Abstract
Langerin CD207 is a type II transmembrane protein. It is responsible for the formation of Birbeck granules, which are intracellular organelles within Langerhans cells, the dendritic cells of stratified squamous epithelia like the epidermis. Because current anti-CD207 antibodies have limitations, we prepared new monoclonals by immunizing rats with the extracellular region of mouse Langerin followed by a boost with enriched Langerhans cells (LCs). We secured a large panel of mAbs, most of which reacted with the carboxy terminal carbohydrate recognition domain. These mAbs could be used to immunoblot and immunoprecipitate mouse Langerin and to stain the cell surface and intracellular pools of CD207 by FACS analysis. Labeling of Birbeck granules was also achieved by immunoelectron microscopy. Anti-CD207 identified LCs in the epidermis and skin draining lymph nodes of BALB/c and C57BL/6 mice, but BALB/c mice had an additional Langerin+ population in spleen, thymus and mesenteric lymph node. This additional subset had higher levels of CD8 and CD205 than epidermal LCs, and also had a less mature phenotype, i.e., lower MHC II, CD40 and CD86. Subcutaneous injection of IgG but not IgM forms of these new anti-CD207 mAbs led to rapid and selective labeling of the Langerin+ cells in skin draining lymph nodes as well as spleen. The new IgG anti-CD207 mAbs should be useful for further research on LCs and dendritic cells including an evaluation of the consequences of antigen delivery within anti-CD207 mAbs in vivo.
Keywords: Monoclonal Antibody, Langerhans Cells, Langerin, CD207, Dendritic Cells
1. Introduction
Dendritic cells (DCs), including epidermal Langerhans cells (LCs), are potent antigen-presenting cells. DCs are found at several interfaces between the organism and its environment, where they function as sentinels, efficiently capturing and responding to foreign antigens, and transporting them to draining lymph nodes for presentation of antigenic peptides to naïve T cells (Banchereau and Steinman, 1998).
In the periphery, DCs enhance their recognition of antigens through several surface receptors including C-type lectins. C-type lectins bind carbohydrates in a calcium-dependent manner via conserved carbohydrate recognition domains (CRD) (Figdor et al., 2002; Geijtenbeek et al., 2004). Langerin, now termed CD207, is a C-type lectin that is expressed at high levels by LCs. Langerin localizes to the plasma membrane as well as Birbeck granules (BGs), and it also induces BGs formation when Langerin cDNA is transduced into fibroblasts (Valladeau et al., 2002a; Valladeau et al., 2000). Birbeck granules are endocytic vesicles with a distinctive pentalamellar extension or handle that may contribute to presentation of antigens on CD1a molecules (Hunger et al., 2004; Mc Dermott et al., 2002).
The Langerin molecule is comprised of extracellular CRD and neck domains in addition to transmembrane and intracellular portions (Takahara et al., 2002; Valladeau et al., 2002a). The neck domain of Langerin likely mediates trimer formation, while the CRD domain binds preferentially to mannose, n-acetylglucosamine and fucose, as well as select sulfated sugars (Galustian et al., 2004; Stambach and Taylor, 2003; Takahara et al., 2002). Langerin also may bind to pathogens such as HIV (Turville et al., 2002), zymosan and Candida albicans (Takahara et al., 2004), but the characterization and elucidation of endogenous ligands need to be addressed (Tada et al., 2006).
Several DC subsets are being characterized in the lymphoid organs of mice on the basis of their distinct cell-surface markers. Over the past five years, different approaches were developed with the aim of producing antibodies against one of these markers, mouse Langerin (mLangerin). Although those approaches generated mAbs that appear to be useful tools for detecting different DCs populations, no mAb for targeting mLangerin in vivo is available. mAbs that react with the extracellular domain of mLangerin have proved useful for identifying LCs in the epidermis and a major population of Langerhans derived-cells in the skin draining LN (929F3, (Stoitzner et al., 2003)), characterized by the high expression of DEC205 and MHC II and low expression of CD8 (Henri et al., 2001). In addition, the development of a monoclonal antibody IgM has allowed the detection of Langerin in the CD8hi DEC205+ subset of DCs in skin draining LN (205C1, (Douillard et al., 2005)). However, these two mAbs have characteristics (recognition of the intracellular domain or IgM in isotype) that are not desirable for using them to deliver antigens to the corresponding endocytic receptors in vivo, as a means to probe receptor function. This prompted us to generate IgG mAbs to the extracellular domain of mLangerin.
Here we characterized one of these new mAb L31, obtained following immunization of rats with soluble mLangerin, and fresly isolated live and lysates of LCs and DCs. MAb L31 specifically recognized two subsets of Langerin+ cells in peripheral LN of BALB/c mice, based on high vs. intermediate expression of CD11c, and one subset of Langerin+ cells in other lymphoid tissues. Importantly, L31 is quickly captured by DCs and LCs in vivo, indicating that it can be a valuable tool for targeting antigens through this receptor.
2. Materials and methods
2.1. Animals
Wistar Furth rats were purchased from Charles River Laboratories (Wilmington, MA). C57BL/6, BALB/c and CB6 (C57BL/6 × BALB/c F1) mice were purchased from Taconic Farms (Hudson, NY) and Charles River Labs, and used at 6–8 wks of age. CD207-deficient mice were obtained from Dr. Bjoern Clausen (Bennett et al., 2005). All animals were maintained under specific pathogen-free conditions. Animal care and use followed institutional guidelines of the Rockefeller University and Memorial Sloan-Kettering Cancer Center.
2.2. Cell lines
Chinese hamster ovary (CHO) cells and 293T cells were cultured in DMEM with 7 % FBS or 5 % Ultra-Low IgG FBS supplemented with 2mM glutamine (Gibco BRL, Invitrogen, Carlsbad, CA), antibiotics (Invitrogen) and non-essential amino acids (Invitrogen).
2.3 Reagents
Anti-CD16/32, PE-conjugated anti-I-Ek, I-Ab, CD40, CD86, CD80, B7-DC, B7-H1, CCR7, CD11c, FITC-conjugated anti-CD11c, CD8α and I-Ad, allophycocyanin-streptavidin, biotin conjugated anti-CD3, B220, DX5, Thy 1.2, and Gr1 and Alexa 647 conjugated anti-B220 were obtained from BD Biosciences (San Jose, CA) or eBiosciences (San Diego, CA). Streptavidin microbeads were obtained from Miltenyi Biotec. The other reagents were Hank’s solution (Invitrogen), FBS (Sigma-Aldrich), ultra low IgG FBS (Invitrogen), ACK buffer (Biosource, Invitrogen) and 30 % BSA solution (Sigma-Aldrich).
2.4. Vector constructions and expression of mouse Langerin
The full-length open reading frame (ORF) sequence of mouse Langerin (mLangerin, (Takahara et al., 2002)) was amplified by PCR from mouse spleen cDNA followed by cloning and sequencing. This mLangerin ORF cDNA was cloned into the pCMV expression vector (Clontech, Mountain View, CA) to make pCMV-mLangerin ORF, and stably transfected into CHO cells, named CHO/mLangerin cells, as described previously (Kang et al., 2003; Kang et al., 2004). To produce soluble mLangerin protein, the hIgG1Fc-mLangerinECD (GenBank accession no. DQ917567) was generated by PCR, sequenced, and cloned into the pCMV expression vector, and then stably transfected into CHO cells. The soluble hIgG1Fc-mLangerinECD fusion protein was purified from the culture supernatants of CHO/hIgG1Fc-mLangerinECD cells, by affinity to Protein A Sepharose™ (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) as described previously (Galustian et al., 2004).
