Abstract
The prefrontal cortex projects to many thalamic nuclei, in pathways associated with cognition, emotion, and action. We investigated how multiple projection systems to the thalamus are organized in prefrontal cortex after injection of distinct retrograde tracers in the principal mediodorsal (MD), the limbic anterior medial (AM), and the motor-related ventral anterior/ventral lateral (VA/VL) thalamic nuclei in rhesus monkeys. Neurons projecting to these nuclei were organized in interdigitated modules extending vertically within layers VI and V. Projection neurons were also organized in layers. The majority of projection neurons to MD or AM originated in layer VI (~80%), but a significant proportion (~20%) originated in layer V. In contrast, prefrontal neurons projecting to VA/VL were equally distributed in layers V and VI. Neurons directed to VA/VL occupied mostly the upper part of layer V, while neurons directed to MD or AM occupied mostly the deep part of layer V. The highest proportions of projection neurons in layer V to each nucleus were found in dorsal and medial prefrontal areas. The laminar organization of prefrontal cortico-thalamic projections differs from sensory systems, where projections originate predominantly or entirely from layer VI. Previous studies indicate that layer V cortico-thalamic neurons innervate through some large terminals thalamic neurons that project widely to superficial cortical layers. The large population of prefrontal projection neurons in layer V may drive thalamic neurons, triggering synchronization by recruiting several cortical areas through widespread thalamo-cortical projections to layer I. These pathways may underlie the synthesis of cognition, emotion and action.
Keywords: Macaca mulatta, mediodorsal thalamic nucleus, anterior thalamic nuclei, layer V pyramidal neurons, cortico-thalamic pathway
Introduction
The prefrontal cortex in macaque monkeys has lateral, orbitofrontal and medial sectors that have complementary roles in behavior. The lateral prefrontal sector has a key role in working memory, and the orbitofrontal and medial sectors are associated with distinct aspects of emotional processes [reviewed in (Goldman-Rakic, 1988; Fuster, 1989; Petrides, 1994; Barbas, 2000)]. The entire prefrontal cortex is connected with several thalamic nuclei including the mediodorsal, the anterior medial, the ventral anterior, the medial pulvinar, midline and intralaminar nuclei [e.g. (Kievit and Kuypers, 1977; Goldman-Rakic and Porrino, 1985; Preuss and Goldman-Rakic, 1987b; Barbas et al., 1991; Morecraft et al., 1992; Dermon and Barbas, 1994; Bachevalier et al., 1997; Cavada et al., 2000; Xiao and Barbas, 2002a; Xiao and Barbas, 2002b; Xiao and Barbas, 2004; Zikopoulos and Barbas, 2007)]. However, the organization of multiple projections from prefrontal areas to several thalamic nuclei is not well understood. We sought to address this issue by investigating prefrontal cortical projections to its principal thalamic mediodorsal (MD) nucleus, the limbic anterior medial (AM) nucleus, and the motor related ventral anterior (VA) nucleus in the same animals. These nuclei are robustly connected with prefrontal cortices, and have some related and complementary functions [reviewed in (Jones, 2007)]. The MD and AM receive projections from the amygdala, which is associated with emotional processing. The AM nucleus receives robust projections from the hippocampal formation associated with memory, and the VA and MD have a key role in motor and cognitive functions through connections with the basal ganglia and prefrontal cortex (Alexander et al., 1986; Armstrong, 1990; Groenewegen et al., 1990; Mitchell et al., 1999; Middleton and Strick, 2000; Graybiel, 2000; Eichenbaum, 2000; Haber and McFarland, 2001; Xiao and Barbas, 2002b; Xiao and Barbas, 2004).
Previous studies have shown that the majority of projection neurons to the thalamic MD or AM nuclei originate from layer VI (Giguere and Goldman-Rakic, 1988; Yeterian and Pandya, 1994; Xiao and Barbas, 2002b). Layer VI gives rise to the large majority of all cortico-thalamic projections [reviewed in (Steriade et al., 1997; Jones, 2007)]. However, a significant proportion of prefrontal neurons projecting to the thalamus originate in layer V, constituting about 20% of neurons projecting to AM or MD, and making up nearly half of projection neurons directed to the VA (Xiao and Barbas, 2002b; Xiao and Barbas, 2004; Zikopoulos and Barbas, 2007). Cortical pathways emanating from layers VI and V are thought to innervate distinct classes of thalamo-cortical neurons [reviewed in (Guillery, 1995; Rouiller and Welker, 2000)]. In the reverse pathway, thalamic neurons innervated by cortical layer VI project focally to the middle cortical layers. In contrast, thalamic neurons innervated by cortical layer V project widely to the superficial cortical layers, and likely participate in high-order association processes [reviewed in (Jones, 1998a; Rouiller and Welker, 2000; Jones, 2003)]. Reciprocal pathways linking the cortex with the thalamus are thought to have distinct functions and are affected in a variety of neurologic and psychiatric diseases (Scheibel, 1997; Aggleton and Brown, 1999; Llinas et al., 1999; Van Der Werf et al., 2003; Llinas and Steriade, 2006).
Here we investigated the organization of prefrontal projection neurons directed to its principal MD nucleus, the limbic AM nucleus, and the motor related VA nucleus. We provide evidence that multiple prefrontal projection systems to distinct thalamic nuclei have a dual organization into modules and layers. These prefrontal pathways may underlie the functional specialization of thalamo-cortical interactions in recruiting cortical and other thalamic structures associated with cognition, emotion and action.
Experimental procedures
Surgical procedures
Experiments were conducted on five young adult rhesus monkeys (Macaca mulatta) of both sexes under sterile procedure, according to the NIH guide for the Care and Use of Laboratory Animals (DHEW Publication no. [NIH] 80-22, revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD). Procedures involving animals were designed to minimize animal suffering.
To inject tracers in the VA, AM and MD nuclei it was necessary to first obtain a map of the thalamus using magnetic resonance imaging (MRI). We used the interaural line as a reference by filling hollow ear bars of the stereotaxic apparatus with betadine salve (containing polymyxin B sulfate, bacitracin zinc and pramoxine HCl, Purdue Frederick Company, Norwalk, CT), which is visible in MRI. Brain scans were obtained from monkeys anesthetized with a mixture of ketamine hydrochloride (10 mg/kg, intramuscularly) followed by sodium pentobarbital, administered intravenously through a femoral catheter (to effect). The stereotaxic coordinates for the VA, AM and MD were calculated in three dimensions using the interaural line as reference.
One week after MRI surgery was performed to inject neural tracers. Monkeys were anesthetized first with ketamine hydrochloride (10 mg/kg, intramuscularly), intubated, and then anesthetized with gas anesthetic (isoflurane) until a surgical level of anesthesia was achieved. Overall physiological condition was monitored, including heart rate and temperature. A small hole was made above the injection site for penetration of the injection needle. Small amounts of fluorescent retrograde tracers were delivered to the thalamic nuclei (Table 1). The types and amount of tracers injected were as follows: diamidino yellow (which fluoresces yellow and labels only the nucleus; Sigma; 3% solution, volume of 0.3–1.6 μl); fast blue (fluoresces blue and labels the soma and proximal dendrites; Sigma, St. Louis, MO; 1% solution, volume of 0.8 –2 μl); fluoro-emerald (fluoresces green and labels the soma and proximal dendrites; Molecular Probes; 10% solution, volume of 3–4 μl); and fluoro-ruby (fluoresces red and labels the soma and proximal dendrites; dextran tetramethylrhodamine, Molecular Probes; 10% solution, volume of 3–4 μl).
Table 1.
Injection sites, cases and tracer types in thalamic VA, MD and AM nuclei
| Cases | case BD (male) | case BE (male) | case BG (female) | case BB (female) | case AZ (male) |
|---|---|---|---|---|---|
| Injection sites | |||||
| VA or VA/VL | FE (L) | FR (L) | FR (R) | ||
| MD | FB (L) | DY (L) | FB (R) | FE (L) | FR (L) |
| AM | FR (L) | DY (L) | DY (L) |
Abbreviations: FB, fast blue; FE, fluoro-emerald; FR, fluoro-ruby; DY, diamidino yellow. L, left side; R, right side.
After injection of tracers the wound was closed in anatomic layers and the skin sutured. At the completion of surgical procedures the animals were monitored until recovery from anesthesia, and they were given antibiotics and analgesic (Buprenex, intramuscularly) every 12 hours, or as needed.
Perfusion and tissue processing
Animals were given an overdose of anesthetic (sodium pentobarbital, >50 mg/kg, to effect) and perfused through the heart with a fixative (4% paraformaldehyde in 0.1 M sodium phosphate buffer, PB, pH 7.4) 18 days after injection of tracers. The brain was then removed from the skull, photographed and transferred through a graded series of sucrose solutions for cryoprotection (10%, 15%, 20%, 25% and 30% in 0.1 M PB with 0.05% azide). After cryoprotection the brain was frozen in −75°C isopentane for 2 hrs, and cut on a freezing microtome coronally at 50 μm in ten matched series. Adjacent sections in each series were thus separated by 500 μm. Two matched series of sections were mounted, dried under darkness, and stored at 4°C. One series was used to map labeled neurons in prefrontal cortices and the other was coverslipped with Krystalon (EM Science) for long-term storage and photography.
Data analysis
Mapping labeled neurons in areas and layers
After injection of retrograde tracers in MD, VA/VL and AM, we mapped all labeled neurons in prefrontal cortices in one series of sections, using unbiased, uniform random sampling (1 in 10 sections), and exhaustive sampling within the entire prefrontal cortex. Section outlines and the location of labeled neurons in coronal sections through the ipsilateral prefrontal cortex were viewed with a microscope under fluorescence illumination (Nikon, Optiphot). Maps of projection neurons were plotted on paper using a digital plotter (Hewlett Packard, 7475A), which is electronically coupled to the stage of the microscope and to a PC computer (all cases, except case BG). Movement of the stage was recorded through linear potentiometers (Vernitech, Axsys, San Diego, CA) mounted on the X and Y axes of the microscope stage. Analog signals were converted to digital signals via an analog-to-digital converter (Data Translation, Marlboro, MA). Software designed in our laboratory ensured that each neuron was counted only once, as described previously (Barbas and De Olmos, 1990). In case BG, tissue sections were viewed with a microscope under fluorescence illumination (Olympus Optical BX60, Thornwood, NY) and plotted using a commercial system (Neurolucida, Microbrightfield, Williston, VT). After plotting, sections were counterstained with thionin and returned to the microscope to delineate layers, and count labeled neurons by layer in individual prefrontal areas. The neuron profile counts estimated by this exhaustive plotting were used to calculate density and laminar proportions of prefrontal projection neurons within and between different prefrontal areas.
