Abstract
In patients with fibromyalgia syndrome (FMS) and temporomandibular disorder (TMD), stress and pain may chronically enhance sympathetic activity, altering cardiovascular responses and worsening pain. This study examined cardiovascular, epinephrine (EPI), norepinephrine (NE), cortisol and clinical pain responses in 54 female patients with these disorders and 34 controls. In a subsample of 10 FMS, 10 TMD patients and 16 controls, using a counterbalanced, double-blind, cross-over design, the same responses were assessed after intravenous administration of low dose propranolol vs. placebo. Testing included baseline, postural, speech and ischemic pain stressors. FMS patients showed lesser heart rate (HR) increases to posture challenge but greater blood pressure (BP) increases to postural and speech tasks than Controls, as well as higher overall BP and greater total vascular resistance (TVR) than TMDs or Controls. TMDs showed higher overall cardiac output and lower TVR than Controls. Both FMS and TMD groups showed lower baseline NE than Controls, and TMDs showed lower overall EPI and NE levels. Group differences in HR, EPI and NE were abolished after propranolol although BP, CO and TVR differences persisted. In both FMS and TMD, number of painful body sites and ratings of total clinical pain obtained 4 times during each session were significantly lower after beta-blockade vs. placebo.
Perspective
These findings support the hypothesis that both FMS and TMD may frequently involve dysregulation of beta-adrenergic activity that contributes to altered cardiovascular and catecholamine responses and to severity of clinical pain. Acute treatment with low dose propranolol led to short-term improvement in all these domains.
Descriptors: fibromyalgia, temporomandibular, pain, propranolol, sympathetic, beta-adrenergic
Introduction
Chronic musculoskeletal pain is a common and debilitating health problem, especially in women. Temporomandibular disorders (TMD) involve persistent pain in the muscular regions of the jaw, neck and head, and have the highest prevalence of all chronic orofacial pain conditions, estimated at 10% worldwide.1,9,20,31 Fibromyalgia (FMS), characterized by more widespread pain, has a prevalence rate estimated at from 2–5%,60 and shares many features with TMD. First, for both FMS and TMD, 80%–90% of the sufferers are female.1,13,20,60 Second, threshold levels for both pressure pain and heat pain are significantly lower in FMS and in TMD patients compared to controls, not only at standard tender points defining these disorders but also at minimally symptomatic body sites.27,37,38,47 Third, both disorders are thought to result from dysregulation of pain modulatory systems involving altered interactions among the central (CNS) and peripheral nervous systems and the immune system 9,12,34,47,56 Fourth, TMD and FMS disorders co-occur in up to 50% of cases.1,9,31
Several lines of evidence indicate that sympathetic nervous system (SNS) function is altered in patients with FMS and TMD.21,45,46,49,55 In some FMS patients, this dysregulation involves increased SNS drive at rest, together with deficient SNS-mediated responses to certain challenges, such as postural change or exercise.25,49 Decreased heart rate (HR) variability at baseline and during sleep indicates lesser parasympathetic and greater beta-adrenergic tone in FMS patients vs. controls.14,49,55 During tests of orthostatic reflex responses, many FMS patients demonstrate deficient HR and blood pressure (BP) responses.6,12,23 Decreased plasma epinephrine (EPI) and norepinephrine (NE) responses to stressors such as exercise or hypoglycemia as well as decreased NE metabolite in cerebrospinal fluid have been reported in FMS patients.25,30,39,63 In TMD patients, de Abreu et al.17 reported lower ambulatory BP than controls, and we15 observed lesser plasma NE increases to a speech stressor.
To address SNS dysregulation in FMS and TMD and its possible role influencing clinical pain, our investigation included the following elements. First, the cardiovascular measures under study included BP, HR and also impedance-derived measures of cardiac output (CO) and total vascular resistance (TVR) at rest and during stressors. Second, we included plasma EPI and NE as well as cortisol as blood-based biomarkers of SNS and hypothalamic-pituitary-adrenal (HPA) activity. Third and most important, smaller subgroups of our FMS and TMD patients and controls were retested in a double-blind, counterbalanced crossover design comparing effects of a single low intravenous (i.v.) dose of the beta-adrenergic antagonist propranolol vs. placebo on cardiovascular, neuroendocrine and clinical pain rating measures during baseline and stressors. We hypothesized that pretreatment with propranolol, which antagonizes both beta-1 and beta-2 adrenergic receptors, would reduce myalgic pain in patients with FMS and/or TMD and would “normalize” catecholamine and cardiovascular responses to stress. We further hypothesized that any reduction in pain after propranolol vs. placebo would be greater in those FMS and TMD patients showing greater beta-adrenergic drive prior to treatment and greater reduction in such drive after treatment, indexed by HR increase to a speech task.8,24,42 Responses to an orthostatic challenge and to an experimental pain sensitivity task (arm ischemia) were also compared.
Materials and Methods
Subjects
A total of 54 female patients including 29 with TMD only and 25 with FMS and 34 healthy, pain-free controls aged 20–59 participated; the FMS group included 14 with FMS alone and 11 who met criteria for both FMS and TMD. Recruitment sources included dental and rheumatology clinics at University of North Carolina and local advertising. Because more than 80% of patients presenting with TMD or FMS in our clinics are female, only women were included in our study sample. As in the general clinical population, our FMS Patients were significantly older (mean age [standard error or SE] = 46.4 [3.1] years) than the TMD Patients (33.1 [3.8] years, P < .01). Because Controls were recruited to match both TMD and FMS Patients, the mean age for Controls (40.6 [3.1] years) fell between those of TMD and FMS Patients, and did not differ significantly from the combined patient group (39.4 [2.7] years).
Of the 88 participants in the larger study, 10 TMD and 10 FMS Patients and 16 healthy Controls completed a second substudy repeating the same stress testing protocol in two sessions approximately one week apart (mean 7[1] days), once after pretreatment with i.v. propranolol and once after pretreatment with i.v. saline placebo, in a double-blind, counterbalanced crossover design. Mean age and body mass index of 8 Controls were matched to those of the 10 TMD Patients (32.7 vs. 31.3 years and 23.3 vs. 26.3 kg/m2, respectively) while the other 8 Controls were matched to the 10 FMS Patients (45.9 vs. 44.5 years and 27.8 vs. 28.1 kg/m2, respectively). The dose of propranolol was deliberately chosen to be low (0.1 mg/kg) to minimize any reduction in mean BP which might alter endogenous pain modulation occurring via baroreceptor activity, thereby increasing rather than decreasing pain symptoms. 17,52
At an initial screening session, candidates for participation gave informed consent for this protocol as approved by our institutional review board, and underwent a medical history and physical examination to confirm their diagnoses. TMD Patients were required to meet the research diagnostic criteria for TMD established by Dworkin et al.19 presenting either with myofascial pain or with mixed myofascial pain and arthralgia for at least 3 months; patients with only arthralgia were excluded. FMS Patients had to meet the 1990 criteria of the American College of Rheumatology (widespread pain for more than 3 months, and pain with 4 kg/cm of manual pressure reported for 11 or more of 18 tender points).54,61 Patients meeting criteria for TMD who also met criteria for FMS were classified as FMS, the more generalized pain condition, after preliminary analyses indicated that patients with both FMS and TMD vs. with FMS alone did not differ reliably in any cardiovascular or neuroendocrine measure.
Volunteers also underwent a structured clinical psychiatric interview, and those meeting DSM criteria for current depression or other psychiatric disorder were allowed to participate in the larger study but were excluded from the propranolol substudy. Also, for the propranolol substudy only, individuals currently taking any prescription medications were withdrawn from them for 3 weeks under their physician’s guidance while use of over-the-counter pain medications was terminated 48 hours prior to testing. These steps allowed us to examine effects of beta blockade on FMS and TMD independent of confounding due to effects of clinical depression or medications. It was recognized that this might make the subsample less representative, but comparisons of subsample patients tested using the same protocol after placebo vs. those tested in the larger and more representative original sample could verify whether similar responses were obtained. Excluded from all testing were subjects on antihypertensive or cardiovascular medications (including beta blockers), or with a history of hypertension, coronary artery disease, neurological disorder, thyroid disease, diabetes mellitus, recent substance abuse, pulmonary disease, bronchitis or asthma, kidney or liver disease or glaucoma, or an ECG positive for serious cardiac arrhythmia. To minimize risk of hypotensive responses to beta-blockade, two potential subjects with resting BP levels less than 95/60 mmHg were excluded from the substudy.
