Abstract
Botulinum neurotoxins (BoNTs) are extremely potent neuromuscular poisons that act through soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein cleavage to inhibit neurotransmitter release. The ability of BoNT serotype A (BoNT/A) to eliminate localized transmitter release at extremely low doses is well characterized. In the current study, we investigated the less understood characteristic of BoNT/A to induce nerve outgrowth, sometimes referred to as sprouting. This phenomenon is generally considered a secondary response to the paralytic actions of BoNT/A, and other potential factors that may initiate this sprouting have not been investigated. Alternatively, we hypothesized that BoNT/A induces sprouting through presynaptic receptor activation that is independent of its known intracellular actions on the soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) synaptosomal associated protein of 25 kDa (SNAP-25). To test this, the effects of BoNT/A application on neurite outgrowth were examined using primary cultures enriched with motor neurons isolated from embryonic mouse spinal cord. In this system, BoNT/A potently stimulated neuritogenesis at concentrations as low as 0.01 nM. The neuritogenic effects of BoNT/A exposure were concentration dependent and antagonized by Triticum vulgaris lectin, a known competitive antagonist of BoNT. Similar results were observed with the isolated BoNT/A binding domain, revealing that neuritogenesis could be initiated solely by the binding actions of BoNT/A. In addition, the presence or absence of SNAP-25 cleavage by BoNT/A was not a determinant factor in BoNT/A-induced neuritogenesis. Collectively, these results suggest that binding of BoNT/A to the motor neuronal membrane activates neuritogenesis through as yet undetermined intracellular pathway(s), independent of its known action on vesicular release.
Botulinum neurotoxin (BoNT), notorious as the agent responsible for the potentially fatal paralytic disease botulism, is an extremely potent neuromuscular poison (Arnon et al., 2001). Ironically, the toxin's ability to virtually eliminate transmitter release within a relatively confined area at extremely low doses makes it highly desirable as a therapeutic agent (Jankovic, 2004; Erbguth, 2008). The seven BoNT serotypes (A–G) are zinc metalloproteases that, once activated, exist as dichain molecules linked by a disulfide bond. These dichains consist of a 100-kDa heavy chain (HC) that includes the toxin binding domain and a 50-kDa light chain that includes the enzymatic domain (Kozaki et al., 1981; Das-Gupta, 1989). After receptor binding and internalization, BoNTs selectively cleave specific nerve terminal proteins known as SNAREs (Schiavo et al., 2000). These SNAREs form the core fusion machinery necessary to initiate vesicular neurotransmitter release. Selective proteolytic inactivation of SNARE proteins at the mammalian neuromuscular junction (NMJ) inhibits acetylcholine release causing flaccid muscle paralysis (Kalandakanond and Coffield, 2001a,b).
Although BoNTs have been studied for well over 50 years, their targeting of SNAREs was revealed less than 20 years ago. SNARE protein inactivation is now widely considered the primary intracellular action of the paralytic BoNTs. Since then, only a limited number of other potential intracellular actions have been investigated (Coffield et al., 1994; Ishida et al., 2004). An intriguing corollary to the paralytic activity of the toxins is the observation that after intoxication, some BoNT-poisoned nerve endings extend or “sprout” neurites. A number of studies have examined the importance of sprouting in toxin duration of action and subsequent NMJ remodeling (Angaut-Petit et al., 1990; Comella et al., 1993; Juzans et al., 1996; de Paiva et al., 1999). In addition, a few studies have reported the importance of molecular factors such as neural cell adhesion molecule, laminin, and insulin-like growth factor on BoNT-induced sprouting (Booth et al., 1990; Caroni et al., 1994; Schäfer and Wernig, 1998; Lee et al., 1999). Furthermore, the effects of BoNT on neurite outgrowth in cell culture have been reported by a few groups, with varying results (Bonner et al., 1994; Igarashi et al., 1996; Osen-Sand et al., 1996). None examined these effects on cultured spinal motor neurons. BoNT-induced sprouting is generally considered an indirect response secondary to denervation, and direct presynaptic events mediating this process have been largely ignored. It is possible, however, that the phenomenon is actually a result of intracellular signaling initiated by the direct interaction of BoNT with the presynaptic membrane. Thus, an examination of the potential actions of BoNT on motor neuron neuritogenesis merits further consideration.
In the current study, we examined the effects of intact BoNT serotype A (also known as BoNT/A holotoxin) and its isolated binding domain HC on neurite outgrowth using motor neuron-enriched primary cultures isolated from the embryonic mouse spinal cord. Both BoNT/A holotoxin and BoNT/A HC potently stimulated neuritogenesis. These neuritogenic effects were concentration dependent and abolished by the presence of Triticum vulgaris lectin (TVL), a plant protein that has affinity for sialic acid containing membrane components such as polysialogangliosides. TVL was chosen because it is a ligand for complex sialogangliosides (e.g., GTb1) known to act as BoNT receptors (Kozaki et al., 1998). In addition, TVL has been shown to competitively antagonize the binding of BoNT/A to central nervous system preparations, to antagonize BoNT-induced paralysis, and to inhibit BoNT-induced cleavage of SNAP-25, the SNARE protein target of BoNT/A (Bakry et al., 1991; Blasi et al., 1993; Schiavo et al., 1993; Kalandakanond and Coffield, 2001b). SNAP-25 may also participate in neurite growth, although its exact role remains to be elucidated (Osen-Sand et al., 1996; Morihara et al., 1999; Kimura et al., 2003). Herein, we report that SNAP-25 was expressed in the cultured motor neurons and subsequently cleaved by BoNT/A. It is interesting that neurite outgrowth occurred regardless of whether SNAP-25 was intact or cleaved, indicating that this known intracellular target was not relevant to BoNT/A-induced neuritogenesis in embryonic motor neurons (eMNs). Collectively, these results suggest that binding of BoNT/A to the motor neuronal membrane activates neuritogenesis through undetermined intracellular pathway(s), independent of its known action on vesicular release.