To produce SF-mLangerinECD (GenBank accession no. DQ917568) or SF-mLangerinCRD (GenBank accession no. DQ917569), the cDNAs for the ECD and CRD of mLangerin, generated by PCR and sequenced, were fused to the sequence containing a signal peptide and a FLAG epitope tag. These constructs were cloned into pCMV expression vector and stably transfected into CHO cells. SF-mLangerinECD and SF-mLangerinCRD soluble proteins were purified from the culture supernatants of CHO/SF-mLangerinECD or CHO/SF-mLangerinCRD cells, by Anti-FLAG® M1 Affinity Gel (Sigma-Aldrich, St. Louis, MO) following the manufacturer’s instruction. Soluble SIGN-R1 was produced and purified as previously described (Galustian et al., 2004; Kang et al., 2006; Kang et al., 2004).
2.5. Animal immunization and monoclonal antibody production
Two protocols of immunization were carried out. In protocol 1, Wistar Furth rats at 4~6 wks of age were immunized subcutaneously (s.c.) with 50 μg of hIgG1Fc-mLangerinECD protein mixed with adjuvant TiterMax® (TiterMax USA, Inc., Norcross, GA) following the manufacturer’s instruction. Each rat was immunized 5 times at 3 wk intervals. Three months after the last immunization, the rats were boosted s.c. two times at 3 wk intervals with 50 μg of SF-mLangerinECD protein mixed with adjuvant. In protocol 2, one rat immunized as above was further boosted intraperitoneally (i.p.) with a cell lysate of 5×105 cutaneous LCs and DCs from mouse ear skin explants cultures (Ortner et al., 1996), prepared by repeated freezing (−80 °C) and thawing (37 °C) in PBS. On the same day, a fresh cell preparation of live 5×105 cutaneous LCs and DCs in 100 μl PBS was given intravenously (i.v.). Seven days after each immunization, sera were screened by Western blot analysis or ELISA as described below to determine which mice had the highest anti-mLangerin specific responses.
One to two months after the last round of immunization, one rat from each protocol group was selected to receive a final i.v. boost of 50 μg SF-mLangerinECD in the absence of adjuvant. After 4 days, the cells from spleen were used for hybridoma fusion at the Monoclonal Antibody Core Facility of the Rockefeller University and Memorial Sloan-Kettering Cancer Center. In brief, cells of the murine myeloma line P3X63Ag8.653 (ATCC, Manassas, VA) were fused with spleen cells from the donor rat at a 1:4 ratio in 50 % polyethylene glycol (PEG; EM Science; Germany). Stable hybrids were selected by growth in RPMI 1640 medium containing 15 % FBS, hypoxanthine, aminopterin and thymidine, according to standard protocols, and distributed into 96-well plates. Supernatants from the hybridoma culture medium were screened by ELISA (as described below).
Hybridoma cultures whose supernatants showed antibody activity against mouse Langerin were expanded and cloned twice by limiting dilutions. Some hybridomas were grown in RPMI 1640 containing 5 % ultra low IgG FBS. The culture supernatants were collected and the mAbs were purified with protein G (Pierce, Rockford, IL; GE Healthcare, Piscataway, NJ), according to the manufacture’s instructions. The purified mAbs were labeled with Alexa 488 (Molecular Probes, Invitrogen), Alexa 647 (Molecular Probes, Invitrogen) or EZ-Link Biotin (Pierce) reagents following the manufacture’s instructions.
2.6. Characterization of anti-mouse Langerin mAbs
SDS-PAGE and Western blot analysis. To test for anti-mLangerin antibodies in the samples of rat serum and hybridoma supernatants, a serial dilution of samples were evaluated by Western blot analysis on lysates of cells expressing mLangerin. In brief, control CHO and stable CHO/mLangerin cells as well as 293T cells transiently transfected with control or pCMV-mLangerin vectors (or pCMV vectors expressing soluble mLangerin proteins) by Lipofectamine™ 2000 reagent (Invitrogen) were lysed with RIPA lysis buffer (150 mM NaCl, 50 mM Tris-HCl pH 8.0, 1 % NP-40, 0.5 % desoxycholate, 0.1 % SDS) including protease inhibitor cocktail (Sigma-Aldrich). Negative and positive cell lysates were separated on 10 % SDS-PAGE, blotted onto Hybond™-P polyvinylidine difluoride (PVDF) membrane (GE Healthcare, Piscataway, NJ), incubated with serum/supernatant samples, detected by anti-rat IgG secondary antibodies conjugated with HRP (Southern Biotech, Birmingham, AL), and visualized with ECL Plus™ reagents (GE Healthcare).
ELISA. For the screening of hybridomas, the purified SF-mLangerinECD protein was used for ELISA as described previously (Park et al., 2000). In brief, high-binding ELISA plates (BD Falcon, Bedford, MA) were coated overnight with 50 μl of 2 μg/ml of SF-mLangerinECD in PBS. Plates were then washed with PBS with 0.1 % Tween 20 (PBS-T) and blocked with 5 % goat serum (Sigma-Aldrich) in PBS-T (blocking solution) for 1hr at 37°C. Serial dilutions of the sera in blocking solution or undiluted hybridoma supernatants were incubated for 1 hr at 37 °C and were visualized with anti-rat IgG conjugated to HRP (Southern Biotech, Birmingham, AL), followed by colorimetric assay using OPD (o-phenylenediamine dihydrochloride; Sigma-Aldrich) in CPB solution, i.e. 10 mg OPD tablet in 25 ml CPB buffer (25 mM citric acid, 50 mM Na2HPO4) with 10 μl of 30 % H2O2 solution. Optical density at 450 nm was measured using an Opsys MR microplate reader (Thermo Labsystems, Chantilly, VA).
Isotyping. The isotypes of mAb heavy and light chains were determined by the Western blot analysis using mAb supernatant as primary Ab and rat isotype specific HRP-conjugated Abs (Southern Biotech) as secondary Ab.
Immunofluorescence. Epidermal sheets were prepared as previously described (Ortner et al., 1996). In brief, ears were separated with forceps into dorsal and ventral leaflets. Dorsal halves were floated for 30 min at room temperature on 0.5 M EDTA (Invitrogen). Then, epidermis and dermis were separated from each other with thin forceps. Epidermal sheets were fixed with cold acetone for 20 min at room temperature, incubated with purified L31 followed by Alexa 488 conjugated anti-rat IgG and biotin-labeled anti-I-Ad, followed by streptavidin-Alexa 555. Peripheral lymph nodes were harvested and frozen in Tissue-Tek OCT® (optimal cutting temperature) compounds (Sakura Finetek USA, Torrance, CA). Frozen tissue was sectioned 10 μm in thickness on a microtome and fixed 20 min with cold acetone. Sections were blocked with 5 % mouse serum in FACS buffer (2 % FBS in PBS). The staining was performed in humidified chamber with purified L31 mAb followed by anti-rat IgG Alexa 488 and anti-B220 Alexa 647. Sections were mounted in Aqua-Poly Mount (Polysciences, Warrington, PA) and were stored at 4°C until microscopic examination. The images were acquired with a deconvolution microscopy (Olympus, Melville, NY) or with a Zeiss LSM 510 system (Carl Zeiss MicroImaging, Thornwood, NY) at the Rockefeller University Bio-Imaging Resource Center.
Skin explant culture. Mouse ears were split in dorsal and ventral halves by means of strong forceps and the dorsal halves were cultured in RPMI 1640 supplemented with 5 % FBS in 24 well tissue culture plates as described (Ortner et al., 1996; Stosel et al., 1997; Weinlich et al., 1998). 16 hrs post-incubation, microscopic examination was performed after staining as described in (iv).
FACS analysis. Spleen, thymus, mesenteric LNs and peripheral LNs (popliteal, cervical, inguinal, axillary and branchial) were collected from CO2-sacrificed mice. Organs were cut in small pieces and incubated at 37 °C for 30 min in Hank’s medium supplemented with 400 U/ml Collagenase D (Roche Diagnostics, Indianapolis, IN). EDTA (5 mM in final concentration) was added for the last 5 min of incubation. Digested fragments were filtered through a cell-strainer, and the cell suspension was centrifuged for 5 min at 1200 rpm. The resulting suspension was floated in 30 % BSA (Sigma-Aldrich) and centrifuged for 30 min at 2200 rpm to enrich for DCs in the low-density fraction. This was washed with PBS and stained with antibodies to CD11c, Langerin (L31), I-Ek and DEC205 (NLDC145). In some experiments, cells were fixed and permeabilized with cytofix/cytoperm buffer (BD Biosciences). Alternatively, the Langerin+ population was enriched by negative selection. In brief, digested cell suspensions were incubated with biotin-labeled anti-CD3, B220, Thy 1.2, CD19, Gr-1 and DX5 for 20 min at 4°C. The cells were then incubated for 20 min with streptavidin MACS® microbeads and negatively selected by application of a magnetic field.