Measurement of modules of labeled neurons through layers VI and V
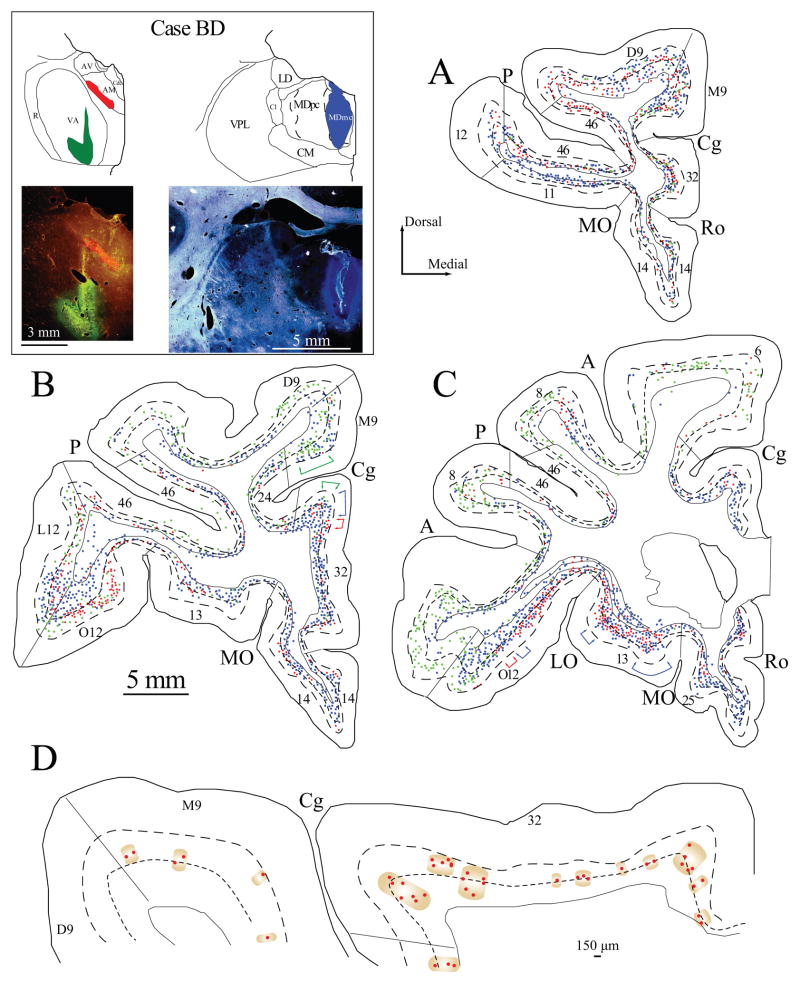
We also estimated the width of columnar modules of neurons projecting to each of the thalamic nuclei throughout the prefrontal cortex, using exhaustive sampling in one series (1 in 10 sections) in every case. For each injection site, the modules consisted of clusters of projection neurons that extended vertically within layers VI and V. We operationally defined modules as clusters of neurons separated by a distance of at least 150 μm, as illustrated in Figure 1D. Adjacent modules of labeled neurons directed to two or three thalamic nuclei overlapped especially at the fringes, as shown in Figure 1.
Figure 1.
Projection neurons in prefrontal cortices directed to the VA, AM and MD nuclei. Inset (top left), shows maps and photomicrographs of the injection sites in VA, AM and MDmc in two coronal sections through the thalamus (colored areas, case BD). A–C, Distribution of projection neurons in a series of coronal sections in rostral (A) to caudal (C) prefrontal cortices after injection of fluoro-emerald in the VA (green), fluoro-ruby in the AM (red) and fast blue in MDmc (blue). Each colored dot represents one neuron. Colored brackets in (B–C) show examples of the modular distribution of prefrontal projection neurons to AM (red), VA (green) and MD (blue). D, Neurons projecting to AM (enlarged from B) are shown separately to illustrate the clustering of neurons into modules of variable widths, separated by at least 150 μm. Dotted lines in A–D show the top of cortical layers V and VI.
Three-Dimensional reconstruction of labeling
We compared the topography and distribution of retrogradely labeled neurons across prefrontal areas and cases, by reconstructing in three dimensions a reference rhesus monkey brain using the free, open source software Reconstruct (Fiala, 2005). We then imported the traced outlines of prefrontal areas, containing three-dimensional information about the quantitative topography of labeling in Reconstruct, aligned and registered them with corresponding levels of the reference brain, and generated three-dimensional models as described (Medalla and Barbas, 2006; Zikopoulos and Barbas, 2006; Zikopoulos and Barbas, 2007; Barbas and Zikopoulos, 2007; Fiala et al., 2007). As a result, all markers and traces were stereotaxically registered and superimposed on the three-dimensional model.
Photography
Photographs of labeled neurons in prefrontal cortices were captured with a CCD camera using a software system (Neurolucida, Microbrightfield, Williston, VT). Images were transferred into Adobe Photoshop (Adobe Systems Inc., San Jose, CA) for arrangement and adjusting of contrast, but were not retouched. Images of sections with multiple labeling were captured sequentially for each label using the appropriate filter for each dye, and the images were merged.
Statistical analysis
The percentage of projection neurons by layer was calculated by dividing the number of projection neurons in layer V or layer VI by the number of projection neurons in both layers for each prefrontal area. We employed linear correlation coefficient Pearson ρ to test the relationship in the paired percentage of layer V projection neurons for each prefrontal area directed to MD and VA/VL, or directed to MD and AM.
Results
Overview of prefrontal cortex
The prefrontal cortex in rhesus monkeys is large and architectonically heterogeneous [reviewed in (Barbas, 2000; Barbas et al., 2002)]. It has lateral, basal (orbital) and medial sectors, and each sector has several architectonic areas, listed briefly below to describe their location. References to architectonic areas are according to the map of Barbas and Pandya (Barbas and Pandya, 1989), modified from the map of Walker (Walker, 1940).
On the lateral surface area 10 encompasses the entire frontal pole, and is adjoined dorsally by area 9 (D9), ventrally by lateral area 12 (L12), and centrally by area 46. The latter is located within the dorsal bank of the principal sulcus and the adjoining cortex above (D46), and within the ventral bank of the principal sulcus and the adjoining cortex below (V46). Area 46 is bordered by area D9 above and area L12 below, and caudally by area 8, which is parcellated into dorsal (D8) and ventral (V8) subdivisions, situated respectively within the rostral bank of the upper and lower limbs of the arcuate sulcus and the adjacent lateral cortex.
On the basal surface, the prefrontal cortex includes orbital area 12 (O12), which extends from the inferior prefrontal convexity to the orbital surface. Area O12 is bordered by area 13 in the central part of the orbital surface. Area 13 shares a border with area 11 anteriorly, and with areas OPro and area OPAll posteriorly, which are the most caudal orbitofrontal areas [for review see (Barbas and Zikopoulos, 2006; Barbas, 2007)]. On the medial part of the orbital surface area 13 abuts area 14 rostrally, which has orbital (O14) and medial (M14) subdivisions. Orbital area 25 (O25) is situated behind area O14 on the basal surface and extends on the medial surface below the corpus callosum (M25). This is the most caudal extent of the medial prefrontal cortex, and is bordered above by the subcallosal part of area 24. The latter skirts the rostral part of the corpus callosum, and shares a border with area 32 anteriorly. Area 32 is situated below medial area 9 (M9), above area M14 and behind area 10.
The entire prefrontal cortex has bidirectional connections with MD, its principal thalamic nucleus, but also with a large number of other thalamic nuclei, including the VA and AM, whose connections are more restricted within the prefrontal cortex, as described below. The nomenclature of the thalamus is according to the map of Jones (Jones, 1985) as modified from the map of Olszewski (Olszewski, 1952).
Injection sites in thalamic nuclei
Data on projection neurons to the above prefrontal cortices were obtained from five animals after injection of distinct retrograde tracers in the VA, MD and AM nuclei, totaling 11 injection sites (Table 1). The tracers were largely restricted to the intended nuclei, with some spread to neighboring nuclei or adjacent tracts in some cases, as elaborated below. None of the affected tracts are connected with the prefrontal cortex. In addition, there was no leakage of tracer in overlying cortical areas in any case, confirmed by the absence of labeled neurons in layers II and III, contrasted by densely distributed labeled neurons in layers V and VI, which project to the thalamus. There was no evidence of spread of the dyes to other subcortical structures. Spread of the tracer within the thalamus was observed in case AZ (injection intended for AM spread to MD) and the injections in VA in cases BE and BG, which spread to VL, another motor-related thalamic nucleus. The VL has a similar pattern of projection as the VA (Kievit and Kuypers, 1977), but its connections with prefrontal cortices are generally sparser and found mostly in VLm (Barbas et al., 1991; Dermon and Barbas, 1994). We conservatively refer to injection sites in VA as VA/VL, unless the VL was not involved. The distribution of labeled projection neurons in the prefrontal cortex after injections in VA/VL, MD, or AM was similar and their relative numbers within layers were comparable among cases.
