Abstract
Background
Metabolic syndrome (MetS) has been linked with an increased risk of developing cancer; however the association between MetS and cancer mortality remains less clear. Little research has focused on pre-cancer risk factors that may affect the outcome of treatment. The purpose of this study was to examine the association between MetS and all-cancer mortality in men.
Methods
The participants included 33,230 men aged 20-88 years who were enrolled in the Aerobics Center Longitudinal Study and free of known cancer at baseline.
Results
At baseline 28% of all the participants had MetS. During an average of 14 years follow-up there were a total of 685 deaths due to cancer. MetS at baseline was associated with a 56% greater age-adjusted risk in cancer mortality.
Conclusion
These data show that MetS is associated with an increased risk of all-cause cancer mortality in men. Based on these findings it is evident that successful interventions should be identified to attenuate the negative effects of MetS.
Keywords: Insulin resistance, epidemiology, hypertension, obesity, dyslipedemia, lung cancer, colorectal cancer
1. Introduction
Metabolic syndrome (MetS) is defined as having any 3 of the following 5 components: abdominal obesity, high blood pressure, high fasting triglycerides, low fasting high density lipoprotein-cholesterol (HDL-C), and high fasting glucose (1, 2). Recent evidence indicates that one in five adults in the U.S. have coexisting risk factor abnormalities that meet the criteria for MetS (1, 3). Several studies have shown that the presence of MetS increases risk for type II diabetes, cardiovascular disease (CVD), as well as all-cause and CVD mortality(4-6).
There is growing interest in a potential etiological role for MetS and the development of cancer (1, 4, 7-12). Studies have shown that each of the MetS components may independently increase the risk for developing certain types of cancer (2, 3, 10, 12, 13). For example, investigations have demonstrated abdominal obesity to be a positive risk factor for an increased risk of endometrium, breast, colon, liver, gallbladder, esophagus, and kidney cancers (7). In addition, impaired glucose regulation and dyslipidemia (suppressed HDL-C and/or elevated triglycerides) have also been shown to increase one's risk of colon, breast, and/or prostate cancers (1, 9, 10, 13).
Findings from a small number of studies have suggested a positive association between MetS and risk of colorectal cancer mortality (12, 14). Other studies have shown a greater risk of colorectal cancer mortality associated with the presence of MetS compared to that associated with the individual MetS components alone (1). Additional prospective data are needed to further examine the role of MetS in cancer prognosis. The purpose of this study was to examine the association between MetS and all-cause cancer mortality in a large cohort of men enrolled in the Aerobics Center Longitudinal Study (ACLS).
2. Materials and Methods
2.1 Study Population
Participants were 33,230 men aged 20-88 years who, at baseline, were free of known cancer, completed a preventive medical examination at the Cooper Clinic (Dallas, TX) between 1977 and 2003, and are enrolled in the ACLS. Study participants came to the clinic for periodic preventive health examinations and for counseling regarding diet, exercise, and other lifestyle factors associated with increased risk of chronic disease. Many participants were referred by their employers for the examination, others were referred by their personal physicians, and some were self-referred. All participants in the current analysis had complete measures for each metabolic syndrome component. Most of the participants were white, well-educated, and from middle to upper socioeconomic strata. All participants provided written informed consent to participate in the follow-up study and the Cooper Institute institutional review board approved the study protocols annually.
