Abstract
A2A adenosine receptor (A2AAR) has been shown to suppress superoxide generation in leukocytes via the cAMP-protein kinase A (PKA) pathway. However, no study has yet explored the role of A2AAR in relation to NADPH oxidase in murine tracheas in vitro, which may lead to altered smooth muscle relaxation in asthma. Therefore, the present study evaluated the effects of A2AAR deficiency on the NADPH oxidase pathway in tracheas of A2A wild-type (WT) and A2A knockout (KO) mice. A2AWT mice were sensitized with ovalbumin (30 μg i.p.) on days 1 and 6, followed by 5% ovalbumin aerosol challenge on days 11, 12, and 13. A2AAR (gene and protein expression), cAMP, and phosphorylated PKA (p-PKA) levels were decreased in A2AWT sensitized mice compared with controls. A2AKO mice also showed decreased cAMP and p-PKA levels. A2AWT sensitized and A2AKO control mice had increased gene and protein expression of NADPH oxidase subunits (p47phox and gp91phox) compared with the controls. Tracheal relaxation to specific A2AAR agonist, 4-[2-[[6-amino-9-(N-ethyl-β-d-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid hydrochloride (CGS 21680), decreased in A2AWT sensitized mice compared with the controls, although it was absent in A2AKO mice. Pretreatment with NADPH oxidase inhibitors apocyanin/diphenyliodonium reversed the attenuated relaxation to CGS 21680 in A2AWT sensitized tracheas, whereas specific PKA inhibitor (9S,10S,12R)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i] [1,6]benzodiazocine-10-carboxylic acid hexyl ester (KT 5720) blocked CGS 21680-induced relaxation. Tracheal reactive oxygen species (ROS) generation was also increased in A2AWT sensitized and A2AKO control mice compared with the controls. In conclusion, this study shows that A2AAR deficiency causes increased NADPH oxidase activation leading to decreased tracheal relaxation via altered cAMP-PKA signaling and ROS generation.
Asthma, a chronic airways disease, is characterized by inflammation, airway hyper-responsiveness, and altered tracheal responsiveness to relaxing agents. These effects are mediated largely by the release of histamine, prostaglandins, cytokines, adenosine, and reactive oxygen species (ROS) from various cells, which include inflammatory leukocytes, epithelial cells, and airway smooth muscle cells (Busse and Lemanske, 2001; Barnes and Drazen, 2002; Nadeem and Mustafa, 2006). Production of ROS is associated both with intracellular signaling and the reproduction of many pathophysiologic features associated with asthma by altering the organization and function of cell membranes and increasing airway reactivity, airway secretions, vascular permeability, and the release of chemoattractants (Nadeem et al., 2008; van der Vliet, 2008).
Adenosine-mediated effects are exerted through the activation of the four different G-protein-coupled transmembrane cell surface adenosine receptors (ARs). Of these four ARs, most of the anti-inflammatory actions are attributed to A2AR activation (Lappas et al., 2005; Nadeem and Mustafa, 2006). A2AAR is coupled to a heterotrimeric Gs-protein that stimulates adenylyl cyclase, leading to intracellular cAMP accumulation and protein kinase A (PKA) activation (Fredholm et al., 2001). A2AAR-mediated responses in tracheal smooth muscle have not been explored previously, but other seven transmembrane receptors, such as β2-adrenergic receptor (β2-AR), have mechanisms of action similar to A2AAR. β2-AR has been reported to induce airway smooth muscle relaxation via PKA-induced phosphorylation of a number of specific target proteins. Phosphorylation of these proteins results in smooth muscle relaxation through effects on potassium and calcium channels, sodium/potassium ATPases, and calcium sensitivity of myosin (Shore and Moore, 2003). A2AAR-mediated relaxation via cAMP-PKA has been reported in smooth muscle from various organs (Gopalakrishnan et al., 2002; Huang et al., 2003; Radenković et al., 2005).
It is well known that A2AAR limits inflammation by inhibition of the proinflammatory cytokines, superoxide anion generation, and oxidative stress from monocytes, neutrophils, and other airway inflammatory cells (Lappas et al., 2005; Nadeem and Mustafa, 2006; Nadeem et al., 2007). However, no study has yet evaluated the effects of A2AAR on ROS/superoxide generation in murine tracheal smooth muscle. A2AAR-mediated suppression of ROS is mediated via cAMP-PKA signaling pathway in leukocytes (Sullivan et al., 2001), with some studies also showing the involvement of cAMP-PKA pathway in inhibition of NADPH oxidase (Bengis-Garber and Gruener, 1996; Kim et al., 2007); however, whether this pathway is functional in murine tracheal smooth muscle is not known. Moreover, previous studies in isolated leukocytes also did not investigate the effects of A2AAR on NADPH oxidase, which may be the main source of superoxide/ROS generation. Involvement of NADPH oxidase in regulation and production of ROS has now been shown in different smooth muscle cells, including airway smooth muscle cell (Thabut et al., 2002; Sturrock et al., 2007; van der Vliet, 2008). Moreover, it has also been shown that cAMP-mediated relaxation of smooth muscle is impaired by ROS (Erdös et al., 2004; Bubolz et al., 2005), so it is likely that ROS generated by NADPH oxidase has an inhibitory effect on A2AAR-mediated tracheal relaxation in allergic mice.
Therefore, this study was designed to investigate the effects of A2AAR on tracheal smooth muscle relaxation in a murine model of asthma (Nadeem et al., 2007; Ponnoth et al., 2008). Furthermore, effects of cAMP-PKA and NADPH oxidase pathways were evaluated on tracheal smooth muscle by using this model of asthma. Our data show for the first time that A2AWT sensitized mice have decreased A2AAR expression, cAMP, and phosphorylated PKA levels and concurrently have increased NADPH oxidase expression and superoxide/ROS generation leading to decreased tracheal relaxation. A2AKO control mice also show effects similar to A2AWT sensitized mice. This suggests that decreased A2AAR-cAMP-PKA signaling leads to decreased tracheal relaxation via increased NADPH oxidase activation in allergen-sensitized and -challenged mice.
Materials and Methods
Mice Sensitization and Challenge. A2AKO mice were obtained from the Institute of Experimental Medicine (C. Ledent, Universite Libre de Bruxelles, Brussels, Belgium). C57BL/6 (A2AWT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). A2AKO mice were backcrossed 12 generations to the C57BL/6 background. A2AKO mice were generated and genotyped by polymerase chain reaction (PCR) as described previously (Ledent et al., 1997).
