Abstract
PM02734 (elisidepsin) is a novel marine-derived cyclic peptide belonging to the Kahaladide family of compounds currently under phase I development with early evidence of a positive therapeutic index. The cytotoxicity of PM02734 has been determined in a panel of human NSCLC (non-small cell lung cancer) cell lines. Western blot analysis showed a direct correlation between ErbB3 expression and cell sensitivity to PM02734. Furthermore, PM02734 was more effective in the induction of ErbB3 degradation and dephosphorylation than that of ErbB2 and ErbB1 in human NSCLC cell lines. The combination of PM02734 and erlotinib was synergistic in all NSCLC cell lines tested, including erlotinib resistant cell lines, with combination indexes ranging between 0.59 and 0.81. The combination of PM02734 and erlotinib was more effective than either drug alone in mice inoculated i.v. with A549 cells. The combination of PM02734 and erlotinib was more effective in inhibiting AKT than either single agent alone in H322 cells. These results have provided a rational basis for an ongoing clinical trial to explore this combination in patients with advanced malignant solid tumours.
Keywords: PM02734, elisidepsin, erlotinib, NSCLC, ErbB3
Introduction
PM02734 (elisidepsin) is a novel marine-derived cyclic peptide belonging to the Kahaladide family of compounds1 currently under late phase I development with preliminary evidence of antitumor activity and a favourable therapeutic index. There is solid evidence that sensitivity to Kahalalide compounds correlates with the expression of ErbB receptors. The natural compound Kahalalide F (KF) was shown to be the third compound, among 49,000 compounds tested in the NCI database, showing the highest correlation between cytotoxicity and high c-erbB2 mRNA expression levels; this information was generated in a study in which EGF receptor, c-erbB2 and TGF-α mRNA expression levels in the 60 cell lines comprising the NCI anticancer drug screen were analyzed and correlated by COMPARE analysis with drug sensitivity2.
Based on this observation, a number of confirmatory studies were implemented. The sensitivity to KF in a panel of tumour cell lines (including NSCLC, breast, ovarian and hepatic carcinomas) was found to significantly correlate with ErbB3 protein expression levels; no correlation was observed with EGFR, ErbB2 and ErbB4 expression levels3. In addition to the general down-regulation of ErbB family members observed after long-term exposure to KF, a 4 h treatment with KF resulted in the selective downregulation of ErbB3 in a sensitive, high ErbB3-expressing cell line; moreover, ectopic expression of ErbB3 increased the sensitivity of a resistant cell line to KF. KF decreased the expression of phosphorylated AKT and induced the redistribution of p27/kip1 from the cytosol to the nucleus. Nuclear p27 inhibits cyclin/CDK complexes resulting in G1 cell-cycle arrest and the concomitant induction of a hypodiploid population. Similar effects have been observed with tyrosine-kinase ErbB inhibitors and anti-ErbB mAbs4.
A recent report indicates that overexpression of fatty acid 2-hydroxylase (FA2H) and exogenous addition of 2-hydroxylated fatty acids increase the sensitivity of mammalian cells to PM027345. These authors show that PM02734 affects intracellular vesicle trafficking and induces a rapid necrosis-like cell death.
Sensitivity of NSCLC cells to EGFR inhibition by erlotinib has been shown to correlate more with phospho-ErbB3 and total ErbB3 levels than with total EGFR or ErbB2 expression6. ErbB3 has been also shown to be a biomarker of sensitivity to erlotinib in a panel of pancreatic and colorectal cancer cell lines7,8. Cancer cells showing HER family signalling, either directly through EGFR-ErbB3 heterodimer formation or indirectly through Src family kinase activation of ErbB3, tend to be more sensitive to EGFR inhibition9. Moreover, ErbB3 is used to couple EGFR to the PI3K/AKT pathway in gefitinib-sensitive cell lines10. The ErbB2/ErbB3 heterodimer has been shown to be the most transforming and mitogenic receptor complex of the ErbB family11. These observations suggest that cancer cell lines driven by a member of the ErbB receptor system often couple to ErbB3 to activate the PI3K/AKT pathway and promote the cancer phenotype.
In this study we sought to characterize the cytotoxic effect of PM02734 in a panel of human NSCLC cell lines and studied the cytotoxicity of the combination of PM02734 and erlotinib. Our results indicate that the combination of concurrent PM02734 and erlotinib is synergistic at pharmacologically achievable concentrations in NSCLC cell lines and in in vivo NSCLC xenograft models. These results have provided a rationale to study the synergism of the combination of PM02734 and ErbB inhibitors in an ongoing clinical trial.
Materials and Methods
Chemicals
PM02734 was manufactured by PharmaMar (Madrid, Spain). The drugs were dissolved in DMSO at a stock concentration of 10 mM, and diluted to the indicated concentrations with medium. Polyclonal Anti-EGFR, anti-p-EGFR, anti-ErbB2, anti-p-ErbB2, anti-ErbB3, anti-p-ErbB3, anti-AKT and anti-p-AKT antibodies were purchased from Cell Signalling Technology (Danvers, MA). Polyclonal anti-ERK and anti-p-ERK antibodies were obtained from Promega (Madison, WI). Anti-cyclin A, anti-cyclin E, anti-cyclin D and anti-cyclin B antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell lines and cell culture
Human non-small-cell lung cancer cell lines (H322, A549, H661, H1299, H1975, H358, H460 and H1650) were obtained from American Type Culture Collection (ATCC, Manassas, VA). H3255 NSCLC cell line was a gift from Dr. Pasi A. Jänne (Dana-Farber Cancer Institute, Boston MA). Cell lines were maintained in RPMI1640 medium with 10% fetal bovine serum and maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Cell growth assay
Exponentially growing cells (1×105/ml) were plated in 96-well plates and allowed to attach overnight. Cells were exposed to various concentrations of PM02734 at 37°C for 72 h. After treatment, the cell survival fraction was assessed by the reduction of tetrazolium bromide (MTT) assay or the cell viability was assessed by cell count using trypan blue exclusion12. IC50 value resulting from 50% cell growth inhibition was calculated graphically.
