 414.4>364 414.4>382 |
- | 33 | - | 8.9 | NBUP-d3 |
| NBUP-d3 | 417.3 | 417.3>343 417.3>399 |
- | 33 | - | 8.9 | - |
| BUP | 468.4 | 468.4>396 468.4>414 468.4>426 |
- | 36 | - | 10.8 | BUP-d4 |
| BUP-d4 | 472.4 | 472.4>400 472.4>415 |
- | 36 | - | 10.8 | - |
SIM (Selected ion monitoring); SRM (Selected ion monitoring); CRM (Consecutive reaction monitoring); BUP-d4 (buprenorphine-d4); and NBUP-d3 (norbuprenorphine-d3).
Validation
Validation parameters included linearity, limits of detection (LOD) and quantification (LOQ), imprecision, analytical recovery, extraction efficiency, matrix effect, process efficiency, selectivity, carryover, dilutional integrity and stability studies. Linearity was determined by least-squares regression with 1/x weighting to compensate for heteroscedasticity. Acceptable linearity was achieved when the coefficient of determination was at least 0.99 and calibrators quantified within ±20% at the LOQ and ±15% at other concentrations. LOD and LOQ were evaluated with decreasing analyte concentrations in drug-fortified umbilical cord (1, 0.8, 0.5, 0.2 and 0.1 ng/g). LOD was defined as the lowest concentration with acceptable chromatography, presence of all qualifier ions with signal-to-noise ratios of at least 3, and a retention time (RT) within ± 0.2 min of average calibrator RT. Eight umbilical cord samples from different sources (n=8) were fortified at the LOD, and these parameters checked. LOQ was the lowest concentration that met LOD criteria with signal-to-noise ratio of at least 10, imprecision less than 20%, and analytical recovery between 80–120% of target concentration (n=20, 5 replicates on 4 different days).
Imprecision and analytical recovery were determined at three concentrations (3, 15 and 40 ng/mL) across the linear dynamic range by preparing and analysing 5 replicates on four different days (n=20). Imprecision, expressed as % relative standard deviation (%RSD) of measured concentrations, was expected to be less than 15%, except for the low QC, for which 20% was acceptable. The guidelines given by Krouwer and Rabinowitz [47] were followed for calculation of pooled intra-day, inter-day and total imprecision. Analytical recovery was evaluated as % of target concentration (n=20), required to be between 85–115%, except for the low QC for which 80–120% was acceptable.
Extraction efficiency for each analyte was measured at each QC concentration. Blank umbilical cord was fortified with QC and internal standard solution before and after SPE. Percent extraction efficiency from umbilical cord was expressed as mean analyte area of samples (n=5) fortified with control solution before extraction divided by mean area of samples (n=5) with control solution added after SPE. Matrix effect was assessed by comparing analyte peak areas in eight unique blank extracted umbilical cords fortified with QC and internal standard solutions after SPE to peak areas of samples at the same nominal concentrations prepared in an 85:15 v/v mixture of mobile phase A and mobile phase B (neat). Matrix suppression or enhancement was calculated as follows: (100 × mean peak area of fortified umbilical cord tissue after SPE/mean peak area of fortified mobile phase) −100.
Process efficiency examines the overall effect of SPE extraction efficiency and matrix effect together on the quantification of analytes of interest. It was determined by comparing mean analyte peak areas of five samples fortified before SPE to mean peak areas of five neat samples prepared in mobile phase at the same concentration.
Interferences from endogenous matrix components were evaluated by analyzing umbilical cord specimens from eight healthy subjects who were not exposed to BUP fortified with IStd. Endogenous interferences were considered insignificant if analytes were not detected or quantified below the LOD in these eight umbilical cords. Method selectivity was demonstrated by adding high concentrations (500 ng/g) of potentially interfering licit and illicit drugs to low QC samples. The following drugs and metabolites were examined: cocaine, benzoylecgonine, norcocaine, norbenzoylecgonine, ecgonine ethyl ester, ecgonine methyl ester, anhydroecgonine methyl ester, ecgonine, methadone, EMDP, EDDP, methadol, Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC, 11-nor-9-carboxy-THC, morphine, normorphine, morphine 3-β-glucuronide, morphine 6-β-glucuronide, codeine, norcodeine, 6-acetylmorphine, 6-acetylcodeine, diazepam, lorazepam, oxazepam, alprazolam, imipramine, clomipramine, fluoxetine, norfluoxetine, clonidine, ibuprofen, pentazocine, caffeine, diphenhydramine, chlorpheniramine, brompheniramine, acetylsalicylic acid, acetaminophen, and phencyclidine. In addition, umbilical cord specimens from seven opioid-dependent women maintained on methadone were analyzed after fortification with BUP and metabolites at low QC concentrations. Many of these women relapsed to heroin and cocaine use during methadone treatment, providing the opportunity to test specificity of these drugs and metabolites in authentic specimens. Sufficient specificity was achieved if BUP, NBUP, BUP-Gluc and NBUP-Gluc quantified within ±20% of low QC concentrations.