A2AKO and A2AWT mice of mixed sexes, 12 to 16 weeks of age, were used in all experiments. All mice were housed in a pathogen-free facility with 12-h day and night cycle. All experimental animals used in this study were subject to a protocol approved by the Institutional Animal Care and Use Committee of West Virginia University.
Sensitization was performed according to the protocol from this laboratory described previously with slight modification (Oldenburg and Mustafa, 2004; Mustafa et al., 2007; Nadeem et al., 2007; Ponnoth et al., 2008). Mice were sensitized on days 1 and 6 with intraperitoneal injections of ovalbumin (Sigma-Aldrich, St. Louis, MO) at 30 μg per dose with 200 μl of Imject Alum (Pierce Chemical, Rockford, IL). Nonsensitized control animals only received the Imject alum with the same volumes. Ten days after sensitization, the mice were placed in a Plexiglas chamber and challenged with 5% aerosolized ovalbumin, or with 0.9% saline as a control, by use of an ultrasonic nebulizer (DeVilbiss Healthcare, Somerset, PA) for 20 min, both in the morning and afternoon, for 3 days. On day 14, mice were sacrificed for organ bath, molecular, and biochemical studies on the trachea. The aerosolization of allergen was performed at a flow rate of 2 ml/min, and the aerosol particles had a median aerodynamic diameter of less than 4 μm (De Vilbiss Healthcare).
Mice were assigned to the following groups: wild-type control group (A2AWT Control), mice received only vehicle for sensitization and challenge; wild-type sensitized group (A2AWT Sensitized), mice were sensitized and challenged with ovalbumin by use of the above-described protocol; and knockout control group (A2AKO Control), mice received only vehicle for sensitization and challenge. A2AWT mice sensitized and challenged with allergen is denoted as “A2AWT sensitized” throughout this article.
Relaxation and Contraction Experiments in Isolated Trachea. In brief, mice were sacrificed by intraperitoneal injection (0.1 ml of pentobarbitone sodium, 200 mg/ml), tracheas were excised, dissected free of surrounding tissue, and cut into two transverse rings (approximately 3–4 mm). Tracheal rings were then mounted on two stainless steel hooks in organ baths containing normal Krebs-Henseleit buffer, pH 7.4 (composition, 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 1.8 mM CaCl2, and 11 mM glucose), continuously aerated with 95% O2 and 5% CO2, and maintained at 37°C. During the dissection, tissue was immersed in ice-cold oxygenated buffer. Tracheal rings were suspended with a resting tension of 800 mg and were allowed to equilibrate for 60 min, with a complete change of buffer every 20 min. After tracheal rings were stimulated with 60 mM KCl several times to produce reproducible contractions, the rings were allowed to return to baseline for 20 min and then stimulated with KCl again to produce a submaximal contraction until it reached a stable plateau. Relaxation to selective A2AAR agonist CGS 21680 (10-11-10-6 M) was then performed by a cumulative concentration response curve (CRC). Pretreatments of equilibrated rings included selective A2AAR antagonist ZM 241385 (10-5 M) (Ansari et al., 2007), NADPH oxidase inhibitors apocynin and diphenyliodonium (10-5 M) (Thabut et al., 2002), and specific PKA inhibitor KT 5720 (10-7 M) (Ay et al., 2006). These inhibitors were added 30 min before CRC with A2AAR agonist and were present throughout the experiment. CRC with pinacidil (10-10-10-5 M; K+ATP channel opener) was also run with and without PKA inhibitor KT 5720 (10-7 M). For tracheal contractility experiments, responsiveness of tracheal rings to methacholine was also assessed (positive control). Concentrations of the agonists were not increased until the response to the previous concentration had stabilized, typically 7 to 8 min after administration of previous concentration. Relaxation/contraction responses of the tracheal rings were expressed as a percentage increase/decrease in the contraction with respect to KCl (100%) in response to each concentration of agonist used. Isometric tension was measured by force displacement transducers (BIOPAC Systems Inc., Santa Barbara, CA) connected to BIOPAC MP100 data acquisition and analysis hardware from BIOPAC Systems Inc. (Obiefuna et al., 2005; Ansari et al., 2007; Ponnoth et al., 2008).
Tracheal Reactive Oxygen Species and Superoxide Generation. For reactive oxygen species generation in tracheal rings, the rings in organ bath buffer were incubated with 100 μM 6-carboxy-2′,7′-dichlorofluorescin diacetate (Sigma-Aldrich) for 30 min at 37°C. 6-Carboxy-2′,7′-dichlorofluorescin diacetate forms a fluorescent product, dichlorofluorescein (DCF), on oxidation with ROS. Fluorescence caused by DCF in each well was measured and recorded for 15 min at 485 nm (excitation) and 530 nm (emission) by using a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT), with temperature maintained at 37°C (Wang and Joseph, 1999). The background fluorescence caused by buffer and DCF was subtracted from the total fluorescence in each well caused by tracheal rings in the presence of DCF. Fluorescence intensity units were then normalized by milligrams of tissue for each tracheal ring and expressed as arbitrary fluorescence units per milligram of tissue. In addition, some tracheal rings for ROS generation experiments were treated with PKA inhibitor KT 5720 (10-7 M), A2AAR antagonist ZM 241385 (10-5 M), NADPH oxidase inhibitor diphenyliodonium (DPI) (10-5 M), and polyethylene glycol-conjugated superoxide dismutase (PEG-SOD; 100 U/ml).
For superoxide generation in tracheal rings, the rings in organ bath buffer were incubated with 25 μM dihydroethidium (DHE; Invitrogen, Carlsbad, CA) for 30 min at 37°C. DHE fluorescence caused by superoxide generation in each well was measured and recorded for 15 min at 480 nm (excitation) and 570 (emission) using a Synergy HT Multi-Mode Microplate Reader (BioTek Instruments Inc.) with temperature maintained at 37°C (Peshavariya et al., 2007). The background fluorescence caused by buffer and DHE were subtracted from the total fluorescence in each well caused by tracheal rings in the presence of DHE. Fluorescence intensity units were then normalized by milligrams of tissue for each tracheal ring and expressed as arbitrary fluorescence units per milligram of tissue.