Protocol
The protocol was essentially the same for the larger sample who did not receive any drug and for the smaller crossover substudy comparing propranolol vs. placebo. Patients were instructed not to consume caffeine on the day of testing and not to smoke within 2 hours before testing. To control for diurnal variation, in the propranolol substudy, each subject completed both sessions (lasting 3 hours) at the same time of day. First, each subject had an indwelling venous catheter placed by the nurse for blood sampling and for delivery of the drug or placebo, and was instrumented for BP measurement and impedance cardiography. A baseline 18 minute rest period was then begun with the subject in a reclining chair in the seated position. In the substudy, during the first 5 minutes of rest, drug infusion (either saline placebo or 0.1 mg/kg propranolol; minimal dose = 5 mg) was carried out by a nurse. Neither the patient nor the experimenters were informed by the nurse on which day the drug vs. placebo was administered. The subject was reclined and continued resting for the final 9 minutes of the 18-minute baseline. Systolic BP (SBP), diastolic BP (DBP) and HR were obtained during minutes 1, 3, 5, 7, 9, 10, 12, 14, 16 and 18, after which the first blood sample for catecholamines and cortisol was drawn. Following baseline, three laboratory stressors were administered—a postural challenge, a speech task and ischemic forearm pain test—each of which was followed by an 8-minute rest period.
Clinical pain ratings
After the baseline rest, subjects were then shown Margoles diagrams of the human head and body 48 with which they had been familiarized during the screening session, and asked to circle any sites in which they were currently feeling pain. They were then shown a visual analog scale numbered from 0 (“NO PAIN AT ALL”) to 100 (“MOST INTENSE PAIN IMAGINABLE”) and asked to rate their pain at each circled body site. Two clinical pain measures were derived from these: 1) the number of pain sites and 2) the total of the pain ratings summed across all pain sites. Pain measures were also recorded in this same way three more times in each session, immediately after completion of each of the three stressors.
Postural challenge (Stand)
After 10 minutes of reclining, the subject moved rapidly to stand for 5 minutes. Cardiovascular measures were obtained at minutes 1, 3 and 5 and the blood draw began after minute 1.
Speech
The subject was presented with a printed description of one of two hypothetical situations in which she would be required to play a role: [A] on one day, defending a young teenager in an argument against a verbally aggressive man who was wrongfully accusing him of causing a traffic accident, or [B] on the other day defending a dog against a neighbor who wanted it put to death for biting a teenager who had abused and teased it. Each accuser was played by an actor who was presented to the subject on videotape. Analyses of data previously acquired using these two versions of the task have shown that both elicit equivalent increases in BP, HR and catecholamines.7,15 The two scenarios were counterbalanced across groups, and subjects in the propranolol substudy were tested with a different scenario for each session to reduce habituation. The task lasted 6 minutes: 1 minute to review the scenario and prepare, 2 minutes to watch the videotape, and 3 minutes for active speech. Cardiovascular measures were recorded during minutes 1 and 3 and the blood draw after minute 1 of active speech.
Ischemic task
A modified submaximal effort tourniquet procedure47 was used to evoke ischemic arm pain. The BP cuff on the arm without the in-dwelling needle was replaced by another cuff positioned above the elbow to make the arm ischemic; thus, no BP readings could be obtained during the experimental pain task. This new cuff was inflated and maintained at 230 mmHg, after which the subject’s arm was lowered to horizontal and a stopwatch was started. The subject squeezed a hand-grip dynamometer at 1/3 of maximum grip force for 20 repetitions of 2 seconds duration each, with an intersqueeze interval of 2 seconds. The subject was instructed to say “pain” when it first became painful (time to pain onset) and to say “stop” when she could no longer tolerate the pain (time to pain tolerance; maximum= 20 minutes). A blood draw was initiated 1 minute into the task.
Blood sample analyses
Blood was centrifuged for plasma separation, then frozen at −80°C for later assay. Plasma NE and EPI were determined using the high performance liquid chromatography (HPLC) technique. Serum cortisol was measured by radioimmunoassay using commercially available kits (ICN Biomedical Inc). Blood samples were unable to be obtained in 3 control subjects due to clotting in the catheter, and assay failure led to partial data loss in 8 other subjects (6 patients, 2 controls).
Cardiovascular assessment
The Suntech 4240 BP Monitor was used for collection of SBP, DBP and mean BP, using a standard auscultation cuff placed on the arm not chosen for phlebotomy. Using a Hutcheson Impedance Cardiogaph (Model HIC-1), stroke volume was estimated using the Kubicek equation, HR was derived from the ECG, and our primary hemodynamic measures of cardiac output (CO) and total vascular resistance (TVR) were then calculated from HR, stroke volume and mean BP levels using standard formulas.8,42
Statistical Analyses
Due to non-normal (skewed) distributions, NE and EPI data were log transformed prior to analysis, yielding the variables log NE and log EPI. Differences between the FMS, TMD and Control groups (Diagnostic Groups) in cardiovascular, catecholamine, cortisol and clinical pain measures were analyzed for each Event in the larger no-drug study (Baseline, Stand, Speech, and Ischemic tasks) and the smaller propranolol substudy with repeated measures analyses using SAS PROC MIXED (SAS Institutes, Raleigh NC). Preliminary analyses incorporating age as a covariate showed that this factor was non-significant (P > .05) in all but one analysis involving HR, and in no instances were significant findings altered after adjusting for age differences; thus, final analyses excluded age. Specific group comparisons following significant Diagnostic Groups X Event interactions were assessed by pairwise comparisons of least squares means. Simple regression tests (Pearson correlations) were used to determine relationships between the pain and the cardiovascular measures in the Patients; in particular, we focused on correlations of pain and HR reactivity to the speech task as our best index of cardiovascular sympathetic responsivity. Data are presented as group means + standard errors, with alpha set at P < .05.
Results
Cardiovascular Responses
In the larger, more representative sample, repeated measures analyses of variance yielded main effects of Diagnostic Group for SBP, DBP, CO and TVR, F’s (2,85) ≥ 3.96, P < .025, and significant Diagnostic Group x Event interactions were obtained for SBP, DBP and HR, F’s (6,238) ≥ 2.51, P< .05. No significant main effect involving HR was obtained (P > .65). Subsequent group comparisons showed that FMS Patients had higher SBP and TVR levels across all resting and stressor conditions than either TMD patients or Controls (see Table 1). TMD Patients had higher overall CO and lower TVR levels than Controls as well as FMS Patients, lower overall SBP and DBP levels than FMS Patients, and lower baseline DBP than Controls. In terms of cardiovascular changes from baseline levels during Stand and Speech tasks, FMS Patients showed lesser HR increases but greater SBP increases to Stand (3.9 vs. 12.1 bpm and 7.4 vs. 4.5 mmHg, P < .01 and .05) and also greater SBP increases to Speech (23.8 vs. 17.1 mmHg, P < .025) than Controls.
Table 1.
