Abstract
Neurocognitive studies have observed rIFC involvement in motor, cognitive, and affective inhibition, suggesting that rIFC is a common inhibitory mechanism across psychological domains. If true, intentional inhibition in one domain may have unintended inhibitory effects (‘spillover’) in other domains. The present study used an emotional go/no-go task that produces responses in both motor and affective domains, but induces intentional inhibition in only the motor domain. Data support the hypothesis that intentional inhibition in the motor domain, via rIFC, produces inhibitory spillover in the affective domain. Specifically, we observed increased rIFC along with reduced amygdala activity when participants intentionally inhibited motor responses during the presentation of negatively-valenced stimuli, and greater inverse connectivity between these regions during motor inhibition in a PPI analysis. Given the absence of intentional affect regulation, these results suggest that intentional inhibition of a motor response dampens the amygdala activation coincident with affective stimuli to the extent that rIFC activation is higher.
The human ability to inhibit unwanted thoughts, feelings, and behaviors is central to effective goal pursuit in daily life. On a process level, it might be efficient for inhibition of motor, cognitive, and affective responses to share a neurological mechanism, but the subjective experience of inhibiting each of these responses feels different from one another. It is therefore unclear whether these different forms of inhibition depend on common or disparate neurocognitive systems. Recent cognitive neuroscience research in each domain independently implicates right inferior frontal cortex (rIFC) and suggests that this region may be a point of convergence involved in various forms of inhibition. If rIFC is a common inhibitory mechanism across various domains, it is possible that intentional inhibition in one domain (e.g. inhibiting a motor response) may lead to incidental inhibitory “spillover” in another domain (e.g. suppressing affective responses). However, because the research to date has typically been limited to a single domain in each investigation, the spillover hypothesis remains untested.
The role of rIFC in inhibition is best established in the domain of motor inhibition. Studies of motor inhibition have consistently identified a network of brain regions including rIFC, anterior cingulate cortex (ACC), and dorsolateral prefrontal cortex (DLPFC). These studies commonly use the go/no-go task during which participants press a button on most trials (‘go’) and thus form a prepotent response to press the button. However, on some trials (‘no-go’) a cue indicates that the response should be withheld. These and other similar motor inhibition studies (Aron, Robbins, & Poldrack, 2004; Menon, Adleman, White, Glover, & Reiss, 2001) typically find greater rIFC, ACC, and DLPFC (BA 9/46, e.g. [−34 50 32] and [26 46 30] as reported by Menon et al., 2001) during the no-go trials than the go trials, suggesting these regions are involved in detecting the need to inhibit a response and in the inhibition itself. A recent meta-analysis of studies using the go/no-go task implicates the rIFC in response inhibition during no-go trials relative to baseline (Simmonds, Pekar, & Mostofsky, 2008). Multiple neuropsychological studies (Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003; Picton et al., 2007) and a transcranial magnetic stimulation study (Chambers et al., 2006) have also demonstrated that permanent and temporary lesions to the rIFC impair response inhibition.
Studies of cognitive inhibition have also implicated rIFC, although some forms of cognitive inhibition may depend more on left IFG (Jonides, Smith, Marshuetz, Koeppe, & Reuter-Lorenz, 1998; Menon et al., 2001). Similar to the motor inhibition findings, recent studies of thought suppression (Anderson & Green, 2001; Mitchell et al., 2007) have observed increased activation in the rIFC, DLPFC, and ACC network during intentional attempts at suppressing a thought. Additionally, Goel and Dolan (2003) found that rIFC was the only brain region associated with overcoming the ‘belief bias’ in which syllogisms that are not logically sound, because one of their premises is false, must still be judged as logically valid. In this task, participants must inhibit the common initial response that the syllogism is illogical because one of its premises is false.
Finally, most studies that have examined inhibition of affective processing report rIFC or the overlapping region of right lateral orbitofrontal cortex, with a number of these studies also reporting left IFC activations. Several of the studies on ‘reappraisal’, or the conscious inhibition of affective responses by cognitive reinterpretation, have observed increased rIFC (Kim & Hamann, 2007; Ochsner et al., 2004) and when it has been measured, activity in rIFC is typically associated with decreased limbic activity and diminished self-reported distress (Ochsner et al., 2004; Phan et al., 2005). A related series of studies demonstrated that negative face stimuli presented during the delay period of a working memory task disrupted performance, but participants who showed increased rIFC activity during the delay period reported feeling less distracted by the negative (but not neutral) faces (Dolcos & McCarthy, 2006). Additionally, when individuals experience negative personal events such as social rejection (Eisenberger, Lieberman, & Williams, 2003), physical pain (Wager et al., 2004) or unfair treatment in a bargaining game (Tabibnia, Satpute, & Lieberman, 2008) increased rIFC activity is associated with decreased limbic activity and diminished self-reported distress, suggesting affective inhibition.
Thus, numerous studies have examined inhibitory processes within a single domain and have implicated rIFC in the inhibitory processes within each separate domain. These studies are conducted by creating a prepotent response or activation in the domain of interest and then examining which brain regions are more active when this prepotent response is overcome. If rIFC serves as a common inhibitory mechanism, one might expect that recruiting this region via intentional inhibition in one domain might simultaneously, and unintentionally, inhibit responses across other domains (i.e. inhibitory spillover). Given that the previous studies have systematically created responses only in the domain under investigation, it is unlikely that there would be evidence of unintended inhibition in other domains. For example, if motor tasks do not produce affective responses, there would be no affective response to incidentally inhibit when explicitly inhibiting a motor response. To address this, in the current study we used a modified version of the emotion go/no-go task in order to simultaneously induce a prepotent motor response and an incidental affective response. Producing two responses during the task, only one of which was related to the task and intentionally inhibited, allowed us to examine whether intentional inhibition in one domain (motor) also produced inhibitory spillover in the other domain (affect).