In summary, the findings presented herein support a neuritogenic action for BoNT/A on cultured motor neurons. Further studies are necessary to: 1) clarify the intracellular pathways mediating this function, 2) determine whether this phenomenon holds true in older neurons, and 3) correlate these in vitro findings with BoNT-induced sprouting in vivo.
Materials and Methods
Cell Culture. All procedures involving animals were approved by the Institutional Animal Care and Use Committee. The protocol used was modified from Anderson et al. (2004). In brief, embryos were collected from timed pregnant NIH Swiss mice (gestation days 13–14). After removal from the dam, the embryonic spinal cords were isolated, cleaned of meninges, and then minced with scissors. The minced tissue was transferred to 0.025% trypsin solution for dissociation for 25 min at 37°C, with frequent agitation. The trypsinization step was terminated with the addition of soybean trypsin inhibitor in DNase-containing isolation buffer. After centrifugation, the dissociated tissue was triturated until evenly suspended. Tissue debris was removed by further centrifugation of the dissociated suspension through a bed of 4% bovine serum albumin at 300g for 10 min. The preparation was enriched with motor neurons by loading the cell suspension gently onto a Histodenz gradient (6.7%; Sigma-Aldrich, St. Louis, MO) that was then spun at 500g for 20 min. The motor neuron-enriched interface was collected by gentle aspiration and cleared of cellular debris by centrifugation through another bed of 4% bovine serum albumin at 300g for 10 min. The neuron containing fraction was resuspended in L-15 medium supplemented with 10% glucose, 2 mM l-glutamine, 5% horse serum, 7.5% sodium bicarbonate, 1% penicillin-streptomycin, 5 μg/ml insulin, 100 μg/ml putrescine, 100 μg/ml conalbumin, 3 μM sodium selenite, and 2 μM progesterone.
Neurite Outgrowth Assay. eMNs were plated in 24-well plates coated with poly-d-lysine plus laminin (BD Biosciences, San Jose, CA) at a density of 1 × 105 cells/ml. The cultures were maintained in a humidified incubator (5% CO2) at 37°C for 4 h to ensure cell attachment. After 4 h, the cells were treated with intact BoNT/A (holotoxin) or BoNT/A HC plus or minus TVL and returned to the 37°C incubator for 24, 48, or 72 h. After treatment, the cultures were washed, and the cells were fixed in 4% paraformaldehyde, pH 7.4, for 30 min. After fixation, the cells were washed with phosphate-buffered saline, blocked with 5% goat serum for 30 min to prevent nonspecific binding, and then incubated overnight with a polyclonal antibody to protein gene product 9.5 (1:400; Serotec, Oxford, UK), a protein marker for neurons. Labeled neurons were detected by fluorescence using an fluorescein isothiocyanate-conjugated secondary IgG.
Neurons were visualized using an Olympus (Tokyo, Japan) inverted microscope, and images were captured with a charge-coupled device camera. IPLab Software was used to measure neurite length and to count primary and secondary neurite numbers for each treatment. Total neurite length was defined as the sum of the lengths of all neurites of a given neuron excluding the soma. Primary neurites were defined as neurites that arose directly from the neuronal soma; secondary neurites were defined as branches arising directly from primary neurites. One-way analysis of variance, followed by Bonferroni or Dunnett post hoc tests, was used to test statistical significance (GraphPad Prism 5; GraphPad Software Inc., San Diego, CA).
Immunoblots. Cells were harvested from culture plates at desired time points using T-PER protein extraction reagent (Pierce Chemical, Rockford, IL) plus protease inhibitor cocktail. Cell extracts were sonicated briefly followed by centrifugation. Supernatants were collected and processed for SDS-polyacrylamide gel electrophoresis followed by Western blot using standard protocols. Protein samples (10 μg/lane) were resolved on 12.5% Tris-HCl gels. Resolved proteins were transferred to polyvinylidene difluoride membranes and probed for SNAP-25 using a rabbit SNAP-25 primary antibody (1:15,000; Sigma-Aldrich). SNAP-25 immunoreactivity was visualized with chemiluminescence.
Biochemicals. BoNT/A holotoxin and BoNT/A HC were purchased from Metabiologics, Inc. (Madison, WI). TVL (also known as WGA) was purchased from Sigma-Aldrich and used at 1 μM.