Mean (± SE) Cardiovascular Responses in Larger Sample (n=88)
| Baseline | Stand | Recovery 1 | Speech | Recovery 2 | |
|---|---|---|---|---|---|
| SBP | |||||
| Control | 115.0 (2.0) | 119.5 (2.4) | 114.8 (2.0) | 132.1 (2.9) | 116.0 (2.1) |
| TMDa | 110.0 (1.7) | 116.2 (2.3) | 110.5 (1.6) | 130.3 (2.3) | 111.9 (1.6) |
| FMSc | 118.6 (2.2) | 126.0 (3.4)e | 120.3 (3.3) | 142.4 (3.3)e | 122.7 (3.0) |
| DBP | |||||
| Control | 68.0 (1.4) | 74.9 (1.7) | 71.3 (1.3) | 82.1 (2.0) | 71.8 (1.5) |
| TMDa | 63.2 (1.1)d | 72.0 (1.3) | 67.6 (0.9) | 82.9 (1.5) | 67.7 (1.1) |
| FMS | 68.5 (1.6) | 76.2 (2.8) | 72.2 (2.0) | 87.5 (2.5) | 74.3 (2.0) |
| HR | |||||
| Control | 70.3 (1.9) | 82.4 (2.0) | 71.6 (1.7) | 84.9 (2.4) | 73.9 (1.6) |
| TMD | 70.4 (1.7) | 86.8 (1.8) | 74.6 (1.6) | 89.6 (2.0) | 76.5 (2.0) |
| FMS | 72.5 (2.1) | 76.4 (2.5)e | 73.5 (2.3) | 87.0 (2.9) | 74.9 (2.4) |
| CO | |||||
| Control | 6.97 (0.37) | 5.54 (0.28) | 6.00 (0.30) | 7.25 (0.47) | 6.22 (0.32) |
| TMDa,b | 7.91 (0.42) | 6.34 (0.35) | 6.67 (0.35) | 7.54 (0.38) | 6.80 (0.36) |
| FMS | 6.33 (0.29) | 5.22 (0.36) | 5.36 (0.32) | 6.90 (0.47) | 5.39 (0.33) |
| TVR | |||||
| Control | 1039 (66) | 1416 (82) | 1237 (68) | 1217 (70) | 1215 (70) |
| TMDa,b | 882 (61) | 1215 (72) | 1058 (58) | 1099 (71) | 1064 (72) |
| FMSc | 1244 (68) | 1628 (96) | 1437 (99) | 1389 (83) | 1464 (99) |
TMD group significantly different from FMS group across all events (P < .015).
TMD group significantly different from Control group across all events (P < .025 for TVR, P < .05 for CO).
FMS group significantly different from Control group across all events (P < .05).
TMD group significantly different from Control group (P < .025 for DBP at Baseline).
FMS group significantly different from Controls in change from Baseline (P < .01 for HR reactivity to Stand, P <.05 for SBP reactivity to Stand and P <.025 for SBP reactivity to Speech).
Note: No cardiovascular measures could be obtained during the Ischemic Pain task since blood flow to the arm with the BP cuff was occluded throughout that task.
Among the 36 subjects participating in the propranolol substudy, essentially all of the same group differences described above were observed after placebo treatment (see Table 2). Repeated measures analyses of variance again yielded main effects of Diagnostic Groups for SBP, DBP, CO and TVR, F’s (2,33) ≥ 3.43, P< .05. Although effects were slightly less definitive with the smaller cell sizes, after placebo the FMS group tended to have higher overall TVR and SBP than the other two groups (P < .025 and P < .10 vs. TMDs and Controls respectively), as well as lesser HR increase to Stand (P< .10) and greater SBP increase to both Stand and Speech tasks than Controls (P < .025). The TMD group again had higher overall CO and lower TVR than the other two groups, lower BP levels than the FMS group, and lower baseline and recovery DBP than Controls. After placebo, there were two new cardiovascular findings in the subsample that were not seen in the larger sample: the TMD group showed greater SBP increases to Stand than Controls (9.7 vs. 1.7 mmHg, P <.05), and the FMS group had marginally higher overall DBP levels as well as SBP and TVR levels (P <.10, see Table 2) when only the latter two differed in the larger sample.
Table 2.
Mean (± SE) Cardiovascular Responses After Placebo vs. Propranolol in Subsample (n=36)
| Baseline | Stand | Recovery 1 | Speech | Recovery 2 | |
|---|---|---|---|---|---|
| SBP Placebo | |||||
| Control | 116.5 (3.6) | 118.2 (4.1) | 115.4 (3.3) | 133.2 (4.2) | 116.4 (3.4) |
| TMDa | 107.4 (2.7) | 117.1 (4.5)b | 110.2 (3.5) | 125.9 (3.5) | 111.2 (3.3) |
| FMS | 123.8 (5.8) | 134.9 (6.7)c | 126.8 (7.2) | 148.7 (7.2)c | 129.8 (6.5) |
| SBP Propranolold | |||||
| Control | 113.2 (4.2) | 115.3 (4.7) | 113.5 (3.7) | 130.9 (3.7) | 115.8 (3.9) |
| TMDa | 106.8 (2.9) | 112.6 (4.3) | 107.1 (3.7) | 119.9 (2.8) | 107.6 (3.5) |
| FMSc | 120.8 (6.3) | 129.3 (6.5)c | 121.9 (6.4) | 146.4 (6.2)c | 124.0 (5.6) |
| DBP Placebo | |||||
| Control | 69.1 (2.1) | 75.6 (2.8) | 72.6 (2.0) | 83.2 (2.9) | 73.8 (2.2) |
| TMDa | 62.5 (1.8) | 72.7 (2.3) | 66.4 (1.8) | 80.5 (2.5) | 67.0 (2.0) |
| FMSc | 73.2 (2.0) | 82.4 (3.5) | 77.0 (3.1) | 92.6 (4.1) | 78.7 (4.0) |
| DBP Propranolol | |||||
| Control | 68.9 (1.6) | 75.5 (2.7) | 72.2 (1.6) | 85.6 (2.7) | 72.1 (1.7) |
| TMDa | 64.3 (1.5) | 72.1 (2.5) | 66.6 (2.0) | 83.4 (2.8) | 66.2 (2.7) |
| FMS | 71.2 (3.1) | 79.2 (4.1) | 74.7 (4.0) | 92.1 (4.4) | 75.9 (3.6) |
| HR Placebo | |||||
| Control | 70.5 (2.8) | 83.7 (2.9) | 71.2 (2.7) | 85.0 (3.7) | 73.7 (2.6) |
| TMD | 69.2 (3.2) | 85.7 (3.6) | 71.9 (93.5) | 87.1 (4.6) | 74.8 (3.6) |
| FMS | 74.3 (3.9) | 80.7 (2.4)c | 75.0 (4.3) | 90.4 (4.2) | 77.3 (4.3) |
| HR Propranolold | |||||
| Control | 63.8 (2.4) | 72.5 (2.6) | 64.1 (1.86) | 70.3 (2.4) | 66.3 (1.9) |
| TMD | 65.6 (2.8) | 74.4 (2.8) | 67.1 (2.3) | 77.1 (2.9) | 69.1 (2.8) |
| FMS | 66.5 (3.7) | 71.6 (3.1) | 66.2 (2.8) | 73.6 (2.6) | 68.6 (2.7) |
| Placebo | |||||
| Control | 6.48 (0.47) | 5.20 (0.37) | 5.48 (0.39) | 6.33 (0.47) | 5.63 (0.41) |
| TMDa,b | 8.09 (0.70) | 6.47 (0.63) | 6.72 (0.57) | 7.24 (0.63) | 6.88 (0.60) |
| FMS | 5.59 (0.27) | 4.49 (0.27) | 4.74 (0.30) | 6.62 (0.52) | 4.92 (0.35) |
| CO Propranolold | |||||
| Control | 5.84 (0.42) | 4.57 (0.32) | 4.96 (0.36) | 5.09 (0.35) | 4.85 (0.35) |
| TMDa,b | 7.60 (0.75) | 5.73 (0.54) | 6.21 (0.54) | 6.54 (0.61) | 6.47 (0.59) |
| FMS | 5.20 (0.30) | 4.28 (0.31) | 4.61 (0.34) | 5.22 (0.34) | 4.73 (0.34) |
| TVR Placebo | |||||
| Control | 1149 (109) | 1503 (137) | 1373 (116) | 1358 (98) | 1360 (122) |
| TMDa,b | 853 (102) | 1184 (107) | 1021 (88) | 1116 (85) | 1002 (79) |
| FMSc | 1333 (111) | 1886 (141) | 1662 (144) | 1438 (147) | 1650 (140) |
| TVR Propranolold | |||||
| Control | 1242 (107) | 1666 (130) | 1482 (105) | 1696 (123) | 1646 (122) |
| TMDa,b | 904 (103) | 1296 (131) | 1112 (116) | 1266 (134) | 1073 (121) |
| FMS | 1407 (131) | 1928 (137) | 1684 (138) | 1770 (139) | 1650 (134) |
TMD group significantly different from FMS group for SBP, DBP, Co and TVR across all events on both Placebo and Propranolol (P < .015).