In previous neuroimaging studies of the emotional go/no-go task (Hare, Tottenham, Davidson, Glover, & Casey, 2005; Shafritz, Collins, & Blumberg, 2006), participants responded to pictures of faces via a button press (‘go’) or by withholding a button press (‘no-go’) based on the emotional valence of the face (e.g. “press for sad; don’t press for happy”). In these studies, motor inhibition was experimentally yoked to the valence of the stimulus and thus confounded with affect. In our task, responses are based on the gender of the face, and importantly, the affective valence of the face is independent of gender. Participants view affective stimuli, which should produce an affective response in the limbic system, even though the emotional content of the stimuli is incidental to the task. This alteration of the emotional go/no-go task is critical to separating cases where affective stimuli incidentally recruit rIFC (e.g. Dolcos & McCarthy, 2006) from those where rIFC activity related to motor inhibition might nonetheless modulate amygdala activation. In other words, this task structure allows us to examine whether increased rIFC activity during motor inhibition produces decreased limbic responses despite the absence of intentional affect inhibition.
Based on previous work on motor inhibition (Aron et al., 2004), we expected inhibition during the no-go trials, regardless of stimulus valence, to be associated with activation in an inhibitory network including rIFC, ACC, and DLPFC. Additionally, negatively valenced images were expected to produce amygdala activation (Costafreda, Brammer, David, & Fu, 2008; Morris et al., 1996; Phan, Wager, Taylor, & Liberzon, 2002), even though participants are attending to gender and not to affect explicitly (Costafreda et al., 2008; Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003). If rIFC produces inhibitory spillover into the affective domain during intentional inhibition of motor responses, then amygdala responses to negative images should be diminished during no-go trials relative to go trials, because no-go trials should recruit rIFC in the process of motor inhibition. Further, if amygdala activation is reduced specifically by the motor inhibition during no-go trials (as opposed to reductions due to increased task difficulty during no-go trials relative to go trials), then amygdala activation should also be reduced during no-go relative to resting baseline. Finally, to establish functional connectivity that is selective to no-go trials, time course of activation in rIFC and the amygdala should also be more strongly inversely correlated during negative no-go than go trials.
Method
Subjects
Fourteen right-handed participants (6 male; ages 21–35, M =25.6 ± 3.8) were recruited from the UCLA community and paid $25 for participating. Data from one male and one female were excluded due to excessive head motion during scanning, yielding twelve participants in the analyses. All participants provided written informed consent that was approved by the UCLA Office for Protection of Research Subjects.
Stimuli
The images presented during the go/no-go task were photographs drawn from the NimStim face set (Tottenham, Borscheid, Ellertsen, Marcus, & Nelson, 2002). Four negative (fear closed-mouth, fear open-mouth, anger closed-mouth, anger open-mouth) and 1 positive (happy closed-mouth) images were selected from each of 18 male and 23 female actors. Seven of the actors do not have one of the negative images, yielding a total of 157 unique negative images and 41 unique positive images. In order to reduce amygdala habituation to the negative images (Wright, Fischer, Whalen, McInerney, Shin, & Rauch, 2001), the stimuli were grouped into blocks that contained half positive and half negative images. Across the experiment, each negative image was presented no more than twice (and never more than once per block), and each positive image was presented no more than 6 times (and never more than once per block). Because the inhibitory spillover hypothesis is focused on responses to negative stimuli in this paradigm, there was less concern over having matched negative and positive stimuli. The positive images were included to minimize habituation to the negative images. Each block was either 80% male (“go male / no-go female” blocks) or 80% female (“go female / no-go male” blocks).
Procedure
Participants performed a modified version of the emotional go/no-go task. They saw a series of male and female faces, each expressing a positive or negative emotion. For half of the blocks, participants were instructed to press a button each time a female face was presented (‘go’) and not to respond when a male face was presented (‘no-go’). The gender instructions were reversed for the other half of the blocks. Within each block the ‘go’ gender was presented on 80% of the trials and the ‘no-go’ gender was presented on 20% of the trials. Blocks were composed of 50% positive facial expression trials and 50% negative facial expression trials. Valence of the facial expressions was incidental to the task and did not co-vary with target gender or go/no-go instructions. This design yielded eight conditions: go positive (go+), go negative (go−), no-go positive (no-go+), and no-go negative (no-go−), with male and female target versions of each. There were 100 no-go and 400 go trials evenly divided among positive and negative faces.
The task was divided across 2 functional runs, each with 5 blocks. The order of the blocks within each run alternated which gender was the ‘go’ gender, and was counterbalanced across participants. There was a 16-second fixation rest between the blocks, where the final 2 seconds of the rest period contained instructions for the following block to either “Press for Males” or “Press for Females.” Each block contained 50 1-second trials, separated by a fixation-cross baseline ISI that varied randomly in duration according to a gamma distribution from 0 to 8000ms (M = 1500ms). The order of the trials within blocks was optimized for event-related analysis using the OptimizeDesign algorithm (Wager & Nichols, 2003).
Foam padding was placed around participants’ heads to reduce motion. Stimuli were presented on LCD goggles, and responses were recorded on a magnet-safe button box placed in the right hand.
Image acquisition and analysis
Data were acquired on a Siemens Allegra 3T scanner at the UCLA Ahmanson-Lovelace Brainmapping Center. High-resolution structural T2-weighted echo-planar images (spin-echo; TR = 5000 ms; TE = 33 ms; matrix size 128 × 128; 36 sagittal slices; FOV = 20 cm; 3 mm thick, skip 1 mm) were acquired coplanar with the functional scans. Two functional scans lasting 2 min, 50 sec were acquired during the task (echo-planar T2*-weighted gradient-echo, TR = 2000 ms, TE = 25 ms, flip angle = 90°, matrix size 64 × 64, 34 axial slices, FOV = 20 cm; 3 mm thick, skip 1 mm).
