Abstract
Death of mechanosensory cells in the inner ear results in two profound disabilities: hearing loss and balance disorders. Although mammals lack the capacity to regenerate hair cells, recent studies in mice and other rodents have offered valuable insight into strategies for stimulating hair cell regeneration in mammals. Investigations of model organisms that retain the ability to form new hair cells after embryogenesis, such as fish and chicks, are equally important and have provided clues as to the cellular and molecular mechanisms that may block hair cell regeneration in mammals. Here, we summarize studies on hair cell regeneration in the chicken and the zebrafish, discuss specific advantages of each model, and propose future directions for the use of non-mammalian models in understanding hair cell regeneration.
Keywords: hair cell regeneration, chick, zebrafish, ear, lateral line
Overview
More than 50% of individuals over the age of 60 suffer hearing loss as the result of aging, genetic predisposition or environmental exposure to noise or ototoxic drugs (Beisel et al., 2008). Most hearing and many balance deficiencies spring from damage or loss of sensory hair cells, which are highly specialized cells with elaborate microvillar arrays called hair bundles. The hair bundle is responsible for transducing sound energy or head movements into neural signals that are the initial input to the auditory and vestibular nervous system.
Two broad strategies can be envisioned to treat hair cell loss: prevention and/or replacement. Pharmacological approaches for preventing hair cell loss have been identified in model systems and in human patients (reviewed in Guthrie, 2008 and Cotanche, 2008). Genes contributing to hair cell protection or susceptibility have also been discovered via genetic screens and may be targets for gene therapy in the future (Lang et al., 2006; Friedman et al., 2008; Owens et al., 2008). These results are promising first steps to the eventual goal of preventing hair cell loss. However, to treat individuals already suffering from hair cell loss, strategies for cellular replacement must be investigated. The only currently available treatment is a biomechanical approach to compensating for hair cell loss via cochlear implants. These implants bypass the need for hair cell transduction and directly stimulate the auditory nerve, attempting to replicate the interactions of inner hair cells and their associated nerves (reviewed in Rubinstein, 2004). Despite advances in cochlear implants, they lack the precise tuning and sensitivity of a functioning ear and regeneration of hair cells an appealing alternative for re-establishing auditory function.
Mammals, in general, lack the ability to regenerate hair cells (reviewed in Matsui and Cotanche, 2004). In contrast, new hair cell production is common among cold-blooded vertebrates following amputation (Stone, 1933, 1937), as a normal part of body growth (Corwin, 1981; Popper and Hoxter, 1984; Corwin, 1985), and after hair cell lesion (Balak et al., 1990; Lombarte et al., 1993). It was the unexpected discovery of hair cell regeneration in the auditory system of birds following experimental damage that spurred scientific investigation in this area (Corwin and Cotanche, 1988; Ryals and Rubel, 1988). As discussed below, studies in avian models over the last 20 years have revealed the progenitor cell identity, the time-course of regeneration, and the cellular processes involved. So far, however, they have provided modest information about how hair cell regeneration is controlled. The principal obstacle is the identification of genes and signaling pathways that direct progenitor cell behavior.
Genetic analyses are needed to reveal which regulatory pathways have been conserved and lost across evolution. This information may unveil molecular strategies for re-activating hair cell regeneration in mammals. Indeed, several studies of the mature mammalian inner ear suggest non-sensory cells can be induced to give rise to hair cells. For example, a small number of purified supporting cells from the adult mouse can differentiate into hair cells in vitro (White et al., 2006), and non-sensory cells from the mature end organs may have this capacity (Li et al., 2003; Oshima et al., 2007). Further, misexpression of the transcription factor Atoh1 is sufficient to induce ectopic hair cell formation in the adult Guinea pig organ of Corti or rat utricle (Zheng and Gao, 2000; Kawamoto et al., 2003; Shou et al., 2003; Izumikawa et al., 2005). However, activation of Atoh1 does not induce proliferation of supporting cells necessary to maintain hair cell function (Shou et al., 2003). Nonetheless, these experiments highlight the potential for activating hair cell regeneration in mammals, and they underscore the importance of characterizing the molecules that direct and integrate the cellular processes associated with new hair cell production in birds and other animals capable of hair cell regeneration. These processes include re-initiation and termination of cell proliferation, differentiation of precursor cells into hair cells and supporting cells, and innervation of new hair cells.
One powerful strategy for identifying proteins that are critical regulators of hair cell regeneration is to analyze regeneration in animals subjected to unbiased mutagenesis. Historically, such forward genetic screens have provided breakthrough information for understanding control of cellular processes in other tissues. For example, several genes regulating programmed cell death, and now known to have human orthologs, were first identified during forward genetic screens of the invertebrate nematode, C. elegans (Putcha and Johnson, 2004). Among vertebrates, mice have been used extensively for genetic analysis. However, mice do not spontaneously form new hair cells, and therefore they are only useful for identifying genes that single-handedly trigger hair cell regeneration, a formidable task! Zebrafish, another genetically tractable model, has recently emerged as a powerful tool with the potential to identify molecular regulators of hair cell regeneration.
In this review, we will address recent studies of hair cell regeneration in chickens and zebrafish, and discuss how these animal models are likely to make substantial contributions to our comprehension of hair cell regeneration in non-mammals and to open up avenues for new study in mammals.
Lessons from the feathered
Birds offer great opportunities to examine hair cell regeneration in its different forms. By far, most studies have been conducted in chickens. In the chicken vestibular epithelia (utricle, saccule, lagena, and cristae), there is continuous and asynchronous low-level cell proliferation (Jorgensen and Mathiesen, 1988; Roberson et al., 1992; Kil et al., 1997). This proliferation is driven by periodic hair cell apoptosis which appears to occur after hair cells reach 3 months of age (Kil et al., 1997; Stone et al., 1999; Matsui et al., 2002). Rates of supporting cell division in chicken utricles are increased when hair cell death is experimentally increased (Weisleder and Rubel, 1993). In contrast, no ongoing hair cell production occurs in the chicken auditory epithelium (Oesterle and Rubel, 1993). Rather, progenitor cells are mitotically quiescent by mid-embryogenesis and cellular differentiation is completed by hatching (Cohen and Fermin, 1978; Cotanche and Sulik, 1984; Tilney et al., 1986). Production of new hair cells is only triggered by hair cell damage (Cruz et al., 1987; Corwin and Cotanche, 1988; Ryals and Rubel, 1988; Oesterle and Rubel, 1993), and the replacement of hair cells leads to near-complete recovery of auditory and vestibular function within 1–2 months (reviewed in Bermingham-McDonogh and Rubel, 2003). Hair cell regeneration even occurs in the inner ears of senescent birds (Ryals and Rubel, 1988). The remarkable ability of the avian auditory epithelium to jump-start cellular growth despite long periods of quiescence places it in stark contrast to the mammalian organ of Corti, in which no signs of spontaneous regeneration have been noted (Roberson and Rubel, 1994; Forge et al., 1998).
In both auditory and vestibular epithelia, regenerated hair cells emerge during the first week after damage (Cotanche, 1987; Janas et al., 1995; Stone et al., 1996). At this time, some new hair cells already possess well differentiated cytoplasm and hair bundles, and they form synapses with afferent and efferent terminals that remained nearby after damage (Ryals and Westbrook, 1994; Hennig and Cotanche, 1998). By 3–4 weeks after damage, auditory and vestibular function has recovered substantially, and by 2 months, recovery is near-complete (reviewed in Bermingham-McDonogh and Rubel, 1999), although some small deficits in epithelial structure and sensory function can persist for longer periods (Marean et al., 1993).
Studies looking at regeneration in avian models use several methods for damaging hair cells. In the earliest studies, investigators used acoustic overstimulation (Cotanche, 1987), which kills hair cells in different regions of the cochlea, depending on the frequency of the stimulus and often has high levels of variability in the extent of cell death. Ototoxic drugs, including aminoglycoside antibiotics, such as gentamicin, also damage the chicken inner ear epithelia (Cruz et al., 1987). The primary advantage of aminoglycosides is that they produce a broader, more homogeneous field of hair cell damage than noise damage. Other ototoxins, such as cisplatin or heavy metals, have been poorly studied in birds.