Lack of carryover was demonstrated by injecting IStd-fortified blank umbilical cord extract immediately after a sample fortified with 100 ng/g of all analytes. Carryover was considered negligible to 100 ng/g if analytes were not detected in the blank umbilical cord or if calculated concentrations were below the LOD. Dilution integrity was evaluated by diluting umbilical cord samples (n=2) containing 160 ng/g of each analyte with blank umbilical cord to achieve a 1:4 dilution. Internal standard was added to diluted samples, and were extracted as described. Dilutional integrity was maintained if specimens quantified at 40 ng/g ± 20%.
Analyte stability was investigated under a variety of conditions. Autosampler stability was determined by duplicate QC samples at low, medium and high concentrations, stored at 10°C in the autosampler for 48 and 72 h after preparation. In addition, stability was tested for drug-fortified umbilical cord stored at room temperature (22°C) for 16 h, in the refrigerator (4°C) for 72 h, and after three freeze-thaw cycles for each QC in triplicate. Stability was considered acceptable if QC samples quantified within ±20% of target.
Identification criteria
Identification criteria included RT within ± 0.2 min of the average calibrator RT, presence of the precursor ion, presence of 3 MS2 products for BUP and NBUP, presence of 2 MS3 products for BUP-Gluc and NBUP-Gluc, and relative ion intensities (% of base peak) within ±20% if the relative ion intensity is >50%, ±25% if between 20–50%, ±30% if between 10–20%, and ±50% if 10% [48]. For BUP and NBUP two relative ion intensities were calculated based on the ion ratios (most abundant product ion divided by less abundant ion) 414/396 and 414/426 for BUP, and 340/364 and 340/382 for NBUP. In the case of glucuronides, one relative ion intensity was calculated based on the ion ratio 414/396 for BUP-Gluc, and 340/382 for NBUP-Gluc. These values were compared to the mean relative ion intensity of all of the calibrators.
Proof of Method
In order to demonstrate method applicability, an authentic umbilical cord from an opioid-dependent pregnant woman receiving controlled buprenorphine treatment was analyzed. The specimen was collected as part of a protocol providing BUP pharmacotherapy to opioid-dependent pregnant women approved by the Johns Hopkins Bayview and National Institute on Drug Abuse Institutional Review Boards. Subjects provided written informed consent to participate.
RESULTS AND DISCUSSION
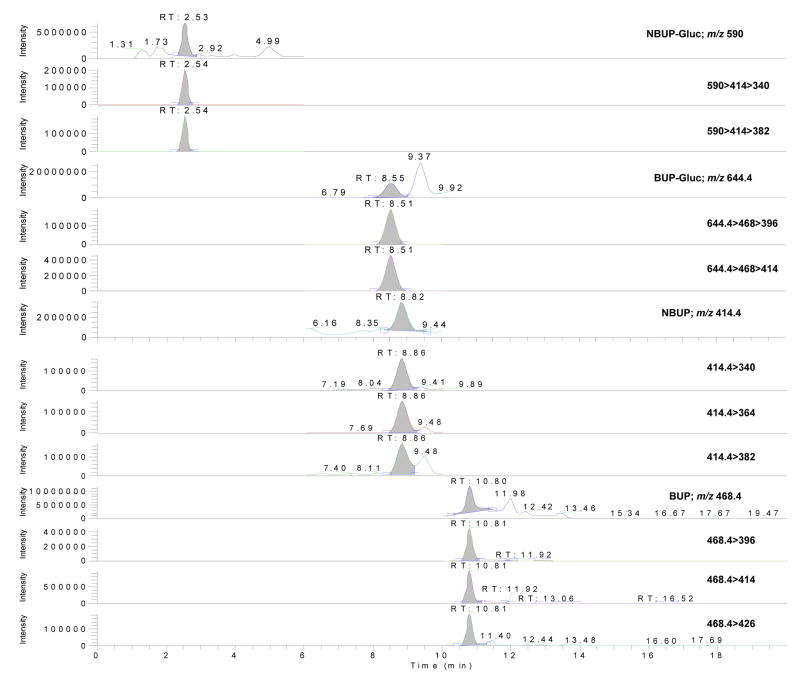
The linearity of compound-to-IS peak ratios versus theoretical concentrations was verified in umbilical cord using 1/x-weighted linear regression. Curvature was tested on a set of four calibration curves on four different days with determination coefficients (r2) ≥ 0.99 (BUP intercept = 0.039±0.018, slope = 0.039±0.002, r2 = 0.996 ±0.002; NBUP intercept = 0.072±0.073, slope = 0.04±0.001, r2 = 0.995 ±0.003; BUP-Gluc intercept = 0.168±0.021, slope = 0.068±0.018, r2 = 0.993 ±0.003; NBUP-Gluc intercept = 0.039±0.014, slope = 0.024±0.006, r2 = 0.996 ±0.002), and residuals within ±20% at the LOQ and ±15% at other calibrator concentrations. The linear dynamic range was 1–50 ng/g with a LOD of 0.8 ng/g for all analytes. Figure 1 shows a chromatogram of a blank umbilical cord sample fortified at the low QC concentration.
