Abstract
The objectives of the study were to determine the pharmacokinetics of oxymorphone (oxy) and of ammonium sulfate-loaded, liposome-encapsulated oxymorphone (LE-ASG oxy) and to evaluate the behavioral effects of both opioid preparations by using ethographic evaluation specific to rhesus monkeys. Rhesus monkeys (n = 8) were injected with 2.0 mg/kg LE-ASG oxy s.c.. Blood samples were collected at serial time points up to 144 h in six monkeys and up to 456 h in two monkeys. Separate groups of monkeys were injected with 0.1 mg/kg oxy s.c. (n = 4) or i.v. (n = 5). Blood samples were collected at serial time points up to 24 h after injection. Pharmacokinetic parameters were calculated by using commercially available software. Behavior was recorded in a different group of 10 monkeys administered LE-ASG oxy (2.0 mg/kg s.c.) or oxy (0.1 mg/kg s.c.) on separate occasions. Behavioral evaluations were made at serial time points while monkeys were in an extended cage with a compatible stimulus animal. Oxymorphone was rapidly eliminated from the serum in the oxy group. Measurable drug was present in serum for up to 4 h after oxy was administered subcutaneously or intravenously. LE-ASG oxy was present in serum in measurable concentrations for more than 2 weeks. Neither oxy nor LE-ASG oxy produced observable sedation. LE-ASG oxy decreased some environmentally directed behaviors, but this drug formulation increased watchfulness, decreased self-directed and elimination behaviors, increased nonspecific social contact, and decreased threat behaviors. LE-ASG oxy persisted for an extended period in rhesus monkey serum and produced behavioral changes consistent with this opioid.
Most currently available opioids exhibit short in vivo half-lives that require repeated dosing every few hours to maintain therapeutic blood concentrations and analgesic effects in laboratory animals (Hawk and Leary, 2005). Extended release opioid preparations have milder adverse effects, because there is less bolus release into the system after their administration than for the standard pharmaceutical preparations of the same drugs, and they can provide longer periods of analgesia (Krugner-Higby et al., 2003; Smith et al., 2003). The decreased adverse effects may be caused by lower peak drug concentrations and less fluctuation in serum concentrations over a dosing interval.
Opioid drugs are known to produce similar physiologic and behavioral effects in human beings and nonhuman primates (Jaffe and Matin, 1985). Monkeys, especially rhesus macaques, are widely used in behavioral pharmacology and addiction research (Weerts et al., 2007). Behaviors exhibited in a novel environment and in a social context may identify more differences in behavioral repertoire than observations of monkeys in their home cages (152 × 71 × 79 cm) (Capitanio, 1999; Capitanio et al., 2006). Thus, monkeys provide a sophisticated behavioral model for assessing the potential utility of opioid formulations for human use.
In our previous work, we developed liposomal formulations of hydromorphone, oxymorphone, and butorphanol that can provide pain control for periods as long as 3 to 4 days. Such preparations are appropriate for analgesia in perioperative patients (Smith et al., 2004, 2006, 2008; Sladky et al., 2006). These formulations can be administered subcutaneously and do not require epidural administration to provide extended analgesia, as is the case with other less stable liposomal formulations (Viscusi et al., 2005, 2006). The inclusion of cholesterol and the use of high-phase-transition temperature phospholipids were necessary to achieve a 4-day release period (Smith et al., 2008). Opioid formulations that can be administered systemically and have even longer release characteristics would be useful in treating chronic pain. This has led us to explore methods to further improve the stability and release characteristics of liposomal opioid preparations (Webb et al., 2007).
In the present study, we hypothesized that the use of ammonium sulfate gradient loading would considerably extend the effective release period of an opioid drug (oxymorphone) encapsulated into liposomes. We tested our hypothesis by determining serum concentrations of the drug over time after it was administered to healthy rhesus macaques and compared serum concentration versus time curves with those after standard pharmaceutical oxymorphone was administered. The study was a partial crossover design because it was impossible to know a priori what time interval should be used after administration of LE-ASG oxy before administration of another drug. We also recorded the behavioral effects of a single subcutaneous injection of LE-ASG oxy compared with a subcutaneous injection of the standard pharmaceutical preparation of oxymorphone.
Materials and Methods
Animals. Subjects were rhesus macaques of Indian origin from the breeding colony at the Harlow Center for Biological Psychology at the University of Wisconsin-Madison. The University of Wisconsin Animal Care and Use Committee approved all experiments described, and all studies were conducted within U.S. Department of Agriculture and Association for Assessment of Laboratory Animal Care guidelines. Monkeys used for pharmacokinetic studies were male or female and more than 3 years old (n = 4–5 for oxy; n = 10 for LE-ASG oxy). All of the animals used for behavioral studies were male or female, more than 3 years old (n = 10/group), and currently living in a compatible pair, so they could be placed with their familiar con-specific in the extended cage without physical aggression. The monkeys were healthy and apparently free of infectious disease at the time of experimental assignment.