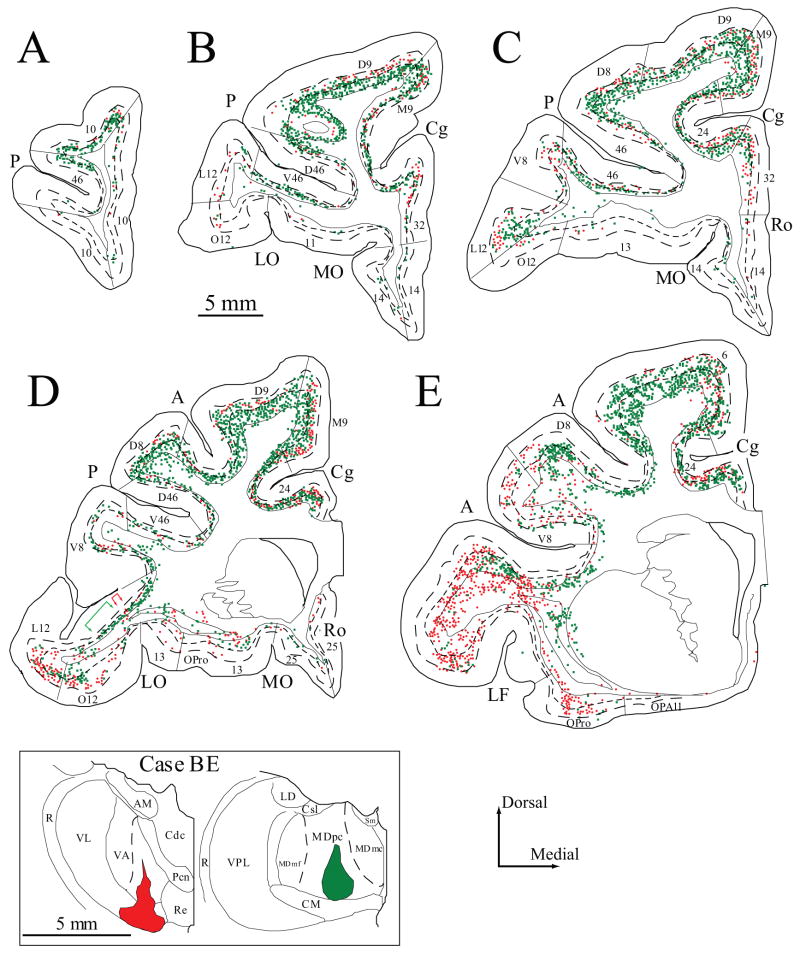
In case BD, three distinct tracers were injected (Fig. 1, inset). The core of the fluoro-emerald injection was in the magnocellular part of VA (VAmc) and the ventral part of its parvicellular part (VApc). The halo of the injection was in the ventral part of the neighboring motor-related ventral lateral nucleus, pars oralis (VLo). In previous studies we found no evidence of connections between the VLo and prefrontal cortices (Barbas and Mesulam, 1981; Barbas et al., 1991; Dermon and Barbas, 1994). Injection of a second tracer, fast blue, was in the caudal part of the magnocellular portion of MD (MDmc) and stria medullaris (Sm). The injection of a third tracer, fluoro-ruby, was restricted to the ventral edge of the AM nucleus. The halo of the injection impinged on the dorsal part of the mammillothalamic tract, and the needle tract passed through the ventral part of the fornix.
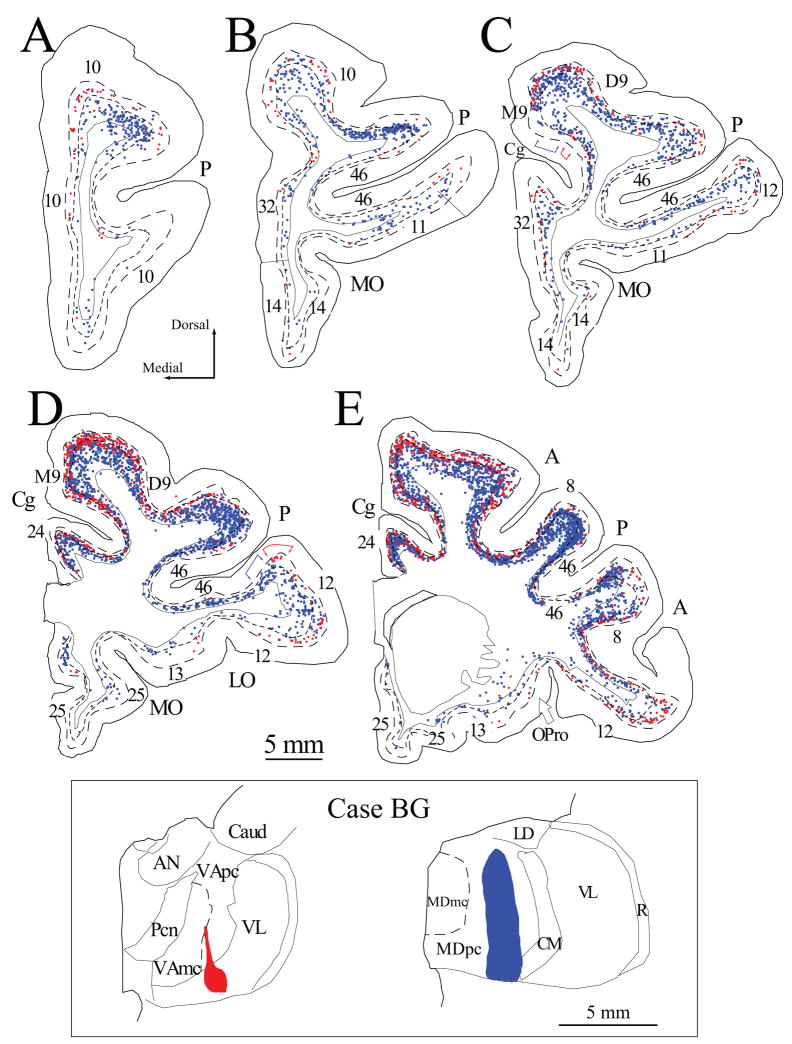
In two cases, two distinct tracers were injected in VA and MD. In case BE, the injection of fluoro-ruby covered the caudal half of VAmc and the ventral part of VLo (Fig. 2, inset). The dye impinged on the medial part of the ventral lateral nucleus, pars medialis (VLm) and the ventral part of the posterior ventral lateral nucleus (VLp). Injection of fluoro-emerald was in the posterior two thirds of the ventral part of MDpc (Fig. 2, inset). In case BG, the injection of fluoro-ruby covered the caudal fourth of VApc and the central part of VL (Fig. 3, inset, left). In the same animal the fast blue injection site included the central part of MDpc throughout its dorso-ventral extent (Fig. 3, inset, right).
Figure 2.
Projection neurons in prefrontal cortices directed to the VA/VL and MD thalamic nuclei. A–E, Distribution of projection neurons in a series of coronal sections in rostral (A) to caudal (E) prefrontal cortices after injection of fluoro-ruby in the VA/VL (red) and diamidino yellow in MDpc (green). Colored brackets in (D) show examples of the modular distribution of prefrontal projection neurons to VA/VL (red), and MD (green). Dotted lines in A–E show the top of cortical layers V and VI. Inset, Injection sites in VA/VL and MDpc shown in coronal sections through the thalamus (colored areas, case BE).
Figure 3.
Projection neurons in prefrontal cortices directed to the VA/VL and MD nuclei. A–E, Distribution of projection neurons in a series of coronal sections in rostral (A) to caudal (E) prefrontal cortices after injection of fluoro-ruby in the VA/VL (red) and fast blue in MDpc (blue). Colored brackets in (C, D) show examples of the modular distribution of prefrontal projection neurons to VA/VL (red), and MD (blue). Dotted lines in A–E show the top of cortical layers V and VI. Inset, Injection sites in VA/VL and MDpc shown in coronal sections through the thalamus (colored areas, case BG).
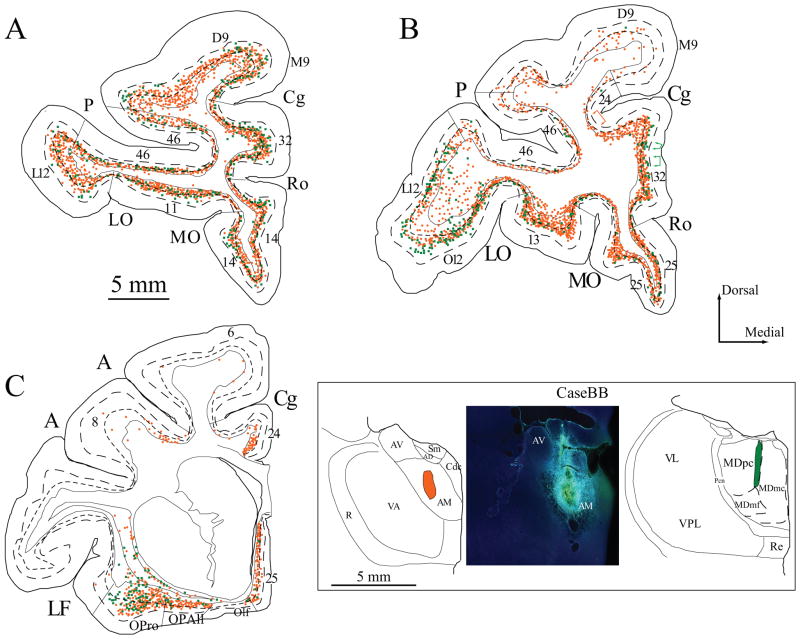
In two other cases, two distinct tracers covered parts of AM and MD. In case BB the core of the injection of diamidino yellow was restricted to the central portion of the AM nucleus (Fig. 4, inset, left and center). Injection of fluoro-emerald was in the rostral half of MDmc at the border of MDmc and MDpc (Fig. 4, inset, right). In case AZ (not shown) the core of the injection was in caudal AM and spread to a small portion of the anterior part of MD, the anterior ventral nucleus (AV), the central denso cellular (Cdc), and central superior lateral (Csl) nuclei, and the stria medullaris. Injection of fluoro-ruby was restricted to the caudal part of MDmc.
Figure 4.
Projection neurons in prefrontal cortices directed to the AM and MD thalamic nuclei. A–C, Distribution of projection neurons in a series of coronal sections in rostral (A) to caudal (C) prefrontal cortices after injection of diamidino yellow in the AM (orange) and fluoro-emerald in MD (green). Colored brackets in (B) show examples of the modular distribution of prefrontal projection neurons to AM (orange), and MD (green). Dotted lines in A–C show the top of cortical layers V and VI. Inset, Injection sites in AM (left and center) and MD (right) in coronal sections through the thalamus (colored areas, case BB).
