Abstract
Multiple controlled clinical trials support the efficacy of nortriptyline as a smoking cessation agent. While therapeutic plasma nortriptyline concentrations (PNCs) are known for the treatment of depression, little is known about PNCs in smoking cessation treatment. PNCs from three randomized, placebo-controlled smoking cessation trials (N = 244) were analyzed, both pooled and separately. PNCs normalized for dose and weight were not associated with sex or age, but were associated with cigarettes/day and race. Greater smoking was associated with decreased normalized PNCs. In addition, both Asians and Blacks had significantly higher normalized PNCs than Whites. Weak and inconsistent associations between PNCs and self-reported side effects were observed. PNCs were linearly related to end of treatment and long-term biochemically verified smoking abstinence. Maximum therapeutic effects were observed at a range of plasma concentrations somewhat lower than those found effective for the treatment of depression.
Keywords: nortriptyline, smoking cessation, plasma nortriptyline concentration, therapeutic drug monitoring
The tricyclic antidepressant, nortriptyline, has shown consistent efficacy as a smoking cessation agent[1, 2]. Unlike other smoking cessation pharmacotherapies, therapeutic drug monitoring (TDM) has played an important role in nortriptyline therapy. A given dose of nortriptyline may produce plasma concentrations of the drug that differ by as much as 30-fold[3]. Based on a known therapeutic range or window for depression of 50 to 150 ng/mL[4], most nortriptyline smoking cessation studies have measured drug concentrations and titrated dosage to place smokers in this range[5-9].
Using depression dosing guidelines for these initial nortriptyline smoking cessation studies was reasonable. However, a database now exists to empirically address several important topics concerning plasma nortriptyline concentrations (PNCs) in smokers, including describing variability in PNCs by dosage, identifying patient characteristics associated with PNCs, relating PNCs to self-reported side effects, and defining a smoking-cessation specific therapeutic range for nortriptyline. With respect to the last point, defining a separate nortriptyline therapeutic range for smokers may be necessary for several reasons. The original work that informed the therapeutic range for depression was conducted in a relatively small set of patients, most of whom experienced severe endogenous depression[10, 11]. The degree of affective dysregulation observed in smoking cessation is comparatively mild and transient relative to major depression[12, 13], so lower PNCs may be sufficient to achieve smoking abstinence. At the same time, lower PNCs would help to decrease the frequency and severity of side effects common to tricyclics including dry mouth, blurred vision, weight gain, constipation, and orthostatic hypotension.
The current paper uses data from three randomized, placebo-controlled trials that evaluated nortriptyline for smoking cessation in 244 individuals[5-7]. We had three goals that we accomplished using both pooled and parallel analyses. First, we described concentrations of plasma nortriptyline during dose run-up (weeks 2 and 4 of treatment) and at 2 weeks post-cessation (week 6 of treatment) by intended dosage. We further attempted to account for the wide variability in PNCs through explanatory modeling, including the factors of sex, age, cigarettes/day, and race, while accounting for medication compliance. Second, we evaluated the association between nortriptyline concentrations and self-reported side effects, both before and after cessation. Third, we used initial post-cessation plasma nortriptyline concentrations (week 6) to predict end of treatment (week 12) and long-term (week 24) biochemically-confirmed smoking abstinence. We attempted to determine if the shape of the plasma nortriptyline concentration response would assume an inverted u-shape as observed for depression[14].
Methods
Complete details about study protocols and results are presented elsewhere[5-7]. We identify the published reports as follows: Study 1[7], Study 2[6], and Study 3[5]. Similar methods were employed for the three studies, with the exception of study design and psychotherapeutic interventions described in full detail in the parent publications.
Participants
In all three studies, smokers of 10 or more cigarettes per day were recruited through a multimedia campaign. Following telephone screening, eligible individuals visited our clinic to complete an informed consent procedure, and to undergo baseline assessment to determine final study eligibility. Multiple exclusion criteria were applied including, use of a monoamine oxidase inhibitor within 2 weeks, major medical conditions (e.g., cardiovascular disease,) major psychiatric conditions (e.g., major depressive disorder, bipolar disorder), recent alcohol or substance abuse treatment, and pregnancy, intended pregnancy or lactation.
Procedures
Medication
Prior to smoking cessation, participants in each study underwent a 4-week medication induction period with the goal of achieving PNCs in the range of 50 to 150 ng/mL prior to cessation. Medications were taken once each day in the evening hours. During week 1 of medication, participants took 25 mg/day for 3 days followed by 4 days of 50 mg/day of nortriptyline (except for 1 subject who remained on 25 mg/day due to side effects). At the second week of treatment, plasma concentrations were first assessed, and those not yet in the therapeutic range received a dose increment of 25 mg/day for a total of 75 mg/day. At the fourth week of treatment, plasma concentrations were once more assessed; if required, the dose was increased by 25 mg/day to 100 mg/day. In study 1, a final plasma concentration was obtained at week 6, but no additional dose increment was made. In studies 2 and 3, a final plasma concentration was obtained at week 6 (week two of cessation) with a final 25 mg/day increment if needed for a possible maximum dosage of 125 mg/day. After week 7, patients were maintained on their dose for the remaining 5 weeks of treatment, followed by a one-week tapering-off phase to no medication. One exception was Study 3[5] in which half of subjects received an additional 40 weeks of medication therapy. In Study 3, all participants began transdermal nicotine replacement (21 mg/day) at week 5 of nortriptyline therapy and continued for an additional 8 weeks.
Psychotherapy
Psychotherapy conditions are considered as covariates for the purposes of the current analyses. During the first twelve weeks of treatment, therapy was provided in a group milieu. Briefly, in Study 1, participants received either a health education intervention or a cognitive behavioral intervention[7]. Study 2[6] compared a medical management intervention to a cognitive behavioral intervention. All participants in Study 3 received cognitive-behavioral treatment[5].