Electron microscopy. Cells were fixed with a mixture of 4 % formaldehyde and 0.075 % glutaraldehyde in 0.1 M sodium cacodylate·HCl buffer (pH 7.4) and then were enrobed in 10 % gelatin and infiltrated with 2.3 M sucrose overnight at 4 °C. Cells were then mounted on metal nails and frozen in liquid nitrogen. Ultrathin cryosections (ca. 100 nm) were cut with glass knives on a Leica EM FC-6 cryo-ultramicrotome, picked up with a drop of 2.3 M sucrose, and placed on coated nickel grids. Ultrathin cryosections were incubated with L31 (1 μg in 200 μl), and detection of primary antibody was achieved with goat anti-rat 12 nm colloidal gold particles (Electron Microscopy Sciences, Hatfield, PA) diluted 1:20. Sections were fixed in 0.1 % glutaraldehyde and post-fixed in reduced osmium (2 % osmium tetroxide and 1.6 % potassium ferrocyanide). After several washes in distilled water, the grids were contrasted in neutral uranyl acetate followed by acidic uranyl acetate. Then after 5 min of wash in 2 % polyvinyl alcohol (PVA), the grids were picked up with a loop and excess PVA were removed by a piece of filter paper. The cryosections were evaluated with a Tecanai G2 transmission EM operated at 80KV in the Rockefeller University Bio-Imaging Resource Center.
3. Results
3.1. Production of IgM mAbs against mouse Langerin
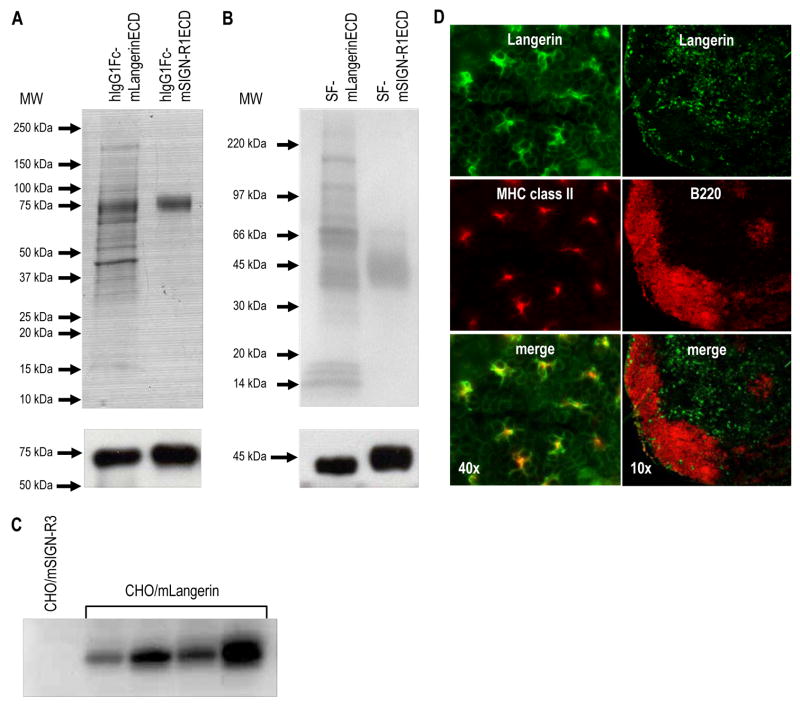
We designed an approach to produce mAbs to the extracellular (ECD) portion of mouse Langerin, consisting of neck and CRD domains. This was fused to a human IgG1 Fc cassette and was stably transfected into CHO cells. The fusion protein was purified from cultured supernatant using Protein A Sepharose. In addition, for the screening of immune serum by ELISA, the ECD of mLangerin was stably expressed in CHO cells as a tagged fusion protein (Soluble FLAG-mLangerinECD or SF-mLangerinECD). SF-mLangerinECD was purified from supernatant using anti-FLAG M1 affinity gel. The purity of the soluble forms of mouse Langerin (hIgG1Fc-mLangerinECD and SF-mLangerinECD) was tested by Coomassie blue staining, and the identity of the proteins was confirmed by Western blotting with HRP-conjugated anti-human IgG (Figure 1A) or HRP-conjugated anti-FLAG (Figure 1B). Interestingly, both soluble purified preparations contained a significant number of associated proteins other than the recombinant mLangerinECD protein itself (Figures 1A and 1B, upper panel). In contrast, the preparation of other lectins, e.g. mouse SIGN-R1 (purified hIgG1Fc-mSIGN-R1ECD and SF-mSIGN-R1ECD; right lanes of Figures 1A and 1B) showed more than 90 % of purity in Coomassie blue stains, as compared to <50 % purity for mouse Langerin (left lanes of Figures 1A and B). These results imply that the soluble mLangerinECD can bind many different proteins in the CHO cell culture supernatant, while soluble mSIGN-R1ECD cannot.
Figure 1. Soluble forms of mouse Langerin and rat IgM mAbs.
(A) Purification of hIgG1Fc-mLangerinECD and hIgG1Fc-mSIGN-R1ECD proteins. The recombinant proteins were stably expressed in CHO cells and purified from the culture medium by Protein A Sepharose. The purified recombinant proteins were analyzed on 10 % SDS-PAGE by Coomassie blue staining (upper panel) or transferred onto PVDF membrane and immunoblotted using anti-human IgG (lower panel).
(B) Purification of SF-mLangerinECD and SF-mSIGN-R1ECD proteins. The recombinant proteins were stably expressed in CHO cells and purified from the culture medium by anti-FLAG M1 affinity gels. The purified recombinant proteins were analyzed on 10 % SDS-PAGE by Coomassie blue staining (upper panel) or transferred onto PVDF membrane and immunoblotted using anti-FLAG (lower panel).
(C) Screening of rat anti-mLangerin immune serum by Western blot. A CHO cell-line stably expressing full-length mSIGN-R3 (negative control) and 4 different CHO cell-lines stably expressing full-length mLangerin were lysed and used to screen diluted sera from rats immunized with soluble mLangerin. The blots were incubated with the sera in 1:2000 dilutions.
(D) MAb ML11 stains Langerin+ cells in situ. Ear epidermis (left panels) was separated from dermis, fixed with acetone for 20 min at RT, and double stained with anti-MHC II-PE and ML11 mAb followed by biotinylated anti-rat IgM and Alexa 488-labeled streptavidin. Skin draining lymph nodes (right panels) were embedded in OCT, sectioned at 10 μm, and stained with anti-B220-PE and ML11 mAb followed by biotinylated anti-rat IgM and Alexa 488-labeled streptavidin.
The rats were immunized 5 times at 3 wk intervals with 50 μg of hIgG1Fc-mLangerinECD protein and were screened for Langerin-specific antibody responses in the serum at 7 days after each injection. The serum samples were screened for reactivity in Western blot analysis on cell lysates from stably transfected CHO/mLangerin cells. All the sera showed similar strong reactivity with Langerin but not with a lysate from stably transfected cells expressing another C-type lectin, CHO/mSIGN-R3 cells (Figure 1C). To boost the immunization, we injected rats with SF-mLangerinECD protein. Also, this SF-mLangerinECD protein allowed us to screen the Ab responses easily by ELISA.