The imaging data were analyzed using SPM5 (Wellcome Department of Cognitive Neurology, Institute for Neurology, London, UK). Images from each participant were realigned to correct for head motion, slice-time corrected to adjust for relative timing within each TR, normalized into the Montreal Neurological Institute standard stereotactic space, and smoothed with an 8 mm Gaussian kernel, full width at half maximum. The design was modeled as an event-related 2 (target gender: male/female) × 2 (response: go/no-go) × 2 (valence: positive/negative) factorial with the jittered ISIs and fixation rests excluded to form a baseline. No effects of target gender were observed so we collapsed across male-target and female-target trials. The final design included in analyses had 4 conditions: go+, go−, no-go+, and no-go−. Linear contrasts were computed to assess neural activity during each condition and main effects compared to baseline, and among the experimental conditions compared with each other.
Finally, psychophysiological interactions (PPIs) were computed for each subject to examine differences in functional connectivity among neural regions between task conditions. In these analyses, an interaction between neural activity (deconvolved from the hemodynamic response) in a seed region (e.g. amygdala) and task condition (e.g. no-go− versus go−) was generated for each participant (Friston et al., 1997). Whole-brain parameter estimates were then regressed onto this interaction to search for other regions that were differentially associated with the seed region between the two task conditions. The results can be interpreted as a functional, task-dependent association between the two regions.
For all analyses, individual participant contrasts were generated with fixed-effects models and then grouped into a random-effects model to allow for greater generalizability. An uncorrected p-value of .001 combined with a cluster size threshold of 10 voxels to control for multiple comparisons was used (Forman et al., 1995). Because of the a priori hypotheses of inverse connectivity between rIFC and amygdala, we generated an 8-mm spherical ROI around the maximum amygdala voxel identified in the main effect analysis of go− > no-go−. We used this ROI with a .05 significance threshold to identify functional connectivity between the rIFC seed and the amygdala in a PPI analysis. All neuroimaging results are reported in Montreal Neurological Institute (MNI) coordinates.
Results
Behavioral results of motor inhibition
Participants were able to successfully inhibit motor responses on 98.4% of the no-go trials. Neither the average error rate on the no-go trials nor the average response time on the go trials was different between positively- and negatively-valenced faces (paired t13 = 1.24 and 0.84, respectively, both ns). The inaccurate trials were discarded for all further analyses.
Neural activations during motor inhibition
Replicating findings from previous motor inhibition studies (Menon et al., 2001), no-go trials (as compared to go trials) activated rIFC (BA 47), DLPFC (BA 9/10), and ACC (BA 32). Table 1 provides the size and maximal voxel activations for each of these clusters. The precise location of the rIFC activation in the go/no-go task varies from study to study (Simmonds et al., 2008), but activity in this ventral location has been identified by several other groups (Horn, Dolan, Elliott, Deakin, & Woodruff, 2003; Liddle, Kiehl, & Smith, 2001). The only region more active during go than no-go trials was the precentral gyrus (BA 4/6).
Table 1.
Neural regions that showed increased activity during motor inhibition
| Region | x | y | z | Cluster size | t-val | |
|---|---|---|---|---|---|---|
| No-go > go | rIFC (BA 47) | 38 | 24 | −12 | 135 | 4.96 |
| Anterior cingulate (BA 32) | −6 | 12 | 44 | 41 | 4.48 | |
| DLPFC (BA 9/10) | −26 | 48 | 22 | 74 | 5.64 | |
| 48 | 42 | 26 | 45 | 6.35 | ||
| Superior temporal (BA 22) | 54 | −42 | 8 | 11 | 5.47 | |
| Occipital (BA 17/18) | 2 | −94 | −2 | 389 | 7.94 | |
|
| ||||||
| Go > No-go | Precentral gyrus (BA 4/6) | 12 | −24 | 68 | 24 | 6.34 |
Note: All activations: p < .001, uncorrected, 10-voxel extent threshold. BA = Brodmann’s area; DLPFC = dorsolateral prefrontal cortex; IFC = inferior frontal cortex.
Neural activations to emotional faces
Next, we examined the effect of target face valence on brain activity. Replicating previous findings (Morris et al., 1996), viewing negative faces (compared to baseline) activated the amygdala and other limbic structures including ventral striatum and anterior insula. Viewing positive faces (compared to baseline) activated multiple limbic regions, including amygdala and ACC (Table 2). Though amygdala is commonly associated with threatening emotional faces, amygdala activation in response to positive emotional faces has been observed in multiple previous studies (Hamann, Ely, Hoffman, & Kilts, 2002; Yang et al., 2002) and is consistent with the growing consensus that the amygdala is more sensitive to emotional intensity or relevance rather than valence, per se (Cunningham, Raye, & Johnson, 2004). Due to this increased activation to both positive and negative emotional faces, there were no differences in the direct contrast between these two conditions.
Table 2.