Measures
Nortriptyline concentrations were evaluated during the daytime hours following the preceding evening's dose administration. Nortriptyline concentrations have been shown to be stable throughout the day due to its half-life of 36 hours[15]. Blood plasma samples were analyzed by commercial laboratories, e.g., Quest Diagnostics, Inc., using liquid chromatography and tandem mass spectrometry.
Each week, participants rated themselves on side effects. Known and high frequency side effects of nortriptyline therapy in depression were incorporated into a broader list of side effects evaluated in our other clinic studies (i.e., dry mouth, rash, weight gain, lightheadedness, shaky hands, constipation, blurry vision, sexual problems, difficulty urinating, racing heart, swollen legs, chest pain or pressure, shortness of breath, and other).
In the smoking cessation literature, the conventional definition of treatment success is biochemically-confirmed, 7-day point-prevalence abstinence[16]. Biochemical indices are typically expired carbon monoxide or cotinine (a longer-lived metabolite of nicotine). Smoking abstinence was determined 2 weeks post-quit based on no self-reported smoking in the preceding 7 days and an expired carbon monoxide concentration ≤ 10 parts-per-million. At end of treatment (8 weeks post-quit) and long-term follow-up (20 weeks post-quit), smoking abstinence was defined as no self-reported smoking in the preceding 7 days and a plasma cotinine concentration ≤ 60 ng/mL for studies 1 and 2. Smoking abstinence was not examined in study 3 due to use of the nicotine patch.
Baseline demographic and smoking history variables were captured using an author-constructed inventory. Nicotine dependence was measured using the Fagerstrom Test of Nicotine Dependence (FTND)[17]. Mood was measured using the Profile of Mood States (POMS)[18] and the Beck Depression Inventory (BDI)[19].
Analyses
Assumptions
All analyses were conducted using the Statistical Analysis System, Version 9.13[20]. Values of p<.05 were considered statistically significant. Type I error rate in all post-hoc comparisons was controlled with Bonferroni adjustments. Due to sub-sample analyses, participant attrition or infrequent missing data, the number of subjects or data points available for statistical analysis varied from the maximum of 244 to a minimum 43.
Analytic Methods
Comparability of study groups on baseline demographic, smoking history, and psychological variables was evaluated using ANOVA for continuous variables and chi-square tests for categorical variables. Differences in PNCs by subject factors were evaluated using multivariate linear regression; for this analysis, PNCs were normalized by dose and weight (PNC /[dose/weight]). Associations between PNCs and side effects were expressed as point-biserial correlation coefficients. In order to evaluate the predictive relationship of PNCs to smoking abstinence at 8 and 20 weeks post-quit, a repeated-measures, multivariate logistic regression model was constructed using a subsample pooled from Studies 1 and 2 (subjects in study 2 who received a final dose increment at week 6 were excluded). To account for potential bias from the use of a non-randomized subsample, correlates of abstinence (i.e., age, sex, FTND score, ethnicity, body mass index, psychological therapy condition, and medication compliance) were included as covariates. In order to assess the possibility of a non-linear relationship of nortriptyline concentrations to outcome[10, 11], a quadratic term was also included.
Results
Sample Characteristics
Sample characteristics of participants in the three studies are presented in Table 1.
Table 1.
Sample Characteristics of Three Nortriptyline Smoking Cessation Trials
| Study 1a | Study 2b | Study 3c | P-value | |
|---|---|---|---|---|
| N = 94 | N = 71 | N = 79 | ||
| Demographic | ||||
| Age | 41.2 (9.6) | 39.2 (11.7) | 38.2 (10.2) | n.s. |
| Sex (% female, n) | 51.1 (48) | 45.1 (32) | 38.0 (30) | n.s |
| Race (% White, n) | 87.2 (82) | 88.7 (63) | 74.7 (59)d | .0325 |
| BMI | 24.6 (3.3) | 25.8 (5.5) | 26.2 (4.7) | n.s. |
| Smoking and Mood | ||||
| Cigarettes/Day | 23.4 (11.4) | 20.3 (8.4) | 19.6 (7.5)e | .0214 |
| CO Concentration | 24.6 (11) | 25.9 (10.9) | 21.8 (8.8) | n.s. |
| FTND | 5.6 (2.2)f | 4.6 (2.1) | 4.6 (2.0) | .0038 |
| POMS | 31.9 (36) | 27.7 (33.5) | 26.5 (34.6) | n.s. |
| BDI | 9.2 (7.4) | 8 (7.4) | 9.1 (7.7) | n.s. |
N = 244.
Hall et al., 1998[7].
Hall et al., 2002[6].
Hall et al., 2004[5].
Percentage of Whites in Study 3 lower than Study 1, although not significant after Bonferroni adjustment.
Cigarettes per/day significantly lower in Study 3 than Study 1.
FTND score significantly higher in Study 1 than Studies 2 and 3.
Apart for some small differences in proportion of Whites, cigarettes/day, and FTND, the three study samples were generally comparable.
