Abstract
Background
Small intensive pharmacokinetic (PK) studies of medications in early-phase trials cannot identify the range of factors that influence drug exposure in heterogeneous populations. We performed PK studies in large numbers of HIV-infected women on nonnucleoside-reverse-transcriptase-inhibitors (NNRTIs) under conditions of actual use to assess patient characteristics that influence exposure and evaluated the relationship between exposure and response.
Methods
225 women on NNRTI-based antiretroviral regimens from the Women’s Interagency HIV Study (WIHS) were enrolled into 12 or 24-hour PK studies. Extensive demographic, laboratory and medication covariate data was collected before and during the visit to be used in multivariate models. Total NNRTI drug exposure was estimated by area-under-the-concentration-time curves (AUC).
Results
Hepatic inflammation and renal insufficiency were independently associated with increased nevirapine (NVP) exposure in multivariate analyses; crack cocaine, high fat diets, and amenorrhea were associated with decreased levels (n=106). Higher efavirenz (EFV) exposure was seen with increased transaminase, albumin levels, and orange juice consumption; tenofovir use, increased weight, being African-American and amenorrhea were associated with decreased exposure (n=119). With every 10-fold increase in NVP or EFV exposure, participants were 3.3 and 3.6 times as likely to exhibit virologic suppression, respectively. Patients with higher drug exposure were also more likely to report side effects on therapy.
Conclusions
Our study identifies and quantitates previously unrecognized factors modifying NNRTI exposure in the “real-world” setting. Comprehensive PK studies in representative populations are feasible and may ultimatley lead to dose optimization strategies in patients at risk for failure or adverse events.
Keywords: HIV, antiretrovirals, nevirapine, efavirenz, pharmacokinetics, drug exposure, women
INTRODUCTION
Despite the continuing successes of highly active antiretroviral therapy (HAART) in treated populations, these therapies have limitations. As many as 50% of patients fail to achieve sustained virologic responses on HAART, even in the era of more potent combination regimens1, and viral resistance is increasingly problematic1, 2. ARVs have a range of adverse effects, resulting in high rates of regimen switching or discontinuation3. Treatment failures and adverse events are reported more frequently in cohort or clinic-based settings than in clinical trials for many drugs (including ARVs), which may reflect systematic differences in trial participants from “real world” populations. Clinical trial enrollees may be healthier4, and, in the HIV setting, be less likely to be women and minorities5, than treated populations.
Pharmacokinetic (PK) studies are often embedded within clinical trials investigating drug safety and efficacy to inform formulation and dosing of new medications. Typically, twelve to 24 hour intensive PK studies are performed in small subsets of patients to determine blood concentrations of drug after dosing at steady state. These focused intensive PK studies in relatively homogenous patient populations or non-HIV-infected volunteers are very important to determine ideal dosing and typical PK curves for new drugs. Conscribed sample sizes and restricted eligibility6, 7 however, limit the generalizability of these PK findings to heterogeneous patient populations. The typical PK component of clinical trials does not thoroughly investigate the range of individual characteristics (e.g. concurrent medical conditions, dietary patterns, weight differences, ethnicity and gender, use of concomitant medications or recreational drugs) common among patients who will eventually receive drug prescriptions. The end result can be the revelation of unanticipated adverse effects and treatment failures after drug approval and dissemination8.
We present here the largest intensive PK study performed to date to assess modifiers of exposure for two commonly used nonnucleoside-reverse-transcriptase-inhibitor (NNRTIs) in a diverse cohort of HIV-infected women. In addition to its size and representation of actual HIV-infected populations, the study was performed under conditions of actual use, where participants took their regular concomitant medications, consumed a typical diet, smoked cigarettes as usual, etc., during PK sampling. We also report on the association between drug exposure and virologic response and self-reported side effects in this unselected cohort.
METHODS
Study population
The Women’s Interagency HIV Study (WIHS) is a large multicenter, prospective cohort study of HIV-infected (and at-risk uninfected) women established in 19949. The cohort is highly representative of HIV-infected women in the United States in terms of age, race, ethnicity, socioeconomic status, rates of substance use, degree of infirmity, and coinfections. All WIHS participants are seen biannually for structured interviews, physical examinations, and specimen collection. For those on HAART, antiretroviral therapy is prescribed by participants’ primary providers and not by the observational study. The participating WIHS sites are located in Washington DC, Bronx, Brooklyn, Chicago, and Northern California. Simulation methods were used to evaluate sample size for identifying a large number of predictors of drug exposure, as estimated by area-under-the-plasma-concentration-time-curve (AUC) from intensively studied subjects, and we found that approximately 110 patients per drug should be sufficient to identify a range of important factors in PK variability10.
Intensive PK protocol methods
Enrollment for the “WIHS Intensive PK Study” was initiated in April 2003 and all participants on efavirenz (EFV) or nevirapine (NVP)-based HAART regimens were offered enrollment. The only eligibility criteria for participation in the intensive PK protocol were use of a target ARV for at least six months and participant informed consent. Committees on Human Research at all participating institutions approved the study.
Participants were brought into clinical research centers associated with each of the WIHS sites for 12 or 24-hour sampling of the ARV under conditions of routine use. Participants were seen for the PK visit within six weeks of their core WIHS visit since data collected at both the preceding core visit and substudy visit were used in subsequent exposure models. NVP is usually dosed at 200mg twice a day and drug concentrations were obtained over 12 hours; EFV is typically dosed at 600mg daily and drug levels were measured over a 24 hour period. During the intensive PK visit, a series of plasma samples were collected at various time points relative to the dosing of the target ARV (0, 0.5, 1, 2, 4, 6, 8, and 12 hours after witnessed dosing for NVP; 0, 4, 8, 15, 18, and 24 hours after witnessed dosing of EFV). Actual times of plasma collection were recorded. The participant’s usual diet was ascertained by phone prior to the PK visit and simulation of her typical diet was undertaken during the visit. Concomitant medications were administered as usual during PK sampling. While recreational drugs were not permitted during the visit, their use prior to initiation of the protocol, if routine for the subject, did not preclude participation and was recorded as data. If routine for the participant, cigarette smoking was allowed during the visit between blood draws.
On the day of the study visit, participants were administered a series of questionnaires, including details on their current ARV regimen and degree of adherence, use of concomitant medications, recent or current symptoms and illnesses, current menstrual, contraceptive, and obstetric events, substance use patterns, and diet. Weights and urine pregnancy tests were performed during the visit. The longitudinal WIHS core data set included measures of height, fat free mass as measured by bioelectrical impedance analysis, renal, hepatic and other laboratory measurements over time, further menstrual history, and hepatitis B and C coinfection status.