TMD group significantly different from Control group across all events on both Placebo and Propranolol (P < .025 for TVR, P < .05 for CO) and in SBP change from Baseline during Stand on Placebo (P < .05).
FMS group significantly different from Control group (P < .025 for SBP change from Baseline during Stand and Speech across both Placebo and Propranolol conditions, and marginally different for SBP, DBP and TVR levels on Placebo across all events and for HR change from Baseline during Stand on Placebo (P < .10).
Main effect of Placebo vs. Propranolol significant across all Groups and Events (P < .02 for SBP, P < .0005 for HR, CO and TVR, P =NS for DBP).
Surprisingly, most of these group differences in cardiovascular responses persisted after propranolol. Although propranolol did lead to the expected significant decreases in SBP, HR and CO and increases in TVR across all Events, these changes were generally quite similar in all 3 groups. However, one normalizing effect was seen with propranolol: HR and SBP responses to the postural task in the FMS Patients no longer differed from Controls. It should be noted that with this low dose of propranolol, there was no reliable reduction in DBP, and SBP decreases, while statistically significant, averaged only 3 mmHg.
Adrenal Responses
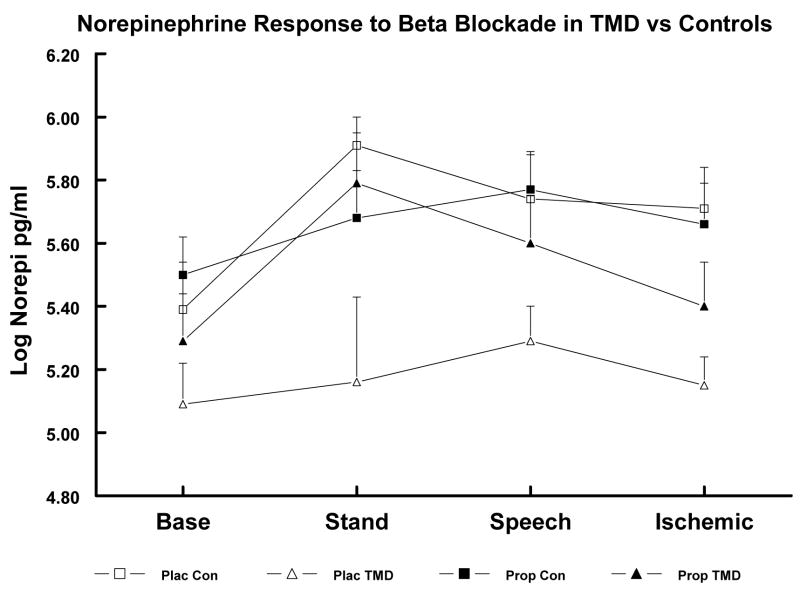
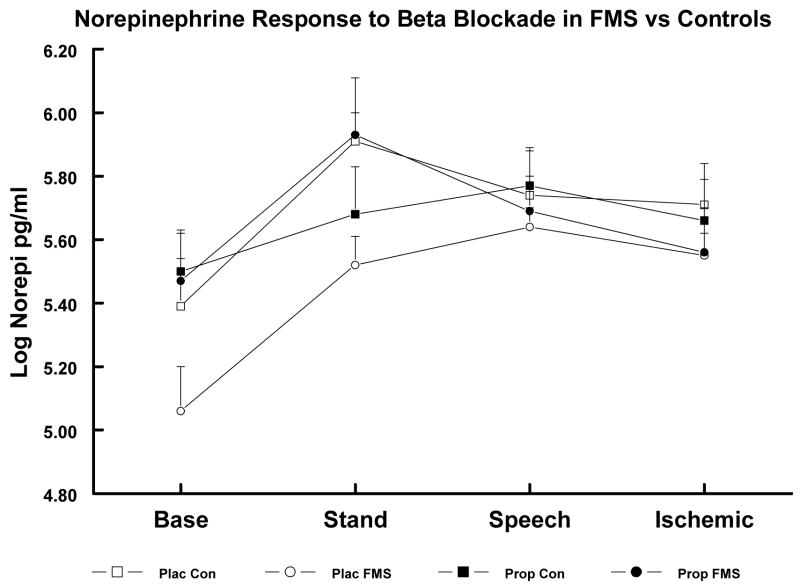
In the larger sample, main effects of Diagnostic Groups were obtained for both log NE and log EPI, F’s (2,80), P ≥ 3.67, and a significant Diagnostic Groups x Event interaction was obtained for log NE, F (6,212) ≥ 2.88, P < .025, while no significant effects were obtained for cortisol. Based on subsequent least squares mean comparisons, TMD Patients showed lower log NE and log EPI levels than Controls across all events (P < .01 and < .05, respectively; see Table 3). FMS Patients had lower baseline log NE levels than Controls (P < .05) but did not differ significantly in NE levels during stressors or in EPI levels at any point. In the substudy after placebo, these same group differences between TMDs and Controls were obtained, but here the FMS group had lower log NE levels during Baseline and Stand than Controls (P < .05; see Table 4 and Figures 1 and 2). After propranolol, these group differences were abolished due to significant increases in log NE and log EPI levels in the FMS and TMD groups with no reliable changes in the Control group.
Table 3.
Mean (± SE) Log Transformed Levels of Stress Hormones in Larger Sample (n=88)
| Baseline | Stand | Speech | Ischemic | |
|---|---|---|---|---|
| Log Epinephrine | ||||
| Control | 2.50 (0.13) | 2.83 (0.13) | 3.22 (0.17) | 2.97 (0.18) |
| TMDa | 2.25 (0.14) | 2.55 (0.14) | 3.48 (0.17) | 3.00 (0.17) |
| FMS | 2.61 (0.16) | 2.85 (0.16) | 3.29 (0.20) | 3.04 (0.22) |
| Log Norepinephrine | ||||
| Control | 5.27 (0.09) | 5.69 (0.07) | 5.52 (0.08) | 5.48 (0.07) |
| TMDa | 5.07 (0.09) | 5.37 (0.09) | 5.45 (0.09) | 5.22 (0.07) |
| FMS | 5.07 (0.09)b | 5.54 (0.07) | 5.50 (0.07) | 5.41 (0.07) |
| Log Cortisol | ||||
| Control | 2.10 (0.12) | 2.05 (0.13) | 2.03 (0.13) | 1.88 (0.14) |
| TMD | 2.12 (0.13) | 2.03 (0.13) | 1.94 (0.13) | 1.96 (0.14) |
| FMS | 2.12 (0.13) | 2.17 (0.14) | 2.23 (0.14) | 2.11 (0.15) |
TMD group differs from Controls across all events (P< .05 for EPI and P < .01 for NE).
FMS group differs from Controls at Baseline only (P< .05).
Table 4.
Mean (± SE) Log Transformed Levels of Cortisol and Epinephrine After Placebo vs. Propranolol in Subsample (n=36)
| Baseline | Stand | Speech | Ischemic | |
|---|---|---|---|---|
| Log Epinephrine Placebo | ||||
| Control | 3.00 (0.33) | 3.37 (0.30) | 3.78 (0.30) | 3.33 (0.40) |
| TMDa | 2.13 (0.10) | 2.27 (0.18) | 3.17 (0.18) | 2.70 (0.18) |
| FMS | 2.70 (0.28) | 3.06 (0.28) | 3.28 (0.38) | 3.13 (0.33) |
| Log Epinephrine Propranolol | ||||
| Control | 3.11 (0.30) | 3.54 (0.29) | 4.16 (0.28) | 3.80 (0.30) |
| TMD | 2.64 (0.30) | 3.40 (0.44) | 4.00 (0.30) | 3.22 (0.25) |
| FMS | 2.43 (0.20) | 3.59 (0.54) | 3.58 (0.31) | 3.30 (0.26) |
| Log Cortisol Placebo | ||||
| Control | 2.13 (0.18) | 1.97 (0.17) | 2.04 (0.17) | 2.02 (0.16) |
| TMD | 1.74 (0.27) | 1.57 (0.27) | 1.64 (0.25) | 1.66 (0.26) |
| FMS | 1.80 (0.18) | 1.73 (0.21) | 1.80 (0.22) | 1.78 (0.22) |
| Log Cortisol Propranolol | ||||
| Control | 2.18 (0.18) | 2.34 (0.15) | 2.31 (0.15) | 2.32 (0.15) |
| TMD | 1.94 (0.29) | 1.70 (0.22) | 1.81 (0.21) | 2.09 (0.17) |
| FMS | 1.94 (0.23) | 2.09 (0.17) | 2.23 (0.17) | 2.13 (0.24) |
TMD group differs from Controls across all events only under Placebo condition (P < .05).
