Abstract
In the adult dentate gyrus, radial glia-like cells represent putative stem cells generating neurons and glial cells. Here, we combined patch-clamp recordings, biocytin filling, immunohistochemistry, single-cell transcript analysis, and mouse transgenics to test for connexin expression and gap junctional coupling of radial glia-like cells and its impact on neurogenesis. Radial glia-like cells were identified in mice expressing EGFP under control of the nestin and gfap promoters. We show that a majority of Radial glia-like cells are coupled and express Cx43. Neuronal precursors were not coupled. Mice lacking Cx30 and Cx43 in GFAP-positive cells displayed almost complete inhibition of proliferation and a significant decline in numbers of radial glia-like cells and granule neurons. Inducible virus-mediated ablation of connexins in the adult hippocampus also reduced neurogenesis. These findings strongly suggest the requirement of connexin expression by radial glia-like cells for intact neurogenesis in the adult brain and point to possible communication pathways of these cells.
Keywords: adult neurogenesis, coupling, gap junction, astrocyte, patch clamp
Astrocytes have emerged as beguiling cell types involved in the modulation of synaptic transmission and cell growth. Importantly, astrocytes can give rise to neurons within circumscribed regions of the adult brain. In the subgranular zone (SGZ) of the dentate gyrus (DG), astrocytes with a radial glia (RG)-like morphology are considered stem cells (1). They extend a long branching process through the granule cell layer (GCL), electrophysiologically display a passive current pattern (2–4), and express GFAP and nestin. How these proliferating RG-like cells are integrated into the hippocampal network is poorly understood. Epileptic seizures or ischemic insults can trigger proliferation of RG-like cells, while the transit-amplifying progenitors in the DG are less affected (3, 5). However, RG-like precursor cells neither receive functional GABAergic nor glutamatergic synaptic input (6, 7). Therefore, alternative environmental cues must be involved in the control of proliferation and differentiation of RG-like cells in the DG. One possible pathway of how they might receive signals is coupling via gap junctions, which provide conduits for transfers of ions or metabolites between coupled cells. In the developing embryonic neocortex, the expression of connexins or formation of gap junctions modulate proliferation and differentiation of RG cells (8, 9). Moreover, gap junctional communication is required to maintain murine neural progenitor cells in a proliferative state in vitro (10, 11). Recent work has shown that during embryonic development, gap junctions mediate neuronal migration through adhesive interactions with the cytoskeleton (12). Until now, no data exist regarding connexin expression and the coupling status of RG-like cells in the adult brain and its possible impact on neurogenesis.
In the present study, we addressed the question whether RG-like cells in the DG are coupled and, if so, which connexins might form gap junctions between these precursor cells. In a second step we investigated proliferation and neurogenesis in the adult DG, both after constitutive or inducible deletion of connexin expression in RG-like cells. Combining patch-clamp techniques with different genetic approaches, we demonstrate here that a significant proportion of RG-like cells in the DG are coupled through gap junctions and predominantly express connexin 43 (Cx43) and connexin 30 (Cx30). Intriguingly, ablation of connexin expression in RG-like cells leads to a dramatic decrease of proliferation and reduced numbers of RG-like cells and granule neurons in the adult DG.
Results
Identification of RG-Like Cells.
RG-like cells reveal a characteristic morphology with the soma located in the SGZ and a long process extending through the GCL. Typically, they express GFAP as well as nestin (2, 4, 13). Transgenic hGFAP–EGFP and nestin-EGFP mice were used to identify these RG-like cells in situ [Fig. 1 and supporting information (SI) Fig. S1]. Because in both mouse lines different fluorescent cell types have been described, only cells meeting the following criteria were considered putative RG-like cells: (i) having EGFP expression, (ii) having cell soma located in the SGZ, and (iii) having a radial process spanning the GCL, as revealed through postrecording immunocytochemistry (see below). Based on these criteria, 34 out of 48 (age p9–p12) and 8 out of 8 (age p90–p120) recorded cells were classified as RG-like cells in hGFAP-EGFP mice. In nestin-EGFP mice (p90–p120), 23 out of 29 recorded cells were categorized as RG-like cells using the same criteria (Table S1).
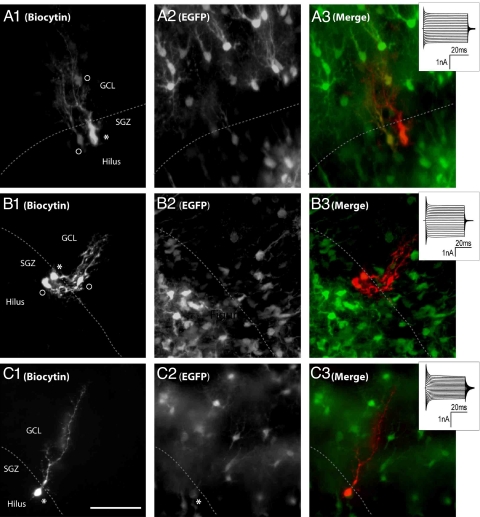
Fig. 1.