Nortriptyline Dosages and Plasma Concentrations
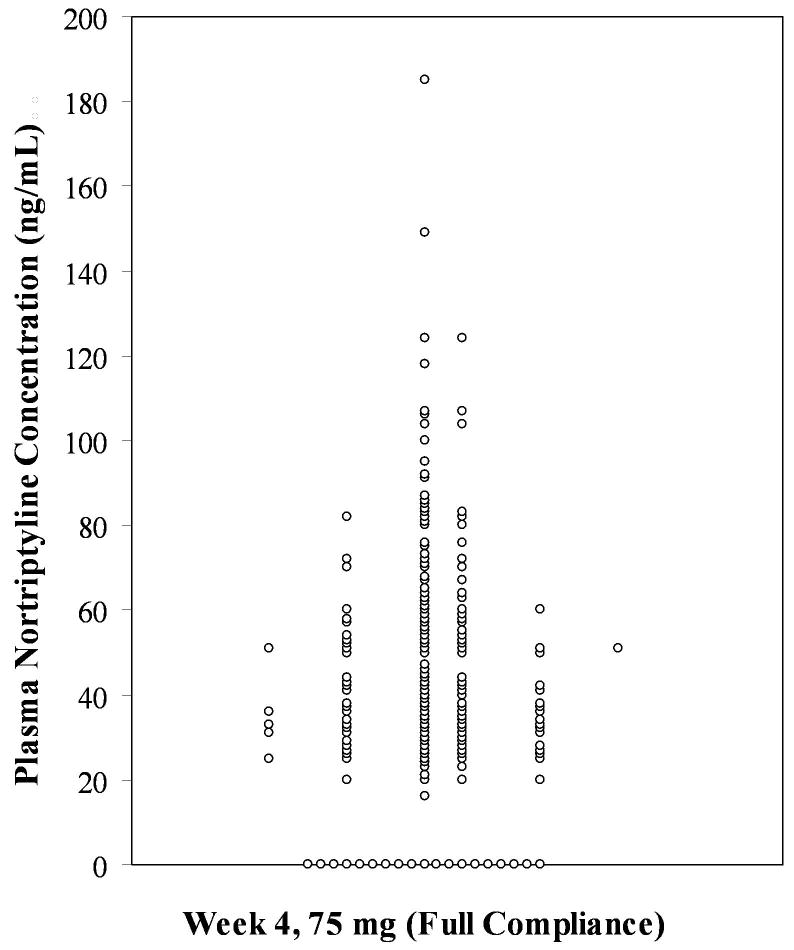
The considerable variability in plasma nortriptyline concentrations within a fixed dose is illustrated in Figure 1. Data for 183 subjects reporting full compliance with the 75 mg dose at week 4 are presented.
Figure 1.
A vertical dot-plot with jittering of coincident observations. Plasma nortriptyline concentrations at for 183 subjects reporting full compliance with the 75 mg dose at week 4.
Importantly, 19 subjects had undetectable concentrations despite self-reported full compliance. Moreover, plasma concentrations ranged from 16 ng/mL to 185 ng/mL.
Intended dosages for all subjects still enrolled in the study at each time point are presented in Table 2. In addition, mean PNCs are given only for subjects reporting full compliance (i.e., taking all medication as directed) in the week preceding sampling.
Table 2.
Frequency of Intended Dosages and Mean Plasma Nortriptyline Concentrations by Dosages
| Week 2a | Week 4 | Week 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N = 164b/142c | N = 230/211c | N = 219/195c | |||||||
| Nortriptyline | nb | nc | M (SD)d | nb | nc | M (SD)d | nb | nc | M (SD)d |
| 25 mg/day | 1 | 1 | 28.0 (–) | 1 | 1 | 72.0 (–) | 1 | 1 | 57.0 (–) |
| 50 mg/day | 164 | 141 | 23.9 (25.1) | 20 | 18 | 63.0 (34.9) | 18 | 12 | 80.8 (76.2) |
| 75 mg/day | – | – | – | 209 | 184 | 47.1 (29.9) | 101 | 82 | 68.9 (33.0) |
| 100 mg/day | – | – | – | – | – | – | 99 | 87 | 46.5 (24.3) |
Plasma nortriptyline concentrations only available for studies 1 and 2.
Total number of subjects at dosage.
Number of subjects who self-reported full compliance in the preceding week.
Mean and standard deviation plasma nortriptyline concentrations in ng/mL.
Increases from 50 mg to 75 mg from week 2 to week 4 significantly elevated PNCs by 24.4 ng/mL (SE = 2.5), t(132) = 9.85, p <.0001. A 25 mg increase to 100 mg from week 4 to week 6 also significantly raised PNCs by 17.8 ng/mL (SE = 2.5), t(94) = 7.91, p <.0001. Although increasing dosages generally resulted in increased PNCs, there was considerable variability within dosages. The lower observed plasma nortriptyline concentrations in the higher dosages of 75 mg and 100 mg are expected since these higher dosages were given to subjects who had not initially achieved therapeutic plasma concentrations. The frequency of dosages at week 7 (when the final dosages for the remainder of treatment were set) were for the remaining sample (N = 211): 25 mg (1%, n = 2), 50 mg (8.5%, n = 18), 75 mg (45.0%, n = 95), 100 mg (41.2%, n = 87), and 125 mg (4.3%, n = 9).
Factors Related to Plasma Nortriptyline Concentrations
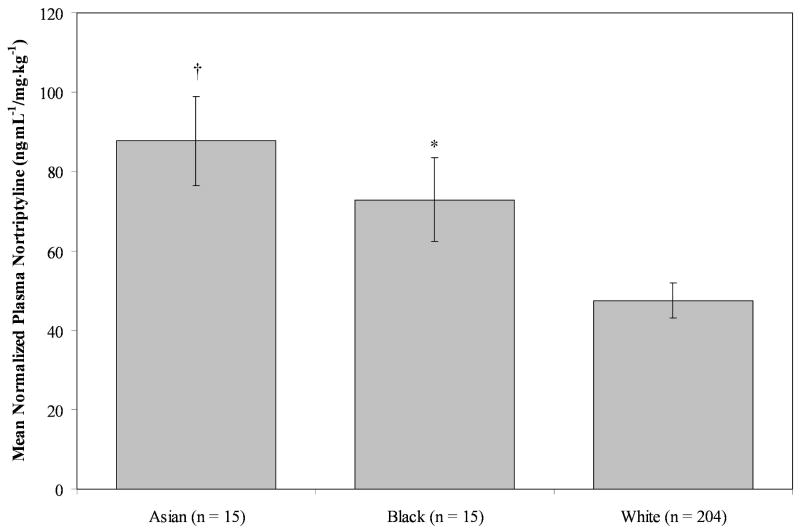
The relationship of sex, age, cigarettes/day in the two weeks preceding plasma sampling, and race (White, Black or Asian) to normalized nortriptyline concentrations (ng·mL-1/mg· kg-1) at week 4 (prior to cessation) were evaluated, using a multivariate linear regression model, including full compliance in the prior week (0 = non-compliance, 1 = compliance) as a covariate. Normalized plasma nortriptyline concentrations at week 4 were significantly related to cigarettes/day, F(1, 197) = 4.89, p = .0281. Each additional cigarette smoked decreased normalized plasma nortriptyline concentrations by .57 ng·mL-1/mg· kg-1 (SE = .26), controlling for the other terms in the model. Further, normalized PNCs also significantly differed by race, F(2, 197) = 9.83, p<.0001 (see Figure 2).
