Abstract
The B cell receptor (BCR) transduces antigen binding into alterations in the activity of intracellular signaling pathways through its ability to recruit and activate the cytoplasmic protein-tyrosine kinase Syk. The recruitment of Syk to the receptor, its activation and its subsequent interactions with downstream effectors are all regulated by its phosphorylation on tyrosine. This review discusses our current understanding of how this phosphorylation regulates the activity of Syk and its participation in signaling through the BCR.
Keywords: Syk, B cell activation, antigen receptor, protein-tyrosine kinase, protein phosphorylation, signal transduction
1. Introduction
B cells are essential components of the adaptive immune response, producing antigen-specific antibodies for the targeting of foreign molecules and cells. Foreign particles are recognized by the B cell receptor (BCR) for antigen, which comprises a membrane-bound immunoglobulin that binds the antigen and an associated heterodimer of disulfide-linked Ig-α (CD79a) and Ig-β (CD79b) subunits that are required for transducing this binding into alterations in intracellular signaling pathways [1,2]. Although none of the components of the BCR complex has intrinsic enzymatic activity, their engagement leads to the enhanced phosphorylation of multiple intracellular proteins on tyrosine, which occurs through the recruitment and activation of cytoplasmic protein-tyrosine kinases [1,3–6]. Spleen tyrosine kinase (Syk) is a critical component of this signaling machinery. Studies in “knockout” mice and cell lines indicate that Syk is essential for most of the biochemical responses to BCR engagement [7–10].
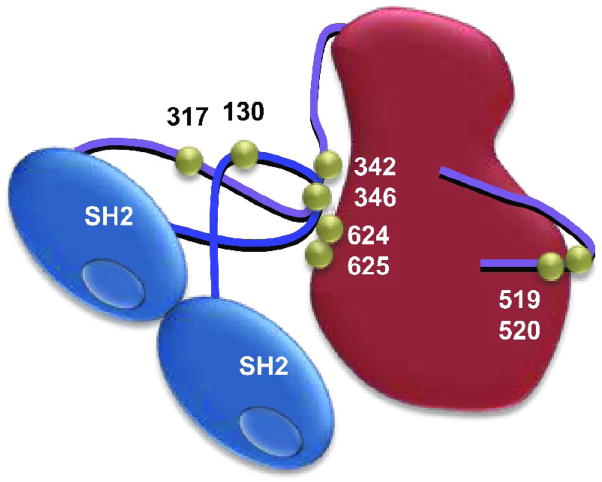
Syk was originally identified at the protein level as a 40-kDa proteolytic fragment containing the catalytic domain that was isolated from bovine thymus based on its ability to phosphorylate a synthetic peptide substrate [11]. A kinase of the same size was isolated from porcine spleen [12]. Antibodies against this active fragment identified the full-length enzyme as a 72-kDa protein, a size confirmed when the cDNA was sequenced [13,14]. Syk has at its amino terminus a tandem pair of Src homology 2 (SH2) domains separated by a 60-amino acid linker (linker A) (Fig. 1). A C-terminal catalytic domain is connected to the tandem SH2 domains by a stretch of 106-amino acids (linker B) that contains multiple sites of phosphorylation [15,16]. A role for Syk in cellular signaling was first identified in B lymphocytes [17–19], but Syk is expressed in many cell types including most cells of the hematopoietic system, and at lower levels in some epithelial cells, fibroblasts, hepatocytes, vascular smooth muscle cells, endothelial cells and neuronal cells [20,21]. A variant of Syk, SykB, which lacks a 23 amino acid “linker insert” from the linker B region due to alternative splicing of exon 7, also is expressed in a variety of cell types [22–25]. The ζ-chain associated protein of 70 kDa (Zap-70) is a Syk homolog expressed in T cells and NK cells and is the only other member of the Syk-family of cytoplasmic protein-tyrosine kinases [26]. Zap-70, like SykB, lacks a linker insert region.
Fig. 1.
Model of Syk showing approximate locations of major sites of tyrosine-phosphorylation.
2. Activation of Syk in B lymphocytes by binding to the antigen receptor
2.1 Phosphorylation of tyrosines in the receptor ITAM
Signaling is initiated in B cells when BCR complexes become aggregated. Rapidly following aggregation, the cytoplasmic tails of Ig-α and Ig-β are phosphorylated on tyrosines located in a stretch of amino acids known as an immunoreceptor tyrosine-based activation motif (ITAM) [1,3,27–29]. ITAMs are found not only within these two components of the BCR complex, but also within subunits of many additional immune cell receptors—examples include the T cell antigen receptor (TCR), the immunoglobulin receptors FcεRI, FcγRI, and FcγRIIa, and the DAP12 component of NK cell activating receptors and integrins—and in cytoplasmic proteins of the ERM family [30–36]. ITAMs have the consensus sequence (D/E)X2YX2LX7–10YX2(L/I). For Ig-α, this sequence is ENLYEGLNLDDCSMYEDI. The phosphorylation of the two tyrosines within the ITAM leads to the physical recruitment of Syk to the site of the clustered receptor in an interaction mediated by its tandem pair of SH2 domains [37,38,39]. Interestingly, a number of virally encoded proteins also contain ITAM sequences, which they use to co-opt Syk for their own nefarious purposes [35,40,41].
The initial phosphorylation of the ITAM is catalyzed by a member of the Src-family of cytoplasmic protein-tyrosine kinases. Several members of this family are expressed in B cells, but, of these, Lyn is thought to be particularly important and its elimination from mice yields a pronounced B cell phenotype [42–48]. Lyn associates with the clustered BCR [49–51] and catalyzes the phosphorylation of the first (N-terminal) tyrosine of the ITAM. This specificity can be demonstrated through in vitro analyses of kinase-substrate interactions and in a cellular model of BCR-signaling reconstituted in insect cells [52,53]. Structural studies indicate that this specificity for the first ITAM tyrosine is dictated by the glutamate at the P-3 position and the glycine at the P+2 position [52]. The glutamate provides side chain hydrogen bonding interactions with residues in the catalytic cleft of Lyn while the glycine allows for the conformational flexibility needed for favorable binding of this region of the ITAM to the kinase. Accordingly, the stoichiometry of phosphorylation of the first tyrosine of Ig-α in activated B cells is much greater than that of the second [54].
Optimal signaling, however, requires the phosphorylation of both tyrosines of the ITAM as two phosphotyrosines are needed for a high-affinity interaction with Syk’s tandem pair of SH2 domains. The kinase that phosphorylates the second tyrosine of the ITAM is less certain, but a prime candidate is Syk, itself. Syk is capable of phosphorylating Ig-α in vitro and, in the reconstituted insect cell model system, its expression leads to the phosphorylation of both ITAM tyrosines even in the absence of Lyn [53]. Consistent with this observation, BCR-mediated signaling and the recruitment of Syk to the receptor can and does occur, albeit at a slower initial rate, in B cells that lack Lyn [7,44,55–57].
2.2. Activation of Syk by binding to phosphorylated ITAMs
Syk binds with high affinity to the doubly phosphorylated ITAM through its tandem pair of SH2 domains. These two domains are juxtaposed to form a Y-shaped structure that is held together and oriented properly through domain-domain interactions and through linker A for the simultaneous engagement of both ITAM phosphotyrosines. Linker A contains 3 α–elices, two of which form a coiled-coil [58]. Each SH2 domain recognizes one of the two pYXXL/I sequences within the phosphorylated ITAM via two major binding pockets, one that accommodates the phosphotyrosine and another that binds the hydrophobic side chain of the P+3 residue. Overall binding occurs in a head-to-tail orientation with the N-terminal SH2 domain of Syk interacting with the second phosphotyrosine of the ITAM and the C-terminal domain binding the first. The interaction surface between the two SH2 domains is sufficiently small and linker A sufficiently flexible to allow the tandem SH2 domains to exist in either an open or a closed conformation [58–61]. This facilitates Syk’s interactions with ITAMs that vary in the degree of spacing between the two pYXXL/I binding motifs. Thus, Syk can bind with high affinity to immunoreceptors that have as few as 10 or as many as 15 residues separating the two ITAM phosphotyrosines [60].
Each of the SH2 domains contains all of the amino acid residues required to bind independently to one of the pYXXL/I sequences of the ITAM [58]. Thus, it has been suggested that Syk can interact also with proteins containing only one phosphotyrosine. The C-terminal SH2 domain, when expressed independently of the N-terminal domain and linker A, does, in fact, retain the ability to recognize and bind phosphopeptides on its own [62]. It is reasonable to speculate that the C-terminal SH2 domain mediates the initial recruitment of Syk to the BCR after phosphorylation of the first ITAM tyrosine by Lyn. Inactivation of the C-terminal SH2 domain does block the ability of Syk to signal when expressed in Syk-deficient DT40 B cells [37]. Subsequent phosphorylation of the second ITAM tyrosine by Syk would then allow for a much higher affinity interaction in which both SH2 domains are engaged. Interestingly, Syk also is recruited to and activated by engagement of a class of receptors exemplified by Dectin-1 and C-type lectin-like receptor 2 (CLEC-2) [36] that contain only a single pYXXL motif, suggesting an interaction mediated by a single SH2 domain. However, the mechanism by which Syk binds to these receptors is not completely understood as mutagenesis studies indicate that both SH2 domains are still required [63].
The binding of Syk to the phosphorylated ITAMs of clustered BCR complexes leads to its activation. In vitro studies indicate that the simple binding to Syk in solution of a dually phosphorylated peptide with the sequence of an ITAM is sufficient to fully activate the kinase [64–66]. In the X-ray crystal structure of the Syk homolog, Zap-70, the linker A region makes close contacts with the C-terminal lobe of the kinase domain and with residues in linker B forming what is referred to as a “linker-kinase sandwich” [67]. These interactions likely restrict the flexibility of the hinge region of the kinase domain preventing it from transitioning from an inactive to an active conformation. Engagement of the tandem SH2 domains by a dually phosphorylated ITAM reorients the domains and alters the conformation of linker A to disrupt these interactions. An analysis of Syk’s structure by electron microscopy suggests an autoinhibited conformation comparable to that of Zap-70 indicating that Syk is likely to be regulated and activated in an analogous fashion [68]. Consequently, the binding of Syk to its reaction product, a dually phosphorylated ITAM, activates the enzyme and generates a positive feedback loop to promote the phosphorylation of additional ITAM tyrosines to recruit even more Syk molecules to the clustered BCR complexes [53,66].
Syk, recruited to the BCR, can catalyze the phosphorylation of ITAM tyrosines and at least one non-ITAM tyrosine (Y204) on Ig-α [69]. These residues lie within predicted AP-2 binding sites and the conversion of all three to non-hydrophobic residues severely impairs receptor internalization [69]. If the activity of Syk is then inhibited, the Syk-receptor complex rapidly dissociates and the receptor is internalized most likely due to its rapid dephosphorylation by protein tyrosine phosphatases [70]. Thus, phosphorylated receptors that bind and activate Syk are retained at the cell surface while clustered, but nonphosphorylated receptors are internalized. The persistence of Syk-BCR complexes at the plasma membrane likely plays an important role in determining the length of time that Syk remains active following the initial engagement of the receptor. The prolonged activation of Syk is required for some receptor-stimulated events such as the activation of the NFAT transcription factor, which requires Syk to remain active for more than one hour following receptor ligation [70]. As we will see below, the binding of Syk to the adaptor protein BLNK/SLP-65 at the plasma membrane also provides a mechanism for its prolonged activation [71].
3. The phosphorylation of Syk on tyrosine
Shortly following BCR-engagement, Syk that has been recruited to the receptor becomes phosphorylated on multiple tyrosines through both autophosphorylation and phosphorylation by Lyn [15–18]. In solution in the presence of ATP, Syk readily catalyzes an autophosphorylation reaction, the rate of which is enhanced if Lyn is present [66]. Thus, the recruitment of Syk to a receptor complex where Lyn is resident and active provides an additional mechanism to increase the rate at which it becomes phosphorylated and activated. Interestingly, like ITAM-binding, phosphorylation alone is sufficient to fully activate the kinase [66]. The ability of either ITAM-binding or phosphorylation to activate Syk allows the kinase to be active within cells both when phosphorylated, but released from the receptor, and when not phosphorylated, but bound to the receptor. Active kinase that has dissociated from the receptor can be found in the non-membrane fraction of cells [72]. In fact, active, tyrosine-phosphorylated Syk and Zap-70 have even been observed in the nucleus of activated B and T cells, respectively [73,74]. While a role for these kinases in the nucleus of immune cells is not known, Syk has been reported to function as a transcriptional repressor in breast cancer cells by binding to SP-1 and recruiting histone deacetylases to a subset of SP-1-regulated promoters [75]. However, the phosphorylation of Syk is much more complicated than simple activation as it occurs on multiple tyrosines, some of which modify the activity of the kinase directly through conformational changes and many of which serve dual roles as docking sites for proteins that contain SH2 or related phosphotyrosine-recognition domains.
3.1 Phosphorylation of tyrosine-130
Of the sites of tyrosine-phosphorylation on Syk that have been characterized, the one closest to the N-terminus is Y130 (numbered according to its position in the murine enzyme; this is Y131 in human Syk). This tyrosine is located in one of the three α-helices of linker A and is a prominent site of autophosphorylation in vitro [5]. It would be expected that this site is exposed to solution and available for phosphorylation based on the X-ray crystal structure of Zap-70, which has an equivalent tyrosine that also is a site of autophosphorylation [67,76]. The phosphorylation of Y130 can be detected at a low level on Syk isolated from cells following receptor crosslinking or at a higher level on Syk isolated from cells in which protein-tyrosine phosphatases have been inhibited [16,77]. The phosphorylation of Y130 or its replacement with glutamate to mimic phosphorylation has interesting effects on the activity of Syk as it both disrupts its interactions with the phosphorylated ITAMs of the BCR complex and enhances its catalytic activity [78]. The presence of an acidic amino acid at position 130 partially uncouples contacts between the two SH2 domains by disordering the structure of linker A [77]. The resulting more extended conformation of the tandem SH2 domains lacks the proper orientation for a high affinity, two-site interaction with a dually phosphorylated ITAM and instead exhibits a reduced binding affinity similar to that of a single SH2 domain. The disordering of linker A caused by the phosphorylation of Y130 likely also accounts for the elevated kinase activity as this would be expected to destabilize the interactions of linker A with linker B and the catalytic domain. Thus, the phosphorylation of Y130 and consequent decrease in affinity for ITAMs results in the release of a bound, but active, kinase from the receptor and decreases the ability of a phosphorylated, active kinase to bind to the receptor [78]. A physiological role for Y130-phosphorylation is not known, but it could contribute to the activation of Syk in response to oxidative stress since it is readily observed in cells treated with hydrogen peroxide [77,78]. The expression of a mutant form of Syk with Y130 replaced by glutamate enhances the ability of the kinase to bind to and phosphorylate centrosomal components in breast cancer cells and activates integrins through “inside-out” signaling in B cells [79,80]. Thus, this mutant can be a useful tool for identifying roles for active Syk in the absence of its association with an ITAM-bearing receptor.