Overview of prefrontal cortico-thalamic projections
Injections in thalamic nuclei labeled numerous projection neurons in prefrontal cortices, whose relative distribution within areas varied, consistent with the location of the injection site and the topographic nature of cortico-thalamic projections [reviewed in (Jones, 2007)]. The overall number of labeled neurons also varied, which likely is related to the size of the injection site. Detailed accounts of normalized densities of projection neurons in prefrontal cortices directed to AM and VA were reported in previous studies (Xiao and Barbas, 2002b; Xiao and Barbas, 2004). Here we provide examples of the density of projection neurons in prefrontal cortices for one case where all three thalamic nuclei were injected with distinct tracers (case BD). In this case with a large injection of fast blue in MDmc the highest density of projection neurons was found in medial prefrontal (area 25, n=818/mm3; area 32, n=803/mm3; area 24, n=321/mm3; M14, n=240/mm3; M9, n=225/mm3) and orbitofrontal areas (area OPro, n=492/mm3; area 11, n=397/mm3; area 13, n=321/mm3; O12, n=259/mm3; O14, n=250/mm3), and the lowest density was found in lateral areas (D9, n=150/mm3; D46, n=188/mm3; L12, n=135/mm3; area 10, n=150/mm3; V46, n=120/mm3; V8, n=59/mm3; D8, n=26/mm3). These findings are consistent with the predominant connections of medial and orbitofrontal cortices with MDmc and lateral areas with MDpc (Goldman-Rakic and Porrino, 1985; Siwek and Pandya, 1991; Barbas et al., 1991; Dermon and Barbas, 1994). In the same case, a small injection of fluoro-ruby in the thalamic AM nucleus labeled neurons in medial (area 32, n=195/mm3; area 24, n=108/mm3 M9, n=88/mm3; M14, n=69/mm3; area 25, n=43/mm3), orbitofrontal (O12, n=113/mm3; area 13, n=98/mm3; area 11, n=83/mm3; O14, n=82/mm3; area OPro, n=25/mm3), and lateral areas (area 10, n=105/mm3; L12, n=88/mm3; D46, n=114/mm3; V46, n=145/mm3; D9, n=52/mm3; D8, n=18/mm3; V8, n=18/mm3). In the same case an injection of fluoro-emerald in VA labeled neurons in medial areas (M9, n=84/mm3; area 32, n=58/mm3; area 24, n=29/mm3), and lateral areas (V8, n=86/mm3; D8, n=65/mm3; L12, n=83/mm3; D9, n=62/mm3; area 10, n=28/mm3; v46, n=24/mm3; D46, n=19/mm3). In orbitofrontal areas the highest density of projection neurons was noted in area O12 (n=41/mm3), and the density was lower in other areas (OPro, n=6/mm3; area 13, n=7/mm3).
Laminar organization of prefrontal cortico-thalamic projections
Projection neurons directed to the motor related nuclei VA/VL, overlapped with those projecting to MD, especially in dorso-medial and lateral prefrontal areas (e.g., Figs. 1, 3). Projection neurons to AM and MD overlapped especially in medial and orbitofrontal cortices (e.g., Fig. 1, 4). Supplementary Figure 1 shows the regional and laminar distribution of labeled neurons projecting to all three thalamic nuclei on a reconstructed brain (case BD).
We first determined the laminar distribution of cortico-thalamic neurons in prefrontal cortices that projected to each of the three thalamic nuclei for all cases, and the results are shown in Figure 5. This analysis included data from all injection sites and for all prefrontal areas, except for a few areas that did not have labeled neurons in a sufficient number of cases to conduct statistical analysis (e.g., areas 11 and 14 after injections in VA/VL; or area 11 after injection in MDpc). The prefrontal projection to area 11 is consistent with its predominant connections with MDmc (Barbas et al., 1991; Dermon and Barbas, 1994), as seen here for one case (case BD).
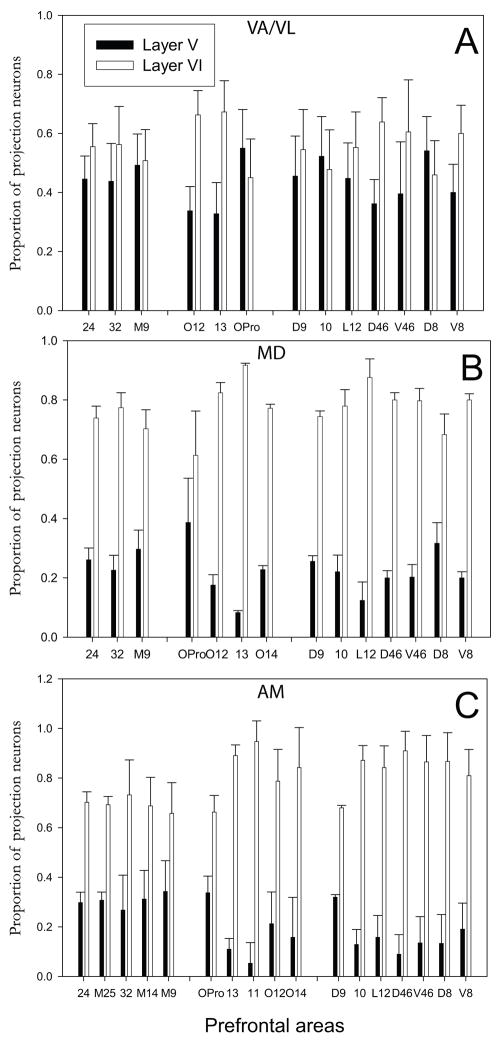
Figure 5.
Laminar origin of projection neurons in prefrontal cortices directed to VA (or VA/VL), MD, and AM nuclei. A, Proportion of projection neurons in layer V and layer VI directed to VA (or VA/VL). B, Proportion of projection neurons in layer V and layer VI directed to MD. C, Proportion of projection neurons in layer V and layer VI directed to AM. Vertical lines on bars show standard errors. Prefrontal areas are grouped based on their topography: Medial areas, left; Orbital areas, middle; Dorsolateral areas, right.
Most projection neurons in prefrontal cortices were found in layer VI. However, we found significant proportions of projection neurons in layer V that were directed to each of these nuclei as well (Fig. 5). About half of the projection neurons in prefrontal cortices directed to VA/VL originated from layer V (44%) and the rest (56%) were found in layer VI (Fig. 5A). About one fifth of the projection neurons directed to MD or AM originated from layer V and the majority (~80%) originated from layer VI (Fig. 5B, C).
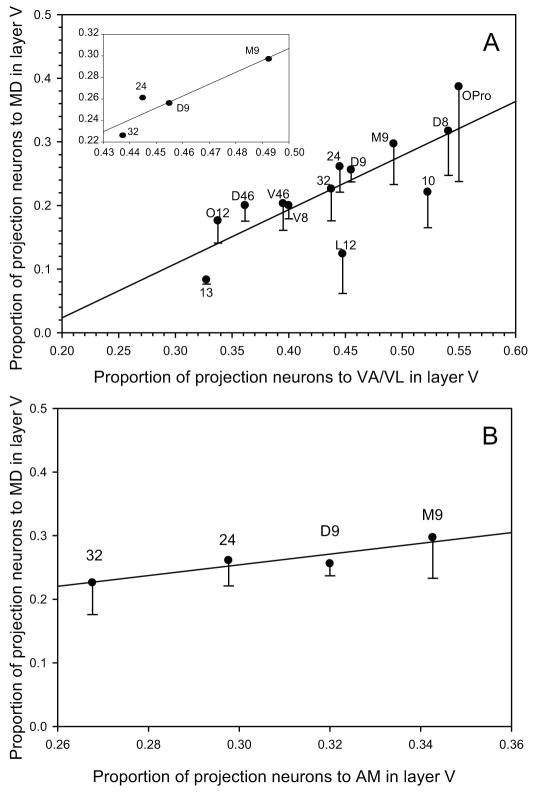
Moreover, the prevalence of projection neurons in layer V varied regionally. There was a higher proportion of projection neurons directed to AM in dorsal and medial prefrontal areas 24, 25, 32, 14 and 9 (about 30%) than in lateral and orbitofrontal areas (5–20%, area OPro was an exception). This regional bias was also seen for projections to MD and VA/VL, confirmed by a significant correlation (Pearson ρ=0.78, p<0.01, Fig. 6A), with dorsal and medial prefrontal areas (9, 32 and 24) showing consistently higher percentages of projection neurons in layer V (Pearson ρ=0.86, p<0.01, Fig. 6A and inset). The relative proportions of projection neurons from layer V to AM and MD also showed a high correlation for dorso-medial areas 9, 24 and 32 (Pearson ρ=0.86, p<0.01, Fig. 6B).
Figure 6.
Relationship of the proportions of projection neurons in layer V in prefrontal cortices directed to pairs of thalamic nuclei. A, Correlation of the proportion of layer V projection neurons in prefrontal areas directed to VA/VL and MD (Pearson ρ=0.78); the correlation was higher for dorso-medial prefrontal areas considered separately (Pearson ρ=0.86, inset). B, Correlation of the proportion of layer V projection neurons in prefrontal areas directed to AM and MD in dorso-medial prefrontal areas (Pearson ρ=0.86). Vertical lines show standard error.