Figure 2.
Effects of race on plasma nortriptyline concentrations normalized for dose and weight at week 4 (pre-cessation) controlling for sex, age, cigarettes/day in the two weeks preceding sampling, and full compliance in the week preceding sampling. Ten subjects not included were of Latino, other, or mixed ethnicity.
Whites had normalized PNCs (M = 47.5 ng·mL-1/mg· kg-1, SE = 4.37) lower than both Asians, (M = 87.7 ng·mL-1/mg·kg-1, SE = 11.2), t(197) = -3.79, p = .0006, and Blacks, (M = 72.9 ng·mL-1/mg· kg-1, SE = 10.6), t(197) = -2.45, p = .0451.
Side Effects
The five most common side effects were evaluated in the pooled sample prior to cessation at weeks 2 (Studies 1 and 2) and weeks 4 (Studies 1 and 3) of nortriptyline therapy. Only plasma concentrations for those self-reporting full compliance in the week preceding sampling are included. Side effects were also evaluated at week 6 of therapy (2 weeks post-quit) in Studies 2 and 3 that had data on concurrent abstinence (see Table 3).
Table 3.
Correlation between Plasma Nortriptyline Concentrations and Major Nortriptyline Side Effects
| Pre-Cessation | Post-Cessation | |||||||
|---|---|---|---|---|---|---|---|---|
| Studies 1,2ab | All Studiesac | Study 2b | Study 3c | |||||
| Week 2 | Week 4 | Alld | Ae | Sf | Alld | Ae | Sf | |
| N | 142 | 139 | 43 | 20 | 23 | 54 | 44 | 10 |
| Dry Mouth | .20* | .35† | .01 | .00 | .04 | .07 | .46 | .03 |
| Lightheadedness | .01 | .11 | -.09 | .18 | -.31 | .17 | .14 | .18 |
| Shaky Hands | .14 | .19* | -.32 | –g | -.49* | -.13 | .14 | -.17 |
| Constipation | .05 | .10 | .03 | -.08 | .13 | .04 | .37 | .00 |
| Blurry Vision | .05 | .12 | .28 | –g | .43* | .21 | –g | .21 |
p <.05.
p <.01 Correlations computed for subjects who self-reported full compliance in the week preceding sampling.
Hall et al., 1998[7].
Hall et al., 2002[6].
Hall et al., 2004[5].
All subjects.
Abstinent subjects.
Smoking subjects.
Rate of side effect was constant at 0%, preventing calculation of correlation coefficient.
Prior to cessation, self-reported dry mouth (weeks 2 and 4) and shaking hands showed small positive associations with PNCs. After cessation, few reliable associations between PNCs and side effects were observed.
Abstinence
The relationship of plasma nortriptyline concentration to abstinence at both end of treatment, week 12 (8 weeks post-quit) and at long-term follow-up, week 24 (20 weeks post-quit) in Studies 1 and 2 was evaluated (due to the inclusion of extended treatment conditions and the nicotine patch, Study 3 was not used). The effect of time (week 8 or 20) and its interaction with PNC were included. In addition, to assess the possibility of a curvilinear relationship of nortriptyline concentration to abstinence, a quadratic term was included. In order to control for other factors known to correlate with abstinence[21], age, gender, FTND score, ethnicity (White, Black or Asian), and BMI, were included as covariates, as well as psychotherapy condition and compliance in the week preceding sampling. Initial model evaluation showed no reliable effect of an interaction between time and PNC or evidence for curvilinearity, so the final analysis was re-run excluding these terms.
Results of the multivariate logistic regression are shown in Table 4. Plasma nortriptyline concentrations at week 6 were positively associated with abstinence at both weeks 8 and 20. For every 10 ng/mL increase in PNC, the odds of being abstinent from smoking increased by a factor of 1.21. Abstinence rates declined from 51% at week 8 to 36% at week 20.
Table 4.
Effect of Plasma Nortriptyline Concentration on Abstinence at Weeks 8 and 20 Post-quit
| Predictor | d.f., F, p-value | OR | 95% CI |
|---|---|---|---|
| PNCa | F(1,103) = 7.59, .0069 | 1.020 | (1.006, 1.034) |
| Timeb | F(1,103) = 19.43, <.0001 | 0.928 | (0.900, 0.960) |
| Covariate | |||
| Age | F(1, 103) = 1.92, n.s | – | – |
| Sex | F(1, 103) = 2.45, n.s | – | – |
| FTND | F(1, 103) = 6.17, .0168 | 0.796 | (0.663, 0.960) |
| Ethnicity | F(2, 103) = 1.79, n.s | – | – |
| BMI | F(1, 103) = 0.24, n.s | – | – |
| Therapy | F(3, 103) = 2.64, n.s. | – | – |
| Compliance | F(1, 103) = 0.46, n.s. | – | – |
Repeated-measures, logistic regression. PNC×Time and PNC×PNC (quadratic) terms were excluded from the final model due to non-significance.
Plasma nortriptyline level taken at week 6 (week 2 post-quit).
Time = week 8 and week 20 post-quit.