2.2 Clinical Data
Participants completed a comprehensive health evaluation that included self-reported personal and family health histories; a standardized medical examination by a physician; anthropometric measures; a maximal treadmill exercise test; and a fasting venous blood draw. Examination procedures followed a standard manual of operations and have been previously described in greater detail (15, 16). Briefly, body mass index (BMI, kg/m2) was computed from measured height and weight. Waist circumference was measured at the level of the umbilicus. Resting blood pressure was recorded as the first and fifth Korotkof sounds by auscultatory methods (17). Serum samples were analyzed for total cholesterol, HDL-C, triglyceride, and glucose using standardized automated bioassays that met Centers for Disease Control and Prevention standards of the Lipid Standardization Program. Information on smoking habits (never, current, or past smoker), alcohol intake (drinks per week), personal history of hypertension, diabetes, hypercholesterolemia, and cardiovascular disease (myocardial infarction or stroke), and family history of cancer was obtained from a standardized questionnaire. Physical inactivity was assessed using a questionnaire and was defined as reporting no leisure-time physical activity in the 3 months before the baseline examination. Cardiorespiratory fitness was quantified as the duration of a symptom-limited maximal treadmill exercise test using a modified Balke protocol (16, 18). The duration of the test on this protocol is highly correlated (r = 0.92) with directly measured maximal oxygen uptake in men (19). To standardize exercise test performance, we computed maximal metabolic equivalents (METs, 1 MET = 3.5 ml O2 uptake/kg/min) of cardiorespiratory fitness based on the final treadmill speed and grade (20).
2.3 Metabolic Syndrome Definition
Participants were classified as having metabolic syndrome using criteria of the National Cholesterol Education Program Adult Treatment Panel III, and were based on the presence of 3 or more of the following risk factors (21) : 1) abdominal obesity (waist girth > 102 cm); 2) fasting hypertriglyceridemia (≥ 150 mg/dL); 3) low HDL cholesterol (<40 mg/dL); 4) high blood pressure (≥130/85 mmHg); and 5) high fasting glucose (≥110 mg/dL). History of physician-diagnosed hypertension and diabetes also were used to identify men who met criteria for high blood pressure and glucose, respectively, as done in other epidemiological studies (22, 23) and in our previous report (24).
2.4 Mortality Surveillance
Participants were followed from the date of their baseline examination until the date of death or until the time of censoring, December 31, 2003. Person-years of exposure were computed as the sum of follow-up time among decedents and survivors. The National Death Index (NDI) was the primary data source for mortality surveillance. The underlying cause of death was determined from the NDI report or by a nosologist's review of official death certificates obtained from the department of vital records in the decedent's state of residence at time of death. Causes of cancer death were identified using International Classification of Diseases, Ninth Revision (ICD-9) codes for deaths occurring before 1999 and Tenth Revision (ICD-10) codes (in parentheses) for deaths during 1999-2003: Our primary outcome for this analysis was total cancer mortality, 140-208 (C00-C97); and secondary outcomes were: Lung, 1622-1625, 1628-1629 (C340-C343, C348-C349); Colon, 153 (C18); Rectum,154 (C19-C21); and Prostate, 185 (C60) cancer mortality. These secondary outcomes were chosen to confirm results of other investigators. Additionally they were the only cancers reported in our population to make a strong comparison.
2.5 Statistical Analysis
Characteristics of the cohort at baseline were compared by exposure status using t- tests and χ2 tests. Kaplan-Meier survival curves were generated for MetS and according to the number of prevalent MetS risk factors. Cox proportional hazards regression analysis was used to estimate multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CI). Tests for linear trend of HRs across exposure groups were obtained using ordinal scoring. Two types of Cox regression models were fit to the follow-up data. First, we estimated the associations adjusting only for baseline age (years). Second, we adjusted for baseline age (years), examination year (calendar year), height (cm), smoking status (never, past, or current smoker), alcohol intake (≥5 drinks/wk or not), physically inactive (yes/no), hypercholesterolemia (yes/no), cardiovascular disease (yes/no), family history of cancer (yes/no), and cardiorespiratory fitness (treadmill test duration in minutes). These are common covariates adjusted for in cancer risk investigations. However, cardiorespiratory fitness and/or physical inactivity were not generally included in the adjusted models, and appear to be a novel inclusion in the current investigation. Cumulative hazard plots grouped by exposure suggested no appreciable violations of the proportional hazards assumption. Because of the known correlation between BMI and several of the MetS components, we elected not to include it as a covariate in our models, but we do consider the combined effect of BMI and other exposures on mortality risk in joint analyses described below.