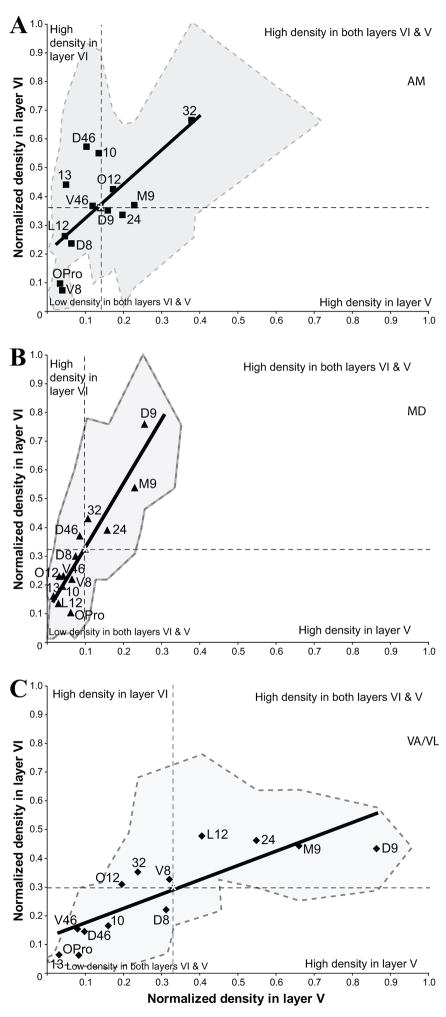
We then compared the relative proportion of neurons in layers V and VI for each injection site within each prefrontal area, which revealed quantitative differences in the laminar distribution of projection neurons directed to AM, MD, and VA/VL (Fig. 7). As shown in Figure 7, MD was targeted heavily by layer VI neurons (steeper slope; Fig. 7B), VA/VL was targeted equally by both layers V and VI (smallest slope; Fig. 7C), and AM was targeted mainly by layer VI but also a significant number of layer V neurons (intermediate slope; Fig. 7A).
Figure 7.
Laminar comparison of the relative density of prefrontal neurons projecting to three thalamic nuclei: (A), AM; (B), MD; (C), VA/VL. Asterisks in each scatter plot indicate the position of the average relative density of projection neurons in layers V and VI for all prefrontal areas, separating the plot area in quadrants. Prefrontal areas that cluster in the top left or bottom right quadrant included a higher than average density of projection neurons in layer VI or V, respectively. Prefrontal areas that cluster in the top right or bottom left quadrant included respectively a higher or a lower than average density of projection neurons in both layers VI and V. The slope of the fit (solid lines) shows that MD was targeted heavily by layer VI neurons (steeper slope), VA was targeted equally by both layers V and VI (smallest slope), and AM was targeted mainly by layer VI but also by a significant number of layer V neurons (intermediate slope). The shaded areas in each plot indicate the variation in the density of projection neurons in layer V (X-axis) and layer VI (Y-axis) for each prefrontal area.
Sublaminar distribution of prefrontal cortico-thalamic projection neurons in layer V
We found that prefrontal neurons that projected to the three thalamic nuclei showed a spatial bias within layer V. The significant overlap of prefrontal cortico-thalamic neurons projecting to different thalamic nuclei made it possible to directly compare their sublaminar distribution within layer V. As shown in Figures 1–4 and summarized on a reconstructed brain in Supplementary Figure 1, the projection systems to the three thalamic nuclei overlapped, especially in lateral areas 9, 10, 12, 46 and 8, and in medial areas 24, 32, 14 and 9. In these areas, projection neurons in layer V directed to VA/VL were found mostly superficial to projection neurons directed to MD, as shown in photomicrographs in Figure 8, and in the maps of coronal sections through the prefrontal cortex (Figs. 1–3). Similarly, projection neurons directed to VA/VL were found above neurons projecting to AM (Fig. 1A–C). In general, projection neurons directed to AM and MD were found at similar laminar depths, with the vast majority found in the deep parts of layer V, close to layer VI (Fig. 4). There was no apparent sublaminar organization of labeled neurons in layer VI.
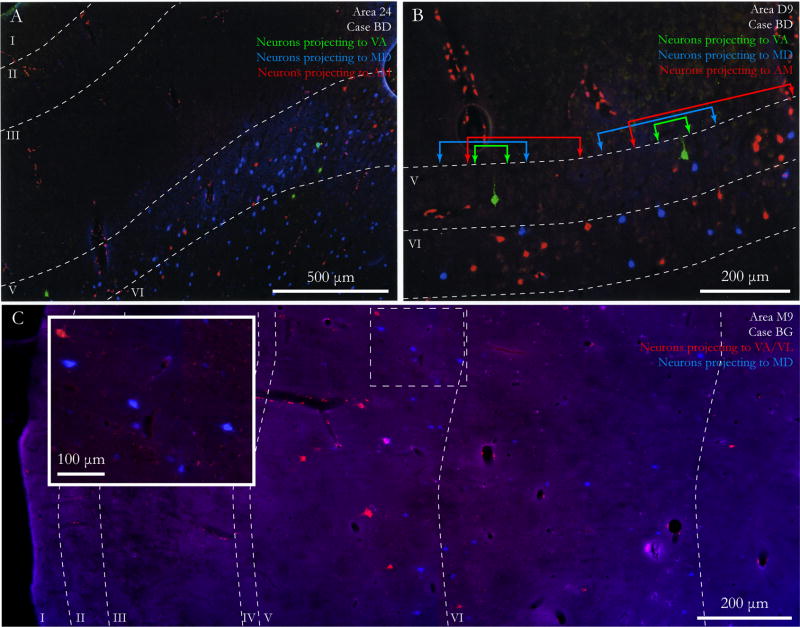
Figure 8.
Modular and laminar organization of prefrontal cortico-thalamic projection neurons. A, Low power photomicrograph of all layers of area 24 from pia to white matter (case BD) shows labeled prefrontal neurons in layers V and VI projecting to VA (green), AM (red), and MD (blue). B, Laminar and modular organization of projection neurons in area D9 (case BD). Modules of neurons projecting to each thalamic nucleus are separated by a minimum distance of 150 μm and are delineated by colored lines with arrowheads. C, Photomontage of area M9 (case BG) shows the laminar distribution of prefrontal neurons projecting to VA/VL (red) and MD (blue). Layer V neurons projecting to VA/VL (red) are found mainly in the upper part, while neurons projecting to MD (blue) are found deeper within layer V. Inset, Higher magnification of delineated site at the bottom of layer V.
Modular organization of prefrontal cortico-thalamic projection neurons
The above evidence shows a distinct laminar and sublaminar distribution of projection neurons within prefrontal areas directed to the three thalamic nuclei. In addition, within each prefrontal area the three projection systems showed a modular organization, which was apparent throughout the prefrontal cortex. Cortico-thalamic projections to VA/VL and MD were organized in interdigitated modules perpendicular to the pial surface, extending through layers VI and V. Figure 1D illustrates at a higher magnification the modules for a single projection (red labeled neurons projecting to AM) in area 32 (from 1B). The modules are seen in maps of projection neurons on diagrams of coronal sections through the prefrontal cortex (colored brackets in Fig. 1B; Fig. 2D show examples in areas 32, M9 and L12; Fig. 3C–D plots show examples in area M9 and area 46). Photomicrographs in Figure 8A, B show examples of modules of labeled neurons in areas 24 and dorsal 9.
The modular organization was also evident for projection neurons directed to AM and MD, which were found at the same laminar depth (Figs. 1; 4; 8). For example, fast blue labeled neurons to MD were found in modules next to clusters of fluoro-ruby labeled neurons projecting to AM (colored brackets in Fig. 1B, C show examples in areas 32 and O12). Similarly, fluoro-emerald labeled neurons directed to MD were found in large numbers in orbital and medial prefrontal areas, where they were interdigitated with diamidino yellow labeled neurons directed to AM (colored brackets in Fig. 4B show examples from area 32).
Module sizes of projection neurons in each prefrontal area targeting MD, AM, and VA/VL varied. Modules targeting VA/VL had generally smaller width, with the exception of modules in area 12. The average width of modules was: MD, 660 (mean ± 30 μm (SEM); range, 160 – 2020 μm); AM, 560 (mean ± 40 μm; range, 170 – 1230 μm); and of VA/VL, 490 (mean ± 30μm; range 170 – 1160 μm).
Discussion
Projections from prefrontal cortex to MD, the principal thalamic nucleus for the prefrontal cortex, the motor-related nuclei VA/VL, and the limbic nucleus AM, showed a dual organization into modules and layers. Prefrontal cortices issued projections in significant numbers from layer V, especially when directed to the VA/VL nuclei. Moreover, there was consistently a higher proportion of projection neurons in layer V in dorsal and medial than in ventral prefrontal areas.
Organization of prefrontal cortico-thalamic projections into modules and layers
Projection neurons to the three thalamic nuclei originated from many prefrontal areas, and overlapped extensively, especially in dorso-medial and lateral prefrontal areas, which issued robust projections to MD and VA/VL, and in medial and orbitofrontal cortices, which projected to all three nuclei. In the areas of overlap, projection neurons to MD, VA/VL and AM were organized into interdigitated modules extending through layers VI and V, suggesting some degree of segregation of prefrontal projections to distinct thalamic nuclei. The modular organization and sizes found are in agreement with previous observations regarding ocular dominance columns in areas 17 and 18, which ranged between 500 and 2000 μm in width (Friedman et al., 1989), or the width of afferent fiber columns in the prefrontal cortex, which was reported to average 685 μm (Bugbee and Goldman-Rakic, 1983), and the width of interdigitated columns of contralateral and ipsilateral projections from the parietal cortex to the prefrontal cortex, which ranged from 300 to 750 μm (Goldman-Rakic and Schwartz, 1982; Goldman-Rakic, 1984).
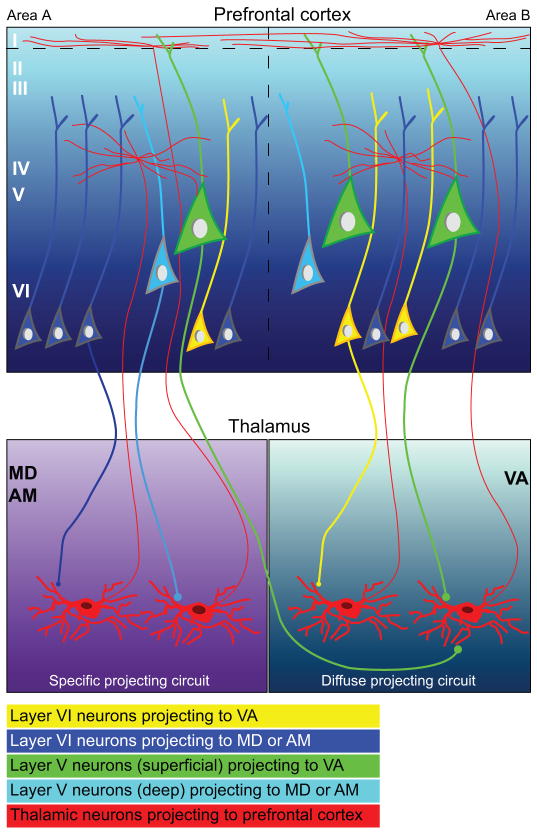
Projection neurons to the three thalamic nuclei were also organized in layers, as summarized in Figure 9. Most projection neurons in all cases were found in layer VI, which gives rise to cortico-thalamic projections in all cortical systems [reviewed in (Steriade et al., 1997; Jones, 2007)]. However, significant proportions of prefrontal cortico-thalamic neurons were found in layer V, in a pattern that differed depending on thalamic destination. Among the nuclei studied, the proportion of projection neurons from layer V directed to the VA/VL was higher than to MD or AM. Moreover, within layer V, projection neurons directed to the VA/VL occupied primarily the upper part of layer V, whereas projection neurons directed to MD or AM occupied the deep part of layer V, showing a sublaminar organization.