Results
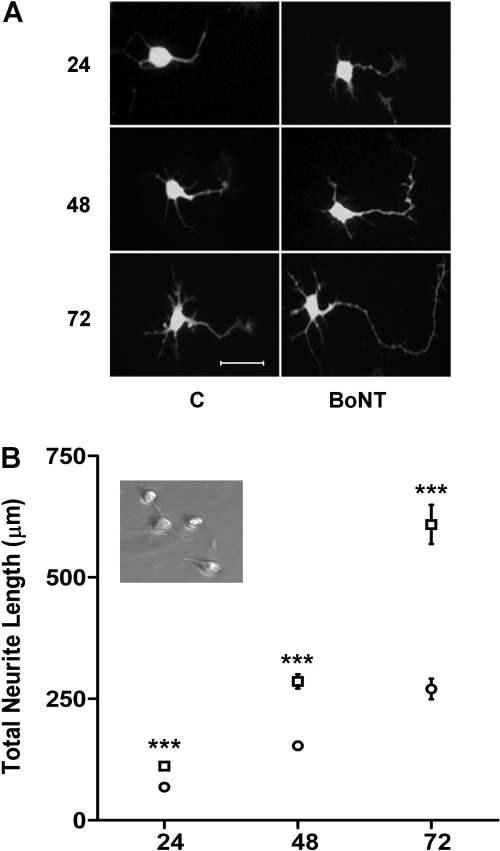
BoNT/A Promotes Neurite Outgrowth of eMNs. Neurite outgrowth in vitro is dependent on several factors, including but not limited to cell origin, age, and culture conditions (e.g., plating density, media, substrate, growth factors, etc.). In our initial experiments, we examined neurite outgrowth from day 13 eMNs under control and BoNT/A-treated conditions at three time points (Fig. 1). Neurons were allowed to attach to laminin-coated plates before treatment. Substantial numbers of cells were attached and initiating primary neurite extension by 4 h after plating (Fig. 1B, inset). Between 24 and 48 h in culture, total neurite length in untreated control neurons more than doubled, with a mean increase of 124%. An additional 76% increase was measured between 48 and 72 h (Fig. 1B, open circles). Altogether, total neurite length in untreated control eMNs increased by 295% between 24 and 72 h.
Fig. 1.
Neurite outgrowth of cultured eMNs. Comparison of a single dose of BoNT/A at three time points. A, fluorescent images of individual eMNs taken from cultures treated with 0.1 nM BoNT/A (BoNT) or without (C) for 24, 48, and 72 h. Neurons were imaged using an antibody to protein gene product 9.5. Scale bar, 50 μm. B, comparisons of total neurite length in μm (mean ± S.E.M.) between control (○, n = 30) and BoNT/A (□, n = 30)-treated neurons at 24, 48, and 72 h. ***, value is significantly different from control at p < 0.001. Inset, phase-contrast images of neurons at 4 h postplating just before treatment.
Initial assessment of neurite outgrowth in eMNs treated with a single concentration of BoNT/A holotoxin revealed a stimulatory effect on neurite outgrowth. In cultures treated with 0.1 nM BoNT/A, total neurite length was significantly greater (p < 0.001) in the BoNT/A-treated eMNs compared with time-matched, untreated control neurons at each of the three time points (Fig. 1, A and B, open squares). Furthermore, the rate of neurite outgrowth was greater in the BoNT/A-treated cultures. Total neurite length increased by 156% between 24 and 48 h of treatment and by 174% between 48 and 72 h of treatment. Total neurite length in BoNT/A-treated eMNs increased by 444% between 24 and 72 h.
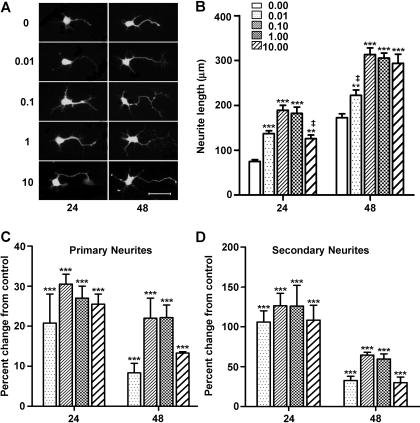
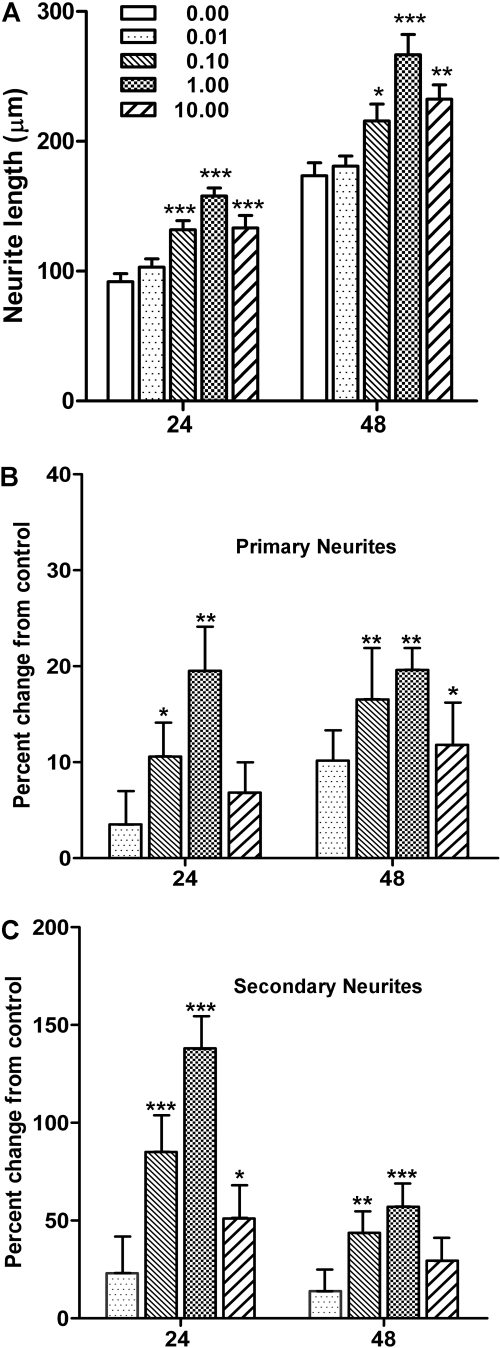
Neuritogenic Effects of BoNT/A Are Concentration-Dependent. The responses of neurite outgrowth to different concentrations of BoNT/A holotoxin were examined at 24 and 48 h and compared with time-matched untreated control neurons (Fig. 2). In general, the stimulatory effect on total neurite length increased with increasing concentrations of BoNT/A, with the maximal responses observed at 0.1 nM (Fig. 2, A and B). This was followed by a stabilization of the response, with a slight depression in response at the highest concentration (10 nM) at 24 h; however, this response was still significantly greater than untreated controls. These concentration-dependent increases in neurite length ranged from 68 to 152% at 24 h and from 29 to 82% at 48 h. To examine whether BoNT treatment selectively stimulated primary or secondary neurite growth, primary and secondary neurite lengths were measured separately for two toxin concentrations (data not illustrated). As might be expected, total primary neurite length was greater than secondary neurite length at 24 h, even in untreated cultures. BoNT/A treatment strongly stimulated both primary and secondary neurite growth; however, there was no evidence of a selective effect of the toxin on the two classes of neurites. After 24-h BoNT/A treatment, primary neurite length increased significantly by 75.3 and 54.6% at 0.1 and 1.0 nM BoNT/A, respectively, whereas secondary neurite length increased by 41.8 and 77.2%, respectively. By 48 h, primary neurite length increased by 10.8 and 75.4% at 0.1 and 1.0 nM BoNT, respectively, whereas secondary neurite length increased by 109.9 and 110%, respectively.