Biocytin tracing and coupling in the SGZ of transgenic hGFAP-EGFP mice. (A1–A3) Confocal images of a coupled RG-like cell (p9–p12). (A1) Tracer spread from the recorded cell (asterisk) to 2 neighbouring cells (°). (A2) EGFP expression in the same section. (A3) Merged image. (B1–B3) Another example of a coupled RG-like cell (p90–p120). (B1) Biocytin spread from the recorded cell (asterisk) to 2 neighboring cells (°). (B2) EGFP expression. (B3) Merged image. (C1–C3) Confocal images of a noncoupled RG-like cell (p9–p12). (C1) Biocytin was confined to the recorded cell (asterisk). (C2) EGFP expression. (C3) Merged image. Cells were filled with biocytin during recording (20 min). Insets give the current profiles of the recorded cells (+20 to –160 mV; Vh = −70 mV). Broken lines indicate the border between SGZ and GCL. (Scale bar, 50 μm.)
Consistent with the literature (2, 4), in the adult brain all RG-like cells in both transgenic models expressed a voltage- and time-independent current pattern, similar to the “passive astrocytes” described previously (14, 15). However, in the juvenile DG of hGFAP-EGFP mice, 10 out of 34 fluorescent RG-like cells expressed a complex current pattern (Fig. S2 and see Table S1), possibly indicating different developmental stages.
Passive RG-Like Cells Are Gap-Junction Coupled.
During recording, cells were filled with biocytin to allow for reidentification after pipette withdrawal. Following biocytin immunostaining, tracer spread was quantified using confocal z-stack micrographs. In juvenile hGFAP-EGFP mice, RG-like cells with a passive current pattern displayed a dye coupling ratio of about 46% (11 out of 24 cells) (see Table S1 and Fig. 1 A and C). Three of these 11 cells (27%) were weakly coupled with 1 to 5 neighboring cells, while the remainder (n = 8, 73%) showed stronger coupling (6–13 cells). To determine whether the coupling ratio of RG-like cells changes during development, the same protocols were applied to adult hGFAP-EGFP mice. As mentioned above, at this stage all RG-like cells displayed a passive current pattern. Quantification of biocytin spread identified a coupling ratio of 63% (5 out of 8 cells), with the tracer spreading to up to 7 neighboring cells (see Fig. 1B and Table S1).
Next we addressed the question whether in hGFAP-EGFP and nestin-EGFP mice the same pool of RG-like cells is labeled. The latter mouse model has been used to characterize different precursor cell types in the SGZ, including RG-like cells (2, 4, 6). Fluorescent RG-like cells in adult nestin-EGFP mice consistently express GFAP (2), a finding that has also been confirmed with postrecording staining (4). Immunostaining of hGFAP-EGFP mice revealed that 75.9% of the fluorescent RG-like cells were nestin-positive (85 out of 112 cells, n = 3 mice; not shown), indicating significant overlap among fluorescent RG-like cells in both mouse lines. Biocytin filling during recording detected a coupling ratio of 74% among nestin-EGFP-positive RG-like cells (17 out of 23 cells). Quantification of tracer spread consistently revealed a low coupling strength (1–5 coupled cells) (see Fig. S1 and Table S1). Nonradial cells and RG-like cells with complex current patterns were never coupled (see SI Text and Figs. S2 and S3).
In conclusion, both in the juvenile and adult DG, a significant proportion of passive RG-like cells are coupled through gap junctions.
RG-Like Cells Express Different Connexin Isoforms.
In astrocytes, Cx30 and Cx43 are widely present in the adult brain, while Cx26 is expressed predominantly by subpial and subependymal astrocytes (16). While RG cells in the prenatal brain express Cx26 and Cx43 (8), no information is available about RG-like cells in the postnatal DG. To clarify whether RG-like cells express Cx43, we used a transgenic approach. Cx43fl/+/hGFAP-Cre/hGFAP-EGFP mice (p90) were generated expressing β-galactosidase (β-Gal) under control of regulatory elements of the Cx43 gene in hGFAP-Cre-positive cells (17), allowing indirect analysis of Cx43 expression in hGFAP-EGFP-positive cells (Fig. S4). Immunohistochemistry of nuclear β-Gal showed Cx43 expression in 53% of the fluorescent RG-like cells (10 out of 19 cells from 6 hippocampi) (see Fig. S4). Interestingly, this figure roughly matches the ratio of gap-junction coupling as revealed through biocytin filling of RG-like cells (see above). We noted that β-Gal immunoreactivity was also present in non-RG-like cells (n = 28) and in EGFP-negative cells. As previously suggested (17), activation of hGFAP-Cre at one time point is sufficient to delete floxed DNA, even in cells no longer showing hGFAP promoter activity. Thus, at the time of analysis, some cells may well express Cx43/β-Gal but not hGFAP-EGFP. The number of EGFP-positive cells in the SGZ of these mice was generally low.