Laboratory procedures
Procedures for measuring ARV blood levels have been described previously11. Plasma samples (0.1 mL) were prepared for injection by adding A-86093 (Abbott Laboratories, Abbott Park, IL) as an internal standard, adding acetonitrile (0.4 mL) to precipitate the protein, mixing, centrifuging, transferring the supernatant to an autosampler vial, and diluting if necessary. Plasma was analyzed for nevirapine and efavirenz by standard techniques of liquid chromatography/tandem mass spectrometry12. Nevirapine was analyzed with a ZORBAX Eclipse XDB-C8 (4.6 × 50 mm, 3.5 μm particle size) analytical column and an XDB-C8 (4.6 × 12.5 mm) guard column (Agilent Technologies; Palo Alto, CA). Efavirenz was analyzed with a BDS Hypersil C18 (4.6 × 50 mm, 5 μm) analytical column and a 3 mm × 2 mm ODS guard column (Thermo Electron Corp.; Waltham, MA). Data analysis was performed with MassLynx 3.5 software (Micromass, Manchester, UK). The absolute recovery of NNRTIs from plasma was 94.2% for nevirapine and 99.8% for efavirenz. Intra- and interday precision was <11.7% for both NNRTIs and accuracies ranged from −2.9% to 0.7% for NVP and −6.0% to 14.8% for EFV11.
Study measurements
Outcome variables
The outcome variable for the intensive PK study pharmacokinetic analyses is total drug exposure. The dose-adjusted parameter used to define exposure was “AUC/dose”, where AUC is the area-under-the-plasma concentration-time curve and dose is the target ARV dose witnessed at the start of the PK sampling. Since our study was observational in nature, each participant brought in her usual dose of the target ARV for witnessed consumption during the study. Four of the women on EFV and 10 of the women on NVP were prescribed or used doses disparate than the standard unit-doses for each of these agents. AUCs were calculated using the trapezoidal rule and the other exposure metrics were calculated using traditional equations programmed in Stata/SE version 9.2. If a missing datapoint occurred before the first or after the last observation, it did not contribute to the calculations; if the missing time or concentration occurred between two observed datapoints, it was extrapolated from a straight line between those points. The outcome variable of AUC/dose was log transformed to reduce skewness in the data. The outcome variables for the pharmacodynamic analyses were HIV viral load measurements at the time of the intensive PK study visit and self report of the drug leading to “any” side effects.
Statistical analyses
The drug exposure outcome was analyzed in relation to a number of factors that may influence NNRTI PK measurements. Categorical variables and continuous variables that were categorized included race (African American compared to other, including Caucasian, Hispanic, Native American, Asian); age (categorized by decade); hepatitis C infection status; chronic hepatitis B infection (as defined by positive hepatitis B surface antigen); platelet count (<150/mL versus ≥150) as a marker of liver dysfunction; stage in menstrual cycle or menopausal status; pregnancy status; renal dysfunction (creatinine clearance (CrCl) calculated by either the Cockcroft-Gault13 or Modification of Diet in Renal Disease (MDRD) equation14 and dichotomized by <60ml/min versus ≥60 and <80ml/min/1.73 m2 versus ≥80, respectively); smoking (yes/no) or alcohol use (categorized into mild, moderate, severe); percentage of fat in the usual diet as ascertained by a validated dietary questionnaire15 (<30%, 30–35%, 36–40% fat or >40% usual fat intake in the preceding 30 days); persistent diarrhea in the past 30 days; concurrent symptoms or infections; use of medications known to increase or decrease target ARV exposure by inhibition or induction of cytochrome P450 or P-glycoprotein levels (including concomitant protease inhibitors); and self-reported adherence measurements. Continuous variables included hepatitis C RNA levels in hepatitis C-infected patients, creatinine clearance as measured using the two methods above, body mass index and fat free mass measurements, as well as serum hepatic transaminase levels (aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma glutamyl-transferase (GGT)) as markers of liver inflammation. Since measures of lean body mass are typically used to predict drug dosages16, ideal body weight, lean body weight, adjusted body weight, and predicted normal weight were estimated from height and weight parameters using standard equations17 and assessed for their independent relationships to the outcome.
Univariate analyses were performed by linear regression between the log-transformed outcomes and the categorical or continuous variables of interest. Multivariable models were constructed by manual forward stepwise selection, starting with the predictor that had the smallest p-value on univariate analysis. At each step, each remaining predictor was examined as a possible addition to the model, and the one with the smallest p-value was added, until no remaining predictor had a p-value of less than 0.10. Each candidate model was run separately to avoid excessive casewise deletion of observations that had missing values on other unselected candidate predictors. Covariates with obvious collinearity were not included in the same models. Age, race, and ideal body weight were included as variables in all multivariate models.
The pharmacodynamic analyses used NNRTI exposure as the predictor and assessed its relationship to important outcomes, including HIV viral load suppression at the time of sampling and self-reported side effects on the medication. All analyses were performed using the Stata/SE 9.2 statistical package.