Difficulty in accessing structures of the inner ear in situ has led investigators to develop other techniques for analyzing hair cell regeneration after damage and understanding how regeneration is altered by modifying the cellular environment. These techniques include cell culture methods to isolate supporting cells from auditory (Stone et al., 1996) and vestibular epithelia (Warchol, 1995). Organotypic cultures of cochlear ducts (Oesterle et al., 1993; Navaratnam et al., 1996; Warchol and Corwin, 1996; Frenz et al., 1998; Chen et al., 2003; Daudet et al., 2009) or of the utricle or the saccule (Oesterle et al., 1993; Warchol and Corwin, 1993) provide a method for easier access to a cells in the intact organ. These in vitro preparations make it feasible to do studies using pharmaceutical inhibition or activation of signaling pathways by adding drugs directly to culture media. In addition, these preparations provide opportunities for gene misexpression analyses by electroporating DNA into the sensory epithelium. To date, only small numbers of supporting cells have been transfected using this approach (Daudet et al., 2009). For future studies, it will be important to develop methods for broader conditional transgene activation/inhibition in chicken sensory epithelia.
Techniques have also been developed to alter the environment of the inner ear by in ovo manipulation. Wide-spread misexpression of genes can be achieved by transfecting progenitor cells in the embryonic inner ear in ovo (Morgan and Fekete, 1996) using either viral transduction (Fekete et al., 1998) or plasmid electroporation (Daudet and Lewis, 2005). Alternatively, very young embryos can be broadly transfected in ovo, and embryo mosaics can be hatched out and studied. Data generated as a result of many of these approaches are discussed below and has been vital to progress in understanding the process of avian hair cell regeneration.
Hair cell progenitors
In birds and cold-blooded animals, the predecessors to new hair cells during normal turnover and during damage-induced regeneration are supporting cells, the non-sensory cells that surround hair cells and serve structural and physiological auxiliary functions in the tissue. Although regeneration of hair cells in birds was first described as a process requiring supporting cell division (Corwin and Cotanche, 1988; Jorgensen and Mathiesen, 1988; Ryals and Rubel, 1988), the earliest phase of hair cell regeneration in fact involves a very different cellular process: the phenotypic conversion of supporting cells into hair cells without cell division called direct transdifferentiation (Fig. 1a), (Adler and Raphael, 1996; Baird et al., 1996; Roberson et al., 1996; Roberson et al., 2004). During this rather unusual process, supporting cells undergo a dramatic set of morphological and molecular changes to acquire all of the properties of sensory hair cells. The conversion begins as early as 15 hours after gentamicin administration, and converting cells have acquired several hair cell characteristics two days later (Roberson et al., 2004; Cafaro et al., 2007). Studies in the chicken auditory epithelium, in which nucleotide analogs were provided to track all cells that divided over the course of regeneration, showed that a substantial proportion of new hair cells were not labeled for the nucleotide and were therefore not derived from mitotically active progenitor cells (Roberson et al., 1996; Roberson et al., 2004). Short systemic treatments with the mitotic inhibitor, AraC, did not prevent the formation of new hair cells in chickens after noise damage, and further support the dispensability of cell division for new hair cell formation in the ear (Adler and Raphael, 1996). Similar results were found using a different mitotic inhibitor, aphidicolin, in drug-damaged saccules of frog ears (Baird et al., 1996; Baird et al.,) and newt ears (Taylor and Forge, 2005). However, it is not established that direct transdifferentiation occurs in the vestibular epithelium of chickens, primarily due to the difficulty of ruling out normal maturation of undifferentiated precursor cells since this epithelium has continual cellular turnover.
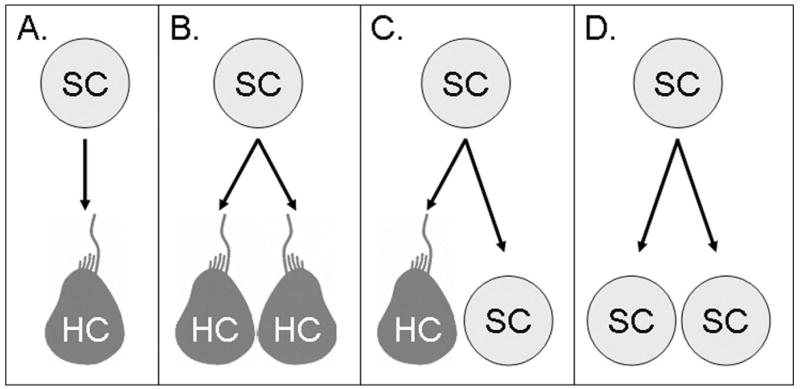
Fig. 1.
Methods of Hair Cell Replacement.
The production of hair cells (depicted here as fish hair cells, which retain kinocillia) may occur by several methods. (A) Supporting Cells (SC) may rapidly produce hair cells by direct transdifferentiation; direct, phenotypic conversion to a hair cell (HC) without the requirement for mitosis. When HC replacement depends on mitosis there are several possible mechanisms. (B) Symmetric division of one SC produces two HCs, rapidly replacing HC but eventually leading to a depletion of SC. (C) Asymmetric SC division produces one HC and one SC, replacing lost HC more slowly but replenishing the SC pool. (D) Symmetric SC division may produce two SC as a method of maintaining the SC population. This symmetric division could occur in tandem with, or following symmetric SC divisions resulting in two hair cells. One final alternative, not depicted, is that SCs produce HC precursors distinct from a fully differentiated HC and thus introduce a middle stage to all of the methods depicted above.
It is important to stress that there is no evidence for latent, undifferentiated hair cell precursors in the chicken auditory epithelium and therefore, this process is likely triggered in fully differentiated supporting cells. Further, although approximately 30–50% of new hair cells are formed by direct transdifferentiation in the chicken basilar papilla (Roberson et al., 1996; Roberson et al., 2004) it is not clear when directly transdifferentiated hair cells become functional or the degree to which their production contributes to the early phases of functional recovery.
Later phases of hair cell regeneration are driven by supporting cell division. Supporting cells first re-enter the cell cycle between 2 and 3 days after damage and they continue to proliferate until the second week after damage (Corwin and Cotanche, 1988; Ryals and Rubel, 1988). It has been suggested that supporting cell proliferation may only be needed when significant supporting cell depletion has occurred due to direct transdifferentiation (Roberson et al., 2004). However, since both new hair cells and supporting cells differentiate from supporting cell progeny (Corwin and Cotanche, 1988; Ryals and Rubel, 1988); this proliferative phase clearly serves more purpose than to simply replace supporting cells that have converted (irreversibly) into hair cells.
During cell division, supporting cell nuclei, which normally reside near the basal lamina, migrate toward the lumen, where they undergo mitosis (Raphael, 1992; Tsue et al., 1994). Cell progeny then differentiate into either hair cells or supporting cells. Statistical analysis of mitotic events shows a strong propensity for asymmetric differentiation in the chicken utricle during ongoing hair cell regeneration (Roberson et al., 1992; Stone et al., 1999) but considerably less predictability for cell fate outcomes of sibling cells in the damaged chicken basilar papilla (Stone and Rubel, 2000). These observations suggest there are no separate cell lineages for hair cells and supporting cells in either epithelium but rather, that cell fate is determined by signals in the microenvironment, such as numbers or patterning of existing hair cells.
Regulation of supporting cell behaviors after hair cell damage
One looming challenge is to identify the intrinsic and extrinsic factors that direct supporting cell behavior in animals capable of regeneration and then make comparisons with mammals to determine why supporting cells in their inner ears lie dormant after hair cell damage. Mechanisms that direct supporting cells to undergo mitotic or non-mitotic production of hair cells, or to remain quiescent, remain largely unknown. Supporting cells may be a heterogeneous population: subpopulations of supporting cells may be pre-programmed to regenerate hair cells via either mechanism while other supporting cells are incapable of changing their phenotype and do not contribute to hair cell replacement. It is clear that cells across the sensory epithelia are not identical, in quiescence or after damage. Expression of transcription factors such as Gata3 and Prox1 define subpopulations of supporting cells in the undamaged utricular and auditory epithelia, respectively (Stone et al., 2004; Warchol and Speck, 2007). The expression of Prox1, limited to hair cells in the quiescent (undamaged) basilar papilla, is strongly upregulated in ~50% of supporting cells following hair cell damage (Stone et al., 2004). In the regenerating auditory epithelia, those cells with high levels of nuclear Prox1 expression differentiate as hair cells rather than supporting cells, suggesting a role for Prox1 in the hair cell fate specification or differentiation. Gata3 is a second example of a marker for subpopulations of supporting cells (Warchol and Speck, 2007). In the avian utricle, Gata3-positive supporting cells are localized to the striolar reversal zone, a central stripe where hair cell bundle polarity is 180° to hair cells on either side. Unlike Prox1, Gata3 is strongly expressed in both quiescent and regenerating tissues. The specific expression pattern of Gata3 is not downregulated after damage, in contrast to other, more ubiquitous transcription factors in the same tissue. This suggests a role for the Gata3 positive subset of supporting cells in defining the orientation of hair cell polarity in both development and regeneration. Both Gata3 and Prox1 expression provide support for the idea that there are specializations in supporting cells that regulate their behavior during regeneration.