To reveal the expression pattern of connexin isoforms in RG-like cells, Cx26, Cx30, and Cx43 were investigated on the transcript level. After biocytin filling, the cytoplasm of individual nestin-EGFP-positive RG-like cells was harvested and subjected to single-cell RT-PCR (Fig. S5, n = 16). Ten of these cells (63%) expressed at least 1 of the 3 isoforms (see Fig. S5). Detailed analysis yielded the following expression pattern: 8 out of 16 cells were Cx43 positive, 5 out of 16 cells expressed Cx30, and 3 out of 16 cells expressed Cx26. It is noteworthy that Cx26 mRNA was detected in some RG-like cells of the DG and that Cx43 was expressed most abundantly.
Deletion of Cx30 and Cx43 Decreases Proliferation and Granule Cell Numbers.
RG-like cells are proliferating although the turnover rate is relatively slow (18, 19) Accordingly, Ki67 immunohistochemistry and confocal analysis of z-stacks in the SGZ of hGFAP-EGFP mice (p11–p12) localized the proliferation marker only in 4.7% (38 out of 812 cells) of the fluorescent RG-like cells (Fig. S6). This finding was in agreement with a report using BrdU incorporation to estimate proliferation of RG-like cells in hGFAP-EGFP mice (3). Interestingly, the vast majority (118 out of 156 cells, 76%) of the EGFP/Ki67-positive (i.e., proliferative active cells) had a nonradial morphology.
To investigate whether connexin expression may influence proliferative activity in the adult SGZ, we assessed mice lacking Cx30 and Cx43 in GFAP-positive cells (Cx30–/–/Cx43fl/fl/hGFAP-Cre/dko mice), which exhibit complete uncoupling of astrocytes in the hippocampus (20). To check whether coupling between RG-like cells in dko mice was likewise affected, the biocytin filling protocol was applied to identified RG-like cells (see Materials and Methods). None of 6 RG-like cells tested in dko mice displayed tracer coupling (see Fig. S5F), demonstrating that genetic deletion of Cx30 and Cx43 not only inhibits tracer coupling between astrocytes in the hippocampus proper but between RG-like cells in the SGZ as well.
In adult dko mice, uncoupling of RG-like cells led to a dramatic (87%) reduction of Ki67-positive nuclei in the SGZ, as compared with wildtype C57BL/6 mice (Fig. 2 A and B; Fig. 3D, and Table S2). To confirm this finding we analyzed the incorporation of 5-bromo-2-deoxyuridine (BrdU) into the SGZ. Consistent with the Ki67 quantification we found a significant decrease (78%) of BrdU positive cells in the SGZ of dko versus C57BL/6 mice (Fig. 4 A and B).
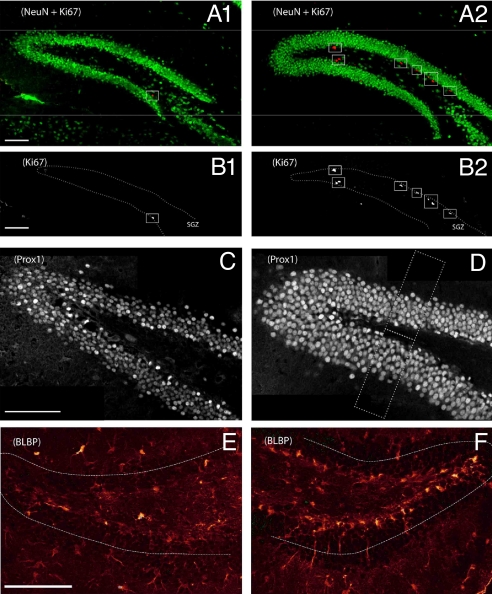
Fig. 2.
Reduced proliferation and cell numbers in the DG of dko mice (p60). Confocal images of the DG of a dko (A1, B1, C, E) and a C57BL/6 mouse (A2, B2, D, F). Note that the coronal slices represent approximately the same z-position in rostrocaudal extension of the hippocampus, respectively. (A1, A2) Ki67 (red) and NeuN (green) immunostaining. (B1, B2) Ki67 staining (positive cells are framed) in gray scale of the same section as in (A1, A2) indicates lower proliferative activity in the dko mouse (for quantification, see Table S2). (C, D) Prox1 staining of coronal sections. Reduced numbers of Prox1-positive cells were observed in the dko mouse (C) vs. control (D). Frames in (D) indicate counting boxes. (E and F) BLBP staining, dotted lines indicate position of the GCL. (Scale bars, 100 μm.)
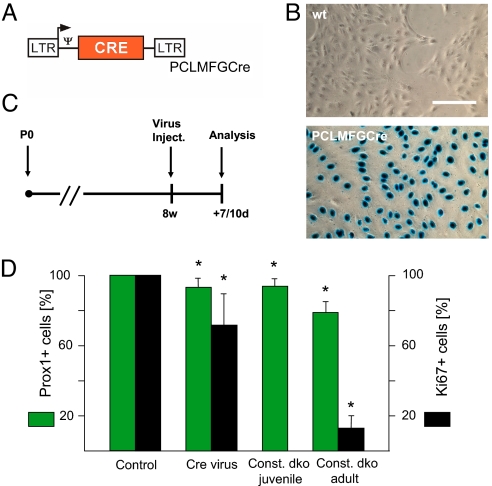
Fig. 3.