Liposome Encapsulation by Ammonium Sulfate Gradient Loading. A mixture of 80 μmol of dipalmitoylphosphatidylcholine and 40 μmol of cholesterol (Avanti Polar Lipids, Alabaster, AL) was dried by removing trichloromethane, dissolved in 1 ml of sterile tert-butanol (Sigma-Aldrich, St. Louis, MO) by heating to 55°C in a water bath, frozen in a dry ice-isopropyl alcohol mixture, and lyophilized for 24 h. Oxymorphone (42.2 mg; U.S. Pharmacopeia, Rockville, MD) was dissolved in double-processed tissue culture water (Sigma-Aldrich) and brought to pH 7.0 (42.2 mg/ml, 1.0 ml) and sterilized by using a 0.22-mm filter (Millipore, Billerica, MA). Ammonium sulfate (240 mM or 3.17 g/100 ml; AMEND Drug and Chemical Company, Irvington NJ) was dissolved in double-processed tissue culture water (Sigma Aldrich). Then the solution was filtered by using a 250-ml, 0.22-mm filter (Nalgene, Rochester, NY). The solution (31.7 mg/ml, 1.0 ml) was used to swell liposomes in a 55°C water bath for 1 h. The liposomes were frozen in dry ice-isopropyl alcohol for 2 min and stored at -20°C overnight. The liposomes were thawed at room temperature, washed in 0.9% sodium chloride, pH 5.5 (Baxter Healthcare Corporation, Deerfield, IL), and sedimented at 100,000g for 30 min at 4°C in an ultracentrifuge (model L8M; Beckman Coulter, Fullerton, CA). The supernatant was removed and the liposome pellet was resuspended in 1 ml of oxymorphone (42.2 mg) and placed in a 55°C water bath for 1 h. After this, the excess oxymorphone was removed by washing the liposomes with more 0.9% sodium chloride, pH 5.5 (Baxter Healthcare Corporation) and sedimented at 100,000g for 30 min at 4°C in the Beckman ultracentrifuge. The supernatant was removed, and the liposome pellet was resuspended in 1 ml of 10 mM sodium ethanoate buffer, pH 4.0. Encapsulation efficiency of oxymorphone was quantified via spectrophotometric analysis at 281 nm against a known concentration of oxymorphone. Each batch was determined to have a specific milligram per milliliter concentration before animal experiments. Injectable oxymorphone was obtained from a commercial source (Endo Laboratories, Chadds Ford, PA).
Drug Administration and Sample Collection. A single dose of a liposomal drug is higher, in milligrams per kilogram, than a single dose of an immediate release drug, but the cumulative dose over the extended time period is similar. Liposomal opioid drugs are typically administered at dosages that are at least 10 times the parenteral dose for the free drug. The dose used in this study was based on published data in dogs (Smith et al., 2008) and preliminary studies in rhesus macaques (L. Krugner-Higby, unpublished data). Oxy was administered as an intravenous injection into a saphenous vein by an experienced person (B. Schmidt). Subcutaneous injections were made into the loose skin caudal to the scapula. All injections were made with a 3-ml syringe attached to a 22-gauge 1-inch needle. Monkeys administered 2 mg/kg LE-ASG oxy were not treated with another drug preparation because we did not know what the appropriate washout period between drug treatments should be for these animals until after the pharmacokinetic data had been analyzed. All other monkeys had at least a 3-week washout period between drug treatments. Monkeys were not used for behavior testing at the same time as they were used for pharmacokinetics studies.
Blood samples were collected from conscious monkeys in a squeeze restrainer from the saphenous or femoral veins into serum separator tubes (Vacutainer SST, 5 ml; BD Medical, Franklin Lakes, NJ). The serum was separated after centrifugation at 1000g for 15 min at 10°C. The serum was stored frozen at -70°C until analysis. Blood samples from monkeys administered 0.1 mg/kg oxy, either intravenously (n = 4) or subcutaneously (n = 5) on separate occasions, were collected before drug administration and at 5, 10, 15, 20, 30, and 45 min and 1, 1.5, 2, 4, 6, and 8 h after intravenous drug administration. Blood samples were collected from monkeys (n = 8) receiving 2.0 mg/kg LE-ASG oxy before drug administration and at daily intervals for 144 h. Two additional monkeys were sampled to 456 h.
Liquid Chromatography Tandem Mass Spectrometry Analysis for Serum Oxymorphone Concentration. Serum samples were analyzed by liquid chromatography tandem mass spectrometry (Acquity TQD; Waters, Milford, MA) for oxymorphone (m/z 302→283.7) according to published methods (KuKanich et al., 2008a,b). Solid-phase extraction (Varian Bond Elut C18; Varian, Inc., Palo Alto, CA) of the serum (200 μl) was used to extract drug from serum. The mobile phase consisted of ammonium acetate and methanecarbonitrile with a linear gradient. Oxymorphone d3 (m/z 305→286.8) was used as an internal standard. The lower limit of quantification of the assay was 0.1 ng/ml. The accuracy (mean ± S.D.) was within 8 ± 5% of the actual concentration, and the coefficient of variation was 5 ± 1% on replicates of five each at 0.1, 5, and 50 ng/ml.