Immunohistochemistry for A2AAR on Tracheal Smooth Muscle. Lungs along with tracheas taken from all of the groups were removed and fixed in picric acid formaldehyde fixative for 3 h followed by three rinses with phosphate-buffered saline (PBS; 100 mM, pH 7.8)-Tx (0.3%). After embedding the tracheal tissue in OCT Tissue-Tek (Sakura Finetek Europe, Zoeterwoude, The Netherlands), it was then rapidly frozen by immersion in isopentane (Thermo Fisher Scientific, Waltham, MA) cooled by liquid nitrogen. Next, 10-μm sections of the tracheal dorsal surface (the dorsal surface of the trachea was oriented uppermost on the cork supports so that tracheal smooth muscle could be sectioned in a coronal plane) were cut from the cryostat blocks onto poly-l-lysine-coated glass slides (Thermo Fisher Scientific). Slides with tracheal smooth muscle sections were incubated with an anti-A2AAR polyclonal rabbit IgG (Marala and Mustafa, 1998) developed in our laboratory for detection of A2AAR protein at a dilution of 1:100 in PBS-Tx + 1% bovine serum albumin (PBS-Tx-BSA, pH 7.8) in a humidified chamber at 4°C overnight. They were then rinsed three times with PBS-Tx, allowing 5 min per rinse, and covered with fluorescein isothiocyanate-labeled goat anti-rabbit IgG (Zymed Laboratories, South San Francisco, CA) diluted 1:100 in PBS-Tx-BSA and incubated at 37°C for 45 min. After that, the coverslips were rinsed three times in PBS-Tx and were mounted on glass slides in Fluoromount (Southern Biotechnology Associates, Birmingham, AL). The coverslips with tracheal smooth muscle were then observed with an Olympus AX70 fluorescence microscope (Olympus America, Melville, NY) equipped with fluorescein (excitation wavelengths, 455–500 nm; emission wavelengths, >510 nm). Nonspecific background labeling was determined by omission of primary antisera.
Immunoblotting for Tracheal A2AAR, Phosphorylated PKA, and Subunits of NADPH Oxidase (p47phox and gp91phox). Tracheas from different groups were homogenized with six volumes of ice-cold tissue lysis buffer consisting of 0.05 M Tris-buffered saline, pH 7.4, 1% Triton X-100, 0.25% sodium deoxycholate, 150 mM sodium chloride, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM sodium orthovanadate, and 1 mM sodium fluoride (Sigma-Aldrich). Homogenized samples were centrifuged for 30 min at 12,000g at 4°C and the supernatant was stored at -80°C for Western blot experiments.
Aliquots of the tracheal supernatant (40 μg protein/well) were separated on 10% SDS-PAGE. Prestained protein molecular markers (20–112-kDa low range) were run in parallel. Proteins were transferred to nitrocellulose membranes and then probed with either anti-phospho-PKA rabbit polyclonal IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for detection of the active form of catalytic PKA or anti-gp91phox mouse monoclonal IgG (BD Biosciences, San Jose CA), anti-p47phox rabbit polyclonal IgG (Santa Cruz Biotechnology, Inc.), anti-A2AAR rabbit polyclonal IgG (Marala and Mustafa, 1998) at a dilution of 1:1000, or anti-β-actin rabbit polyclonal IgG (Santa Cruz Biotechnology, Inc.) at a dilution of 1:5000. This was followed by the incubation with the secondary horseradish peroxidase-conjugated antibodies (anti-mouse and anti-rabbit immunoglobulins from goat for gp91phox and others, respectively; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) for 1 h at room temperature. For detection of bands, the membranes were treated with enhanced chemiluminescence reagent (GE Healthcare) for 1 min and subsequently exposed to ECL Hyperfilm. Relative band intensities were quantified by densitometry (Alpha Innotech Corp., San Leandro, CA), and each sample was normalized to the β-actin values. Western blot values are expressed as percentage of control after densitometric analysis.
Real-Time PCR for Tracheal A2AAR, PKA, and Subunits of NADPH Oxidase Subunits (p47phox and gp91phox). Total RNA was isolated from the trachea by using the TRIzol reagent from Invitrogen followed by DNase treatment to eliminate potential genomic DNA contamination as described previously (Ansari et al., 2007; Nadeem et al., 2007; Ponnoth et al., 2008). This was followed by conversion of 0.5 μg of total RNA into cDNA by using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions in a total volume of 100 μl. Real-time PCR was performed on an ABI PRISM 7300 Detection System (Applied Biosystems) using Taqman Universal Mastermix (Applied Biosystems). In brief, the reaction volume (25 μl) included 12.5 μl of 2× Taqman Universal Mastermix, 1 μl of cDNA, and 1.25 μl of 20× FAM-labeled Taqman gene expression assay master mix solution. For the real-time PCR for A2AAR, PKA, and subunits of NADPH oxidase (p47phox and gp91phox) genes, the Taqman inventoried assays-on-demand gene expression products were purchased from Applied Biosystems, and 18S rRNA (Ribosomal RNA; GenBank accession number X03205) was used as an endogenous control. In addition, some tracheas from A2AWT control and A2AWT sensitized mice were denuded of epithelium (epithelium was denuded by inserting a thin wire into the lumen of the trachea and rubbing it over a soaked blotting paper) to delineate the role of smooth muscle and epithelium on A2AAR, gp91phox, and p47phox expression. The fold difference in expression of target cDNA was determined by using the comparative CT method. The fold difference in gene expression of the target was calculated as described previously (Livak and Schmittgen, 2001).
Measurement of Tracheal cAMP and Protein Concentration. cAMP and protein levels in tracheal homogenates were measured by using an enzyme immunoassay kit from Assay Designs (Ann Arbor, MI) and a Bradford assay kit from Bio-Rad (Hercules, CA), respectively.
Materials. Unless stated otherwise, all chemicals were of the highest grade available and were purchased from Sigma-Aldrich. CGS 21680, ZM 241385, KT 5720, pinacidil, and DPI were dissolved in dimethyl sulfoxide, whereas apocynin and methacholine were dissolved in distilled water.
Statistical Analysis. The data were expressed as mean ± S.E.M. Comparisons among different groups were analyzed by analysis of variance followed by Tukey's multiple comparison tests. Comparison between two groups was assessed by unpaired t test. A p value of less than 0.05 was considered significant for all statistical tests. All statistical analyses were performed by using GraphPad Prism statistical package (GraphPad Software, San Diego, CA).