Fig. 2.
Concentration-dependent effects of BoNT/A on three different parameters of neurite outgrowth. A, fluorescent images of eMNs taken from cultures treated with increasing concentrations of BoNT/A (0–10 nM) at 24 and 48 h. Neurons imaged using an antibody to protein gene product 9.5. Scale bar, 50 μm. B, comparisons of total neurite length in micrometers (mean ± S.E.M., n = 30) from the lowest (0 nM) to the highest (10 nM) concentrations at 24 and 48 h. Exception: n = 28 for 48-h 0.01 nM group. C and D, comparisons of the numbers of primary (C) and secondary (D) neurites from 0.01 to 10 nM BoNT/A at 24 and 48 h. Data are represented as the mean percentage change from control (0 nM) values ± S.E.M., n = 30. B to D, ***, **, and *, values are significantly different from control (0 nM) at p < 0.001, p < 0.01, and p < 0.05, respectively; ‡, value is significantly different from all other concentrations at p < 0.001.
In addition to neurite length, the numbers of primary neurite extensions and secondary branches were counted and compared with untreated controls (Fig. 2, C and D). At 24 h, the number of primary neurites in toxin-treated cultures increased by an average of 29% across all four concentrations. The individual concentration responses were significantly different from untreated eMNs and demonstrated a similar pattern of concentration dependence as total neurite length (p < 0.001). The number of primary neurites was also increased at 48 h, albeit to a lesser extent. As previously noted for total neurite length, the primary neurite response observed at the 10 nM concentration was also depressed at 48 h relative to the lower concentrations but was still significantly increased compared with controls. Lastly, BoNT/A treatment also significantly increased the number of secondary branches (p < 0.001). The pattern of this response was very similar to that of the primary neurite response, although the magnitude of the branching response was substantially greater (note that the x-axis for Fig. 2D is 5 times that of Fig. 2C). Collectively, these data suggest that BoNT/A treatment promotes neuritogenesis in eMNs.
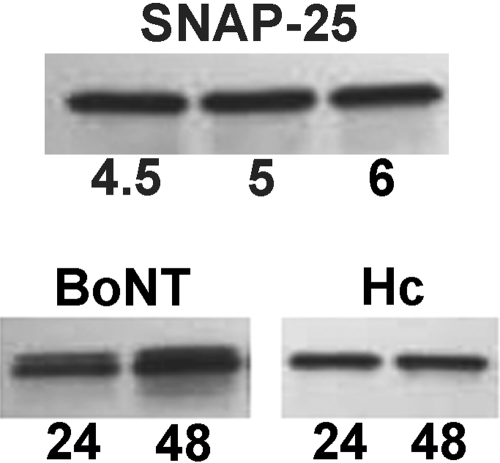
SNAP-25 Is Present in eMNs and Cleaved by BoNT/A. SNAP-25, the SNARE protein intracellular target of BoNT/A may participate in neurite growth in developing neurons, although its exact role is unclear. In the current study, Western blot analyses revealed the presence of stable SNAP-25 protein expression in the 13-day eMN cultures as early as 4.5 h postplating (Fig. 3, top). It is interesting that exposure to intact BoNT/A (0.1 nM) resulted in cleavage of SNAP-25 in the eMNs, coincident with promotion of neurite outgrowth at 24 and 48 h (Fig. 3, bottom). In this case, cleavage was evident by the appearance of a second band of slightly lower molecular weight (∼24 kDa) detectable just below the parent SNAP-25 band. These data demonstrate that neurite outgrowth in these embryonic neurons was not inhibited by cleavage of SNAP-25 and, further, confirm the functional binding and uptake of BoNT/A by the eMNs in culture.
Fig. 3.