Figure 1.
LCMS, LCMS2 and LCMS3 chromatograms of an umbilical cord sample fortified at 3 ng/g (low quality control sample) for buprenorphine, norbuprenorphine, buprenorphine-glucuronide and norbuprenorphine-glucuronide.
Quantification was performed with the parent ion because MS2 and MS3 product ion curves had coefficients of determination less than 0.99 and residuals higher than ±20% at LOQ and ±15% at other levels. Similarly, investigators employing an ion trap mass spectrometer performed quantification in the same manner as the present work [27], while other investigators employed product ions [9, 26, 28, 36]. In these cases, a coefficient of determination of 0.98 and residuals within ±20% across the entire calibration range [9], or no information about the value of each calibrator against the curve was reported [26, 28, 36].
Imprecision and analytical recoveries were satisfactory at all tested concentrations. For all analytes, imprecision was <14.5%, and analytical recovery between 94.1% and 112.3% (Table 2). Extraction efficiencies ranged from 66.3% to 94%, and process efficiency from 73.4% to 95.5% for all three QC concentrations (Table 3). No significant matrix effect was detected for NBUP, but at the low QC concentration for BUP (24.1%) and NBUP-Gluc (22.7%), and low and high QC for BUP-Gluc (27.4 and 22.2%, respectively) ion enhancement >20% was detected (Table 3). In these cases %RSD (n=8) was less than 20%, demonstrating similar effects in different umbilical cords.
Table 2.
Imprecision and analytical recoveries of buprenorphine (BUP) and metabolites, norbuprenorphine (NBUP); buprenorphine glucuronide (BUP-Gluc) and norbuprenorphine glucuronide (NBUP-Gluc) in human umbilical cord by LCMS.
| Analyte | Quality control Concentrations (ng/g) | Total mean (n=20) ng/g | Imprecision (n=20, %RSD) |
Analytical recovery(n=20, % of target) | ||
|---|---|---|---|---|---|---|
| Pooled intra-day | Inter-day | Total | ||||
| BUP | 3 | 3 | 6.7 | 9 | 11.2 | 100.7 |
| 15 | 16.3 | 5.9 | 0 | 5.9 | 108.8 | |
| 40 | 40.3 | 5.7 | 7.2 | 9.2 | 100.9 | |
|
| ||||||
| NBUP | 3 | 2.9 | 8.9 | 6.4 | 11 | 96.7 |
| 15 | 16.1 | 4.1 | 9.5 | 10.3 | 107.1 | |
| 40 | 37.6 | 6.5 | 3.2 | 7.2 | 94.1 | |
|
| ||||||
| BUP-Gluc | 3 | 3 | 10.1 | 10.4 | 14.5 | 100 |
| 15 | 16.4 | 6.1 | 13.1 | 14.4 | 109.6 | |
| 40 | 44.9 | 6.9 | 10 | 12.1 | 112.3 | |
|
| ||||||
| NBUP-Gluc | 3 | 3 | 7.8 | 6.4 | 10 | 99 |
| 15 | 15.1 | 9.8 | 8.3 | 12.8 | 100.9 | |
| 40 | 39.9 | 12.3 | 6 | 13.7 | 99.8 | |
Table 3.
Extraction efficiency, process efficiency and matrix effect for buprenorphine (BUP) and metabolites, norbuprenorphine (NBUP); buprenorphine glucuronide (BUP-Gluc) and norbuprenorphine glucuronide (NBUP-Gluc) in human umbilical cord by LCMS.