Results
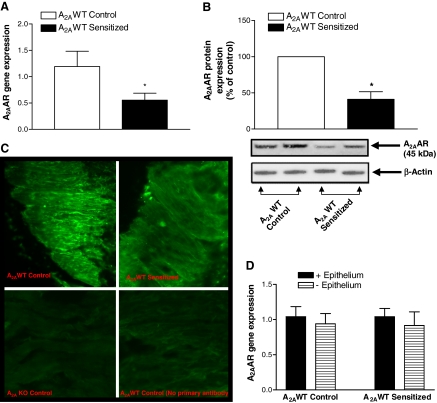
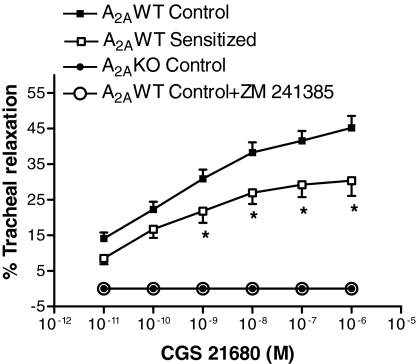
Tracheal Expression of A2AAR, Relaxation Responses to CGS 21680, a Specific A2AAR Agonist, and Tracheal Reactivity to Methacholine. As shown in Fig. 1, A and B, tracheal expression of A2AAR was significantly lower in A2AWT sensitized mice than in A2AWT control mice, as assessed by real-time PCR and Western blot analysis, respectively. Expression of A2AAR was also confirmed by immunohistochemistry, which showed A2AAR immunostaining in A2AWT control. A2AAR immunostaining was decreased in A2AWT sensitized tracheal smooth muscle (Fig. 1C). However, A2AKO tracheal smooth muscle showed no immunostaining for A2AAR as shown in Fig. 1C. Real-time PCR and Western blot analysis did not detect any A2AAR mRNA transcripts and protein expression, respectively, in trachea of A2AKO mice (data not presented). Epithelial denudation did not affect the expression of A2AAR both in A2AWT control and A2AWT sensitized tracheas (Fig. 1D). Organ bath studies showed concentration-dependent tracheal relaxation to CGS 21680, a specific agonist for A2AAR in A2AWT control mice (maximal relaxation reaching 45% at 10-6 M). CGS 21680-induced tracheal relaxation was lower in A2AWT sensitized (maximal relaxation reaching 30% at 10-6 M) compared with A2AWT control mice (Fig. 2). Pretreatment of tracheal rings with specific A2AAR antagonist, ZM 241385 (10-5 M) abolished CGS 21680-induced relaxation in A2AWT control mice. However, A2AKO tracheal rings showed no relaxation response to CGS 21680 (Fig. 2). These data show that the presence of A2AAR on tracheal smooth muscle is associated with relaxation. Furthermore, A2AWT sensitized mice have decreased CGS 21680-induced tracheal relaxation because of down-regulation of A2AAR.
Fig. 1.
Expression of A2AAR by real-time PCR (n = 7–10) (A) and Western blot analysis (n = 3–4) (B) in tracheas of A2AWT control and A2AWT sensitized mice. C, expression of A2AAR by immunohistochemistry (n = 4) in tracheas of A2AWT control, A2AWT sensitized, and A2A KO control mice. D, expression of A2AAR before and after epithelial denudation in A2AWT control and A2AWT sensitized tracheas (n = 4). For gene expression by the comparative CT method using real-time PCR, the A2AWT Control column was used as the calibrator against which all other groups were compared. C represents fluorescent photomicrographs for A2AAR immunostaining (green staining) on tracheal smooth muscle (magnification, 400×). There was no expression of message for A2AAR either at gene or protein level in A2A KO mice. Values are expressed as mean ± S.E.M. *, p < 0.05 compared with A2AWT control.
Fig. 2.
Concentration-response curve for A2AAR agonist CGS 21680 in tracheas of A2AWT control (n = 25), A2AWT sensitized mice (n = 12; □) and A2A KO control mice (n = 6). ZM 241385, a specific A2AAR antagonist (10-5 M), completely abolished CGS 21680-induced tracheal relaxation in A2AWT control mice (n = 6; ○). Values are expressed as mean ± S.E.M. *, p < 0.05 compared with A2AWT control.
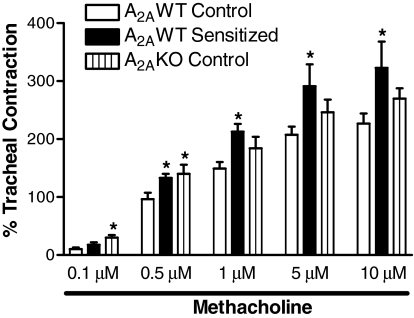
Because A2AAR might also affect airway reactivity through different mechanisms, we tested the effects of methacholine on tracheal reactivity in the three study groups. A2AWT sensitized mice showed increased tracheal contraction to methacholine compared with A2AWT control mice (Fig. 3). Further, A2AKO control trachea also showed increased tracheal contraction compared with A2AWT control mice, thus suggesting that A2AAR deficiency also increases tracheal responsiveness to nonspecific contractile stimuli.
Fig. 3.
Effect of methacholine on tracheal reactivity in A2AWT control (n = 12), A2AWT sensitized (n = 8), and A2A KO control mice (n = 8). Values are expressed as mean ± S.E.M. *, p < 0.05 compared with A2AWT control.
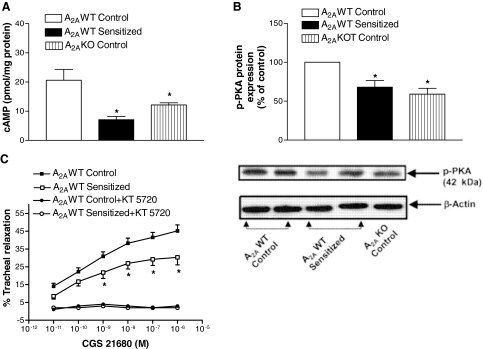
Tracheal Protein Kinase A Expression, cAMP Levels, and the Effect of PKA Inhibitor on CGS 21680-Induced Relaxation. We investigated the molecular mechanism of A2AAR-mediated tracheal relaxation in mice. Decreased expression of A2AAR in A2AWT sensitized mice and deletion in A2AKO control mice were associated with decreased cAMP levels compared with A2AWT control (Fig. 4A). Treatment of A2AWT control tracheas with CGS 21680 (10-6 M) led to an increase of 171 ± 12% in cAMP levels over A2AWT control-untreated tracheas. ZM 241385 (10-5 M) reversed CGS 21680-induced increase in cAMP levels (105 ± 5%). We also assessed the effects of a specific activator of adenylate cyclase, forskolin (10-5 M), to test the possibility of whether decreased cAMP in A2AWT sensitized tracheas is due to alteration in adenylate cyclase activity. Forskolin-induced increase in A2AWT control and A2AWT sensitized tracheas was almost equal (271 ± 9 and 263 ± 22%, respectively) from corresponding baseline, suggesting that down-regulation of A2AAR is responsible for decreased cAMP production in A2AWT sensitized mice. cAMP is typically associated with activation of PKA, a family of Ser/Thr kinases that plays diverse roles in regulation of smooth muscle function; therefore, the effects of decreased cAMP levels were assessed on PKA gene expression and protein phosphorylation. Decreased tracheal cAMP levels in the A2AWT sensitized and A2AKO control mice led to decreased phosphorylation of PKA (Fig. 4B), whereas PKA gene expression by real-time PCR was equal in A2AWT control, A2AWT sensitized, and A2AKO control tracheas (data not shown). KT 5720 (10-7 M), a specific inhibitor of PKA, almost completely abolished the CGS 21680-induced relaxation both in A2AWT control and A2AWT sensitized mice (Fig. 4C), suggesting that cAMP-PKA signaling is responsible for A2AAR-mediated tracheal relaxation in mice. To rule out the nonspecific effects of PKA inhibitor KT 5720 on tracheal relaxation, pinacidil (K+ATP channel opener) was used. Pinacidil is a smooth muscle relaxant that does not elicit its effects through cAMP-PKA pathway. Pinacidil-induced tracheal relaxation in the A2AWT control group was almost similar in the presence (EC50 = 1.01 × 10-6 M) and absence of KT 5720 (EC50 = 1.18 × 10-6 M). Overall, these data suggest that decreased CGS 21680-mediated relaxation in A2AWT sensitized tracheas is caused by decreased cAMP-PKA signaling.