Alternatively, supporting cells may not be specialized in their response to damage and instead may have equal potential for any behavior, with their ultimate responses dependent on cues in the microenvironment. In the drug-damaged chicken auditory epithelium, only one of seven supporting cells divide, and such cells are concentrated in the neural half of the damaged epithelium, despite complete hair cell loss in all regions (Cafaro et al., 2007). While this latter finding suggests the neural region could be enriched in hair cell progenitors or in cells programmed for proliferation, it is also possible that mitogenic signals are stronger in the neural region. Mitogens for supporting cells in the neural region, and their cellular sources, have yet to be identified.
Two general tactics have been used to establish candidate regulators of hair cell regeneration in birds. First, investigators have chosen to study genes or proteins known to be important for cellular production in other tissues, such as those that regulate the developing inner ear, assuming they may have similar functions in the regenerating inner ear (e.g., (Navaratnam et al., 1996). Second, investigators have used genomic queries to identify gene transcripts that are up- or down-regulated after damage. For example, Bermingham-McDonogh et al. (2001) used differential display to analyze transcripts for receptor tyrosine kinases in the chicken auditory epithelium. They determined that, in supporting cells, fibroblast growth factor (FGF) receptor 3 is highly transcribed in normal tissue and is down-regulated after damage. Analysis of FGF in cochlear duct cultures showed that, in fact, FGF inhibits supporting cell division after damage (Oesterle et al., 2000). More recently, Warchol, Lovett, and colleagues have analyzed transcripts in chicken auditory and vestibular epithelia using large microchip gene arrays (Hawkins et al., 2003; Hawkins et al., 2007). This analysis revealed hundreds of genes encoding transcription factors and a limited number of genes encoding other potential regulatory proteins that are transcriptionally up- or down-regulated during the early phases of regeneration in mature chickens. Analyses of gene networks can reveal signaling pathways that are activated or suppressed during regeneration, paving the way for further analyses, such as cellular localization of specific transcripts and functional tests of specific genes or signaling pathways. This important information will undoubtedly help to elucidate the complex molecular regulation of hair cell regeneration in chickens, and perhaps in other animals as well.
The role of specific molecules in regulating supporting cell behavior after hair cell damage has been tested in cultures of explanted sensory organs or epithelia from the chicken inner ear. In the auditory epithelium, stimulation of cAMP promotes supporting cell division (Navaratnam et al., 1996), while application of bFGF inhibits it (Oesterle et al., 2000; Bermingham-McDonogh et al., 2001). In the vestibular epithelium, additional molecules appear to promote supporting cell division, including IGF-1 (Oesterle et al., 1997), PI3-kinase, TOR, MAP Kinase, (Witte et al., 2001), and immune cytokines such as TNF-alpha and TGF-alpha (Warchol, 1999).
Very few studies have addressed the factors that promote avian supporting cells to convert directly into hair cells. The Notch signaling pathway regulates cellular differentiation controlled by cell-cell interactions. A recent study by Daudet et al. (2009) examined the role of Notch signaling in supporting cell behavior in the chicken auditory epithelium. In undamaged conditions, the Notch receptor is transcribed in supporting cells, suggesting Notch signaling could maintain supporting cell quiescence (Stone and Rubel, 1999). However, inhibition of gamma secretase, which blocks all Notch activity, did not cause supporting cells to convert into hair cells. After damage, several genes in the Notch pathway, including ligands Serrate1 and Delta1 and the Notch effector, Hes5, are upregulated. If Notch signaling is inhibited in damaged tissue, via inhibition of gamma secretase activity, substantial overproduction of hair cells via both mitotic and non-mitotic mechanisms occurs. Gamma secretase inhibition has no direct effect on supporting cell division, and overproduction of hair cells occurs at the expense of supporting cell depletion. Further, overexpression of Notch’s signaling intracellular domain prevented supporting cells from converting into hair cells. Taken together, these findings demonstrate that Notch activity modulates the number of supporting cells and post-mitotic precursor cells that acquire the hair cell fate, regardless of the mechanism, but only when original hair cells are damaged or absent.
These studies leave open the question of how supporting cells are maintained in their differentiated state prior to damage and suggest a role for Notch-independent pathways. It has recently been shown that pillar cells, a subset of supporting cells in the mouse organ of Corti, display Notch-independent differentiation and maintenance during development (Doetzlhofer et al., 2009). This subset of supporting cells is distinguished by expression of the transcription factor Hey2 that, unlike most other Hes/Hey transcription factors, is Notch-independent. When Hey2 is activated by FGF, pillar cells are specifically prevented from differentiating into hair cells, even if Notch signaling is inactivated. Conversely, when Hey2 activity is disrupted, pillar cells differentiate into hair cells. Although the activity of Hey2 during hair cell regeneration has not been tested, it is tempting to speculate that it may be similarly important for preventing excessive differentiation of supporting cells into hair cells after damage in mature hair cell epithelia.
Lessons from the finned
Zebrafish, Danio rerio, are relative newcomers to the field of hair cell regeneration and an exciting model due to a unique combination of regenerative capacity and genetic tractability. In addition to these traits, small size and high fecundity minimize storage demands while external embryogenesis and transparency as embryos and young larvae facilitate live imaging. The promise of these two features in a single model has led to careful characterization of zebrafish hair cell development, structure and regeneration and comparison to other models. Although the majority of this review is dedicated to regeneration research in zebrafish, there is also a rich history of other (fin-free) non-mammalian vertebrates, in the field. Regeneration studies using salamanders, newts and frogs established the importance of non-mammalian vertebrate models in regeneration (reviewed in Corwin and Oberholtzer, 1997). Together with zebrafish, cold-blooded models of hair cell regeneration are making vital contributions to understanding which regenerative processes are conserved in vertebrates and importantly, which are lost in mammals along with their ability to regenerate hair cells.
Hair cell structure, function and development are well conserved from fish to mammals (reviewed in Nicolson, 2005b). Conserved hair cell features include distinctive cellular structure of cilia organized with a specific cell polarity (Lopez-Schier et al., 2004), mechanotransduction (reviewed in Nicolson, 2005a), and development. Furthermore, genetic conservation is also evident in the wide range of fish orthologs identified based on human deafness genes such as those responsible for Usher syndrome (reviewed in Whitfield, 2002). Finally, the cellular response to ototoxic stimuli is also conserved in zebrafish. Hair cells respond to a wide range of toxic stimuli that have been characterized in mammalian models as well as in patients. Aminoglycoside antibiotics, including, neomycin and gentamicin, rapidly cause lateral-line hair cell death in a dose dependent manner (Harris et al., 2003; Murakami et al., 2003; Lopez-Schier and Hudspeth, 2006; Santos et al., 2006; Ma et al., 2008). Platinum-based drugs used to treat cancer (Ton and Parng, 2005; Ou et al., 2007; Owens et al., 2008), and other metals including copper and silver (Hernandez et al., 2006; Linbo et al., 2006; Olivari et al., 2008) also cause dose-dependent hair cell death.
In addition to hair cells in the inner ear, zebrafish have a set of easily accessible hair cells in lateral line neuromasts, similar to that in other cold-blooded vertebrates such as salamanders, newts and tadpoles. The lateral line is located on the surface of the fish where hair cells, clustered into neuromasts, sense water movement (Coombs and Montgomery, 1999; Montgomery et al., 2003). Information about water movement is critical for a range of behaviors such as localization of predators or prey, orientation against currents, and interactions with other fish (Coombs and Montgomery, 1999). The structure and function of lateral line hair cells is very similar to that of the avian inner ear (Coombs et al., 1989). Moreover, genes identified as orthologs of human deafness genes that functioned in the zebrafish ear also disrupt lateral line hair cell function, reinforcing the conservation of functional mechanisms at the cellular level. Due to their location on the surface of the body, and the stereotyped positions of neuromasts, hair cells in the zebrafish lateral line can be rapidly screened for regeneration in live animals and the majority of zebrafish studies on hair cell regeneration have utilized the lateral line system.