One rat was selected based on the highest ELISA titer and subjected to hybridoma preparation. A total of 1800 hybridomas were screened initially by ELISA against SF-mLangerinECD. From 38 positive hybridomas, only three (ML11, ML14 and ML20) were found to specifically react with mLangerin expressing CHO cells by FACS and Western blotting (data not shown). Isotype determination revealed that all three were rat IgMs and reacted against the ECD of mLangerin. Moreover, all three mAbs stained Langerin+ cells in mouse epidermis and in the T cell areas of skin draining lymph nodes, e.g., Figure 1D for ML11.
We then evaluated the targeting of these IgM mAbs to mouse Langerin after s.c. inoculation. In contrast to NLDC145, a rat IgG anti-mDEC205 mAb that targeted DCs in vivo (Hawiger et al., 2001), the injected ML11, ML14 or ML20 mAbs were unable to label Langerin+ cells in vivo (data not shown). Presumably, the large size of the IgM molecules impeded their use to target CD207 in situ.
3.2. Production of IgG mAbs against mouse Langerin
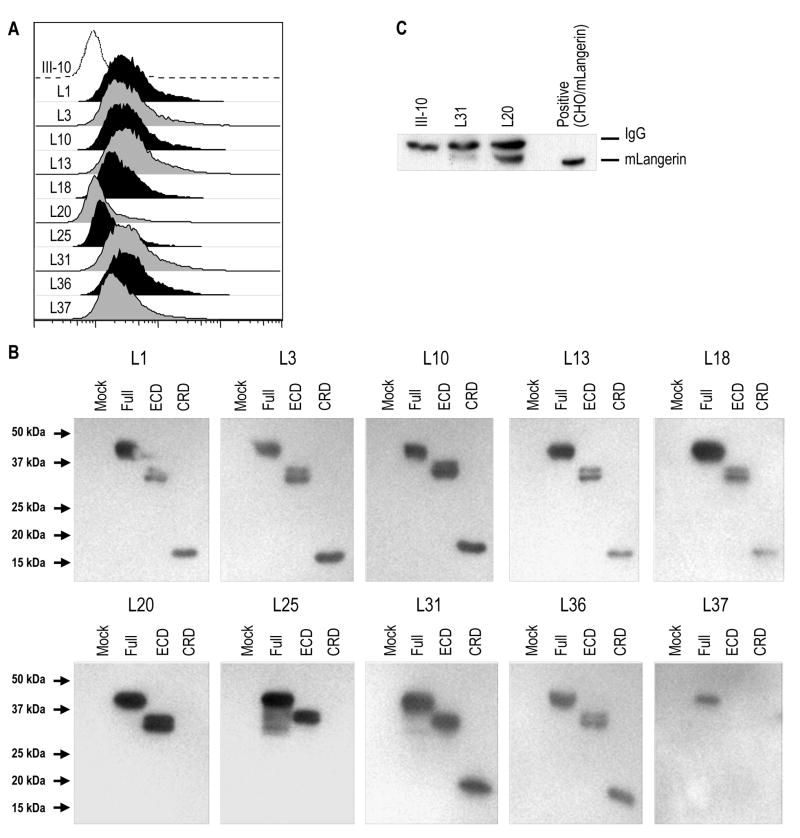
To obtain IgG mAbs better able to target mLangerin in vivo, we slightly modified the strategy. One of the rats, immunized with hIgG1Fc-mLangerinECD and SF-mLangerinECD as above, was further inoculated with a mix of a cell lysate of epidermal DCs (LCs and dermal DCs) that migrated from skin explant cultures and freshly isolated live DCs. After 2 months, this rat was boosted with SF-mLangerinECD and subjected to hybridoma formation. A total of 1400 hybridomas were obtained, and their supernatants were screened by ELISA using anti-rat IgG heavy (γ) chain as secondary Ab. Ten antibody-secreting hybridomas reacted against CHO or 293T cells expressing different forms of mLangerin by FACS or Western blotting (Figures 2A and 2B). As shown in Figure 2B, L20 and L25 reacted with the ECD but not with the CRD of mouse Langerin, implying reactivity with the membrane proximal neck domain of Langerin. Moreover, L37 was able to detect only cell lysates of 293T cells transfected with full-length mLangerin, suggestion weak binding affinity. Isotype determination revealed that only L8 secreted rat IgG2b, while all the remaining 9 hybridomas secreted rat IgG2a (data not shown).
Figure 2. Characterization of rat IgG mAb to mouse Langerin.
(A) FACS analysis of stable CHO/mLangerin cells with new rat IgG anti-CD207 mAbs (filled histograms) or with rat IgG isotype control III-10 mAb (open histogram), detected with PE-labeled anti-rat IgG Ab.
(B) Binding of anti-mLangerin mAbs to different domains of Langerin. Whole cell lysates of 293T cells transfected with full-length mouse Langerin (Full), or with its extracellular domain (ECD) or its carbohydrate recognition domain (CRD), were applied to 10 % SDS-PAGE and blotted with the 10 new rat IgG anti-CD207 mAbs (L1 to L37) followed by HRP-conjugated anti-rat IgG.
(C) Whole cell lysates of skin draining LNs (from C57BL/6 mice) were immunoprecipitated with mAbs of L31, L20 or III-10 isotype control. Samples were resolved in 10 % SDS-PAGE and immunoblotted with L31.
All 10 new rat IgG anti-mLangerin mAbs successfully stained Langerin in sections from mouse spleen, lymph nodes, and epidermis, and the mAbs could be used to immunoprecipitate and immunoblot Langerin expressed in cell-lines or lymph nodes of wild type but not Langerin deficient mice (data not shown). Comparison of all the clones revealed that L20 was the best for immunoprecipitation (Figure 2C) and L31 for FACS analyses and immunofluorescence (see below).
3.3. The L31 rat IgG anti-mouse CD207 mAb detects Langerin in Langerhans cells
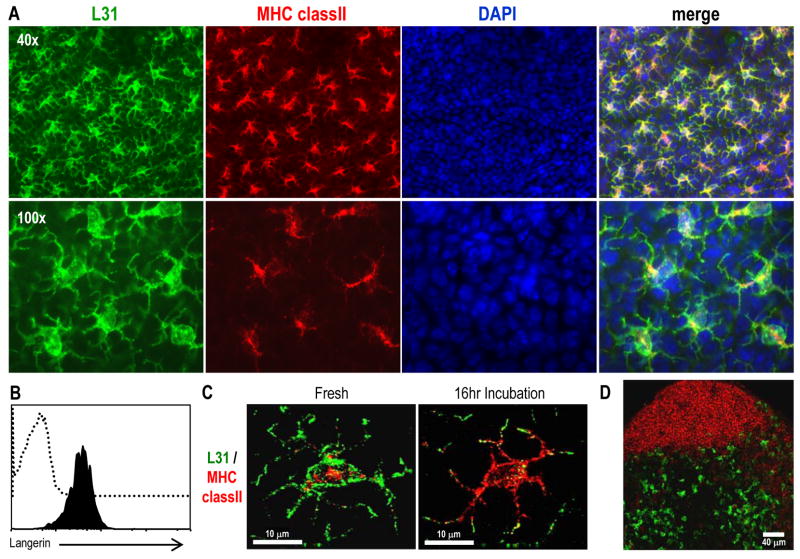
To validate the new rat IgG L31 anti-CD207 mAb, we prepared epidermal sheets from the ears of BALB/c mice (Figure 3) or C57BL/6 mice (data not shown). Since LCs are the only epidermal cells that constitutively express MHC II molecules (Tsuruta et al., 1999), the sheets were double stained with MHC II and L31 and were examined by immunofluorescence. L31 mAb specifically stained MHC II+ cells in the epidermal sheets (Figure 3A). There was no detectable expression of Langerin in MHC II− cells. In addition, as shown in Figure 3B, L31 specifically stained cell surface Langerin displayed in freshly isolated MHC II+ LCs obtained after trypsinization of ear epidermis (Figure 3B).
Figure 3. Staining of epidermal Langerhans cells with L31 mAb.