Pharmacokinetic parameters were estimated with computer software (WinNonlin 5.0; Pharsight, Mountain View, CA) using noncompartmental analysis. The estimated variables included the area under the curve from time 0 to infinity (AUC0-inf) and the area under the curve from 0 to the last time point (AUC0-last) by using the linear trapezoidal rule. The area under the first moment curve from time 0 to infinity (AUMC0-inf), and area under the first moment curve from time 0 to the last measured time point (AUMC0-last), serum clearance (Cl), apparent volume of distribution at steady state (Vss), apparent volume of distribution of the area during the elimination phase (Vz), first-order rate constant (λz), terminal half-life (t1/2 λz), mean residence time extrapolated to infinity (MRT0-inf), mean residence time from 0 to the last measured time point (MRT0-last), maximum serum concentration (CMAX), and time to maximum serum concentration (TMAX) were also estimated. The Vz per fraction of the dose absorbed (Vz · F-1) and clearance per fraction of the dose absorbed (Cl · F-1) were calculated for subcutaneous treatments. The concentration at time 0 (C0) was calculated by log-linear regression using the first two time points.
Playroom Social Interaction and Behavioral Analysis. Pharmacologic experiments indicated that clinically relevant subcutaneous doses of oxy and LE-ASG oxy did not produce gross behavioral changes associated with opioid sedation. Therefore, behavioral experiments were designed by use of broad, generalized ethographic scoring techniques rather than an ethogram that was focused toward grading opioid-induced sedative effects. Differences from baseline (expressed as percentage change) were used for data analysis to control for individual variation between animals and for the different amounts of habituation to the testing environment because both members of compatible pairs were tested sequentially.
Monkeys were presented with a familiar conspecific between 8:00 AM and 12:00 PM in an extended enclosure measuring 304.2 × 71.1 × 78.7 cm. The monkeys were allowed to interact in the enclosure for 1 h, during which time they were observed and scored by using a standard ethographic program designed for rhesus macaques (Hypercard, 2.3) on a laptop computer (iBook G4; Apple Computer, Cupertino, CA). Similar ethograms have been used for behavioral toxicology studies (Ferguson et al., 1993). Behaviors were scored for frequency of occurrence during the testing period and for duration if applicable. Observations were done unblinded by B. Schmidt and four other observers, all of whom were trained by the same person (B. Schmidt). Liposomal opioid preparations were white, required refrigeration, were drawn into the syringe at the time of administration, and were difficult to disguise from observers. Observers were trained for several weeks. After memorizing the behavior key, observers practiced real-time observations with groups of monkeys in their home cage. Observers began data collection only after they were proficient in both behavior recognition and the scoring program. Data were reviewed periodically throughout the study to detect any inconsistencies. A t test (p > 0.05) performed on baseline data from the same monkeys did not detect significant differences between observers. Monkeys were observed in the playroom daily (three baseline values) for 1 h between 8:00 AM and 12:00 PM before any drug administration. Monkeys were baseline-tested in the weeks before drug administration. Monkeys were introduced to the extended cage on at least 3 and up to 11 occasions before testing, often as a compatible stimulus animal for the monkey that was being tested. Monkeys were never tested for baseline or post-treatment after having been in the extended cage the same day in the role of a stimulus animal. This ensured that monkeys were never in the extended cage for more than 1 h per day. Pairs were tested on separate occasions immediately after subcutaneous injection with either 0.1 mg/kg oxy or 2.0 mg/kg LE-ASG oxy (0 h) and then for the next 4 days after drug administration. Monkeys administered LE-oxy were tested for 4 days because, according to the pharmacokinetic data, the serum concentration of drug had reached a plateau by that time. Differences (expressed as percentage change) from baseline values for ethographic data were analyzed by using Wilcoxon's signed rank test run on SPSS (SPSS Inc., Chicago, IL). Significance was inferred at p < 0.05.
The following are generic or chemical names of some compounds used in these studies: oxymorphone, 4,5α-epoxy-3,14-dihydroxy-17-methylmorphinan-6-one; dipalmitoylphosphatidylcholine, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; and cholesterol, (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl 17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol. The chemical name is used in the text for all other compounds.
Results
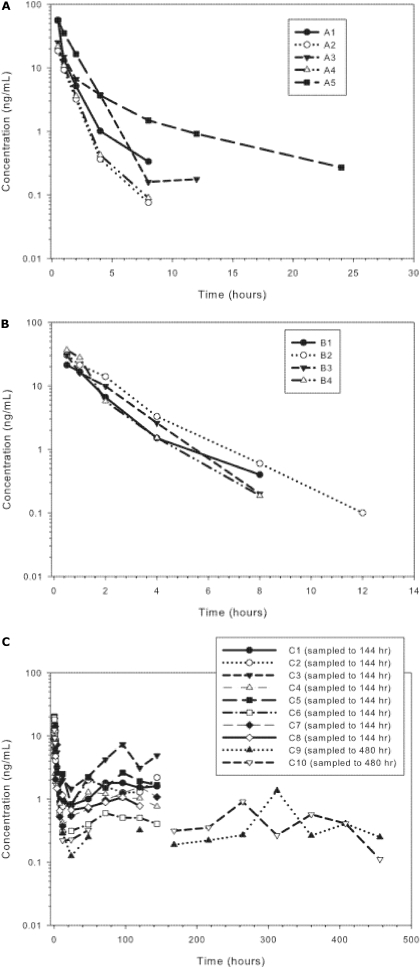
Pharmacokinetics of Oxy in Rhesus Macaques. oxy had a half-life of approximately 1.5 h and an MRT of 1.7 h (Fig. 1A and Table 1) in rhesus macaques after administration of 0.1 mg/kg s.c. Drug concentrations decreased rapidly and were measurable (limit of quantification = 0.1 ng/ml) in only one animal 12 h postinjection. Injection of 0.1 mg/kg i.v. standard oxymorphone resulted in a plasma profile similar to standard oxymorphone injected subcutaneously (Fig. 1B and Table 2).