RESULTS
Data collection
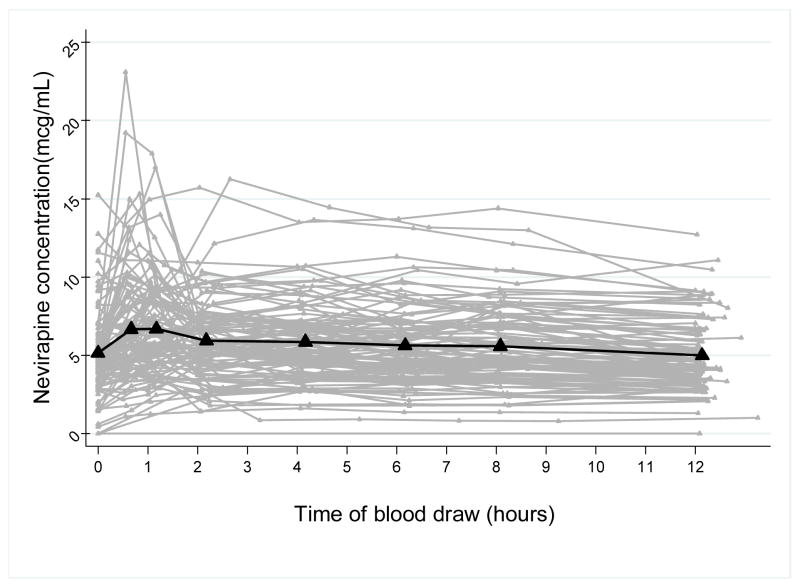
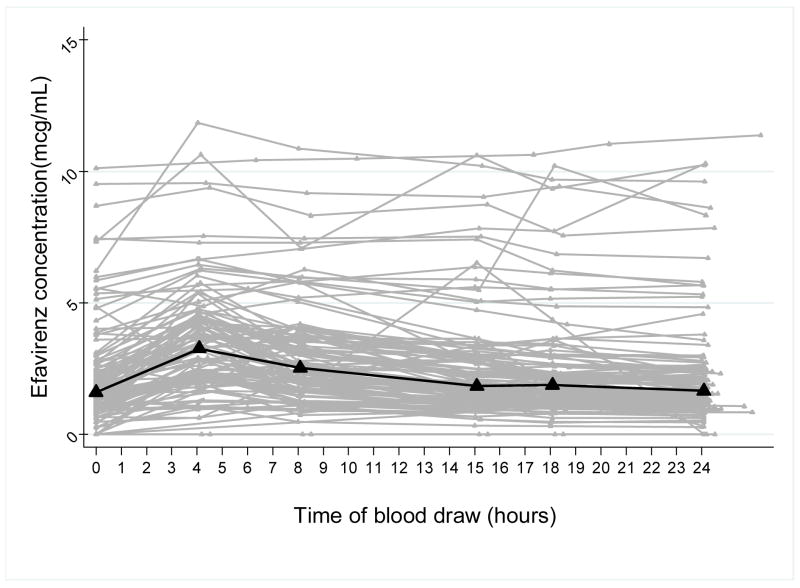
Enrollment and data collection was completed for 106 WIHS participants on NVP-containing regimens and 119 WIHS participants on EFV-containing regimens. Time versus concentration curves for all of the intensive PK study participants on NVP are depicted in Figure 1a with a median PK curve superimposed on top of the spaghetti plot. Figure 1b shows the time-concentration curves for the 119 participants on EFV with the median curve. Both plots illustrate the marked interindividual variability in the PK curves for this “real world” study population. Table 1 shows the summary of the PK parameters or exposure metrics for NVP and EFV.
Figure 1.
Figure 1a: Time-concentration curves for 106 participants on NVP with superimposed median PK curve (bold line)
Figure 1b: Time-concentration curves for 119 participants on EFV with superimposed median PK curve (bold line)
TABLE 1.
Summary exposure metrics in the WIHS Intensive PK study
| Drug | AUC (mcg1 × h/mL) | Cmin (mcg/mL) | Cmax (mcg/mL) | tmax (hours) | CL/F (ml/h) |
|---|---|---|---|---|---|
|
Nevirapine (n=106) | |||||
| Median | 63.4 | 4.15 | 7.57 | 1.2 | 3.16 |
| Range | (14.2 – 212.0) | (0 – 14.3) | (2.77 – 23.1) | (0–12.6) | (0.94 – 16.5) |
| CV (%) | 47 | 53 | 47 | 110 | 62 |
|
| |||||
|
Efavirenz (n=119) | |||||
| Median | 55.6 | 1.46 | 3.79 | 4.2 | 10.8 |
| Range | (11.4 – 639.8) | (0 – 25.2) | (1.9 – 27.5) | (0–24.1) | (0.94 – 52.6) |
| CV (%) | 108 | 133 | 85 | 70 | 62 |
mcg = micrograms; h = hours; mL = milliliter
Distribution of covariates in the population under study
Table 2 shows the prevalence of each of the possible variables investigated in this study. The sample is racially diverse (62% and 73% African Americans in the NVP and EFV groups, respectively) and factors that could potentially influence ARV exposure based on previous literature or plausibility were well represented: high BMI (mean of 29 kg/m2 in both groups); active smoking (61% in the group on EFV); renal insufficiency (10% in the NVP group with calculated CrCl < 60ml/min); liver function abnormalities (21.4% in the NVP group had transaminases above reference values); persistent diarrhea over preceding 30 days (22% in the EFV group); concurrent hepatitis C infection (41% in the EFV group); and high fat diets (56% of participants on NVP had greater than 30% fat in their diets).
TABLE 2.
Distributions of covariates in the WIHS Intensive PK Study for participants on NVP and EFV-containing HAART regimens
| ARV under study (Sample size) | Nevirapine (106) | Efavirenz (119) |
|---|---|---|
| Race distribution | Caucasian (22%); Hispanic (10%); African-American (62%); Other (6%) | Caucasian (8%); Hispanic (18%); African-American(73%); Other (1%) |
| Age distribution | 20–29 years (12%); 30–39 years (31%); 40–49 years (38%); 50–59 years (15%); ≥60 years (4%) | 20–29 years (8%); 30–39 years (29%); 40–49 years (45%); 50–59 years (17%); ≥60 years (1%) |
| Adherence to ARVs (self-report) in past month | ≤35% adherence (1%); 36–65% adherence (4%); 66–80% adherence (6%); 81–94% adherence (36%); ≥95% adherence (53%) |
≤35% adherence (4%); 36–65% adherence (3%); 66–80% adherence (10%); 81–94% adherence (19%); ≥95% adherence (64%) |
| Hepatitis C positive | 33% | 41% |
| Chronic Hepatitis B | 4% | 3% |
| Thrombocytopenia (platelets<150/mL) | 17% | 15% |
| Menstruating at time of PK sampling | 18% | 13% |
| Amenorrhea for ≥ 12 months (self-report) | 19% | 26% |
| Pregnancy | 2% | 0% |
| Renal insufficiency1 (CrCl <60ml/min, calc. using Cockcroft-Gault equation) | 10% | 7% |
| Renal insufficiency2 (GFR <80ml/min, calculated using MDRD equation) | 40% | 21% |
| Smoker | 53% | 61% |
| Crack cocaine use more than 1–2 times per month | 7% | 8% |
| Percent fat consumption in diet in past month | <30% fat (44%); 30–35% fat (9%); 36–40% fat (35%); >40% fat (12%) | <30% fat (47%); 30–35% fat (9%); 36–40% fat (25%); >40% fat (18%) |
| Active diarrhea (3 or more soft stools a day within last 30 days) | 13% | 22% |
| Concomitant meds known to increase target ARV levels (e.g. CYP3A4 inhibitors) | 5% | 10% |
| Concomitant meds known to decrease target ARV levels (CYP3A4 inducers) | 0% | 4% |
| Use of tenofovir | 16% | 23% |
| Oranges or orange juice consumption in the preceding 5 days | 63% | 67% |
| Mean AST level (range) IU/L | 35 (11–125) | 33(10–209) |
| Mean ALT level (range) IU/L | 29 (4–95) | 27(8–117) |
| Mean GGT level (range) IU/L | 125 (8–1032) | 119(13–1371) |
| Mean BMI (range) kg/m2 | 29.8 (17.3–55.4) | 28.9 (14.0–57.8) |
| Fat free mass (range) kg as measured by impedance | 45.7 (33.5–64.8) | 45.6 (33.8–111.5) |
| Mean ideal body weight3 (range) kg | 54.6 (29.6–70.3) | 54.1 (38.6–70.3) |
| Mean creatinine clearance (ml/min) (range)1 | 105 (39–234) | 110 (11–273) |
Crockfeld-Gault equation: (0.85 if female) × (140-age)(weight/’kg)/(serum creatinine × 72)
MDRD equation: GFR = 186 × (serum creatinine)−1.154 × (age)−0.203 × (0.742 if female) × (1.210 if black)
IBW(kg) = 45.4 + 0.89 × (height(cm) − 152.4)17
Univariate analysis between the exposure outcome and various predictors
Tables 3a and 3b show the univariate relationships between the specified variables and exposure (AUC/dose) for NVP and EFV, respectively. The variables associated with a statistically significant increase in nevirapine AUC were hepatitis C coinfection, renal insufficiency, orange or orange juice consumption in the preceding 5 days, and increases in serum AST, ALT, and GGT levels (Table 3a). Increases in lean body mass, higher CrCl, and a greater percentage of recent dietary fat consumption were associated with lower NVP AUC levels.