Analysis of Combined Drug Effects
H322, A549, H1299 and H460 cells were plated in 96-well plates as described above. After overnight incubation at 37°C, attached cells were treated with various concentrations of PM02734 or erlotinib alone or combination of both compounds using concomitant or sequential schedules at various concentrations of PM02734 and erlotinib with 1:1 molar ratio at 37°C for 72 h. Cell survival fractions were determined by MTT assay as described above, and the combinational effects were analyzed by the median effect method of Chou and Talalay13. The synergistic effects (CI index) were analyzed by software CalcuSyn. CI values: <1, or >1 represent synergism or antagonism.
Immunoblot analysis
Exponentially growing cells were harvested by trypsinization. After centrifugation, the cell pellet was lysed by lysis buffer containing 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X100 v/v, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 1 mM DTT, 1 mM PMSF, and 1 μg/ml of protease inhibitor cocktail. An equal amount of cell lysate was subjected to a 7.5% or 12% SDS-PAGE w/v. After transfer to a membrane, the protein blots were incubated in the presence of primary antibody at 0°C overnight. After incubation with horseradish peroxidase-conjugated secondary antibody, the signals of detected protein were visualized by using an ECL reaction as described before14. For quantitative analysis, the ECL signals were scanned by a laser scanning densitometer (Kodak Image Station 440, New Haven, CT).
Mutation analysis of EGFR and k-Ras genes
Genomic DNA was isolated from each tested cell line by the phenol/chloroform extraction method (Invitrogen), The primers that were specific for amplifying the cDNA fragments of the EGFR tyrosine kinase domain and Ras cDNA fragments containing codons 12, 13 and 61 were synthesized as described15,16. After 35 cycles of PCR amplification, PCR products were purified by a PCR purification kit (Qiagen) and sequenced at the Albert Einstein Cancer Centre DNA Sequencing Shared Resource.
In vivo analysis of Combined Drug Effects
Nude mice (NUR-NU-F-M, female, 5-6 weeks old, Taconic Farm, Germantown NY) were inoculated intravenously with the human A549 NSCLC cell line (4.2 × 106 or 8.4 × 106 cells/mouse). Under these experimental conditions i.v. inoculated A549 cells grow only in the lungs but not in other organs, and the survival of mice is directly related to the tumour burden. Nine days after tumour inoculation mice were randomly divided into 4 groups with 7 mice in the combination group and 5 mice in the other groups. One group of mice received the combination treatment (PM02734: 0.1 mg/kg × 3/week for 2 weeks, i.v.; erlotinib: 50 mg/kg × 5/week for 2 weeks, p.o.). Other groups were treated with PM02734 alone (0.1 mg/kg ×3/week for 2 weeks, i.v.), erlotinib alone (50 mg/kg × 5/week for 2 weeks, p.o.) or without treatment. Mice were observed daily and survival or % Increased Life Span was determined. Kaplan-Meier curves and log-rank test were used for the statistical analysis.
Results
Determination of PM02734-induced cytotoxicity in human NSCLC cell lines and correlation with the expression of ErbB family proteins
Nine human NSCLC cell lines were chosen as models for determination of cell sensitivity to PM02734 and EGFR tyrosine kinase inhibitor erlotinib. First we investigated the EGFR, and Ras gene status, because these genes have been proposed to be the molecular determinants of cell sensitivity to the EGFR tyrosine kinase inhibitor erlotinib. Table 1 summarizes the histological and molecular characteristics and cytotoxicity to PM02734 and erlotinib of nine human NSCLC cell lines. As shown in Table 1, NSCLC cell lines exhibited a narrow range of sensitivity to PM02734 with IC50 values ranging from 0.3 μM ut to >5 μM. Interestingly, two of the PM02734 sensitive cell lines (H322 and H3255) were the only two cell lines sensitive to erlotinib.
Table 1.
Characteristics of gene status of EGFR and k-RAS and cytotoxic effect of PM02734 or erlotinib in human NSCLC cell lines.
| Cell lines | Cell type | EGFR gene | k-Ras gene | PM02734 IC50 (μM) | Erlotinib IC50 (μM) |
|---|---|---|---|---|---|
| H322 | Adenocarcinoma | WT | WT | 0.30±0.15 | 1.25±0.30 |
| A549 | Adenocarcinoma | WT | G12S | 0.58±0.25 | >10 |
| H3255 | Adenocarcinoma | L858R | WT | 1.00±0.25 | 0.05±0.02 |
| H661 | Large cell | WT | WT | 1.50±0.85 | >10 |
| H1299 | Not specified | WT | N-Ras(Q61K) | 2.80±0.85 | >10 |
| H1975 | Adenocarcinoma | L858R,T790M | WT | 2.00±0.65 | >10 |
| H1650 | Bronchoaveolar | Del exon 19 | WT | >5 | 10.0±3.0 |
| H358 | Bronchoaveolar | WT | WT | >5 | 2.10±0.83 |
| H460 | Adenocarcinoma | WT | WT | >5 | 8.30±1.50 |
Note: The mutation of EGFR and k-Ras genes was determined by direct sequencing as described in Materials and Methods. The cytotoxicity of PM02734 or erlotinib was determined by MTT assay after 72 h of incubation, and the IC50 value resulting from 50% of cell growth inhibition was calculated as described in Materials and Methods. All data are the mean ± S. D. of three independent experiments.