3.2 Phosphorylation of tyrosine-290
The phosphorylation of Y290 (Y296 in human Syk) occurs in vitro during an autophosphorylation reaction [15] and has been reported also in a mass spectrometric analysis of proteins that are phosphorylated on tyrosine in lung cancer cells [81]. It does not appear to be a major site of phosphorylation and its modification has not yet been reported in intact B cells activated through the BCR. Y290 lies in the linker insert region of Syk, the section near the N-terminus of linker B that is missing from SykB or Zap-70. This linker insert is interesting in that it has been reported to affect the subcellular localization of Syk in breast epithelial cells by serving as a nuclear localization signal [82]. However, in B cells, a SykB-enhanced green fluorescent protein (EGFP) fusion protein or a Syk-EGFP fusion protein lacking the N-terminal 2/3 of linker B is still able to enter the nucleus due to the presence of a separate shuttling sequence located at the C-terminal end of linker B [73]. A role for the phosphorylation of Y290 has not been identified as the replacement of this residue with phenylalanine has no noticeable effect on the ability of Syk to signal through either FcεRI or the TCR [26]. Thus, it is unlikely that it is the phosphorylation of Y290 that underlies the intrinsic differences in the activities of Syk and SykB. Compared to Syk, SykB exhibits a reduced ability to bind to and mediate signaling from ITAM-bearing receptors [26].
3.3 Phosphorylation of tyrosine-317
Y317 (Y323 in human Syk) is in the linker B region and is a major site of autophosphorylation and of phosphorylation in intact B cells following engagement of the antigen receptor [15,16]. It has also been identified in mass spectrometric analyses of phosphoproteins in B cells and mast cells [83,84]. The analogous residue in Zap-70, Y292, is present in a flexible, disordered region of linker B not visible in the X-ray crystal structure [67]. Thus, Y317 is probably exposed, which is consistent with the rapid rate at which it is autophosphorylated in vitro [78]. However, a catalytically inactive Syk is phosphorylated on Y317 in cells that express Lyn and the phosphorylation of Y317 is reduced substantially in DT40 B cells that lack Lyn [16,85]. Thus, Src-family kinases are major contributors to the phosphorylation of this site in cells. The effects of phosphorylation at Y317 are interesting as they inhibit the ability of the kinase to signal from the BCR in B cells or from FcεRI in mast cells [16,85–88]. Thus, the substitution of Y317 with phenylalanine yields an enzyme with a greatly enhanced capacity to couple the BCR to the increased phosphorylation of several downstream targets including phospholipase C-γ2 (PLC-γ2) and the B cell linker protein/SH2 domain-containing leukocyte protein of 65 kDa (BLNK/SLP-65) (also known as BASH) [85]. As a consequence, the production of inositol 3,4,5-trisphosphate (IP3) is enhanced, which increases the amplitude and duration of calcium mobilization. A similar increase in the receptor-stimulated production of IP3 is observed in B cells that lack Lyn where the phosphorylation of Y317 is reduced as a consequence [85]. The replacement of Y292 of Zap-70 with phenylalanine also creates a gain-of-function mutant that enhances signaling from the TCR [89].
The phosphorylation of Syk on Y317 enhances its interactions with Casitas B-lineage lymphoma (Cbl)-family proteins. c-Cbl is the cellular homolog of v-Cbl, the product of the oncogene of the Cas NS-1 retrovirus, which causes pre- and pro-B cell lymphomas in infected mice[90]. As first described in mast cells, c-Cbl is an inhibitor of Syk-dependent signaling [91]. Several proximal BCR-stimulated signaling events, including the phosphorylation of Syk, Ig-α, PLC-γ and Vav-1 and the mobilization of calcium are elevated in B cells from mice lacking both c-Cbl and its homolog Cbl-b [92]. Both yeast two-hybrid analyses and pull-down assays indicate that the interaction of c-Cbl with Syk is enhanced by its phosphorylation on Y317 [86,93,94]. Furthermore, the overexpression of c-Cbl inhibits Syk-dependent signaling in B cells and the activity of Syk ectopically expressed in COS-7 cells in a manner dependent on the presence of Y317 [86,94].
The binding of c-Cbl and Cbl-b to pY317 of Syk is mediated by their N-terminal tyrosine kinase binding (TKB) domains. The TKB domain is constructed from three subdomains: a four-helix bundle, a calcium-binding EF hand and a variant SH2 domain. A consensus target sequence for the TKB domain has been identified as (N/D)XpY(S/T)XXP based on analyses of libraries of phosphopeptide ligands and the sequences of the binding regions of Cbl-interacting proteins [90,95,96]. The amino acid sequences surrounding pY317 in murine and human Syk are NPpYEPTG and NPpYEPEL, respectively. Phosphopeptides of this type are bound by the TKB domain in an overall orientation similar to that of a phosphopeptide bound to a conventional SH2 domain [97]. The asparagine (N) at position pY-2 makes major hydrogen bonding contacts not only with residues in the TKB domain, but also with one of the oxygens on the phosphate group of the phosphotyrosine [97]. This intrapeptidyl hydrogen bond is essential for binding. While it is not known if this intramolecular bond forms prior to or following the binding of the peptide to the TKB domain, it is interesting to note that the phosphorylation of Y317 results in a reduced electrophoretic mobility of Syk or of the isolated linker B region of Syk on an SDS-polyacrylamide gel suggesting that phosphorylation results is a local conformational change [16.98].
In addition to the TKB domain, Cbl-family proteins contain a Zn-binding RING finger domain and a C-terminal region containing multiple proline-rich sequences and several sites of tyrosine-phosphorylation that govern their interactions with a wide array of proteins that possess either SH3 or SH2 domains [90,96]. In general, TKB domains bind their targets with relatively low affinity—the Kd for the binding of a Syk-derived phosphopeptide to the c-Cbl domain is approximately 1.5 μM—and the C-terminal regions of the Cbl-proteins make major contributions to their interactions with activated tyrosine kinases [99,100]. In fact, interactions between Syk and c-Cbl have been described that are independent of pY317 and/or the TKB domain [86,91,93,101].
The RING finger domains recruit E2 ubiquitin conjugating enzymes and confer on Cbl-family proteins E3 ubiquitin ligase activity [102]. Consequently, the interaction of Cbl-family proteins with activated Syk leads to its ubiquitination [103,104]. The attachment of polyubiquitin chains to proteins by E3 ligases is well known to target proteins for degradation through the 26S proteosome; and, indeed, Cbl and/or ubiquitination-dependent decreases in the concentration of Syk have been described that can be reversed by the application to cells of proteosome inhibitors [79,103–107]. However, mono- and polyubiquitination can have many effects on proteins separate from proteosomal degradation. These include changes in enzymatic activity, the promotion or inhibition of protein-protein associations and alterations in protein trafficking [108]. For example, c-Cbl catalyzes the extensive monoubiquitination of the receptors for epidermal growth factor and platelet-derived growth factor leading to their internalization and trafficking to the lysosome [109,110]. Syk is extensively ubiquitinated following its activation via the engagement of the BCR in primary B cells or the collagen receptor GPVI in platelets [92,111]. This ubiquitination is abrogated in B cells lacking both c-Cbl and Cbl-b and in platelets lacking c-Cbl resulting in enhanced receptor-mediated activation of Syk and downstream signaling. However, in both cell types, Cbl-dependent ubiquitination occurs in the absence of any observable decrease in the level of Syk indicating a role for ubiquitination that is independent of protein degradation. In c-Cbl-deficient platelets, the phosphorylation of Syk following platelet aggregation is prolonged, suggesting that either Syk’s ubiquitination or its association with c-Cbl promotes its interactions with protein tyrosine phosphatases that would normally dephosphorylate and inactivate the kinase [111].
The recruitment of proteins to phosphorylated Y317 on Syk is not restricted to Cbl-family proteins. In our hands, the most commonly encountered binding partner for Syk in a yeast two-hybrid screen is p85, the regulatory subunit of phosphoinositide-3-kinase (PI3K) [98]. p85 has two SH2 domains that mediate its interactions with phosphorylated kinases or adaptor proteins to both redirect the lipid kinase within the cell—typically to the plasma membrane—and relieve inhibition of the constitutively associated 110 kDa catalytic subunit. The more C-terminal of the two SH2 domains of p85 interacts directly with Syk when it is phosphorylated on Y317 [98]. Despite the lack of similarity between the sequence of amino acids that surrounds Y317 and that of a classical ligand for a p85 SH2 domain (pYXXM), molecular modeling supports a reasonable fit between pY317 and the SH2 domain. In fact, this interaction is more robust than the interaction of Syk with the c-Cbl TKB domain [98]. Thus, the phosphorylation of Syk on Y317 by Syk itself or by Src-family kinases leads to the formation of a complex between Syk and PI3K.
While the activation of PI3K through the BCR in B cells is dependent on Syk [112], it is not clear whether or not the phosphorylation of Y317 plays an important role as p85 is well known to interact with other phosphorylated adaptor proteins such as BCAP, CD19, or Gab1 [113–115]. However, it is becoming increasingly clear that this interaction does play a positive role in a subset of Syk’s functions. For example, both Syk and PI3K are required for phagocytosis mediated by IgG-receptors that bear cytoplasmic ITAMs [116–120]. In COS cells transfected to express FcγRIIA, the phagocytosis of IgG-coated red blood cells requires the ectopic expression of catalytically active Syk, but is not supported by the expression of a Syk mutant lacking Y317 [98]. This Syk(Y317F) mutant also fails to support FcγRIIA-mediated activation of the kinase Akt, which is a downstream target of PI3K. Furthermore, the phagocytosis of opsonized particles through FcγR’s promotes a direct association between Syk and PI3K [121]. In differentiated, polarized HL-60 cells, Syk localizes to the lamellipodum at the leading edge of cells migrating on fibronectin and recruits PI3K p110δ to this location in an interaction dependent on Y317, suggesting an important role for a Syk-PI3K interaction in integrin-mediated leukocyte motility [122]. Also, the HIV Nef protein activates a Src-family kinase in the Golgi that phosphorylates Zap-70 on Y292 in T cells or Syk on Y317 in promonocytic cells to promote the binding and activation of PI3K leading to the down-regulation of cell surface MHC-1 [123]. In atypical myelodisplastic syndrome, a gene rearrangement generates a TEL-Syk fusion protein that is oligomerized, phosphorylated on tyrosine and bound to p85, leading to the constitutive activation of PI3K and Akt [124]. Syk-PI3K complexes also are important for the inflammatory response of neutrophils to monosodium urate crystals [125] and the internalization of human rhinovirus via ICAM-1 in epithelial cells [126].
Thus, both Cbl-family proteins and p85 have the capacity to bind to Syk when it is phosphorylated on Y317. In fact, competition between the two proteins for binding has been hypothesized to determine the pathway by which IgG-receptors internalize immune complexes of different sizes [121]. The phagocytosis of large, IgG-coated particles through FcγR’s requires PI3K activity and promotes a direct association between Syk and p85. In contrast, the endocytosis of small immune complexes does not require active PI3K and is accompanied by a decrease in the Syk-p85 interaction. This leads to the interesting hypothesis that phagocytosis proceeds with an interaction of PI3K with pY317 while endocytosis occurs in concert with an interaction of c-Cbl with pY317 [121].
3.4 Phosphorylation of tyrosines-342 and 346
Tyrosines 342 and 346 (348 and 352 in the human enzyme) lie within linker B and are major sites modified both through autophosphorylation in vitro and by phosphorylation in intact B cells following the crosslinking of the BCR [15,16]. In conjunction with Y317, these three tyrosines account for the bulk of the phosphorylation that occurs in the interdomain B region of Syk. Since Y342 and Y346 are present on the same tryptic peptide, it can be shown by peptide mapping or mass spectrometry that a single molecule of Syk can become phosphorylated on both sites [15,16,81,83,84,127]. BCR engagement leads to a mixture of forms of phosphorylated Syk, some modified within this region on only one of these two tyrosines and others phosphorylated on both. Studies in RBL-2H3 mast cells activated through FcεRI indicate that, of these two sites, Y342 is the predominant site of phosphorylation as determined through the use of phosphopeptide-specific antibodies [128]. Little or no phosphorylation of Syk on Y346 is observed in this system. However, several mass spectrometric analyses of phosphopeptides derived from a variety of cell types have identified both the dually phosphorylated tryptic peptide as well as a singly phosphorylated peptide, but the one modified on Y346 [81,83,84,127]. Also, Y346 is the predominant site of autophosphorylation in vitro [15]. Thus, more work needs to be done to establish firmly the conditions under which one or the other or both tyrosines become phosphorylated. Both sites on Syk can be phosphorylated following BCR crosslinking in DT40 B cells that lack Lyn; and the rate of autophosphorylation at these sites in vitro is enhanced by ITAM-binding [16,66]. However, both also can be phosphorylated on a catalytically inactive mutant of Syk expressed in cells where Lyn is present [16]. Thus, both autophosphorylation and phosphorylation in trans by a Src-family kinase can contribute to the modification of Y342 and Y346.
Unlike the phosphorylation of Y317, the modification of Y342 and Y346 plays a positive role in Syk-dependent signaling. Consequently, their elimination or replacement by phenylalanines reduces signaling downstream of ITAM-bearing receptors. In general, the elimination of both Y342 and Y346 has more profound effects on kinase function than the elimination of either single site. When expressed in B cells or mast cells, Syk mutants lacking both tyrosines exhibit a reduced ability to couple the BCR or FcεRI to the phosphorylation of PLC-γ and the mobilization of calcium [85,88,128,129]. In mast cells or RBL-2H3 cells expressing the double mutant, there is a decrease in the receptor-induced phosphorylation of LAT and SLP-76 [88,128] and in mast cells from Syk-deficient mice, a decrease in the FcεRI-stimulated phosphorylation of Vav-1 [88]. The elimination of each individual tyrosine has both redundant and unique effects. The replacement of Y342 with phenylalanine results in a more substantial decrease in the receptor-stimulated phosphorylation of PLC-γ in B cells and mast cells and of LAT, SLP-76 and Vav-1 in mast cells than does the replacement of Y346 [88,128,129]. In contrast, the elimination of Y346 more strongly decreases the FcεRI-stimulated phosphorylation of ERK and Akt in primary mast cells than does the elimination of Y342 [88]. Zap-70 has an analogous pair of tyrosines at positions 315 and 319 that also are important for its activity. Zap-70 lacking Y315 is unable to restore BCR-stimulated signaling to Syk-deficient DT40 B cells and Zap-70 lacking Y319 is unable to mediate TCR-dependent signaling in Jurkat T cells [130–132]. Elimination of either Y315 or Y319 impairs both positive and negative selection of T cells in mice [133,134].