Fig. 4.
cAMP levels (n = 6) (A) and protein expression of p-PKA (n = 4) (B) in tracheas of A2AWT control, A2AWT sensitized, and A2A KO control mice. C, effect of specific PKA inhibitor KT 5720 (10-7 M) on A2AAR agonist CGS 21680-induced relaxation responses in tracheas of A2AWT control (n = 6) and A2AWT sensitized mice (n = 6). KT 5720 almost completely abolished CGS 21680-induced tracheal relaxation in both A2AWT control and A2AWT sensitized mice. Values are expressed as mean ± S.E.M. Values are expressed as mean ± S.E.M. *, p < 0.05 compared with A2AWT control.
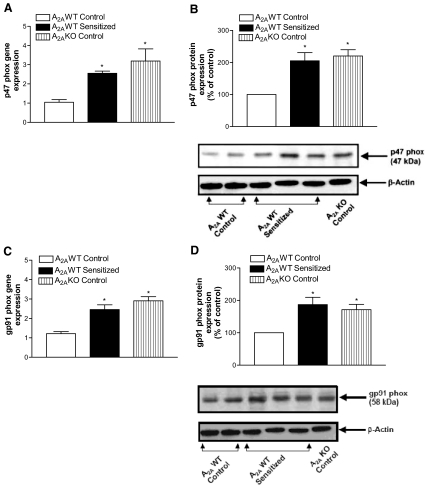
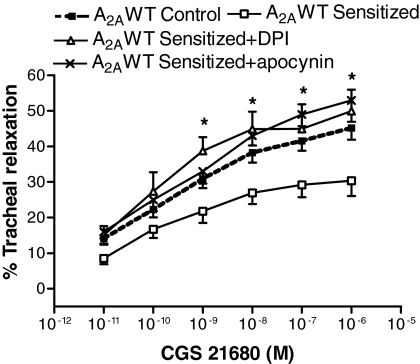
Tracheal Expression of NADPH Oxidase Subunits (gp91phox and p47phox) and Effect of NADPH Oxidase Inhibitors on CGS 21680-Induced Relaxation. Because cAMP-PKA signaling is known to be involved in regulation of reactive oxygen species, the effects of A2AAR deficiency were assessed on NADPH oxidase, the enzyme responsible for superoxide production. Decreased cAMP and p-PKA levels led to increased gene and protein expression for p47phox (Fig. 5, A and B) and gp91phox (Fig. 5, C and D), subunits of NADPH oxidase in both A2AWT sensitized and A2AKO control mice compared with A2AWT control. Epithelial denudation both in A2AWT control and A2AWT sensitized tracheas did not make any significant difference in the expression of NADPH oxidase subunits (gp91phox and p47phox; data not shown). Organ bath experiments showed that pretreatment with NADPH oxidase inhibitor DPI or apocynin (10-5 M) reversed the attenuation in CGS 21680-mediated tracheal relaxation in A2AWT sensitized tracheas to that observed in A2AWT control tracheas (Fig. 6). These data suggest that decreased A2AAR-mediated tracheal relaxation is through increased NADPH oxidase expression and is uniquely coupled with this receptor.
Fig. 5.
Expression of NADPH oxidase subunits, p47phox (A and B) and gp91phox (C and D) by real-time PCR (n = 7–10) and Western blot analysis (n = 4–6), respectively, in tracheas of A2AWT control, A2AWT sensitized, and A2A KO control mice. For gene expression by comparative CT method using real-time PCR, the A2AWT control column was made as the calibrator against which all other groups were compared. Values are expressed as mean ± S.E.M. *, p < 0.05 compared with A2AWT control.
Fig. 6.
Effect of NADPH oxidase inhibitors apocynin (10-5 M)/diphenyliodonium (DPI, 10-5 M) on A2AAR agonist CGS 21680-induced relaxation responses in tracheas of A2AWT sensitized mice (n = 8). Values are expressed as mean ± S.E.M. *, p < 0.05 compared with A2AWT sensitized mice.
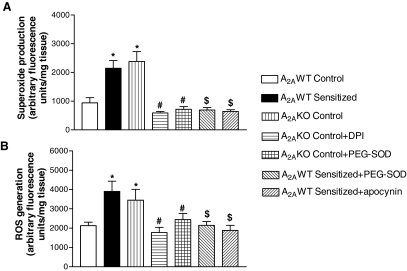
Tracheal Reactive Oxygen Species and Superoxide Generation. Increased activity of NADPH oxidase due to deficiency of A2AAR was also confirmed by measuring ROS and superoxide generation from tracheal rings. A2AWT sensitized and A2AKO control mice had increased tracheal superoxide (Fig. 7A) and ROS generation (Fig. 7B) compared with A2AWT control. PEG-SOD (100 U/ml) decreased tracheal ROS and superoxide generation both in A2AWT sensitized and A2AKO control, thus confirming superoxide as the source of increased ROS/superoxide production (Fig. 7, A and B). ROS production in epithelium-intact trachea (2228 ± 176 arbitrary fluorescence units/mg tissue; n = 6) did not differ significantly from epithelium-denuded trachea (2407 ± 143 arbitrary fluorescence units/mg tissue; n = 6) in A2AWT control mice. Increased ROS/superoxide generation in A2AWT sensitized and A2AKO control mice (Fig. 7, A and B) was also restored to normal levels by pretreating the tracheal rings with DPI/apocynin (NADPH oxidase inhibitors). Effects of A2AAR antagonism and PKA inhibition were also assessed on ROS generation. Treatment of tracheal rings with PKA inhibitor KT 5720 (10-7 M) or A2AAR antagonist ZM 241385 (10-5 M) led to 195 ± 18 and 182 ± 13% increased ROS generation, respectively, from the baseline in A2AWT control mice. These data suggest that both PKA inhibition and A2AAR deficiency/antagonism are associated with increased ROS generation. Overall, these data suggest that decreased A2AAR-cAMP-PKA signaling leads to increased tracheal ROS/superoxide generation in mice.