The expression of endogenous ErbB family receptors was analyzed and correlated with cell sensitivity to PM02734. Western blot analysis indicated a correlation between high ErbB expression levels, in particular ErbB3/HER3 (r = 0.62) and less noticeably ErbB2 (r = 0.48) and ErbB1/EGFR (r = 0.49), and increased cell sensitivity to PM02734 (Fig 1). This finding is in agreement with previous observations in which a correlation between sensitivity to the parent compound KF and ErbB3 expression has been observed in a panel of tumor cell lines (including NSCLC, breast, ovarian and hepatic carcinomas)2.
Figure 1. Relationship between cell sensitivity to PM02734 and expression of ErbB family proteins in human NSCLC cell lines.
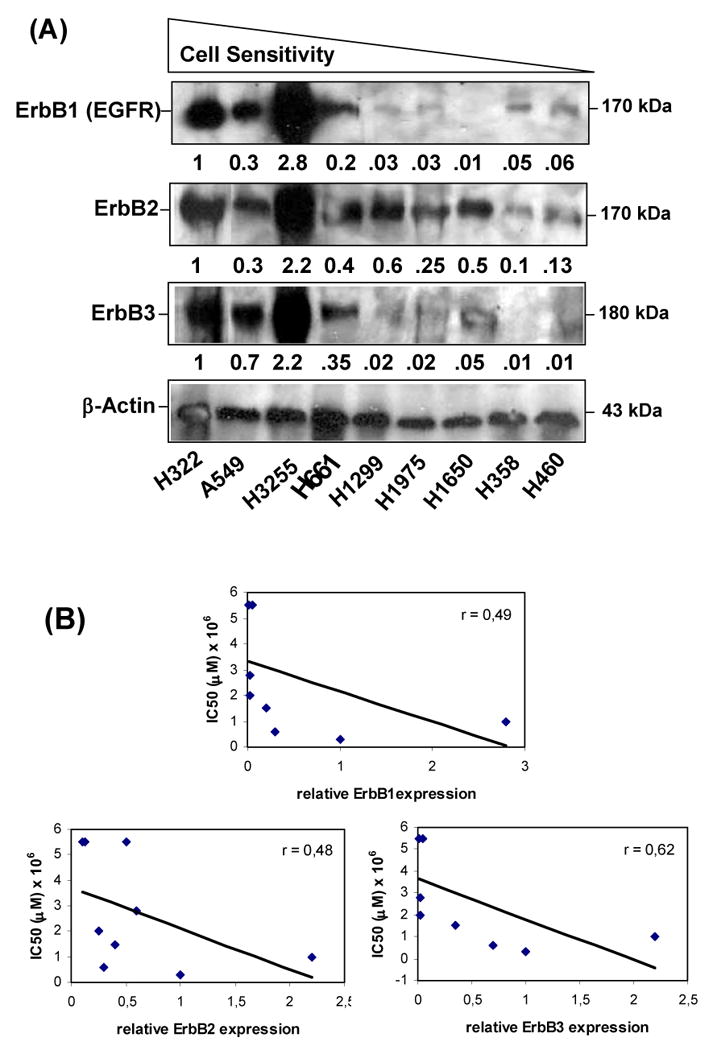
(A) An equal amount (30 μg of protein) of cell lysate from exponentially growing cells was subjected to a 7.5% SDS-PAGE. After transferring to a membrane, ErbB1 (EGFR), ErbB2 and ErbB3 were detected by immunoblots using corresponding antibodies. β-actin was used as a loading control. The values represent the relative densities as compared to the value of H322 cells and normalized with β-actin expression. (B) ErbB receptor expression levels were plotted against PM02734 IC50 values shown in Table 1. Pearson's correlation coefficient (r) was calculated by linear regression.
Effect of PM02734 on the expression and phosphorylation of ErbB family proteins in human NSCLC cell lines
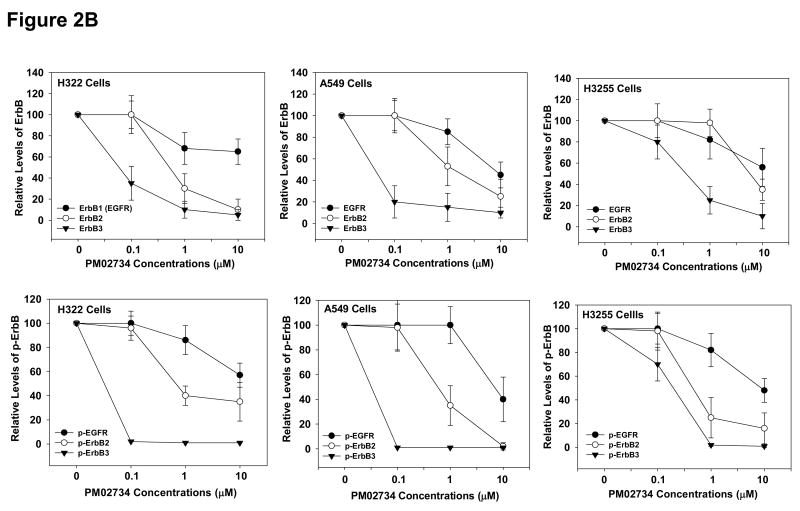
Since a correlation between ErbB protein expression and cell sensitivity to PM02734 was found in the tested panel of human NSCLC cell lines, we sought to determine whether this compound could selectively affect the expression and activation of the different kinds of ErbB receptors. To this end, we utilized three PM02734 sensitive cell lines, H322, A549 and H3255 cell lines, all of them expressing high levels of ErbB family proteins. Cells were incubated overnight in serum-free medium and then exposed to different concentrations of PM02734 for 2 h. After stimulation with 100 ng/ml of EGF, cells were harvested and lysed. Total and phosphorylated ErbB proteins were detected by immunoblot analysis. As shown in Fig. 2A, PM02734 was more effective in reducing ErbB3 and dephosphorylated ErbB3 protein expression and dephosporylation than the equivalent changes in ErbB2 and ErbB1 expression in the tested cell lines. The quantitative analysis presented in Fig. 2B indicates a 70-80% total ErbB3 protein downregulation and about a 98% reduction of phosphorylated ErbB3 expression in H322 and A549 cells following treatment with 0.1 μM PM02734; similar extents of ErbB2 protein downregulation required cell treatment with a 10-fold higher concentration (1 μM PM02734). In contrast, downregulation of ErbB1 expression required as much as 10 μM of PM02734. These findings indicate the differential sensitivity of ErbB components to PM02734 stress. ErbB3 is the most sensitive, ErbB2 has an intermediate sensitivity, and ErbB1 (EGFR) is mostly resistant to PM02734-induced stress. These results suggest that ErbB receptors, and in particular ErbB3, may be major determinants of cell sensitivity to this compound.