| Analyte | Quality control Concentrations (ng/g) | Extraction Efficiency (%, n=5) | Process Efficiency (%, n=5) | Matrix Effect (n=8) |
|
|---|---|---|---|---|---|
| % Effect | % RSD | ||||
| BUP | 3 | 77.2 | 90.2 | 24.1 | 8.4 |
| 15 | 87.5 | 88.9 | 4.6 | 16.2 | |
| 40 | 80.6 | 86 | 13 | 13.3 | |
|
| |||||
| NBUP | 3 | 94 | 89.1 | 8.8 | 21.8 |
| 15 | 81.8 | 92.9 | 15.5 | 14 | |
| 40 | 80.9 | 85.4 | 3.7 | 10.5 | |
|
| |||||
| BUP-Gluc | 3 | 75.2 | 93.1 | 27.4 | 6.9 |
| 15 | 85.7 | 95.4 | 11.3 | 15.8 | |
| 40 | 81.1 | 95.5 | 22.2 | 11.1 | |
|
| |||||
| NBUP-Gluc | 3 | 66.3 | 80.9 | 22.7 | 8.6 |
| 15 | 91.4 | 90 | 6.1 | 12.5 | |
| 40 | 87.3 | 73.4 | −12.7 | 18 | |
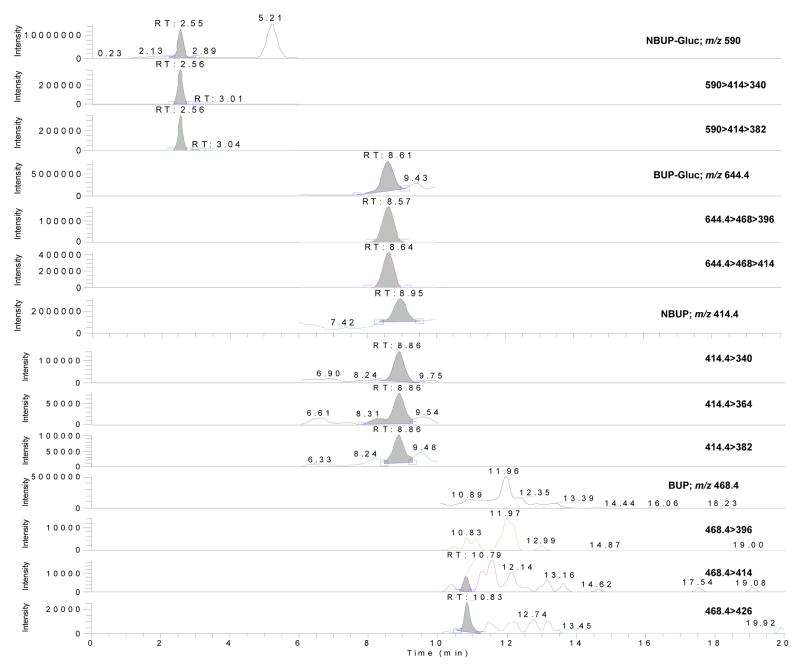
Deuterated analogs of glucuronides couldn’t be employed as IStd because they are not commercially available. NBUP-d3 was used as IStd for BUP-Gluc and NBUP-Gluc instead of BUP-d4 because its retention time was closer. Neither NBUP-d3 nor BUP-d4 showed matrix effect (<6%, RSD<19.8%), and all compounds showed good linearity, imprecision and analytical recovery. Under the described conditions, there was no interference with any extractable endogenous compound in umbilical cord (Figure 2). Method selectivity was demonstrated by adding high concentrations (500 ng/g) of 41 potentially interfering licit and illicit drugs to low QC samples. In all test samples, low QC quantified within ±20%, indicating no interference with the four analytes of interest. In addition, seven umbilical cords from pregnant women under methadone treatment also were fortified at low QC, and all specimens quantified within ±20%.
Figure 2.
LCMS, LCMS2 and LCMS3 chromatograms of a blank umbilical cord.
No analytes of interest were detected in a blank sample injected immediately following analysis of a 100 ng/g QC sample, indicating no carryover at this concentration. The ability of the method to accurately quantify specimens containing high concentrations of analytes was evaluated by diluting 160 ng/g samples (n=2) with blank umbilical cord. In order to achieve a 1:4 dilution, 1.5 g of blank umbilical cord were added to 0.5 g of a 160 ng/g sample. Samples quantified within ±20% of 40 ng/g confirming dilution integrity.
No significant loss or deterioration was observed for any of the analytes in extracted samples stored on the autosampler for 48 or 72 h as QC samples quantified within ±20% of target. Table 4 summarizes stability data of the analytes in fortified umbilical cord, reported as percent difference between mean fresh control concentrations and mean concentrations following storage. Analytes were stable under all storage conditions with percent differences from fresh controls of 0.7 to 20.9%.
Table 4.
Stability of buprenorphine (BUP) and metabolites, norbuprenorphine (NBUP); buprenorphine glucuronide (BUP-Gluc) and norbuprenorphine glucuronide (NBUP-Gluc) in human umbilical cord at different conditions.
| Analyte | Fresh (n=3) | RT 16 h (n=3) | 4°C 72 h (n=3) | 3 F/T (n=3) | |||
|---|---|---|---|---|---|---|---|
| Mean (ng/g) | Mean (ng/g) | Diff % | Mean (ng/g) | Diff % | Mean (ng/g) | Diff % | |
| BUP | 3.2 | 3.3 | 4 | 3.4 | 6.6 | 3.2 | 0.9 |
| 16.6 | 16.7 | 1.1 | 13.5 | −18.3 | 16 | −3.4 | |
| 40.2 | 40.7 | 1.1 | 36.1 | −10.3 | 38.4 | −4.7 | |
| NBUP | 3.3 | 3.3 | −0.7 | 3 | −10.4 | 3 | −8.75 |
| 17 | 13.5 | −20.9 | 14.7 | −13.5 | 16 | −6.4 | |
| 38.9 | 43 | 10.4 | 39.9 | 2.5 | 38.6 | −0.8 | |
| BUP-Gluc | 3 | 3.1 | 4.6 | 2.8 | −4.5 | 2.9 | −4.1 |
| 16.4 | 17.1 | 3.9 | 14.4 | −12.5 | 17.4 | 5.8 | |
| 37.5 | 38.1 | 1.6 | 39.3 | 4.9 | 41.7 | 11.3 | |
| NBUP-Gluc | 3.2 | 2.8 | −12 | 2.7 | −14.6 | 3 | −6.7 |
| 16.2 | 16.6 | 3.1 | 14.3 | −11.2 | 15.3 | −5.4 | |
| 40.6 | 38.6 | −5 | 39.6 | −2.3 | 41.2 | 1.6 | |
RT (Room temperature); 3 F/T (3 freeze/thaw cycles); Diff % (percentage difference from fresh controls).