We estimated cancer mortality risk based on joint exposure to MetS (present or not) and age (20-39, 40-59, ≥ 60 yrs), BMI (< 25, ≥ 25 kg/m2), and smoking status (never, past, and current smoker). Multiplicative interaction was tested by fitting each of these terms and their cross-product along with the covariates in the multivariate-adjusted model. We also completed our primary analyses after excluding deaths that occurred in the first five years of follow-up. Similar patterns of association were observed in these analyses. Statistical analyses were performed using SAS (version 9.1, SAS Institute, Cary, NC) software. All p values were obtained from two-sided hypothesis tests.
3. Results
At baseline, the prevalence of MetS was 27.9%. A comparison of baseline characteristics of those with and without MetS are presented in Table 1. Those without MetS were younger, had a more favorable lipids and glucose profile, were less likely to be physically inactive or a current smoker, consumed 5 or more alcoholic beverages per week, had higher maximal cardiorespiratory fitness, a lower prevalence of any of the five MetS components, as well as fewer cases of chronic medical conditions compared to those with MetS.
Table 1. Baseline Characteristics According to Metabolic Syndrome Status.
| Metabolic Syndrome | |||
|---|---|---|---|
| No | Yes | P value | |
| n | 23962 | 9268 | |
| Age, y | 44.0 ± 9.6 | 47.2 ± 9.8 | <0.0001 |
| Maximal cardiorespiratory fitness, METs | 12.4 ± 2.4 | 10.1 ± 2.1 | <0.0001 |
| Body mass index, kg/m2 | 25.4 ± 2.9 | 29.6 ± 4.5 | <0.0001 |
| Height, cm | 178.8 ± 6.5 | 179.3 ± 8.2 | <0.0001 |
| Waist, cm | 90.5 ± 8.6 | 103.1 ± 12.0 | <0.0001 |
| Fasting lipids, mmol/L | |||
| Total cholesterol | 5.3 ± 1.0 | 5.8 ± 1.1 | <0.0001 |
| HDL-C | 1.3 ± 0.3 | 1.0 ± 0.2 | <0.0001 |
| Triglycerides | 1.2 ± 0.7 | 2.5 ± 2.0 | <0.0001 |
| Fasting blood glucose, mmol/L | 5.4 ± 0.7 | 6.1 ± 1.4 | <0.0001 |
| Blood pressure, mmHg | |||
| Systolic | 119 ± 13 | 128 ± 14 | <0.0001 |
| Diastolic | 79 ± 9 | 86 ± 10 | <0.0001 |
| Physically inactive, % | 20.6 | 35.1 | <0.0001 |
| Smoking status, % | |||
| Never smoker | 71.4 | 62.8 | |
| Past smoker | 12.7 | 16.8 | <0.0001 |
| Current smoker | 15.9 | 20.4 | |
| ≥ 5 Alcohol drinks per week, % | 36.9 | 30.7 | <0.0001 |
| Chronic medical condition*, % | |||
| Hypercholesterolemia | 16.5 | 30.3 | <0.0001 |
| Hypertension | 20.9 | 58.4 | <0.0001 |
| Diabetes | 2.1 | 11.0 | <0.0001 |
| Cardiovascular disease | 1.5 | 3.4 | <0.0001 |
| Family history of cancer, % | 23.6 | 31.0 | <0.0001 |
| Metabolic syndrome criteria met, % | |||
| Abdominal obesity† | 7.2 | 55.9 | <0.0001 |
| High blood pressure‡ | 32.1 | 76.8 | <0.0001 |
| High triglycerides§ | 13.6 | 75.7 | <0.0001 |
| Low HDL-C¶ | 19.7 | 70.3 | <0.0001 |
| High glucose ‖ | 33.6 | 76.9 | <0.0001 |
HDL-C= high-density lipoprotein cholesterol.