Zebrafish hair cell regeneration has been demonstrated following ototoxic treatments in a range similar to those affecting avian, mouse models, and human patients. In nearly all zebrafish studies on hair cell death and regeneration, animals have been analyzed at 3 to 10 days post-fertilization. Despite the young age of these animals, the process of regeneration appears strikingly conserved in comparison to other models. It remains to be established whether regeneration in larval zebrafish relies on lingering developmental plasticity or uses distinct regenerative pathways. One approach to answering this question is to identify genes that alter hair cell regeneration without affecting development.
In all the larval stages analyzed, hair cell regeneration in the zebrafish lateral line is robust and rapid (Williams and Holder, 2000; Harris et al., 2003; Hernandez et al., 2006; Lopez-Schier and Hudspeth, 2006; Ma et al., 2008). Within 48 hours, hair cells have regenerated, reestablished both mechanotransduction, hair cell bundle polarity, and synapses with the auditory nervous system (Hernandez et al., 2006; Lopez-Schier and Hudspeth, 2006). Little is known about hair cell regeneration in terms of fish behavior, although several groups are actively pursuing methods for studying functional recovery of the zebrafish lateral line.
Regeneration in zebrafish has been analyzed using a variety of well established techniques including antibody labeling, RNA in situ hybridization labeling and electron microscopy. These techniques have been particularly important for the validation of live imaging approaches, which are a major advantage of the zebrafish over other models for hair cell regeneration. In live animals, the presence or absence of hair cells can be detected by a variety of vital dyes, including DASPEI, Yo-Pro and FM1-43 (Seiler and Nicolson, 1999; Harris et al., 2003; Murakami et al., 2003; Collazo et al., 2005; Santos et al., 2006). Dyes added to fish media are rapidly and specifically taken up by hair cells. Although the precise mechanism of vital dye uptake remains unclear, uptake of FM1-43, for example, depends on functional mechanotransduction and so is a good indicator of hair cell maturity in a live animal.
Hair cells in live zebrafish can also be analyzed using a variety of transgenic lines. Developing animals remain optically clear until the formation of metamorphic pigment cells at ~15–20 days (Budi et al., 2008) and fluorescent proteins expressed in the lateral line can be easily observed during this period. Transgenic strains include markers specific to hair cells, afferent and efferent nerves, and supporting cells (Parinov et al., 2004; Scott et al., 2007). Combining transgenic lines with vital dyes and live imaging provides a powerful tool for dissecting the process of hair cell regeneration.
Hair Cell Progenitors
Several studies in the zebrafish lateral line suggest that the majority of trauma-induced hair cell regeneration is the result of mitotic proliferation (Fig. 1b,c). The proliferation of supporting cells, as measured by BrdU labeling, increases significantly following aminoglycoside- or copper-induced hair cell damage and prior to the appearance of new hair cells (Williams and Holder, 2000; Hernandez et al., 2006; Hernandez et al., 2007; Ma et al., 2008). The majority of regenerated hair cells develop from BrdU labeled precursors (Ma et al., 2008). Furthermore, if supporting cells are damaged, as appears to be the case with high, but not low doses of copper, hair cell regeneration does not occur (Hernandez et al., 2006). Mitotic proliferation is also the major mechanism for hair cell regeneration in the axolotl salamander lateral line (Jones and Corwin, 1996).
These results suggest a predominant role for mitotic regeneration of hair cells in cold-blooded vertebrates. However, it is difficult to draw conclusions from these data alone since there are continuous, low levels of hair cell production in the zebrafish lateral line. It is difficult to distinguish between direct transdifferentiation (Fig. 1a), as observed in avian models, and differentiation of pre-existing hair cell precursors. The possibility of low levels of direct transdifferentiation in zebrafish and other cold-blooded vertebrates remains an open question.
While these studies clearly establish the importance of mitotic regeneration in the lateral line, significant questions remain about the identity of the proliferating progenitors. In comparison to supporting cells in chick and mammalian models, zebrafish supporting cells are poorly defined and the term often refers to any non-hair cells found in neuromasts. In fact, these cells can be further defined on the basis of morphology and location. The outermost supporting cells in a neuromast, often called mantle cells, are thin, elongated and form the external surface of the neuromast. Beneath the mantle cells and above the basement membrane are a second set of supporting cells with nuclei on the basal side of the neuromast and thin cytoplasmic processes extending apically to intercalate with individual hair cells and prevent hair cell-hair cell contact (Metcalfe et al., 1985). As the role of supporting cells during regeneration is better understood, it is likely that the definition of supporting cell in zebrafish will be refined. For example, following hair cell damage, supporting cells closest to the center of a neuromast begin to divide more rapidly that those near the periphery (Ma et al., 2008) hinting at the possibility of functional specialization. Similarly, differentiation of sibling progeny may be symmetric, producing two hair cell precursors (Fig. 1b) or two supporting cells (Fig. 1d) or asymmetric, producing one hair cell precursor and a supporting cell (Fig. 1c). Time-lapse imaging studies have begun to address this question. Following neomycin-induced hair cell death, only symmetric hair cell regeneration was detected during live imaging of zebrafish (Lopez-Schier and Hudspeth, 2006). This is in contrast to studies in salamanders where, following laser ablation, hair cells were replaced by asymmetric divisions resulting in one supporting cell and one hair cell (Fig. 1d), (Jones and Corwin, 1996).
These data provide additional support to the prevailing hypothesis that the majority of hair cell regeneration in the lateral line is proliferative. However, symmetric divisions producing hair cells do not replenish the pool of putative stem-cells (Fig. 1b). This suggests that a second stage of supporting cell division, either symmetric producing two supporting cells (Fig. 1d), asymmetric (Fig. 1c) or by a mechanism, as yet unknown, is responsible for maintaining the population of supporting cells within regenerating neuromasts.
Regulation of hair cell numbers
Not only do hair cells regenerate following aminoglycoside-induced damage, they regenerate just the right number of cells. Regenerating neuromasts maintain their relative sizes suggesting active regulation for termination of hair cell regeneration (Ma et al., 2008). The Notch pathway has recently been implicated in hair cell regeneration (for review see (Collado et al., 2008) although it is best known for its role in fate determination via lateral inhibition during development (reviewed in Lewis, 2008). In the zebrafish lateral line, members of the Notch pathway (notch3, deltaA, and atoh1a) are up-regulated in the 24 hours following aminoglycoside-induced damage, when supporting cell division is most active and new hair cells are being specified (Ma et al., 2008), similar to studies in other systems (Stone and Rubel, 1999; Hori et al., 2007). Pharmacological block of the Notch pathway has no effect on lateral line hair cell number in the absence of injury, but following damage, leads to an excess of regenerated hair cells (Ma et al., 2008), as was later observed in avian cell culture models (Daudet et al., 2009). While the overproduction of regenerating hair cells following inhibition of the Notch pathway occurs in both zebrafish and the chick basilar papilla, their methods differ. In damaged zebrafish neuromasts, both the rate and the duration of supporting cell division are elevated when Notch activity is inhibited. These data suggest that in the zebrafish lateral line, increased Notch signaling during regeneration plays a role in the return to quiescence, ensuring that the right number of hair cells are generated. In avian auditory epithelia recovering from aminoglycoside-induced damage, supporting cell division does not increase after inhibition of the Notch signaling pathway. Instead, excessive numbers of precursor cells are induced to differentiate into hair cells, regardless of their origin by mitosis or direct transdifferentiation. Therefore, although the exact mechanisms for regulating hair cell numbers are different between the two models, inhibition of Notch signaling results in overproduction of hair cells in both zebrafish and chick, further emphasizing the extent of genetic conservation during hair cell regeneration amongst different species.
Re-innervation
Functional regeneration requires more than replacement of hair cells. It also requires re-establishing afferent and efferent synapses. This is further complicated by hair cell polarity. Within a neuromast, hair cells are oriented with their cilia in parallel with or perpendicular to the lateral line (Flock and Wersall, 1962; Lopez-Schier et al., 2004). A single afferent nerve innervates multiple hair cells and multiple neuromasts, but only hair cells of the same polarity (Nagiel et al., 2008). During regeneration, hair cell polarity is first re-established (Lopez-Schier and Hudspeth, 2006) and then innervation follows; only cells of the same polarity are re-innervated by a given neuron (Nagiel et al., 2008). At this time, little is known about the molecular signals regulating polarity-specific re-innervation of hair cells, nor have any studies explored efferent re-innervation in the zebrafish.