Neural regions that showed increased activity during emotion perception
| Region | x | y | z | Cluster size | t-val | |
|---|---|---|---|---|---|---|
| Negative > baseline | Amygdala | −22 | 0 | −24 | 116 | 5.42 |
| 28 | −4 | −24 | 77 | 4.92 | ||
| Ventral striatum | 28 | 8 | −2 | 69 | 3.41 | |
| Dorsal striatum | −18 | −2 | 16 | 53 | 3.37 | |
| Anterior insula | −36 | 24 | 2 | 98 | 4.73 | |
| 40 | 20 | 6 | 142 | 4.70 | ||
| DLPFC (BA 45/46) | −50 | 38 | 26 | 19 | 4.13 | |
| 48 | 26 | 22 | 123 | 3.71 | ||
| Precentral gyrus (BA 6) | 48 | 2 | 40 | 80 | 4.50 | |
| −56 | 8 | 42 | 119 | 3.94 | ||
| Supplementary motor area (BA 6) | −10 | 6 | 64 | 449 | 6.68 | |
| Occipital (BA 17/18) | 24 | −78 | −10 | 4054 | 15.91 | |
| −20 | −86 | −10 | 4003 | 12.13 | ||
|
| ||||||
| Positive > baseline | Amygdala | −28 | −8 | −22 | 183 | 5.46 |
| 28 | −6 | −22 | 65 | 5.04 | ||
| Dorsal striatum | −10 | −10 | 8 | 87 | 4.67 | |
| Hippocampus | −22 | −16 | −16 | 292 | 10.23 | |
| dACC (BA 32) | 10 | 14 | 40 | 302 | 6.47 | |
| −6 | 12 | 44 | 385 | 6.81 | ||
| DLPFC (BA 9/10) | 44 | 52 | 22 | 142 | 6.80 | |
| −38 | 54 | 24 | 111 | 5.77 | ||
| Anterior insula | 42 | 18 | 0 | 330 | 5.66 | |
| −36 | 24 | −2 | 423 | 7.43 | ||
| Brain stem | 10 | −24 | −16 | 241 | 15.29 | |
| −10 | −24 | −18 | 224 | 8.15 | ||
| SMA (BA 6) | −8 | 6 | 64 | 446 | 8.60 | |
| Precentral gyrus (BA 6) | −44 | 6 | 32 | 298 | 6.71 | |
| 52 | 4 | 40 | 224 | 5.59 | ||
| Occipital (BA 18/19) | 24 | −76 | −10 | 3005 | 16.35 | |
| −22 | −82 | −18 | 2380 | 11.84 | ||
Note: All activations: p < .001, uncorrected, 10-voxel extent threshold. BA = Brodmann’s area; dACC = dorsal anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; SMA = supplementary motor area.
Effects of motor inhibition on negatively-valenced trials
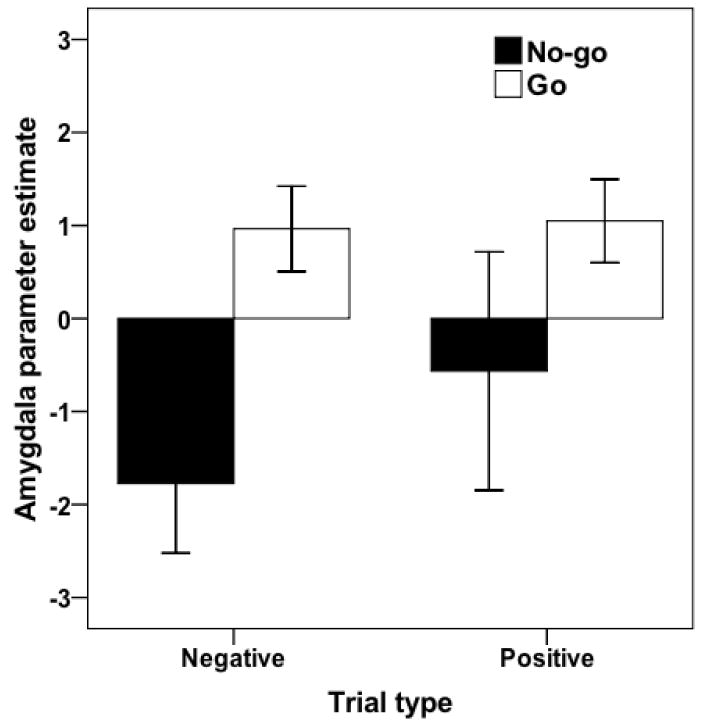
Emotion trials were divided by task condition to further clarify the preceding results. Extending previous work on motor inhibition, we observed increased activity during no-go− (relative to go−) in rIFC, DLPFC, and ACC (Table 3; Figure 1), suggesting that motor inhibition during viewing of negative stimuli recruits the same inhibitory network observed in other research. Next, two results emerged that together are consistent with the inhibitory spillover hypothesis. First, amygdala and ventral striatum were less active during no-go− relative to go− trials (Figure 2), suggesting that during intentional motor inhibition there is decreased limbic activity to a stimulus that would otherwise produce substantial limbic activity (Morris et al., 1996). Second, amygdala activity was significantly reduced during no-go− relative to baseline (Table 4; Figure 2). This reduction relative to baseline is important because incidental presentation of emotional stimuli has reliably produced increased amygdala activity in numerous previous studies (Liddell et al., 2005; Morris, Ohman, & Dolan, 1998; c.f. Pessoa, McKenna, Gutierrez, & Ungerleider, 2002). Here, we observed a reduction in amygdala activity during no-go relative to go trials and also relative to baseline. Taken together, these findings suggest that the difference in amygdala activity between the no-go and go trials is driven at least in part by a reduction in amygdala activity during inhibition, and not just by inattention to the affective content of the stimuli during inhibition or a relative increase in amygdala activity during go trials. Limbic reductions to negative stimuli during intentional motor inhibition coupled with increased rIFC activity are consistent with the inhibitory spillover hypothesis.
Table 3.