Many of the signaling defects exhibited by Syk mutants in which Y342 and Y346 have been replaced with phenylalanines can be reversed by the simultaneous elimination of the inhibitory tyrosine at Y317. Consequently forms of Syk with all three tyrosines (Y317, Y342 and Y346) replaced by phenylalanines can reconstitute much of BCR- or FcεRI-mediated signaling when expressed in Syk-deficient B cells or mast cells, respectively [85,88]. In turn, Zap-70 can reconstitute many aspects of TCR-stimulated signaling in T cells even if the entire linker B region is eliminated [135]. However, even in the absence of Y317, maximal signaling by Syk in B cells still requires Y342 and Y346 [85]. This dependence is especially apparent when low doses of activating anti-IgM antibody are used to crosslink the BCR or if alternative pathways such as those mediated through PI3K are inhibited. Under these conditions, the phosphorylation of Y342 and Y346 are needed for a robust receptor-mediated phosphorylation of PLC-γ2, generation of IP3 and mobilization of calcium.
The phosphorylation of Y342 and Y346 enhances signaling both by increasing the activity of Syk and by generating docking sites that mediate protein-protein interactions. Several proteins, including PLC-γ, Vav-1 and Vav-2, the Src-family kinases Lck and Fgr, the p85 subunit of PI3K and Grb2, have SH2 domains that can that bind to one or both of these phosphotyrosines. Interestingly, these residues act individually and in concert to determine the identity of the protein that binds, with some proteins binding when only one or the other tyrosine is phosphorylated and others binding preferentially when both are modified. The interaction between Syk and PLC-γ1 or 2 is mediated by the C-terminal SH2 domain of the phospholipase [136–138] and is blocked when both Y342 and Y346 are substituted with phenylalanines as first shown for an interaction between PLC-γ1 and a CD8-Syk fusion protein [138]. In vitro phosphopeptide-binding assays indicate that the PLC-γ SH2 domain actually binds when both Y342 and Y346 are phosphorylated and binds poorly when only a single site is modified [129].
Guanine nucleotide-exchange factors of the Vav-family interact with Syk in a variety of cell types [139–142]. Binding of the Vav-1 SH2 domain is nearly completely disrupted by the replacement of Y342 with phenylalanine [139]. Consequently, the replacement of Y342 inhibits the recruitment of Vav-1 to Syk localized to lamellipodia of migrating, differentiated HL-60 cells and inhibits the phosphorylation of Vav-1 in mast cells [88,142]. The substitution of Y346 also decreases the binding of Vav-1, but to a lesser extent (60%), and the replacement of both tyrosines essentially eliminates the interaction [139]. Phosphopeptide-binding assays indicate that Vav-1 can interact with a peptide containing only pY342, fails to bind one containing only pY346, but interacts most strongly with a peptide containing both phosphotyrosines [129].
Lck, which binds to Zap-70 in T cells [131,143], and Fgr, which binds Syk in monocytes to inhibit β2 integrin-mediated cell spreading [144], exhibit a similar preference for peptide ligands containing phosphotyrosines at both Y342 and Y346 [129]. In contrast, Grb2 binds preferentially to a Syk-derived phosphopeptide modified only on Y346, which may help explain why the deletion of this tyrosine negatively affects the coupling of FcεRI to the activation of ERK in mast cells [88,129]. Thus, the nature of the proteins capable of binding to Syk is dependent on the site of phosphorylation as well as the stoichiometry of phosphorylation within the linker B region.
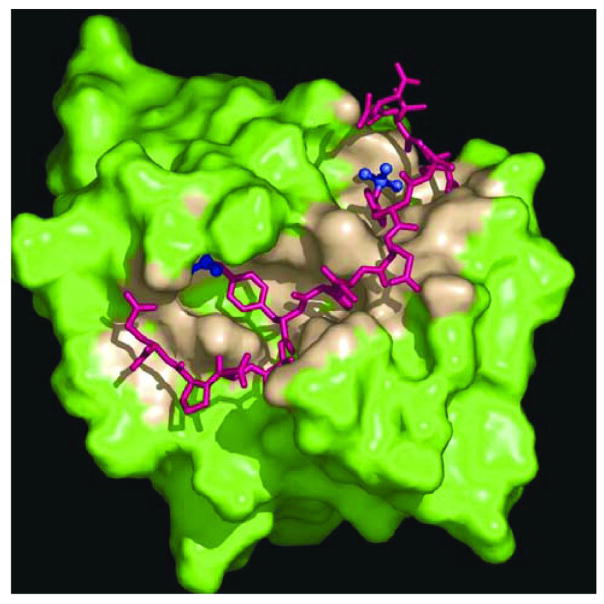
A structural analysis of the C-terminal SH2 domain of PLC-γ1 bound to a dually phosphorylated peptide derived from Syk linker B explains the molecular basis for the unusual interaction of a single SH2 domain with a site containing two phosphotyrosines [129]. The SH2 domain binds pY342 in the canonical phosphotyrosine-binding pocket that is common to all SH2 domains. However, it then undergoes a substantial conformational change to accommodate the pY346 residue within a second phosphotyrosine-binding pocket. Two lysine residues from the SH2 domain, K54 and K56, interact with pY346 within this second pocket, which actually buries more of the phosphotyrosine residue than is buried in the primary pocket (Fig. 2). The formation of this second phosphotyrosine-binding site increases the affinity of the SH2 domain for the phosphopeptide over sevenfold. Of these two lysines, K56 is conserved in the sequences of all of the SH2 domains known to be capable of binding this region of Syk when it contains two phosphotyrosines, suggesting that it is particularly important for forming this second binding pocket (Table 1). Consistent with this idea, substitution of K56 with a glutamine results in a sevenfold reduction in the affinity of the PLC-γ1 SH2 domain for the dually phosphorylated peptide. An analogous lysine is absent from the SH2 domains of Grb2 and of a number of other domains that do not interact strongly with a phosphopeptide containing both pY342 and pY346 [129].
Fig. 2.
A stick representation (magenta) of the Syk linker B peptide showing the two phosphoryl groups by ball-and-sticks (blue). The pY342 residue is on the right and pY346 on the left. The SH2 domain is shown using a solvent accessible surface representation where the contact region within 5 angstrom of the peptide is colored tan.
Table 1.
SH2 domains that have been tested for their ability to bind to a dually phosphorylated peptide containing both pY342 and pY346. The position of K65 is underlined.
| SH2 domain | Sequence | Binding |
|---|---|---|
| Grb2 | NDVQHFKVLRDGAG | No |
| Abl | GRVYHYRINTASDG | No |
| Fgr | DHVKHYKIRKLDMG | Yes |
| Lck | EVVKHYKIRNLDNG | Yes |
| SHP1N | DQVTHIRIQNSGDF | No |
| SHP1C | LRVTHIKVMCEGGR | No |
| p85N | GNNKLIKIFHRD-G | Yes |
| Vav1 | VEVKHIKIMTAE-G | Yes |
| Vav3 | NEAKHIKILTRD-G | Yes |
| PLC-γN | GKVQHCRIHSRQDA | No |
| PLC-γC | GKIKHCRVQQE--G | Yes |
The phosphorylation of Y342 and Y346 also enhances the catalytic activity of Syk and likely accounts for much of the ability of Lyn to activate the enzyme. The phosphorylation of the analogous tyrosines on Zap-70 is catalyzed by Lck and is an important step in the activation of Zap-70 following engagement of the TCR [67,145]. The ability of Lck to phosphorylate and activate Zap-70 is abrogated by the replacement of Y315 and Y319 with phenylalanine [145]. In contrast, direct phosphorylation by Lck is not needed for the activation of a Zap-70 mutant in which both tyrosines are replaced by alanines. In the X-ray crystal structure of Zap-70, both Y315 and Y319 have been replaced by phenylalanines, which participate in aromatic-aromatic interactions with residues in both linker A and the catalytic domain [67]. Unlike phenylalanines or perhaps tyrosines, neither alanines nor phosphotyrosines would be able to stabilize the linker-kinase sandwich that preserves the autoinhibited form of the kinase. Thus, phosphorylation on these residues would lead to the activation of the kinase. A similar mechanism is suggested for Syk since the replacement of Y342 and Y346 with phenylalanines reduces its ability to phosphorylate LAB/NTAL when it is co-expressed with the kinase in HEK 293T cells [145].
3.5 Phosphorylation of tyrosines-519 and 520
Tyrosines 519 and 520 (Y525 and 526 in the human enzyme) are located in the activation loop within the catalytic domain. Both residues are phosphorylated in an in vitro autophosphorylation reaction and in B cells following engagement of the BCR [15,16]. Syk also is phosphorylated on both activation loop tyrosines following clustering of FcεRI in mast cells [146,147]. We were unable to detect the phosphorylation of either Y519 or Y520 of a catalytically inactive mutant of Syk in B cells expressing Lyn suggesting that Syk itself is the major catalyst of activation loop phosphorylation [16]. However, in mast cells some Lyn-dependent phosphorylation of the activation loop has been observed and is thought to be important for the initial activation of Syk; and then the bulk of the phosphorylation is catalyzed by Syk itself [147].
The activation loops of many protein kinases contain residues whose phosphorylation leads to the upregulation of kinase activity most often through alterations in substrate binding and/or enhancement of the rate of phosphoryl transfer [148]. Thus, for many kinases, catalytic activity is profoundly affected by the status of phosphorylation of the activation loop. For Syk, however, the situation is less clear. Mutations in one or both of these tyrosines have little or no effect on the catalytic activity of the enzyme when measured in an in vitro kinase assay [37, 149–151]. For example, the kinetics of substrate phosphorylation catalyzed by the kinase domain of unphosphorylated Syk are indistinguishable from those of either Syk pre-phosphorylated on the activation loop tyrosines or Syk that has both tyrosines replaced by phenylalanine [151]. These observations are consistent with the orientation of the activation loop in the X-ray crystal structure of the catalytic domain of Syk [152]. In this structure, the activation loop adopts the same “loop-out” conformation that is typically observed in active protein-tyrosine kinases with phosphorylated activation loops. Similarly, the activation loop of the isolated catalytic domain of Zap-70 is in the activated conformation [153]. Thus, the unphosphorylated activation loop of Syk does not appear to restrict access to the ATP or substrate binding pockets and does not disturb the proper orientation of important catalytic residues within the active site.
Despite the apparent lack of involvement of Y519 and Y520 in regulating the catalytic activity of Syk, mutant forms of the kinase in which these residues have been replaced with phenylalanines still exhibit major signaling deficits in cells [37,149, 150,154]. In Syk-deficient DT40 B cells reconstituted with a form of Syk lacking both activation loop tyrosines, the BCR-stimulated phosphorylation of cellular proteins is reduced and the phosphorylation of PLC-γ2 is abrogated [37]. Consequently, BCR ligation fails to enhance the production of IP3 and mobilization of calcium (however, this defect is less robust when higher levels of the mutant kinase are expressed [155]). Defective signaling also is observed in RBL-2H3 mast cells expressing the Syk double mutant, which cannot support the FcεRI-induced phosphorylation of cellular proteins—including PLC-γ2—or histamine release [150]. Thus, there is an intriguing lack of correlation between the effects of activation loop-phosphorylation in vitro and in intact cells. One interesting suggestion is that the phosphorylation of Y519 and Y520 generates a binding site for one or more intracellular proteins such that the effects of this phosphorylation on these interactions would be observed only in cells and not in purified preparations of the kinase [149,150,154].
3.6 The C-terminal tyrosines of Syk
Three adjacent tyrosines (Y623, 624 and 625 in murine Syk; Y629, Y630 and Y631 in the human enzyme) are located near the C-terminus of the protein. Two of these, Y624 and Y625, are phosphorylated, albeit at a relatively low initial rate, during an autophosphorylation reaction in vitro [15,16]. We did not detect these in our initial analysis of phosphorylation sites modified in cells following BCR engagement [16]. However, the phosphorylation of Syk on Y624 does occur in a model of BCR signaling reconstituted in S2 insect cells as detected by a phospho-specific antibody [71]. Furthermore, an analysis of the phosphoproteome of mast cells identified a doubly phosphorylated peptide derived from Syk that was modified on both Y624 and 625 [83]. The level of this phosphopeptide increased about two-fold following activation of cells through the high affinity IgE-receptor.
A pair of tyrosines (Y597 and 598) are located in an analogous position near the C-terminus of Zap-70. These lie in an α-helix within the large C-terminal lobe of the catalytic domain and play an important role in the formation of the kinase domain/linker A interaction that stabilizes the autoinhibited form of the kinase. These two tyrosines form a cleft that accommodates the side chain of a proline residue from linker A [67]. Disruption of this interaction occurs if both residues are replaced by phenylalanines resulting in the activation of the kinase [67,71,156]. The replacement of the three C-terminal tyrosines of Syk also results in a gain-of-function mutant suggesting a similar role for these residues [156]. The efficient phosphorylation of these tyrosines likely would require prior disassembly of the linker-kinase sandwich either through the autophosphorylation of linker B tyrosines or the binding of the tandem SH2 domains to a phosphorylated ITAM. Likewise, the phosphorylation of the C-terminal tyrosines would be expected to stabilize the more active, open conformation of the kinase by disrupting the interaction of linker A with the catalytic domain.
The sequence surrounding pY624, pYYDV, matches that of a ligand for the SH2 domains of adaptor proteins of the SLP-family [157]. In B cells, BLNK/SLP-65 is a major substrate of Syk whose phosphorylation generates docking sites for a variety of effectors of BCR signaling including PLC-γ2 and Bruton’s tyrosine kinase (Btk) [157–163]. The formation of this protein complex or “signalsome” alters the subcellular localization of and enhances the phosphorylation and activation of PLC-γ2 to generate IP3 and diacylglycerol leading to the activation of protein kinase C and the mobilization of calcium. BLNK/SLP-65 is associated with the plasma membrane in an interaction requiring its N-terminal leucine zipper motif [164] and is recruited to the BCR via its SH2 domain binding to the phosphorylated, non-ITAM tyrosine Y204 of Ig-α [165,166]. This interaction is important for the initial phase of calcium mobilization that follows clustering of BCR complexes [71]. The activation of Syk and its subsequent phosphorylation on Y624 generates another docking site for the SH2 domain of BLNK/SLP-65 [71]. The resulting physical interaction between Syk and its substrate maintains the kinase in an active conformation and results in the retention of active Syk at the plasma membrane where it can persist even following BCR internalization [71,167]. The resulting complex provides for the signals required for sustained calcium influx. Interestingly, the amino acids surrounding the analogous C-terminal tyrosines of Zap-70 are not consensus sequences for binding BLNK/SLP-65. Consequently, the TCR can signal through ectopically expressed BLNK/SLP-65 only if Syk is co-expressed [167]. In normal T cells that lack BLNK/SLP-65, signaling through the TCR via Zap-70 is transduced instead by a combination of SLP76 (SH2-domain-containing leukocyte protein of 76 kDa) and LAT (linker for activation of T-cells) [168].