Besides retention time, identification criteria included the presence of 1 precursor and 3 MS2 fragments for BUP and NBUP, and 1 precursor and 2 MS3 fragments for BUP-Gluc and NBUP-Gluc, yielding 6.5 identification points. According to the EU Official Agency a minimum of 3 identification points is required [48]. The relative ion intensities obtained satisfied the EU criteria that calculates different tolerance windows depending on the value of the relative ion intensity [48]. Sauvage et al. published an interesting work describing the different relative ion intensity variations permitted by several organizations [49].
The validated method was applied to the analysis of an umbilical cord specimen collected at birth from a pregnant woman treated with up to 18 mg/day BUP (mean daily dose 15.9 mg/day) for 111 days. The cumulative dose over this period was 1,760 mg BUP. Interestingly, BUP was not detected, but concentrations of metabolites were NBUP-Gluc 13.4 ng/g, BUP-Gluc 3.5 ng/g and NBUP 1.2 ng/g (Figure 3). Although the woman received BUP daily, concentrations of buprenorphine and metabolites in the umbilical cord were low, especially if we compare these results with concentrations found in meconium that in this case were NBUP 730.6 ng/g, BUP 23.9 ng/g and BUP-Gluc 8.2 ng/g [50]. This raises questions as to whether umbilical cord is an adequate alternative matrix for detecting in utero drug exposure, and how the window of drug detection in umbilical cord compares to detection in meconium. Montgomery et al. [11] obtained good concordance between meconium and umbilical cord for opiates (94.9%), cocaine (99.2%), and cannabis (90.7%), but unfortunately the concentrations found in these paired matrices were not reported. In the literature, only cocaine and metabolites’ concentrations in umbilical cord has been published. Moore et al. [44] reported one case with a concentration of 1200 ng/g for benzoylecgonine in umbilical cord, and Winicker et al. [46] 13 specimens where the main metabolite was benzoylecgonine (up to 1237 ng/mL) followed by ecgonine methyl ester (up to 52ng/mL). Keeping in mind these data, it is possible that compounds are concentrated in meconium or umbilical cord according to physicochemical properties (less lipophilic umbilical cord, more lipophilic meconium). More research on this issue is needed and future analyses will determine the advantages and disadvantages of the two matrices.
Figure 3.
LCMS, LCMS2 and LCMS3 chromatograms of an authentic umbilical cord specimen positive for norbuprenorphine (1.2ng/g), buprenorphine-glucuronide (3.5ng/g) and norbuprenorphine-glucuronide (13.4ng/g). No buprenorphine was detected. The cumulative maternal buprenorphine dose over the 111 day treatment period was 1,760 mg.
CONCLUSION
For the first time, a novel LCMS method simultaneously quantifies buprenorphine and its main metabolites in human umbilical cord with good selectivity and sensitivity. This method was employed successfully to analyze umbilical cord collected in a clinical study investigating buprenorphine as substitution therapy for opioid treatment during pregnancy.