Data shown as mean±SD unless specified otherwise
Chronic medical condition was defined as the following: hypercholesterolemia (history of physician diagnosed high cholesterol or measured fasting total cholesterol ≥ 240 mg/dL [6.20 mmol/L]); hypertension (history of physician diagnosis or resting SBP ≥140 mmHg or DBP ≥90 mmHg); diabetes (history of physician diagnosis, or use of insulin or measured fasting glucose ≥ 126 mg/dL [7.0 mmol/L]); cardiovascular disease (personal history of physician diagnosed myocardial infarction or stroke).
Abdominal obesity was defined as waist girth >102 cm [40 in]
High blood pressure was defined as systolic blood pressure≥ 130 mm Hg, diastolic blood pressure ≥85 mm Hg, or history of physician-diagnosed hypertension
High triglycerides was defined as triglycerides ≥1.69 mmol/L (150 mg/dL)
Low HDL-C was defined as HDL <1.04 mmol/L (40 mg/dL)
High glucose was defined as glucose≥100 mg/dL or history of physician-diagnosed diabetes
There were 685 deaths due to cancer during an average of 14 ± 7 years follow-up. Table 2 presents the hazard ratios and confidence intervals for all-cause cancer mortality associated with MetS. Baseline MetS was associated with a 56% greater age-adjusted risk of cancer mortality compared with absence of this condition (P < 0.0001). After multivariate adjustment, this association remained significant (P < 0.001). In addition, there was a graded increase in risk associated with a greater number of prevalent MetS components (P for trend < 0.001). Compared to men with 0 components, those with 3 or more components had an 83% higher risk of cancer death.
Table 2. HRs and 95% CIs for All-cause Cancer Mortality Associated with Metabolic Syndrome.
| Age-adjusted model | Fully adjusted model † | |||||
|---|---|---|---|---|---|---|
| N | Deaths | HR (95% CI) | P | HR (95% CI) | P | |
| Metabolic syndrome* | ||||||
| No | 23962 | 420 | 1.0 (Referent) | 1.0 (Referent) | ||
| Yes | 9268 | 265 | 1.56 (1.34-1.82) | <0.0001 | 1.41 (1.19-1.66) | <0.0001 |
| No. of Metabolic syndrome risk factors | ||||||
| 0 | 6484 | 86 | 1.0 (Referent) | 1.0 (Referent) | ||
| 1 | 9489 | 149 | 1.05 (0.84-1.37) | 1.02 (0.78-1.34) | ||
| 2 | 7989 | 185 | 1.41 (1.09-1.83) | 1.33 (1.01-1.73) | ||
| ≥3 | 9268 | 265 | 1.83 (1.43-2.34) | 1.62 (1.24-2.10) | ||
| Linear Trend P | <0.0001 | <0.0001 | ||||
| Individual metabolic syndrome components | ||||||
| Abdominal obesity | ||||||
| No | 26315 | 505 | 1.0 (Referent) | 1.0 (Referent) | ||
| Yes | 6915 | 180 | 1.51 (1.27-1.79) | <0.0001 | 1.28 (1.06-1.55) | 0.01 |
| High blood pressure | ||||||
| No | 18422 | 327 | 1.0 (Referent) | 1.0 (Referent) | ||
| Yes | 14808 | 358 | 1.16 (0.99-1.35) | 0.06 | 1.11 (0.95-1.30) | 0.18 |
| High triglycerides | ||||||
| No | 22946 | 426 | 1.0 (Referent) | 1.0 (Referent) | ||
| Yes | 10284 | 259 | 1.37 (1.17-1.60) | <0.0001 | 1.25 (1.06-1.47) | 0.009 |
| Low HDL-C | ||||||
| No | 21985 | 395 | 1.0 (Referent) | 1.0 (Referent) | ||
| Yes | 11245 | 290 | 1.36 (1.17-1.59) | <0.0001 | 1.25 (1.06-1.46) | 0.007 |
| High glucose | ||||||
| No | 18055 | 307 | 1.0 (Referent) | 1.0 (Referent) | ||
| Yes | 2353 | 378 | 1.26 (1.09-1.47) | 0.003 | 1.22 (1.04-1.42) | 0.01 |
HR, hazard ratios; CI, confidence interval.