4. Dephosphorylation of Syk
The phosphorylation of Syk following receptor clustering occurs rapidly due to its activation, its association with the receptor and Lyn and the transient inhibition of protein-tyrosine phosphatases resulting from BCR-stimulated increases in H2O2 [169–171]. Once the activity of Syk is terminated, both the kinase and its substrates are rapidly dephosphorylated and the activation of downstream signaling pathways ends [70]. Thus, the continued activity of Syk is necessary to maintain the kinase in an active state. The full identity of the repertoire of protein tyrosine phosphatases that regulate the state of phosphorylation of Syk is most likely not completely known, but several enzymes that alter the state of phosphorylation of the kinase have been identified. SH2 domain-containing protein tyrosine phosphatase-1 (SHP-1) is recruited to immunoreceptor tyrosine-based inhibitor motifs (ITIMs; which have a consensus sequence I/L/V-X-pY-X-X-L/V) on inhibitory receptors such as CD22 or PIR-B following their phosphorylation by Lyn [172–177]. This accounts, in part, for the inhibitory effects of Lyn’s expression on B cell signaling as the receptor-associated SHP-1 becomes activated and attenuates BCR signaling by dephosphorylating multiple components the BCR-signaling network including Syk. In fact, a direct interaction between SHP-1 and Syk has been reported [178,179]. Consequently, the expression of a dominant-negative mutant of SHP-1 leads to the hyperphosphorylation of Syk [178]. Syk also is a direct substrate of protein tyrosine phosphatase receptor–type O truncated (PTPROt), a phosphatase whose expression is regulated during B cell development [180]. An inactive, substrate-trapping mutant of PTPROt binds phosphorylated Syk from lysates of pervanadate-treated cells. Interestingly, the overexpression of PTPROt inhibits the BCR-stimulated phosphorylation of Syk and attenuates signaling initiated through BCR engagement as well as the tonic BCR signaling that is required for lymphoma cell proliferation. The proline-, glutamic acid-, serine- and threonine-enriched protein tyrosine phosphatase, PEP, also has been implicated as a negative regulator of Syk-family kinases [181]. PEP associates with the SH3 domain of Csk (C-terminal Src kinase), a negative regulator of Src-family kinases and of T and B cell signaling. The enhanced expression of PEP in T cells suppresses signaling from the TCR and reduces the phosphorylation of Zap-70. A substrate-trapping mutant of PEP binds directly to tyrosine-phosphorylated Zap-70. Syk also has been reported to bind and serve as a substrate for T-cell ubiquitin ligand-2/suppressor of T-cell receptor signaling, TULA-2/STS-2, which is an unconventional phosphatase with a catalytic domain related to the active site of phosphoglyceromutase [182]. The state of tyrosine-phosphorylation of Syk is reduced in cells overexpressing TULA-2, but is enhanced in cells overexpressing TULA-1, an inactive and putative dominant negative inhibitor of TULA-2. Finally, Zap-70 also serves as a substrate for the low molecular weight phosphotyrosine phosphatase LMPTP, in an interaction that actually stimulates the kinase through the selective dephosphorylation of the inhibitory pY292 [183]. Thus, it appears likely that Syk is subject to the regulatory influences of multiple phosphatases that can catalyze its dephosphorylation, which may account for the very rapid loss of phosphotyrosine from the kinase that occurs following its inhibition [70].
5. Substrate specificity
Once activated, Syk catalyzes the phosphorylation of multiple protein substrates that are important for transducing the antigen-receptor interaction into the appropriate physiological response [184]. The consequences of a protein’s phosphorylation by Syk vary depending on the nature of the substrate and the site that is modified. For a subset of Syk’s substrates, the addition of a phosphate group induces conformational changes that lead to alterations in the intrinsic activity of the phosphorylated protein. The phosphorylation on tyrosine of PLC-γ2, Btk, hematopoietic progenitor kinase-1 (HPK1) and the Vav1 guanine nucleotide exchange factor leads to their activation [185–192]. For many substrates, phosphorylation on tyrosines instead promotes protein-protein associations by generating docking sites that are recognized by proteins that have SH2 domains or other phosphotyrosine-binding motifs. In fact, it has been observed that Syk demonstrates a preference for the phosphorylation of tyrosines within motifs that can then be recognized by group I SH2 domains [193]. Thus, the phosphorylation of many substrates for Syk including BLNK/SLP-65, LAB/NTAL/LAT2, 3BP2, BCAP, BANK and GCET generates scaffolds for the assembly of larger signaling complexes [114,194–203]. For example, the phosphorylation of BLNK/SLP-65, a major Syk substrate in B cells, creates docking sites that bind Btk and PLC-γ to generate a protein complex that regulates the mobilization of calcium [157–163].
It is less well recognized that phosphorylation also can inhibit rather than promote protein-protein associations. For example, in red blood cells the acidic cytoplasmic tail of the anion transport channel protein, band 3, binds to and inhibits the activities of several of the glycolytic enzymes including aldolase and glyceraldehyde-3-phosphate dehydrogenase (G3PDH). The phosphorylation of Y8 on band 3 by Syk blocks these interactions and relieves the inhibition [204]. Structural analyses indicate that the region surrounding Y8 forms a loop with the tyrosine located in the center where it interacts with the hydrophobic side chains of neighboring amino acids [205,206]. The side chains of the surrounding acidic amino acids are oriented outward and mediate electrostatic interactions with basic residues in the active sites of both aldolase and G3PDH to inhibit their activities. The phosphorylation of this tyrosine by Syk destabilizes the loop through electrostatic repulsion and abrogates the band 3/enzyme interactions. This type of loop structure has been termed a phosphorylation sensitive interaction (PSI) loop. It is reasonable to speculate that this mechanism will be important to the regulation of additional protein-protein interactions. It is interesting to note, for example, that the acidic C-terminus of α-tubulin also binds glycolytic enzymes and is an excellent substrate for Syk [72,207].
Many of the substrates of Syk can be recruited directly to the kinase or to kinase-adaptor or kinase-receptor complexes through interactions mediated by structural motifs such as SH2 or SH3 domains. These interactions that occur outside of Syk’s active site serve to bring the kinase and substrates together in close proximity. However, the specific tyrosine that is phosphorylated is also determined by the nature of the amino acids in its immediate vicinity. Syk exhibits a marked preference for the phosphorylation of tyrosines surrounded by multiple acidic amino acids. This was first demonstrated for band 3, which Syk readily phosphorylates both in vitro and in intact erythrocytes [11,204,208]. The highly acidic, cytoplasmic fragment of band 3 or substrates based on its sequence are frequently used in kinase assays for the detection and quantification of Syk’s catalytic activity. Studies on the specificity of Syk using as substrates members of a phage display library identify a preferred recognition sequence of E/DE/DEE/DYEE, consistent with a preference for tyrosines embedded within highly acidic sequences [209]. For several of Syk’s protein substrates, phosphorylation sites with these characteristics also have been identified (Table 2). A comparison of these sites yields a consensus sequence consistent with the results of the peptide library screen. Thus, the same substrate specificity for Syk extends to both peptides and proteins.
Table 2.
Syk substrates and sites of phosphorylation
| Substrate | Site(s) | Ref | Substrate | Site | Ref |
|---|---|---|---|---|---|
| 3BP2 | YPMDNEDYEHEDEDD | 201 | GS | PEEDGERYDEDEEAA | 213 |
| HEDEDDSYLEPDSPG | HPK1 | LSDSDDDYDDVDIPA | 189 | ||
| EEDSDEDYEKVPLPN | HS1 | EPEPENDYEDVEEMD | 214–216 | ||
| Band 3 | MEELQDDYEDMMEEN | 208, 210 | EDEPEGDYEEVLEPE | ||
| ENLEQEEYEDPDIPE | LAB | EDDDANSYENVLICK | 217 | ||
| AKPDSSFYKGLDLNG | EDEESEDYQNSASIH | ||||
| EEEGRDEYDEVAMPV | EEDGEPDYVNGEVAA | ||||
| BLNK/SLP65 | SDDFDSDYENPDEHS | 158, 163 | PKCA | SDFEGFSYVNPQFVH | 218 |
| EHSDSEMYVMPAEEN | PLC-γ1 | IGTAEPDYGALYEGR | 173 | ||
| EENADDSYEPPPVEQ | SLP76 | SSFEEDDYESPNDDQ | 219 | ||
| LLEDEADYVVPVEDN | DGEDDGDYESPNEEE | ||||
| VEDNDENYIHPTESS | SYNA | VDPDNEAYEMPSEEG | 220 | ||
| Btk | RYVLDDEYTSSVGSK | 188 | EMPSEEGYQDYEPEA | ||
| Cbl | EGEEDTEYMTPSSRP | 211 | SEEGYQDYEPEA | ||
| SENEDDGYDVPKPPV | TUBA | MAALEKDYEEVGVDS | 72 | ||
| CD79a | EYEDENLYEGLNLDD | 53 | Vav1 | EAEGDEIYEDLMRSE | 193 |
| NLDDCSMYEDISRGL | |||||
| GCET2 | GNSAEEYYENVPCKA | 203 | Consensus | EXXDEEDYEXPXEPX | |
| FcγRIIA | LGGTETEYSLLHMPS | 212 | |||
| LEETNNDYETADGGY | |||||
| TDDDKNIYLTLPPND |
Abbreviations: 3BP2, SH3-domain binding protein 2; Band 3, erythrocyte anion transport channel; BLNK/SLP-65, B cell linker protein/SH2 domain-containing leukocyte protein of 65 kDa; Btk, B Bruton’s tyrosine kinase; Cbl, Casitas B-lineage lymphoma protein; GCET2, germinal center expressed transcript 2; GS, glycogen synthase; HPK1, hematopoietic progenitor kinase-1; HS1, hematopoietic lineage cell-specific protein-1; LAB, linker for activation of B cells; PKCA, protein kinase C-α; PLC-γ1; phospholipase C-γ1; SYNA, α-synuclein; TUBA, α-tubulin.
7. Conclusions
Aggregation of the B cell antigen receptor leads to its association with Lyn and the subsequent phosphorylation of the first ITAM tyrosines of Ig-α and Ig-β. The recruitment of Syk, likely via its C-terminal SH2 domain, to the phosphorylated ITAM allows Syk to catalyze the phosphorylation of the more C-terminal tyrosines of each ITAM allowing the recruitment of additional Syk molecules to the clustered BCR complexes. This binding of Syk to the dually phosphorylated ITAM and its phosphorylation by Lyn and/or its autophosphorylation on Y342 and Y346 in linker B (and perhaps of Y519 and 520 in the activation loop) fully activates the kinase. Depending on the sites that are phosphorylated and the stoichiometry of their phosphorylation, the phosphotyrosines within linker B serve as sites for protein-protein interactions that help to amplify weak signals. The phosphorylation of Y317 by Lyn, in turn, dampens signaling in a Cbl-dependent manner, but is important for signaling to PI3K through other receptors involved in such processes as phagocytosis and motility. Once activated, Syk phosphorylates protein substrates on tyrosines located within highly acidic regions. These include enzymes whose activity is enhanced as well as adaptor proteins whose phosphorylation promotes the assembly of signaling complexes through the recruitment of proteins containing phosphotyrosine-interacting domains. The binding of one of these adaptor proteins, BLNK/SLP-65, to the phosphorylated C-terminal tyrosines of Syk anchors the active kinase to the plasma membrane to promote more extended signaling. Activated Syk can also dissociate from the receptor and appear in an active form in locations within the cell other than the plasma membrane including the nucleus. The phosphorylation of Y130 provides one mechanism for this dissociation. Signaling is terminated through the down-regulation of membrane-bound receptors and through the dephosphorylation of Syk and its substrates by one or more of several candidate phosphatases. Thus, multiple factors act in concert to influence the activity of Syk in order to regulate the quality and quantity of the signal that is sent from the BCR, which ultimately determines the physiological outcome of receptor engagement.