Fig. 7.
Detection of end products of NADPH oxidase activation by measuring superoxide (n = 6–8) (A) and ROS production (n = 6–8) (B) in tracheas of A2AWT control, A2AWT sensitized, and A2A KO control mice. The effect of NADPH oxidase inhibitors apocynin (10-5 M)/DPI (10-5 M) and PEG-SOD (100 U/ml) is also shown. Values are expressed as mean ± S.E.M. *, p < 0.05 compared with A2A WT control; $, p < 0.05 compared with A2AWT sensitized; #, p < 0.05 compared with A2AKO control.
Discussion
This is the first study to show that A2AAR down-regulation as assessed by real-time PCR, Western blot analysis, and immunohistochemistry leads to decreased tracheal relaxation through NADPH oxidase activation in allergen-sensitized and -challenged trachea in mice. This was confirmed by increased production of ROS/superoxide and expression of NADPH oxidase subunits in tracheal rings from A2AWT sensitized mice compared with A2AWT control mice. Pretreatment with NADPH oxidase inhibitors (apocyanin/diphenyliodonium) also reversed the attenuated tracheal relaxation to CGS 21680 in A2AWT sensitized trachea. Furthermore, decreased CGS 21680-induced tracheal relaxation in A2AWT sensitized mice was due to decreased cAMP-PKA signaling. Epithelial denudation had no effect on the expression of A2AAR and NADPH oxidase subunits (gp91phox and p47phox) in both A2AWT control and A2AWT sensitized trachea. A2AKO control mice showed no gene and protein expression for A2AAR and, as expected, no relaxation response to CGS 21680.
The seven transmembrane-spanning receptors (7-TSRs) represent the largest signaling family in the genome. It is estimated that the lung expresses 25 to 50 7-TSRs in airway epithelial cells, airway smooth muscle, pulmonary vasculature, alveolar walls, and resident immune cells (Green and Liggett, 1996). In regard to asthma, several 7-TSRs play established roles in bronchoconstriction (M3-muscarinic receptor) and bronchodilation (β2-adrenergic receptor). Despite identification of the endogenous ligands and receptor localization, there are a number of 7-TSRs expressed in the airways whose functions are unknown in relaxation that include A2AAR. This lack of understanding of A2AAR function has impeded our ability to ascertain the role of this receptor in the modulation of airway relaxation/contraction. Thus, the biochemical basis of bronchial hyper-reactivity and bronchoconstriction in asthma remains only partially understood. Expression of A2AAR on tracheal smooth muscle was confirmed by real-time PCR and by immunoblotting and immunohistochemical analyses in A2AWT control and A2AWT sensitized mice. Tracheal smooth muscle of A2AKO mice showed the absence of this gene and protein expression. However, A2AWT sensitized mice showed significantly less protein and gene expression for A2AAR in tracheal smooth muscle compared with A2AWT control mice. CGS 21680, a specific A2AAR agonist caused concentration-dependent tracheal relaxation in A2AWT control tracheas, whereas A2AWT sensitized tracheas showed decreased relaxation to this agonist. This study shows for the first time that A2AAR, which is one of the members of 7-TSRs, is expressed on tracheal smooth muscle and is functionally involved in the relaxation of smooth muscle. Furthermore, it shows that A2AWT sensitized mice have decreased tracheal relaxation because of the down-regulation of A2AAR.
The role of A2AAR-PKA signaling was also evaluated in tracheal relaxation. Pretreatment of tracheal rings with KT 5720, a specific inhibitor of PKA, almost completely abolished CGS 21680-induced tracheal relaxation both in A2AWT control and A2AWT sensitized mice. Earlier studies have also shown the involvement of A2AAR in smooth muscle relaxation from different organs via the cAMP-PKA pathway (Cushing et al., 1991; Haynes, 2000; Gopalakrishnan et al., 2002; Radenković et al., 2005), but this is the first study to show its involvement in tracheal smooth muscle relaxation. A2AWT sensitized and A2AKO control had decreased cAMP and p-PKA levels, thus suggesting that the deficiency of A2AAR leads to decreased tracheal relaxation due to altered cAMP-PKA signaling. Specificity of PKA pathway in A2AAR-mediated tracheal relaxation was also confirmed by using pinacidil (K+ATP channel opener) in the presence and absence of PKA inhibitor KT 5720. Pinacidil-induced tracheal relaxation was similar both in the presence and absence of KT 5720, further strengthening our observation that A2AAR acts specifically through the cAMP-PKA pathway.
Because A2AAR-induced cAMP elevation is coupled not only to relaxation, as shown in the present study, but also to the inhibition of inflammation that could alter airway reactivity (Lappas et al., 2005; Nadeem and Mustafa, 2006), we assessed the effect of A2AAR deficiency on tracheal reactivity. Our data showed that the deficiency of A2AAR also leads to increased tracheal reactivity to methacholine not only in A2AWT sensitized but also in A2AKO control groups. This could be due to the increased release of ROS/oxidative stress and proinflammatory cytokines, both of which have been shown to enhance airway reactivity (Nadeem and Mustafa, 2006; Nadeem et al., 2008). An earlier study from our laboratory has also reported an increase in Penh in vivo to methacholine both in A2AWT sensitized and A2AKO control mice (Nadeem et al., 2007).