Neural regions that showed increased activity during motor inhibition
| Region | x | y | z | Cluster size | t-val | |
|---|---|---|---|---|---|---|
| No-go− > go− | rIFC (BA 47) | 40 | 16 | −18 | 53 | 3.93 |
| DLPFC (BA 45) | 52 | 30 | 24 | 131 | 3.66 | |
| VMPFC (BA 11) | 18 | 36 | −12 | 15 | 5.58 | |
| dACC (BA 32) | 2 | 12 | 44 | 114 | 3.48 | |
| rACC (BA 33) | 2 | 24 | −4 | 338 | 4.11 | |
| Superior temporal (BA 38) | 56 | 10 | −12 | 20 | 3.59 | |
| −54 | 8 | −12 | 43 | 3.91 | ||
| Inferior parietal (BA 40) | 56 | −30 | 32 | 35 | 4.34 | |
| Anterior insula | 40 | 24 | 6 | 52 | 3.84 | |
| Hippocampus | 28 | −26 | −10 | 122 | 4.79 | |
|
| ||||||
| Go− > no-go− | Amygdala | −18 | −4 | −28 | 12 | 3.77 |
| Ventral striatum | −22 | 4 | −4 | 63 | 6.20 | |
| SMA (BA 6) | 16 | −22 | 70 | 10 | 3.99 | |
|
| ||||||
| No-go+ > go+ | rIFC (BA 47) | 48 | 20 | −22 | 157 | 7.37 |
| lIFC (BA 46) | −48 | 42 | 2 | 63 | 4.38 | |
| DLPFC (BA 9/10) | 46 | 48 | 18 | 249 | 5.31 | |
| dACC (BA 32) | 4 | 32 | 18 | 508 | 4.74 | |
| Superior temporal (BA 22) | 54 | −4 | −4 | 43 | 3.73 | |
|
| ||||||
| Go+ > no-go+ | SMA (BA 6) | 12 | −24 | 66 | 50 | 6.70 |
Note: All activations: p < .001, uncorrected, 10-voxel extent threshold. BA = Brodmann’s area; dACC = dorsal anterior cingulate cortex; DLPFC = dorsolateral prefrontal cortex; IFC = inferior frontal cortex; rACC = rostral anterior cingulate cortex; SMA = supplementary motor area; VMPFC = ventromedial prefrontal cortex.
Figure 1.
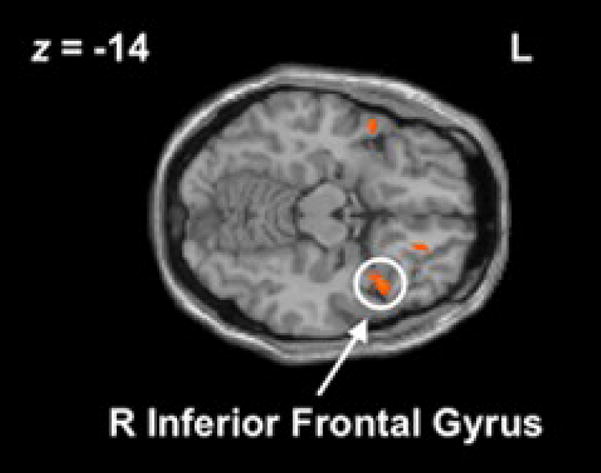
Right inferior frontal cortex (x=40, y=16, z=−18) activation observed during no-go− > go−. This region was used as a seed to identify target regions more negatively associated with rIFC during no-go− than go−.
Figure 2.
Parameter estimates for amygdala (x=−18, y=−4, z=−28) in no-go−, go−, no-go+, and go+. There was a significant reduction during no-go− trials (t = −5.12, p < .001) trials, and significant increases during go− and go+ trials (ts = 4.55, 5.07, respectively, both ps < .001). There was significantly greater activity during go− than no-go− (t = 6.57, p < .001). Error bars depict 95% confidence interval.
Table 4.
Neural regions that showed increased activity during negative stimuli
| Region | x | y | z | Cluster size | t-val | |
|---|---|---|---|---|---|---|
| No-go− > base | IFC | 44 | 16 | −6 | 374 | 5.77 |
| −48 | 18 | 10 | 464 | 4.25 | ||
| DLPFC (BA 9) | −28 | 62 | 26 | 63 | 7.26 | |
| 40 | 52 | 28 | 40 | 6.06 | ||
| Superior temporal (BA 38) | −50 | 12 | −10 | 21 | 4.08 | |
| Hippocampus | −30 | −24 | −10 | 316 | 5.70 | |
| 32 | −28 | −10 | 54 | 3.15 | ||
| Anterior insula | 40 | 20 | 4 | 122 | 4.82 | |
| Cingulate (BA 31/32) | −6 | 12 | 48 | 150 | 6.52 | |
| SMA (BA 6) | −2 | 4 | 66 | 264 | 5.05 | |
| Precentral gyrus (BA 6) | 40 | 6 | 62 | 136 | 8.16 | |
| Occipital (BA 17/18) | −24 | −80 | −12 | 11358 | 15.32 | |
| 40 | −84 | −12 | 11358 | 12.81 | ||
|
| ||||||
| Go− > baseline | Amygdala | −20 | −4 | −24 | 20 | 4.46 |
| 22 | −2 | −24 | 38 | 3.84 | ||
| Dorsal striatum | 24 | 2 | 8 | 248 | 3.58 | |
| −22 | 4 | 2 | 79 | 3.50 | ||
| DLPFC | −54 | 30 | 18 | 143 | 5.11 | |
| −56 | 10 | 40 | 12 | 3.69 | ||
| Medial OFC | −6 | 48 | −22 | 67 | 5.86 | |
| Dorsal cingulate (BA 32) | −8 | 16 | 46 | 68 | 3.89 | |
| SMA (BA 6) | −8 | 6 | 68 | 468 | 5.43 | |
| Occipital (BA 17/18) | −16 | −88 | −12 | 2068 | 11.87 | |
| 14 | −90 | −14 | 1464 | 8.92 | ||
|
| ||||||
| Base > no-go− | Amygdala | −14 | −6 | −30 | 17 | 4.25 |
| Superior temporal (BA 22) | −52 | −8 | 4 | 25 | 3.90 | |
| Ventral striatum | −10 | 2 | −4 | 16 | 3.98 | |
| Medial prefrontal cortex (BA 10) | −10 | 68 | 14 | 10 | 4.61 | |
|
| ||||||
| Base > go− | Hippocampus | −30 | −20 | −26 | 26 | 4.99 |
| Postcentral gyrus (BA 2) | −22 | −32 | 74 | 55 | 3.83 | |
| Superior parietal gyrus (BA 7) | 24 | −54 | 72 | 247 | 4.17 | |
| −8 | −52 | 74 | 55 | 4.05 | ||
Note: All activations: p < .001, uncorrected, 10-voxel extent threshold. BA = Brodmann’s area; DLPFC = dorsolateral prefrontal cortex; IFC = Inferior frontal cortex; OFC = orbitofrontal cortex; SMA = supplementary motor area.