Acknowledgments
Work on Syk in B cells has been supported by United States Public Health Services grant CA037372 awarded by the National Cancer Institute. The author thanks Carol B. Post, Purdue University, for the generating the structure and drawing shown in Fig. 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Campbell KS, Cambier JC. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide-linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990;9:441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cambier JC, Pleiman CM, Clark MR. Signal transduction by the B cell antigen receptor and its coreceptors. Annu Rev Immunol. 1994;12:457–486. doi: 10.1146/annurev.iy.12.040194.002325. [DOI] [PubMed] [Google Scholar]
- 3.Gold MR, Law DA, DeFranco AL. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990;345:810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- 4.Campbell MA, Sefton BM. Protein tyrosine phosphorylation is induced in murine B lymphocytes in response to stimulation with antiimmunoglobulin. EMBO J. 1990;9:2125–2131. doi: 10.1002/j.1460-2075.1990.tb07381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 6.Skaggs BJ, Clark MR. Proximal B cell receptor signaling pathways. Signal Transduction. 2004;5–6:173–194. [Google Scholar]
- 7.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VLJ. Perinatal lethality and a block in the development of B cells in mice lacking the tyrosine kinase p72syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;16:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 10.Cornall RJ, Cheng AM, Pawson T, Goodnow CC. Role of Syk in B-cell development and antigen-receptor signaling. Proc Natl Acad Sci USA. 2000;97:1713–1718. doi: 10.1073/pnas.97.4.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zioncheck TF, Harrison ML, Geahlen RL. Purification and characterization of a protein-tyrosine kinase from bovine thymus. J Biol Chem. 1986;261:15637–15643. [PubMed] [Google Scholar]
- 12.Kobayashi T, Nakamura S-i, Taniguchi T, Yamamura H. Purification and characterization of a cytosolic protein-tyrosine kinase from porcine spleen. Eur J Biochem. 1990;188:535–540. doi: 10.1111/j.1432-1033.1990.tb15433.x. [DOI] [PubMed] [Google Scholar]
- 13.Zioncheck TF, Harrison ML, Isaacson C, Geahlen RL. Generation of an active lymphocyte protein-tyrosine kinase by proteolysis. J Biol Chem. 1988;263:19195–19202. [PubMed] [Google Scholar]
- 14.Taniguchi T, Kobayashi T, Kondo J, Takahashi K, Nakamura H, Suzuki J, Nagai K, Yamada T, Nakamura S, Yamamura H. Molecular cloning of a porcine gene Syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991;266:15790–15796. [PubMed] [Google Scholar]
- 15.Furlong MT, Mahrenholz AM, Kim KH, Ashendel CL, Harrison ML, Geahlen RL. Identification of the major sites of autophosphorylation of the murine protein-tyrosine kinase Syk. Biochim Biophys Acta. 1997;1355:177–190. doi: 10.1016/s0167-4889(96)00131-0. [DOI] [PubMed] [Google Scholar]
- 16.Keshvara LM, Isaacson C, Yankee TM, Sarac R, Harrison ML, Geahlen RL. Syk- and Lyn-dependent phosphorylation of syk on multiple tyrosines following B-cell activation includes a site that negatively regulates signaling. J Immunol. 1998;161:5276–5283. [PubMed] [Google Scholar]
- 17.Hutchcroft JE, Harrison ML, Geahlen RL. B Lymphocyte activation is accompanied by phosphorylation of a 72 kda protein-tyrosine kinase. J Biol Chem. 1991;266:14846–14849. [PubMed] [Google Scholar]
- 18.Hutchcroft JE, Harrison ML, Geahlen RL. Association of the 72 kDa protein-tyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992;267:8613–8619. [PubMed] [Google Scholar]
- 19.Yamada T, Taniguchi T, Yang C, Yasue S, Saito H, Yamamura H. Association with B-cell antigen receptor with protein-tyrosine kinase p72syk and activation by engagement of membrane IgM. Eur J Biochem. 1993;213:455–459. doi: 10.1111/j.1432-1033.1993.tb17781.x. [DOI] [PubMed] [Google Scholar]
- 20.Yanagi S, Inatome R, Takano T, Yamamura H. Syk expression and novel function in a wide variety of tissues. Biochem Biophys Res Commun. 2001;288:495–498. doi: 10.1006/bbrc.2001.5788. [DOI] [PubMed] [Google Scholar]
- 21.Ulanova M, Puttagunta L, Marcet-Palacios M, Duszyk M, Steinhoff U, Duta F, Kim MK, Indik ZK, Schreiber AD, Befus AD. Syk tyrosine kinase participates in β1-integrin signaling and inflammatory responses in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L497–L507. doi: 10.1152/ajplung.00246.2004. [DOI] [PubMed] [Google Scholar]
- 22.Yagi S, Suzuki K, Hasegawa A, Okumura K, Ra C. Cloning of the cDNA for the deleted syk kinase homologous to ZAP-70 from human basophilic leukemia cell line (KU812) Biochem Biophys Res Commun. 1994;200:28–34. doi: 10.1006/bbrc.1994.1409. [DOI] [PubMed] [Google Scholar]
- 23.Rowley RB, Bolen JB, Fargnoli J. Molecular cloning of rodent p72syk. Evidence of alternative mRNA splicing. J Biol Chem. 1995;270:12659–12664. doi: 10.1074/jbc.270.21.12659. [DOI] [PubMed] [Google Scholar]
- 24.Latour S, Chow LML, Veillette A. Differential intrinsic enzymatic activity of Syk and Zap-70 protein tyrosine kinases. J Biol Chem. 1996;271:22782–22790. doi: 10.1074/jbc.271.37.22782. [DOI] [PubMed] [Google Scholar]
- 25.Latour S, Zhang J, Siraganian RP, Veillette A. A unique insert in the linker domain of Syk is necessary for its function in immunoreceptor signaling. EMBO J. 1998;17:2584–2595. doi: 10.1093/emboj/17.9.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 27.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- 28.Gold MR, Matsuuchi L, Kelly RB, DeFranco AL. Tyrosine phosphorylation of components of the B-cell antigen receptors following receptor crosslinking. Proc Natl Acad Sci USA. 1991;88:3436–3440. doi: 10.1073/pnas.88.8.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cambier JC. New nomenclature for the Reth motif (or ARH1/TAM/ARAM/YXXL) Immunol Today. 1995;16:110. doi: 10.1016/0167-5699(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 30.Cambier JC. Antigen and Fc receptor signaling. The awesome power of the lmmunoreceptor tyrosine- based activation motif (ITAM) J Immunol. 1995;155:3281–3285. [PubMed] [Google Scholar]
- 31.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 32.Rozsnyay Z, Sarmay G, Zoller M, Gergely J. Membrane-bound ezrin is involved in B-cell receptor-mediated signaling: potential role of an ITAM-like ezrin motif. Immunol Lett. 1996;54:163–169. doi: 10.1016/s0165-2478(96)02667-3. [DOI] [PubMed] [Google Scholar]
- 33.Urzainqui A, Serrador J, Viedma F, Yáñez-Mó M, Rodríguez A, Corbí A, Alonso-Lebrero J, Luque A, Deckert M, Vázquez J. ITAM-based interaction of ERM proteins with Syk mediates signaling by the leukocyte adhesion receptor PSGL-1. Immunity. 2002;17:401 – 412. doi: 10.1016/s1074-7613(02)00420-x. [DOI] [PubMed] [Google Scholar]
- 34.Mócsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nature Immunol. 2006;7:1326 – 1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grande SM, Bannish G, Fuentes-Panana EM, Katz E, Monroe JG. Tonic B-cell and viral ITAM signaling: context is everything. Immunol Rev. 2007;218:214–234. doi: 10.1111/j.1600-065X.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 36.Underhill DM, Goodridge HS. The many faces of ITAMs. Trends Immunol. 2007;28:66–73. doi: 10.1016/j.it.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Kurosaki T, Johnson SA, Pao L, Sada K, Yamamura H, Cambier JC. Role of the Syk authophosphorylation site and SH2 domains in B cell antigen receptor signaling. J Exp Med. 1995;182:1815–1823. doi: 10.1084/jem.182.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ottinger EA, Botfield MC, Shoelson SE. Tandem SH2 domains confer high specificity in tyrosine kinase signaling. J Biol Chem. 1998;273:729–735. doi: 10.1074/jbc.273.2.729. [DOI] [PubMed] [Google Scholar]
- 39.Koch CA, Anderson D, Moran MF, Ellis C, Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991;252:668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- 40.Lanier LL. Viral immunoreceptor tyrosine-based activation motif (ITAM)-mediated signaling in cell transformation and cancer. Trends Cell Biol. 2006;16:388–390. doi: 10.1016/j.tcb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Tomlinson CC, Damania B. Critical role for endocytosis in the regulation of signaling by the Kaposi’s sarcoma-associated herpesvirus K1 protein. J Virol. 2008;82:6514–6523. doi: 10.1128/JVI.02637-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burkhardt AL, Brunswick M, Bolen JB, Mond JJ. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci USA. 1991;88:7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Koizumi T, Watanabe T. Altered antigen receptor signaling and impaired Fas-mediated apoptosis of B cells in Lyn-deficient mice. J Exp Med. 1996;184:831–838. doi: 10.1084/jem.184.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan VWF, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 45.Chan VWF, Lowell CA, DeFranco AL. Defective negative regulation of antigen receptor signaling in Lyn-deficient B lymphocytes. Current Biol. 1998;8:545–553. doi: 10.1016/s0960-9822(98)70223-4. [DOI] [PubMed] [Google Scholar]
- 46.Smith KGC, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT. Inhibition of the B Cell by CD22: A Requirement for Lyn. J Exp Med. 1998;187:807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeFranco AL, Chan VWF, Lowell CA. Positive and negative roles of the tyrosine kinase Lyn in B cell function. Seminars Immunol. 1998;10:299–307. doi: 10.1006/smim.1998.0122. [DOI] [PubMed] [Google Scholar]
- 48.Gauld SB, Cambier JC. Src-family kinases in B-cell development and signaling. Oncogene. 2004;23:8001–8006. doi: 10.1038/sj.onc.1208075. [DOI] [PubMed] [Google Scholar]
- 49.Yamanashi Y, Kakiuchi T, Mizuguchi J, Yamamoto T, Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991;251:192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- 50.Clark MR, Johnson SA, Cambier JC. Analysis of Ig-alpha-tyrosine kinase interaction reveals two levels of binding specificity and tyrosine phosphorylated Ig-alpha stimulation of Fyn activity. EMBO J. 1994;13:1911–1919. doi: 10.1002/j.1460-2075.1994.tb06460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burg DL, Furlong MT, Harrison ML, Geahlen RL. Interactions of Lyn with the antigen receptor during B cell activation. J Biol Chem. 1994;269:28136–28142. [PubMed] [Google Scholar]
- 52.Gaul BS, Harrison ML, Geahlen RL, Burton RA, Post CB. Substrate recognition by the Lyn protein-tyrosine kinase: NMR structure of the immunoreceptor tyrosine-based activation motif signaling region of the B-cell antigen receptor. J Biol Chem. 2000;275:16174–16182. doi: 10.1074/jbc.M909044199. [DOI] [PubMed] [Google Scholar]
- 53.Rolli V, Gallwitz M, Wossning T, Flemming A, Schamel WWA, Zürn C, Reth M. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol Cell. 2002;10:1057–1069. doi: 10.1016/s1097-2765(02)00739-6. [DOI] [PubMed] [Google Scholar]
- 54.Pao LI, Famiglietti SJ, Cambier JC. Asymmetrical phosphorylation and function of immunoreceptor tyrosine-based activation motif tyrosines in B cell antigen receptor signal transduction. J Immunol. 1998;160:3305–3314. [PubMed] [Google Scholar]
- 55.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in Lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 56.Li HL, Davis WW, Whiteman EL, Birnbaum MJ, Puré E. The tyrosine kinases Syk and Lyn exert opposing effects on the activation of protein kinase Akt/PKB in B lymphocytes. Proc Natl Acad Sci USA. 1999;96:6890–6895. doi: 10.1073/pnas.96.12.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma H, Yankee TM, Hu J, Asai DJ, Harrison ML, Geahlen RL. Visualization of Syk-antigen receptor interactions using green fluorescent protein: differential roles for Syk and Lyn in the regulation of receptor capping and internalization. J Immunol. 2001;166:1507–1516. doi: 10.4049/jimmunol.166.3.1507. [DOI] [PubMed] [Google Scholar]
- 58.Fütterer K, Wong J, Grucza RA, Chan AC, Waksman G. Structural basis for Syk tyrosine kinase ubiquity in signal transduction pathways revealed by the crystal structure of its regulatory SH2 domains bound to a dually phosphorylated ITAM peptide. J Mol Biol. 1998;281:523–537. doi: 10.1006/jmbi.1998.1964. [DOI] [PubMed] [Google Scholar]
- 59.Grucza RA, Futterer K, Chan AC, Waksman G. Thermodynamic study of the binding of the tandem-SH2 domain of the Syk kinase to a dually phosphorylated ITAM peptide: evidence for two conformers. Biochemistry. 1999;38:5024–5033. doi: 10.1021/bi9829938. [DOI] [PubMed] [Google Scholar]
- 60.Grucza RA, Bradshaw JM, Mitaxov V, Waksman G. Role of electrostatic interactions in SH2 domain recognition: salt-dependence of tyrosyl-phosphorylated peptide binding to the tandem SH2 domain of the Syk kinase and the single SH2 domain of the Src kinase. Biochemistry. 2000;39:10072–10081. doi: 10.1021/bi000891n. [DOI] [PubMed] [Google Scholar]
- 61.Kumaran S, Grucza RA, Waksman G. The tandem Src homology 2 domain of the Syk kinase: A molecular device that adapts to interphosphotyrosine distances. Proc Natl Acad Sci USA. 2003;100:14828–14833. doi: 10.1073/pnas.2432867100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Narula SS, Yuan RW, Adams SE, Green OM, Green J, Philips TB, Zydowsky LD, Botfiled MS, Hatada M, Laird ER, Zoller MJ, Karas JL, Dalgarnol DC. Solution structure of the C-terminal SH2 domain of the human tyrosine kinase Syk complexed with a phosphotyrosine pentapeptide. Structure. 1995;3:1061–1073. doi: 10.1016/s0969-2126(01)00242-8. [DOI] [PubMed] [Google Scholar]