Genetic screens for novel genes in hair cell regeneration
Reliable regeneration combined with access to hair cells of the lateral line, and the high level of genetic conservation in the processes of hair cell development and regeneration, are all factors that make zebrafish a unique model for discovering new genes specific to regeneration. Furthermore, zebrafish have an established record of successful genetic screens, including screens for mutations that affect development of the inner ear and lateral line (Granato et al., 1996; Malicki et al., 1996; Whitfield et al., 1996; Nicolson et al., 1998; Kappler et al., 2004; Obholzer et al., 2008) and screens for mutations in lateral line hair cells that provide resistance to ototoxic drugs (Owens et al., 2008). Forward genetic screens to identify animals with abnormal regeneration of lateral line hair cells are ongoing in several labs. Identification and characterization of genes regulating zebrafish hair cell regeneration will be followed by studies to determine whether mammalian orthologs can be co-opted to promote regeneration. As with any model system (even mouse), there are caveats about whether mechanisms will directly translate to humans. Some aspects of zebrafish development and regeneration are likely to diverge from other species; for example zebrafish hair cell regeneration shows little of the direct transdifferentiation seen in other systems. However, it is likely that fundamental mechanisms will be conserved and it is also important to determine which mechanisms are not conserved. Comparing the genetics of zebrafish vs. mammalian hair cell development and regeneration may provide critical insights as to why zebrafish regularly regenerate hair cells while mammals cannot.
Other mechanisms requiring hair cell addition
Most zebrafish studies have examined hair cell regeneration after ablation of specific subsets of hair cells with or without supporting cell ablation. However, hair cells need to be replaced in a number of additional situations. Turnover of hair cells is a normal process of aging in fish. In 10 day old zebrafish, low levels of apoptotic cell death, appear to be localized to hair cells within neuromasts (Williams and Holder, 2000). Although hair cell turnover in adult (sexually mature) zebrafish has not been reported, turnover in other fish occurs even at 9 years of age (Popper and Hoxter, 1984; Lombarte and Popper, 1994). We do not yet know whether hair cell replacement as a result of normal turnover following processes distinct from regeneration or shares common mechanisms.
In addition to turnover, neuromasts continue to be added as the fish grows. This requires two additional processes: intercalation and stitch formation. Intercalation is the process of inserting neuromasts into the lateral line in the anterior-posterior axis while stitch formation is the addition of neuromasts dorsoventrally (reviewed in Ghysen and Dambly-Chaudiere, 2007). During intercalation neuromasts are added between existing neuromasts from latent precursors established during the initial development of the lateral line (Grant et al., 2005). Following intercalation the entire lateral line migrates dorsoventrally then expands. The expansion, or addition of stitches, is a dorsoventral addition of neuromasts at each previously established neuromast in the in the lateral line (Metcalfe et al., 1985). Early studies on salamander development describe the addition of neuromasts by budding; existing neuromasts generate additional neuromasts that eventually migrate away (Stone, 1933). Following these studies, performed well before development of live imaging and molecular markers, it was generally assumed that the process of stitch formation was the same in zebrafish. More recently, stitch formation was examined in sexually mature zebrafish (Ledent, 2002; Sapede et al., 2002). When new stitches appear in zebrafish, they are already innervated suggesting that addition does occur by budding (Ledent, 2002). However, stitch formation progresses in anterior to posterior waves, rather than simultaneously, suggesting additional levels of regulation. Revisiting the process of neuromast migration and stitch formation may reveal whether there are significant differences between neuromast addition and regeneration. This question, in combination with genetic screen, will address the question of whether regeneration in the zebrafish is distinct from developmental processes.
Hair cell regeneration also occurs as a part of fin or tail regeneration. In zebrafish, fin regeneration includes regenerating a portion of the lateral line as the fin itself is regenerated. This process differs from regeneration following hair cell-specific ablation since multiple neuromasts, (including hair cells, a variety of supporting cells and neuronal innervation) must be recapitulated in a stereotyped pattern. Following fin amputation in sexually mature zebrafish, mantle cells of the neuromast remaining immediately anterior to the plane of amputation begin to proliferate. These mantle cells essentially recapitulate the process observed during development: they form a small primordium that migrates onto the regenerated fin and deposits new neuromasts. Neuromasts generated during fin regeneration are deposited in the absence of innervation, in contrast to stitch formation where neuromasts migrating dorsoventrally carry along innervating processes (Ledent, 2002; Dufourcq et al., 2006). This two-step process of regeneration, fin before neuromasts, is strikingly similar to that described in other cold-blooded vertebrates included salamanders regenerating their tail tips (Stone, 1933; Stone, 1937; Corwin, 1986; Corwin et al., 1989; Jones and Corwin, 1993), and tadpoles regenerating tails (Speidel, 1947; Wright, 1947). It is worth noting that lateral line regeneration depends on a pool of neuromast-precursors distinct from the fin blastema; the source of regeneration for all other fin structures. While many genes involved in formation and differentiation of the blastema have been identified (reviewed in Akimenko et al., 2003; Iovine, 2007) little is known about the genetics of lateral line regeneration following amputation in the zebrafish. The relationships between precursors that generate new hair cells after damage and those that generate new neuromasts during lateral line growth, stitch formation or during fin regeneration are currently unknown. Establishing whether the replacement of entire neuromasts differs significantly from the replacement of hair cells within an existing neuromast may provide the opportunity to determine the full extent of stem-cell like properties of supporting cells.
Conclusions and Future directions
The chick is now a classic model for hair cell regeneration with a wealth of studies on a variety of structures, both auditory and vestibular, in the inner ear. In avian models, the time-course of hair cell regeneration, the identity of hair cell precursors and a range of other cellular processes have been characterized. Studies in birds have generated important questions that will define the direction of future research. The zebrafish, a relative upstart by comparison, is now also established as a hair cell regeneration model, displaying a consistent time-course of regeneration in response to a range of damaging conditions that also function in avian and mammalian models. In zebrafish, work remains to clarify the identity of hair cell progenitors and the processes leading to hair cell and supporting cell replacement. However, this line of examination is significantly aided by the accessibility of lateral line hair cells for manipulation and live imaging. Due to a wide range of existing transgenic lines, mutants and the opportunity for unbiased, forward genetic screens to identify genes involved in regeneration, the zebrafish is an exciting new model for addressing questions that remain about molecular regulation of hair cell regeneration.
Acknowledgments
The authors would like to thank Dr. A. Suli and Dr. S. Garcia for comments on the manuscript and E. Ma for art contributions. Our work is supported by the Hearing Regeneration Initiative and the National Institutes of Health Grants DC008973, DC005987, and DC05361 (DWR, HRB) and DC003696 and DC04661 (JSS).