Some previous studies reported the effects of A2AAR on superoxide/ROS production but they were mostly conducted on isolated leukocytes. These studies showed that A2AAR-mediated suppression of superoxide/ROS is mainly via cAMP-PKA pathway (Visser et al., 2000; Sullivan et al., 2001; Varani et al., 2002, 2005); however, these studies did not investigate the source of ROS generation. Because activation of PKA is coupled to multiple pathways, one of them being inhibition of NADPH oxidase (Bengis-Garber and Gruener, 1996; Kim et al., 2007), it was hypothesized that decreased relaxation in A2AWT sensitized mice could be due to increased NADPH oxidase activation via altered cAMP-PKA signaling. Real-time PCR and Western blot data showed increased tracheal expression of p47phox and gp91phox, subunits of NADPH oxidase in A2AWT sensitized and A2AKO control mice compared with A2AWT control mice. Organ bath data showed that pretreatment with NADPH oxidase inhibitors apocynin and DPI restored the impaired tracheal relaxation to CGS 21680 in A2AWT sensitized mice. Concurrently, ROS/superoxide generation, which are end products of NADPH oxidase activation, were assessed biochemically. Our data showed that ROS/superoxide generation was higher in A2AWT sensitized and A2AKO control tracheas than in A2AWT control tracheas. Pretreatment of A2AWT control tracheas with the PKA inhibitor KT 5720 also led to increased ROS/superoxide production, thus suggesting that PKA inhibition is involved in the activation of NADPH oxidase and impaired tracheal relaxation. However, these experiments were performed in whole-tissue samples, without taking into account the effect of infiltrating cells. Future experiments using airway smooth muscle cells will be important in delineating the contribution of airway smooth muscle without the infiltrating cells.
cAMP-PKA signaling leading to relaxation has been shown to be mediated by different mechanisms, one of these mechanisms being activation of ion channels, including potassium channels that are involved in the hyperpolarization and hence relaxation of smooth muscle in various organs (Miura et al., 1992; Haynes, 2000; Huang et al., 2003). Several studies have shown that ROS production in many diseases, such as diabetes and hypertension, is responsible for decreased relaxation to cAMP via inhibition of ion channels (Liu and Gutterman, 2002; Erdös et al., 2004; Bubolz et al., 2005). Our study also suggests that decreased cAMP-PKA signaling and subsequent activation of NADPH oxidase and ROS production in allergic mice could lead to decreased tracheal relaxation, possibly via inactivation/alteration of ion channels. However, this remains to be confirmed in future studies.
β2-ARs are similar to A2AAR because they are 7-TSRs; and both animal and human studies have shown that their density and activity are decreased by allergen challenge, leading to decreased responsiveness to relaxing agents (Motojima et al., 1989; Sato et al., 1990; Song et al., 1997). Therefore, increased airway reactivity and altered relaxation responses in patients with asthma may result from decreased density and expression of these two cAMP-elevating receptors that counteract inflammation and airway reactivity. In conclusion, this is the first study to show the presence of A2AAR on tracheal smooth muscle and its functional role in causing relaxation. Furthermore, it shows that allergen-sensitized and -challenged mice have decreased A2AAR-cAMP-PKA signaling leading to impaired tracheal relaxation via increased NADPH oxidase activation and ROS/superoxide generation.
Acknowledgments
We thank Kevin Roush, Shilpa Sakhalkar, and Courtney Williamson for technical assistance.
This work was supported in part by the National Institutes of Health [Grants HL027339, HL094447]; and by Bridge Grant Funding from the Health Sciences Center of West Virginia University.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.151613.
ABBREVIATIONS: ROS, reactive oxygen species; AR, adenosine receptor; β2-AR, β2-adrenergic receptor; KO, knockout; PKA, protein kinase A; 7-TSR, seven transmembrane-spanning receptor; WT, wild-type; CRC, concentration response curve; PCR, polymerase chair reaction; DCF, dichlorofluorescein; DHE, dihydroethidium; PBS, phosphate-buffered saline; BSA, bovine serum albumin; DPI, diphenyliodonium; ZM 241385, 4-(2-[7-amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol; KT 5720, (9S,10S,12R)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid hexyl ester; CGS 21680, 4-[2-[[6-amino-9-(N-ethyl-β-d-ribofuranuronamidosyl)-9H-purin-2-yl]amino]ethyl]benzenepropanoic acid hydrochloride; PEG-SOD, polyethylene glycol-conjugated superoxide dismutase; Tx, Triton-X.
References
- Ansari HR, Nadeem A, Talukder MA, Sakhalkar S, and Mustafa SJ (2007) Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol 292 H719-H725. [DOI] [PubMed] [Google Scholar]
- Ay B, Iyanoye A, Sieck GC, Prakash YS, and Pabelick CM (2006) Cyclic nucleotide regulation of store-operated Ca2+ influx in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 290 L278-L283. [DOI] [PubMed] [Google Scholar]
- Barnes PJ and Drazen JM (2002) Pathophysiology of asthma, in Asthma and COPD (Barnes PJ, Drazen JM, Rennard S eds) pp 343-359, Academic Press, London.
- Bengis-Garber C and Gruener N (1996) Protein kinase A downregulates the phosphorylation of p47 phox in human neutrophils: a possible pathway for inhibition of the respiratory burst. Cell Signal 8 291-296. [DOI] [PubMed] [Google Scholar]
- Bubolz AH, Li H, Wu Q, and Liu Y (2005) Enhanced oxidative stress impairs cAMP-mediated dilation by reducing Kv channel function in small coronary arteries of diabetic rats. Am J Physiol Heart Circ Physiol 289 H1873-H1880. [DOI] [PubMed] [Google Scholar]
- Busse WW and Lemanske RF Jr (2001) Asthma. N Engl J Med 344 350-362. [DOI] [PubMed] [Google Scholar]
- Cushing DJ, Brown GL, Sabouni MH, and Mustafa SJ (1991) Adenosine receptor-mediated coronary artery relaxation and cyclic nucleotide production. Am J Physiol 261 H343-H348. [DOI] [PubMed] [Google Scholar]
- Erdös B, Simandle SA, Snipes JA, Miller AW, and Busija DW (2004) Potassium channel dysfunction in cerebral arteries of insulin-resistant rats is mediated by reactive oxygen species. Stroke 35 964-969. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, and Linden J (2001) International union of pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53 527-552. [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan SM, Buckner SA, Milicic I, Groebe DR, Whiteaker KL, Burns DJ, Warrior U, and Gopalakrishnan M (2002) Functional characterization of adenosine receptors and coupling to ATP-sensitive K+ channels in Guinea pig urinary bladder smooth muscle. J Pharmacol Exp Ther 300 910-917. [DOI] [PubMed] [Google Scholar]
- Green SA and Liggett SB (1996) G protein coupled receptor signalling in the lung, in The Genetics of Asthma (Liggett S and Meyers D eds) pp 67-90, Marcel Dekker Inc., New York.