SNAP-25 protein in eMNs. Top, immunoblot of SNAP-25 protein isolated from eMN cultures at 4.5, 5, and 6 h after plating. Bottom, immunoblots of eMN cultures treated with either 0.1 nM BoNT/A holotoxin (left) or 0.1 nM BoNT/A HC (right) at 24 and 48 h. The presence of two bands in blots from holotoxin-treated cultures indicates toxin-induced cleavage, with the lower band representing cleaved SNAP-25. Note the absence of the cleaved SNAP-25 bands in the HC-treated cultures. Protein samples (10 μg/lane) were resolved on 12.5% Tris-HCl gels. SNAP-25 immunoreactivity was detected with rabbit SNAP-25 primary antibody and visualized by chemiluminescence.
Effects of BoNT/A Holotoxin Are Mimicked by the Toxin Binding Domain and Blocked by TVL. To determine whether the stimulatory effect of BoNT/A on eMN neuritogenesis could be mediated by binding of the toxin alone or required internalization of the toxin enzymatic domain, separate experiments were performed using the toxin HC minus the light chain. BoNT/A HC-induced concentration-dependent increases in total neurite outgrowth that were qualitatively similar to BoNT/A holotoxin in response pattern, although somewhat less in response magnitude and sensitivity (Fig. 4A). In this case, the percentage increases in neurite length ranged from 5 to 61% at 24 h and from 3 to 38% at 48 h, with the maximal responses observed at 1 nM. Likewise, BoNT/A HC-induced concentration-dependent increases in both the number of primary neurites and secondary branches at both time points (Fig. 4, B and C). The depressed response induced by 10 nM holotoxin relative to the lower concentrations was also evident with the toxin HC. As confirmed by Western blot (Fig. 3, bottom), the BoNT/A HC lacked enzymatic activity. Thus, binding of the toxin HC alone was sufficient to induce neurite outgrowth, and cleavage of SNAP-25 did not play any role.
Fig. 4.
Concentration-dependent effects of BoNT/A binding domain (HC) on neurite outgrowth. A, comparisons of total neurite length in micrometers (mean ± S.E.M., n = 30) from the lowest (0 nM) to the highest (10 nM) concentrations of HC at 24 and 48 h. B and C, comparisons of the numbers of primary (B) and secondary (C) neurites from 0.01 to 10 nM HC at 24 and 48 h. Data are represented as the mean percentage change from control (0 nM) values ± S.E.M., n = 30. A to C, ***, **, and *, values are significantly different from control (0 nM) at p < 0.001, p < 0.01, and p < 0.05, respectively.
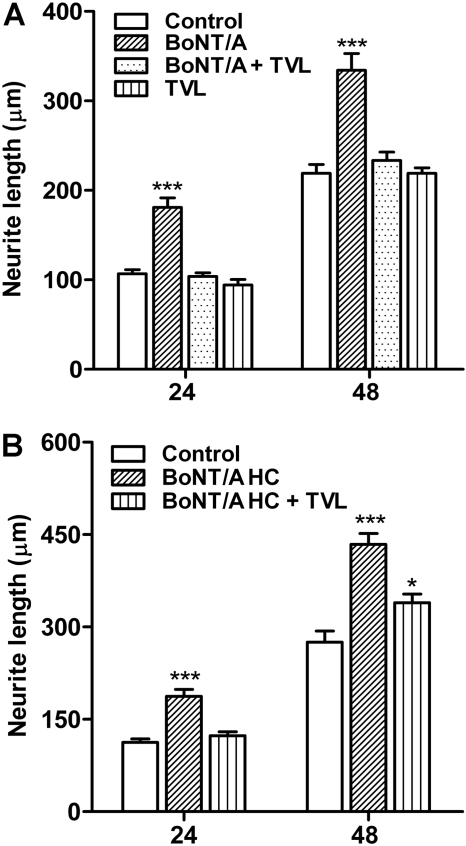
T. vulgaris lectin was used to investigate the potential role of complex ganglioside binding in the enhancement of neurite outgrowth by BoNT/A. The concentration of TVL chosen for this study is well within the range of concentrations shown by Bakry et al. (1991) to competitively antagonize BoNT binding to central nervous system membranes and to antagonize BoNT paralytic action at the NMJ. As illustrated in Fig. 5A, treatment with TVL (1 μM) completely abolished the stimulatory effects of BoNT/A holotoxin on total neurite length at both 24 and 48 h. TVL alone had no effect on neurite outgrowth. In addition, TVL treatment abolished the stimulatory effects of BoNT/A on primary and secondary neurite numbers (data not shown). Finally, treatment with TVL also abolished the stimulatory effects of BoNT/A HC at 24 h and significantly reduced the response at 48 h (Fig. 5B).
Fig. 5.
Antagonism of BoNT/A holotoxin (A) and HC (B) effects on total neurite length by ganglioside blockade using TVL. For each group, data are presented as mean ± S.E.M. (n = 30). TVL used at 1 μM. *** and *, values are significantly different from controls at p < 0.001, and p < 0.05, respectively.