Effects of motor inhibition on positively-valenced trials
As with the negatively-valenced trials, we observed increased rIFC activation during no-go+ relative to go+, as well as increases in DLPFC and dACC (Table 3), suggesting that motor inhibition recruits a similar network regardless of the valence of affective faces that are incidental to the task. However, unlike the relative limbic decreases observed during the negatively-valenced trials, there were no comparable reductions in limbic regions during go+ relative to no-go+ (Figure 2). The interaction of stimulus valence (positive/negative) and motor task (go/no-go) on activation in left amygdala (x=−18, y=−4, z=−28) was marginally significant (F1,13=2.44, p<.08 one-tailed). It is possible that the relatively smaller number of unique positive face images (1 per actor) compared to negative face images (4 per actor) caused more habituation during positive trials. Because we failed to find support for the inhibitory spillover in positive face trials, we focused the rest of our analyses on the consequences of motor inhibition during the incidental presentation of negatively-valenced faces.
Functional connectivity when viewing negative emotions
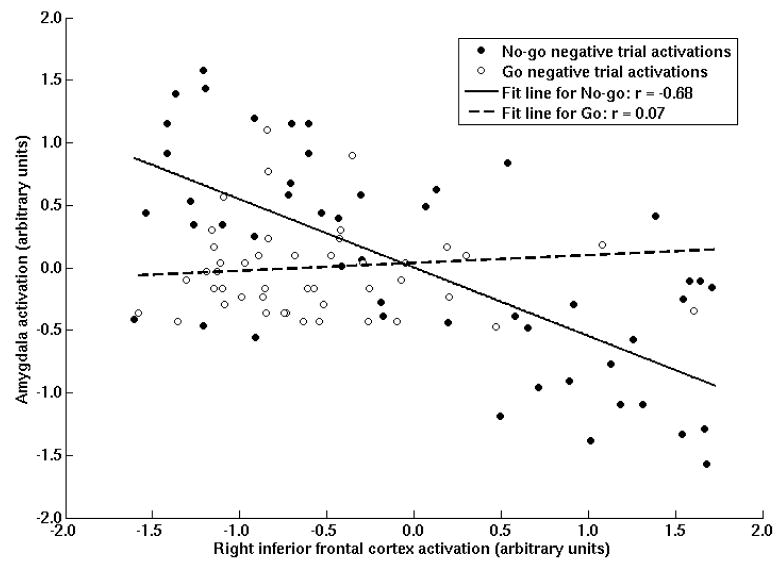
The finding that less amygdala activity was present during no-go− trials relative to both go− trials and baseline is consistent with the hypothesis that the rIFC activity during intentional motor inhibition was leading to inhibitory spillover effects in the amygdala. To further examine the inhibitory spillover hypothesis, we conducted a series of psychophysiological interaction (PPI) analyses to examine the relation of the time series of hemodynamic responses in rIFC and the amygdala. Specifically, we examined whether the time courses in these regions were more strongly inversely correlated during no-go− trials than during go− trials, as predicted by the inhibitory spillover hypothesis. This hypothesis was tested using a PPI generated from a seed region in the rIFC that was active during the “no-go− > go−” contrast (x = 40, y = 16, z = −18; Figure 1). Though either the amygdala or the rIFC could have been used as a seed region, this region was selected to be consistent with theoretical models of down-regulation of limbic regions originating in rIFC. Using the method described by Friston and colleagues (1997), the activity from this voxel was deconvolved into an imputed neural response, and then used to create a psychophysiological regressor during no-go− trials and go− trials each compared to baseline, and no-go− compared to go− trials. These analyses were conducted within-subject, then used to test for regions commonly associated with rIFC at the group level. These regressions revealed that the rIFC time course was inversely related to amygdala and insula time course during no-go− trials, but these relationships were not observed during go− trials (Table 5; Figures 3 and 4). Furthermore, the ROI around the amygdala voxel observed in the “go− > no-go−” contrast (x = ±18, y = −4, z = −28) reveals greater negative connectivity between rIFC and amygdala time courses during no-go− relative to go− trials (see Table 5; Figure 4), with 10 of the 12 participants showing the effect.
Table 5.
Neural regions negatively associated with rIFC during negative trials
| Region | x | y | z | Cluster size | t-val | |
|---|---|---|---|---|---|---|
| No-go− | Amygdala | −26 | −6 | −22 | 11 | 3.64 |
| 22 | −10 | −22 | 61 | 4.52 | ||
| Insula | 42 | 18 | −6 | 30 | 3.44 | |
| −36 | 18 | −6 | 106 | 3.48 | ||
| SMA (BA 6/8) | −12 | 18 | 50 | 66 | 4.63 | |
| rIFC (BA 11) | 40 | 50 | −6 | 104 | 4.13 | |
| lIFC (BA 46) | −36 | 24 | −6 | 106 | 3.58 | |
| Posterior temporal (BA 37) | 38 | −56 | −30 | 124 | 3.91 | |
| −44 | −54 | −22 | 536 | 4.48 | ||
|
| ||||||
| Go− | OFC (BA 11) | 36 | 46 | −10 | 17 | 3.45 |
| Middle temporal (BA 21) | −56 | −8 | −16 | 38 | 3.89 | |
| Posterior temporal (BA 37) | 44 | −60 | −10 | 22 | 3.52 | |
|
| ||||||
| No-go− > Go− | Amygdala1 | 20 | −6 | −24 | 2 | 2.42 |
| −28 | −10 | −24 | 12 | 2.33 | ||
Note: All activations: p < .001, uncorrected, 10-voxel extent threshold. BA = Brodmann’s area. Seed region is the rIFC voxel from no-go− > go−: x = 40, y = 16, z = −18.