- Haynes JM (2000) A(2A) adenosine receptor mediated potassium channel activation in rat epididymal smooth muscle. Br J Pharmacol 130 685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Chan FL, Lau CW, Tsang SY, Chen ZY, He GW, and Yao X (2003) Roles of cyclic AMP and Ca2+-activated K+ channels in endothelium-independent relaxation by urocortin in the rat coronary artery. Cardiovasc Res 57 824-833. [DOI] [PubMed] [Google Scholar]
- Kim JS, Diebold BA, Babior BM, Knaus UG, and Bokoch GM (2007) Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14–3-3 binding. J Biol Chem 282 34787-34800. [DOI] [PubMed] [Google Scholar]
- Lappas CM, Sullivan GW, and Linden J (2005) Adenosine A2A agonists in development for the treatment of inflammation. Expert Opin Investig Drugs 14 797-806. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, and Parmentier M (1997) Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2A receptor. Nature 388 674-678. [DOI] [PubMed] [Google Scholar]
- Liu Y and Gutterman DD (2002) The coronary circulation in diabetes: influence of reactive oxygen species on K+ channel-mediated vasodilation. Vascul Pharmacol 38 43-49. [DOI] [PubMed] [Google Scholar]
- Livak KJ and Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25 402-408. [DOI] [PubMed] [Google Scholar]
- Marala RB and Mustafa SJ (1998) Immunological characterization of adenosine A2A receptors in human and porcine cardiovascular tissues. J Pharmacol Exp Ther 286 1051-1057. [PubMed] [Google Scholar]
- Miura M, Belvisi MG, Stretton CD, Yacoub MH, and Barnes PJ (1992) Role of potassium channels in bronchodilator responses in human airways. Am Rev Respir Dis 146 132-136. [DOI] [PubMed] [Google Scholar]
- Motojima S, Yukawa T, Fukuda T, and Makino S (1989) Changes in airway responsiveness and beta- and alpha-1-adrenergic receptors in the lungs of guinea pigs with experimental asthma. Allergy 44 66-74. [DOI] [PubMed] [Google Scholar]
- Mustafa SJ, Nadeem A, Fan M, Zhong H, Belardinelli L, and Zeng D (2007) Effect of a specific and selective A(2B) adenosine receptor antagonist on adenosine agonist AMP and allergen-induced airway responsiveness and cellular influx in a mouse model of asthma. J Pharmacol Exp Ther 320 1246-1251. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Fan M, Ansari HR, Ledent C, and Jamal Mustafa S (2007) Enhanced airway reactivity and inflammation in A2A adenosine receptor-deficient allergic mice. Am J Physiol Lung Cell Mol Physiol 292 L1335-L1344. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Masood A, and Siddiqui N (2008) Oxidant–antioxidant imbalance in asthma: scientific evidence, epidemiological data and possible therapeutic options. Ther Adv Respir Dis 2 215-235. [DOI] [PubMed] [Google Scholar]
- Nadeem A and Mustafa SJ (2006) Adenosine receptor antagonists and asthma. Drug Discov Today Ther Strat 3 269-275. [Google Scholar]
- Obiefuna PC, Batra VK, Nadeem A, Borron P, Wilson CN, and Mustafa SJ (2005) A novel A1 adenosine receptor antagonist, L-97-1 [3-[2-(4-aminophenyl)-ethyl]-8-benzyl-7-{2-ethyl-(2-hydroxyethyl)-amino]-ethyl}-1-propyl-3,7-dihydro-purine-2,6-dione], reduces allergic responses to house dust mite in an allergic rabbit model of asthma. J Pharmacol Exp Ther 315 329-336. [DOI] [PubMed] [Google Scholar]
- Oldenburg P and Mustafa SJ (2004) Involvement of A1 adenosine receptors in adenosine induced bronchoconstriction in an allergic mouse model [Abstract]. Am J Respir Crit Care Med 169 A197. [Google Scholar]
- Peshavariya HM, Dusting GJ, and Selemidis S (2007) Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res 41 699-712. [DOI] [PubMed] [Google Scholar]
- Ponnoth DS, Nadeem A, and Mustafa SJ (2008) Adenosine-mediated alteration of vascular reactivity and inflammation in a murine model of asthma. Am J Physiol Heart Circ Physiol 294 H2158-H165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radenkoviæ M, Grboviæ L, Pesiæ S, and Stojiæ D (2005) Isolated rat inferior mesenteric artery response to adenosine: possible participation of Na+/K+-ATPase and potassium channels. Pharmacol Rep 57 824-832. [PubMed] [Google Scholar]
- Sato T, Bewtra AK, Hopp RJ, Nair N, and Townley RG (1990) Alpha- and beta-adrenergic-receptor systems in bronchial asthma and in subjects without asthma: reduced mononuclear cell beta-receptors in bronchial asthma. J Allergy Clin Immunol 86 839-850. [DOI] [PubMed] [Google Scholar]
- Shore SA and Moore PE (2003) Regulation of beta-adrenergic responses in airway smooth muscle. Respir Physiol Neurobiol 137 179-195. [DOI] [PubMed] [Google Scholar]
- Song P, Milanese M, Crimi E, Rehder K, and Brusasco V (1997) Allergen challenge of passively sensitized human bronchi alters M2 and beta2 receptor function. Am J Respir Crit Care Med 155 1230-1234. [DOI] [PubMed] [Google Scholar]
- Sturrock A, Huecksteadt TP, Norman K, Sanders K, Murphy TM, Chitano P, Wilson K, Hoidal JR, and Kennedy TP (2007) Nox4 mediates TGF-beta1-induced retinoblastoma protein phosphorylation, proliferation, and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 292 L1543-L1555. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Rieger JM, Scheld WM, Macdonald TL, and Linden J (2001) Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A(2A) receptor agonists. Br J Pharmacol 132 1017-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabut G, El-Benna J, Samb A, Corda S, Megret J, Leseche G, Vicaut E, Aubier M, and Boczkowski J (2002) Tumor necrosis factor-alpha increases airway smooth muscle oxidants production through a NADPH oxidase-like system to enhance myosin light chain phosphorylation and contractility. J Biol Chem 277 22814-22821. [DOI] [PubMed] [Google Scholar]
- van der Vliet A (2008) NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med 44 938-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani K, Gessi S, Merighi S, Iannotta V, Cattabriga E, Spisani S, Cadossi R, and Borea PA (2002) Effect of low frequency electromagnetic fields on A2A adenosine receptors in human neutrophils. Br J Pharmacol 136 57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani K, Portaluppi F, Gessi S, Merighi S, Vincenzi F, Cattabriga E, Dalpiaz A, Bortolotti F, Belardinelli L, and Borea PA (2005) Caffeine intake induces an alteration in human neutrophil A2A adenosine receptors. Cell Mol Life Sci 62 2350-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser SS, Theron AJ, Ramafi G, Ker JA, and Anderson R (2000) Apparent involvement of the A(2A)subtype adenosine receptor in the anti-inflammatory interactions of CGS 21680, cyclopentyladenosine, and IB-MECA with human neutrophils. Biochem Pharmacol 60 993-999. [DOI] [PubMed] [Google Scholar]
- Wang H and Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27 612-616. [DOI] [PubMed] [Google Scholar]