Acknowledgments
This research was supported by the National Institutes of Health, Intramural Research Program, National Institute on Drug Abuse, and funding for Marta Concheiro, Ph.D. from the Ministerio de Educación y Ciencia of Spain (Grant number EX-2007-1194). Authors would like to thank Dr. Fred Askin, Gerrun March, and Leigh Ruane from Johns Hopkins Bayview Medical Center (Baltimore, MD) for their help in obtaining umbilical cord samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.D’Apolito K. Substance abuse: infant and childhood outcomes. J Pediatr Nurs. 1998;13:307–316. doi: 10.1016/S0882-5963(98)80017-1. [DOI] [PubMed] [Google Scholar]
- 2.Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Della Grotta S, Liu J, Lester BM. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006;118:1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 3.Lozano J, Garcia-Algar O, Vall O, de la Torre R, Scaravelli G, Pichini S. Biological matrices for the evaluation of in utero exposure to drugs of abuse. Ther Drug Monit. 2007;29:711–734. doi: 10.1097/FTD.0b013e31815c14ce. [DOI] [PubMed] [Google Scholar]
- 4.Gray T, Huestis M. Bioanalytical procedures for monitoring in utero drug exposure. Analytical and Bioanalytical Chemistry. 2007;388:1455–1465. doi: 10.1007/s00216-007-1228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kintz P, Mangin P. Determination of gestational opiate, nicotine, benzodiazepine, cocaine and amphetamine exposure by hair analysis. J Forensic Sci Soc. 1993;33:139–142. doi: 10.1016/s0015-7368(93)72996-5. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin RS, Wilkins DG, Averin O, Choo RE, Schroeder JR, Jasinski DR, Johnson RE, Jones HE, Huestis MA. Buprenorphine and Norbuprenorphine in Hair of Pregnant Women and Their Infants after Controlled Buprenorphine Administration. Clin Chem. 2007;53:2136–2143. doi: 10.1373/clinchem.2007.091413. [DOI] [PubMed] [Google Scholar]
- 7.Kelly T, Gray TR, Huestis MA. Development and validation of a liquid chromatography-atmospheric pressure chemical ionization-tandem mass spectrometry method for simultaneous analysis of ten amphetamine-, methamphetamine- and 3,4-methylenediozymethamphetamine-related (MDMA) analytes in human meconium. J Chromatogr B: Analyt Technol Biomed Life Sci. 2008;867:194–204. doi: 10.1016/j.jchromb.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray TR, Shakleya DM, Huestis MA. Quantification of nicotine, cotinine, trans-3′-hydroxycotinine, nornicotine and norcotinine in human meconium by liquid chromatography tandem mass spectrometry. J Chromatogr B. 2008;863:107–114. doi: 10.1016/j.jchromb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kacinko SL, Shakleya DM, Huestis MA. Validation and application of a method for the determination of buprenorphine, norbuprenorphine, and their glucuronide conjugates in human meconium. Anal Chem. 2008;80:246–252. doi: 10.1021/ac701627q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choo RE, Murphy CM, Jones HE, Huestis M. Determination of methadone, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, 2-ethyl-5-methyl-3,3-diphenylpyraline and methadol in meconium by liquid chromatography atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B. 2005;814:369–373. doi: 10.1016/j.jchromb.2004.10.068. [DOI] [PubMed] [Google Scholar]
- 11.Montgomery D, Plate C, Alder SC, Jones M, Jones J, Christensen RD. Testing for fetal exposure to illicit drugs using umbilical cord tissue vs meconium. J Perinatol. 2006;26:11–14. doi: 10.1038/sj.jp.7211416. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery DP, Plate CA, Jones M, Jones J, Rios R, Lambert DK, Schumtz N, Wiedmeier SE, Burnett J, Ail S, Brandel D, Maichuck G, Durham CA, Henry E, Christensen RD. Using umbilical cord tissue to detect fetal exposure to illicit drugs: a multicentered study in Utah and New Jersey. J Perinatol. 2008;28:750–753. doi: 10.1038/jp.2008.97. [DOI] [PubMed] [Google Scholar]
- 13.Drug Addiction Treatment Act of 2000 (2000). p. Page 111 STAT. 1101.
- 14.Lejeune C, Simmat-Durand L, Gourarier L, Aubisson S. Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenophine substitution. Drug Alcohol Depend. 2006;82:250–257. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Fischer G. Treatment of opioid dependence in pregnant women. Addiction. 2000;95:1141–1144. doi: 10.1046/j.1360-0443.2000.95811411.x. [DOI] [PubMed] [Google Scholar]
- 16.Dunlop A, Panjari M, O’Sullivan H, Henschke P, Love V, Ritter A, Lintzeris N. Clinical guidelines for the use of buprenorphine in pregnancy. Turning Point Alcohol and Drug Centre; Fitzroy: 2003. [Google Scholar]