Defined as the presence of ≥3 of the 5 metabolic risk factors.
Adjusted for age, examination year, height, smoking status, alcohol intake, physically inactive, hypercholesterolemia, cardiovascular disease, family history of cancer, and cardiorespiratory fitness.
We also evaluated cancer mortality risk associated with individual MetS components (Table 2). We found a positive multivariable-adjusted association between cancer mortality and four of the five MetS components: abdominal obesity increased risk by 28% (P = 0.01); high triglycerides increased risk by 25% (P = 0.009); low HDL-C increased risk by 25% (P = 0.007); and high glucose increased risk by 22% (P = 0.01). High blood pressure was the only component that did not increase risk (P = 0.18) in the fully adjusted model.
Kaplan-Meier plots for the presence of MetS and according to the number of prevalent MetS components are shown in Figure 1. Prognosis was worse among those with MetS (Panel 1A) and with increasing number of prevalent MetS components (Panel 1B; P < 0.0001 for each).
Figure 1. Survival free of all-cause cancer across metabolic syndrome status. MetSyn=metabolic syndrome.
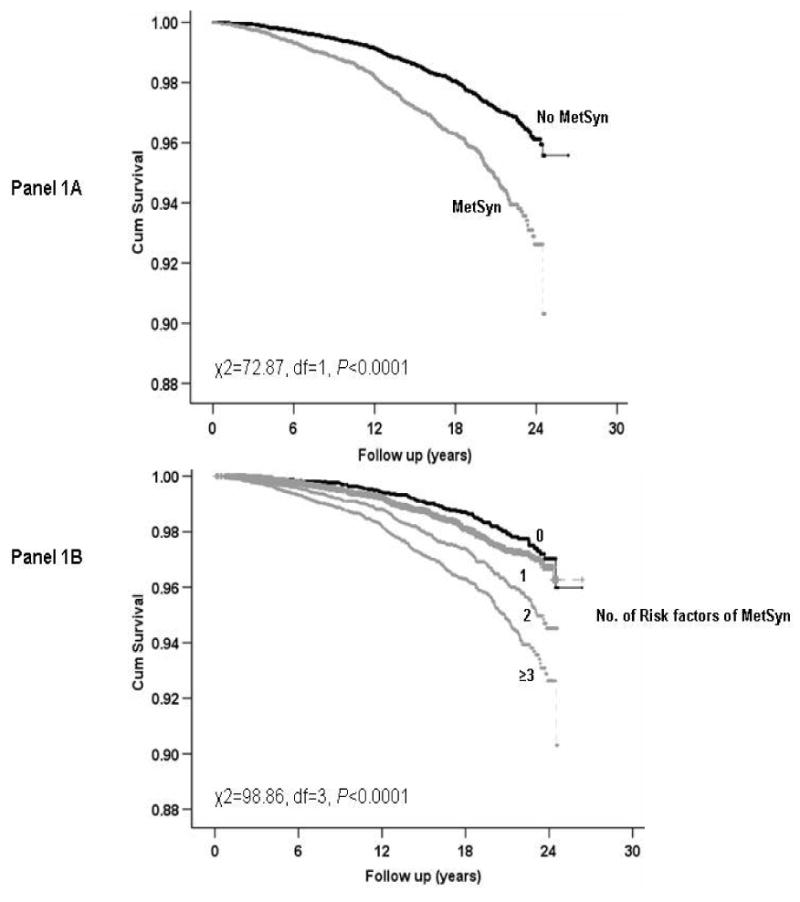
When evaluating cause-specific cancer mortality (Table 3), the presence of MetS was significantly associated with increased risk for lung (P < 0.0001) and colorectal cancer (P = 0.004), but not prostate cancer (P = 0.31). However, the positive associations were attenuated in the fully adjusted models.