Figure 9.
Schematic diagram summarizes the laminar origin of prefrontal cortico-thalamic pathways to MD, AM, and VA and the reciprocal thalamo-cortical projections. Prefrontal projection neurons directed to all three thalamic nuclei originate from interdigitated modules of pyramidal neurons spanning layers VI and V. Left panels, Projection neurons directed to MD and AM originate mainly from layer VI pyramidal neurons (dark blue), which have been shown, in previous studies to terminate through small boutons onto thalamic neurons that project focally to the middle cortical layers (left red neuron in MD/AM panel). Most layer V projection neurons directed to MD and AM are pyramidal neurons situated in the deep part of the layer (light blue), and have been shown to terminate through small and large boutons onto thalamic neurons that project to the superficial cortical layers (right red neuron in MD/AM panel). Right panels, The VA receives projections from a higher proportion of layer V neurons than MD or AM, which constitute nearly as many as from layer VI. Projections to VA (right panels) that originate from layer VI pyramidal neurons (yellow) have also been shown, in previous studies, to terminate through small boutons onto thalamic neurons that project focally to the middle cortical layers (left red neuron in VA panel). The majority of layer V pyramidal neurons projecting to VA are situated in the upper part of the layer (green). These large layer V pyramidal neurons terminate through small and large boutons onto thalamic neurons that send widespread projections to the superficial layers of the prefrontal cortex (right red neuron in VA panel). Widespread thalamic projections to layer I impinge on the apical dendrites of pyramidal neurons in neighboring columns or areas (areas B and A), and may thus spread activation across multiple areas.
Cortical layer V neurons make up a small proportion of the population of neurons projecting to high-order thalamic nuclei, and also project to motor-related subcortical structures (Guillery, 1995). The upper part of layer V in prefrontal cortex includes the majority of projection neurons directed to the striatum in monkeys, cats and dogs (Selemon and Goldman-Rakic, 1985; Arikuni and Kubota, 1986; Goldman-Rakic and Selemon, 1986; Tanaka, 1987; Yeterian and Pandya, 1994; Thomson and Bannister, 2003). The upper part of layer V also projects to the motor-related VA/VL nuclei of the thalamus, as shown here, providing additional evidence for the preferential involvement of this sub-layer in motor circuits. On the other hand, neurons in the deep part of layer V of association cortices project to several thalamic nuclei, such as the intralaminar, which then project widely to several cortical areas (Catsman-Berrevoets and Kuypers, 1978; Thomson and Bannister, 2003). This evidence suggests specificity in layer V projection neurons directed to VA/VL, on one hand, and to MD and AM, on the other hand, which may be differentially recruited in behavior.
Circuit differences of laminar-specific projections
Connections within cortical columns
The significance of the differences in the relative distribution of prefrontal cortico-thalamic projection neurons within layers VI and V that were directed to MD, VA/VL and AM is based on the specific anatomic interactions of each of these layers within cortical columns, on one hand, and in circuits linking the cortex with the thalamus, on the other hand. At the level of cortical columns, pyramidal neurons in the upper part of cortical layer V, which included the majority of projection neurons to the VA/VL, are generally large, and have long apical dendrites that ascend to layer I, and long horizontal dendrites that stretch laterally over several millimeters within layer V (Mountcastle, 1997). In the visual cortex, horizontal connections across cortical columns are thought to integrate contour information of objects and bind visual inputs to form much larger receptive fields (Wiesel and Gilbert, 1989; Stettler et al., 2002). The axonal branches of neurons in layer V also ascend vertically to all layers above. The lower part of layer V, where most layer V neurons directed to MD and AM were found, is populated mostly by small pyramidal neurons, whose apical dendrites ascend to layer III, and their branched axon terminals project vertically to layers II, III, IV and V. Small pyramidal neurons are also found in layer VI, and their apical dendrites ascend to the upper part of layer IV, which receives the majority of thalamo-cortical projections. Some axonal branches of layer VI neurons project to layers III–VI (Thomson and Bannister, 2003). The sublaminar segregation of prefrontal projections into upper layer V, directed to the VA/VL, and into deep layer V, directed to MD or AM, suggests differential effects on the cortical microarchitecture of columns by each of these layer V projection systems.
Connections with the thalamus
Cortical neurons in layers V and VI also have distinct patterns of projection to the thalamus. Neurons from layer V of sensory association cortices terminate as large and round clusters of terminals in sensory thalamic nuclei and innervate the proximal dendrites of thalamic neurons (Jones and Hendry, 1989; Guillery, 1995; Rockland, 1996; Jones, 1998a; Jones, 1998b; Rockland et al., 1999; Rouiller and Welker, 2000; Guillery and Sherman, 2002). Projection neurons directed to the motor-related VA/VL nuclei, half of which originate from layer V, terminate mainly through small but also some large terminals that synapse with calbindin positive thalamic projection neurons with perisynaptic ionotropic glutamate receptors, which, in turn, project to cortical layer I [Fig. 9; (Zikopoulos and Barbas, 2007)].
In contrast, axons from neurons in layer VI terminate as small boutons in continuous distributions and innervate mainly the distal dendrites of thalamic neurons, forming synapses predominantly with parvalbumin positive thalamic projection neurons enriched with metabotropic glutamate receptors (Reichova and Sherman, 2004; Zikopoulos and Barbas, 2007). In turn, these thalamo-cortical neurons project to the middle cortical layers, including layer IV. Our findings of differences in the prevalence of layer V prefrontal neurons projecting to different thalamic nuclei also suggest differences in recruitment of thalamo-cortical pathways.
Functional implications of laminar-specific connections
The prevalence of layer V cortico-thalamic projections varied regionally, being higher in dorsal prefrontal areas (e.g., area 9) and medial areas (e.g., areas 24 and 32). Area 9 is situated in front of the premotor areas and has a role in planning sequential tasks requiring response monitoring and attentional control (Goldman-Rakic, 1987; Fuster, 1993; Petrides, 1995; Petrides, 1996; Hikosaka et al., 1999; Sakai et al., 1999; Levy and Goldman-Rakic, 2000). Anterior cingulate areas (24 and 32) are engaged when cognitive demands are high, such as in monitoring performance and errors during conflicting events (Devinsky et al., 1995; Botvinick et al., 1999; Carter et al., 2000; Miller and Cohen, 2001; Paus, 2001; Milham et al., 2001; Walton et al., 2007; Brown and Braver, 2008). Neurons from layer V of area 32 in rhesus monkeys project to lateral prefrontal cortices (Barbas and Rempel-Clower, 1997). In dorsolateral area 9 axons from area 32 form synapses with spines of excitatory neurons and also innervate inhibitory neurons through large terminals (Medalla and Barbas, 2009). The high proportion of projection neurons in layer V in dorsal and medial prefrontal areas may have a role in neuronal processing of sequential events and motor outcomes.
The significant proportion of projection neurons in layer V directed to the VA/VL, MD, and AM thalamic nuclei provides a mechanism for interlinking prefrontal areas through the thalamus (Haber and McFarland, 2001; McFarland and Haber, 2002; Zikopoulos and Barbas, 2007). This linkage may be effected through thalamo-cortical projections to layer I, which stretch parallel to the pial surface over long distances, where they impinge on the apical dendrites of layer V neurons and likely activate them (von Stein et al., 2000; Larkum et al., 2004). Through this linkage, fast firing driver neurons in layer V may transmit signals across several cortical areas and induce oscillations (Guillery, 1995; Sherman and Guillery, 1998; Rouiller and Welker, 2000; Guillery and Sherman, 2002). There is evidence that in prefrontal cortex, activation of layer V pyramidal neurons may initiate slow wave oscillations that propagate vertically to other layers (Sanchez-Vives and McCormick, 2000; Ulbert et al., 2004). Through these extensive horizontal thalamo-cortical projections to layer I of prefrontal cortex, circuits through the VA may interact with circuits through MD and AM to process information in the context of emotion and memory.
In addition, the prefrontal cortex receives projections from MDmc, VAmc and AM in both hemispheres (Preuss and Goldman-Rakic, 1987a; Dermon and Barbas, 1994), which are part of the circuits through the basal ganglia and the prefrontal cortex (Groenewegen et al., 1990; Xiao and Barbas, 2002b). Bilateral connections between prefrontal cortex and these thalamic nuclei may coordinate bilateral motor activity through communication within local circuits in the prefrontal cortex. Moreover, within the context of the modular and laminar organization of cortico-thalamic projections, reciprocal connections between prefrontal cortices and thalamic nuclei may initiate brain wave oscillations, associated with consciousness, attention and executive control (Smythies, 1997; Steriade, 2000; Llinas and Steriade, 2006; Vertes, 2006; Schiff, 2008). Disturbance in this transthalamic cortico-cortical communication may underlie the pathology in psychiatric diseases (Llinas et al., 1999; Lewis et al., 2001; Rubenstein and Merzenich, 2003) and movement disorders (Goldman-Rakic et al., 1992; Hoover and Strick, 1993; Bergman et al., 1998; Sabatini et al., 2000; Garcia-Cabezas et al., 2007), affecting specific nodes in this intricate circuitry.