Discussion
Botulinum neurotoxin serotype A, marketed as Botox in the United States, is used currently for an ever-expanding list of neurological disorders characterized by neuromuscular hyperactivity (Jankovic, 2004). The cellular mechanism of action that underlies BoNT/A's extraordinary ability to eliminate localized transmitter release at extremely low doses is well characterized and will not be further discussed here. Less well appreciated is the inherent ability of BoNT/A to induce axonal sprouting at poisoned nerve terminals (Holland and Brown, 1981; Hopkins et al., 1981; Diaz et al., 1989; Pamphlett, 1989; Angaut-Petit et al., 1990; Holds et al., 1990; Bonner et al., 1994; de Paiva et al., 1999). Such sprouting has also been reported after poisoning by BoNT serotypes D and F (Comella et al., 1993; Meunier et al., 2003). A number of studies have examined the importance of sprouting in toxin duration of action and subsequent NMJ remodeling (Angaut-Petit et al., 1990; Comella et al., 1993; Juzans et al., 1996; de Paiva et al., 1999). Although a few studies have examined this sprouting phenomenon in cell culture with variable results (Bonner et al., 1994; Igarashi et al., 1996; Osen-Sand et al., 1996). However, there has been little study to examine the cellular basis of this sprouting, which has been largely considered a nonspecific response to chemical denervation. In contrast, we hypothesized that this phenomenon may actually be a direct presynaptic neuritogenic effect of the toxin.
In the current study, BoNT/A stimulated neuritogenesis from eMNs grown in culture by increasing the number of primary neurites extended, the number of secondary branches formed, and, thus, total neurite length. These stimulatory effects of BoNT/A were concentration dependent and, more importantly, initiated solely by BoNT/A binding as evidenced by qualitatively similar responses observed after exposure to the isolated toxin binding fragment. The toxin's actions did not appear to stimulate selectively primary or secondary neurite growth. Rather, the effects seemed to be more widely distributed because both were strongly stimulated. As might be expected, the effects were biphasic, with the stimulatory effects on primary neurites occurring earlier (24-h time point), followed by stimulatory effects on secondary neurites by 48 h.
The stimulatory actions are in general agreement with the work of Bonner et al. (1994) in which chick ciliary ganglion neurons were cocultured with skeletal muscle. In that study, BoNT/A exposure increased both primary and secondary neurite branching frequency; however, in contrast to our work, they did not observe an increase in the numbers of primary neurites. Conversely, our results are at variance with other studies reporting that BoNT/A actually inhibited neuritogenesis because of its cleavage of SNAP-25 (Osen-Sand et al., 1996; Morihara et al., 1999). In fact, in our study, neuritogenesis was promoted despite SNAP-25 cleavage.
Antagonism of the BoNT neuritogenic actions by TVL is in keeping with the previously recognized role of complex polygangliosides as universal membrane receptors for the various BoNT serotypes (Bakry et al., 1991; Kozaki et al., 1998; Schiavo et al., 2000). Early studies revealed the importance of gangliosides in BoNT binding, whereas later studies attempted to clarify the nature of ganglioside-BoNT interactions and their relevance to the more selective binding of BoNT to protein receptors. As a consequence, it has been proposed that gangliosides may act to increase toxin receptor affinity or to promote lateral interactions between BoNT coreceptors acting in a receptor array (Montecucco et al., 2004). Gangliosides themselves are reported to mediate neurite outgrowth, possibly through modulation of calcium channels/calcium influx in growth cones (Ledeen and Wu, 2002). Thus, the precise function of gangliosides in the BoNT-induced outgrowth reported here, beyond their known role as toxin receptors, remains unclear. In any event, the fact that the neuritogenic actions of BoNT/A do not require the toxin enzymatic domain but can be mediated solely by its binding domain suggests that the interactions of the toxin with gangliosides and/or other potential membrane receptors initiate intracellular signals that ultimately modulate neurite formation. This is somewhat reminiscent of known neurotrophins and is a novel finding because intracellular signaling by BoNT/A has not been recognized previously.
Although the concentration-dependent responses to the toxin binding fragment were qualitatively similar to those of the holotoxin, the magnitude was somewhat less, suggesting potential differences in potency between the intact toxin and the toxin fragment. The reasons for this difference are unclear. One potential explanation may be that separation of the binding fragment from the enzymatic domain alters somewhat the conformation of its binding pocket(s) such that its interaction with membrane receptors is affected. In any event, the stimulatory actions of the isolated binding domain confirm the significance of a receptor-initiated event in the promotion of neuritogenesis by BoNT.
It is interesting that both the holotoxin and the HC binding fragment concentration responses displayed inverse U-shaped curves. This biphasic response is reminiscent of hormesis. Hormesis, a biphasic dose-response model characterized by low-dose stimulation and high-dose inhibition, typically displays a U-shaped curve. The inverse or reciprocal U shape is an alternative way to illustrate this response. Beginning at the low dose, the magnitude of the stimulatory or positive effect increases with dose, until at some high dose the magnitude diminishes, becoming less positive and displaying an inverse U shape. In fact, this type of response has been demonstrated recently for a variety of neuroactive substances, including neurotoxins, on both neurite outgrowth and neuronal survival (Calabrese, 2008). In the current study, the reduction in the stimulatory effect on neurite outgrowth at the highest dose was notable for both the holotoxin and the HC. The reasons for the diminished response at the highest dose are unknown. Although speculative, the biphasic effect might be explained by the activation of different receptor-mediated mechanisms at low dose versus high dose. In support of this, early radioligand binding studies revealed that BoNT binding was characterized by both high- and low-affinity binding. From these early studies, it was proposed that the toxin had two different binding sites, which potentially interact with two different binding partners in a dose-dependent manner (Montecucco, 1986). One possible explanation for the biphasic response in the current study might be that the higher dose of toxin recruited low-affinity binding sites, resulting in the activation of receptor mechanism(s) that in some way opposed the mechanism(s) activated by high-affinity binding at the lower doses.