Table 3. HR (95% CI) for Cause-specific Cancer Mortality Associated with Metabolic Syndrome*.
| Age-adjusted model | Fully adjusted model † | |||||
|---|---|---|---|---|---|---|
| Outcomes (anatomic site) | Presence of Metabolic syndrome | Deaths | HR (95% CI) | P | HR (95% CI) | P |
| Lung | No | 89 | 1.0 (Referent) | 1.0 (Referent) | ||
| Yes | 68 | 1.87 (1.36-2.57) | 0.0001 | 1.47 (1.04-2.06) | 0.03 | |
| Colon | No | 27 | 1.0 (Referent) | 1.0 (Referent) | ||
| Yes | 20 | 1.72 (0.97-3.08)) | 0.066 | 1.51 (0.81-2.83) | 0.20 | |
| Colon and rectum | No | 30 | 1.0 (Referent) | 1.0 (Referent) | ||
| Yes | 27 | 2.15 (1.27-3.62) | 0.004 | 1.71 (0.98-3.01) | 0.06 | |
| Prostate | No | 21 | 1.0 (Referent) | 1.0 (Referent) | ||
| Yes | 13 | 1.43 (0.71-2.85) | 0.31 | 1.32 (0.63-2.77) | 0.47 | |
HR, hazard ratios; CI, confidence interval.
Defined as the presence of ≥3 of the 5 metabolic risk factors.
Adjusted for age, examination year, height, smoking status, alcohol intake, physically inactive, hypercholesterolemia, cardiovascular disease, family history of cancer, and cardiorespiratory fitness.
Finally, we examined the risk of cancer mortality based on the joint effects of MetS and other risk factors (Table 4). For this analysis, the referent group was comprised of men who did not have MetS and were at lowest risk stratum for the other factor. Among men without MetS, older men (age 40-59 years) and current smokers were at higher risk than younger men (age <40 years) and those who were past smokers. There was no elevation in risk in men without MetS who had a BMI ≥25.0. Men with MetS had higher risk for cancer death than men in the reference category for all comparisons except for past smokers.
Table 4. HRs and 95% CIs for All-cause Cancer Mortality Associated with Metabolic Syndrome According to Other Risk Factors.
| Presence of Metabolic Syndrome |
||||
|---|---|---|---|---|
| No | Yes | |||
| N | Deaths | HR (95% CI) | ||
| Body mass index (kg/m2) | ||||
| <25 | 12458 | 244 | 1.00 | 1.44 (1.02-2.02) |
| ≥25 | 20772 | 441 | 1.09 (0.90-1.32) | 1.48 (1.20-1.83) |
| Age (years) | ||||
| 20-39 | 11902 | 78 | 1.00 | 1.03 (0.60-1.78) |
| 40-59 | 18623 | 425 | 1.11 (0.78-1.57) | 1.68 (1.16-2.43) |
| ≥60 | 2705 | 182 | 1.20 (0.70-2.08) | 1.54 (0.89-2.66) |
| Smoking status | ||||
| Never smoker | 22924 | 454 | 1.00 | 1.43 (1.16-1.74) |
| Past smoker | 4606 | 52 | 0.91 (0.59-1.40) | 1.35 (0.85-2.13) |
| Current smoker | 5700 | 179 | 1.70 (1.35-2.14) | 2.29 (1.74-3.00) |
HR, hazard ratios; CI, confidence interval.