TABLE 3.
| TABLE 3a: Univariate analyses between covariates and nevirapine exposure, as measured by AUC/dose, for 106 HIV-infected women on NVP-containing HAART | ||||
|---|---|---|---|---|
| Variable | Estimated effect on AUC (↑ or ↓) | 95% lower C.I. | 95% upper C.I. | P value |
| Categorical variables | ||||
| Race (African American vs. other) | ↑ 1.04 fold | 0.96 | 1.25 | 0.69 |
| Adherence to ARVs (≥95% vs. <95%) in past month | ↑ 1.03 fold | 0.86 | 1.23 | 0.74 |
| Hepatitis C positive | ↑ 1.26 fold | 1.04 | 1.53 | 0.017 |
| Chronic Hepatitis B | ↑ 1.14 fold | 0.70 | 1.87 | 0.59 |
| Low platelet count | ↑ 1.16 fold | 0.88 | 1.55 | 0.27 |
| Menstruating at time of PK sampling | ↓ 0.80 fold | 0.64 | 1.02 | 0.069 |
| Pregnancy | ↓ 0.83 fold | 0.43 | 1.61 | 0.59 |
| Amenorrhea for 12 months | ↓ 0.92 fold | 0.73 | 1.16 | 0.49 |
| Renal insufficiency(CrCl, calculated <60ml/min vs. ≥60; Cockcroft-Gault) | ↑ 1.47 fold | 1.10 | 1.95 | 0.009 |
| Renal insufficiency (GFR, calculated <80ml/min vs ≥80ml/min; MDRD equation) | ↑ 1.23 fold | 1.03 | 1.48 | 0.023 |
| Smoker | ↑ 1.09 fold | 0.91 | 1.30 | 0.37 |
| Percent fat in diet (>40% fat versus ≤40% fat) in past 30 days | ↓ 0.75 fold | 0.57 | 0.98 | 0.03 |
| Diarrhea | ↓ 0.85 fold | 0.66 | 1.11 | 0.24 |
| Drugs known to increase NVP levels (CYP3A4 inhibitors) | ↑ 1.34 fold | 0.88 | 2.04 | 0.17 |
| Oranges or orange juice in past 5 days | ↑ 1.23 fold | 1.02 | 1.48 | 0.027 |
| Crack cocaine use | ↓ 0.80 fold | 0.56 | 1.14 | 0.213 |
| Continuous variables | ||||
| Age (in decades) | ↑ 1.0 fold | 0.96 | 1.14 | 0.29 |
| Per doubling of AST level | ↑ 1.21 fold | 1.09 | 1.35 | <0.0005 |
| Per doubling of ALT level | ↑ 1.24 fold | 1.12 | 1.36 | <0.0005 |
| Per doubling of GGT level | ↑ 1.14 fold | 1.08 | 1.21 | <0.0005 |
| Per doubling of body mass index (BMI), which includes fat mass | ↓ 0.79 fold | 0.62 | 1.00 | 0.053 |
| Per doubling of fat free mass | ↓ 0.54 fold | 0.34 | 0.85 | 0.009 |
| Per doubling of creatinine clearance | ↓ 0.78 | 0.67 | 0.92 | 0.003 |
| TABLE 3b: Univariate analyses between covariates and efavirenz exposure, as measured by AUC/dose, for 119 HIV-infected women on EFV-containing HAART | ||||
|---|---|---|---|---|
| Variable | Estimated effect on AUC (↑ or ↓) | 95% lower C.I. | 95% upper C.I. | P value |
| Categorical variables | ||||
| Race (African American vs. other) | ↑ 1.05 fold | 0.79 | 1.39 | 0.75 |
| Adherence to ARVs (≥95% vs. <95%) in past month | ↑ 1.32 fold | 1.02 | 1.71 | 0.033 |
| Hepatitis C positive | ↑ 1.15 fold | 0.89 | 1.45 | 0.29 |
| Chronic Hepatitis B | ↓ 0.60 fold | 0.27 | 1.34 | 0.21 |
| Low platelet count | ↑ 1.42 fold | 1.00 | 2.01 | 0.05 |
| Menstruating at time of PK sampling | ↓ 0.84 fold | 0.58 | 1.22 | 0.36 |
| Amenorrhea for >12 months | ↑ 1.27 fold | 0.96 | 1.69 | 0.09 |
| Renal insufficiency (Calculated creatinine clearance <60ml/min vs. ≥60ml/min) | ↑ 1.26 fold | 0.77 | 2.06 | 0.36 |
| Smoker | ↓ 0.97 fold | 0.75 | 1.26 | 0.84 |
| Use of tenofovir | ↓ 0.71 fold | 0.53 | 0.95 | 0.022 |
| Diarrhea | ↑ 1.05 fold | 0.78 | 1.43 | 0.74 |
| Concomitant use of LPV or RTV, which can increase EFV levels | ↑ 1.19 fold | 0.79 | 1.80 | 0.40 |
| Concomitant use of meds which can decrease EFV levels | ↓ 0.68 fold | 0.37 | 1.26 | 0.22 |
| Oranges or orange juice in the past 5 days | ↑ 1.31 fold | 1.01 | 1.71 | 0.044 |
| Continuous variables | ||||
| Age (in decades) | ↑ 1.08 fold | 0.92 | 1.26 | 0.34 |
| Per doubling of AST level | ↑ 1.29 fold | 1.11 | 1.49 | 0.001 |
| Per doubling of ALT level | ↑ 1.21 fold | 1.05 | 1.41 | 0.012 |
| Per doubling of GGT level | ↑ 1.12 fold | 1.03 | 1.23 | 0.013 |
| Per doubling of bilirubin level | ↑ 1.10 fold | 0.97 | 1.25 | 0.13 |
| Per doubling of albumin level | ↑ 1.88 fold | 0.88 | 4.02 | 0.10 |
| Per doubling of body mass index (BMI), which includes fat mass | ↓ 0.75 fold | 0.54 | 1.03 | 0.073 |
| Per doubling of ideal body weight | ↓ 0.47 fold | 0.21 | 1.07 | 0.072 |
| Per doubling of creatinine clearance | ↓ 0.93 fold | 0.75 | 1.15 | 0.49 |
Statistically significant associations with a higher EFV AUC in univariate analyses were seen with greater self reported ARV adherence, thrombocytopenia, consumption of orange juice, and increases in hepatic transaminases (Table 3b). A decreased EFV AUC was statistically significantly associated with concomitant use of the nucleoside reverse transcriptase inhibitor (NRTI), tenofovir, and marginally associated with larger ideal body weights.