- 63.Fuller GLJ, Williams JAE, Tomlinson MG, Eble JA, Hanna SL, Pöhlmann S, Suzuki-Inoue K, Ozaki Y, Watson SP, Pearce AC. The C-type lectin receptors CLEC-2 and Dectin-1, but not DC-SIGN, signal via a novel YXXL-dependent signaling cascade. J Biol Chem. 2007;282:12397–12409. doi: 10.1074/jbc.M609558200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rowley RB, Burkhardt AL, Chao HG, Matsueda GR, Bolen JB. Syk protein-tyrosine kinase is regulated by tyrosine phosphorylated Ig alpha/Ig beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J Biol Chem. 1995;270:11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- 65.Shiue L, Green J, Green OM, Karas JL, Morgenstern JP, Ram MK, Taylor MK, Zoller MJ, Zydowsky LD, Bolen JG. Interaction of p72syk with the gamma and beta subunits of the high-affinity receptor for immunoglobulin E, Fc epsilon RI. Mol Cell Biol. 1995;15:272–281. doi: 10.1128/mcb.15.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsang E, Giannetti AM, Shaw D, Dinh M, Tse JKY, Gandhi S, Ho H, Wang S, Papp E, Bradshaw JM. Molecular mechanism of the Syk activation switch. J Biol Chem. 2008;283:32650–32659. doi: 10.1074/jbc.M806340200. [DOI] [PubMed] [Google Scholar]
- 67.Deindl S, Kadlecek TA, Brdicka T, Cao X, Weiss A, Kuriyan J. Structural basis for the inhibition of tyrosine kinase activity of ZAP-70. Cell. 2007;129:735–746. doi: 10.1016/j.cell.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 68.Arias-Palomo E, Recuero-Checa MA, Bustelo XR, Llorca O. 3D structure of Syk kinase determined by single-particle electron microscopy. Biochim Biophys Acta. 2007;1774:1493–1499. doi: 10.1016/j.bbapap.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou P, Araujo E, Zhao T, Zhang M, Massenburg D, Veselits M, Doyle C, Dinner AR, Clark MR. B cell antigen receptor signaling and internalization are mutually exclusive events. PLoS Biol. 2006;4:1147–1158. doi: 10.1371/journal.pbio.0040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oh H, Ozkirimli E, Shah K, Harrison ML, Geahlen RL. Generation of an analog-sensitive Syk tyrosine kinase for the study of signaling dynamics from the B cell antigen receptor. J Biol Chem. 2007;282:33760–33768. doi: 10.1074/jbc.M704846200. [DOI] [PubMed] [Google Scholar]
- 71.Kulathu Y, Hobeika E, Turchinovich G, Reth M. The kinase Syk as an adaptor controlling sustained calcium signalling and B-cell development. EMBO J. 2008;27:1333–1344. doi: 10.1038/emboj.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peters JD, Furlong MT, Asai DJ, Harrison ML, Geahlen RL. Syk, activated by cross- linking the B-cell antigen receptor, localizes to the cytosol where it interacts with and phosphorylates α-tubulin on tyrosine. J Biol Chem. 1996;271:4755–4762. doi: 10.1074/jbc.271.9.4755. [DOI] [PubMed] [Google Scholar]
- 73.Zhou F, Hu J, Ma H, Harrison ML, Geahlen RL. Nucleocytoplasmic trafficking of the Syk protein-tyrosine kinase. Mol Cell Biol. 2006;26:3478–3491. doi: 10.1128/MCB.26.9.3478-3491.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sloan-Lancaster J, Zhang W, Presley J, Williams BL, Abraham RT, Lippincott-Schwartz J, Samelson LE. Regulation of ZAP-70 intracellular localization: visualization with the green fluorescent protein. J Exp Med. 1997;186:1713–1724. doi: 10.1084/jem.186.10.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang L, Devarajan E, He J, Reddy SP, Dai JL. Transcription repressor activity of spleen tyrosine kinase mediates breast tumor suppression. Cancer Res. 2005;65:10289–10297. doi: 10.1158/0008-5472.CAN-05-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watts JD, Affolter M, Krebs DL, Wange RL, Samelson LE, Aebersold R. Identification by electrospray ionization mass spectrometry of the sites of tyrosine phosphorylation induced in activated Jurkat T cells on the protein tyrosine kinase ZAP-70. J Biol Chem. 1994;25:29520–29529. [PubMed] [Google Scholar]
- 77.Zhang Y, Oh H, Burton RA, Burgner JW, Geahlen RL, Post CB. Tyr130 phosphorylation triggers Syk release from antigen receptor by long-distance conformational uncoupling. Proc Natl Acad Sci USA. 2008;105:11760–11765. doi: 10.1073/pnas.0708583105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keshvara LM, Isaacson C, Harrison ML, Geahlen RL. Syk activation and dissociation from the B-cell antigen receptor is mediated by phosphorylation of tyrosine-130. J Biol Chem. 1997;272:10377–10381. doi: 10.1074/jbc.272.16.10377. [DOI] [PubMed] [Google Scholar]
- 79.Zyss D, Montcourrier P, Vidal B, Anguille C, Mérezègue F, Sahuquet A, Mangeat PH, Coopman PJ. The Syk tyrosine kinase localizes to the centrosomes and negatively affects mitotic progression. Cancer Res. 2005;65:10872–10880. doi: 10.1158/0008-5472.CAN-05-1270. [DOI] [PubMed] [Google Scholar]
- 80.Stupack DG, Li E, Silletti S, Kehler JA, Geahlen RL, Hahn K, Nemerow GR, Cheresh DA. Matrix valency regulates integrin-mediated lymphoid adhesion via Syk kinase. J Cell Biol. 1999;144:777–788. doi: 10.1083/jcb.144.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, MacNeill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu T-L, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 82.Wang L, Duke L, Zhang PS, Arlinghaus RB, Symmans WF, Sahin A, Mendez R, Dai JL. Alternative splicing disrupts a nuclear localization signal in spleen tyrosine kinase that is required for invasion suppression in breast cancer. Cancer Res. 2003;63:4724–4730. [PubMed] [Google Scholar]
- 83.Cao L, Yu K, Banh C, Nguyen V, Ritz A, Raphael BJ, Kawakami Y, Kawakami T, Salomon AR. Quantitative time-resolved phosphoproteomic analysis of mast cell signaling. J Immunol. 2007;179:5864–5876. doi: 10.4049/jimmunol.179.9.5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goss VL, Lee KA, Moritz A, Nardone J, Spek EJ, MacNeill J, Rush J, Comb MJ, Polakiewicz RD. A common phosphotyrosine signature for the Bcr-Abl kinase. Blood. 2006;107:4888–4897. doi: 10.1182/blood-2005-08-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong JJ, Yankee TM, Harrison ML, Geahlen RL. Regulation of signaling in B cells through the phosphorylation of Syk on linker region tyrosines. A mechanism for negative signaling by the Lyn tyrosine kinase. J Biol Chem. 2002;277:31703–31714. doi: 10.1074/jbc.M201362200. [DOI] [PubMed] [Google Scholar]
- 86.Yankee TM, Keshvara LM, Sawasdikosol S, Harrison ML, Geahlen RL. Inhibition of Signaling through the B cell antigen receptor by the proto-oncogene product, c-Cbl, requires Syk tyrosine 317 and the c-Cbl phosphotyrosine-binding domain. J Immunol. 1999;163:5827–5835. [PubMed] [Google Scholar]
- 87.Sada K, Zhang J, Siraganian RP. Point mutation of a tyrosine in the linker region of Syk results in a gain of function. J Immunol. 2000;164:338 –344. doi: 10.4049/jimmunol.164.1.338. [DOI] [PubMed] [Google Scholar]
- 88.Simon M, Vanes L, Geahlen RL, Tybulewicz VLJ. Distinct roles for the linker region tyrosines of Syk in FcεRI signaling in primary mast cells. J Biol Chem. 2005;280:4510–4517. doi: 10.1074/jbc.M410326200. [DOI] [PubMed] [Google Scholar]
- 89.Zhao Q, Weiss A. Enhancement of lymphocyte responsiveness by a gain-of-function mutation of ZAP-70. Mol Cell Biol. 1996;16:6765–6774. doi: 10.1128/mcb.16.12.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thien CBF, Langdon WY. c-Cbl and Cbl-b ubiquitin ligases: substrate diversity and the negative regulation of signalling responses. Biochem J. 2005;391:153–166. doi: 10.1042/BJ20050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ota Y, Beitz LO, Scharenberg AM, Donovan JA, Kinet JP, Samelson LE. Characterization of Cbl tyrosine phosphorylation and a Cbl-Syk complex in RBL-2H3 cells. J Exp Med. 1996;184:1713–1723. doi: 10.1084/jem.184.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kitaura Y, Jang IK, Wang Y, Han YC, Inazu T, Cadera EJ, Schlissel M, Hardy RR, Gu H. Control of the B cell-intrinsic tolerance programs by ubiquitin ligases Cbl and Cbl-b. Immunity. 2007;26:567–578. doi: 10.1016/j.immuni.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deckert M, Elly C, Altman A, Liu YC. Coordinated regulation of the tyrosine phosphorylation of Cbl by Fyn and Syk tyrosine kinases. J Biol Chem. 1998;273:8867–8874. doi: 10.1074/jbc.273.15.8867. [DOI] [PubMed] [Google Scholar]
- 94.Lupher ML, Rao N, Lill NL, Andonioui CE, Miyake S, Clark EA, Druker B, Band H. Cbl-mediated Negative Regulation of the Syk Tyrosine Kinase. A critical role for Cbl phosphotyrosine-binding domain binding to Syk phosphotyrosine 323. J Biol Chem. 1998;273:35273–35281. doi: 10.1074/jbc.273.52.35273. [DOI] [PubMed] [Google Scholar]
- 95.Lupher ML, Songyang Z, Shoelson SE, Cantley LC, Band H. The Cbl phosphotyrosine-binding domain selects a D(N/D)XpY motif and binds to the TyrP292 negative regulatory phosphorylation site of ZAP-70. J Biol Chem. 1997;272:33140–33144. doi: 10.1074/jbc.272.52.33140. [DOI] [PubMed] [Google Scholar]
- 96.Swaminathan G, Tsygankov AY. The Cbl family proteins: ring leaders in regulation of cell signaling. J Cell Physiol. 2006;209:21–43. doi: 10.1002/jcp.20694. [DOI] [PubMed] [Google Scholar]
- 97.Ng C, Jackson RA, Buschdorf JP, Sun Q, Guy GR, Sivaraman J. Structural basis for a novel intrapeptidyl H-bond and reverse binding of c-Cbl-TKB domain substrates. EMBO J. 2008;27:804–816. doi: 10.1038/emboj.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moon KD, Post CB, Durden DL, Zhou Q, De P, Harrison ML, Geahlen RL. Molecular basis for a direct interaction between the Syk protein-tyrosine kinase and phosphoinositide 3-kinase. J Biol Chem. 2005;280:1543–1551. doi: 10.1074/jbc.M407805200. [DOI] [PubMed] [Google Scholar]
- 99.Yokouchi M, Kondo T, Sanjay A, Houghton A, Yoshimura A, Komiya S, Zhang H, Baron R. Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. J Biol Chem. 2001;276:35185–35193. doi: 10.1074/jbc.M102219200. [DOI] [PubMed] [Google Scholar]
- 100.Waterman H, Katz M, Rubin C, Shtiegman K, Lavi S, Elson A, Jovin T, Yarden Y. A mutant EGF receptor defective in ubiquitylation and endocytosis unveils a role for Grb2 in negative signalling. EMBO J. 2002;21:303–313. doi: 10.1093/emboj/21.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ota Y, Samelson LE. The product of the proto-oncogene c-cbl: a negative regulator of the Syk tyrosine kinase. Science. 1997;276:418–420. doi: 10.1126/science.276.5311.418. [DOI] [PubMed] [Google Scholar]
- 102.Joazeiro CAP, Wing SS, Huang HK, Leverson JD, Hunter T, Liu YC. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–312. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 103.Rao N, Ghosh AK, Ota S, Zhou P, Reddi AL, Hakezi K, Druker BK, Wu J, Band H. The non-receptor tyrosine kinase Syk is a target of Cbl-mediated ubiquitylation upon B-cell receptor stimulation. EMBO J. 2001;20:7085–7095. doi: 10.1093/emboj/20.24.7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paolini R, Molfetta R, Beitz LO, Zhang J, Scharenberg AM, Piccoli M, Frati L, Siraganian R, Santoni A. Activation of Syk tyrosine kinase is required for c-Cbl-mediated ubiquitination of FcεRI and Syk in RBL cells. J Biol Chem. 2002;277:36940–36947. doi: 10.1074/jbc.M204948200. [DOI] [PubMed] [Google Scholar]
- 105.Paolini R, Molfetta R, Piccoli M, Frati L, Santoni A. Ubiquitination and degradation of Syk and ZAP-70 protein tyrosine kinases in human NK cells upon CD16 engagement. Proc Natl Acad Sci USA. 2001;98:9611–9616. doi: 10.1073/pnas.161298098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Youssef LA, Wilson BS, Oliver JM. Proteasome-dependent regulation of Syk tyrosine kinase levels in human basophils. J Allergy Clin Immun. 2002;110:366–373. doi: 10.1067/mai.2002.127562. [DOI] [PubMed] [Google Scholar]
- 107.Peruzzi G, Molfetta R, Gasparrini F, Vian L, Morrone S, Piccoli M, Frati L, Santoni A, Paolini R. The adaptor molecule CIN85 regulates Syk tyrosine kinase level by activating the ubiquitin-proteasome degradation pathway. J Immunol. 2007;179:2089–2096. doi: 10.4049/jimmunol.179.4.2089. [DOI] [PubMed] [Google Scholar]
- 108.Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- 109.Mosesson Y, Shtiegman K, Katz M, Zwang Y, Vereb G, Szollosi J, Yarden Y. Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J Biol Chem. 2003;278:21323–21326. doi: 10.1074/jbc.C300096200. [DOI] [PubMed] [Google Scholar]
- 110.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 111.Dangelmaier CA, Quinter PG, Jin J, Tsygankov AY, Kunapuli SP, Daniel JL. Rapid ubiquitination of Syk following GPVI activation in platelets. Blood. 2005;105:3918–3924. doi: 10.1182/blood-2004-09-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beitz LO, Fruman DA, Kurosaki T, Cantley LC, Scharenberg AM. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J Biol Chem. 1999;274:32662–32666. doi: 10.1074/jbc.274.46.32662. [DOI] [PubMed] [Google Scholar]
- 113.Buhl AM, Cambier JC. Phosphorylation of CD19 Y484 and Y515, and linked activation of phosphatidylinositol 3-kinase, are required for B cell antigen receptor-mediated activation of Bruton’s tyrosine kinase. J Immunol. 1999;162:4438–4446. [PubMed] [Google Scholar]
- 114.Okada T, Maeda A, Iwamatsu A, Gotoh K, Kurosaki T. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity. 2000;13:817–827. doi: 10.1016/s1074-7613(00)00079-0. [DOI] [PubMed] [Google Scholar]
- 115.Ingham RJ, Santos L, Dang-Lawson M, Holgado-Madruga M, Dudek P, Maroun CR, Wong AJ, Matsuuchi L, Gold MR. The Gab1 docking protein links the B cell antigen receptor to the phosphatidylinositol 3-kinase/Akt signaling pathway and to the SHP2 tyrosine phosphatase. J Biol Chem. 2001;276:12257–12265. doi: 10.1074/jbc.M010590200. [DOI] [PubMed] [Google Scholar]