Fig. 1.
A, serum profile of oxymorphone after 0.1 mg/kg s.c. oxymorphone HCl to rhesus monkeys. B, serum profile of oxymorphone after 0.1 mg/kg i.v. oxymorphone HCl to rhesus monkeys. C, serum concentrations of oxymorphone after administration of 2 mg/kg s.c. in rhesus monkeys (n = 8 sampled to 144 h, and n = 2 sampled to 456 h).
TABLE 1.
Pharmacokinetic parameters of oxymorphone HCl (0.1 mg/kg s.c.) to healthy rhesus monkeys (n = 4)
|
Parameter
|
Units
|
Geometric Mean
|
Animal
|
|||
|---|---|---|---|---|---|---|
| 89040 | AN96 | AS60 | AT62 | |||
| AUCextrapolated | % | 0.88 | 0.62 | 0.55 | 2.52 | 0.71 |
| AUC0-last | h · ng · ml–1 | 45.08 | 50.10 | 64.69 | 38.20 | 33.35 |
| AUC0-inf | h · ng · ml–1 | 45.58 | 50.41 | 65.05 | 39.19 | 33.59 |
| AUMC0-inf | h · h · ng · ml–1 | 78.23 | 86.01 | 132.32 | 71.61 | 45.95 |
| AUMC0-last | h · h · ng · ml–1 | 72.77 | 83.03 | 127.12 | 60.96 | 43.59 |
| Cl · F–1 | ml · min–1 · kg–1 | 32.54 | 29.42 | 22.80 | 37.85 | 44.15 |
| Cmax | ng · ml–1 | 26.01 | 30.48 | 30.46 | 21.26 | 23.20 |
| t1/2 λz | h | 1.47 | 1.09 | 1.76 | 1.94 | 1.27 |
| λz | h–1 | 0.47 | 0.64 | 0.39 | 0.36 | 0.55 |
| MRT0-inf | h | 1.72 | 1.71 | 2.03 | 1.83 | 1.37 |
| MRT0-last | h | 1.61 | 1.66 | 1.97 | 1.60 | 1.31 |
| Tmax | h | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Vz · F–1 | liters · kg–1 | 4.15 | 2.78 | 3.47 | 6.36 | 4.85 |
TABLE 2.
Pharmacokinetic parameters of oxymorphone HCl 0.1 mg/kg i.v. to healthy rhesus monkeys (n = 5)
|
Parameter
|
Units
|
Geometric Mean
|
Animal
|
||||
|---|---|---|---|---|---|---|---|
| AO61 | AS26 | AS31 | AX59 | AX63 | |||
| AUCextrapolated | % | 0.52 | 0.21 | 0.61 | 0.76 | 0.63 | 1.97 |
| AUC0-last | h · ng · ml–1 | 62.26 | 111.41 | 31.56 | 56.62 | 36.67 | 127.22 |
| AUC0-inf | h · ng · ml–1 | 62.75 | 111.65 | 31.75 | 57.05 | 36.90 | 129.78 |
| AUMC0-inf | h · h · ng · ml–1 | 76.80 | 67.65 | 28.90 | 98.17 | 33.33 | 393.96 |
| AUMC0-last | h · h · ng · ml–1 | 69.50 | 64.02 | 26.85 | 91.91 | 30.88 | 308.30 |
| C0 | ng · ml–1 | 68.38 | 245.10 | 36.95 | 42.72 | 43.37 | 89.07 |
| Cl | ml · min–1 · kg–1 | 23.64 | 13.29 | 46.72 | 26.00 | 40.20 | 11.43 |
| t1/2 λz | h | 2.51 | 2.15 | 1.77 | 1.70 | 1.78 | 6.57 |
| λz | h–1 | 0.28 | 0.32 | 0.39 | 0.41 | 0.39 | 0.11 |
| MRT0-inf | h | 1.22 | 0.61 | 0.91 | 1.72 | 0.90 | 3.04 |
| MRT0-last | h | 1.12 | 0.57 | 0.85 | 1.62 | 0.84 | 2.42 |
| Vss | liters · kg–1 | 1.74 | 0.48 | 2.55 | 2.68 | 2.18 | 2.08 |
| Vz | liters · kg–1 | 5.13 | 2.48 | 7.15 | 3.82 | 6.20 | 6.50 |
Pharmacokinetics of LE-ASG Oxy in Rhesus Macaques. LE-ASG oxy resulted in prolonged serum drug concentrations, with serum concentrations measurable out to 456 h postinjection. The MRT of LE-ASG oxy was approximately 190 h, compared with 1.7 h for the standard preparation of oxy. Serum concentrations of the two monkeys sampled for 456 h after receiving 2.0 mg/kg LE-ASG oxy did not decline below 0.5 ng/ml until after 350 h (Fig. 1C and Table 3).