Therapeutic range
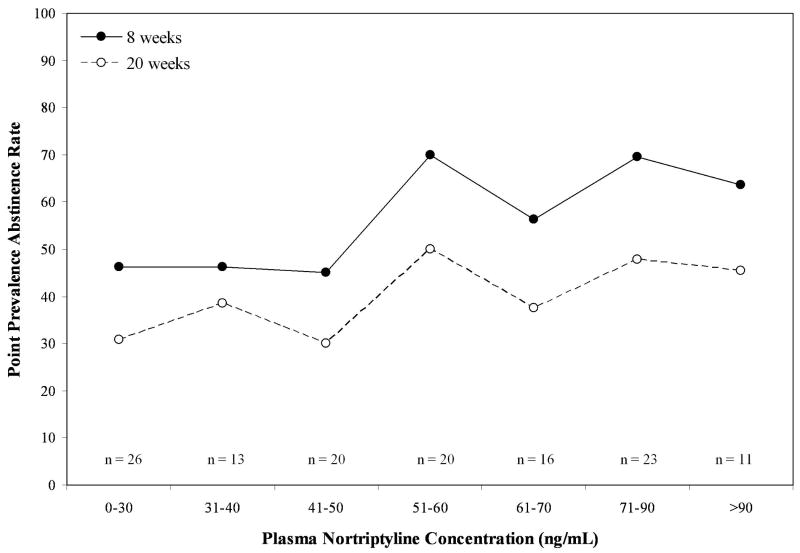
In order to make recommendations about a possible therapeutic range of PNCs for smoking cessation, predicted abstinence rates from the foregoing model were plotted by PNCs at week 6, categorized after Asberg et al.,[14] who originally suggested an inverted u-shaped response function (0 to 30, 31-40, 41-50, 51-60, 61-70, 71-90, and 91 or more ng/mL). At both weeks 12 and 24 (see Figure 3), a linear relationship between plasma nortriptyline concentrations and abstinence is apparent, with little suggestion of non-linearity in evidence. In fact, abstinence rates were largely stable above 40 ng/mL.
Figure 3.
7-day, biochemically confirmed smoking abstinence as a function of plasma nortriptyline concentration at week 12 (week 8 post-quit) and week 24 (week 20 post-quit).
Discussion
We used data from three randomized, placebo-controlled, double-blind trials of nortriptyline for smoking cessation to describe plasma nortriptyline concentrations and to evaluate their relationship to clinical outcomes. Increases in intended dosage yielded increases in plasma nortriptyline concentrations; however, marked variability in concentrations for fixed doses was seen. Amount of smoking showed a small but reliable association with normalized PNCs, but cessation did not result in marked increases in this quantity. In our sample, Asian and Black smokers had normalized PNCs significantly higher than Whites. Self-reported side effects bore little relationship to PNCs. End of treatment and long-term abstinence were significantly related to PNCs taken at 2-weeks post-cessation, after controlling for relevant covariates. No evidence for a curvilinear plasma nortriptyline response curve was observed.
The fact that fixed or mostly fixed dosages of nortriptyline yielded a broad range of PNCs is not surprising. Alexanderson[3] showed that for a fixed dose of nortriptyline, plasma concentrations of the drug can range as much as 30-fold. The primary cause of this variability appears to be genetic, based on studies of monozygotic and dizygotic twins[22], mostly due to differences in the polymorphic cytochrome P-450 enyzme, CPY2D6[23]. Even including only subjects with self-reported full compliance in the week preceding sampling, we still observed some test concentrations falling below the concentration of detectability (e.g., at week 6, 7% were < 20 ng/mL). The most likely explanation for these observations is medication non-compliance. However, it is also possible some of these subjects were ultra-rapid nortriptyline metabolizers[24].
In our sample, we observed significantly higher normalized plasma nortriptyline concentrations in Asians and Blacks compared to Whites (see Figure 2). Ethnic differences in the metabolism of tricyclic antidepressants have been observed in Asians as well as in Africans and African-Americans[25, 26]. Data specific to nortriptyline are few but are concordant with our findings[15, 27]. Kishimoto and Hollister[27] found that after a single 100 mg dose of nortriptyline in 20 males (Japanese, N = 10; American, N = 10 [race not specified]), Japanese participants achieved numerically superior maximum plasma concentrations (39.3 vs. 32.0 ng/mL) and significantly higher exposure, as assessed by area-under-the curve (1150 vs. 730 ng/hour/mL). Evidence of slower metabolism or clearance for another tricyclic antidepressant, desipramine, has been seen in Chinese or mixed Asian samples compared to Caucasians[28, 29]. Ziegler et al., (1977) observed that nortriptyline concentrations were nearly 50% higher in African-Americans than Whites (113.5 vs.75.7 ng/mL). The potential genetic basis for ethnic differences in PNCs likely relates to differences in the frequency of defective CPY2D6 alleles (i.e., so-called “poor metabolizers”), with a higher rate of defective alleles for this enzyme generally, but not always, observed in Asians and those of African ancestry[30-35]. For a given dose of nortriptyline, higher plasma concentrations of the drug will be achieved due to slower metabolism.
Smokers have been observed to have lower concentrations of nortriptyline than non-smokers[36, 37], although not always[38]. In our sample, greater smoking was associated with smaller nortriptyline concentrations, although this effect was slight. Several potential mechanisms may account for this relationship. Nortriptyline is primarily metabolized by CYP2D6 (90%), while the balance of metabolism is accomplished by CYP1A2 and CYP2C19[39]. Since CYP2D6 has generally not been shown to be inducible [40, 41], this enzyme is unlikely to be involved in reduced PNC with greater smoking. Cigarette smoking has been shown to induce CYP1A2 [42] while the inductive effects of cigarette smoke on CYP2C19 await further evaluation [43]. Overall, the role of smoking on nortriptyline concentrations in our sample appeared modest.