Figure 2. Effect of PM02734 on proteolysis and phosphorylation of ErbB family proteins in H322, A549, and H3255 cell lines.
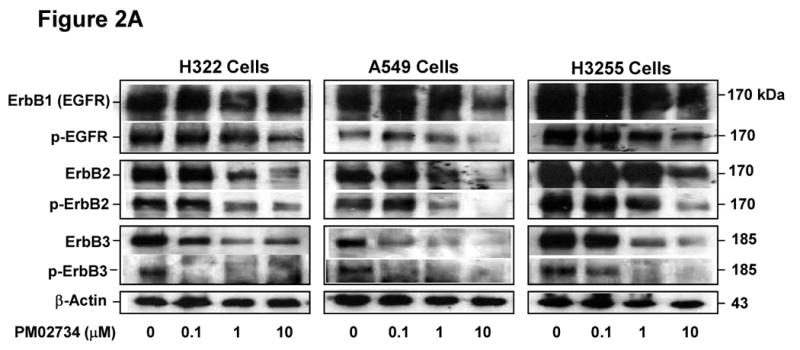
(A) Cells were incubated in serum-free medium overnight, and then exposed to the indicated concentrations of PM02734 for 2 h. Following stimulation with 100 ng/ml EGF for 5 min, ErbB protein expression and phosphorylation were determined by immunoblot analysis. (B), Quantitative analysis of ErbB proteolysis and dephosphorylation was measured by a laser scanning densitometer. Data represent the mean ± S.D of three independent experiments.
Characterization of the combination of PM02734 and erlotinib in human NSCLC cell lines
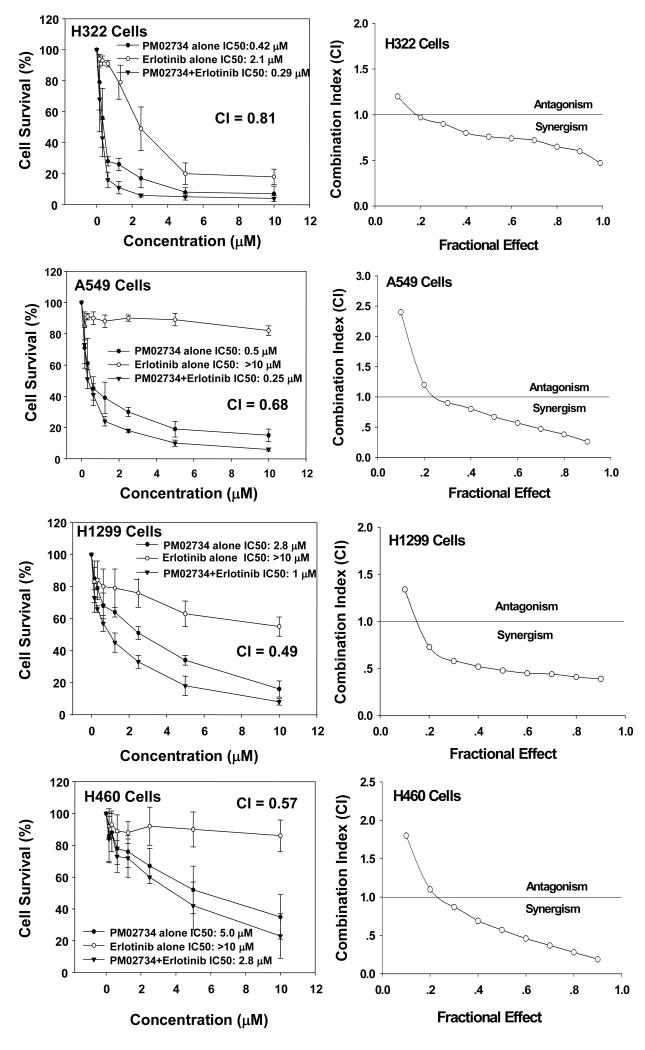
Based on the observation that ErbB3 is a determinant of cell sensitivity to PM02734 and that short term exposure of sensitive cell lines to the parent compound KF selectively induced downregulation of ErbB33, we tried to determine whether the combination of PM02734 and erlotinib, a potent EGFR tyrosine kinase inhibitor, could be synergistic. Four cell lines with varying degrees of sensitivity to both drugs were selected from the above described panel. H322, A549, H1299 and H460 cells were exposed to increasing concentrations of PM02734 or erlotinib alone or to various concentrations of PM02734 and erlotinib with 1:1 molar ratio at 37°C for 72 h. The combination of PM02734 and erlotinib was synergistic in all cell lines with combination indexes ranging between 0.49 and 0.81 (Fig. 3). Interestingly, the combination was found to be synergistic even in cell lines resistant to erlotinib (A549, H1299) or to PM02734 and erlotinib (H460).