All analyses were adjusted for age, examination year, height, smoking status, alcohol intake, physically inactive, hypercholesterolemia, cardiovascular disease, family history of cancer, and cardiorespiratory fitness.
4. Discussion
In this large prospective study, a positive association was found between MetS and all-cause cancer mortality in men. We also found that MetS was associated with a higher mortality risk for lung and colorectal cancers. These associations were robust even after adjusting for a number of potential confounders; however, only lung cancer remained statistically significant. Cancer mortality risk was significantly higher with an increasing number of MetS components present at baseline. Assessment of the joint associations between MetS and other risk factors indicated that cancer mortality risk associated with MetS was particularly higher for men who were over 40 years of age, overweight or obese, or current smokers.
Previous epidemiological investigations have found that individual components of MetS are associated with higher risk of developing cancer; however, the data examining risk of cancer mortality following exposure to MetS are more sparse. Only three epidemiological investigations have looked at the risk of cancer mortality associated with MetS. These studies focused on site-specific mortality such as colorectal (12, 14) and prostate (25) cancer, and different criteria have been used to define MetS across studies. Trevisan et al. (12) pooled data from The Risk Factors and Life Expectancy Project, which represents nine different large-scale epidemiological studies with a total of 21,311 men and 15,991 women included in data analysis. They found that both men and women who exhibited MetS had a nearly 3-fold increase in risk of dying from colon cancer compared to those without MetS. In another report, Colangelo et al. (14) studied 20,433 men and 15,149 women and showed that the risk of colorectal cancer mortality was 67% greater in men and 29% greater in women who had 3-4 MetS components as compared with their peers who had no prevalent MetS components. Our findings are consistent with these studies regarding an increased risk of colorectal cancer mortality in men with MetS.
Recently, Hsing et al. (8, 25) reviewed published studies on MetS components and prostate cancer. They found only one investigation in which fatal cases of prostate cancer had a higher BMI, waist to hip ratio, and systolic blood pressure. However, since each of these components was analyzed individually the investigators were unable to determine if the presence of MetS, per se, or simply a greater number of prevalent MetS components was associated with the risk of prostate cancer mortality. Thus, it was concluded that the epidemiological evidence is insufficient to suggest a link between MetS and prostate cancer mortality. Our findings are consistent with the conclusions of Hsing et al. (25).
MetS is a complex multi-factorial condition, and, therefore, several plausible mechanisms may be involved with the higher risk of all-cause cancer mortality. Numerous investigations have shown that many of the individual MetS components increase the risk of developing cancer (i.e., obesity, insulin resistance, hypertension and high triglycerides), some more specific to certain types of cancers than others (9, 13, 26-28). For example, it is well known that obesity is an important risk factor for cancer mortality, and one large prospective study indicated that obesity accounted for 14% of all cancer deaths in men and 20% in women (26).
Although the mechanisms behind the associated increase in cancer risk and/or mortality are not completely understood, previous data suggests insulin resistance to be one of the underlying mechanisms of MetS, and possibly cancer cell proliferation. Additionally, many metabolic abnormalities related to insulin resistance are closely associated with obesity and weight change (29). In a literature review by Giovannuci (29), strong evidence in animal models was compiled directly implicating a cancer-enhancing effect of insulin. Findings from the investigations included in the review demonstrated growth of aberrant crypt foci, a colorectal cancer precursor, as well as increasing the number and size of tumors, was enhanced by insulin injections. These findings were later replicated in a more natural setting in which rats were fed a high energy, high fat diet which led to insulin resistance and impaired glucose tolerance (29, 30).