Figure 1.
Log Norepinephrine (NE) levels in substudy TMD Patients and Controls (Con) after Placebo (Plac) vs. after Propranolol (Prop). Plac TMD < Plac Con across all events (P < .05). Group differences abolished after Prop.
Figure 2.
Log Norepinephrine (NE) levels in substudy FMS Patients and Controls (Con) after Placebo (Plac) vs. after Propranolol (Prop). Plac FMS < Plac Con at Baseline and Stand (P < .05). Group differences abolished after Prop.
As in the larger sample, cortisol levels did not differ reliably between the Patients vs. Controls in the substudy during either session. However, propranolol did induce a slight but significant increase in cortisol levels across all Groups and Events (P< .05; see Table 4).
Pain Responses
In the full sample, number of clinical pain sites and total pain across sites at Baseline were higher in the FMS vs. TMD group, as expected, (means = 4.1 vs. 2.0 for pain sites, P< .05 and 136 vs. 50 for total pain scores, P < .001). Mean pain rating per pain site was slightly but not significantly higher in the FMS vs. TMD groups (33 vs. 26). The same patterns were seen in the propranolol substudy between the FMS and TMD groups for placebo session, although pain sites and total pain levels at baseline on the placebo day tended to be slightly higher in the subsample than in the full sample patient groups (compare values above to 4.7 and 2.7 mean pain sites and 160 and 83 mean total pain scores for FMS and TMD patients in the substudy), probably because all substudy patients were tested after discontinuing all medications. Based on a priori hypotheses, we performed additional analyses to determine whether the total whole-body clinical pain at Baseline among all substudy Patients was related to individual differences in either NE, EPI or to the magnitude of HR increase from Baseline during this Speech task. Correlation analyses showed that Patients reporting greater total pain at Baseline exhibited greater HR increases to Speech (r = +.55, P < .015). When subdivided by diagnosis, this relationship was slightly stronger in the TMD group (r = +.82) than in the FMS group (r = +.47). FMS Patients reporting greater pain at Baseline also had significantly lower Baseline log NE levels (r = −.44, P < .05). These relationships between greater baseline pain and both greater HR reactivity and lower Baseline log NE were present in the propranolol subsample of FMS Patients as well (r = +.66 and −.71 respectively, P< .05).
Experimental pain sensitivity was assessed as time to pain onset and time to pain tolerance in the affected arm during the ischemic task. TMD Patients showed shorter times to ischemic arm pain onset than the FMS or the Control groups (92 vs. 204 and 180 sec, P < .025). No group differences in time to arm pain tolerance were seen. In Patients (but surprisingly not in Controls), higher Baseline SBP and Baseline TVR were correlated with longer times to arm pain threshold (r = +.67 and +.55, P < .01).
Effects of Beta-blockade on Pain Responses
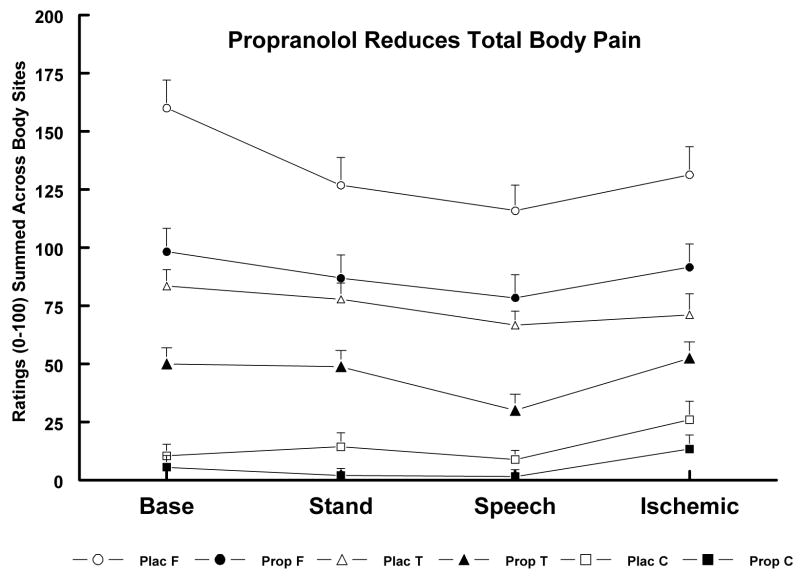
To examine our hypothesis that low-dose propranolol may lead to decreases in clinical pain, within-patient comparisons were made for clinical pain ratings obtained after Baseline, Stand, Speech and Ischemic tasks on both test days. The number of painful body sites were fewer by 1.3 and 1.4 sites after propranolol vs. placebo (P < .05) for TMD and FMS groups respectively, and the sum of pain severity ratings was lower in both groups after propranolol vs. placebo summed across all events (P < .05; see Figure 3). Even the Control group (where a minority of subjects reported some body pain in the placebo session) showed a non-significant tendency to report less total pain during the propranolol vs. the placebo session. Across all groups and both sessions, pain was lowest after the Speech, when BP levels were at their peak.
Figure 3.
Ratings of Total Clinical Pain in FMS (F), TMD (T) and Control (C) Groups after Placebo (Plac) vs. Propranolol (Prop). Plac F > Prop F and Plac T > Prop T, (P < .05).
To provide a second test that the reduction in total whole-body clinical pain was linked to the reduction in beta-adrenergic activity with propranolol, we once again examined relationships involving HR reactivity to the Speech task. Patients with higher HR reactivity to Speech during the Placebo session showed greater decreases in their total pain ratings after Beta-blockade (r = +.52, P < .04). Also, those Patients showing greater reductions in Speech HR from the placebo to the propranolol session showed greater reductions in total pain (r = + .57, P < .02).
In contrast to whole-body clinical pain reductions after propranolol, times to ischemic arm pain onset and arm pain tolerance in our experimental pain sensitivity task were significantly shorter after propranolol vs. after saline in FMS Patients only (192 vs. 81 sec and 388 vs. 196 sec, P < .05). Experimental arm pain responses in Controls and TMD Patients did not differ significantly after placebo vs. propranolol; however, after propranolol, both TMD and FMS groups showed shorter times to arm pain onset than Controls (75 and 81 vs. 216 sec, P < .05).
Discussion
The present study provides new evidence confirming that adrenergic function is dysregulated in females with FMS, TMD or both disorders. These patients showed lower plasma EPI and/or NE levels than healthy women of similar ages, and the lower the NE level, the greater the clinical whole-body pain. Hemodynamic patterns were altered in different ways in these patients: FMS patients showed higher overall BP levels and greater BP increases to stress due to greater vasoconstriction, whereas TMD patients showed heightened cardiac output with lower BP and vascular resistance. Although these different hemodynamic patterns cannot be definitively explained, we believe that the greater and more widespread pain in the FMS group may be directly linked to their greater vasoconstriction and BP. The findings also provide initial support for the interpretation that beta-blockade with low-dose propranolol may partially normalize adrenergic dysregulation and temporarily reduce the clinical pain severity by 40% in both FMS and TMD patients. Finally, our findings indicated important subgroup differences in beta-adrenergic activity; those patients who showed greater adrenergic dysregulation (indexed by greater HR reactivity and lower NE levels) had greater total whole-body pain after placebo and greater pain reduction after propranolol.