Multivariate models
The results of the multivariate models for prediction of NVP and EFV exposure are shown in Tables 4a and 4b, respectively. Increases in ALT level remained statistically significantly associated with an increase in NVP AUC/dose (Table 4a), as did increases in AST level and GGT level in independent models. Lower creatinine clearance was also associated with increases in NVP exposure. Routine high fat diets, regular crack cocaine use (at least once a week) and self-reported amenorrhea for at least 12 months were independently associated with decreases in NVP exposure in multivariate modeling.
Table 4.
| Table 4a: Multivariate model depicting effects of various predictors on the outcome of NVP exposure when controlled for other factors in the model1 | ||||
|---|---|---|---|---|
| Predictor | Estimated effect on AUC (↑ or ↓) | 95% lower C.I. | 95% upper C.I. | P value |
| Per 2-fold increase in ALT | ↑ 1.25 fold | 1.14 | 1.38 | <0.001 |
| Per 2-fold decrease in creatinine clearance | ↑ 1.22 fold | 1.01 | 1.47 | 0.036 |
| Percent fat in diet (>40% vs ≤40% fat) in past 30 days | ↓ 0.69 fold | 0.54 | 0.87 | 0.002 |
| Crack cocaine use | ↓ 0.70 fold | 0.51 | 0.96 | 0.028 |
| Amenorrhea for > 12 months | ↓ 0.77 fold | 0.61 | 0.97 | 0.026 |
| Table 4b: Multivariate model depicting effects of various predictors on the outcome of EFV exposure when controlled for other factors in the model1 | ||||
|---|---|---|---|---|
| Predictor | Estimated effect on AUC (↑ or ↓) | 95% lower C.I. | 95% upper C.I. | P value |
| Per 2-fold increase in ALT | ↑ 1.22 fold | 1.05 | 1.41 | 0.009 |
| Per 2-fold increase in albumin level | ↑ 2.47 fold | 1.19 | 5.10 | 0.015 |
| Oranges or orange juice in the past 5 days | ↑ 1.39 fold | 1.09 | 1.78 | 0.009 |
| Amenorrhea for >12 months | ↓ 0.73 fold | 0.55 | 0.97 | 0.030 |
| Use of tenofovir | ↓ 0.75 fold | 0.57 | 0.99 | 0.045 |
| Per 2-fold increase in ideal body weight (IBW) | ↓ 0.38 fold | 0.18 | 0.83 | 0.015 |
| African-American vs. other | ↓ 0.75 fold | 0.56 | 1.00 | 0.05 |
Controlled for age, race, ideal body weight
Controlled for age
Higher EFV exposure was seen with increased serum ALT and serum albumin levels, or consumption of oranges or orange juice in the preceding 5 days (Table 4b) in multivariate models. Factors associated with decreased EFV exposure were self-reported amenorrhea for at least 12 months, being African-American, the concomitant use of tenofovir, and larger ideal body weights.
Association of exposure with virologic suppression and side effects
In order to assess the relationship between drug exposure and virologic response in the cohort, logistic regression models examined the odds of virologic suppression. Among the patients on EFV, 39 (33%) had detectable HIV viral loads at the time of intensive PK sampling despite being on the drug for more than 6 months. The higher the EFV exposure, the greater the likelihood of having an undetectable viral load at the time of sampling. With every 10-fold increase in EFV AUC, the odds ratio for virologic suppression was 3.58 (95% CI 1.28–14.07) when controlled for self-reported adherence, age, race and ideal body weight. For the patients on NVP, 51 (43%) had detectable HIV viral loads at the time of sampling and the likelihood of exhibiting virologic suppression increased with higher NVP exposure. With every 10-fold increase in NVP AUC, the odds ratio for virologic suppression was 3.34 (95% CI 1.49–17.54) when controlled for adherence, age, race and ideal body weight.
In terms of self-reported side effects, patients with higher AUCs were more likely to report that they felt that their drug “gave any side effects” or was “toxic or harmful”. Those with EFV exposure in the top median were 2.6 (95% CI 1.5–4.9) times as likely to report side effects than those with an EFV AUC in the lower half for the group. Similarly, those with NVP exposure in the top median were 1.9 (95% CI 1.4–2.9) times as likely to report toxicities than those in the lower median. Of note, patients with side effects had markedly reduced odds of adhering to their therapies on a routine basis than who reported no side effects: the odds of ≥95% adherence was 0.05 (95% CI 0.02–0.16) for those reporting side effects on EFV and 0.25 (0.09–0.71) for those on NVP.