- 17.Jones HE, Johnson RE, Jasinski DR, O’Grady EK, Chisholm CA, Choo RE, Crocetti M, Dudas R, Harrow C, Huestis MA, Jansson LM, Lantz M, Lester BM, Milio L. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Marquet P. Pharmacology of high-dose buprenorphine. In: Kintz P, Marquet P, editors. Buprenorphine Therapy of Opiate Addiction. Humana Press; Totowa, New Jersey: 2002. pp. 1–11. [Google Scholar]
- 19.Kobayashi K, Tamamoto T, Chiba K, Tani M, Shimada N, Ishizaki T, Kuroiwa Y. Human buprenorphine n-dealkylation is catalyzed by cytochrome P450 3A4. Drug Metab Dispos. 1998;26:818–821. [PubMed] [Google Scholar]
- 20.Hegstad S, Khiabani HZ, Oiestad EL, Berg T, Christophersen AS. Rapid quantification of buprenorphine-glucuronide and norbuprenorphine-glucuronide in human urine by LC-MS-MS. J Anal Toxicol. 2007;31:214–219. doi: 10.1093/jat/31.4.214. [DOI] [PubMed] [Google Scholar]
- 21.Kronstrand R, Selden TG, Josefsson M. Analysis of buprenorphine, norbuprenorphine, and their glucuronides in urine by liquid chromatography-mass spectrometry. J Anal Toxicol. 2003;27:464–470. doi: 10.1093/jat/27.7.464. [DOI] [PubMed] [Google Scholar]
- 22.Cirimele V, Etienne S, Villain M, Ludes B, Kintz P. Evaluation of the One-Step ELISA kit for the detection of buprenorphine in urine, blood, and hair specimens. Forensic Sci Int. 2004;143:153–156. doi: 10.1016/j.forsciint.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 23.Cirimele V, Kintz P, Lohner S, Ludes B. Enzyme immunoassay validation for the detection of buprenorphine in urine. J Anal Toxicol. 2003;27:103–105. doi: 10.1093/jat/27.2.103. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Moody DE, McCance-Katz EF. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-electrospray ionization-tandem mass spectrometry. Ther Drug Monit. 2006;28:245–251. doi: 10.1097/01.ftd.0000197094.92559.b4. [DOI] [PubMed] [Google Scholar]
- 25.Tracqui A, Kintz P, Mangin P. HPLC-MS determination of buprenorphine and norbuprenorphine in biological fluids and hair samples. J Forensic Sci. 1997;42(1):111–114. [PubMed] [Google Scholar]
- 26.Miller EI, Torrance HJ, Oliver JS. Validation of the Immunalysis microplate ELISA for the detection of buprenorphine and its metabolite norbuprenorphine in urine. J Anal Toxicol. 2006;30:115–119. doi: 10.1093/jat/30.2.115. [DOI] [PubMed] [Google Scholar]
- 27.Favretto D, Frison G, Vogliardi S, Ferrara SD. Potentials of ion trap collisional spectrometry for liquid chromatography/electrospray ionization tandem mass spectrometry determination of buprenorphine and nor-buprenorphine in urine, blood and hair samples. Rapid Commun Mass Spectrom. 2006;20:1257–1265. doi: 10.1002/rcm.2444. [DOI] [PubMed] [Google Scholar]
- 28.Liu AC, Lin TY, Su LW, Fuh MR. Online solid-phase extraction liquid chromatography-electrospray-tandem mass spectrometry analysis of buprenorphine and three metabolites in human urine. Talanta. 2008;75:198–204. doi: 10.1016/j.talanta.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 29.Hull MJ, Bierer MF, Griggs DA, Long WH, Nixon AL, Flood JG. Urinary buprenorphine concentrations in patients treated with suboxone as determined by liquid chromatography-mass spectrometry and CEDIA immunoassay. J Anal Toxicol. 2008;32:516–521. doi: 10.1093/jat/32.7.516. [DOI] [PubMed] [Google Scholar]
- 30.Kacinko S, Concheiro-Guisan M, Shakleya D, Huestis M. Development and Validation of a Liquid Chromatography-Tandem Mass Spectrometric Assay for the Simultaneous Quantification of Buprenorphine, Norbuprenorphine, Buprenorphine Glucuronide and Norbuprenorphine Glucuronide in Human Urine. Anal Bioanal Chem. 2008;392:903–911. doi: 10.1007/s00216-008-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Rosas ME, Lofwall MR, Strain EC, Siluk D, Wainer IW. Simultaneous determination of buprenorphine, norbuprenorphine and the enantiomers of methadone and its metabolite (EDDP) in human plasma by liquid chromatography/mass spectrometry. J Chromatogr B: Analyt Technol Biomed Life Sci. 2007;850:538–543. doi: 10.1016/j.jchromb.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Musshoff F, Trafkowski J, Kuepper U, Madea B. An automated and fully validated LC-MS/MS procedure for the simultaneous determination of 11 opioids used in palliative care, with 5 of their metabolites. J Mass Spectrom. 2006;41:633–640. doi: 10.1002/jms.1021. [DOI] [PubMed] [Google Scholar]