Based on an 8-mm ROI around the amygdala voxel from go− > no-go−: x = ±18, y = −4, z = −28, p < .05 uncorrected.
Figure 3.
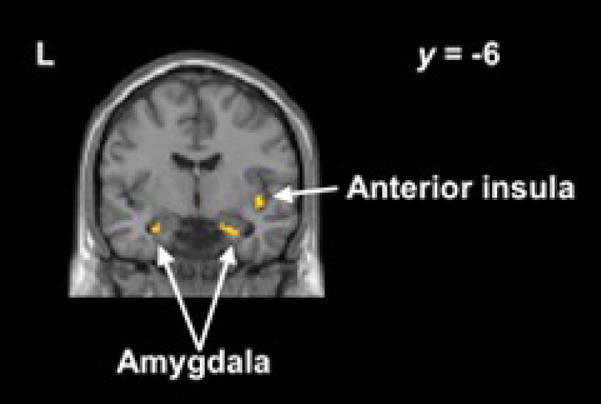
Regions with greater inverse association with right inferior frontal cortex (x=40, y=16, z=−18) during no-go− than baseline include amygdala (x=−26, y=−6, z=−22; x=22, y=−10, z=−22) and anterior insula (x=42, y=18, z=−6).
Figure 4.
An example of the inverse functional connectivity between right inferior frontal cortex (x=40, y=16, z=−18) and left amygdala (x=−26, y=−6, z=−22) for a typical subject. Here, the trial-by-trial correlation between rIFC and amygdala was −0.68 during no-go− trials and was 0.07 during go− trials. The psychophysiological analysis (PPI) is a group-level test of the difference of these betas between no-go− and go− trials.
Discussion
Neurocognitive studies have consistently observed rIFC involvement in inhibition across a number of domains (e.g. motor, cognitive, affective), which raises the possibility that rIFC might act as a common inhibitory region across each of those domains. If rIFC has inhibitory outputs that impact each domain, then intentional inhibition in one domain should produce inhibitory spillover into other domains to the extent that rIFC is activated and responses in other domains are available to be inhibited. However, extant studies of inhibition have each been limited to a single domain, rendering these studies unable to identify evidence for inhibition in one domain spilling over into others. The current investigation is the first to address this issue by inducing a prepotent motor response together with an affective response, but inducing intentional inhibition in only the motor domain. The results support the inhibitory spillover hypothesis and suggest that rIFC serves as a more coarse brake system than typically assumed.
During an emotional go/no-go task for which there was intentional motor inhibition, but where the affective elements were incidental to the task, we observed increased rIFC and reduced amygdala activity when participants engaged in response inhibition during the presentation of negatively-valenced stimuli. Amygdala activity during intentional motor inhibition was significantly below resting levels on these trials, but not during similar trials without motor inhibition (see Figure 2). Connectivity analyses revealed significantly greater inverse correlations between the time courses of rIFC and amygdala during no-go− trials than go− trials, suggesting that when participants intentionally inhibited a motor response in the presence of an affective stimulus that would typically activate the amygdala, amygdala activity was dampened to the extent that the rIFC response was stronger.
Because subjective ratings were not collected after each trial, it is impossible to assess whether changes in amygdala activity correlated with changes in experienced affect. Such ratings would have contaminated the task, but their absence makes it more difficult to assess the psychological meaning of the amygdala reductions during motor inhibition (Poldrack, 2006). Although it is still somewhat contentious as to what type of affective computations the amygdala is performing, it is generally agreed upon that the primary function of the amygdala relates to one or more aspects of affective processing. One might suggest that perhaps the amygdala is playing a role in facilitating motor responses during the go trials which is absent during the no-go trials. In essence, this view would suggest that the amygdala reductions represent a form of motor inhibition rather than inhibitory spillover into affective responses. Arguing against this point, in all of the previous non-affective go/no-go studies, the amygdala has never been reported as active in any comparison. The fact that it is present in our go trials is therefore more consistent with incidental affective processing of the target facial expressions than motor processing. Likewise, the reduction of amygdala activity during no-go trials is more consistent with inhibitory spillover than a motor amygdala account.
Inhibitory spillover was observed during negatively-valenced but not positively-valenced trials. The fact that motor inhibition-related reductions were not found in limbic regions during the presentation of positive stimuli, despite an increase in amygdala activation during the presentation of positive faces compared to baseline, suggests that any inhibitory spillover in the affective domain may be specific to negatively-valenced stimuli. This finding is consistent with decades of research on affect inhibition suggesting that negative affect is far more frequently down-regulated than positive affect (Gross, Richards, & John, 2006). Though there are theoretical accounts of the intentional inhibition of positive affect (e.g. Parrott, 1993), almost all empirical accounts of affect regulation involve negative affect. It thus might be expected that inhibitory spillover into the affective domain would be more potent for negatively-valenced stimuli.
One major implication of these results is that individuals who are impaired in one form of inhibition might be impaired in multiple forms because the different forms rely on common neural mechanisms such as rIFC. In this case, an individual with motor inhibition deficits might also demonstrate impaired regulation of other types of impulses. Existing data indirectly support this possibility. For example, methamphetamine abusers have a specific deficit in inhibiting prepotent motor responses during the stop-signal task (Monterosso, Aron, Cordova, Xu, & London, 2005). Consistent with the notion of impairment across multiple forms of inhibition, methamphetamine abusers have also shown deficits in cognitive inhibition on the Stroop task (Salo et al., 2002) and deficits in mood and emotion regulation independent of psychiatric comorbidity (Payer et al., 2008). Furthermore, relative to normal controls, methamphetamine abusers have shown structural deficits in rIFC (Thompson et al., 2004) consistent with rIFC being a convergent contributor to these multiple forms of inhibitory impairments.