DISCUSSION
Study significance
In this study, we identify factors that may influence NNRTI exposure in a diverse population of HIV-infected women and show that exposure is associated with virologic suppression and self report of adverse effects on therapy. NNRTIs are increasingly used in first-line treatment regimens in the developed and developing world, especially with their global roll-out, the use of NVP to prevent perinatal transmission18, and the co-formulation of EFV into a once daily combination regimen (Atripla®). Adequate exposure to these medications is paramount in preventing drug resistance19. Although PK studies in selected cohorts are valuable in defining the typical exposure metrics of a medication, intensive PK studies in large unselected cohorts such as ours may identify covariates that influence exposure in “real world” populations. Table 5 lists the characteristics of our study design that distinguish it from smaller intensive PK studies performed to date for these NNRTIs, including our large sample size and the racial and ethnic diversity of the cohort.
TABLE 5.
Unique attributes of the study method for assessing contributors to antiretroviral exposure
|
Our work demonstrates that detailed studies of representative and diverse patient groups are feasible. Most population PK studies of ARVs to date have employed sparse sampling, extrapolation and simulation methods20 justified by citing feasibility issues on performing intensive PK measurements on large samples of HIV-infected patients. Full 12 or 24 hour PK studies for antiretrovirals have been reported in relatively small numbers of patients (40–50 participants in the largest published studies)21–27. Because many of these intensive PK studies restrict eligibility, the resultant PK models are limited in the number of covariates examined. We chose to employ a unique study design for intensive PK sampling in a large unselected observational cohort of HIV-infected patients in order to assess the influence of a more comprehensive set of factors on drug exposure. Although the mean exposure metrics for our intensive PK studies are similar to those reported in the literature for both drugs28, 29, the wider coefficients of variation for our calculated parameters (Table 1 and Figure 1) reflect the variability in an unrestricted study population,
Factors that contribute to NVP exposure
In this unselected population, hepatic inflammation and renal insufficiency were independently associated with increased NVP exposure in multivariate analyses, whereas crack cocaine, high fat diets, and amenorrhea were associated with decreased levels. Although NVP is cleared hepatically, the effects of uremic toxins on relevant hepatic transporters or metabolizing enzymes30 may explain the influence of renal insufficiency on NVP clearance. While consumption of a single fatty meal did not influence NVP exposure among 24 adult volunteers studied prior to drug licensure31, the effects of chronic fat consumption have not been studied. Dietary fat can inhibit the hepatic p-glycoprotein efflux transporter over time32, and thus lead to increased hepatocyte NVP concentrations33 with increased metabolism by the cytochrome p450 3A4 (CYP3A4) and 2B6 (CYP2B6) systems34. The fact that decreased NVP levels are not seen with the administration of a single high fat meal31 may reflect the fact that CYP2B6 is found in the liver, but not in the intestine35; chronic exposure to high fat may lead to transporter effects and changes in hepatic, but not enteric, metabolism.
Use of crack cocaine and amenorrhea are examples of factors not likely to be examined in more focused intensive PK studies. The decreased exposure to NVP found among participants who reported recent recreational use of crack in our study could be explained by induction of the CYP3A4 metabolic system by cocaine36. Crack cocaine use in HIV-infected populations is not infrequent37 and its effects on exposure may contribute to treatment failure apart from the crack’s effect on adherence38. Neither age (which should be related to postmenopausal status) nor use of exogenously administered hormones (which can be related to prolonged amenorrhea) was independently associated with decreased NNRTI exposure. Previous reports have shown higher NVP39 and EFV concentrations40 in pre-menopausal women than men, but a comparison of NNRTI exposure between postmenopausal women and either premenopausal women or age-matched men has not been performed. Since a systemic analysis of the effects of menstrual status on the disposition of ARVs is lacking in the literature to date, intensive PK studies in unselected cohorts of women are important.
Factors that contribute to EFV exposure
Hepatic transaminase levels, albumin levels, recent consumption of oranges or orange juice, prolonged amenorrhea, concomitant use of tenofovir, ideal body weight, and race contributed to EFV exposure in multivariate models. The association of increased albumin levels with increased EFV levels is likely explained by the fact that EFV is primarily protein-bound (in contradistinction to NVP)41. Inhibition of intestinal p-glycoprotein transport or down-regulation of enteric CYP3A4 by citrus components in oranges or orange juice42 could lead to enhanced bioavailability and increased exposure to efavirenz33. Concomitant use of tenofovir was associated with decreased levels of efavirenz in our multivariate model; an unrelated study failed to demonstrate this relationship in univariate analysis43. This drug-drug interaction may be important given the availability of a fixed dose combination containing tenofovir and efavirenz and may be modified by competing factors in the model.
The association of efavirenz exposure with ideal body weight probably reflects increased hepatic clearance in individuals with larger lean and hepatic mass16. In previous studies, higher EFV concentration in patients of African descent have been linked to an increased frequency of specific polymorphisms in the multidrug resistance transporter (MDR) and CYP2B6 genes44. The association between African-American race and decreased EFV exposure in our multivariate models, however, may reflect the importance of assessing a host of variables in predictions of drug exposure for an individual patient.
Limitations
The observational nature of our study is not a limitation, but a strength, since modeling the pharmacokinetics of chronically administered medications under conditions of actual use allows for the identification of “real-world” factors that contribute to drug exposure and efficacy. Although we could have included more precise measures of dietary intake, gastrointestinal absorption, metabolic activity or renal function in our models, we chose to focus only on factors readily measured in routine clinical care. However, although our models explain a much larger proportion of NNRTI exposure variability than more limited models in the literature, a large amount of unexplained variation still exists. One major limitation of our current PK models is the lack of data on genetic polymorphisms in the host. In view of the increasing awareness that variability in transporter and metabolic enzymes may have a large contribution to plasma drug levels44, host pharmacogenomic parameters will be incorporated into the PK models in upcoming analyses.
The ultimate aim of identifying factors that contribute to medication exposure in representative populations with chronic diseases is to provide quantitative data for dose optimization within an individual after data on the exposure-response relationship is assessed longitudinally. Although we explored some pharmacodynamic relationships between AUCs and virologic suppression and self-reported side effects cross-sectionally at the time of intensive PK sampling, longitudinal analyses using population PK models can explore the exposure-response relationship over time. Population PK methods45–47 employ sparse PK data from an individual and the characteristics identified by intensive PK sampling in a representative population to produce more robust measures of exposure (using techniques of nonlinear mixed effects modeling) for predicting treatment responses. The next step is to model estimates of exposure using sparse level data and these intensive datasets in order to measure the relationship of exposure and response over time and, ultimately, aid in dose individualization parameters.