Ablation of connexins in RG-like cells decreases proliferation and neurogenesis in the adult DG. (A) Scheme depicting the Cre-expressing retroviral vector used. CRE, coding region of the Cre recombinase; LTR, long terminal repeat; arrow, transcription start site; Ψ, packaging signal. (B) Efficiency of Cre-mediated recombination after retroviral delivery in cultured cells. (Top) wildtype (wt); (Bottom) PCLMFGCre-infected CV1–5B cells. (Scale bar, 100 μm.) (C) Time schedule for Cre retrovirus application and subsequent analysis. (D) Histogram comparing the relative effects of inducible Cre retrovirus-mediated vs. constitutive ablation of connexins in Cx30–/–/Cx43fl/fl mice on numbers of Prox1- and Ki67-positive cells. Asterisks mark significant differences between ipsi- and contralateral side (virus injection; Cx30–/–/Cx43fl/fl mice) or dko and C57BL/6 mice (juvenile and adult, respectively).
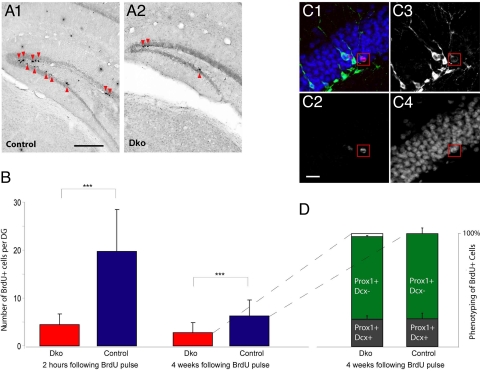
Fig. 4.
Decreased BrdU incorporation and neurogenesis in the DG of dko mice (p60). (A) Peroxidase based immunohistochemical BrdU-detection revealed significantly lower numbers of labeled cells (arrowheads) in dko (A2) vs. control mice (A1). Representative images were obtained from 40-μm thick coronal sections through the hippocampus 2 h after the last BrdU injection. (Scale bar, 250 μm.) (B) Quantification of BrdU-positive cells in the SGZ in the rostrocaudal extension. Bar graphs illustrate the number of BrdU-positive cells per DG of dko (red) and control mice (blue), 2 h and 4 weeks after the last BrdU injection. At both time points significant reduction of BrdU labeling was detected. (C) Confocal images of a cell expressing BrdU (C2), Dcx (C3), and Prox1 (C4), taken 4 weeks after the last BrdU injection. (C1) Merged image. (Scale bar, 20 μm.) (D) Percentage of BrdU-positive cells expressing Prox1 only, or Prox1 and Dcx 4 weeks after the last BrdU injection. Note that almost all BrdU-positive cells adopted a neuronal phenotype, and that the proportion of Prox1-positive/Dcx-positive cells (black) did not differ between dko and control mice. In dko mice 0 to 4%, of the BrdU-positive cells expressed neither Prox1 nor Dcx (Left bar, open).
RG-like cells in the adult SGZ give rise to neurons that differentiate and migrate into the GCL. To test whether the lack of astroglial connexin expression has an impact on the number of granule neurons in the DG, Prox1 and NeuN stainings were used. Comparative counting of Prox1 positive cells demonstrated a significant (21%) reduction of granule cells in dko vs. C57BL/5 mice. (Fig. 2 C and D, Fig. 3D, Table S3).
In addition, we mapped the fate of proliferating cells employing BrdU labeling. This revealed a 54% lower total number of BrdU labeled cells also expressing the neuronal marker Prox1 in dko mice, indicating a significant decrease of neurogenesis (Fig. 4 B and C). Importantly, almost all of the BrdU-positive cells expressed Prox1 (with or without Dcx) in dko and wild-type mice (Fig. 4D). The proportion of Prox1-positive/Dcx-positive cells among the BrdU-positive cells in dko and control mice remained unchanged (see Fig. 4D).
To investigate whether lack of Cx43 and Cx30 directly affects adult neurogenesis or rather causes indirect, developmental defects long before adult neurogenesis, Prox1 staining was performed in the DG of juvenile (i.e., 2-week-old) dko mice (n = 3 animals). In these mice, we found a much smaller (i.e., 6%) decrease of Prox1-positive neurons in the GCL as compared to C57BL/6 control mice, confirming a critical requirement of connexin expression for the generation of new granule neurons at postnatal stages later than p14 (see Fig. 3D and Table S3)
Inducible Ablation of Connexin Expression Decreases Proliferation and Granule Cell Numbers.