Figure 3. Synergistic effect between PM02734 and erlotinib in human H322 A549, H1299 and H460 NSCLC cell lines.
Cells were treated with varying concentrations of PM02734 or erlotinib alone or with the combination of both agents with 1:1 molar ratio at 37°C for 72 h. After treatment, cell survival was determined by MTT assay. Data represent the mean ± S.D. of three independent experiments. CI (combination index) vs. fractional effect was analyzed by software CalcuSyn.
In addition, we investigated the effect of different sequence schedules of PM02734 and erlotinib on cell growth. H322 or H1299 cells were plated in a 96-well plate overnight, and then exposed to various concentrations of PM02734 or erlotinib alone, or to the combination of both agents using simultaneous or sequential exposure schedules. The concurrent exposure to both agents for 72 h was more effective in inhibiting cell growth than the sequential schedules: PM02734 followed by erlotinib, or erlotinib followed by PM02734 (data not shown).
In vivo antitumor activity of the combination of PM02734 and erlotinib in an in vivo NSCLC model of experimental lung metastases
The efficacy of the combination of PM02734 and was assessed in vivo in a model of experimental lung metastases. The NSCLC cell line A549 was used for determining the growth inhibitory effects of PM02734 and erlotinib on the establishment of metastatic tumors. A549 was selected because it grows in the lung after i.v. inoculation, partially mimicking lung invasion and metastasis in humans. Initially, the MTD of single PM02734 was determined in CD1 mice (6-7 weeks, male and female); mice were randomly divided into 4 groups of 10 mice per group, each group receiving a single i.v. administration of PM02734 at different doses (0.3, 0.6, 1.2, and 2.4 mg/kg) via tail vein. The highest dose causing <10% death was defined as the MTD. The estimated MTD of PM02734 ranged between 0.6 and 1.2 mg/kg. A similar method was used to determine the MTD of the combination. The following schedule of administration was used: PM02734 3 qod injections/week × 2 weeks, i.v. and erlotinib 5 daily p.o. doses/week × 2 weeks. The dose range of PM02734 was 0.18 (0.03 × 6) ∼ 0.9 (0.15 × 6) mg/kg. The dose for erlotinib was fixed at 50 mg/kg × 10, since it has been described17 as the optimal effective dose in nude mouse cancer models. The MTD for the combination under these experimental conditions was PM02734 0.1 mg/kg i.v. qod × 6 and erlotinib 50 mg/kg p.o. daily × 10 in a period of 2 weeks. The combination was found to be superior to either agent alone in the A549 NSCLC experimental lung metastases model A549 NSCLC (Figure 4). In a first experiment in which 4.2 × 106 cells/animal were inoculated, median survival was not reached (follow-up >150 days) in the group treated with the PM02734 + erlotinib combination, as compared to median survivals of 54, 39 and 32 days for the PM02734 alone, erlotinib alone, and no treatment groups, respectively (Figure 4A; p = 0.0003 for the combination group vs. the other groups). In a second experiment, in which a higher number of cells were inoculated (8.4 × 106), median survival times were 35, 27, 24 and 23 days for the combination, PM02734 alone, erlotinib alone, and no treatment groups, respectively (Figure 4B; p = 0.002 for the combination group vs. the other groups). No significant animal weight loss was observed in any of the treatment groups (data not shown).
Figure 4. In vivo efficacy of the combination of PM02734 and erlotinib in an orthotopic NSCLC xenograft model.
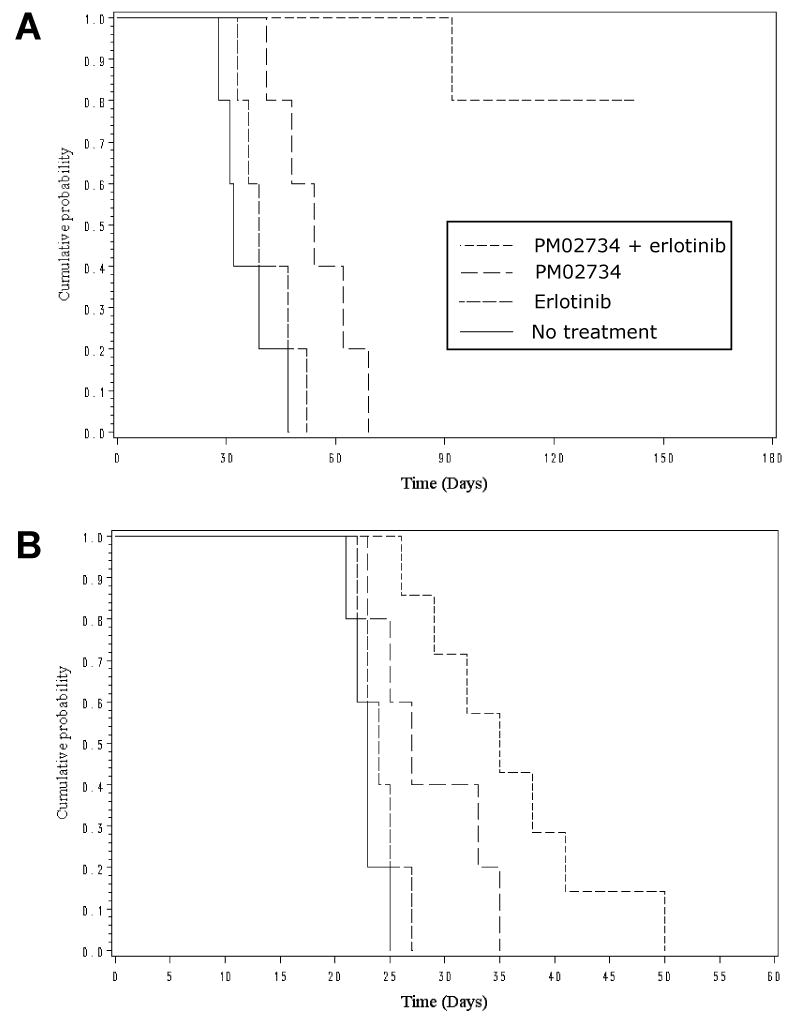
Nude mice were implanted intravenously with 4.2 × 106 (A) or 8.4 ×106 (B) human A549 NSCLC cells. Nine days later, the mice were randomly divided into 4 groups with 5 (in A) or 7 (in B) mice per group. One group of mice received the combination treatment; the other groups were treated with PM02734 alone or erlotinib alone or without treatment. The survival or % Increased Life Span was determined. Log-rank test was used for the statistical analysis: p = 0.0003 (A) and p = 0.002 (B) for the combination group vs. the other groups.