Conclusions
By performing intensive PK analyses of NNRTIs in a large, diverse unrestricted sample of HIV-infected women, we were able to identify key previously unidentified factors that contribute to drug exposure in multivariate models. As ARVS are increasingly being provided to diverse populations (in terms of age, sex, ethnicity, coinfections, and other factors), assessing the influence of individual patient characteristics to drug exposure may help optimize responses and diminish adverse effects for these chronically administered therapies in the “real world’ setting.
Acknowledgments
Sources of support: Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group. The WIHS is funded by the National Institute of Allergy and Infectious Diseases (NAIAD) with supplemental funding from the National Cancer Institute, the National Institute on Drug Abuse (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590). Funding is also provided by the National Institute of Child Health and Human Development (UO1-CH-32632) and the National Center for Research Resources (MO1-RR-00071, MO1-RR-00079, MO1-RR-00083). Dr. Gandhi was supported by a Mentored Patient-Oriented Research Career Development Award (K23 AI067065) from NIAID.
The authors wish to recognize the late Lewis B. Sheiner MD for his many contributions to the field of pharmacokinetic modeling, and the valuable advice he provided during the design of this study. We also wish to thank the WIHS participants who contributed to this study.
Footnotes
Meeting where work was presented: 13th Conference on Retroviruses and Opportunistic Infections (CROI), Denver, Colorado, 2006, February 5–8 (abstract 592)
DISCLOSURES
None of the authors have commercial or other associations that might pose a conflict of interest
References
- 1.Bartlett JA, Buda JJ, von Scheele B, et al. Minimizing resistance consequences after virologic failure on initial combination therapy: a systematic overview. J Acquir Immune Defic Syndr. 2006;41(3):323–331. doi: 10.1097/01.qai.0000197070.69859.f3. [DOI] [PubMed] [Google Scholar]
- 2.Vella S, Palmisano L. The global status of resistance to antiretroviral drugs. Clin Infect Dis. 2005;41:S239–S246. doi: 10.1086/430784. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Youle M, Moore A, et al. Reasons for modification and discontinuation of antiretrovirals: results from a single treatment centre. Aids. 2001;15(2):185–194. doi: 10.1097/00002030-200101260-00007. [DOI] [PubMed] [Google Scholar]
- 4.Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C. Threats to applicability of randomised trials: exclusions and selective participation. J Health Serv Res Policy. 1999;4(2):112–121. doi: 10.1177/135581969900400210. [DOI] [PubMed] [Google Scholar]
- 5.Gifford AL, Cunningham WE, Heslin KC, et al. Participation in research and access to experimental treatments by HIV-infected patients. N Engl J Med. 2002;346(18):1373–1382. doi: 10.1056/NEJMsa011565. [DOI] [PubMed] [Google Scholar]
- 6.Williams JA, Johnson K, Paulauskis J, Cook J. So many studies, too few subjects: establishing functional relevance of genetic polymorphisms on pharmacokinetics. J Clin Pharmacol. 2006;46(3):258–264. doi: 10.1177/0091270005283463. [DOI] [PubMed] [Google Scholar]
- 7.Huang SM, Lesko LJ, Williams RL. Assessment of the quality and quantity of drug-drug interaction studies in recent NDA submissions: study design and data analysis issues. J Clin Pharmacol. 1999;39(10):1006–1014. doi: 10.1177/00912709922011764. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services, Food and Drug Administration (FDA) Guidance for Industry: Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment. 2005 http://www.fda.gov/Cder/guidance/6359OCC.htm.
- 9.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. Epidemiology. 1998;9(2):117–125. [PubMed] [Google Scholar]
- 10.Hooker AC, Foracchia M, Dodds MG, Vicini P. An evaluation of population D-optimal designs via pharmacokinetic simulations. Ann Biomed Eng. 2003;31(1):98–111. doi: 10.1114/1.1533074. [DOI] [PubMed] [Google Scholar]
- 11.Egge-Jacobsen W, Unger M, Niemann CU, et al. Automated, fast, and sensitive quantification of drugs in human plasma by LC/LC-MS: quantification of 6 protease inhibitors and 3 nonnucleoside transcriptase inhibitors. Ther Drug Monit. 2004;26(5):546–562. doi: 10.1097/00007691-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Volosov A, Alexander C, Ting L, Soldin SJ. Simple rapid method for quantification of antiretrovirals by liquid chromatography-tandem mass-spectrometry. Clin Biochem. 2002;35(2):99–103. doi: 10.1016/s0009-9120(02)00286-2. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 14.Manjunath G, Sarnak MJ, Levey AS. Prediction equations to estimate glomerular filtration rate: an update. Curr Opin Nephrol Hypertens. 2001;10(6):785–792. doi: 10.1097/00041552-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1(1):58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Morgan DJ, Bray KM. Lean body mass as a predictor of drug dosage. Implications for drug therapy. Clin Pharmacokinet. 1994;26(4):292–307. doi: 10.2165/00003088-199426040-00005. [DOI] [PubMed] [Google Scholar]
- 17.Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58(2):119–133. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brocklehurst P, Volmink J. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2002;2:CD003510. doi: 10.1002/14651858.CD003510. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez de Requena D, Bonora S, Garazzino S, et al. Nevirapine plasma exposure affects both durability of viral suppression and selection of nevirapine primary resistance mutations in a clinical setting. Antimicrob Agents Chemother. 2005;49(9):3966–3969. doi: 10.1128/AAC.49.9.3966-3969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett JS, Labbe L, Pfister M. Application and impact of population pharmacokinetics in the assessment of antiretroviral pharmacotherapy. Clin Pharmacokinet. 2005;44(6):591–625. doi: 10.2165/00003088-200544060-00003. [DOI] [PubMed] [Google Scholar]