(A) Ear epidermis was obtained, fixed with acetone for 20 min, and double labeled with anti-MHC II-biotin, followed by streptavidin-Alexa 555 and L31 followed by anti-rat IgG Alexa 488. DAPI staining was performed to detect the nucleus of the cells.
(B) Freshly isolated LCs were surface-stained for MHC II-PE, CD11b-FITC and biotin conjugated L31 (filled histogram) or the corresponded isotype control (open histogram) followed by streptavidin-APC. Plots are gated on MHC II+ CD11b+ cells.
(C) Ear skin explants were cultured in RPMI with 5 % FBS for 16 hrs before fixation, and stained for MHC II and Langerin as described in (A).
(D) Sections of peripheral LNs from BALB/c mice were labeled for Langerin with L31 followed anti-rat IgG Alexa 488 (green) and anti-B220 Alexa 647 (red).
It is known that the high expression of Langerin in LCs decreases upon culture (Valladeau et al., 2002b) while MHC II increases and moves to the cell surface (Pierre et al., 1997). We, therefore, used the L31 mAb to analyze LCs in whole skin explants after 16 hrs of culture by immunofluorescence (Figure 3C). Double-labeling revealed that virtually all cells were strongly MHC II+ but expressed low if any Langerin on surface after 16 hrs of culture (Figure 3C, right panel).
When we stained sections of peripheral lymph nodes for CD207, Langerin+ cells were also noted in the T cell areas (as defined by anti-CD3 staining, not shown) but not in the B follicles (as defined by B220 staining, Figure 3D). This confirms prior work localizing CD207 to a major subset of DC in skin draining LN (Kissenpfennig et al., 2005). Thus, the L31 mAb to the ECD of CD207 (as well as the other new mAbs) can be used to identify LCs in the epidermis and in skin draining LNs.
3.4. L31 mAb detects Langerin in Birbeck granules
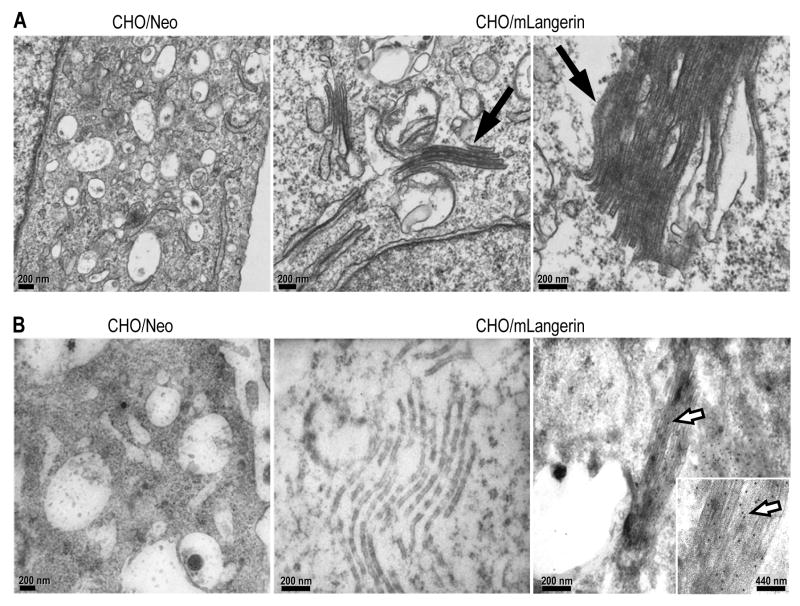
Transfection of Langerin cDNA into fibroblasts induces structures with features of BGs and, in these cells Langerin is not randomly distributed, but instead accumulates in these structures (Valladeau et al., 2002b). The transfection of mLangerin cDNA in CHO cells also induced the formation of BG-like structures, consisting of stacks of tightly apposed membranes resembling the “handles” of BGs (Figure 4A, middle and right panels, filled arrow). To assess the capacity of L31 to detect Langerin in BG, we labeled stable CHO/mLangerin cells and analyzed them by immunoelectron microscopy (Figure 4B). We found that the L31 mAb, but not a control mAb, was able to detect Langerin in Birbeck granules of CHO/mLangerin cells by immunoelectromicroscopy (Figure 4B, middle and right panels, open arrow).
Figure 4. Birbeck granules can be visualized by immuno EM with L31.
(A) CHO cells stably transfected with mLangerin (CHO/mLangerin) were processed for electron microscopy and analyzed for the presence of Birbeck granules (middle and right panels, filled arrow). No BGs were detected in control CHO/Neo cells (left panel).
(B) CHO cells transfected with mLangerin (middle and right panels) were processed for electron microscopy and incubated with L31 (right panel) or the matching isotype control (middle panel) followed by 12 nm colloidal gold particles conjugated anti-rat mAbs (open arrow). CHO cells transfected with irrelevant cDNA as negative control (left panel, CHO/Neo) did not display membrane superimposition and were not stained with L31 mAb.
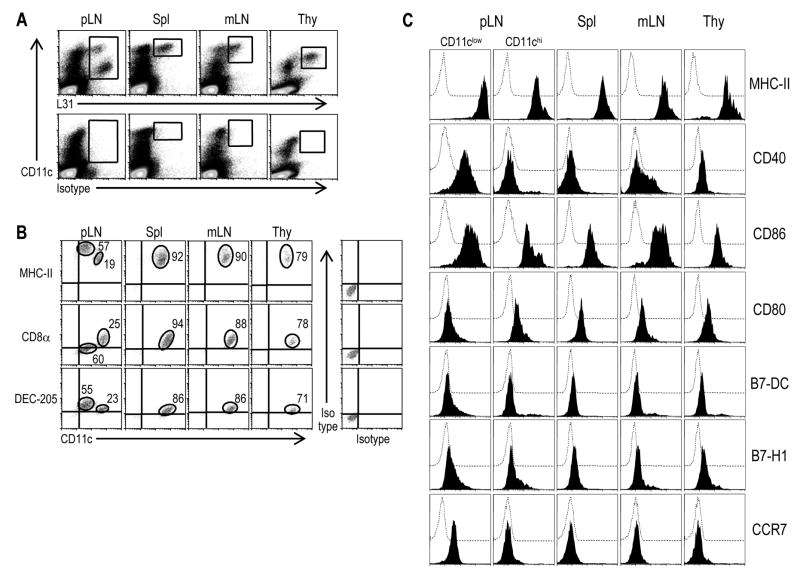
3.5. Phenotype of Langerin+ cells from lymphoid tissues of BALB/c mice, including CD11chigh and CD11clow forms
Two subsets of Langerin+ cells have previously been described in BALB/c mice using IgM mAb (Douillard et al., 2005) and mice expressing EGFP under control of the Langerin promoter (Kissenpfennig et al., 2005). We used the L31 anti-Langerin mAb to analyze the phenotype of these cells. Skin draining peripheral LNs (pLN), spleen, mesenteric LNs (mLNs) and thymus were treated with Collagenase D and low density fractions were enriched for DCs by flotation on 30 % BSA columns. After gating out autofluorescent cells by using the FL3 channel, we analyzed large cells (defined using FSC versus SSC profile) for the expression of total Langerin (surface plus intracellular) and surface CD11c. CD11chi Langerin+ cells were found in each tissue, but in skin draining lymph nodes, we noted an additional CD11c intermediate, Langerin high population, which was presumably derived from the skin (Figure 5A). The Langerin+ cells (boxes in upper panels of Figure 5A) were further analyzed for the expression of CD8α, MHC II and DEC205 in Figure 5B. Two subsets of Langerin+ cells were distinguished in skin draining LNs. The major one represented ~60 % of the Langerin+ cells, and was Langerinhi, CD8−/low, DEC205hi and MHC IIhi. The minor subset (~20 % of the Langerin+ cells) was Langerinlow, DEC205low, CD8+ and MHC IIint (Figure 5B). We further phenotyped the Langerin+ cells in the lymphoid tissues of BALB/c mice. When compared to CD11chi Langerin+ cells, CD11cinter Langerinhi cells in skin draining lymph nodes expressed higher levels of MHC II, CD40, CD86 and CCR7, a phenotype matching that expected for migrating LCs (Figure 5C). We conclude that the L31 mAb identifies Langerin+ cells in the tissues of mice, which in the case of BALB/c mice includes cells in lymphoid organs that do not drain from the skin. The non-skin draining CD207+ DCs express DEC205/CD205 and high levels of CD8 and the CD11c integrin.