The neuritogenic actions of BoNT/A observed in the current study are reminiscent of another group of bacterial enzymes. C3 exoenzymes are transferases that inhibit the activation of RhoA, a small GTPase shown to modulate neurite outgrowth (Vogelsgesang et al., 2007). There are several C3-like transferases produced by different bacteria. The prototype C3bot, which demonstrates the strongest RhoA inhibition, was isolated from Clostridium botulinum strains that also produce the neurotoxin serotypes C1 and D. Because of its greater sensitivity, C3bot is used extensively in studies of RhoA activity, including many examining neuronal development/differentiation. However, it is interesting that one recent study found that C3bot not only modified neurite outgrowth via its intracellular inactivation of RhoA but also demonstrated neurotrophic properties when applied externally that were independent of its enzymatic properties (Ahnert-Hilger et al., 2004). The authors proposed that these neurotrophic actions were receptor-mediated, although, to the best of our knowledge, specific receptors mediating this effect were not characterized.
In conclusion, the findings presented herein support a neuritogenic role for BoNT/A. Further studies are necessary to: 1) clarify the intracellular pathways mediating this function, 2) determine whether this phenomenon holds true in older neurons and nerve terminals of the peripheral nervous system, and 3) correlate these in vitro findings with BoNT-induced sprouting in vivo.
Acknowledgments
We thank Dr. Xia-qing Li for expert advice on cell culture and Dr. Gaylen Edwards and Kimberly Freeman for review of the manuscript.
This work was supported by the National Institutes of Health [Grant R21-AI059705].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.147744.
ABBREVIATIONS: BoNT, botulinum neurotoxin; HC, heavy chain; SNARE, soluble N-ethylmaleimide-sensitive factor attachment receptor; NMJ, neuromuscular junction; BoNT/A, BoNT serotype A; TVL, Triticum vulgaris lectin; SNAP-25, synaptosomal associated protein of 25 kDa.
References
- Ahnert-Hilger G, Höltje M, Grosse G, Pickert G, Mucke C, Nixdorf-Bergweiler B, Boquet P, Hofmann F, and Just I (2004) Differential effects of rho GTPases on axonal and dendritic development in hippocampal neurones. J Neurochem 90 9-18. [DOI] [PubMed] [Google Scholar]
- Anderson KN, Potter AC, Piccenna LG, Quah AK, Davies KE, and Cheema SS (2004) Isolation and culture of motor neurons from the newborn mouse spinal cord. Brain Res Brain Res Protoc 12 132-136. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D, Molgó J, Comella JX, Faille L, and Tabti N (1990) Terminal sprouting in mouse neuromuscular junctions poisoned with botulinum type A toxin: morphological and electrophysiological features. Neuroscience 37 799-808. [DOI] [PubMed] [Google Scholar]
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, et al. (2001) Botulinum toxin as a biological weapon: medical and public health management. JAMA 285 1059-1070. [DOI] [PubMed] [Google Scholar]
- Bakry N, Kamata Y, and Simpson LL (1991) Lectins from Triticum vulgaris and Limax flavus are universal antagonists of botulinum neurotoxin and tetanus toxin. J Pharmacol Exp Ther 258 830-836. [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Südhof TC, Niemann H, and Jahn R (1993) Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 365: 160-163. [DOI] [PubMed] [Google Scholar]
- Bonner PH, Friedli AF, and Baker RS (1994) Botulinum A toxin stimulates neurite branching in nerve-muscle cocultures. Brain Res Dev Brain Res 79 39-46. [DOI] [PubMed] [Google Scholar]
- Booth CM, Kemplay SK, and Brown MC (1990) An antibody to neural cell adhesion molecule impairs motor nerve terminal sprouting in a mouse muscle locally paralysed with botulinum toxin. Neuroscience 35 85-91. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ (2008) Enhancing and regulating neurite outgrowth. Crit Rev Toxicol 38 391-418. [DOI] [PubMed] [Google Scholar]
- Caroni P, Schneider C, Kiefer MC, and Zapf J (1994) Role of muscle insulin-like growth factors in nerve sprouting: suppression of terminal sprouting in paralyzed muscle by IGF-binding protein 4. J Cell Biol 125 893-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffield JA, Considine RV, Jeyapaul J, Maksymowych AB, Zhang RD, and Simpson LL (1994) The role of transglutaminase in the mechanism of action of tetanus toxin. J Biol Chem 269 24454-24458. [PubMed] [Google Scholar]
- Comella JX, Molgo J, and Faille L (1993) Sprouting of mammalian motor nerve terminals induced by in vivo injection of botulinum type-D toxin and the functional recovery of paralysed neuromuscular junctions. Neurosci Lett 153 61-64. [DOI] [PubMed] [Google Scholar]
- DasGupta BR (1989) The structure of botulinum neurotoxin, in Botulinum Neurotoxin and Tetanus Toxin (Simpson LL ed) pp 53-67, Academic Press, New York.