These findings reinforce earlier reports of adrenergic dysregulation in FMS.21,25,45,46,51,55 In one such study, Adler and colleagues2 reported that FMS patients with lower EPI response to hypoglycemia had the worst overall health status. Similarly, Thieme and Turk59 observed that greater clinical pain severity in FMS was correlated with higher DBP, an indirect marker of vasoconstriction. To our knowledge, this is the first study to obtain evidence that FMS patients have greater overall vasoconstriction (higher TVR) than healthy Controls. This observation opens up several possibilities relevant to their chronic pain. One possibility is that this excessive generalized vasoconstriction contributes to the pain by decreasing blood flow to multiple affected body regions 34,45 (allowing build-up of metabolites and/or inflammatory mediators). Baron et al.5 reported that enhancing vasoconstriction by whole-body cooling in patients with complex regional pain syndrome (CRPS) augmented both spontaneous pain and cutaneous sensitivity to mechanical stimuli. Another possibility is that the high TVR is secondary to the FMS patients’ ongoing pain, since painful stimuli like the cold pressor test are known to increase overall vasoconstriction.24,42 A third possibility is that this enhanced TVR reflects an SNS imbalance where alpha-adrenergic (vasoconstrictive) predominate over beta-2 adrenergic (vasodilatory) receptor activity. Several prior investigations have implicated alpha-adrenergic activity in CRPS or other sympathetically maintained pain,5,11,33,53 (although none of these involved FMS patients, and the one microneurographic study in FMS patients yielded negative findings 21). These three tentative explanations linking vasoconstriction to pain in FMS are not mutually exclusive, but may all contribute.
New evidence suggests that there may be a genetic basis for the effects that we observed. In a prospective study of TMD development, Diatchenko and colleagues18 have examined three genetic variants (haplotypes) for the gene encoding catecholamine-O-methyltransferase (COMT), an enzyme that catalyses the O-methylation of all catechol compounds including dopamine, NE and EPI. They found that the haplotype linked to the lowest COMT activity was associated with the highest pain sensitivity and with the greatest risk of developing TMD. Since the main function of COMT in the CNS is the elimination of catechols, low COMT activity could alter central adrenergic tone both to reset pain modulation at the central level and lead peripherally to enhanced sympathetic drive on the heart and vessels, and thereby to possible intramuscular hypoperfusion.26,45 Interestingly, COMT-knockout mice show no obvious increases in catecholamines in the CNS or peripheral circulation, although their function is abnormal.29,32 In our patients, the normalization of their low NE and EPI levels so rapidly after a single dose of propranolol suggests that adrenal stores of catecholamines and their precursors are probably normal, but that the patients’ enhanced SNS drive is in some way inhibiting normal catecholamine release.26
One strength of this investigation is the incorporation of testing a larger, more representative sample without propranolol together with the smaller double-blind, placebo-controlled cross-over propranolol substudy. In this way, we confirmed that cardiovascular and catecholamine responses showed similar dysregulation in the more representative sample as in the subsample when tested after placebo. Although the sample size of 20 patients and 16 healthy participants in the substudy is not large, each subject served as her own comparison so that uncontrolled influences were minimized. Since all substudy patients were nondepressed and were tested after being withdrawn from all medications, potential confounding effects of these important factors were eliminated. A third strength is convergence of information from multiple measures and manipulations. The effect of propranolol in reducing pain while abolishing abnormal cardiovascular responses to postural change and returning plasma NE and EPI to levels similar to those of the Control subjects provides the most definitive evidence that the alterations in beta adrenergic function in these FMS and TMD patients is clinically significant. The propranolol treatment also increased cortisol levels modestly, probably due to a reduction in the negative feedback that normally exists between the adrenomedullary and HPA systems. Although cortisol levels were not significantly different from Controls in our FMS or TMD patients, dysregulated HPA activity has been reported in other studies.16,36,51
The finding regarding the potential analgesic benefit of propranolol is limited by the protocol itself. After a single dose of propranolol, we observed reductions in total clinical pain in both the TMD and FMS groups for the entire 3 hour period that the subjects remained in the laboratory, but have no further information in regard to how long effects lasted or whether they would be maintained in a chronic treatment regimen. Low-dose propranolol has previously been shown to be efficacious as prophylactic therapy for migraine headache, a disorder in which vasomotor activity is a known trigger.22,44 Patients with FMS have been shown to have abnormal vascular responses to stimuli, leading some authorities to postulate that deficient intramuscular blood flow may play a triggering role in their myalgic pain.34,45 Our findings on pain induced by arm ischemia indicated that sensitivity to this type of experimental pain was enhanced after propranolol in FMS patients but not in TMD patients or Controls. We suggest that this hyperalgesic effect only in the FMS group was due to altering the adrenergic balance in favor of greater alpha- vs. beta-adrenergic activation, inducing higher vasoconstriction and BP which may influence pain sensitivity through hypoperfusion or via baroreceptor-mediated endogenous pain modulation.17,52 This observation also highlights the importance of the decision to use a low dose of propranolol because higher doses might have enhanced this type of hyperalgesic response, countering its beneficial effects on clinical pain.
The present findings encourage researchers to address the specific mechanisms through which low-dose propranolol may reduce clinical pain. Recently, it was reported that propranolol and other beta-adrenergic antagonists may cause analgesia by blocking tetrodotoxin (TTX)-insensitive sodium (Na+) channels in sensory neurons, but this required doses much higher than in the present study.10,58 One of the defining tests of sympathetically-maintained pain syndromes is a positive response to the NE-evoked pain test, and another is that the pain is attenuated by sympathetic blockade. Martinez-Lavin and colleagues49,50 have shown that intramuscular NE injections induce greater pain than placebo injections in 80% of FMS patients compared to only 30% of rheumatoid arthritis patients and 30% of controls. Attenuation of muscle pain by propranolol has been indicated in two recent animal studies. In the rat TMD model described by Rodrigues et al.57, hyperalgesia induced by injection of carrageenan followed one hour later by 5-hydroxytryptamine was reduced by either localized injection of propranolol or a selective beta-2 antagonist. In rats with chronic hyperalgesia from carageenan-induced inflammation of the gastrocnemius muscle, Light and Levine41 observed that intramuscular injection of even low doses of propranolol at 24 hours and 7 days after carageenan reduces nociceptive sensitivity, measured as muscle withdrawal threshold to pressure. Together with prior research by Khasar, Carter and Levine,35 these data strongly suggest that one way in which propranolol is decreasing pain sensitivity is by acting on local sensory receptors within the muscle. Specific types of sensory neurons involved may utilize molecular receptors such as acid-sensing ASIC3 and purinergic P2X4 and P2X5 that work in combination to detect increases in muscle metabolites, and may even include adrenergic receptors themselves.40 Light, White and Light 43 recently reported that patients meeting criteria for both FMS and chronic fatigue syndrome showed increases in ASIC3, P2X4 and both alpha- and beta-adrenergic receptors on leukocytes persisting for 48 hours following moderate exercise, while controls showed no increases in any of these receptors. Central effects of propranolol may also be important, and deserve attention in future research. Pharmacologic interventions which alter central sympathetic activity, including tricyclic antidepressants, venlafaxine and pindolol, have led to improvement in symptoms in patients with FMS and other pain syndromes.13,28,62 Two drugs that have shown convincing success in large randomized placebo-controlled trials of FMS are duloxetine (which equally inhibits both NE- and serotonin reuptake) and pregabalin (which reduces synaptic release of neurotransmitters like NE, as well as glutamate and substance P).3,4
Conclusions
These findings suggest that many FMS and TMD patients have dysregulated activity of the SNS that may directly contribute to their clinical myalgic pain as well as to alterations in their cardiovascular and catecholamine responses at rest and during stressors. Our results also indicate that some aspects of this SNS dysregulation, including pain symptoms, can be temporarily improved through use of low doses of the nonselective beta-antagonist, propranolol. Future mechanistic research should include attention to both the local sensory pathways and CNS actions of propranolol. Intervention research should consider using pharmacological and/or behavioral strategies to normalize beta-adrenergic activity and thereby potentially to reduce symptoms and improve quality of life in patients with TMD and FMS.