Figure 5. Phenotype of Langerin+ cells in lymphoid cell suspensions of BALB/c mice.
(A) Skin draining LNs (pLN), spleen, mesenteric LNs (mLN) and thymus were treated with collagenase D. Low density fractions from a 30 % BSA gradient were used to enrich DCs. The expression of Langerin was evaluated after fixation and permeabilization. After gating out autofluorescent cells on the FL3 channel, large cells (defined by FSC versus SSC) were analyzed with L31 mAb and CD11c mAb.
(B) Langerin+ cells (boxes in upper panels in A) were analyzed for the expression of CD8α, MHC II and DEC205.
(C) Phenotype of the two types of Langerin+ cells found in peripheral LNs in the steady state and the Langerin+ cells in spleen, thymus and mesenteric LNs. Levels of surface MHC-II, CD40, CD86, CD80, B7-DC, B7-H1 and CCR7 expressed in CD11chi are compared to those found on the surface of CD11cinter Langerin+ cells in peripheral LN. Langerin+ cells are identified after fixation and permeabilization. Dotted open histogram corresponds to the isotype control.
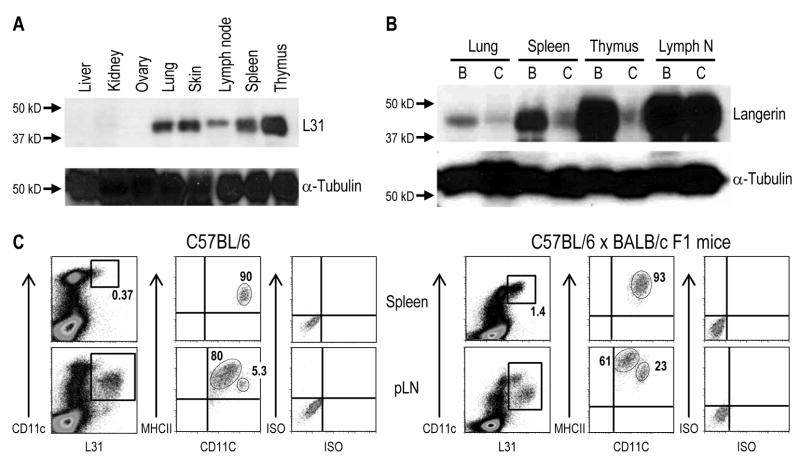
3.6. Different expression of Langerin in mouse strains
We compared the expression of Langerin in organ lysates from 2 different strains of mice. In BALB/c mice, Langerin was noted in the skin, lymphoid organs and lung using the mAb L31 (Figure 6A). For C57BL/6 mice, we detected a strong signal in skin draining lymph nodes, but the signals were much weaker than BALB/c mice in other organs (Figure 6B). Expression of Langerin in spleen and thymus also was low in C57BL/6 compared to BALB/c mice by immunofluorescence (data not shown) and FACS analysis, but positive profiles were noted in the peripheral or skin draining LNs (Figure 6C, left panels). The CD11chi population in pLN of C57BL/6 mice expressed low if any Langerin, but it was readily detected in F1 mice of C57BL/6 × BALB/c (Figure 6, right panels). Moreover, a splenic Langerin+ population in F1 mice (C57BL/6 × BALB/c) was detected (Figure 6C, lower panels) similar to BALB/c mice (Figure 5). Thus, the L31 mAb nicely demonstrates differential expression of Langerin in two different strains of mice.
Figure 6. Expression of Langerin in different mouse strains.
(A) The indicated organs from BALB/c mice were lysed and 30 μg were applied to 10 % SDS-PAGE, transferred onto PVDF membrane and immunoblotted using L31 mAb and anti-tubulin.
(B) Elevated expression levels of Langerin in different tissues of BALB/c (“B”) mouse are detected in comparison to C57BL/6 (“C”) mouse by Western blot analyses with L31 mAb and anti-tubulin as described in (A).
(C) F1 (BALB/c × C57BL/6) mice maintain the CD11chi Langerinlow population that is detected in BALB/c but not C57BL/6 mice. Peripheral LNs and spleen were treated with Collagenase D and the low density fractions from a 30 % BSA gradient were used to enrich DCs. After gating out autofluorescent cells by using the FL3 channel, large cells (defined by FSC versus SSC) were analyzed by flow cytometry for the expression of total Langerin after fixation and permeabilization (with L31), and surface CD11c and MHC II.
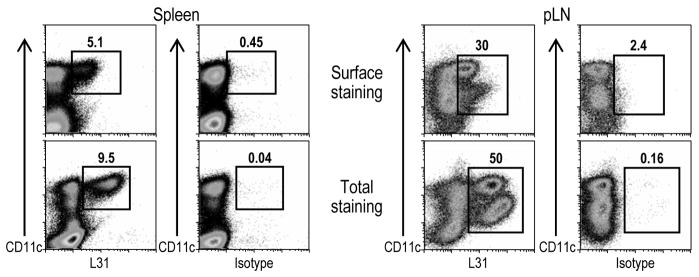
3.7. mAb L31 detects significant levels of surface Langerin in BALB/c mice
Surface Langerin is down-regulated upon maturation of LCs (Valladeau et al., 2002b), what represents a detection problem to most anti-mLangerin mAbs (Stoitzner et al., 2005). We therefore wondered if L31 would still detect low expression of surface Langerin. As shown in Figure 7 splenic Langerin+ cells displayed considerable levels of surface CD207 as detected with L31 in live DCs enriched from spleen cell suspensions. In addition, CD11chi Langerin+ cells in skin draining LNs expressed detectable levels of surface Langerin. However, in accordance with previously published data (Stoitzner et al., 2005; Valladeau et al., 2002b), Langerhans derived-cells (CD11cinter Langerinhi population) in skin draining LNs expressed low amounts of surface CD207, which could still be detected with our new L31 mAb. We conclude that L31 is a strong mAb that can be used to detect the low expression of surface Langerin in addition to large amounts within the cells.
Figure 7. Surface expression of mLangerin as detected with L31 mAb.
DCs from BALB/c mice were enriched by negative selection. In brief, spleen and skin draining LN cell suspensions were incubated with biotin-labeled anti-CD3, B220, CD19, DX5, Gr1 and Thy1.2 mAbs. The non-DCs were removed by the application of a magnetic field after the incubation with streptavidin-beads. Cells were stained with Alexa 647 labeled L31 mAb, or the corresponded isotype control, and CD11c FITC. For the total staining, the cells were fixed and permeabilized with cytofix/cytoperm buffer (BD Biosciences) following the manufacture’s instructions.