To further corroborate that diminished neurogenesis was the result of an inhibitory effect of connexin deletion in the postnatal brain, we performed intrahippocampal injections of a Cre-expressing retrovirus into Cx30–/–/Cx43fl/fl mice, which selectively infected proliferative cells at age p55 (see Fig. 3). Seven and 10 days after injection (n = 6 animals), this led to a significant ipsi- vs. contralateral decrease of Prox1-positive dentate granule cells (7%) and Ki-67 positive cells (29%) in the injected hippocampus of Cx30–/–/Cx43fl/fl mice in which Cre activity led to Cx43 ablation (see Fig. 3D). Notably, this is a rather strong effect, given the short timeframe of analysis. However, using genetic fate mapping, Lagace et al. (21) have shown that about 55% of the progeny derived from nestin-positive precursor cells were Dcx-positive within 12 days, while Ninkovic et al. (22) showed that about 50% of the progeny derived from GLAST-positive precursor cells were already Dcx-positive after 5 days following their Cre-inducible labeling. In control mice (C57BL/6, 129/Ola, n = 5 mice), virus injection had no effect. Likewise, injection of a GFP-expressing control retrovirus in Cx30–/–/Cx43fl/fl mice (n = 5) did not affect Prox1 positive cell numbers (see Tables S2 and S3).
Our findings demonstrate a critical requirement for connexin expression in dividing cells at 2 months of age. Because our analysis did not show coupling of non RG-like cells in the SGZ (Fig. S3 and see Table S1), the effect of the retrovirally delivered Cre recombinase must have been directed at the remaining proliferating cell type in the SGZ (i.e., RG-like cells).
Together, these data strongly suggest that connexins expressed by RG-like cells are involved in the regulation of neuronal cell numbers in the adult DG.
Deletion of Cx43 and Cx30 Affects RG-Like Cells.
To get a first hint as to whether loss of Cx30 and Cx43 affected the number or arrangement of RG-like cells in the adult DG, expression of the RG marker, brain lipid-binding protein (BLBP), was assessed in dko (n = 2 animals) vs. control mice (129/Ola, C57BL/6, n = 4 animals) at p70. The number of BLBP-positive RG-like cells in the SGZ was significantly decreased compared to controls (by 51.9% and 52.7% vs. C57BL/6 and 129/Ola, respectively) (Fig. 2 E and F). In the dko mice, many of the BLBP-positive cells displayed a nonradial morphology and might represent transient amplifying cells which initially may still show BLBP immunoreactivity as well (23).
Discussion
Our data demonstrate that a significant proportion of RG-like cells in the SGZ of the postnatal DG are gap-junction coupled, predominantly express Cx43 and Cx30, and that the deletion of these connexins in RG-like cells leads to impaired neurogenesis in the DG. Notably, decreased proliferation and neurogenesis was not only observed in constitutive dko mice but also after Cre virus-mediated inducible knockout of Cx43 in the adult DG, excluding that these changes were caused by indirect, prenatal developmental defects. To our knowledge this study is unique in identifying connexin expression and functional coupling between RG-like cells and revealing its impact on adult neurogenesis.
Using retroviral labeling, Seri et al. (13, 24) reported that RG-like cells in the SGZ proliferate to generate progeny, which in turn divide and form postmitotic cells differentiating into granule neurons. The formation of clusters called “radial proliferative units” suggests an organization of neurogenesis around RG-like cells and indicates a close interaction of precursor cells (13, 25). Proliferation and differentiation are depending upon the exchange of signals between distinct precursor cell types (26), although the pathways involved have not been clarified. An ultrastructural study supposed that RG-like cells were in contact with other cells through gap junctions (13), albeit a functional approach using tracer filling of individual cells failed to show coupling (4). In the present study we demonstrate that in the juvenile and adult DG, about 50% and 75% of the RG-like cells form functional gap junctions, respectively.
The tracer biocytin used here has a molecular weight of 373 Da and has been used in various previous astrocytic-coupling studies. A critical period of tracer loading is necessary to achieve reproducible steady-state conditions of tracer spread [20 min in case of single cell loading (see refs. 17 and 20)]. Thus, the discrepancy of our findings with those of ref. 4 might be because of shorter recording times and the higher molecular weight of the Alexa dye (731 Da) used in the latter study. Moreover, z-stack confocal analysis was necessary to detect the low number of coupled cells.
Recent evidence suggests that large linear molecules, like polypeptides and siRNA, might “wiggle through” gap junctions formed by Cx43, to control gene expression and other cellular functions (27, 28). Using complementary genetic approaches, we demonstrate that RG-like cells in the postnatal DG predominantly express Cx43 and, at a lower extent, Cx30 and Cx26. In embryogenesis, RG cells express Cx43 as well as Cx26 and couple into ventricular zone clusters (8). These clusters predominantly appear during neurogenesis within the immature brain and gap-junctional coupling is considered to regulate cell division. At this early developmental stage, connexin expression and coupling is a dynamic process related to the cell cycle, with maximal coupling in S and G2 phases (8, 29). According to our findings, RG-like cells in the adult DG form clusters and express connexins similar to embryonic RG cells. It still remains to be clarified whether in RG-like cells there is any relationship between connexin expression and the cell cycle.