TABLE 3.
Pharmacokinetic parameters of liposome encapsulated oxymorphone HCl 2 mg/kg s.c. to healthy rhesus monkeys Group 1 (n = 8) was sampled out to 144 h and group 2 (n = 2) was sampled out to 456 h.
|
Parameter
|
Units
|
Geometric Mean
|
Group 1 Animals
|
Group 2 Animals
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AQ96 | AS79 | AT13 | AT48 | AW57 | AX61 | AY60 | AZ36 | AP88 | AQ74 | |||
| AUCextrapolated | % | 48.49 | 45.77 | 37.12 | 61.05 | 54.01 | 28.43 | 75.83 | 50.35 | 50.22 | 10.86 | 2.74 |
| AUC0-last | h · ng · ml–1 | 201.81 | 98.11 | 299.78 | 215.08 | 124.36 | 178.74 | 228.46 | 566.97 | 151.04 | 210.94 | 231.85 |
| AUC0-inf | h · ng · ml–1 | 424.45 | 180.90 | 476.74 | 552.25 | 270.43 | 249.73 | 945.32 | 1141.88 | 303.42 | 236.63 | 238.37 |
| AUMC0-inf | h · h · ng · ml–1 | 81,317.40 | 33,889.23 | 64,545.36 | 116,387.11 | 51,381.27 | 26,943.43 | 440,437.75 | 195,577.93 | 62,975.84 | 47,242.81 | 60,150.91 |
| AUMC0-last | h · h · ng · ml–1 | 13,277.31 | 5041.46 | 20,275.01 | 15,923.12 | 6431.20 | 12,045.47 | 16,031.44 | 45,242.59 | 10,560.89 | 43,886.70 | 45,786.29 |
| Cl · F–1 | ml · min–1 | 69.89 | 164.00 | 62.23 | 53.72 | 109.70 | 118.80 | 31.38 | 25.98 | 97.77 | 124.46 | 125.37 |
| Cmax | ng · ml–1 | 12.78 | 19.35 | 20.30 | 5.26 | 11.89 | 14.16 | 12.70 | 20.41 | 7.90 | 9.17 | 12.32 |
| MRT0-inf | h | 191.58 | 187.34 | 135.39 | 210.75 | 190.00 | 107.89 | 465.91 | 171.28 | 207.55 | 198.19 | 254.20 |
| MRT0-last | h | 65.79 | 51.39 | 67.63 | 74.03 | 51.71 | 67.39 | 70.17 | 79.80 | 69.92 | 189.29 | 217.06 |
| Tmax | h | 0.57 | 0.25 | 0.50 | 1.00 | 1.00 | 1.50 | 1.00 | 0.25 | 0.25 | 0.50 | 0.50 |
| Vz · F–1 | liters · kg–1 | 672.03 | 2011.52 | 396.36 | 496.24 | 1235.75 | 469.40 | 843.63 | 183.16 | 1173.12 | 438.63 | 776.07 |
Behavioral Effects of Oxy and LE-ASG Oxy. Most behaviors did not differ between macaques administered oxy versus LE-ASG oxy (Table 4). Several behaviors were different at only one time point (Table 5). A few behaviors showed significant and persistent differences between the two groups. Behaviors that differed between the groups of macaques at two or more time points may be categorized as those directed toward the animals' environment, self-directed behaviors, and elimination behaviors (Table 6). The monkeys watched objects, animals, or people more often after LE-ASG oxy than after oxy (at 48 and 96 h), but monkeys administered LE-ASG had less locomotory behavior (at 24 and 72 h) than monkeys administered oxy. At 24 and 48 h, the monkeys administered LE-ASG oxy made significantly more misjudgments of distance when jumping or climbing. There were no significant differences between any of the behaviors at t = 0, immediately after drug administration, except for self-clasp. Monkeys administered LE-AG oxy were observed to engage in this behavior significantly less than monkeys administered oxy at the 0- and 96-h time points (Table 6). Significantly fewer instances of other self-directed behaviors (self-groom and self-mouth) were noted at single time points in monkeys administered LE-ASG oxy (Table 5). Elimination behavior, including defecation and urination, were the behaviors that differed most robustly between groups. Monkeys administered LE-ASG oxy had consistently fewer occurrences of defecation (at 24, 48, and 72 h after injection) and urination (at 24 and 72 h) than monkeys administered oxy (Table 6).
TABLE 4.
Behaviors scored on the ethogram that did not differ at any time point between monkeys administered oxy or LE-ASG oxy
| General Category | Specific Behavior |
|---|---|
| Alimentation | Drink |
| Eat | |
| Social | Lip smack |
| Groom | |
| Huddle | |
| Vocalize | |
| Environmentally directed | Inactive |
| Stereotypic locomotion | |
| Self-directed | Scratch |
TABLE 5.