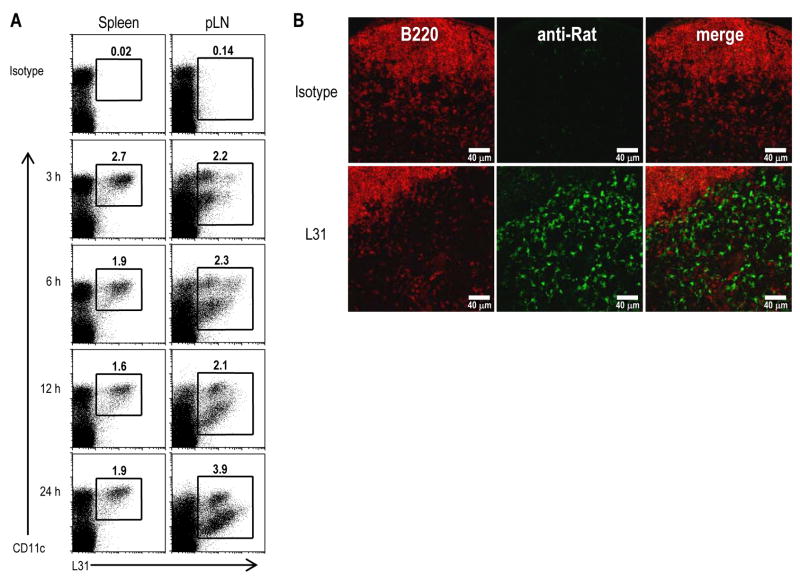
3.8. Systemic distribution of L31 mAb after targeting to DCs
The availability of a rat IgG mAb against the extracellular domain of Langerin allowed us to determine whether this Ab can be used to target the endocytic receptor Langerin in vivo. We injected mice s.c. in the footpads with L31 or the isotype control rat IgG and detected by FACS analysis and immunofluorescence. The isotype matched control III-10 mAb did not bind to DCs at all time points (Figure 8A, top panels). In contrast, within 3 hrs of s.c. inoculation, the first time point studied, Alexa 647-conjugated L31 mAb began to load a sizable fraction of DCs in draining lymph nodes and spleen. Moreover, the uptake of L31 mAb by LCs in the draining lymph nodes increased progressively from 6 to 12 hrs. This was confirmed by immunofluorescence using anti-rat antibodies to detect the targeted L31 in the T cell area of skin draining lymph nodes (Figure 8B). These results indicate that L31 mAb is suitable for targeting the CD207 endocytic receptor in vivo in contrast to IgM anti-Langerin or Abs to the cytosolic domain.
Figure 8. Langerin targeting of mAb L31 after s.c. injection.
(A) BALB/c mice were injected s.c. with 10 μg of Alexa 647-conjugated rat IgG mAbs L31 or III-10, the isotype-matched control. At the indicated time points, DCs were enriched from the draining lymph nodes or spleen by floating in 30 % BSA and analyzed by FACS. The frequencies of DCs capturing the injected, labeled III-10 (top panels) or L31 are indicated.
(B) Langerin+ cells in skin draining lymph nodes are targeted following s.c. injection of 25 μg of L31 or III-10 isotype control. Peripheral LNs were harvested 3 hrs post-inoculation and the injected mAbs visualized by staining with anti-rat IgG Alexa 555 (green) and anti-B220-Alexa 647 (red).
4. Discussion
DCs are comprised of different forms or subsets. Different DC subsets can express different C-type lectins, many of which function as endocytic receptors. Following internalization, antigens can be processed for presentation to MHC and CD1 restricted T cells to initiate defense mechanisms against pathogens and to maintain T-cell tolerance. The delivery of antigens to different subsets of DCs through antibodies against C-type lectin receptors is a valuable new approach for the study of the functional specialization of defined DC populations. We report here the production, characterization, and reactivity of IgG mAbs directed against the extracellular domain of mouse Langerin, a C-type lectin present in LCs and DCs. An antibody to the cytosolic domain is available (Stoitzner et al., 2003), as is an IgM mAb to the extracellular domain (Douillard et al., 2005), but neither are suitable for targeting antigens within anti-CD207 mAbs to assess the function of Langerin and Langerin+ cells in vivo.
The most difficult part of this study was the generation of an IgG mAb. We started by immunizing rats with the soluble ECD but only observed IgM mAbs. This might indicate that soluble Langerin antigens aggregate and act as a thymus independent antigen. However, when we boosted the immune rats with freshly isolated live and lysates of LCs and DCs, we were able to prepare many IgG mAbs to the extracellular domain. Of the 10 mAbs, two of them seemed to bind to epitopes in the neck domain and interestingly, these were inefficient to stain CD207 on the surface of CHO transfectants (Figure 2A, L20 and L25). We found that one mAb was more suitable for immunoprecipitation (L20) and another (L31) was more suitable for Western blotting and immunofluorescent staining (sections and cells), but all the new mAbs to the CRD were active in detecting CD207.
Taking advantage of the new mAb L31, we analyzed the expression of Langerin in different organs of BALB/c mice. As reported before (Douillard et al., 2005; Kissenpfennig et al., 2005), we found two populations of DCs expressing Langerin in skin draining LN. We also found that these subpopulations could be clearly differentiated by the expression of CD11c, one being CD11chi and the other CD11clow. The CD11clow subset corresponds to emigrating LCs, and had a more mature surface phenotype (e.g. high expression of CD40, CD86, MHCII and CCR7), whereas the CD11chi population corresponded to the CD8+ DEC205+ subset of DCs in lymphoid tissues. Interestingly, the expression of Langerin by this latter subset was primarily seen in BALB/c and was much reduced in C57BL/6 mice.
The downregulation of the expression of Langerin upon LC’s maturation (Stoitzner et al., 2003) poses a detection problem for anti-CD207 mAbs (Stoitzner et al., 2005). However, the L31 mAb detects appreciable levels of Langerin on Langerhans derived-cells in skin draining LNs of BALB/c and C57BL/6 mice. While our new mAb was being characterized, a rat IgG mAb against the extracellular domain of mLangerin became commercially available (eBiosciences). Comparative studies using both mAb (L31 vs eBioRMUL.2 from eBiosciences) revealed an improved sensitivity of L31. Indeed, low expression of Langerin, e.g. as on the DEC+ DC subset in C57BL/6’s spleen, could be detected by FACS using L31.
The mAb newly described in this study is an IgG against the extracellular domain of mLangerin. Its application in different immunofluorescence settings offers a new tool for the study of LCs and DCs. In addition, this mAb allows for the rapid and selective targeting to LCs in vivo. For example, we are now cloning the heavy and light chains of L31 mAb so that they can be engineered to encode defined protein antigens, to test if ligation of L31 in vivo leads to more efficient antigen presentation, as described previously for engineered mAbs to DEC205/CD205 (Boscardin et al., 2006; Hawiger et al., 2001; Trumpfheller et al., 2006) and DCIR2 (Dudziak et al., 2007; Soares et al., 2007). Ongoing studies will try to determine the independent contributions of different subsets of Langerin+ cells in vivo after the targeting of antigens to CD207 is achieved with anti-Langerin mAbs.
Acknowledgments
We thank Judy Adams for preparing the figures and Lucio Verani for help with the references. We acknowledge Jay Overholser, Bermann Francois, Frances Weis-Garcia, Eleana C. Sphicas, and Alison North for their help with hybridoma production, electron microscopy, and confocal microscopy. We were supported by NIH Grants to RMS (AI 13013, AI 40045, AI 057158) and CGP (AI 057158) and a grant from the Histiocytosis Association of Canada.