The impact of connexin expression on neurogenesis in the mature brain has not been investigated yet. However, previous work yielded indirect evidence for such a link, by demonstrating that epileptiform activity induces proliferation of RG-like cells in the DG (3) as well as enhanced expression of Cx43 and coupling (30). In addition, several in vitro studies suggest a role of coupling in the control of precursor cell proliferation and migration (10, 11, 31). Using both an hGFAP-Cre transgene to target hippocampal progenitor cells from early development on and postnatally induced gene deletion in dividing cells by a Cre-expressing retrovirus, we now demonstrate that expression of Cx43 and Cx30 by RG-like cells is required for normal adult neurogenesis. Specifically, assessment of proliferation with the markers Ki67 and BrdU in adult dko mice revealed a massive decrease in the number of dividing cells. This decrease was much less pronounced in juvenile constitutive dko mice and could be provoked by induced retrovirus-mediated ablation of Cx43 in adult mice. Furthermore, fate analysis of BrdU-positive cells yielded a lower number of newborn neurons (≈50%), which was in line with the finding of an overall decreased number (≈20%) of Prox1-positive cells in the GCL of dko mice. Because the relative expression of Dcx, a marker associated with normal differentiation of newborn neurons in the DG, appeared to be unaffected in dko mice (see Fig. 4D), our findings suggest that survival or differentiation pathways were not significantly altered.
In this context it is important to note that only a subpopulation of the newly generated neurons integrates into the adult GCL, while the majority undergoes programmed cell death. This situation might explain why an almost complete inhibition of proliferation as observed in the dko mice caused only a limited, although significant, decline in Prox1-positive cells.
Which mechanisms might underlie the observed decrease in proliferation and neurogenesis? Gap junction coupling plays a crucial role in regulating neurogenesis, and deletion of Cx43 may disturb cell proliferation (32, 33). Indeed, in the neocortex of embryonic Cx43-deficient mice, changes in migration of neurons have been observed, which were however fully compensated during later development (34). The overall brain architecture of newborn (35) and adult mice lacking Cx43 in astrocytes was normal (36). The DG of adult constitutive dko mice also looked roughly normal, although our BLBP stainings revealed a clear reduction of RG-like cells in the SGZ, which might well account for the decreased proliferation. A recent study underlines the importance of gap junctions in embryonic development but raises a distinct possibility of their function. Accordingly, they provide dynamic adhesive contacts that interact with the internal cytoskeleton to enable leading process stabilization as well as translocation of the nucleus (12). Thus, it is conceivable that in the absence of Cx30 or Cx43 migration defects contribute to the decreased numbers of granule cells in the adult DG.
We show that combined deletion of Cx30 and Cx43 causes decreased proliferation in the adult DG. These changes may be especially relevant for regenerative processes, for example following epileptic seizures (3), and neurogenesis induced by learning or exposure to enriched environments (see ref. 37). Although the underlying mechanisms still need to be clarified, the requirement of connexin expression for normal adult neurogenesis reported here supports the view that embryonic (see ref. 38 for review) and postnatal proliferation share similar basic processes.
Together, our findings reveal a potential communication pathway of RG-like cells in the adult DG. Connexin expression by RG-like cells is necessary to maintain adult neurogenesis and proper development of the DG. Future work has to reveal the mechanism linking connexin expression and coupling to proliferation in the adult hippocampus.
Materials and Methods
Preparation of Brain Slices.
Experiments were carried out with transgenic hGFAP-EGFP mice (n = 22) (18) aged 9 to 12 and 90 to 120 postnatal days, transgenic nestin-EGFP mice (p90–p120, n = 42) (39), and transgenic Cx43fl/fl/hGFAP-Cre/Cx30–/– mice (p90–p120, n = 6) (20). All experiments were approved by legal authorities (Landesamt für Natur, Umwelt und Verbraucherschutz NRW, record token 9.93.2.10.31.07.139) and conform to national regulations. Coronal slices of the hippocampus were prepared and stored in artificial cerebrospinal fluid containing (in mM): 126 NaCl, 3 KCl, 2 MgSO4, 2 CaCl2, 10 glucose 1.25 NaH2PO4, 26 NaHCO3, equilibrated with 95% O2 and 5% CO2 to a pH of 7.4 at room temperature.
Patch-Clamp Recordings.
Patch-clamp recordings were performed as described (20). Pipettes had resistances of 3.5 to 6 MΩ when filled with recording solution consisting of (in mM) 130 K-gluconate, 20 Hepes, 3 Na2-ATP, 1 MgCl2, 10 EGTA at pH 7.26. Biocytin (0.5%, Nε-biotinyl-L-lysine; Sigma) was added to the pipette solution for intracellular labeling. Voltages were corrected for liquid junction potential and series resistance compensation of up to 60% was performed. Cells with hGFAP or nestin promoter activity were selected by means of their EGFP fluorescence. Visual control was achieved with a microscope equipped with infrared DIC (Axioskop, Zeiss). Only one cell per slice was recorded for 20 min, while the membrane was stepped to potentials between –160 and +70 mV. During voltage-clamp recordings, the holding potential (Vh) was –70 mV, if not stated otherwise.
Tissue Processing for Biocytin Staining.