Acknowledgments
This research was supported by an NIH Center for Inflammatory Disorders grant to the University of North Carolina School of Dentistry and NIH grants NS057821 and NS45685.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aaron LA, Burke MM, Buchwald D. Overlapping conditions among patients with chronic fatigue syndrome, fibromyalgia and temporomandibular disorder. Arch Intern Med. 2000;160:221–227. doi: 10.1001/archinte.160.2.221. [DOI] [PubMed] [Google Scholar]
- 2.Adler GK, Kinsley BT, Hurwitz S, Mossey CJ, Goldenberg DL. Reduced hypothalamic-pituitary and sympathoadrenal responses to hypoglycemia in women with fibromyalgia syndrome. Am J Med. 1999;106:534–543. doi: 10.1016/s0002-9343(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 3.Arnold LM, Pritchett YL, D’Souza DN, Kajdasz DK, Iyengar S, Wernicke JF. Duloxetine for the treatment of fibromyalgia in women: pooled results from two randomized, placebo-controlled clinical trials. J Womens Health (Larchmt) 2007;16:1145–1156. doi: 10.1089/jwh.2006.0213. [DOI] [PubMed] [Google Scholar]
- 4.Arnold LM, Russell IJ, Diri EW, Duan WR, Young JP, Jr, Sharma U, Martin SA, Barrett JA, Haig G. A 14-week randomized, double-blinded, placebo-controlled monotherapy trial of pregablin in patients with fibromyalgia. J Pain. 2008;9:792–805. doi: 10.1016/j.jpain.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Baron R, Schattschneider J, Binder A, Siebrecht D, Wasner G. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case-control study. Lancet. 2002;359:1655–1660. doi: 10.1016/S0140-6736(02)08589-6. [DOI] [PubMed] [Google Scholar]
- 6.Bou-Holaigah I, Calkins H, Flynn JA, Tunin C, Chang HC, Kan JS, Row PC. Provocation of hypotension and pain during upright tilt table testing in adults with fibromyalgia. Clin Exper Rheumatol. 1997;15:239–246. [PubMed] [Google Scholar]
- 7.Bragdon EE, Light KC, Costello NL, Sigurdsson A, Bunting S, Bhalang K, Maixner W. Group differences in pain modulation: pain-free women compared to pain-free men and to women with TMD. Pain. 2002;96:227–237. doi: 10.1016/S0304-3959(01)00451-1. [DOI] [PubMed] [Google Scholar]
- 8.Brownley KA, Hinderliter AL, West SG, Girdler SS, Sherwood A, Light KC. Sympathoadrenergic mechanisms in reduced hemodynamic stress responses after exercise. Med Sci Sports Exercise. 2003;35:978–986. doi: 10.1249/01.MSS.0000069335.12756.1B. [DOI] [PubMed] [Google Scholar]
- 9.Buskila D. Fibromyalgia, chronic fatigue syndrome and myofascial pain syndrome. Curr Opin Rheumatol. 2001;13:117–127. doi: 10.1097/00002281-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Chidlow G, Melena J, Osborne NN. Betaxolol, a beta-1 adrenoceptor antagonist, reduces Na+ influx into cortical synaptosomes by direct interaction with sodium channels; comparison with other beta-adrenoceptor antagonists. Brit J Pharmacol. 2000;130:759–766. doi: 10.1038/sj.bjp.0703369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi B, Rowbotham M. Effect of adrenergic receptor activation on post-herpetic neuralgia pain and sensory disturbances. Pain. 1997;69:55–63. doi: 10.1016/s0304-3959(96)03245-9. [DOI] [PubMed] [Google Scholar]
- 12.Clauw DJ, Chrousos GP. Chronic pain and fatigue syndromes: overlapping clinical and neuroendocrine features and potential pathogenic mechanisms. Neuroimmunomodulation. 1997;4:134–153. doi: 10.1159/000097332. [DOI] [PubMed] [Google Scholar]
- 13.Clauw DJ, Crofford LJ. Chronic widespread pain and fibromyalgia: what we know and what we need to know. Best Pract Res Clin Rheumatol. 2003;17:685–701. doi: 10.1016/s1521-6942(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 14.Cohen H, Neumann L, Shore M, Amir M, Cassuto Y, Buskila D. Autonomic dysfunction in patients with fibromyalgia: application of power spectral analysis of heart rate variability. Semin Arthritis Rheum. 2000;29:217–227. doi: 10.1016/s0049-0172(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 15.Costello NL, Bragdon EE, Light KC, Sigurdsson A, Bunting S, Grewen K, Maixner W. Temporomandibular disorder and optimism: relationships to ischemic pain sensitivity and interleukin-6. Pain. 2002;100:99–110. doi: 10.1016/s0304-3959(02)00263-4. [DOI] [PubMed] [Google Scholar]
- 16.Crofford LJ, Young EA, Engleberg NC, Korszun A, Brucksch CB, McClure LA, Brown MB, Demitrack MA. Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain Behav Immun. 2004;18:314–325. doi: 10.1016/j.bbi.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 17.De Abreu TC, Nilner M, Thulin T, Vallon D. Office and ambulatory blood pressure in patients with craniomandibular disorders. Acta Odontol Scand. 1993;51:161–170. doi: 10.3109/00016359309041162. [DOI] [PubMed] [Google Scholar]
- 18.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer A, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin SF, Fricton JR, Hollender L, Huggins KH, LeResche L, Lund J, Mohi N, Ohrbach R, Palla SF, Sommers EE, Stohler C, Truelove EL, Von Korff M, Widmer CG. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications critique. J Craniomandib Disord Facial Pain Oral Pain. 1992;6:302–355. [PubMed] [Google Scholar]
- 20.Dworkin SF, LaResche L. Temporomandibular disorder pain: epidemiologic data. APS Bull. 1993 April/May;:12. [Google Scholar]
- 21.Elam M, Johansson G, Wallin BG. Do patients with primary fibromyalgia have an altered muscle sympathetic nerve activity? Pain. 1992;48:371–375. doi: 10.1016/0304-3959(92)90086-Q. [DOI] [PubMed] [Google Scholar]
- 22.Geraud G, Lanteri-Minet M, Lucas C, Valade D French Society for the Study of Migraine Headaches (SFEMC) French guidelines for the diagnosis and management of migraine in adults and children. Clin Ther. 2004;26:1305–1318. doi: 10.1016/s0149-2918(04)80161-9. [DOI] [PubMed] [Google Scholar]
- 23.Furlan R, Colombo S, Perego F, Atzeni F, Diana A, Barbic F, Porta A, Pace F, Malliani A, Sarzi-Puttini P. Abnormalities of cardiovascular neural control and reduced orthostatic tolerance in patients with primary fibromyalgia. J Rheumatol. 2005;32:1787–1793. [PubMed] [Google Scholar]
- 24.Girdler SS, Hinderliter AL, Light KC. Peripheral adrenergic receptor contributions to cardiovascular reactivity: Influence of race and gender. J Psychosom Res. 1993;37:177–193. doi: 10.1016/0022-3999(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 25.Giske L, Vøllestad NK, Mengshoel AM, Jensen J, Knardahl S, Røe C. Attenuated adrenergic responses to exercise in women with fibromyalgia--a controlled study. Eur J Pain. 2008;12:351–360. doi: 10.1016/j.ejpain.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharm Exper Therap. 2003;305:800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- 27.Granges G, Littlejohn G. Pressure pain threshold in pain-free subjects, in patients with chronic regional pain syndromes, and in patients with fibromyalgia syndrome. Arthritis Rheum. 1993;36:642–646. doi: 10.1002/art.1780360510. [DOI] [PubMed] [Google Scholar]
- 28.Grothe DR, Scheckner B, Albano D. Treatment of pain syndromes with venlafaxine. Pharmacotherapy. 2004;24:621–629. doi: 10.1592/phco.24.6.621.34748. [DOI] [PubMed] [Google Scholar]
- 29.Haasio K, Huotari M, Nissinen E, Mannisto PT. Tissue histopathology, clinical chemistry and behavior of adult COMT-gene disrupted mice. J Appl Toxicol. 2003;23:213–219. doi: 10.1002/jat.909. [DOI] [PubMed] [Google Scholar]
- 30.Hamaty D, Valentine JL, Howard R, Howard CW, Wakefield V, Patten MS. The plasma endorphin, prostaglandin and catecholamine profile of patients with fibrositis treated with cyclobenzaprine and placebo: a 5-month study. J Rheumatol. 1989;19 (Suppl Nov):164–168. [PubMed] [Google Scholar]