Effects of PM02734 and erlotinib on the activation of ErbB family proteins and downstream signalling pathways in H322 cells and A549 cells
To further explore the mechanism of synergism, we investigated the effect of the combination on the expression of ErbB proteins and their phosphorylated counterparts and the activation of the PI3K/AKT and MAPK/ERK pathways. We starved H322 and A549 cells in serum-free medium for 24 h, and treated cells with PM02734 (0.5 μM and 1 μM, respectively) or 1 μM erlotinib alone or in combination. After 2 h of treatment, cells were stimulated with 100 ng/ml of EGF for 5 min, and then cell lysates were prepared for the determination of total and phosphorylated ErbB1 (EGFR) and ErbB3 proteins and AKT and ERK1/2 by immunoblotting analysis using the corresponding antibodies. As shown in Figure 5, erlotinib caused an effective inhibition of EGFR, AKT and ERK1/2 phosphorylation, specially in the more sensitive H322 cell line, but was less effective in causing inhibition of ErbB3 phosphorylation; in contrast, PM02734 strongly inhibited the phosphorylation of ErbB3 and AKT and, to a lower extent, the phosphorylation of EGFR and ERK1/2. The combination of erlotinib and PM02734 was more effective in inhibiting the phosphorylation of EGFR, ErbB3 and AKT in H322 and A549 cells than either single agent alone, with a lower impact on ERK phosphorylation.
Figure 5. Effects of PM02734, erlotinib, and combination of both agents on the activation of ErbB family proteins and its related signalling pathways in H322 cells and A549 cells.
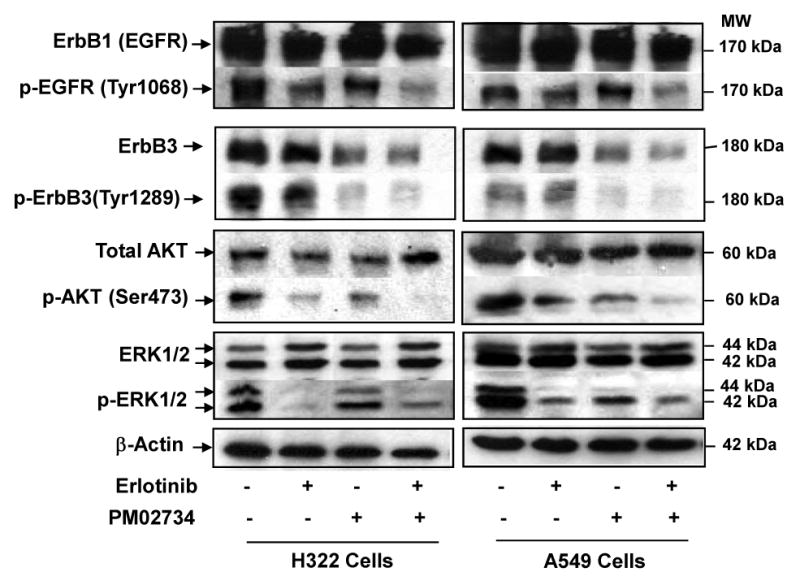
Exponentially growing cells were starved in serum-free medium for 24 h and then treated with 0.5-1 μM PM02734 or 1 μM erlotinib alone or with the combination of both agents at 37°C for 2 h. Following 5 min of EGF stimulation, cells were lysed and subjected to a 10% SDS-PAGE. After transferring to a membrane, proteins were detected by immunoblot analysis using corresponding antibodies. β-actin was used as a loading control.
Discussion
The direct correlation between ErbB3 expression and in vitro sensitivity to PM02734 confirms previous findings with the parent compound KF3 and sustains the notion of a pharmacodynamic effect of this compound on this pathway. The link between this findings and the role of FA2H in PM02734 sensitivity remains unclear. It has been proposed that fatty acid 2-hydroxylation could enhance the formation of sphingolipid rich membrane domains, which may important for drug-membrane interaction5. In this regard, a suggestive observation is the fact that ganglioside GM3, a complex sphingolipid, modulates the phosphorylation and internalization of ErbB2 receptors18,19. For a cell line to be inhibited by EGFR tyrosine kinase inhibitors, EGFR must be the driving force for its growth and survival. When EGFR regulates PI3K/AKT signalling in lung cancer cell lines, it uses ErbB3 to directly link to PI3K; therefore, those tumours that respond to anti-EGFR therapy likely express ErbB3. Moreover, MET amplification has been recently described in 22% of EGFR-mutated NSCLC cases that develop resistance to gefitinib or erlotinib; this amplification causes resistance through c-met partnering with ErbB3 for downstream signaling20. These findings support testing the combination of PM02734 and erlotinib to augment signalling inhibition in cases where ErbB3 partners with other signalling molecules.