After recording, slices were fixed overnight with 4% paraformaldehyde (PFA) in 0.1 M PBS (PBS) (pH 7.4, 4 °C), washed and transferred to PBS. After wash and rinsing, slices were treated with 10% normal goat serum (NGS), 0.5% Triton X-100 in PBS (pH 7.4, 2 h) and subsequently incubated overnight with streptavidin Cy3 (Sigma; 1:300 in 2% NGS, 0.1% Triton X-100 and PBS, pH 7.4, 4 °C) or, for Cx43fl/fl/hGFAP-Cre/Cx30–/– slices, with streptavidin Cy2 (Dianova; 1:200). After washing in PBS, slices were embedded in mounting medium (VectaShield, Vector). Before embedding, Cx43fl/fl/hGFAP-Cre/Cx30–/– slices were incubated in NeuroTrace deep-red fluorescent Nissl stain (Molecular Probes; 1:100 in 0.1% Triton X-100 and PBS, pH 7.4, 4 °C) for 30 min and washed.
Single-Cell RT-PCR.
Amplification of connexin cDNAs.
After recording, the content of the respective RG-like cell was harvested and processed (15). Multiplex 2-round single-cell PCR was performed with primers for Cx26, Cx30, Cx43, and β-actin (Table S4).
Restriction analysis.
The second PCR round was repeated and the product was purified and dissolved in 25-μl water. The PCR products of Cx26, Cx30, and Cx43 were digested with isoform specific restriction endonucleases HpaII (Fermentas), BbvI and AluI (New England Biolabs), respectively. Each product had 1 (Cx30) or 2 recognition sites yielding the predicted cDNA fragments (see Fig. S5A3). For digestion, 8 μl of the purified PCR product were incubated in 10 U restriction enzyme for 4 h at 37 °C. cDNA fragments were analyzed in 2% agarose 1000 using a 50-bp ladder as a length marker (Invitrogen).
Immunohistochemistry.
Coronal sections (50-μm thick) of perfused brains were used for immunohistochemistry and analysis of BrdU incorporation. See SI Text for details.
Application of Cre-Expressing Retrovirus.
Experiments were performed on 60- to 150-day-old C57BL/6J, 129/Ola and Cx30–/–/Cx43fl/fl male mice. Mice were anesthetized with a mixture of medetomidine (0.3 mg/kg, i.p.; CP-Pharma) and ketamine (40 mg/kg, i.p.; Medistar) and placed in a stereotactic frame equipped with a manual microinjection unit (TSE Systems GmbH). One microliter of PCLMFGCre or PCLMFGGFP (control) virus was stereotactically injected into the right hippocampus (anteroposterior, –1.9; mediolateral, −1.5; dorsoventral, −2.1 mm; with bregma as a reference) using a 10-μl microsyringe (Hamilton). Each injection was performed over a period of 10 min using a micrometer screw. After injection, the cannula was left in situ for additional 2 min to limit reflux along the cannula track. Afterward, the scalp incision was sutured and anesthesia was antagonized with atipamezole (300 mg/kg, i.p.; Pfizer). Seven or 10 days following the injection, mice were subjected to perfusion-fixation and immunostaining (see SI Text).
Quantification and Statistical Analysis.
Quantification of proliferating cells was based on regional analysis of the SGZ of the DG. Ki67-positive cells were quantified in a 1-in-5 series of sections totalling to 8 sections (i.e., 16 DG sections) throughout the rostrocaudal extension of the DG. Quantification of BrdU-positive cells in the SGZ and colocalization studies (see Fig. 4) were carried out as described (5). Prox1-positive cells were counted in a 1-in-5 series (1-in-3 for p14 mice) of sections with intervals of 250 μm throughout the rostrocaudal span of the GCL. BLBP-positive cells were also counted in a 1-in-5 series of sections, with a counting box of 220 × 170 × 30 μm that was located 50 μm beyond the tip of the hilus perpendicularly to the longitudinal axis of the GCL.
Numerical analyses were performed using SPSS 12.0 for Windows. Mean values of cells per dentate gyrus (Ki67+, Prox1+ or BrdU+) (see Tables S2 and S3) were calculated and assessed with the Mann−Whitney U or Kruskal-Wallis test for significant differences between groups. The χ2 test was used to compare coupling of passive vs. complex RG-like cells. Data are given as mean ± SD unless otherwise noted. P < 0.05 was considered statistically significant. More information is available in the SI Text .
Supplementary Material
Acknowledgments.
We thank M. Götz for helpful discussions, P. Dublin and L. Kaczmarczyk for help with fate-mapping analysis and validation of connexin deficiency, and I. Fiedler, T. Erdmann, and J. Fischer for excellent technical support. This work was supported by Deutsche Forschungsgemeinschaft SFB/TR3, C1, C9 (to C.S.) and N01, C9 (to M.T.); SFB 645, B2 (to K.W.); and SPP1172, SE 774/3 (to G.S. and C.S.), European Commission FP7-202167NeuroGLIA (to C.S.), Forschungskommission der Medizinischen Fakultät Universitätsklinikum Bonn (to M.R.C.), and Interdisziplinäres Zentrum für Klinische Forschung Jena 1.7 (to A.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813160106/DCSupplemental.