Side effects from nortriptyline and other tricyclic antidepressants can play a major role in medication non-compliance and discontinuation in depressed patients[44]. Rates of nortriptyline discontinuation have been significantly elevated compared to placebo in some smoking cessation trials[8, 9, 45], while others have found shown no difference[5, 6, 46], or a greater rate for placebo[7]. However, the current report showed little association between PNCs and endorsement of side effects. Two previous studies have evaluated the relationship between PNCs and self-reported side effects[47, 48]. Asberg and colleagues[47] evaluated the association between total side effects and plasma nortriptyline concentrations in a sample of depressed patients over the first 4 weeks of treatment. They observed a steady abatement in side effects over time such that by week 4, no association between side effects and PNCs was seen. Ziegler et al., [48] found that two individual side effects bore a consistent relationship to PNCs: dry mouth and perspiration. Perspiration was not assessed in any of the present studies. However, the persistence of dry mouth in the present studies does confirm Ziegler and coworkers' [48] observation, at least in the pre-cessation induction phase. Overall, the nortriptyline concentrations achieved in this multistudy analysis are on the low end of the range recommended for treatment for depression (i.e., 50 to 150 ng/mL), and this may also account for the weak associations seen with side effects. Finally, side effects were measured on a dichotomous scale, and the use of a continuous side effects scale would likely have better informed the foregoing analyses[e.g., 49].
Guidelines for the dosing of nortriptyline in depression have been available for over thirty years[4]. Beginning with an early report[14], evidence of a curvilinear relationship, an inverted u-shape function, between PNCs and depressive remission began to accumulate. When we evaluated PNCs as a continuous predictor of abstinence, we found evidence of a significant linear but not a quadratic (i.e., curvilinear) effect (see Figure 3). Given the lack of side effects seen in the current range of plasma nortriptyline concentrations and the relatively stable abstinence rates with concentrations above 40 ng/mL, the utility of achieving PNCs consistent with depression therapy seems unnecessary. However, in order to offer definitive recommendations on a therapeutic range for nortriptyline in smoking cessation, the current findings require replication in a prospective design where smokers are randomized to specific ranges of PNC. Moreover, formal pharmacokinetic-pharmacodynamic modeling of plasma nortriptyline concentrations will be needed to obtain more accurate estimates of a smoking cessation specific therapeutic range.
Ultimately, the generation of smoking-cessation specific guidelines for therapeutic drug monitoring of nortriptyline is most important for the more than 1 billion smokers without access to other smoking cessation aids [50]. However, as Ozdemir and colleagues[51] recently noted with respect to off-patent medications, lower medication cost does not guarantee greater access and improved treatment outcomes. In the case of nortriptyline, the costs of TDM may limit access in some settings. The possibility of rapid genotyping of CYP2D6 could allow for genotype-specific dosing, eliminating or reducing the need for TDM. Nevertheless, compared to existing smoking cessation medications, nortriptyline is several times less expensive, requires only once-a-dosing, and has an excellent safety profile.
The current study has limitations. First, only total plasma nortriptyline concentrations were evaluated. Free nortriptyline concentrations might have shown a clearer dose response relationship to side effects and abstinence from smoking[52]. Second, we did not conduct phenotypic or genotypic tests of the CYP2D6 gene or enzyme. Third, although we had self-reported data on medication compliance, such data are prone to error and bias. More complete and accurate knowledge of differences in amount of medication taken would have been an important explanatory variable in our analyses, particularly to determine if those with undetectable or elevated medication concentrations were non-compliant or instead were ultra-rapid or poor metabolizers as a function of the CPY2D6 enzyme.
In conclusion, wide variability in plasma nortriptyline concentrations was seen in a pooled sample of cigarette smokers given a narrow range of doses. Therapeutic drug monitoring continues to be important in ensuring that patients receive clinically effective nortriptyline exposure, however, the effective range of PNCs appears lower for smokers than for those with depression. Consideration of racial status, particularly among Asian and African-Americans, may be important in initial dosing. Future research is needed to prospectively confirm the possible optimum therapeutic range of nortriptyline in smoking cessation.
Acknowledgments
This research was supported by National Institute of Drug Abuse grants R01 (DA 23652 DA 02538, DA 002538, DA 015732), and P50 (DA 09253). Support was also provided by the National Cancer Institute through R01 CA 71378. Dr. Mooney is supported by a NIDA Career Development award K01 DA 019446. Dr. Hall is supported through a NIDA Senior Scientist award K05 DA 016752. We thank the participants for taking part in these studies
References
- 1.Wagena EJ, Knipschild P, Zeegers MP. Should nortriptyline be used as a first-line aid to help smokers quit? Results from a systematic review and meta-analysis. Addiction. 2005 Mar;100(3):317–26. doi: 10.1111/j.1360-0443.2005.00998.x. [DOI] [PubMed] [Google Scholar]