- 116.Greenberg S, Chang P, Silverstein SC. Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1993;177:529–534. doi: 10.1084/jem.177.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kiefer F, Brumell J, Al-Alawi N, Latour S, Cheng A, Veillette A, Grinstein S, Pawson T. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol Cell Biol. 1998;18:4209–4220. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cox D, Tseng CC, Bjekic G, Greenberg S. A requirement for phosphatidylinositol 3- kinase in pseudopod extension. J Biol Chem. 1999;274:1240–1247. doi: 10.1074/jbc.274.3.1240. [DOI] [PubMed] [Google Scholar]
- 121.Huang ZY, Barreda DR, Worth RG, Indik ZK, Kim MK, Chien P, Schreiber AD. Differential kinase requirements in human and mouse Fcgamma receptor phagocytosis and endocytosis. J Leukoc Biol. 2006;80:1553–1562. doi: 10.1189/jlb.0106019. [DOI] [PubMed] [Google Scholar]
- 122.Schymeinsky J, Then C, Sindrilaru A, Gerstl R, Jakus Z, Tybulewicz VLJ, Scharffetter-Kochanek K, Walzog B. Syk-mediated translocation of PI3Kδ to the leading edge controls lamellipodium formation and migration of leukocytes. PLoS ONE. 2007;2:e1132. doi: 10.1371/journal.pone.0001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hung CH, Thomas L, Ruby CE, Atkins KM, Morris NP, Knight ZA, Scholz I, Barklis E, Weinberg AD, Shokat KM, Thomas G. HIV-1 Nef assembles a Src family kinase-ZAP-70/Syk-PI3K cascade to downregulate cell-surface MHC-I. Cell Host Microbe. 2007;1:121–133. doi: 10.1016/j.chom.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 124.Kanie T, Abe A, Matsuda T, Kuno Y, Towatari M, Yamamoto T, Saito H, Emi N, Naoe T. TEL-Syk fusion constitutively activates PI3-K/Akt, MAPK and JAK2-independent STAT5 signal pathways. Leukemia. 2004;18:548–555. doi: 10.1038/sj.leu.2403266. [DOI] [PubMed] [Google Scholar]
- 125.Popa-Nita O, Rollet-Labelle E, Thibault N, Gilbert C, Bourgoin SG, Naccache PH. Crystal-induced neutrophil activation. IX. Syk-dependent activation of class Ia phosphatidylinositol 3-kinase. J Leukoc Biol. 2007;82:763–773. doi: 10.1189/jlb.0307174. [DOI] [PubMed] [Google Scholar]
- 126.Lau C, Wang X, Song L, North M, Wiehler S, Proud D, Chow CW. Syk associates with clathrin and mediates phosphatidylinositol 3-kinase activation during human rhinovirus internalization. J Immunol. 2008;180:870 – 880. doi: 10.4049/jimmunol.180.2.870. [DOI] [PubMed] [Google Scholar]
- 127.Salomon AR, Ficarro SB, Brill LM, Brinker A, Phung QT, Ericson C, Sauer K, Brock A, Horn DM, Schultz PG, Peters EC. Profiling of tyrosine phosphorylation pathways in human cells using mass spectrometry. Proc Natl Acad Sci USA. 2003;100:443–448. doi: 10.1073/pnas.2436191100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang J, Berenstein E, Siraganian RP. Phosphorylation of Tyr342 in the linker region of Syk is critical for FceRI signaling in mast cells. Mol Cell Biol. 2002;22:8144–8154. doi: 10.1128/MCB.22.23.8144-8154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Groesch TD, Zhou F, Matilla S, Geahlen RL, Post CB. Structural basis for the requirement of two phosphotyrosine residues in signaling mediated by Syk tyrosine kinase. J Mol Biol. 2006;356:1222–1236. doi: 10.1016/j.jmb.2005.11.095. [DOI] [PubMed] [Google Scholar]
- 130.Wu J, Zhao QH, Kurosaki T, Weiss A. The Vav binding site (Y315) in ZAP-70 is critical for antigen receptor-mediated signal transduction. J Exp Med. 1997;185:1877–1882. doi: 10.1084/jem.185.10.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Williams BL, Irvin BJ, Sutor SL, Chini CCS, Yacyshyn E, Wardenburg JB, Dalton M, Chan AC, Abraham RT. Phosphorylation of Tyr319 in ZAP-70 is required for T- cell antigen receptor-dependent phospholipase C-γ1 and Ras activation. EMBO J. 1999;18:1832–1844. doi: 10.1093/emboj/18.7.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Di Bartolo V, Mege D, Germain V, Pelosi M, Dufour E, Michel F, Magistrelli G, Isacchi A, Acuto O. Tyrosine 319, a newly identified phosphorylation site of ZAP-70, plays a critical role in T cell antigen receptor signaling. J Biol Chem. 1999;274:6285–6294. doi: 10.1074/jbc.274.10.6285. [DOI] [PubMed] [Google Scholar]
- 133.Gong Q, Jin XH, Akk AM, Foger N, White M, Gong QQ, Wardenburg JB, Chan AC. Requirement for tyrosine residues 315 and 319 within ζ chain-associated protein 70 for T cell development. J Exp Med. 2001;194:507–518. doi: 10.1084/jem.194.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Magnan A, Di Bartolo V, Mura AM, Boyer C, Richelme M, Lin YL, Roure A, Gillet A, Arrieumerlou C, Acuto O, Malissen B, Malissen M. T cell development and T cell responses in mice with mutations affecting tyrosines 292 or 315 of the ZAP-70 protein tyrosine kinase. J Exp Med. 2001;194:491–505. doi: 10.1084/jem.194.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao Q, Williams BL, Abraham RT, Weiss A. Interdomain B in ZAP-70 regulates but is not required for ZAP-70 signaling function in lymphocytes. Mol Cell Biol. 1999;19:948–956. doi: 10.1128/mcb.19.1.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sidorenko SP, Law CL, Chandran KA, Clark EA. Human spleen tyrosine kinase p72Syk associates with the Src-family kinase p53/56Lyn and a 120-kDa phosphoprotein. Proc Natl Acad Sci USA. 1995;92:359–363. doi: 10.1073/pnas.92.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sillman AL, Monroe JG. Association of p72syk with the src homology-2 domains of PLCγ1 in B lymphocytes. J Biol Chem. 1995;270:11806–11811. doi: 10.1074/jbc.270.20.11806. [DOI] [PubMed] [Google Scholar]
- 138.Law CL, Chandran KA, Sidorenko SP, Clark EA. Phospholipase C-gamma1 interacts with conserved phosphotyrosyl residues in the linker region of Syk and is a substrate for Syk. Mol Cell Biol. 1996;16:1305–1315. doi: 10.1128/mcb.16.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5:591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 140.Galandrini R, Palmieri G, Piccoli M, Frati L, Santoni A. Role for the Rac1 exchange factor Vav in the signaling pathways leading to NK cell cytotoxicity. J Immunol. 1999;162:3148–3152. [PubMed] [Google Scholar]
- 141.Tedford K, Nitschke L, Girkontaite I, Charlesworth A, Chan G, Sakk V, Barbacid M, Fischer KD. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat Immunol. 2001;2:548–555. doi: 10.1038/88756. [DOI] [PubMed] [Google Scholar]
- 142.Schymeinsky J, Sindrilaru A, Frommhold D, Sperandio M, Gerstl R, Then C, Mócsai A, Scharffetter-Kochanek K, Walzog B. The Vav binding site of the non-receptor tyrosine kinase Syk at Tyr 348 is critical for beta2 integrin (CD11/CD18)-mediated neutrophil migration. Blood. 2006;108:3919–3927. doi: 10.1182/blood-2005-12-030387. [DOI] [PubMed] [Google Scholar]
- 143.Pelosi M, Di Bartolo V, Mounier V, Mège D, Pascussi JM, Dufour E, Blondel A, Acuto O. Tyrosine 319in the interdomain B of ZAP-70 is a binding site for the Src homology 2 domain of Lck. J Biol Chem. 1999;274:14229–14237. doi: 10.1074/jbc.274.20.14229. [DOI] [PubMed] [Google Scholar]
- 144.Vines CM, Potter JW, Xu Y, Geahlen RL, Costello PS, Tybulewicz VL, Lowell CA, Chang PW, Gresham HD, Willman CL. Inhibition of beta 2 integrin receptor and Syk kinase signaling in monocytes by the Src family kinase Fgr. Immunity. 2001;15:507–519. doi: 10.1016/s1074-7613(01)00221-7. [DOI] [PubMed] [Google Scholar]
- 145.Brdicka T, Kadlecek TA, Roose JP, Pastuszak AW, Weiss A. Intramolecular regulatory switch in ZAP-70: analogy with receptor tyrosine kinases. Mol Cell Biol. 2005;25:4924–4933. doi: 10.1128/MCB.25.12.4924-4933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang J, Billingsley ML, Kincaid RL, Siraganian RP. Phosphorylation of Syk activation loop tyrosines is essential for Syk function. An in vivo study using a specific anti-syk activation loop phosphotyrosine antibody. J Biol Chem. 2000;275:35442–35447. doi: 10.1074/jbc.M004549200. [DOI] [PubMed] [Google Scholar]
- 147.El-Hillal O, Kurosaki T, Yamamura H, Kinet JP, Scharenberg AM. Syk kinase activation by a Src kinase-initiated activation loop phosphorylation chain reaction. Proc Natl Acad Sci USA. 1997;94:1919–1924. doi: 10.1073/pnas.94.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Adams JA. Activation loop phosphorylation and catalysis in protein kinases: Is there functional evidence for the autoinhibitor model? Biochemistry. 2003;42:601–607.F. doi: 10.1021/bi020617o. [DOI] [PubMed] [Google Scholar]
- 149.Couture C, Williams S, Gauthier N, Tailor P, Mustelin T. Role of Tyr518 and Tyr519 in the regulation of catalytic activity and substrate phosphorylation by Syk protein-tyrosine kinase. Eur J Biochem. 1997;246:447–451. doi: 10.1111/j.1432-1033.1997.00447.x. [DOI] [PubMed] [Google Scholar]
- 150.Zhang J, Kimura T, Siraganian RP. Mutations in the activation loop tyrosines of protein tyrosine kinase Syk abrogate intracellular signaling but not kinase activity. J Immunol. 1998;161:4366–4374. [PubMed] [Google Scholar]
- 151.Papp E, Tse JK, Ho H, Wang S, Shaw D, Lee S, Barnett J, Swinney DC, Bradshaw JM. Steady state kinetics of spleen tyrosine kinase investigated by a real time fluorescence assay. Biochemistry. 2007;46:15103–15114. doi: 10.1021/bi701596u. [DOI] [PubMed] [Google Scholar]
- 152.Atwell S, Adams JM, Badger J, Buchanan MD, Feil IK, Froning KJ, Gao X, Hendle J, Keegan K, Leon BC, Müller-Dieckmann HJ, Nienaber VL, Noland BW, Post K, Rajashankar KR, Ramos A, Russell M, Burley SK, Buchanan SG. A novel mode of gleevec binding is revealed by the structure of spleen tyrosine kinase. J Biol Chem. 2004;279:55827–55832. doi: 10.1074/jbc.M409792200. [DOI] [PubMed] [Google Scholar]
- 153.Jin L, Pluskey S, Petrella EC, Cantin SM, Gorga JC, Rynkiewicz MJ, Pandey P, Strickler JE, Babine RE, Weaver DT, Seidl KJ. The three-dimensional structure of the ZAP-70 kinase domain in complex with staurosporine. Implications for the design of selective inhibitors. J Biol Chem. 2004;279:42818–42825. doi: 10.1074/jbc.M407096200. [DOI] [PubMed] [Google Scholar]
- 154.Couture C, Baier G, Oetken C, Williams S, Telford D, Marie-Cardine A, Baier-Bitterlich G, Fischer S, Bum P, Altman A, Mustelin T. Activation of p56lck by p72syk through physical association and N-terminal tyrosine phosphorylation. Mol Cell Biol. 1994;14:5249–5258. doi: 10.1128/mcb.14.8.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li HL, Forman MS, Kurosaki T, Puré E. Syk is required for BCR-mediated activation of p90Rsk, but not p70S6k, via a mitogen-activated protein kinase-independent pathway in B cells. J Biol Chem. 1997;272:18200–18208. doi: 10.1074/jbc.272.29.18200. [DOI] [PubMed] [Google Scholar]
- 156.Zeitlmann L, Knorr T, Knoll M, Romeo C, Sirim P, Kolanus W. T cell activation induced by novel gain-of-function mutants of Syk and ZAP-70. J Biol Chem. 1998;273:15445–15452. doi: 10.1074/jbc.273.25.15445. [DOI] [PubMed] [Google Scholar]
- 157.Koretzky GA, Abtahian F, Silverman MA. SLP76 and SLP65: complex regulation of signalling in lymphocytes and beyond. Nat Rev Immunol. 2006;6:67–78. doi: 10.1038/nri1750. [DOI] [PubMed] [Google Scholar]
- 158.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 159.Su YW, Zhang Y, Schweikert J, Koretzky GA, Reth M, Wienands J. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur J Immunol. 1999;29:3702–3711. doi: 10.1002/(SICI)1521-4141(199911)29:11<3702::AID-IMMU3702>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 160.Hashimoto S, Iwamatsu A, Ishiai M, Okawa K, Yamadori T, Matsushita M, Baba Y, Kishimoto T, Kurosaki T, Tsukada S. Identification of the SH2 domain binding protein of Bruton's tyrosine kinase as BLNK--functional significance of Btk-SH2 domain in B-cell antigen receptor-coupled calcium signaling. Blood. 1999;94:2357–2364. [PubMed] [Google Scholar]
- 161.Ishiai M, Sugawara H, Kurosaki M, Kurosaki T. Cutting edge: association of phospholipase C- ***2 Src homology 2 domains with BLNK is critical for B cell antigen receptor signalling. J Immunol. 1999;163:1746–1749. [PubMed] [Google Scholar]
- 162.Fruman DA, Satterthwaite AB, Witte ON. Xid-like Phenotypes: A B Cell Signalosome Takes Shape. Immunity. 2000;13:1–3. doi: 10.1016/s1074-7613(00)00002-9. [DOI] [PubMed] [Google Scholar]
- 163.Chiu CW, Dalton M, Ishiai M, Kurosaki T, Chan AC. BLNK: molecular scaffolding through ‘cis’-mediated organization of signaling proteins. EMBO J. 2002;21:6461–6472. doi: 10.1093/emboj/cdf658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kohler F, Storch B, Kulathu Y, Herzog S, Kuppig S, Reth M, Jumaa H. A leucine zipper in the N terminus confers membrane association to SLP-65. Nat Immunol. 2005;6:204–210. doi: 10.1038/ni1163. [DOI] [PubMed] [Google Scholar]
- 165.Engels N, Wollscheid B, Wienands J. Association of SLP-65/BLNK with the B cell antigen receptor through a non-ITAM tyrosine of Ig-α. Eur J Immunol. 2001;31:2126–2134. doi: 10.1002/1521-4141(200107)31:7<2126::aid-immu2126>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 166.Kabak S, Skaggs BJ, Gold MR, Affolter M, West KL, Foster MS, Siemasko K, Chan AC, Aebersold R, Clark MR. The direct recruitment of BLNK to Ig-α couples the B-cell antigen receptor to distal signaling pathways. Mol Cell Biol. 2002;22:2524–2535. doi: 10.1128/MCB.22.8.2524-2535.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Abudula A, Grabbe1 A, Brechmann M, Polaschegg C, Herrmann N, Goldbeck I, Dittmann K, Wienands J. SLP-65 Signal transduction requires Src homology 2 domain-mediated membrane anchoring and a kinase-independent adaptor function of Syk. J Biol Chem. 2007;282:29059–29066. doi: 10.1074/jbc.M704043200. [DOI] [PubMed] [Google Scholar]