The backbone of this study relates to the positive evidence of synergism when pharmacologically relevant concentrations of PM02734 and erlotinib are used in combination against a panel of human NSCLC cell lines. In fact, synergy has been noted, as per CI<1, on each of the cell lines tested. Moreover, PM02734 has been shown to be active in vitro against A549 and H460 cell lines, that in contrast are resistant to erlotinib.
Such finding, together with the synergistic impact of the combination, suggests that PM02734 lacks complete cross resistance with erlotinib, at least under the experimental frame of this study. Furthermore, the combination of PM02734 and erlotinib showed a statistically significant increase in life span in the A549 model of experimental metastases from NSCLC.
In this work, we have investigated the effect of PM02734 on the expression and phosphorylation of ErbB proteins in H322, A549, and H3255 cell lines and found that PM02734 induces preferentially ErbB3 protein downregulation and dephosphorylation. A concentration as low as 0.1 μM caused about 70-80% ErbB3 protein downregulation, and about 90% inhibition of ErbB3 protein phosphorylation. In contrast, induction of a similar effect on ErbB2 protein downregulation required PM02734 at the IC50 concentration. Whereas ErbB1 (EGFR) appeared to be resistant to PM02734 stress. Of note, 0.1 μM PM02734 does not markedly induce cell growth inhibition or killing, but induces marked ErbB3 downregulation and dephosphorylation. In addition, these effects were less prominent in EGFR-mutated H3255 cells than in EGFR-wild type H322 and A549 cells, suggesting that ErbB1 mutation may make cells less dependent on ErbB3. These results suggest that the pharmacodynamic effect of PM02734 on ErbB components is highly selective; ErbB3 inhibition could be an early event in the cell response to drug stress, although it remains to be further elucidated why ErbB3 protein is so sensitive to PM02734 stress, and what is the molecular basis for PM02734-induced downregulation of ErbB family proteins and the significance of ErbB3 downregulation in drug-induced cell death.
The molecular basis for the reported synergism needs to be elucidated. We have demonstrated that PM02734 was more effective in suppressing the phosphorylation of ErbB3 and ErbB2 as compared with erlotinib, which was more effective in inhibiting ErbB1 phosphorylation, indicating that PM02734 and erlotinib may have a high selectivity to interact with different kinds of ErbB proteins. Consistent with the synergistic effects of the in vitro combination of the two agents (Fig. 3), a greater inhibition of three different kinds of ErbB components was observed in H322 cells treated with the combination of PM02734 and erlotinib at 0.5 μM as compared with that in cells treated with a single agent alone. Furthermore, the inhibition of AKT phosphorylation induced by PM02734 or erlotinib alone was enhanced by the concomitant exposure to both drugs. In a previous study the parent compound KF was shown to decrease AKT phosphorylation only in those cell lines sensitive to the drug3; as an additional marker of AKT activity, KF induces a re-distribution of p27KIP1 from the cytosol to the nucleus. Moreover, constitutive activation of AKT has been shown to protect, in a KF sensitive breast cancer cell line, from KF induced cytotoxicity. AKT represents a major down-stream target of receptor tyrosine kinases that signal via PI3K pathway21 and regulates biological processes, such as proliferation, apoptosis and cell growth. Of note, described mutations leading to constitutive activation of the PI3K/AKT pathway have been identified as a parameter linked to resistance to anticancer agents22,23; such correlations require further studies to seek for patterns of resistance to PM02734. All together the data generated in the current study suggest that the observed synergy of the combination of PM02734 and erlotinib is associated with an inhibition of the PIK3/AKT pathway and an enhancement of the apoptotic effects.
The clinical data of the parent compound KF24 demonstrates the feasibility to deliver this compound at pharmacologically relevant dose levels in heavily pre-treated patients. In fact, long lasting objective responses and tumour control have been noted in lung and pancreatic adenocarcinoma, melanoma and different squamous cell carcinomas. The synthetic analogue PM02734 is under late phase I development. In line with previous data obtained with the parent compound, the results confirm lack of bone marrow toxicity, hair loss, mucositis and severe vomiting. Dose-limiting toxicities were grade 3-4 asymptomatic, reversible transaminase elevations25.
In summary, the results of this study have supported the implementation of a phase I program to define the feasibility, pharmacological interactions and activity of the combination of continuous erlotinib with weekly intravenous infusions of PM02734. In parallel, a set of studies in experimental models will seek to gain information on the molecular determinants of synergism of the combination of PM02734 and different tyrosine kinase receptor interacting agents and on the pharmacodynamics of PM02734 against ErbB receptor pathways. This translational study will provide additional information of relevance for clinical development purposes. Finally the reported PM02734-induced inhibition of erbB receptor signalling provides also with a rationale to explore its potential synergy in combination with radiotherapy26 and with cytotoxic agents27 in solid tumours.
Acknowledgments
This work was supported in part by NIH grant CA84119 and CA96515, and by PharmaMar, Madrid, Spain.