- 2.Hughes JR, Stead LF, Lancaster T. Nortriptyline for smoking cessation: a review. Nicotine Tob Res. 2005 Aug;7(4):491–9. doi: 10.1080/14622200500185298. [DOI] [PubMed] [Google Scholar]
- 3.Alexanderson B. Pharmacokinetics of nortriptyline in man after single and multiple oral doses: the predictability of steady-state plasma concentrations from single-dose plasma-level data. Eur J Clin Pharmacol. 1972 Mar;4(2):82–91. doi: 10.1007/BF00562502. [DOI] [PubMed] [Google Scholar]
- 4.Kragh-Sorensen P, Hansen CE, Baastrup PC, Hvidberg EF. Self-inhibiting action of nortriptylin's antidepressive effect at high plasma levels: a randomized double-blind study controlled by plasma concentrations in patients with endogenous depression. Psychopharmacologia. 1976 Feb 2;45(3):305–12. doi: 10.1007/BF00421145. [DOI] [PubMed] [Google Scholar]
- 5.Hall SM, Humfleet GL, Reus VI, Munoz RF, Cullen J. Extended nortriptyline and psychological treatment for cigarette smoking. Am J Psychiatry. 2004 Nov;161(11):2100–7. doi: 10.1176/appi.ajp.161.11.2100. [DOI] [PubMed] [Google Scholar]
- 6.Hall SM, Humfleet GL, Reus VI, Munoz RF, Hartz DT, Maude-Griffin R. Psychological intervention and antidepressant treatment in smoking cessation. Arch Gen Psychiatry. 2002 Oct;59(10):930–6. doi: 10.1001/archpsyc.59.10.930. [DOI] [PubMed] [Google Scholar]
- 7.Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet G, Hartz DT, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998 Aug;55(8):683–90. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- 8.Prochazka AV, Kick S, Steinbrunn C, Miyoshi T, Fryer GE. A randomized trial of nortriptyline combined with transdermal nicotine for smoking cessation. Arch Intern Med. 2004 Nov 8;164(20):2229–33. doi: 10.1001/archinte.164.20.2229. [DOI] [PubMed] [Google Scholar]
- 9.Prochazka AV, Weaver MJ, Keller RT, Fryer GE, Licari PA, Lofaso D. A randomized trial of nortriptyline for smoking cessation. Arch Intern Med. 1998 Oct 12;158(18):2035–9. doi: 10.1001/archinte.158.18.2035. [DOI] [PubMed] [Google Scholar]
- 10.Razavi D, Mendlewicz J. Tricyclic antidepressant plasma levels: the state of the art and clinical prospects. Neuropsychobiology. 1982;8(2):73–85. doi: 10.1159/000117880. [DOI] [PubMed] [Google Scholar]
- 11.Risch SC, Kalin NH, Janowsky DS, Huey LY. Indications and guidelines for plasma tricyclic antidepressant concentration monitoring. J Clin Psychopharmacol. 1981 Mar;1(2):59–63. doi: 10.1097/00004714-198103000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986 Mar;43(3):289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 13.Tsoh JY, Humfleet GL, Munoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000 Mar;157(3):368–74. doi: 10.1176/appi.ajp.157.3.368. [DOI] [PubMed] [Google Scholar]
- 14.Asberg M, Cronholm B, Sjoqvist F, Tuck D. Relationship between plasma level and therapeutic effect of nortriptyline. Br Med J. 1971 Aug 7;3(770):331–4. doi: 10.1136/bmj.3.5770.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler VE, Knesevich JW, Wylie LT, Biggs JT. Sampling time, dosage schedule, and nortriptyline plasma levels. Arch Gen Psychiatry. 1977 May;34(5):613–5. doi: 10.1001/archpsyc.1977.01770170123013. [DOI] [PubMed] [Google Scholar]
- 16.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003 Feb;5(1):13–25. [PubMed] [Google Scholar]
- 17.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991 Sep;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 18.McNair D, Lorr M, Droppleman L. Manual for Profile of Mood States. San Diego, CA: Edits; 1981. [Google Scholar]
- 19.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 20.SAS Institute Inc. The SAS System for Windows. 9.13. Cary, NC: SAS Institute Inc.; 2005. [Google Scholar]
- 21.Jackson PH, Stapleton JA, Russell MA, Merriman RJ. Nicotine gum use and outcome in a general practitioner intervention against smoking. Addict Behav. 1989;14(3):335–41. doi: 10.1016/0306-4603(89)90064-6. [DOI] [PubMed] [Google Scholar]
- 22.Alexanderson B, Evans DA, Sjoqvist F. Steady-state plasma levels of nortriptyline in twins: influence of genetic factors and drug therapy. Br Med J. 1969 Dec 27;4(686):764–8. doi: 10.1136/bmj.4.5686.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertilsson L, Dahl ML, Tybring G. Pharmacogenetics of antidepressants: clinical aspects. Acta Psychiatr Scand Suppl. 1997;391:14–21. doi: 10.1111/j.1600-0447.1997.tb05954.x. [DOI] [PubMed] [Google Scholar]
- 24.Jerling M, Merle Y, Mentre F, Mallet A. Population pharmacokinetics of nortriptyline during monotherapy and during concomitant treatment with drugs that inhibit CYP2D6--an evaluation with the nonparametric maximum likelihood method. Br J Clin Pharmacol. 1994 Nov;38(5):453–62. doi: 10.1111/j.1365-2125.1994.tb04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin KM, Poland RE, Smith MW, Strickland TL, Mendoza R. Pharmacokinetic and other related factors affecting psychotropic responses in Asians. Psychopharmacol Bull. 1991;27(4):427–39. [PubMed] [Google Scholar]
- 26.Strickland TL, Ranganath V, Lin KM, Poland RE, Mendoza R, Smith MW. Psychopharmacologic considerations in the treatment of black American populations. Psychopharmacol Bull. 1991;27(4):441–8. [PubMed] [Google Scholar]
- 27.Kishimoto A, Hollister LE. Nortriptyline kinetics in Japanese and Americans. J Clin Psychopharmacol. 1984 Jun;4(3):171–2. doi: 10.1097/00004714-198406000-00036. [DOI] [PubMed] [Google Scholar]
- 28.Pi EH, Tran-Johnson TK, Walker NR, Cooper TB, Suckow RF, Gray GE. Pharmacokinetics of desipramine in Asian and Caucasian volunteers. Psychopharmacol Bull. 1989;25(3):483–7. [PubMed] [Google Scholar]