- 168.Wong J, Ishiai M, Kurosaki T, Chan AC. Functional complementation of BLNK by SLP-76 and LAT linker proteins. J Biol Chem. 2000;275:33116–33122. doi: 10.1074/jbc.M004467200. [DOI] [PubMed] [Google Scholar]
- 169.Rolli V, Gallwitz M, Wossning T, Flemming A, Schamel WWA, Zurn C, Reth M. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol Cell. 2002;10:1057–1069. doi: 10.1016/s1097-2765(02)00739-6. [DOI] [PubMed] [Google Scholar]
- 170.Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KVS. The strength of receptor signaling is centrally controlled through a cooperative loop between Ca and an oxidant signal. Cell. 2005;121:281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 171.Irish JM, Czerwinski DK, Nolan GP, Levy R. Kinetics of B cell receptor signaling in human B cell subsets mapped by phosphospecific flow cytometry. J Immunol. 2006;177:1581–1589. doi: 10.4049/jimmunol.177.3.1581. [DOI] [PubMed] [Google Scholar]
- 172.Campbell MA, Klinman NR. Phosphotyrosine-dependent association between CD22 and protein tyrosine phosphatase 1C. Eur J Immunol. 1995;25:1573–1579. doi: 10.1002/eji.1830250616. [DOI] [PubMed] [Google Scholar]
- 173.Law CL, Sidorenko SP, Chandran KA, Zhao Z, Shen SH, Fischer EH, Clark EA. CD22 associates with protein tyrosine phosphatase 1C, Syk, and phospholipase C-gamma(1) upon B cell activation. J Exp Med. 1996;183:547–560. doi: 10.1084/jem.183.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Smith KGC, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT. Inhibition of the B cell by CD22: a requirement for Lyn. J Exp Med. 1998;187:807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maeda A, Scharenberg AM, Tsukada S, Bolen JB, Kinet JP, Kurosaki T. Paired immunoglobulin-like receptor B (PIR-B) inhibits BCR-induced activation of Syk and Btk by SHP-1. Oncogene. 1999;18:2291–2297. doi: 10.1038/sj.onc.1202552. [DOI] [PubMed] [Google Scholar]
- 176.Yohannan J, Wienands J, Coggeshall KM, Justement LB. Analysis of tyrosine phosphorylation-dependent interactions between stimulatory effector proteins and the B cell co-receptor CD22. J Biol Chem. 1999;274:18769–18776. doi: 10.1074/jbc.274.26.18769. [DOI] [PubMed] [Google Scholar]
- 177.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the regulation of cell signaling. Semin Immunol. 2000;12:361–378. doi: 10.1006/smim.2000.0223. [DOI] [PubMed] [Google Scholar]
- 178.Dustin LB, Plas DR, Wong J, Hu YT, Soto C, Chan AC, Thomas ML. Expression of dominant-negative src-homology domain 2-containing protein tyrosine phosphatase-1 results in increased Syk tyrosine kinase activity and B cell activation. J Immunol. 1999;162:2717–2724. [PubMed] [Google Scholar]
- 179.Ganju RK, Brubaker SA, Chernock RD, Avraham S, Groopman JE. β-Chemokine receptor CCR5 signals through SHP1, SHP2, and Syk. J Biol Chem. 2000;275:17263– 17268. doi: 10.1074/jbc.M000689200. [DOI] [PubMed] [Google Scholar]
- 180.Chen L, Juszczynski P, Takeyama K, Aguiar RCT, Shipp MA. Protein tyrosine phosphatase receptor–type O truncated (PTPROt) regulates SYK phosphorylation, proximal B-cell–receptor signaling, and cellular proliferation. Blood. 2006;108:3428–3433. doi: 10.1182/blood-2006-03-013821. [DOI] [PubMed] [Google Scholar]
- 181.Cloutier J, Veillette A. Cooperative inhibition of T cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 1999;189:111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Agrawal R, Carpino N, Tsygankov A. TULA proteins regulate activity of the protein tyrosine kinase Syk. J Cell Biochem. 2008;104:953–964. doi: 10.1002/jcb.21678. [DOI] [PubMed] [Google Scholar]
- 183.Bottini N, Stefanini L, Williams S, Alonso A, Jascur T, Abraham RT, Couture C, Mustelin T. Activation of ZAP-70 through specific dephosphorylation at the inhibitory Tyr-292 by the low molecular weight phosphotyrosine phosphatase (LMPTP) J Biol Chem. 2002;277:24220–24224. doi: 10.1074/jbc.M202885200. [DOI] [PubMed] [Google Scholar]
- 184.Geahlen RL. Syk, AfCS-Nature Molecule Pages. 2007 doi: 10.1038/mp.a000040.01. [DOI] [Google Scholar]
- 185.Rodriguez R, Matsuda M, Perisic O, Bravo J, Paul A, Jones NP, Light Y, Swann K, Williams RL, Katan M. Tyrosine residues in phospholipase C ***2 essential for the enzyme function in B-cell signaling. J Biol Chem. 2001;276:47982–47992. doi: 10.1074/jbc.M107577200. [DOI] [PubMed] [Google Scholar]
- 186.Kim YJ, Sekiya F, Poulin B, Bae YS, Rhee SG. Mechanism of B-cell receptor- induced phosphorylation and activation of phospholipase C-γ2. Mol Cell Biol. 2004;24:9986–9999. doi: 10.1128/MCB.24.22.9986-9999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rawlings DJ, Scharenbert AM, Park H, Wahl MI, Lin S, Kato RM, Fluckiger AC, Witte ON, Kinet JP. Activation of BTK by a phosphorylation mechanism initiated by SRC family kinases. Science. 1996;271:822–825. doi: 10.1126/science.271.5250.822. [DOI] [PubMed] [Google Scholar]
- 188.Baba Y, Hashimoto S, Matsushita M, Watanabe D, Kishimoto T, Kurosaki T, Tsukada S. BLNK mediates Syk-dependent Btk activation. Proc Natl Acad Sci U S A. 2001;98:2582–2586. doi: 10.1073/pnas.051626198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tsuji S, Okamoto M, Yamada K, Okamoto N, Goitsuka R, Arnold R, Kiefer F, Kitamura D. B cell adaptor containing src homology 2 domain (BASH) links B cell receptor signaling to the activation of hematopoietic progenitor kinase 1. J Exp Med. 2001;194:529–539. doi: 10.1084/jem.194.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Crespo P, Schuebel KE, Ostrom AA, Gutkind JS, Bustelo XR. Phosphotyrosine-dependent activation of Rac-1 GDP/GTP exchange by the vav proto-oncogene product. Nature. 1997;385:169–172. doi: 10.1038/385169a0. [DOI] [PubMed] [Google Scholar]
- 191.Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol. 2000;20:1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Amarasinghe GK, Rosen MK. Acidic region tyrosines provide access points for allosteric activation of the autoinhibited Vav1 Dbl homology domain. Biochemistry. 2005;44:15257–15268. doi: 10.1021/bi051126h. [DOI] [PubMed] [Google Scholar]
- 193.Brunati AM, Donella-Deana A, Ruzzene M, Marin O, Pinna LA. Site specificity of p72 protein tyrosine kinase: efficient phosphorylation of motifs recognized by Src homology 2 domains. FEBS Lett. 1995;367:149–152. doi: 10.1016/0014-5793(95)00555-n. [DOI] [PubMed] [Google Scholar]
- 194.Kurosaki T. Regulation of B-cell signal transduction by adaptor proteins. Nat Rev Immunol. 2002;2:354–363. doi: 10.1038/nri801. [DOI] [PubMed] [Google Scholar]
- 195.Togni M, Lindquist J, Gerber A, Kolsch U, Hamm-Baarke A, Kliche S, Schraven B. The role of adaptor proteins in lymphocyte activation. Mol Immunol. 2004;41:615–630. doi: 10.1016/j.molimm.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 196.Simeoni L, Kliche S, Lindquist J, Schraven B. Adaptors and linkers in T and B cells. Curr, Opin Immunol. 2004;16:304–313. doi: 10.1016/j.coi.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 197.Janssen E, Zhang WG. Adaptor proteins in lymphocyte activation. Curr Opin Immunol. 2003;15:269–276. doi: 10.1016/s0952-7915(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 198.Brdicka T, Imrich M, Angelisova P, Brdickova N, Horvath O, Spicka J, Hilgert I, Luskova P, Draber P, Novak P, Engels N, Wienands J, Simeoni L, Osterreicher J, Aguado E, Malissen M, Schraven B, Horejsi V. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J Exp Med. 2002;196:1617–1626. doi: 10.1084/jem.20021405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Janssen E, Zhu M, Zhang W, Koonpaew S, Zhang W. LAB: A new membrane-associated adaptor molecule in B-cell activation. Nat Immunol. 2003;4:117–123. doi: 10.1038/ni882. [DOI] [PubMed] [Google Scholar]
- 200.Ren R, Mayer BJ, Cicchetti P, Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993;259:1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- 201.Maeno K, Sada K, Kyo S, Miah SMS, Kawauchi-Kamata K, Qu X, Shi Y, Yamamura H. Adaptor protein 3BP2 is a potential ligand of Src homology 2 and 3 domains of Lyn protein-tyrosine kinase. J Biol Chem. 2003;278:24912–24920. doi: 10.1074/jbc.M301201200. [DOI] [PubMed] [Google Scholar]
- 202.Yokoyama K, Su I, Tezuka T, Yasuda T, Mikoshiba K, Tarakhovsky A, Yamamoto T. BANK regulates BCR-induced calcium mobilization by promoting tyrosine phosphorylation of IP3 receptor. EMBO J. 2002;21:83–92. doi: 10.1093/emboj/21.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pan Z, Shen Y, Ge B, Du C, McKeithan T, Chan WC. Studies of a germinal centre B-cell expressed gene, GCET2, suggest its role as a membrane associated adapter protein. Br J Haematol. 2007;137:578–590. doi: 10.1111/j.1365-2141.2007.06597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Low PS, Allen DP, Chari P, Zioncheck TF, Geahlen RL, Harrison ML. Tyrosine phosphorylation of band 3 inhibits peripheral protein binding. J Biol Chem. 1987;262:4592–4596. [PubMed] [Google Scholar]
- 205.Schneider ML, Post CB. Solution structure of a Band 3 peptide inhibitor bound to aldolase: A proposed mechanism for regulating binding by tyrosine phosphorylation. Biochemistry. 1995;34:16574–16584. doi: 10.1021/bi00051a005. [DOI] [PubMed] [Google Scholar]
- 206.Eisenmesser EZ, Post CB. Insights into tyrosine phosphorylation control of protein-protein association from the NMR structure of a Band 3 peptide inhibitor bound to glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1998;37:867–877. doi: 10.1021/bi971445b. [DOI] [PubMed] [Google Scholar]
- 207.Volker KW, Knull HR. A glycolytic enzyme binding domain on tubulin. Arch Biochem Biophys. 1997;338:237–243. doi: 10.1006/abbi.1996.9819. [DOI] [PubMed] [Google Scholar]
- 208.Harrison ML, Isaacson CC, Burg DL, Geahlen RL, Low PS. Phosphorylation of human erythrocyte band 3 by endogenous p72syk. J Biol Chem. 1994;269:955–959. [PubMed] [Google Scholar]
- 209.Schmitz R, Baumann G, Gram H. Catalytic specificity of phosphotyrosine kinases Blk, Lyn, c-Src and Syk as assessed by phage display. J Mol Biol. 1996;260:664–677. doi: 10.1006/jmbi.1996.0429. [DOI] [PubMed] [Google Scholar]
- 210.Brunati AM, Bordin L, Clari G, James P, Quadroni M, Baritono E, Pinna LA, Donella-Deana A. Sequential phosphorylation of protein band 3 by Syk and Lyn tyrosine kinases in intact human erythrocytes: identification of primary and secondary phosphorylation sites. Blood. 2000;96:1550–1557. [PubMed] [Google Scholar]
- 211.Feshchenko EA, Langdon WY, Tsygankov AY. Fyn, Yes, and Syk phosphorylation sites in c-Cbl map to the same tyrosine residues that become phosphorylated in activated T cells. J Biol Chem. 1998;273:8323–8331. doi: 10.1074/jbc.273.14.8323. [DOI] [PubMed] [Google Scholar]
- 212.Bewarder N, Weinrich V, Budde P, Hartmann D, Flaswinkel H, Reth M, Frey J. In vivo and in vitro specificity of protein tyrosine kinases for immunoglobulin G receptor (FcgammaRII) phosphorylation. Mol Cell Biol. 1996;16:4735–4743. doi: 10.1128/mcb.16.9.4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mahrenholz AM, Votaw P, Roach PJ, DePaoli-Roach AA, Zioncheck TF, Harrison ML, Geahlen RL. Phosphorylation of glycogen synthase by a bovine protein-tyrosine kinase, p40. Biochem Biophys Res Commun. 1988;155:52–58. doi: 10.1016/s0006-291x(88)81048-9. [DOI] [PubMed] [Google Scholar]
- 214.Gomez TS, McCarney SD, Carrizosa E, Labno CM, Comiskey EO, Nolz JC, Zhu P, Freedman BD, Clark MR, Rawlings DJ, Billadeau DD, Burkhardt JK. HS1 functions as an essential actin-regulatory adaptor protein at the immune synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yamanashi Y, Fukuda T, Nishizumi H, Inazu T, Higashi K, Kitamura D, Ishida T, Yamamura H, Watanabe T, Yamamoto T. Role of tyrosine phosphorylation of HS1 in B cell antigen receptor-mediated apoptosis. J Exp Med. 1997;185:1387–1392. doi: 10.1084/jem.185.7.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brunati AM, Deana R, Folda A, Massimino ML, Marin O, Ledro S, Pinna LA, Donella-Deana A. Thrombin-induced tyrosine phosphorylation of HS1 in human platelets is sequentially catalyzed by Syk and Lyn tyrosine kinases and associated with the cellular migration of the protein. J Biol Chem. 2005;280:21029–21035. doi: 10.1074/jbc.M412634200. [DOI] [PubMed] [Google Scholar]
- 217.Iwaki S, Jiri S, Tkaczyk C, Jensen BM, Furumoto Y, Charles N, Kovarova M, Rivera J, Horejsi V, Metcalfe DD, Gilfillan AM. Kit- and FcεRI-induced differential phosphorylation of the transmembrane adaptor molecule NTAL/LAB/LAT2 allows flexibility in its scaffolding function in mast cells. Cell Signal. 2008;20:195–205. doi: 10.1016/j.cellsig.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kawakami Y, Kitaura J, Yao L, McHenry RW, Kawakami Y, Newton AC, Kang S, Kato RM, Leitges M, Rawlings DJ, Kawakami T. A Ras activation pathway dependent on Syk phosphorylation of protein kinase C. Proc Natl Acad Sci USA. 2003;100:9470–9475. doi: 10.1073/pnas.1633695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shim EK, Moon CS, Lee GY, Ha YJ, Chae SK, Lee JR. Association of the Src homology 2 domain-containing leukocyte phosphoprotein of 76 kD (SLP-76) with the p85 subunit of phosphoinositide 3-kinase. FEBS Lett. 2004;575:35–40. doi: 10.1016/j.febslet.2004.07.090. [DOI] [PubMed] [Google Scholar]
- 220.Negro A, Brunati AM, Donella-Deana A, Massimino ML, Pinna LA. Multiple phosphorylation of alpha-synuclein by protein tyrosine kinase Syk prevents eosin-induced aggregation. FASEB J. 2002;16:210–212. doi: 10.1096/fj.01-0517fje. [DOI] [PubMed] [Google Scholar]