Behaviors scored on the ethogram that differed at a single time point (P < 0.05) between monkeys administered oxy or LE-ASG oxy
| General Category & Specific Behavior | Time Point where Difference Occurred (after Injection) | Direction of Change in ASG-LE Oxy-Treated Monkeys with Respect to Oxy-Treated Monkeys |
|---|---|---|
| h | ||
| Social-interactive | ||
| Threat | 96 | Decreased |
| Nonspecific social ccontact | 24 | Increased |
| Environmentally directed | ||
| Environmental explore | 72 | Decreased |
| Self-directed | ||
| Self-groom | 96 | Decreased |
| Self-mouth | 96 | Decreased |
TABLE 6.
Behaviors scored on the ethogram that differed at two or more time points (P < 0.05) between monkeys administered oxy or LE-ASG oxy
| General Category & Specific Behavior | Time Points Where Difference Occurred (after Injection) | Direction of Change in ASG-LE Oxy-Treated Monkeys with Respect to Oxy-Treated Monkeys |
|---|---|---|
| h | ||
| Environmentally directed | ||
| Watch | 48, 96 | Increased |
| Locomotion | 24, 72 | Decreased |
| Misjudge | 24, 48 | Increased |
| Self-directed | ||
| Self-clasp | 0, 96 | Decreased |
| Elimination | ||
| Urination | 24, 72 | Decreased |
| Defecation | 24, 48, 72 | Decreased |
Discussion
Liposome-encapsulated oxymorphone prepared by ammonium sulfate gradient loading provides extended release characteristics in vivo that are of longer duration than either the commercially available oral extended-release oxymorphone formulation (Matsumoto, 2007) or liposome-encapsulated opioid formulations with release characteristics of 4 to 5 days that have previously been reported by our laboratory (Smith et al., 2008). Previous studies in our laboratory have produced liposomal formulations of oxymorphone and hydromorphone that have release characteristics that are amenable to perioperative use in domestic and laboratory animals (Krugner-Higby et al., 2003; Smith et al., 2003, 2006; L. Krugner-Higby, unpublished data). These preparations were made using either dehydration rehydration vesicles (Kirby and Gregoriadis, 1984) or freeze-thaw vesicles (Smith et al., 2008). Our data suggest that LE-ASG oxy provides release kinetics that makes it appropriate for treating chronic pain.
Ammonium sulfate gradient loading is a method that has been used to produce several types of liposomal chemotherapeutic drug preparations including doxorubicin and vincristine. These formulations are used for extended release after intravenous administration, giving up to 2 weeks of release for doxorubicin and vincristine (Krishna et al., 2001; Webb et al., 2007). After gradient loading, doxorubicin and vincristine are precipitated within the liposomes as insoluble sulfate salts, which considerably slows their release from liposomes. In contrast, oxymorphone sulfate is highly soluble, and we did not expect ammonium sulfate gradient loading to reduce its release rate from the liposomes.
One possible explanation for the reduced leakage rate from these liposomes involves the potential effect of residual ammonium in the liposomes after loading. Both protonated oxymorphone and ammonium will be retained in liposomes, and their leakage will occur indirectly through deprotonation and loss of their respective free bases. Therefore, leakage will generate protons in the liposomes, which will reduce the intraliposomal pH and thereby reduce the rate of leakage. Consequently, leakage will be a self-limiting process, and the leakage of the one species, if inherently faster, will effectively reduce the rate of leakage of the other species. Owing to its higher diffusion coefficient, we would expect ammonia to leak more rapidly than oxymorphone from the liposomes. Therefore, loss of residual ammonium from the liposomes through leakage as ammonia may effectively slow the leakage of oxymorphone.
Most behavioral studies of opioids using nonhuman primates are directed toward reinforcement, substance discrimination, and other parameters associated with drug abuse and addiction (Weerts et al., 2007). Few have examined the behavioral effects of opioid drugs administered in therapeutic concentrations. This is the first report of the behavioral effects of a therapeutic dose of oxy compared with a single dose of an oxymorphone formulation (LE-ASG oxy) that persists for several weeks. A single injection of 2.0 mg/kg LE-ASG oxy in rhesus monkeys maintains concentrations in serum that make it potentially useful for treating chronic pain. This dose of LE-ASG oxy was associated with minimal overt sedative effects. However, it did produce discernible behavioral effects up to 96 h after injection in a social setting, including decreases in locomotory and exploratory behavior. We also observed decreases in self-directed and elimination behaviors that may indicate that monkeys that have low concentrations of opioids in their serum have decreased anxiety associated with a novel cage environment and a social stimulus animal. However, one environmentally directed behavior increased (Table 6, Watch). Reduction of anxiety, including social anxiety, is one of the reasons why opioids have been used recreationally and forms part of the basis for their reinforcing and addictive qualities (Weerts et al., 2007). Morphine and diazepam decrease conditioned startle responses in rhesus monkeys (Winslow et al., 2007). The monkeys administered LE-ASG oxy had fewer aggressive behaviors at 96 h after administration (Threat) than monkeys administered oxy (Table 5). At no time after administration of either oxy or ASG-LE oxy were the monkeys' behavior consistent with dysphoria, and there were no problems with their behavior toward conspecifics or human handlers.