- 33.Ceccato A, Klinkenberg R, Hubert P, Streel B. Sensitive determination of buprenorphine and its N-dealkylated metabolite norbuprenorphine in human plasma by liquid chromatography coupled to tandem mass spectrometry. J Pharm Biomed Anal. 2003;32:619–631. doi: 10.1016/s0731-7085(03)00169-9. [DOI] [PubMed] [Google Scholar]
- 34.Polettini A, Huestis MA. Simultaneous determination of buprenorphine, norburpennorphine, and buprenorphine-glucuronide in plasma by liquid charmatography-tandem mass spectrometry. J Chromatogr B: Biomed Sci Applic. 2001;754:447–459. doi: 10.1016/s0378-4347(01)00029-9. [DOI] [PubMed] [Google Scholar]
- 35.Moody DE, Laycock JD, Spanbauer AC, Crouch DJ, Foltz RL, Josephs JL, Amass L, Bickel WK. Determination of buprenorphine in human plasma by gas chromatography-positive ion chemical ionization mass spectrometry and liquid chromatography-tandem mass spectrometry. J Anal Toxicol. 1997;21:406–411. doi: 10.1093/jat/21.6.406. [DOI] [PubMed] [Google Scholar]
- 36.Murphy CM, Huestis MA. Liquid chromatographic/electrospray ionization tandem mass spectrometric analysis for the quantification of buprenorphine, norbuprenorphine, buprenorphine-3-beta-D-glucuronide and norbuprenorphine-3-beta-D-glucuronide in human plasma. J Mass Spectrom. 2005;40:70–74. doi: 10.1002/jms.776. [DOI] [PubMed] [Google Scholar]
- 37.Scislowski M, Piekoszewski W, Kamenczak A, Florek E. Simultaneous determination of buprenorphine and norbuprenorphine in serum by high-performance liquid chromatography-electrospray ionization-mass spectrometry. J Anal Toxicol. 2005;29:249–253. doi: 10.1093/jat/29.4.249. [DOI] [PubMed] [Google Scholar]
- 38.Hoja H, Marquet P, Verneuil B, Lotfi H, Dupuy JL, Lachatre G. Determination of buprenorphine and norbuprenorphine in whole blood by liquid chromatography-mass spectrometry. J Anal Toxicol. 1997;21:160–165. doi: 10.1093/jat/21.2.160. [DOI] [PubMed] [Google Scholar]
- 39.Wilkins DG, Rollins DE, Valdez AS, Mizuno A, Krueger GC, Cone EJ. A retrospective study of buprenorphine and norbuprenorphine in human hair after multiple doses. J Anal Toxicol. 1999;23:409–415. doi: 10.1093/jat/23.6.409. [DOI] [PubMed] [Google Scholar]
- 40.Kintz P. Determination of buprenorphine and its dealkylated metabolite in human hair. J Anal Toxicol. 1993;17:443–444. doi: 10.1093/jat/17.7.443. [DOI] [PubMed] [Google Scholar]
- 41.Thieme D, Sachs H, Thevis M. Formation of the N-methylpyridinium derivative to improve the detection of buprenorphine by liquid chromatography-mass spectrometry. J Mass Spectrom. 2008;43:974–979. doi: 10.1002/jms.1433. [DOI] [PubMed] [Google Scholar]
- 42.Kintz P, Tracqui A, Mangin P, Edel Y. Sweat testing in opioid users with a sweat patch. J Anal Toxicol. 1996;20:393–397. doi: 10.1093/jat/20.6.393. [DOI] [PubMed] [Google Scholar]
- 43.Grimm D, Pauly E, Poschl J, Linderkamp O, Skopp G. Buprenorphine and Norbuprenorphine Concentrations in Human Breast Milk Samples Determined by Liquid Chromatography-Tandem Mass Spectrometry. Ther Drug Monit. 2005;27:526–530. doi: 10.1097/01.ftd.0000164612.83932.be. [DOI] [PubMed] [Google Scholar]
- 44.Moore CM, Brown S, Negrusz A, Tebbett I, Meyer W, Jain L. Determination of cocaine and its major metabolite, benzoylecgonine, in amniotic fluid, umbilical cord blood, umbilical cord tissue, and neonatal urine: a case study. J Anal Toxicol. 1993;17:62. doi: 10.1093/jat/17.1.62. [DOI] [PubMed] [Google Scholar]
- 45.Le NL, Reiter A, Tomlinson K, Jones J, Moore C. The detection of oxycodone in meconium specimens. J Anal Toxicol. 2005;29:54–57. doi: 10.1093/jat/29.1.54. [DOI] [PubMed] [Google Scholar]
- 46.Winecker RE, Goldberger BA, Tebbett I, Behnke M, Eyler FD, Conlon M, Wobie K, Karlix J, Bertholf RL. Detection of cocaine and its metabolites in amniotic fluid and umbilical cord tissue. J Anal Toxicol. 1997;21:97–104. doi: 10.1093/jat/21.2.97. [DOI] [PubMed] [Google Scholar]
- 47.Krouwer JS, Rabinowitz R. How to improve estimates of imprecision. Clin Chem. 1984;30:290–292. [PubMed] [Google Scholar]
- 48.European Union Decision 2002/657/EC (17/8/2002) Off J Eur Commun. 2002;221:8–36. [Google Scholar]
- 49.Sauvage FL, Gaulier JM, Lachatre G, Marquet P. Pitfalls and prevention strategies for liquid chromatography-tandem mass spectrometry in the selected reaction-monitoring mode for drug analysis. Clin Chem. 2008;54:1519–1527. doi: 10.1373/clinchem.2008.105478. [DOI] [PubMed] [Google Scholar]
- 50.Kacinko S, Jones H, Johnson R, Choo R, Huestis M. Correlations of Maternal Buprenorphine Dose, Buprenorphine, and Metabolite Concentrations in Meconium with Neonatal Outcomes. Clin Pharmacol Ther. 2008;84(5):604–612. doi: 10.1038/clpt.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]