Inhibitory spillover also has implications for treatment. Individuals may have difficulty performing mental exercises meant to strengthen inhibitory control in a relevant domain. It may be difficult to intentionally engage in emotion inhibition for such exercises, given the abstract and often unpredictable nature of emotion. However, it is possible that performing inhibitory exercises in another domain may enhance the efficiency of rIFC-based inhibition in multiple domains and produce long-term benefits for affect regulation. Thus, individuals may be able to start with a more manageable domain and work up to the domain that is actually producing problems in daily life.
The inhibitory spillover results presented here also help illuminate a potential mechanism of ego depletion. Ego depletion is said to have occurred when an extended inhibitory effort in one domain causes subsequent inhibitory impairment in a second domain (Baumeister, Bratslavsky, Muraven, & Tice, 1998). For example, Muraven and colleagues (1998) found that an episode of affective inhibition resulted in a subsequent reduction in the ability to exercise motor control (Study 1), and that prolonged cognitive inhibition resulted in a deficit in inhibiting affect expression (Study 3). Their study and many others have demonstrated that inhibitory control is a limited resource that is shared across domains; the present study provides the first evidence that rIFC may be the point of convergence across those domains. The evidence provided here that rIFC is a coarse inhibitory mechanism—one that spills over into other domains when engaged—helps clarify how ego depletion operates. Engaging in the inhibition of a response in one of several domains recruits rIFC which, when “depleted,” will hinder subsequent inhibition across all domains.
In summary, we found support for the hypothesis that rIFC serves as a common inhibitory mechanism across multiple psychological domains. Intentional inhibition in the motor domain produced unintended inhibitory consequences in the affective domain. To the extent that rIFC was engaged in the service of intentional motor inhibition, there was also a greater reduction in amygdala activity suggesting that inhibitory spillover into the affective domain had also occurred.
Acknowledgments
This research was supported by NIH grants MH071521 to MDL and Neuroimaging Training Grant T90DA022768 to ETB.
Footnotes
Portions of this research were presented at the annual meeting of the Cognitive Neuroscience Society held in San Francisco, CA, in April 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson MC, Green C. Suppressing unwanted memories by executive control. Nature. 2001;410(6826):366–369. doi: 10.1038/35066572. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Bratslavsky E, Muraven M, Tice DM. Ego depletion: Is the active self a limited resource? Journal of Personality and Social Psychology. 1998;74(5):1252–1265. doi: 10.1037//0022-3514.74.5.1252. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, et al. Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci. 2006;18(3):444–455. doi: 10.1162/089892906775990606. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. J Cogn Neurosci. 2004;16(10):1717–1729. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J Neurosci. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Explaining modulation of reasoning by belief. Cognition. 2003;87(1):B11–22. doi: 10.1016/s0010-0277(02)00185-3. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Richards JM, John OP. Emotion Regulation in Everyday Life. In: Snyder DK, Simpson JA, Hughes JN, editors. Emotion regulation in families: Pathways to dysfunction and health. Washington, DC: American Psychological Association; 2006. [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychol Sci. 2002;13(2):135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biol Psychiatry. 2005;57(6):624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Horn NR, Dolan M, Elliott R, Deakin JF, Woodruff PW. Response inhibition and impulsivity: an fMRI study. Neuropsychologia. 2003;41(14):1959–1966. doi: 10.1016/s0028-3932(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Marshuetz C, Koeppe RA, Reuter-Lorenz PA. Inhibition in verbal working memory revealed by brain activation. Proc Natl Acad Sci U S A. 1998;95(14):8410–8413. doi: 10.1073/pnas.95.14.8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, et al. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage. 2005;24(1):235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12(2):100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error- related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12(3):131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Kelley WM, Wyland CL, Wegner DM, Neil Macrae C. Separating sustained from transient aspects of cognitive control during thought suppression. Psychol Sci. 2007;18(4):292–297. doi: 10.1111/j.1467-9280.2007.01891.x. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED. Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend. 2005;79(2):273–277. doi: 10.1016/j.drugalcdep.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393(6684):467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Muraven M, Tice DM, Baumeister RF. Self-control as a limited resource: Regulatory depletion patterns. Journal of Personality and Social Psychology. 1998;74(3):774–789. doi: 10.1037//0022-3514.74.3.774. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Parrott WG. Beyond hedonism: Motives for inhibiting good moods and for maintaining bad moods. In: Wegner DM, Pennebaker JW, editors. Handook of mental control. Edglewood Cliffs, NJ: Prentice Hall; 1993. pp. 278–308. [Google Scholar]
- Payer DE, Lieberman M, Monterosso JR, Xu J, Fong TW, London ED. Differences in cortical activity between methamphetamine-dependent and healthy individuals performing a facial affect matching task. Drug and Alcohol Dependence. 2008;93:93–102. doi: 10.1016/j.drugalcdep.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proc Natl Acad Sci U S A. 2002;99(17):11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural Substrates for Voluntary Suppression of Negative Affect: A Functional Magnetic Resonance Imaging Study. Biological Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cereb Cortex. 2007;17(4):826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends Cogn Sci. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, et al. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res. 2002;111(1):65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Collins SH, Blumberg HP. The interaction of emotional and cognitive neural systems in emotionally guided response inhibition. Neuroimage. 2006;31(1):468–475. doi: 10.1016/j.neuroimage.2005.11.053. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46(1):224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia G, Satpute AB, Lieberman MD. The sunny side of fairness: Fairness preference activates reward circuitry (and disregarding unfairness activates self-control circuitry) Psychological Science. 2008;19:339–347. doi: 10.1111/j.1467-9280.2008.02091.x. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, et al. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24(26):6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, Nelson CA. Categorization of facial expressions in children and adults: Establishing a larger stimulus set. Paper presented at the Cognitive Neuroscience Society.2002. [Google Scholar]
- Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18(2):293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 2001;12(2):379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, et al. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13(14):1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]