- de Paiva A, Meunier FA, Molgó J, Aoki KR, and Dolly JO (1999) Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A 96 3200-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz J, Molgó J, and Pécot-Dechavassine M (1989) Sprouting of frog motor nerve terminals after long-term paralysis by botulinum type A toxin. Neurosci Lett 96 127-132. [DOI] [PubMed] [Google Scholar]
- Erbguth FJ (2008) From poison to remedy: the chequered history of botulinum toxin. J Neural Transm 115 559-565. [DOI] [PubMed] [Google Scholar]
- Holds JB, Alderson K, Fogg SG, and Anderson RL (1990) Motor nerve sprouting in human orbicularis muscle after botulinum A injection. Invest Ophthalmol Vis Sci 31 964-967. [PubMed] [Google Scholar]
- Holland RL and Brown MC (1981) Nerve growth in botulinum toxin poisoned muscles. Neuroscience 6 1167-1179. [DOI] [PubMed] [Google Scholar]
- Hopkins WG, Brown MC, and Keynes RJ (1981) Nerve growth from nodes of Ranvier in inactive muscle. Brain Res 222 125-128. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Kozaki S, Terakawa S, Kawano S, Ide C, and Komiya Y (1996) Growth cone collapse and inhibition of neurite growth by Botulinum neurotoxin C1: a t-SNARE is involved in axonal growth. J Cell Biol 134 205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H, Zhang X, Erickson K, and Ray P (2004) Botulinum toxin type A targets RhoB to inhibit lysophosphatidic acid-stimulated actin reorganization and acetylcholine release in nerve growth factor-treated PC12 cells. J Pharmacol Exp Ther 310 881-889. [DOI] [PubMed] [Google Scholar]
- Jankovic J (2004) Botulinum toxin in clinical practice. J Neurol Neurosurg Psychiatry 75 951-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juzans P, Comella JX, Molgo J, Faille L, and Angaut-Petit D (1996) Nerve terminal sprouting in botulinum type-A treated mouse levator auris longus muscle. Neuromuscul Disord 6 177-185. [DOI] [PubMed] [Google Scholar]
- Kalandakanond S and Coffield JA (2001a) Cleavage of intracellular substrates of botulinum toxins A, C, and D in a mammalian target tissue. J Pharmacol Exp Ther 296 749-755. [PubMed] [Google Scholar]
- Kalandakanond S and Coffield JA (2001b) Cleavage of SNAP-25 by botulinum toxin type A requires receptor-mediated endocytosis, pH-dependent translocation, and zinc. J Pharmacol Exp Ther 296 980-986. [PubMed] [Google Scholar]
- Kimura K, Mizoguchi A, and Ide C (2003) Regulation of growth cone extension by SNARE proteins. J Histochem Cytochem 51 429-433. [DOI] [PubMed] [Google Scholar]
- Kozaki S, Kamata Y, Watarai S, Nishiki T, and Mochida S (1998) Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb Pathog 25 91-99. [DOI] [PubMed] [Google Scholar]
- Kozaki S, Togashi S, and Sakaguchi G (1981) Separation of Clostridium botulinum type A derivative toxin into two fragments. Jpn J Med Sci Biol 34 61-68. [DOI] [PubMed] [Google Scholar]
- Ledeen RW and Wu G (2002) Ganglioside function in calcium homeostasis and signaling. Neurochem Res 27 637-647. [DOI] [PubMed] [Google Scholar]
- Lee RE, Tartell PB, Karmody CS, and Hunter DD (1999) Association of adhesive macromolecules with terminal sprouts at the neuromuscular junction after botulinum treatment. Otolaryngol Head Neck Surg 120 255-261. [DOI] [PubMed] [Google Scholar]
- Meunier FA, Lisk G, Sesardic D, and Dolly JO (2003) Dynamics of motor nerve terminal remodeling unveiled using SNARE-cleaving botulinum toxins: the extent and duration are dictated by the sites of SNAP-25 truncation. Mol Cell Neurosci 22 454-466. [DOI] [PubMed] [Google Scholar]
- Montecucco C (1986) How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem Sci 11 314-317. [Google Scholar]
- Montecucco C, Rossetto O, and Schiavo G (2004) Presynaptic receptor arrays for clostridial neurotoxins. Trends Microbiol 12 442-446. [DOI] [PubMed] [Google Scholar]
- Morihara T, Mizoguchi A, Takahashi M, Kozaki S, Tsujihara T, Kawano S, Shirasu M, Ohmukai T, Kitada M, Kimura K, et al. (1999) Distribution of synaptosomal-associated protein 25 in nerve growth cones and reduction of neurite outgrowth by botulinum neurotoxin A without altering growth cone morphology in dorsal root ganglion neurons and PC-12 cells. Neuroscience 91 695-706. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A, Staple JK, Naldi E, Schiavo G, Rossetto O, Petitpierre S, Malgaroli A, Montecucco C, and Catsicas S (1996) Common and distinct fusion proteins in axonal growth and transmitter release. J Comp Neurol 367 222-234. [DOI] [PubMed] [Google Scholar]
- Pamphlett R (1989) Early terminal and nodal sprouting of motor axons after botulinum toxin. J Neurol Sci 92 181-192. [DOI] [PubMed] [Google Scholar]
- Schäfer R and Wernig A (1998) Polyclonal antibodies against NCAM reduce paralysis-induced axonal sprouting. J Neurocytol 27 615-624. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Matteoli M, and Montecucco C (2000) Neurotoxins affecting neuroexocytosis. Physiol Rev 80 717-766. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Rossetto O, Catsicas S, Polverino de Laureto P, DasGupta BR, Benfenati F, and Montecucco C (1993) Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J Biol Chem 268 23784-23787. [PubMed] [Google Scholar]
- Vogelsgesang M, Pautsch A, and Aktories K (2007) C3 exoenzymes, novel insights into structure and action of Rho-ADP-ribosylating toxins. Naunyn Schmiedebergs Arch Pharmacol 374 347-360. [DOI] [PubMed] [Google Scholar]