Supplementary Material
Supplementary Figure 1. Summary of the modular and laminar organization of prefrontal cortico-thalamic projection neurons. Center, Three-dimensional reconstruction of the right hemisphere of the rhesus monkey cortex, which was rendered transparent to show prefrontal neurons in layers V and VI that project to MD (blue), VA (green), and AM (red). Three representative areas were reconstructed and enlarged to show the modular organization of prefrontal cortico-thalamic neurons: Top left, dorsal area 46. The majority of prefrontal labeled neurons projected to MD, showing widespread distribution along the rostro-caudal and medio-lateral axes of area 46. Neurons projecting to the VA had a similar distribution but were fewer in number. Neurons projecting to AM clustered mainly in the central regions of area 46. Bottom left, lateral area 12; Most of the labeled neurons projected to VA, and were concentrated predominantly in the lateral and caudal parts of area 12. Neurons projecting to MD and AM formed distinct clusters in the central and rostral parts of area 12. Bottom right, area 14. Neurons projecting to AM occupied predominantly central and caudal parts of the area, in the rostro-caudal axis. Neurons projecting to MD and VA were interdigitated, forming clusters, mainly in rostral and caudal parts of the area. Inset, left, Laminar and sublaminar distribution of projection neurons in area 12 directed to the VA and MD. A majority of projection neurons were found in layer VI and were directed predominantly to MD (inset, bottom). In layer V most projection neurons were directed to VA (green and yellow), and most of them were found in the upper parts of the layer (green) and fewer were found in the deep part of layer V (yellow). In contrast, most neurons projecting to MD (dark and light blue) in layer V were found in the deep parts of the layer (light blue), close to layer VI, and fewer were found in the upper part of layer V (dark blue).
Abbreviations
- A
arcuate sulcus
- AM
anterior medial nucleus
- AV
anterior ventral nucleus
- Cdc
central denso-cellular nucleus
- Cg
cingulate sulcus
- Cl
central lateral nucleus
- CM
centromedian nucleus
- Csl
central superior lateral nucleus
- D8
dorsal part of area 8
- D9
dorsal part of area 9
- D46
dorsal part of area 46
- LD
lateral dorsal nucleus
- MD
mediodorsal thalamic nucleus (mc, magnocellular division; mf, multiform division; pc, parvicellular division)
- L12
lateral part of area 12
- LF
lateral fissure
- LO
lateral orbital sulcus
- M9
medial part of area 9
- MO
medial orbital sulcus
- O12
orbital part of area 12
- O14
orbital part of area 14
- Olf
olfactory tubercle
- OPAll
orbital periallocortex (agranular cortex)
- OPro
orbital proisocortex (dysgranular cortex)
- P
principal sulcus
- Pcn
paracentral nucleus
- R
reticular nucleus
- Re
nucleus reuniens
- Ro
rostral sulcus
- Sm
stria medullaris
- V8
ventral subdivision of area 8
- V46
ventral subdivision of area 46
- VA
ventral anterior thalamic nucleus (mc, magnocellular division; pc, parvicellular division)
- VL
ventral lateral nucleus (m, medial division; o, oral division; p, posterior division)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Alexander GE, Delong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arikuni T, Kubota K. The organization of prefrontocaudate projections and their laminar origin in the Macaque monkey: A retrograde study using HRP-Gel. J Comp Neurol. 1986;244:492–510. doi: 10.1002/cne.902440407. [DOI] [PubMed] [Google Scholar]
- Armstrong E. Limbic thalamus: anterior and mediodorsal nuclei. In: Paxinos G, editor. The Human Nervous System. Academic Press, Inc; New York: 1990. pp. 469–481. [Google Scholar]
- Bachevalier J, Meunier M, Lu MX, Ungerleider LG. Thalamic and temporal cortex input to medial prefrontal cortex in rhesus monkeys. Exp Brain Res. 1997;115:430–444. doi: 10.1007/pl00005713. [DOI] [PubMed] [Google Scholar]
- Barbas H. Complementary role of prefrontal cortical regions in cognition, memory and emotion in primates. Adv Neurol. 2000;84:87–110. [PubMed] [Google Scholar]
- Barbas H. Specialized elements of orbitofrontal cortex in primates. Ann N Y Acad Sci. 2007;1121:10–32. doi: 10.1196/annals.1401.015. [DOI] [PubMed] [Google Scholar]
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. J Comp Neurol. 1990;301:1–23. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Rempel-Clower N, Xiao D. Anatomic basis of functional specialization in prefrontal cortices in primates. In: Grafman J, editor. Handbook of Neuropsychology. Elsevier Science B.V; Amsterdam: 2002. pp. 1–27. [Google Scholar]
- Barbas H, Henion TH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1991;313:65–94. doi: 10.1002/cne.903130106. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam MM. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J Comp Neurol. 1981;200:407–431. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN. Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1989;286:353–375. doi: 10.1002/cne.902860306. [DOI] [PubMed] [Google Scholar]
- Barbas H, Rempel-Clower N. Cortical structure predicts the pattern of corticocortical connections. Cereb Cortex. 1997;7:635–646. doi: 10.1093/cercor/7.7.635. [DOI] [PubMed] [Google Scholar]
- Barbas H, Zikopoulos B. Sequential and parallel circuits for emotional processing in primate orbitofrontal cortex. In: Zald David, Rauch Scott., editors. The Orbitofrontal Cortex. Oxford University Press; 2006. pp. 57–91. [Google Scholar]
- Barbas H, Zikopoulos B. The prefrontal cortex and flexible behavior. Neuroscientist. 2007;13:532–545. doi: 10.1177/1073858407301369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. A computational model of risk, conflict, and individual difference effects in the anterior cingulate cortex. Brain Res. 2008;1202:99–108. doi: 10.1016/j.brainres.2007.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugbee NM, Goldman-Rakic PS. Columnar organization of corticocortical projections in squirrel and rhesus monkeys: similarity of column width in species differing in cortical volume. J Comp Neurol. 1983;220:355–364. doi: 10.1002/cne.902200309. [DOI] [PubMed] [Google Scholar]
- Carter CS, Macdonald AM, Botvinick M, Ross LL, Stenger VA, Noll D, Cohen JD. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97:1944–1948. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsman-Berrevoets CE, Kuypers HGJM. Differential laminar distribution of corticothalamic neurons projecting to the VL and the center median. An HRP study in the cynomolgus monkey. Brain Res. 1978;154:359–365. doi: 10.1016/0006-8993(78)90706-0. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Dermon CR, Barbas H. Contralateral thalamic projections predominantly reach transitional cortices in the rhesus monkey. J Comp Neurol. 1994;344:508–531. doi: 10.1002/cne.903440403. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Peters A, Barbas H. Mitochondrial degeneration in dystrophic neurites of senile plaques may lead to extracellular deposition of fine filaments. Brain Struct Funct. 2007;212:195–207. doi: 10.1007/s00429-007-0153-1. [DOI] [PubMed] [Google Scholar]
- Friedman HR, Bruce CJ, Goldman-Rakic PS. Resolution of metabolic columns by a double-label 2-DG technique: interdigitation and coincidence in visual cortical areas of the same monkey. J Neurosci. 1989;9:4111–4121. doi: 10.1523/JNEUROSCI.09-12-04111.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. New York: Raven Press; 1989. [Google Scholar]
- Fuster JM. Frontal lobes. Curr Opin Neurobiol. 1993;3:160–165. doi: 10.1016/0959-4388(93)90204-c. [DOI] [PubMed] [Google Scholar]
- Garcia-Cabezas MA, Rico B, Sanchez-Gonzalez MA, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage. 2007;34:965–984. doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: Areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988;277:195–213. doi: 10.1002/cne.902770204. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Modular organization of prefrontal cortex. Trends Neurosci. 1984;7:419–429. [Google Scholar]
- Goldman-Rakic PS. Motor control function of the prefrontal cortex. Ciba Found Symp. 1987;132:187–200. doi: 10.1002/9780470513545.ch12. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Changing concepts of cortical connectivity: parallel distributed cortical networks. In: Rakic P, Singer W, editors. Neurobiology of cortex. Dahlem Konferenzen; Berhard: 1988. pp. 177–202. [Google Scholar]
- Goldman-Rakic PS, Lidow MS, Smiley JF, Williams MS. The anatomy of dopamine in monkey and human prefrontal cortex. Journal of Neural Transmission. 1992;(Supplementum 36):163-Supplement. doi: 10.1007/978-3-7091-9211-5_8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 1985;242:535–560. doi: 10.1002/cne.902420406. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Schwartz ML. Interdigitation of contralateral and ipsilateral columnar projections to frontal association cortex in primates. Science. 1982;216:755–757. doi: 10.1126/science.6177037. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD. Topography of corticostriatal projections in nonhuman primates and implications for functional parcellation of the neostiatum. In: Jones EG, Peters A, editors. Cerebral Cortex. New York and London: Plenum Press; 1986. pp. 447–466. [Google Scholar]
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–R511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. [DOI] [PubMed] [Google Scholar]
- Guillery RW. Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat. 1995;187 (Pt 3):583–592. [PMC free article] [PubMed] [Google Scholar]
- Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- Haber S, McFarland NR. The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist. 2001;7:315–324. doi: 10.1177/107385840100700408. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K. Parallel neural networks for learning sequential procedures. Trends Neurosci. 1999;22:464–471. doi: 10.1016/s0166-2236(99)01439-3. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. Multiple output channels in the basal ganglia. Science. 1993;259:819–821. doi: 10.1126/science.7679223. [DOI] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. New York (NY): Plenum Press; 1985. [Google Scholar]
- Jones EG. A new view of specific and nonspecific thalamocortical connections. Adv Neurol. 1998a;77:49–71. [PubMed] [Google Scholar]
- Jones EG. Viewpoint: the core and matrix of thalamic organization. Neuroscience. 1998b;85:331–345. doi: 10.1016/s0306-4522(97)00581-2. [DOI] [PubMed] [Google Scholar]
- Jones EG. Chemically defined parallel pathways in the monkey auditory system. Ann N Y Acad Sci. 2003;999:218–233. doi: 10.1196/annals.1284.033. [DOI] [PubMed] [Google Scholar]
- Jones EG. The thalamus. New York: Cambridge University Press; 2007. [Google Scholar]