References
- 1.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Filippov V, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 3.Hüttmann K, et al. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci. 2003;18:2769–2778. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda S, et al. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23:9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunze A, et al. Proliferative response of distinct hippocampal progenitor cell populations after cortical infarcts in the adult brain. Neurobiol Dis. 2006;21:324–334. doi: 10.1016/j.nbd.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Tozuka Y, et al. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Bittman KS, LoTurco JJ. Differential regulation of connexin 26 and 43 in murine neocortical precursors. Cereb Cortex. 1999;9:188–195. doi: 10.1093/cercor/9.2.188. [DOI] [PubMed] [Google Scholar]
- 9.Weissman TA, et al. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron. 2004;43:647–661. doi: 10.1016/j.neuron.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Cheng A, et al. Gap junctional communication is required to maintain mouse cortical neural progenitor cells in a proliferative state. Dev Biol. 2004;272:203–216. doi: 10.1016/j.ydbio.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Duval N, et al. Cell coupling and Cx43 expression in embryonic mouse neural progenitor cells. J Cell Sci. 2002;115:3241–3251. doi: 10.1242/jcs.115.16.3241. [DOI] [PubMed] [Google Scholar]
- 12.Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 13.Seri B, et al. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- 14.Steinhäuser C, Jabs R, Kettenmann H. Properties of GABA and glutamate responses in identified glial cells of the mouse hippocampal slice. Hippocampus. 1994;4:19–36. doi: 10.1002/hipo.450040105. [DOI] [PubMed] [Google Scholar]
- 15.Matthias K, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theis M, Sohl G, Eiberger J, Willecke K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005;28:188–195. doi: 10.1016/j.tins.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Wallraff A, Odermatt B, Willecke K, Steinhäuser C. Distinct types of astroglial cells in the hippocampus differ in gap junction coupling. Glia. 2004;48:36–43. doi: 10.1002/glia.20040. [DOI] [PubMed] [Google Scholar]
- 18.Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- 19.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 20.Wallraff A, et al. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 2006;26:5438–5447. doi: 10.1523/JNEUROSCI.0037-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagace DC, et al. Dynamic contribution of nestin-expressing stem cells to adult neurogenesis. J Neurosci. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ninkovic J, Mori T, Gotz M. Distinct modes of neuron addition in adult mouse neurogenesis. J Neurosci. 2007;27:10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner B, et al. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- 24.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro LA, Korn MJ, Shan Z, Ribak CE. GFAP-expressing radial glia-like cell bodies are involved in a one-to-one relationship with doublecortin-immunolabeled newborn neurons in the adult dentate gyrus. Brain Res. 2005;1040:81–91. doi: 10.1016/j.brainres.2005.01.098. [DOI] [PubMed] [Google Scholar]
- 26.Song H, Stevens C, Gage F. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 27.Neijssen J, et al. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 28.Valiunas V, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bittman K, Owens DF, Kriegstein AR, LoTurco JJ. Cell coupling and uncoupling in the ventricular zone of developing neocortex. J Neurosci. 1997;17:7037–7044. doi: 10.1523/JNEUROSCI.17-18-07037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samoilova M, et al. Epileptiform activity in hippocampal slice cultures exposed chronically to bicuculline: increased gap junctional function and expression. J Neurochem. 2003;86:687–699. doi: 10.1046/j.1471-4159.2003.01893.x. [DOI] [PubMed] [Google Scholar]
- 31.Scemes E, Duval N, Meda P. Reduced expression of P2Y1 receptors in connexin43-null mice alters calcium signaling and migration of neural progenitor cells. J Neurosci. 2003;23:11444–11452. doi: 10.1523/JNEUROSCI.23-36-11444.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sutor B, Hagerty T. Involvement of gap junctions in the development of the neocortex. Biochim Biophys Acta. 2005;1719:59–68. doi: 10.1016/j.bbamem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Wiencken-Barger AE, Djukic B, Casper KB, McCarthy KD. A role for Connexin43 during neurodevelopment. Glia. 2007;55:675–686. doi: 10.1002/glia.20484. [DOI] [PubMed] [Google Scholar]
- 34.Fushiki S, et al. Changes in neuronal migration in neocortex of connexin43 null mutant mice. J Neuropathol Exp Neurol. 2003;62:304–314. doi: 10.1093/jnen/62.3.304. [DOI] [PubMed] [Google Scholar]
- 35.Dermietzel R, et al. Connexin43 null mice reveal that astrocytes express multiple connexins. Brain Res Rev. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 36.Theis M, et al. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci. 2003;23:766–776. doi: 10.1523/JNEUROSCI.23-03-00766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elias LA, Kriegstein AR. Gap junctions: multifaceted regulators of embryonic cortical development. Trends Neurosci. 2008;31:243–250. doi: 10.1016/j.tins.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi M, Saito H, Suzuki M, Mori K. Visualization of neurogenesis in the central nervous system using nestin promoter-GFP transgenic mice. NeuroReport. 2000;11:1991–1996. doi: 10.1097/00001756-200006260-00037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.