Interpretation of elimination behaviors in this study is more complicated. Both urination and defecation were significantly reduced at multiple time points in monkeys receiving LE-ASG oxy compared with oxy (Table 6). Constipation and urinary retention are well documented complications of opioid therapy (Jaffe and Matin, 1985; Plumb, 2005; Anderson and Day, 2008). Urination and defecation are also behaviors that increase in frequency in rhesus macaques under conditions of novelty or social stress (Habib et al., 2000). The monkeys that were administered LE-ASG oxy never had clinically discernible constipation or urinary retention during this study. The association was only recognized after analysis of the data. Therefore, it is more probable that the decreased elimination behavior was associated with decreases in the other anxiety or stress-related behaviors in these animals.
Morphine administered at therapeutic dosages increased spontaneous motor activity in pigtail macaques when the drug was injected at 9:00 AM, and decreased motor activity 10 to 12 h later. This pattern was not observed; however, if the morphine was administered at 3:00 PM, indicating that there are diurnal differences in the behavioral effects of opioid drugs (Weed and Hienz, 2006). In the current study, all drugs were administered between 8:00 and 10:00 AM. Subcutaneously administered oxymorphone decreased locomotor behavior in monkeys that received either oxy or LE-ASG oxy. The magnitude of the change was greater and more consistent in monkeys that received LE-ASG oxy (Table 6).
Differences between the observations of our study and those of other investigators may be attributable to differences in the drug, to the primate species tested, or to the experimental paradigm in which the monkeys were tested. Morphine and oxymorphone are both μ-agonist opioid drugs, but oxymorphone has a higher receptor affinity and also promotes less histamine release than equipotent dosages of morphine (Jaffe and Matin, 1985). Oxymorphone may not produce as much locomotor behavior as morphine because the sedative effects of the drug may predominate, especially at earlier time points. Mild sedative effects, especially in the monkeys administered oxy, may be inferred from the number of misjudgments the animals made at time 0, immediately after drug administration. Monkeys administered oxy made fewer misjudgments on days 1 to 4 after administration, after the drug would have been expected to be cleared from both the central nervous system and the blood. However, the group receiving LE-ASG oxy had significantly more misjudgments at 24 and 48 h after injection (Table 6). Pigtail macaques are closely related to rhesus macaques, but it is sometimes not possible to extrapolate between even fairly closely related species of animals. Finally, pigtail macaques in the other study (Weed and Hienz, 2006) were evaluated for activity in their home cages, unlike the novel physical and social environment that was used in the current study. A greater amount of gross locomotor behavior would be expected (in a novel environment) relative to the home cage, and the higher level of baseline activity in this situation (in our study conditions) may make a small increase in locomotor behavior indiscernible.
A single subcutaneously administered dose of 2 mg/kg LE-ASG oxy persists for over 2 weeks in serum in rhesus macaques. LE-ASG oxy also produced behavioral changes consistent with documented effects of opioid drugs on human beings and macaques in other studies (Jaffe and Matin, 1985; Weed and Hienz, 2006; Winslow et al., 2007). However, the monkeys were never observed to be clinically sedated. Additional studies will be necessary to determine the extent and duration of analgesia provided by LE-ASG oxy, as well as the behavioral effects of the extended-release opioid in painful animals. However, the available data indicate that the preparation may be of long enough duration to be useful in treating chronic pain.
Acknowledgments
We thank Melissa Luck and the staff of the Harlow Center for Biological Psychology for exemplary care of the monkeys used in this study and Andrea Smetana and Laura Wunsch for assistance in data collection.
This work was supported by the National Institutes of Health National Center for Research Resources [Grant 2R01-RR018802].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.150052.
ABBREVIATIONS: LE-ASG oxy, ammonium sulfate gradient loaded liposomal oxymorphone; oxy, oxymorphone hydrochloride, standard pharmaceutical preparation; AUC0-inf, the area under the curve from time 0 to infinity; AUC0-last, area under the curve from 0 to the last time point; AUCExtrapolated, percent of the AUC extrapolated to infinity; AUMC0-inf, area under the first moment curve from time 0 to infinity; AUMC0-last, area under the first moment curve from time 0 to the last measured time point; Cl, serum clearance; Vss, apparent volume of distribution at steady state; Vz, apparent volume of distribution of the area during the elimination phase; λz, first-order rate constant; t1/2 λz, terminal half-life; MRT0-∞, mean residence time extrapolated to infinity; MRT0-last, mean residence time from 0 to the last measured time point; C0, the concentration extrapolated to time 0; Cmax, maximum serum concentration; Tmax, time to maximum serum concentration; Vz · F-1, Vz per fraction of the dose absorbed.
References
- Anderson MK and Day TK (2008) Effects of morphine and fentanyl constant rate infusion on urine output in healthy and traumatized dogs. Vet Anaesth Analg 35 528-536. [DOI] [PubMed] [Google Scholar]
- Capitanio JP (1999) Personality dimensions in adult male rhesus macaques: prediction of behaviors across time and situation. Am J Primatol 47 299-320. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Kyes RC, and Fairbanks LA (2006) Consideration in the selection and conditioning of Old World monkeys for laboratory research: animals from domestic sources. ILAR J 47 294-306. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Medina RO, and Bowman RE (1993) Home cage behavior and lead treatment in rhesus monkeys: a comparison with open-field behavior. Neurotoxicol Teratol 15 145-149. [DOI] [PubMed] [Google Scholar]
- Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, et al. (2000) Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci U S A 97 6079-6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk C and Leary SL (2005) Formulary for Laboratory Animals, 3d ed, Iowa State University Press, Ames.