- Jones EG, Hendry SHC. Differential calcium binding protein immunoreactivity distinguishes classes of relay neurons in monkey thalamic nuclei. Eur J Neurosci. 1989;1:222–246. doi: 10.1111/j.1460-9568.1989.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Kievit J, Kuypers HGJM. Organization of the thalamo-cortical connexions to the frontal lobe in the rhesus monkey. Exp Brain Res. 1977;29:299–322. doi: 10.1007/BF00236173. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Senn W, Luscher HR. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb Cortex. 2004;14:1059–1070. doi: 10.1093/cercor/bhh065. [DOI] [PubMed] [Google Scholar]
- Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133:23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A. 1999;96:15222–15227. doi: 10.1073/pnas.96.26.15222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas RR, Steriade M. Bursting of thalamic neurons and states of vigilance. J Neurophysiol. 2006;95:3297–3308. doi: 10.1152/jn.00166.2006. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22:8117–8132. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Diversity of laminar connections linking periarcuate and lateral intraparietal areas depends on cortical structure. Eur J Neurosci. 2006;23:161–179. doi: 10.1111/j.1460-9568.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- Medalla M, Barbas H. Synapses with inhibitory neurons differentiate anterior cingulate from dorsolateral prefrontal pathways associated with cognitive control. Neuron. 2009;61:609–620. doi: 10.1016/j.neuron.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Brain Res Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mitchell IJ, Cooper AJ, Griffiths MR. The selective vulnerability of striatopallidal neurons. Prog Neurobiol. 1999;59:691–719. doi: 10.1016/s0301-0082(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Geula C, Mesulam M-M. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J Comp Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120 (Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- Olszewski J. An Atlas for Use with the Stereotaxic Instrument. Basel, Switzerland: Karger, S; 1952. The Thalamus of the Macaca mulatta. [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and behaviour. Curr Opin Neurobiol. 1994;4:207–211. doi: 10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Petrides M. Functional organization of the human frontal cortex for mnemonic processing. Evidence from neuroimaging studies. Ann N Y Acad Sci. 1995;769:85–96. doi: 10.1111/j.1749-6632.1995.tb38133.x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Lateral frontal cortical contribution to memory. Seminars in the Neurosciences. 1996;8:57–63. [Google Scholar]
- Preuss T, Goldman-Rakic P. Crossed corticothalamic and thalamocortical connections of macaque prefrontal cortex. J Comp Neurol. 1987a;257:269–281. doi: 10.1002/cne.902570211. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Crossed corticothalamic and thalamocortical connections of macaque prefrontal cortex. J Comp Neurol. 1987b;257:269–281. doi: 10.1002/cne.902570211. [DOI] [PubMed] [Google Scholar]
- Reichova I, Sherman SM. Somatosensory corticothalamic projections: distinguishing drivers from modulators. J Neurophysiol. 2004;92:2185–2197. doi: 10.1152/jn.00322.2004. [DOI] [PubMed] [Google Scholar]
- Rockland KS. Two types of corticopulvinar terminations: round (type 2) and elongate (type1) J Comp Neurol. 1996;368:57–87. doi: 10.1002/(SICI)1096-9861(19960422)368:1<57::AID-CNE5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Andresen J, Cowie RJ, Robinson DL. Single axon analysis of pulvinocortical connections to several visual areas in the macaque. J Comp Neurol. 1999;406:221–250. doi: 10.1002/(sici)1096-9861(19990405)406:2<221::aid-cne7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Rouiller EM, Welker E. A comparative analysis of the morphology of corticothalamic projections in mammals. Brain Res Bull. 2000;53:727–741. doi: 10.1016/s0361-9230(00)00364-6. [DOI] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain. 2000;123 (Pt 2):394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hikosaka O, Miyauchi S, Sasaki Y, Fujimaki N, Putz B. Presupplementary motor area activation during sequence learning reflects visuo-motor association. J Neurosci. 1999;19:RC1. doi: 10.1523/JNEUROSCI.19-10-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Scheibel AB. The thalamus and neuropsychiatric illness. J Neuropsychiatry Clin Neurosci. 1997;9:342–353. doi: 10.1176/jnp.9.3.342. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5:776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. On the actions that one nerve cell can have on another: distinguishing “drivers” from “modulators”. Proc Natl Acad Sci U S A. 1998;95:7121–7126. doi: 10.1073/pnas.95.12.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwek DF, Pandya DN. Prefrontal projections to the mediodorsal nucleus of the thalamus in the rhesus monkey. J Comp Neurol. 1991;312:509–524. doi: 10.1002/cne.903120403. [DOI] [PubMed] [Google Scholar]
- Smythies J. The functional neuroanatomy of awareness: with a focus on the role of various anatomical systems in the control of intermodal attention. Conscious Cogn. 1997;6:455–481. doi: 10.1006/ccog.1997.0315. [DOI] [PubMed] [Google Scholar]
- Steriade M. Corticothalamic resonance, states of vigilance and mentation. Neuroscience. 2000;101:243–276. doi: 10.1016/s0306-4522(00)00353-5. [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA. Thalamus - Organisation and function. Oxford: Elsevier Science; 1997. [Google Scholar]
- Stettler DD, Das A, Bennett J, Gilbert CD. Lateral connectivity and contextual interactions in macaque primary visual cortex. Neuron. 2002;36:739–750. doi: 10.1016/s0896-6273(02)01029-2. [DOI] [PubMed] [Google Scholar]
- Tanaka D., Jr Differential laminar distribution of corticostriatal neurons in the prefrontal and pericruciate gyri of the dog. J Neurosci. 1987;7:4095–4106. doi: 10.1523/JNEUROSCI.07-12-04095.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Bannister AP. Interlaminar connections in the neocortex. Cereb Cortex. 2003;13:5–14. doi: 10.1093/cercor/13.1.5. [DOI] [PubMed] [Google Scholar]
- Ulbert I, Heit G, Madsen J, Karmos G, Halgren E. Laminar analysis of human neocortical interictal spike generation and propagation: current source density and multiunit analysis in vivo. Epilepsia. 2004;45(Suppl 4):48–56. doi: 10.1111/j.0013-9580.2004.04011.x. [DOI] [PubMed] [Google Scholar]
- Van Der Werf YD, Jolles J, Witter MP, Uylings HB. Contributions of thalamic nuclei to declarative memory functioning. Cortex. 2003;39:1047–1062. doi: 10.1016/s0010-9452(08)70877-3. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Interactions among the medial prefrontal cortex, hippocampus and midline thalamus in emotional and cognitive processing in the rat. Neuroscience. 2006;142:1–20. doi: 10.1016/j.neuroscience.2006.06.027. [DOI] [PubMed] [Google Scholar]
- von Stein A, Chiang C, Konig P. Top-down processing mediated by interareal synchronization. Proc Natl Acad Sci U S A. 2000;97:14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AE. A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol. 1940;73:59–86. [Google Scholar]
- Walton ME, Croxson PL, Behrens TE, Kennerley SW, Rushworth MF. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage. 2007;36(Suppl 2):T142–T154. doi: 10.1016/j.neuroimage.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Gilbert CD. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci. 1989;9(7):2432–2442. doi: 10.1523/JNEUROSCI.09-07-02432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D, Barbas H. Pathways for emotions and memory I. input and output zones linking the anterior thalamic nuclei with prefontal cortices in the rhesus monkey. Thalamus and Related Systems. 2002a;2:21–32. [Google Scholar]
- Xiao D, Barbas H. Pathways for emotions and memory II. afferent input to the anterior thalamic nuclei from prefrontal, temporal, hypothalamic areas and the basal ganglia in the rhesus monkey. Thalamus and Related Systems. 2002b;2:33–48. [Google Scholar]
- Xiao D, Barbas H. Circuits through prefrontal cortex, basal ganglia, and ventral anterior nucleus map pathways beyond motor control. Thalamus & Related Systems. 2004;2:325–343. [Google Scholar]
- Yeterian EH, Pandya DN. Laminar origin of striatal and thalamic projections of the prefrontal cortex in rhesus monkeys. Exp Brain Res. 1994;99:383–398. doi: 10.1007/BF00228975. [DOI] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J Neurosci. 2006;26:7348–7361. doi: 10.1523/JNEUROSCI.5511-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikopoulos B, Barbas H. Parallel Driving and Modulatory Pathways Link the Prefrontal Cortex and Thalamus. PLoS One. 2007;2:e848. doi: 10.1371/journal.pone.0000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Summary of the modular and laminar organization of prefrontal cortico-thalamic projection neurons. Center, Three-dimensional reconstruction of the right hemisphere of the rhesus monkey cortex, which was rendered transparent to show prefrontal neurons in layers V and VI that project to MD (blue), VA (green), and AM (red). Three representative areas were reconstructed and enlarged to show the modular organization of prefrontal cortico-thalamic neurons: Top left, dorsal area 46. The majority of prefrontal labeled neurons projected to MD, showing widespread distribution along the rostro-caudal and medio-lateral axes of area 46. Neurons projecting to the VA had a similar distribution but were fewer in number. Neurons projecting to AM clustered mainly in the central regions of area 46. Bottom left, lateral area 12; Most of the labeled neurons projected to VA, and were concentrated predominantly in the lateral and caudal parts of area 12. Neurons projecting to MD and AM formed distinct clusters in the central and rostral parts of area 12. Bottom right, area 14. Neurons projecting to AM occupied predominantly central and caudal parts of the area, in the rostro-caudal axis. Neurons projecting to MD and VA were interdigitated, forming clusters, mainly in rostral and caudal parts of the area. Inset, left, Laminar and sublaminar distribution of projection neurons in area 12 directed to the VA and MD. A majority of projection neurons were found in layer VI and were directed predominantly to MD (inset, bottom). In layer V most projection neurons were directed to VA (green and yellow), and most of them were found in the upper parts of the layer (green) and fewer were found in the deep part of layer V (yellow). In contrast, most neurons projecting to MD (dark and light blue) in layer V were found in the deep parts of the layer (light blue), close to layer VI, and fewer were found in the upper part of layer V (dark blue).