Abbreviations
- BG
Birbeck granules
- CHO cells
Chinese hamster ovary cells
- CRD
carbohydrate recognition domain
- DCs
dendritic cells
- ECD
extracellular domain
- FBS
fetal bovine serum
- i.p
intraperitoneally
- i.v
intravenously
- LCs
Langerhans cells
- LN
lymph node
- mAb
monoclonal antibody
- ORF
open reading frame
- s.c
subcutaneously
- PVDF
polyvinylidine difluoride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bennett CL, van Rijn E, Jung S, Inaba K, Steinman RM, Kapsenberg ML, Clausen BE. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscardin SB, Hafalla JC, Masilamani RF, Kamphorst AO, Zebroski HA, Rai U, Morrot A, Zavala F, Steinman RM, Nussenzweig RS, Nussenzweig MC. Antigen targeting to dendritic cells elicits long-lived T cell help for antibody responses. J Exp Med. 2006;203:599–606. doi: 10.1084/jem.20051639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillard P, Stoitzner P, Tripp CH, Clair-Moninot V, Ait-Yahia S, McLellan AD, Eggert A, Romani N, Saeland S. Mouse lymphoid tissue contains distinct subsets of langerin/CD207 dendritic cells, only one of which represents epidermal-derived Langerhans cells. J Invest Dermatol. 2005;125:983–994. doi: 10.1111/j.0022-202X.2005.23951.x. [DOI] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz V, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- Galustian C, Park CG, Chai W, Kiso M, Bruening SA, Kang YS, Steinman RM, Feizi T. High and low affinity carbohydrate ligands revealed for murine SIGN-R1 by carbohydrate array and cell binding approaches, and differing specificities for SIGN-R3 and langerin. Int Immunol. 2004;16:853–866. doi: 10.1093/intimm/dxh089. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annual review of immunology. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- Hawiger D, Inaba K, Dorsett Y, Guo K, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henri S, Vremec D, Kamath A, Waithman J, Williams S, Benoist C, Burnham K, Saeland S, Handman E, Shortman K. The dendritic cell populations of mouse lymph nodes. J Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- Hunger RE, Sieling PA, Ochoa MT, Sugaya M, Burdick AE, Rea TH, Brennan PJ, Belisle JT, Blauvelt A, Porcelli SA, Modlin RL. Langerhans cells utilize CD1α and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. 2004;113:701–708. doi: 10.1172/JCI19655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YS, Yamazaki S, Iyoda T, Pack M, Bruening S, Kim JY, Takahara K, Inaba K, Steinman RM, Park CG. SIGN-R1, a novel C-type lectin expressed by marginal zone macrophages in spleen, mediates uptake of the polysaccharide dextran. Int Immunol. 2003;15:177–186. doi: 10.1093/intimm/dxg019. [DOI] [PubMed] [Google Scholar]
- Kang YS, Do Y, Lee HK, Park SH, Cheong C, Lynch RM, Loeffler JM, Steinman RM, Park CG. A dominant complement fixation pathway for pneumococcal polysaccharides initiated by SIGN-R1 interacting with C1q. Cell. 2006;125:47–58. doi: 10.1016/j.cell.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Kang YS, Kim JY, Bruening SA, Pack M, Charalambous A, Pritsker A, Moran TM, Loeffler JM, Steinman RM, Park CG. The C-type lectin SIGN-R1 mediates uptake of the capsular polysaccharide of Streptococcus pneumoniae in the marginal zone of mouse spleen. Proc Natl Acad Sci USA. 2004;101:215–220. doi: 10.1073/pnas.0307124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, Laplace-Builhe C, Perrin P, Romani N, Tripp CH, Douillard P, Leserman L, Kaiserlian D, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Mc Dermott R, Ziylan U, Spehner D, Bausinger H, Lipsker D, Mommaas M, Cazenave JP, Raposo G, Goud B, de la Salle H, et al. Birbeck granules are subdomains of endosomal recycling compartment in human epidermal Langerhans cells, which form where Langerin accumulates. Mol Biol Cell. 2002;13:317–335. doi: 10.1091/mbc.01-06-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner U, Inaba K, Koch F, Heine M, Miwa M, Schuler G, Romani N. An improved isolation method for murine migratory cutaneous dendritic cells. J Immunol Methods. 1996;193:71–79. doi: 10.1016/0022-1759(96)00058-0. [DOI] [PubMed] [Google Scholar]
- Park CG, Chwae YJ, Kim JI, Lee JH, Hur GM, Jeon BH, Koh JS, Han JH, Lee SJ, Park JW, et al. Serologic responses of Korean soldiers serving in malaria-endemic areas during a recent outbreak of Plasmodium vivax. Am J Trop Med Hyg. 2000;62:720–725. doi: 10.4269/ajtmh.2000.62.720. [DOI] [PubMed] [Google Scholar]
- Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig M, Steinman RM. DEC-205/CD205+ dendritic cells induce CD4+ T cells to produce IFN-γ by a CD70 dependent, IL-12 independent mechanism. J Exp Med. 2007 doi: 10.1084/jem.20070176. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambach NS, Taylor ME. Characterization of carbohydrate recognition by langerin, a C-type lectin of Langerhans cells. Glycobiology. 2003;13:401–410. doi: 10.1093/glycob/cwg045. [DOI] [PubMed] [Google Scholar]
- Stoitzner P, Holzmann S, McLellan AD, Ivarsson L, Stossel H, Kapp M, Kammerer U, Douillard P, Kampgen E, Koch F, et al. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J Invest Dermatol. 2003;120:266–274. doi: 10.1046/j.1523-1747.2003.12042.x. [DOI] [PubMed] [Google Scholar]
- Stoitzner P, Tripp CH, Douillard P, Saeland S, Romani N. Migratory Langerhans cells in mouse lymph nodes in steady state and inflammation. J Invest Dermatol. 2005;125:116–125. doi: 10.1111/j.0022-202X.2005.23757.x. [DOI] [PubMed] [Google Scholar]
- Stosel H, Koch F, Romani N. Maturation and migration of murine dendritic cells in situ. Observations in a skin organ culture model. Adv Exp Med Biol. 1997;417:311–315. doi: 10.1007/978-1-4757-9966-8_51. [DOI] [PubMed] [Google Scholar]
- Tada Y, Riedl E, Lowenthal MS, Liotta LA, Briner DM, Crouch EC, Udey MC. Identification and characterization of endogenous Langerin ligands in murine extracellular matrix. J Invest Dermatol. 2006;126:1549–1558. doi: 10.1038/sj.jid.5700283. [DOI] [PubMed] [Google Scholar]
- Takahara K, Omatsu Y, Yashima Y, Maeda Y, Tanaka S, Iyoda T, Clausen BE, Matsubara K, Letterio J, Steinman RM, et al. Identification and expression of mouse Langerin (CD207) in dendritic cells. Int Immunol. 2002;14:433–444. doi: 10.1093/intimm/14.5.433. [DOI] [PubMed] [Google Scholar]
- Takahara K, Yashima Y, Omatsu Y, Yoshida H, Kimura Y, Kang YS, Steinman RM, Park CG, Inaba K. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int Immunol. 2004;16:819–829. doi: 10.1093/intimm/dxh084. [DOI] [PubMed] [Google Scholar]
- Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, Huang Y, Schlesinger SJ, Park CG, Nussenzweig MC, et al. Intensified and protective CD4+ T cell immunity at a mucosal surface after a single dose of anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta D, Kaneda K, Teramae H, Ishii M. In vivo activation of Langerhans cells and dendritic epidermal T cells in the elicitation phase of murine contact hypersensitivity. Br J Dermatol. 1999;140:392–399. doi: 10.1046/j.1365-2133.1999.02698.x. [DOI] [PubMed] [Google Scholar]
- Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- Valladeau J, Clair-Moninot V, Dezutter-Dambuyant C, Pin JJ, Kissenpfennig A, Mattei MG, Ait-Yahia S, Bates EE, Malissen B, Koch F, et al. Identification of mouse langerin/CD207 in Langerhans cells and some dendritic cells of lymphoid tissues. J Immunol. 2002a;168:782–792. doi: 10.4049/jimmunol.168.2.782. [DOI] [PubMed] [Google Scholar]
- Valladeau J, Clair-Moninot V, Dezutter-Dambuyant C, Pin JJ, Kissenpfennig A, Mattei MG, Ait-Yahia S, Bates EE, Malissen B, Koch F, et al. Identification of mouse langerin/CD207 in Langerhans cells and some dendritic cells of lymphoid tissues. J Immunol. 2002b;168:782–792. doi: 10.4049/jimmunol.168.2.782. [DOI] [PubMed] [Google Scholar]
- Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, Duvert-Frances V, Vincent C, Schmitt D, Davoust J, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- Weinlich G, Heine M, Stössel H, Zanella M, Stoitzner P, Ortner U, Smolle J, Koch F, Sepp NT, Schuler G, Romani N. Entry into afferent lymphatics and maturation in situ of migrating murine cutaneous dendritic cells. J Invest Dermatol. 1998;110:441–448. doi: 10.1046/j.1523-1747.1998.00161.x. [DOI] [PubMed] [Google Scholar]