Abbreviations
- BrdU
Bromodeoxyuridine
- FGF
fibroblast growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- Akimenko MA, Mari-Beffa M, Becerra J, Geraudie J. Old questions, new tools, and some answers to the mystery of fin regeneration. Dev Dyn. 2003;226:190–201. doi: 10.1002/dvdy.10248. [DOI] [PubMed] [Google Scholar]
- Baird RA, Steyger PS, Schuff NR. Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann N Y Acad Sci. 1996;781:59–70. doi: 10.1111/j.1749-6632.1996.tb15693.x. [DOI] [PubMed] [Google Scholar]
- Baird RA, Burton MD, Fashena DS, Naeger RA. Hair cell recovery in mitotically blocked cultures of the bullfrog saccule. Proc Natl Acad Sci U S A. 2000;97:11722–11729. doi: 10.1073/pnas.97.22.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cell progeny: evidence from phototoxicity and laser ablation experiments in the lateral line system. J Neurosci. 1990;10:2502–2512. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel K, Hansen L, Soukup G, Fritzsch B. Regenerating cochlear hair cells: quo vadis stem cell. Cell Tissue Res. 2008 doi: 10.1007/s00441-008-0639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Stone JS, Reh TA, Rubel EW. FGFR3 expression during development and regeneration of the chick inner ear sensory epithelia. Dev Biol. 2001;238:247–259. doi: 10.1006/dbio.2001.0412. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Rubel EW. Hair cell regeneration: winging our way towards a sound future. Curr Opin Neurobiol. 2003;13:119–126. doi: 10.1016/s0959-4388(03)00018-7. [DOI] [PubMed] [Google Scholar]
- Budi EH, Patterson LB, Parichy DM. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development. 2008;135:2603–2614. doi: 10.1242/dev.019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafaro J, Lee GS, Stone JS. Atoh1 expression defines activated progenitors and differentiating hair cells during avian hair cell regeneration. Dev Dyn. 2007;236:156–170. doi: 10.1002/dvdy.21023. [DOI] [PubMed] [Google Scholar]
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–426. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- Cohen GM, Fermin CD. The development of hair cells in the embryonic chick’s basilar papilla. Acta Otolaryngol. 1978;86:342–358. doi: 10.3109/00016487809107513. [DOI] [PubMed] [Google Scholar]
- Collado MS, Burns JC, Hu Z, Corwin JT. Recent advances in hair cell regeneration research. Curr Opin Otolaryngol Head Neck Surg. 2008;16:465–471. doi: 10.1097/MOO.0b013e32830f4ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collazo A, Bricaud O, Desai K. Use of confocal microscopy in comparative studies of vertebrate morphology. Methods Enzymol. 2005;395:521–543. doi: 10.1016/S0076-6879(05)95027-1. [DOI] [PubMed] [Google Scholar]
- Coombs S, Gorner P, Meunz H. The Mechanosensory Lateral Line: Neurobiology and Evolution. Springer-Verlag; New York: 1989. [Google Scholar]
- Coombs S, Montgomery JC. The enigmatic lateral line system. Comparative hearing. In: Fay RR, Popper AN, editors. Fish and amphibians. Springer; New York: 1999. p. 438. [Google Scholar]
- Corwin JT. Postembryonic production and aging in inner ear hair cells in sharks. J Comp Neurol. 1981;201:541–553. doi: 10.1002/cne.902010406. [DOI] [PubMed] [Google Scholar]
- Corwin JT. Perpetual production of hair cells and maturational changes in hair cell ultrastructure accompany postembryonic growth in an amphibian ear. Proc Natl Acad Sci U S A. 1985;82:3911–3915. doi: 10.1073/pnas.82.11.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin JT. Regeneration and self-repair in hair cell epithelia: experimental evaluation of capacities and limitations. In: Ruben RJ, Van DeWater TR, Rubel EW, editors. The biology of change in otolaryngology. Elsevier; New York: 1986. pp. 291–304. [Google Scholar]
- Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Balak KJ, Borden PC. Cellular Events Underlying the Regenerative Replacement of Lateral Line Sensory Epithelia in Amphibians. In: Coombs S, Gorner P, Munz H, editors. The Mechanosensory Lateral Line: Neurobiology and Evolution. Springer-Verlag; New York: 1989. pp. 161–183. [Google Scholar]
- Corwin JT, Oberholtzer JC. Fish n’ chicks: model recipes for hair-cell regeneration? Neuron. 1997;19:951–954. doi: 10.1016/s0896-6273(00)80386-4. [DOI] [PubMed] [Google Scholar]
- Cotanche DA, Sulik KK. The development of stereociliary bundles in the cochlear duct of chick embryos. Brain Res. 1984;318:181–193. doi: 10.1016/0165-3806(84)90024-5. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Regeneration of hair cell stereociliary bundles in the chick cochlea following severe acoustic trauma. Hear Res. 1987;30:181–195. doi: 10.1016/0378-5955(87)90135-3. [DOI] [PubMed] [Google Scholar]
- Cotanche DA. Genetic and pharmacological intervention for treatment/prevention of hearing loss. J Commun Disord. 2008;41:421–443. doi: 10.1016/j.jcomdis.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz RM, Lambert PR, Rubel EW. Light microscopic evidence of hair cell regeneration after gentamicin toxicity in chick cochlea. Arch Otolaryngol Head Neck Surg. 1987;113:1058–1062. doi: 10.1001/archotol.1987.01860100036017. [DOI] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development. 2005;132:541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Daudet N, Gibson R, Shang J, Bernard A, Lewis J, Stone J. Notch regulation of progenitor cell behavior in quiescent and regenerating auditory epithelium of mature birds. Dev Biol. 2009;326:86–100. doi: 10.1016/j.ydbio.2008.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, Segil N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16:58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourcq P, Roussigne M, Blader P, Rosa F, Peyrieras N, Vriz S. Mechano-sensory organ regeneration in adults: the zebrafish lateral line as a model. Mol Cell Neurosci. 2006;33:180–187. doi: 10.1016/j.mcn.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–7821. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A, Wersall J. A study of the orientation of the sensory hairs of the receptor cells in the lateral line organ of fish, with special reference to the function of the receptors. J Cell Biol. 1962;15:19–27. doi: 10.1083/jcb.15.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Li L, Nevill G. Hair cell recovery in the vestibular sensory epithelia of mature guinea pigs. J Comp Neurol. 1998;397:69–88. [PubMed] [Google Scholar]
- Frenz DA, Yoo H, Liu W. Basilar papilla explants: a model to study hair cell regeneration-repair and protection. Acta Otolaryngol. 1998;118:651–659. doi: 10.1080/00016489850183133. [DOI] [PubMed] [Google Scholar]
- Friedman RA, Van Laer L, Huentelman MJ, Sheth SS, Van Eyken E, Corneveaux JJ, Tembe WD, Halperin RF, Thorburn AQ, Thys S, Bonneux S, Fransen E, Huyghe J, Pyykko I, Cremers CW, Kremer H, Dhooge I, Stephens D, Orzan E, Pfister M, Bille M, Parving A, Sorri M, Van de Heyning PH, Makmura L, Ohmen JD, Linthicum FH, Jr, Fayad JN, Pearson JV, Craig DW, Stephan DA, Van Camp G. grm7 variants confer susceptibility to age-related hearing impairment. Hum Mol Genet. 2008 doi: 10.1093/hmg/ddn402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A, Dambly-Chaudiere C. The lateral line microcosmos. Genes Dev. 2007;21:2118–2130. doi: 10.1101/gad.1568407. [DOI] [PubMed] [Google Scholar]
- Granato M, van Eeden FJ, Schach U, Trowe T, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Genes controlling and mediating locomotion behavior of the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Grant KA, Raible DW, Piotrowski T. Regulation of latent sensory hair cell precursors by glia in the zebrafish lateral line. Neuron. 2005;45:69–80. doi: 10.1016/j.neuron.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Guthrie OW. Aminoglycoside induced ototoxicity. Toxicology. 2008;249:91–96. doi: 10.1016/j.tox.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RD, Bashiardes S, Helms CA, Hu L, Saccone NL, Warchol ME, Lovett M. Gene expression differences in quiescent versus regenerating hair cells of avian sensory epithelia: implications for human hearing and balance disorders. Hum Mol Genet. 2003;12:1261–1272. doi: 10.1093/hmg/ddg150. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Bashiardes S, Powder KE, Sajan SA, Bhonagiri V, Alvarado DM, Speck J, Warchol ME, Lovett M. Large scale gene expression profiles of regenerating inner ear sensory epithelia. PLoS ONE. 2007;2:e525. doi: 10.1371/journal.pone.0000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig AK, Cotanche DA. Regeneration of cochlear efferent nerve terminals after gentamycin damage. J Neurosci. 1998;18:3282–3296. doi: 10.1523/JNEUROSCI.18-09-03282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PP, Moreno V, Olivari FA, Allende ML. Sub-lethal concentrations of waterborne copper are toxic to lateral line neuromasts in zebrafish (Danio rerio) Hear Res. 2006;213:1–10. doi: 10.1016/j.heares.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Hernandez PP, Olivari FA, Sarrazin AF, Sandoval PC, Allende ML. Regeneration in zebrafish lateral line neuromasts: expression of the neural progenitor cell marker sox2 and proliferation-dependent and-independent mechanisms of hair cell renewal. Dev Neurobiol. 2007;67:637–654. doi: 10.1002/dneu.20386. [DOI] [PubMed] [Google Scholar]