- Jaffe JH and Matin WR (1985) Opioid analgesics and antagonists, in Goodman and Gilman's The Pharmacological Basis of Therapeutics (Gilman AG, Rall TW, and Murad F eds) pp 491-531, Macmillan Publishing Company, New York.
- Kirby CJ and Gregoriadis G (1984) A simple procedure for preparing liposomes capable of high encapsulation efficiency under mild conditions, in Liposome Technology (Gregoriadis G ed) pp 19-27, CRC Press, Boca Raton.
- Krishna R, Webb MS, St. Onge G, and Mayer LD (2001) Liposomal and nonliposomal drug pharmacokinetics after administration of liposome-encapsulated vincristine and their contribution to drug tissue distribution properties. J Pharmacol Exp Ther 298 1206-1212. [PubMed] [Google Scholar]
- Krugner-Higby L, Smith L, Clark M, Heath TD, Dahly E, Schiffman B, Hubbard-VanStelle S, Ney D, and Wendland A (2003) Liposome-encapsulated oxymorphone hydrochloride provides prolonged relief of post-surgical visceral pain in rats. Comp Med 53 270-279. [PubMed] [Google Scholar]
- KuKanich B, Hogan BK, Krugner-Higby LA, and Smith LJ (2008a) Pharmacokinetics of hydromorphone hydrochloride in healthy dogs. Vet Anaesth Analg 35 256-264. [DOI] [PubMed] [Google Scholar]
- KuKanich B, Schmidt BK, Krugner-Higby LA, Toerber S, and Smith LJ (2008b) Pharmacokinetics and behavioral effects of oxymorphone after intravenous and subcutaneous administration to healthy dogs. J Vet Pharmacol Ther 31 580-583. [DOI] [PubMed] [Google Scholar]
- Matsumoto AK (2007) Oral extended-release oxymorphone: a new choice for chronic pain relief. Expert Opin Pharmacother 8 1515-1527. [DOI] [PubMed] [Google Scholar]
- Plumb DC (2005) Oxymorphone, in Veterinary Drug Handbook (Plumb DC ed) 2nd ed, pp 840-843, Iowa State University Press, Ames.
- Sladky KK, Krugner-Higby L, Meek-Walker E, Heath TD, and Paul-Murphy J (2006) Serum concentrations and analgesic effects of liposome-encapsulated and standard butorphanol tartrate in parrots. Am J Vet Res 67 775-781. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Krugner-Higby L, Clark M, Wendland A, and Heath TD (2003) A single dose of liposome-encapsulated oxymorphone or morphine provides long-term analgesia in an animal model of neuropathic pain. Comp Med 53 280-287. [PubMed] [Google Scholar]
- Smith LJ, Krugner-Higby L, Trepanier LA, Flaska DE, Joers V, and Heath TD (2004) Comparative sedative effects and serum pharmacokinetics of oxymorphone after subcutaneous administration of a standard or liposome-encapsulated formulation in dogs. J Vet Pharmacol Ther 27 369-372. [DOI] [PubMed] [Google Scholar]
- Smith LJ, KuKanich B, Hogan BK, Brown C, Heath TD, and Krugner-Higby LA (2008) Pharmacokinetics of a controlled-release liposome-encapsulated hydromorphone administered to healthy dogs. J Vet Pharmacol Ther 31 415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LJ, Valenzuela JR, Krugner-Higby LA, Brown C, and Heath TD (2006) A single dose of liposome-encapsulated hydromorphone provides extended relief of hyperalgesia in a rodent model of neuropathic pain. Comp Med 56 487-492. [PubMed] [Google Scholar]
- Viscusi ER, Kopacz D, Hartrick C, Martin G, and Manvelian G (2006) Single dose extended release epidural morphine for pain following hip arthroplasty. Am J Ther 13 423-431. [DOI] [PubMed] [Google Scholar]
- Viscusi ER, Martin G, Hartrick CT, Singla N, and Manvelian G (2005) Forty-eight hours of postoperative pain relief after total hip arthroplasty with a novel, extended release epidural morphine formulation. Anesthesiology 102 1014-1022. [DOI] [PubMed] [Google Scholar]
- Webb MS, Boman NL, Masin D, Yapp D, Ramsay E, Chiu GNC, Cullis PR, and Bally MB (2007) A cationic liposomal vincristine formulation with improved vincristine retention, extended circulation lifetime and increased anti-tumor activity. Lett Drug Des Discov 4 426-433. [Google Scholar]
- Weed MR and Hienz RD (2006) Effects of morphine on circadian rhythms of motor activity and body temperature in pig-tailed macaques. Pharmacol Biochem Behav 84 487-496. [DOI] [PubMed] [Google Scholar]
- Weerts EM, Fantegrossi WE, and Goodwin AK (2007) The value of nonhuman primates in drug abuse research. Exp Clin Psychopharmacol 15 309-327. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, and Davis M (2007) Modulation of fear-potentiated startle and vocalizations in juvenile rhesus monkeys by morphine, diazepam, and buspirone. Biol Psychiatry 61 389-395. [DOI] [PubMed] [Google Scholar]