Footnotes
Conflict of interest statement: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamann MT, Otto CS, Scheuer PJ, Dunbar DC. Kahalalides: bioactive peptides from a marine mollusc Elysia rufescens and its diet algal Briopsys sp. J Org Chem. 1996;61:6594–6600. doi: 10.1021/jo960877+. [DOI] [PubMed] [Google Scholar]
- 2.Wosikowski K, Schuurhuis D, Jonson K, et al. Identification of epidermal growth factor receptor and c-ErbB2 pathway inhibitors by correlation with gene expression patterns. J Natl Cancer Inst. 1997;89:1505–15. doi: 10.1093/jnci/89.20.1505. [DOI] [PubMed] [Google Scholar]
- 3.Janmaat ML, Rodríguez JA, Jimeno J, Kruyt FAE, Giaccone G. Kahalalide F induces necrosis-like death that involves depletion of ErbB3 and inhibition of Akt signalling. Mol Pharmacol. 2005;68:502–10. doi: 10.1124/mol.105.011361. [DOI] [PubMed] [Google Scholar]
- 4.Janmaat ML, Kruyt FAE, Rodríguez JA, Giaccone G. Response to EGFR inhibitors in non-small cell lung cancer cells: Limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin Cancer Res. 2003;9:2316–26. [PubMed] [Google Scholar]
- 5.Herrero AB, Astudillo AM, Balboa MA, Cuevas C, Balsinde J, Moreno S. Levels of SCS7/FA2H-mediated fatty acid 2-hydroxylation determine the sensitivity of cells to antitumor PM02734. Cancer Res. 2008;68:9779–87. doi: 10.1158/0008-5472.CAN-08-1981. [DOI] [PubMed] [Google Scholar]
- 6.Thomson S, Buck E, Petti F, et al. Epithelial to mesenchymal transition is a determinant of sensitivity of non-small-cell lung carcinoma cell lines and xenografts to epidermal growth factor receptor inhibition. Cancer Res. 2005;65:9455–62. doi: 10.1158/0008-5472.CAN-05-1058. [DOI] [PubMed] [Google Scholar]
- 7.Frolov A, Schuller K, Tzeng CW, et al. ErbB3 expression and dimerization with EGFR influence pancreatic cancer cell sensitivity to erlotinib. Cancer Biol Ther. 2007;6:548–54. doi: 10.4161/cbt.6.4.3849. [DOI] [PubMed] [Google Scholar]
- 8.Buck E, Eyzaguirre A, Haley JD, Gibson NW, Canoni P, Iwata KK. Inhibition of Akt by the epidermal growth factor receptor inhibitor erlotinib is mediated by HER-3 in pancreatic and colorectal tumor cell lines and contributes to erlotinib-sensitivity. Mol Cancer Ther. 2006;5:2051–9. doi: 10.1158/1535-7163.MCT-06-0007. [DOI] [PubMed] [Google Scholar]
- 9.Amann J, Kalyankrishna S, Massion PP, et al. Aberrant growth factor receptor signaling and enhanced sensitivity to EGFR inhibitors in lung cancer. Cancer Res. 2005;65:226–35. [PubMed] [Google Scholar]
- 10.Engelman JA, Janne PA, Mermel C, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci USA. 2005;102:3788–93. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100:8933–8. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ling YH, Priebe W, Yang LY, et al. In vitro cytotoxicity, cellular pharmacology, and DNA lesions induced by annamycin, an anthracycline derivative with high affinity for lipid membranes. Cancer Res. 1993;53:1583–9. [PubMed] [Google Scholar]
- 13.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs on enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 14.Ling YH, Li T, Yuan Z, et al. Erlotinib, an effective epidermal growth factor receptor tyrosine kinase inhibitor, induces p27KIP1 up-regulation and nuclear translocation in association with cell growth inhibition and G1/S phase arrest in human non-small-cell lung cancer cell lines. Mol Pharmacol. 2007;72:248–58. doi: 10.1124/mol.107.034827. [DOI] [PubMed] [Google Scholar]
- 15.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone an in combination with erlotinib. J Clin Oncol. 2005;23:5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Ji H, Yuza Y, et al. An alterative inhibitor overcomes resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 17.Friess T, Scheuer W, Hasmann M. Erlotinib antitumor activity in non-small cell lung cancer models is independent of HER1 and HER2 overexpression. Anticancer Res. 2006;26:3505–12. [PubMed] [Google Scholar]
- 18.Sottocornola E, Berra B, Colombo I. GM3 content modulates the EGF-activated p185c-neu levels, but not those of the constitutively activated oncoprotein p185neu. Biochim Biophys Acta. 2003;1635:55–66. doi: 10.1016/j.bbalip.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Sottocornola E, Misasi R, Mattei V, et al. Role of gangliosides in the association of ErbB2 with lipid rafts in mammary epithelial HC11 cells. FEBS J. 2006;273:1821–30. doi: 10.1111/j.1742-4658.2006.05203.x. [DOI] [PubMed] [Google Scholar]
- 20.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 21.Burgering BM, Coffer PJ. Protein Kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 22.Stambolic V, Suzuki A, de la Pompa JL, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 23.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 24.Pardo B, Paz-Ares L, Tabernero J, et al. Phase I clinical and pharmacokinetic study of Kahalalide F administered weekly as a 1-hour infusion to patients with advanced solid tumors. Clin Cancer Res. 2008;14:1116–23. doi: 10.1158/1078-0432.CCR-07-4366. [DOI] [PubMed] [Google Scholar]
- 25.Bruce JY, Geary D, De las Heras B, Soto A, Garcia Paramio P, Yovine A, et al. J Clin Oncol. Suppl. Vol. 26. 2008. Phase I study of PM02734: Association of dose-limiting hepatotoxicity with plasma concentrations. Abstract 2513. [Google Scholar]
- 26.O'Rourke DM, Kao GD, Singh N, et al. Conversion of a radioresistant phenotype to a more sensitive one by disabling erbB receptor signaling in human cancer cells. Proc Natl Acad Sci USA. 1998;95:10842–47. doi: 10.1073/pnas.95.18.10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren W, Korchin B, Zhu QS, et al. Epidermal growth factor receptor blockade in combination with conventional chemotherapy inhibits soft tissue sarcoma cell growth in vitro and in vivo. Clin Cancer Res. 2008;14:2785–95. doi: 10.1158/1078-0432.CCR-07-4471. [DOI] [PubMed] [Google Scholar]