- 31.Hedenberg-Magnussen B, Ernberg M, Kopp S. Presence of orofacial pain and temporomandibular disorder in fibromyalgia. Swed Dent J. 1999;23:185–192. [PubMed] [Google Scholar]
- 32.Huotari M, Gogos JA, Karayiorgou M, Koponen O, Forsberg M, Raasmaja A, Hyttinen J, Mannisto PT. Brain catecholamine metabolism in COMT-deficient mice. Eur J Neurosci. 2002;15:246–256. doi: 10.1046/j.0953-816x.2001.01856.x. [DOI] [PubMed] [Google Scholar]
- 33.Inchiosa MA, Kizelshteyn G. Treatment of complex regional pain syndrome type I with oral phenoxybenzamine: rationale and case reports. Pain Practice. 2008;8:125–132. doi: 10.1111/j.1533-2500.2007.00170.x. [DOI] [PubMed] [Google Scholar]
- 34.Katz DL, Greene L, Ali A, Faridi Z. The pain of fibromyalgia syndrome is due to muscle hypoperfusion induced by regional vasomotor dysregulation. Med Hypotheses. 2007;69:517–525. doi: 10.1016/j.mehy.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–1112. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 36.Korzun A, Young EA, Singer K, Carlson NE, Brown MB, Crofford L. Basal circadian cortisol secretion in women with temporomandibular disorders. J Dent Res. 2002;81:279–283. doi: 10.1177/154405910208100411. [DOI] [PubMed] [Google Scholar]
- 37.Kosek E, Ekholm J, Hansson P. Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and healthy controls. Pain. 1996;64:415–423. doi: 10.1016/0304-3959(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 38.Lautenbacher S, Rollman GB, McCain GA. Multi-method assessment of experimental and clinical pain in patients with fibromyalgia. Pain. 1994;59:45–53. doi: 10.1016/0304-3959(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 39.Legangneux E, Mora JJ, Spreux-Varoquaux O, Thorin I, Herrou M, Alvado G, Gomeni C. Cerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and [3H]imipramine reuptake in the primary fibromyalgia syndrome. Rheumatology. 2001;40:290–296. doi: 10.1093/rheumatology/40.3.290. [DOI] [PubMed] [Google Scholar]
- 40.Light AR, Hughen RW, Zhang J, Rainier J, Zhuqing L, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP and lactate mediated by ASIC, P2X and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Light AR, Levine JD. Diminished carrageenan-induced inflammatory hyperalgesia following direct intramuscular injection of propranolol. Personal communication. 2008 [Google Scholar]
- 42.Light KC, Turner JR, Hinderliter AL, Girdler SS, Sherwood A. Comparison of cardiac versus vascular reactors and ethnic groups in plasma epinephrine and norepinephrine responses to stress. Internat J Behav Med. 1994;3:229–236. doi: 10.1207/s15327558ijbm0103_4. [DOI] [PubMed] [Google Scholar]
- 43.Light KC, White A, Light AR. Post-exercise dysregulation of adrenergic and sensory receptors and altered cytokine profiles in patients with chronic fatigue/fibromyalgia; Presented at: Spring Brain Conference; Palm Springs. 13 March 2008. [Google Scholar]
- 44.Linde K, Rossnagel K. Propranolol for migraine prophylaxis. Cochrane Database Syst Rev. 2004;2:CD003225. doi: 10.1002/14651858.CD003225.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Maekawa K, Clark GT, Kuboki T. Intramuscular hypoperfusion, adrenergic receptors, and chronic muscle pain. J Pain. 2002;3:251–260. doi: 10.1054/jpai.2002.125923. [DOI] [PubMed] [Google Scholar]
- 46.Maekawa K, Twe C, Lotaif A, Chiapelli F, Clark GT. Function of beta-adrenergic receptors on mononuclear cells in female patients with fibromyalgia. J Rhematol. 2003;30:364–368. [PubMed] [Google Scholar]
- 47.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain; evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 48.Margoles MS. Pain charts. Pain. 1980;8:115–117. doi: 10.1016/0304-3959(80)90094-9. [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Lavin M. Biology and therapy of fibromyalgia; stress, the stress response system and fibromyalgia. Arthritis Res Therap. 2007;9:216–222. doi: 10.1186/ar2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez-Lavin M, Vidal M, Barbosa RE, Pineda C, Casanova JM, Nava A. Norepinephrine-evoked pain in fibromyalgia. A randomized pilot study. BMC Musculoskelet Disord. 2002;3:2. doi: 10.1186/1471-2474-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McBeth J, Chiu YH, Silman AJ, Ray D, Morriss R, Dickens C, Gupta A, Macfarlane GJ. Hypothalamic-pituitary-adrenal stress axis function and the relationship with chronic widespread pain and its antecedents. Arthritis Res Ther. 2005;7:R992–R1000. doi: 10.1186/ar1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohn C, Vassend O, Knardahl S. Experimental pain sensitivity in women with temporomandibular disorders and pain-free controls: the relationship to orofacial muscular contraction and cardiovascular responses. Clin J Pain. 2008;24:343–352. doi: 10.1097/AJP.0b013e318162eaf4. [DOI] [PubMed] [Google Scholar]
- 53.Muizelaar JP, Kleyer M, Hertogs IA, DeLange DC. Complex regional pain syndrome (reflex sympathetic dystrophy and causalgia): management with the calcium channel blocker nifedipine and/or the alpha-sympathetic blocker phenoxybenzamine in 59 patients. Clin Neurol Neurosurg. 1997;99:26–30. doi: 10.1016/s0303-8467(96)00594-x. [DOI] [PubMed] [Google Scholar]
- 54.Okifuji A, Turk DC, Sinclair JD, Starz TW, Marcus DA. A standardized manual tender points survey. I. Development and determination of a threshold point for the identification of positive tender points in fibromyalgia syndrome. J Rheumatol. 1997;24:377–383. [PubMed] [Google Scholar]
- 55.Perry F, Heller PH, Kamiya J, Levine JD. Altered autonomic function in patients with arthritic or with chronic orofacial pain. Pain. 1989;39:77–84. doi: 10.1016/0304-3959(89)90177-2. [DOI] [PubMed] [Google Scholar]
- 56.Price DD, Staud R, Robinson ME, Mauderli AP, Cannon R, Vierck CJ., Jr Enhanced temporal summation of second pain and its central modulation in fibromyalgia patients. Pain. 2002;99:49–59. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 57.Rodrigues LLFR, Oliveira MCG, Pelegrini-da-Silva A, de Arruda Veiga MCF, Parada CA, Tambeli CH. Peripheral sympathetic component of the temporomandibular joint inflammatory pain in rats. J Pain. 2006;7:929–936. doi: 10.1016/j.jpain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Tanahashi S, Iida H, Oda A, Osawa Y, Dohi S. Effect of beta-adrenoceptor antagonists on tetrodotoxin-resistant Na+ channels in rat dorsal root ganglion neurons. Anesthesiology. 2003;99:A962. doi: 10.1017/S0265021507000543. [DOI] [PubMed] [Google Scholar]
- 59.Thieme K, Turk DC. Heterogeneity of psychophysiological stress responses in fibromyalgia syndrome patients. Arthritis Res Therap. 2006 doi: 10.1186/ar1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.White KP, Speechley M, Harth M, Ostbye T. The London Fibromyalgia Epidemiology Study: the prevalence of fibromyalgia syndrome in London, Ontario. J Rheumatol. 1999;26:1570–1576. [PubMed] [Google Scholar]
- 61.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, Fam AG, Farber SJ, Fiechtner JJ, Franklin C, Gatter RA, Hamaty D, Lessard J, Lichtbroun AS, Masi AT, McCain GA, Reynolds WJ, Romano TJ, Russell IJ, Sheon RP. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 62.Wood PB, Kablinger AS, Caldito GS. Open trial of pindolol in the treatment of fibromyalgia. Ann Pharmacother. 2005;39:1812–1816. doi: 10.1345/aph.1G014. [DOI] [PubMed] [Google Scholar]
- 63.Yunus MB, Dailey JW, Aldag JC, Masi AT, Jobe PC. Plasma and urinary catecholamines in primary fibromyalgia: a controlled study. J Rheumatol. 1992;19:95–97. [PubMed] [Google Scholar]