- 21.Autar RS, Boffito M, Hassink E, et al. Interindividual variability of once-daily ritonavir boosted saquinavir pharmacokinetics in Thai and UK patients. J Antimicrob Chemother. 2005;56(5):908–913. doi: 10.1093/jac/dki354. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrin I, Breilh D, Birac V, et al. Pharmacokinetics and resistance mutations affect virologic response to ritonavir/saquinavir-containing regimens. Ther Drug Monit. 2001;23(4):332–340. doi: 10.1097/00007691-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Pfister M, Labbe L, Hammer SM, et al. Population pharmacokinetics and pharmacodynamics of efavirenz, nelfinavir, and indinavir: Adult AIDS Clinical Trial Group Study 398. Antimicrob Agents Chemother. 2003;47(1):130–137. doi: 10.1128/AAC.47.1.130-137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brundage RC, Yong FH, Fenton T, Spector SA, Starr SE, Fletcher CV. Intrapatient variability of efavirenz concentrations as a predictor of virologic response to antiretroviral therapy. Antimicrob Agents Chemother. 2004;48(3):979–984. doi: 10.1128/AAC.48.3.979-984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanhove GF, Kastrissios H, Gries JM, et al. Pharmacokinetics of saquinavir, zidovudine, and zalcitabine in combination therapy. Antimicrob Agents Chemother. 1997;41(11):2428–2432. doi: 10.1128/aac.41.11.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fletcher CV, Acosta EP, Cheng H, et al. Competing drug-drug interactions among multidrug antiretroviral regimens used in the treatment of HIV- infected subjects: ACTG 884. Aids. 2000;14(16):2495–2501. doi: 10.1097/00002030-200011100-00011. [DOI] [PubMed] [Google Scholar]
- 27.Floren LC, Wiznia A, Hayashi S, et al. Nelfinavir pharmacokinetics in stable human immunodeficiency virus-positive children: Pediatric AIDS Clinical Trials Group Protocol 377. Pediatrics. 2003;112(3 Pt 1):e220–227. doi: 10.1542/peds.112.3.e220. [DOI] [PubMed] [Google Scholar]
- 28.van Heeswijk RP, Veldkamp AI, Mulder JW, et al. The steady-state pharmacokinetics of nevirapine during once daily and twice daily dosing in HIV-1-infected individuals. Aids. 2000;14(8):F77–82. doi: 10.1097/00002030-200005260-00001. [DOI] [PubMed] [Google Scholar]
- 29.Veldkamp AI, Harris M, Montaner JS, et al. The steady-state pharmacokinetics of efavirenz and nevirapine when used in combination in human immunodeficiency virus type 1-infected persons. J Infect Dis. 2001;184(1):37–42. doi: 10.1086/320998. [DOI] [PubMed] [Google Scholar]
- 30.Sun H, Frassetto L, Benet LZ. Effects of renal failure on drug transport and metabolism. Pharmacol Ther. 2006;109(1–2):1–11. doi: 10.1016/j.pharmthera.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Nevirapine package insert. Boehringer Ingelheim Pharmaceuticals, Inc. Revised: January 11. 2005. [Google Scholar]
- 32.Harris RZ, Jang GR, Tsunoda S. Dietary effects on drug metabolism and transport. Clin Pharmacokinet. 2003;42(13):1071–1088. doi: 10.2165/00003088-200342130-00001. [DOI] [PubMed] [Google Scholar]
- 33.Almond LM, Edirisinghe D, Dalton M, Bonington A, Back DJ, Khoo SH. Intracellular and plasma pharmacokinetics of nevirapine in human immunodeficiency virus-infected individuals. Clin Pharmacol Ther. 2005;78(2):132–142. doi: 10.1016/j.clpt.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Erickson DA, Mather G, Trager WF, Levy RH, Keirns JJ. Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos. 1999;27(12):1488–1495. [PubMed] [Google Scholar]
- 35.Lechevrel M, Casson AG, Wolf CR, et al. Characterization of cytochrome P450 expression in human oesophageal mucosa. Carcinogenesis. 1999;20(2):243–248. doi: 10.1093/carcin/20.2.243. [DOI] [PubMed] [Google Scholar]
- 36.Pellinen P, Stenback F, Kojo A, Honkakoski P, Gelboin HV, Pasanen M. Regenerative changes in hepatic morphology and enhanced expression of CYP2B10 and CYP3A during daily administration of cocaine. Hepatology. 1996;23(3):515–523. doi: 10.1002/hep.510230316. [DOI] [PubMed] [Google Scholar]
- 37.Compton WM, Thomas YF, Conway KP, Colliver JD. Developments in the epidemiology of drug use and drug use disorders. Am J Psychiatry. 2005;162(8):1494–1502. doi: 10.1176/appi.ajp.162.8.1494. [DOI] [PubMed] [Google Scholar]
- 38.Hinkin CH, Barclay TR, Castellon SA, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11(2):185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Regazzi M, Villani P, Seminari E, et al. Sex differences in nevirapine disposition in HIV-infected patients. Aids. 2003;17(16):2399–2400. doi: 10.1097/00002030-200311070-00018. [DOI] [PubMed] [Google Scholar]
- 40.Burger D, van der Heiden I, la Porte C, et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol. 2006;61(2):148–154. doi: 10.1111/j.1365-2125.2005.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boffito M, Back DJ, Blaschke TF, et al. Protein binding in antiretroviral therapies. AIDS Res Hum Retroviruses. 2003;19(9):825–835. doi: 10.1089/088922203769232629. [DOI] [PubMed] [Google Scholar]
- 42.Saito M, Hirata-Koizumi M, Matsumoto M, Urano T, Hasegawa R. Undesirable effects of citrus juice on the pharmacokinetics of drugs: focus on recent studies. Drug Saf. 2005;28(8):677–694. doi: 10.2165/00002018-200528080-00003. [DOI] [PubMed] [Google Scholar]
- 43.Droste JA, Kearney BP, Hekster YA, Burger DM. Assessment of drug-drug interactions between tenofovir disoproxil fumarate and the nonnucleoside reverse transcriptase inhibitors nevirapine and efavirenz in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41(1):37–43. doi: 10.1097/01.qai.0000191997.70034.80. [DOI] [PubMed] [Google Scholar]
- 44.Haas DW. Human genetic variability and HIV treatment response. Curr HIV/AIDS Rep. 2006;3(2):53–58. doi: 10.1007/s11904-006-0018-x. [DOI] [PubMed] [Google Scholar]
- 45.Ette EI, Williams PJ, Lane JR. Population Pharmacokinetics III: Design, Analysis, and Application of Population Pharmacokinetic Studies. Ann Pharmacother. 2004;38(12):2136–2144. doi: 10.1345/aph.1E260. [DOI] [PubMed] [Google Scholar]
- 46.Ette EI, Williams PJ. Population pharmacokinetics II: estimation methods. Ann Pharmacother. 2004;38(11):1907–1915. doi: 10.1345/aph.1E259. [DOI] [PubMed] [Google Scholar]
- 47.Ette EI, Williams PJ. Population pharmacokinetics I: background, concepts, and models. Ann Pharmacother. 2004;38(10):1702–1706. doi: 10.1345/aph.1D374. [DOI] [PubMed] [Google Scholar]