- 29.Rudorfer MV, Lane EA, Chang WH, Zhang MD, Potter WZ. Desipramine pharmacokinetics in Chinese and Caucasian volunteers. Br J Clin Pharmacol. 1984 Apr;17(4):433–40. doi: 10.1111/j.1365-2125.1984.tb02368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bathum L, Skjelbo E, Mutabingwa TK, Madsen H, Horder M, Brosen K. Phenotypes and genotypes for CYP2D6 and CYP2C19 in a black Tanzanian population. Br J Clin Pharmacol. 1999 Sep;48(3):395–401. doi: 10.1046/j.1365-2125.1999.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradford LD, Gaedigk A, Leeder JS. High frequency of CYP2D6 poor and “intermediate” metabolizers in black populations: a review and preliminary data. Psychopharmacol Bull. 1998;34(4):797–804. [PubMed] [Google Scholar]
- 32.Bradford LD, Kirlin WG. Polymorphism of CYP2D6 in Black populations: implications for psychopharmacology. Int J Neuropsychopharmcol. 1998 Dec;1(2):173–85. doi: 10.1017/S1461145798001187. [DOI] [PubMed] [Google Scholar]
- 33.Droll K, Bruce-Mensah K, Otton SV, Gaedigk A, Sellers EM, Tyndale RF. Comparison of three CYP2D6 probe substrates and genotype in Ghanaians, Chinese and Caucasians. Pharmacogenetics. 1998 Aug;8(4):325–33. doi: 10.1097/00008571-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Evans WE, Relling MV, Rahman A, McLeod HL, Scott EP, Lin JS. Genetic basis for a lower prevalence of deficient CYP2D6 oxidative drug metabolism phenotypes in black Americans. J Clin Invest. 1993 May;91(5):2150–4. doi: 10.1172/JCI116441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimoda K, Morita S, Hirokane G, Yokono A, Someya T, Takahashi S. Metabolism of desipramine in Japanese psychiatric patients: the impact of CYP2D6 genotype on the hydroxylation of desipramine. Pharmacol Toxicol. 2000 Jun;86(6):245–9. doi: 10.1111/j.0901-9928.2000.860601.x. [DOI] [PubMed] [Google Scholar]
- 36.Linnoila M, George L, Guthrie S, Leventhal B. Effect of alcohol consumption and cigarette smoking on antidepressant levels of depressed patients. Am J Psychiatry. 1981 Jun;138(6):841–2. doi: 10.1176/ajp.138.6.841. [DOI] [PubMed] [Google Scholar]
- 37.Perry PJ, Browne JL, Prince RA, Alexander B, Tsuang MT. Effects of smoking on nortriptyline plasma concentrations in depressed patients. Ther Drug Monit. 1986;8(3):279–84. doi: 10.1097/00007691-198609000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Norman TR, Burrows GD, Maguire KP, Rubinstein G, Scoggins BA, Davies B. Cigarette smoking and plasma nortriptyline levels. Clin Pharmacol Ther. 1977 Apr;21(4):453–6. doi: 10.1002/cpt1977214453. [DOI] [PubMed] [Google Scholar]
- 39.Olesen OV, Linnet K. Hydroxylation and demethylation of the tricyclic antidepressant nortriptyline by cDNA-expressed human cytochrome P-450 isozymes. Drug Metab Dispos. 1997 Jun;25(6):740–4. [PubMed] [Google Scholar]
- 40.Edwards RJ, Price RJ, Watts PS, Renwick AB, Tredger JM, Boobis AR, et al. Induction of cytochrome P450 enzymes in cultured precision-cut human liver slices. Drug Metab Dispos. 2003 Mar;31(3):282–8. doi: 10.1124/dmd.31.3.282. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Antona C, Jover R, Gomez-Lechon MJ, Castell JV. Quantitative RT-PCR measurement of human cytochrome P-450s: application to drug induction studies. Arch Biochem Biophys. 2000 Apr 1;376(1):109–16. doi: 10.1006/abbi.2000.1697. [DOI] [PubMed] [Google Scholar]
- 42.Schrenk D, Brockmeier D, Morike K, Bock KW, Eichelbaum M. A distribution study of CYP1A2 phenotypes among smokers and non-smokers in a cohort of healthy Caucasian volunteers. Eur J Clin Pharmacol. 1998 Jan;53(5):361–7. doi: 10.1007/s002280050394. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki T, Matsuo K, Sawaki A, Wakai K, Hirose K, Ito H, et al. Influence of smoking and CYP2C19 genotypes on H. pylori eradication success. Epidemiol Infect. 2007 Jan;135(1):171–6. doi: 10.1017/S0950268806006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackwell B. Antidepressant drugs: side effects and compliance. J Clin Psychiatry. 1982 Nov;43(11 Pt 2):14–21. [PubMed] [Google Scholar]
- 45.Wagena EJ, Knipschild PG, Huibers MJ, Wouters EF, van Schayck CP. Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Arch Intern Med. 2005 Oct 24;165(19):2286–92. doi: 10.1001/archinte.165.19.2286. [DOI] [PubMed] [Google Scholar]
- 46.da Costa CL, Younes RN, Lourenco MT. Stopping smoking: a prospective, randomized, double-blind study comparing nortriptyline to placebo. Chest. 2002 Aug;122(2):403–8. doi: 10.1378/chest.122.2.403. [DOI] [PubMed] [Google Scholar]
- 47.Asberg M, Cronholm B, Sjoqvist F, Tuck D. Correlation of subjective side effects with plasma concentrations of nortriptyline. Br Med J. 1970 Oct 3;4(726):18–21. doi: 10.1136/bmj.4.5726.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ziegler VE, Taylor JR, Wetzel RD, Biggs JT. Nortriptyline plasma levels and subjective side effects. British Journal of Psychiatry. 1978;132:53–60. [Google Scholar]
- 49.Rabkin JG, Markowitz JS. Side effect assessment with SAFTEE: pilot study of the instrument. Psychopharmacol Bull. 1986;22(2):389–96. [PubMed] [Google Scholar]
- 50.Ezzati M, Lopez AD. Estimates of global mortality attributable to smoking in 2000. Lancet. 2003 Sep 13;362(9387):847–52. doi: 10.1016/S0140-6736(03)14338-3. [DOI] [PubMed] [Google Scholar]
- 51.Ozdemir V, Aklillu E, Mee S, Bertilsson L, Albers LJ, Graham JE, et al. Pharmacogenetics for off-patent antipsychotics: reframing the risk for tardive dyskinesia and access to essential medicines. Expert Opin Pharmacother. 2006 Feb;7(2):119–33. doi: 10.1517/14656566.7.2.119. [DOI] [PubMed] [Google Scholar]
- 52.Perry PJ, Browne JL, Alexander B, Pfohl BM, Dunner FJ, Sherman AD, et al. Relationship of free nortriptyline levels to therapeutic response. Acta Psychiatr Scand. 1985 Aug;72(2):120–5. doi: 10.1111/j.1600-0447.1985.tb02582.x. [DOI] [PubMed] [Google Scholar]