- Hori R, Nakagawa T, Sakamoto T, Matsuoka Y, Takebayashi S, Ito J. Pharmacological inhibition of Notch signaling in the mature guinea pig cochlea. Neuroreport. 2007;18:1911–1914. doi: 10.1097/WNR.0b013e3282f213e0. [DOI] [PubMed] [Google Scholar]
- Iovine MK. Conserved mechanisms regulate outgrowth in zebrafish fins. Nat Chem Biol. 2007;3:613–618. doi: 10.1038/nchembio.2007.36. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, Raphael Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Janas JD, Cotanche DA, Rubel EW. Avian cochlear hair cell regeneration: stereological analyses of damage and recovery from a single high dose of gentamicin. Hear Res. 1995;92:17–29. doi: 10.1016/0378-5955(95)00190-5. [DOI] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Replacement of lateral line sensory organs during tail regeneration in salamanders: identification of progenitor cells and analysis of leukocyte activity. J Neurosci. 1993;13:1022–1034. doi: 10.1523/JNEUROSCI.13-03-01022.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JE, Corwin JT. Regeneration of sensory cells after laser ablation in the lateral line system: hair cell lineage and macrophage behavior revealed by time-lapse video microscopy. J Neurosci. 1996;16:649–662. doi: 10.1523/JNEUROSCI.16-02-00649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JM, Mathiesen C. The avian inner ear. Continuous production of hair cells in vestibular sensory organs, but not in the auditory papilla. Naturwissenschaften. 1988;75:319–320. doi: 10.1007/BF00367330. [DOI] [PubMed] [Google Scholar]
- Kappler JA, Starr CJ, Chan DK, Kollmar R, Hudspeth AJ. A nonsense mutation in the gene encoding a zebrafish myosin VI isoform causes defects in hair-cell mechanotransduction. Proc Natl Acad Sci U S A. 2004;101:13056–13061. doi: 10.1073/pnas.0405224101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kil J, Warchol ME, Corwin JT. Cell death, cell proliferation, and estimates of hair cell life spans in the vestibular organs of chicks. Hear Res. 1997;114:117–126. doi: 10.1016/s0378-5955(97)00166-4. [DOI] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Zhou D, Smythe N, Spicer SS, Schmiedt RA. Nuclear factor kappaB deficiency is associated with auditory nerve degeneration and increased noise-induced hearing loss. J Neurosci. 2006;26:3541–3550. doi: 10.1523/JNEUROSCI.2488-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledent V. Postembryonic development of the posterior lateral line in zebrafish. Development. 2002;129:597–604. doi: 10.1242/dev.129.3.597. [DOI] [PubMed] [Google Scholar]
- Lewis J. From signals to patterns: space, time, and mathematics in developmental biology. Science. 2008;322:399–403. doi: 10.1126/science.1166154. [DOI] [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Linbo TL, Stehr CM, Incardona JP, Scholz NL. Dissolved copper triggers cell death in the peripheral mechanosensory system of larval fish. Environ Toxicol Chem. 2006;25:597–603. doi: 10.1897/05-241r.1. [DOI] [PubMed] [Google Scholar]
- Lombarte A, Yan HY, Popper AN, Chang JS, Platt C. Damage and regeneration of hair cell ciliary bundles in a fish ear following treatment with gentamicin. Hear Res. 1993;64:166–174. doi: 10.1016/0378-5955(93)90002-i. [DOI] [PubMed] [Google Scholar]
- Lombarte A, Popper AN. Quantitative analyses of postembryonic hair cell addition in the otolithic endorgans of the inner ear of the European hake, Merluccius merluccius (Gadiformes, Teleostei) J Comp Neurol. 1994;345:419–428. doi: 10.1002/cne.903450308. [DOI] [PubMed] [Google Scholar]
- Lopez-Schier H, Starr CJ, Kappler JA, Kollmar R, Hudspeth AJ. Directional cell migration establishes the axes of planar polarity in the posterior lateral-line organ of the zebrafish. Dev Cell. 2004;7:401–412. doi: 10.1016/j.devcel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Lopez-Schier H, Hudspeth AJ. A two-step mechanism underlies the planar polarization of regenerating sensory hair cells. Proc Natl Acad Sci U S A. 2006;103:18615–18620. doi: 10.1073/pnas.0608536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malicki J, Schier AF, Solnica-Krezel L, Stemple DL, Neuhauss SC, Stainier DY, Abdelilah S, Rangini Z, Zwartkruis F, Driever W. Mutations affecting development of the zebrafish ear. Development. 1996;123:275–283. doi: 10.1242/dev.123.1.275. [DOI] [PubMed] [Google Scholar]
- Marean GC, Burt JM, Beecher MD, Rubel EW. Hair cell regeneration in the European starling (Sturnus vulgaris): recovery of pure-tone detection thresholds. Hear Res. 1993;71:125–136. doi: 10.1016/0378-5955(93)90028-y. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Ogilvie JM, Warchol ME. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22:1218–1227. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI, Cotanche DA. Sensory hair cell death and regeneration: two halves of the same equation. Curr Opin Otolaryngol Head Neck Surg. 2004;12:418–425. doi: 10.1097/01.moo.0000136873.56878.56. [DOI] [PubMed] [Google Scholar]
- Metcalfe WK, Kimmel CB, Schabtach E. Organization and development of the zebrafish posterior lateral line. The Mechanosensory Lateral Line. In: Coombs S, Gorner P, Munz H, editors. Neurobiology and Evolution. Springer-Verlag; New York: 1985. pp. 147–159. [Google Scholar]
- Montgomery JC, McDonald F, Baker CF, Carton AG, Ling N. Sensory integration in the hydrodynamic world of rainbow trout. Proc Biol Sci. 2003;270 Suppl 2:S195–197. doi: 10.1098/rsbl.2003.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan BA, Fekete DM. Manipulating gene expression with replication-competent retroviruses. Methods Cell Biol. 1996;51:185–218. doi: 10.1016/s0091-679x(08)60629-9. [DOI] [PubMed] [Google Scholar]
- Murakami SL, Cunningham LL, Werner LA, Bauer E, Pujol R, Raible DW, Rubel EW. Developmental differences in susceptibility to neomycin-induced hair cell death in the lateral line neuromasts of zebrafish (Danio rerio) Hear Res. 2003;186:47–56. doi: 10.1016/s0378-5955(03)00259-4. [DOI] [PubMed] [Google Scholar]
- Nagiel A, Andor-Ardo D, Hudspeth AJ. Specificity of afferent synapses onto plane-polarized hair cells in the posterior lateral line of the zebrafish. J Neurosci. 2008;28:8442–8453. doi: 10.1523/JNEUROSCI.2425-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaratnam DS, Su HS, Scott SP, Oberholtzer JC. Proliferation in the auditory receptor epithelium mediated by a cyclic AMP-dependent signaling pathway. Nat Med. 1996;2:1136–1139. doi: 10.1038/nm1096-1136. [DOI] [PubMed] [Google Scholar]
- Nicolson T, Rusch A, Friedrich RW, Granato M, Ruppersberg JP, Nusslein-Volhard C. Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron. 1998;20:271–283. doi: 10.1016/s0896-6273(00)80455-9. [DOI] [PubMed] [Google Scholar]
- Nicolson T. Fishing for key players in mechanotransduction. Trends Neurosci. 2005a;28:140–144. doi: 10.1016/j.tins.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Nicolson T. The genetics of hearing and balance in zebrafish. Annu Rev Genet. 2005b;39:9–22. doi: 10.1146/annurev.genet.39.073003.105049. [DOI] [PubMed] [Google Scholar]
- Obholzer N, Wolfson S, Trapani JG, Mo W, Nechiporuk A, Busch-Nentwich E, Seiler C, Sidi S, Sollner C, Duncan RN, Boehland A, Nicolson T. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J Neurosci. 2008;28:2110–2118. doi: 10.1523/JNEUROSCI.5230-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterle EC, Rubel EW. Postnatal production of supporting cells in the chick cochlea. Hear Res. 1993;66:213–224. doi: 10.1016/0378-5955(93)90141-m. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Tsue TT, Reh TA, Rubel EW. Hair-cell regeneration in organ cultures of the postnatal chicken inner ear. Hear Res. 1993;70:85–108. doi: 10.1016/0378-5955(93)90054-5. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Tsue TT, Rubel EW. Induction of cell proliferation in avian inner ear sensory epithelia by insulin-like growth factor-I and insulin. J Comp Neurol. 1997;380:262–274. doi: 10.1002/(sici)1096-9861(19970407)380:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Oesterle EC, Bhave SA, Coltrera MD. Basic fibroblast growth factor inhibits cell proliferation in cultured avian inner ear sensory epithelia. J Comp Neurol. 2000;424:307–326. doi: 10.1002/1096-9861(20000821)424:2<307::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Olivari FA, Hernandez PP, Allende ML. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zerafish larve. Brain Research. 2008;1244:1–12. doi: 10.1016/j.brainres.2008.09.050. [DOI] [PubMed] [Google Scholar]
- Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Geleoc GS, Edge A, Holt JR, Heller S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8:18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Raible DW, Rubel EW. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear Res. 2007;233:46–53. doi: 10.1016/j.heares.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Raible DW. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 2008;4:e1000020. doi: 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parinov S, Kondrichin I, Korzh V, Emelyanov A. Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev Dyn. 2004;231:449–459. doi: 10.1002/dvdy.20157. [DOI] [PubMed] [Google Scholar]
- Popper AN, Hoxter B. Growth of a fish ear: 1. Quantitative analysis of hair cell and ganglion cell proliferation. Hear Res. 1984;15:133–142. doi: 10.1016/0378-5955(84)90044-3. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Johnson EM., Jr Men are but worms: neuronal cell death in C elegans and vertebrates. Cell Death Differ. 2004;11:38–48. doi: 10.1038/sj.cdd.4401352. [DOI] [PubMed] [Google Scholar]
- Raphael Y. Evidence for supporting cell mitosis in response to acoustic trauma in the avian inner ear. J Neurocytol. 1992;21:663–671. doi: 10.1007/BF01191727. [DOI] [PubMed] [Google Scholar]
- Roberson DF, Weisleder P, Bohrer PS, Rubel EW. Ongoing production of sensory cells in the vestibular epithelium of the chick. Hear Res. 1992;57:166–174. doi: 10.1016/0378-5955(92)90149-h. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Rubel EW. Cell division in the gerbil cochlea after acoustic trauma. Am J Otol. 1994;15:28–34. [PubMed] [Google Scholar]
- Roberson DW, Kreig CS, Rubel EW. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Auditory Neuroscience. 1996;2:195–205. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Roberson DW, Alosi JA, Cotanche DA. Direct transdifferentiation gives rise to the earliest new hair cells in regenerating avian auditory epithelium. J Neurosci Res. 2004;78:461–471. doi: 10.1002/jnr.20271. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT. How cochlear implants encode speech. Curr Opin Otolaryngol Head Neck Surg. 2004;12:444–448. doi: 10.1097/01.moo.0000134452.24819.c0. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- Ryals BM, Westbrook EW. TEM analysis of neural terminals on autoradiographically identified regenerated hair cells. Hear Res. 1994;72:81–88. doi: 10.1016/0378-5955(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Santos F, MacDonald G, Rubel EW, Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio) Hear Res. 2006;213:25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Sapede D, Gompel N, Dambly-Chaudiere C, Ghysen A. Cell migration in the postembryonic development of the fish lateral line. Development. 2002;129:605–615. doi: 10.1242/dev.129.3.605. [DOI] [PubMed] [Google Scholar]
- Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, Chi NC, Asakawa K, Kawakami K, Baier H. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat Methods. 2007;4:323–326. doi: 10.1038/nmeth1033. [DOI] [PubMed] [Google Scholar]
- Seiler C, Nicolson T. Defective calmodulin-dependent rapid apical endocytosis in zebrafish sensory hair cell mutants. J Neurobiol. 1999;41:424–434. [PubMed] [Google Scholar]
- Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Speidel CC. Correlated studies of sense organs and nerves of the lateral-line in living frog tadpoles. J Comp Neurol. 1947;87:29–55. doi: 10.1002/cne.900870104. [DOI] [PubMed] [Google Scholar]
- Stone JS, Leano SG, Baker LP, Rubel EW. Hair cell differentiation in chick cochlear epithelium after aminoglycoside toxicity: in vivo and in vitro observations. J Neurosci. 1996;16:6157–6174. doi: 10.1523/JNEUROSCI.16-19-06157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone JS, Choi YS, Woolley SM, Yamashita H, Rubel EW. Progenitor cell cycling during hair cell regeneration in the vestibular and auditory epithelia of the chick. J Neurocytol. 1999;28:863–876. doi: 10.1023/a:1007022205821. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Delta1 expression during avian hair cell regeneration. Development. 1999;126:961–973. doi: 10.1242/dev.126.5.961. [DOI] [PubMed] [Google Scholar]
- Stone JS, Rubel EW. Temporal, spatial, and morphologic features of hair cell regeneration in the avian basilar papilla. J Comp Neurol. 2000;417:1–16. doi: 10.1002/(sici)1096-9861(20000131)417:1<1::aid-cne1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Stone JS, Shang JL, Tomarev S. cProx1 immunoreactivity distinguishes progenitor cells and predicts hair cell fate during avian hair cell regeneration. Dev Dyn. 2004;230:597–614. doi: 10.1002/dvdy.20087. [DOI] [PubMed] [Google Scholar]
- Stone L. The development of lateral-line sense organs in amphibians observed in living and vital-stained preparations. J Comp Neurol. 1933;57:507–540. [Google Scholar]
- Stone L. Further experimental studies of the development of lateral-line sense organs in amphibians observed in living preparations. J Comp Neurol. 1937;68:83–115. [Google Scholar]
- Taylor RR, Forge A. Hair cell regeneration in sensory epithelia from the inner ear of a urodele amphibian. J Comp Neurol. 2005;484:105–120. doi: 10.1002/cne.20450. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Saunders JS, DeRosier DJ. Actin filaments, stereocilia, and hair cells of the bird cochlea. III. The development and differentiation of hair cells and stereocilia. Dev Biol. 1986;116:100–118. doi: 10.1016/0012-1606(86)90047-3. [DOI] [PubMed] [Google Scholar]
- Ton C, Parng C. The use of zebrafish for assessing ototoxic and otoprotective agents. Hear Res. 2005;208:79–88. doi: 10.1016/j.heares.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Tsue TT, Watling DL, Weisleder P, Coltrera MD, Rubel EW. Identification of hair cell progenitors and intermitotic migration of their nuclei in the normal and regenerating avian inner ear. J Neurosci. 1994;14:140–152. doi: 10.1523/JNEUROSCI.14-01-00140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME, Corwin JT. Supporting cells in avian vestibular organs proliferate in serum-free culture. Hear Res. 1993;71:28–36. doi: 10.1016/0378-5955(93)90018-v. [DOI] [PubMed] [Google Scholar]
- Warchol ME. Supporting cells in isolated sensory epithelia of avian utricles proliferate in serum-free culture. Neuroreport. 1995;6:981–984. doi: 10.1097/00001756-199505090-00008. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Corwin JT. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J Neurosci. 1996;16:5466–5477. doi: 10.1523/JNEUROSCI.16-17-05466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol ME. Immune cytokines and dexamethasone influence sensory regeneration in the avian vestibular periphery. J Neurocytol. 1999;28:889–900. doi: 10.1023/a:1007026306730. [DOI] [PubMed] [Google Scholar]
- Warchol ME, Speck JD. Expression of GATA3 and tenascin in the avian vestibular maculae: normative patterns and changes during sensory regeneration. J Comp Neurol. 2007;500:646–657. doi: 10.1002/cne.21153. [DOI] [PubMed] [Google Scholar]
- Weisleder P, Rubel EW. Hair cell regeneration after streptomycin toxicity in the avian vestibular epithelium. J Comp Neurol. 1993;331:97–110. doi: 10.1002/cne.903310106. [DOI] [PubMed] [Google Scholar]
- White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Whitfield TT, Granato M, van Eeden FJ, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Nusslein-Volhard C. Mutations affecting development of the zebrafish inner ear and lateral line. Development. 1996;123:241–254. doi: 10.1242/dev.123.1.241. [DOI] [PubMed] [Google Scholar]
- Whitfield TT. Zebrafish as a model for hearing and deafness. J Neurobiol. 2002;53:157–171. doi: 10.1002/neu.10123. [DOI] [PubMed] [Google Scholar]
- Williams JA, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000;143:171–181. doi: 10.1016/s0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Witte MC, Montcouquiol M, Corwin JT. Regeneration in avian hair cell epithelia: identification of intracellular signals required for S-phase entry. Eur J Neurosci. 2001;14:829–838. doi: 10.1046/j.0953-816x.2001.01695.x. [DOI] [PubMed] [Google Scholar]
- Wright MR. Regeneration and degeneration experiments on lateral line nerves and sense organs in anurans. J Exp Zool. 1947;105:221–257. doi: 10.1002/jez.1401050206. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]