Additionally, it is well known that insulin is a major anabolic hormone that can stimulate cell proliferation. It has been proposed that an indirect mechanism may be involved in the effects of insulin on cancer cell proliferation in vivo, such as insulin-like growth factor (IGF)-1 stimulation which plays an important role to cellular proliferation in response to nutrient availability (1). IGF-1 production has been shown to be stimulated by insulin via up-regulation of growth hormones in the liver (29). Furthermore, IGF-1 bioavailability can be increased during hyperinsulinemic conditions by decreasing hepatic secretion of IGF binding protein 1 and 2 so that more IGF-1 is free to bind to its receptor on normal cancerous cells. Over expression of IGF-1 receptors have been observed in breast and colon cancers (31). Human evidence has also demonstrated positive associations among high levels of insulin and IGF-1 and risk of colon cancer (28).
The proposed biologic mechanisms discussed are specific to the development of colon cancer. Although the current investigation focused on risk of cancer mortality, and not specifically incident cancer risk, we found a significantly higher risk of mortality from colon and rectal cancer combined in those with MetS or in the presence of multiple MetS components. This would suggest that similar mechanisms may be responsible for the increased risk of mortality from colorectal cancer in human models.
Based on the findings of this current investigation, it is evident that individuals who exhibited multiple components of MetS had higher risk of cancer mortality. One strength of the current study is that we compared the risk of all-cause cancer mortality associated with MetS, whereas previous investigations focused solely on risk of incident colon, rectum, or prostate cancer. However, an interesting finding that, to our knowledge has not yet been reported, is the significant association of MetS with a higher risk of lung cancer mortality in men. More work needs to be done to replicate or refute this novel finding.
Additional strengths of the current study include the extensive baseline examination to detect subclinical disease, use of measured risk factors, and large number of person-years of follow-up. The associations generally were independent of traditional risk factors, a result that strengthens causal inferences. As discussed earlier, these associations may be biologically plausible, although the precise mechanisms remain unknown.
There also are limitations to this investigation. Due to the analyses of only a male population, we can not conclude whether or not similar results would be observed in women. Past studies have shown that women have a lower risk of cancer mortality following exposure to MetS (1, 12, 14). In addition, the homogeneity of our population sample may limit the generalizability of our findings to men of other races/ethnicities, income levels, or educational attainment, but it should not affect the internal validity of our results. We also did not have sufficient data collection on medications or dietary habits to include in our analysis. It is important to note that when it comes to risk of cancer mortality, consideration must be taken into account for daily changes in lifestyle habits, progressive advancements in medical treatment/prevention, and successful elimination of any MetS component present at baseline. However, we have no such data available. Future studies with such information will need to confirm our findings reported here.
In conclusion, the presence of MetS or a greater number of MetS components were associated with a significantly higher risk for all-cause cancer mortality, as well as lung and colorectal cancer mortality. According to our findings, a dose-response relationship appears to exist between the number of MetS components and the higher risk of all-cause cancer mortality, with the risk associated with the presence of three or more components being particularly evident. In addition, we replicated earlier findings showing that all but one (high blood pressure) of the individual MetS components were positively associated with increased risk of all-cancer death. A reduction in cancer deaths may result from the prevention or control of abnormalities associated with MetS. Future investigations should focus on determining the possible mechanisms associated with the increased risk of cancer mortality, along with the progression to death through which the abnormalities associated with MetS produce their effect. Considering the increasing number of pharmacological therapies becoming available and prescribed for these metabolic abnormalities, it is imperative that the extents to which these treatments can reduce overall cancer mortality are fully understood.
Acknowledgments
Supported by NIH grants AG06945 and HL62508, and by the Communities Foundation of Texas. We thank the Cooper Clinic physicians and technicians for collecting baseline data, and staff at the Cooper Institute for data entry and data management.
Footnotes
Conflict of Interest Statement: None Declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? Am J Pathol. 2006 Nov;169(5):1505–22. doi: 10.2353/ajpath.2006.051090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among u.s. Adults. Diabetes Care. 2004 Oct;27(10):2444–9. doi: 10.2337/diacare.27.10.2444. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007 Sep;86(3):s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004 Jan 27;109(3):433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 6.Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med. 2004 May 24;164(10):1092–7. doi: 10.1001/archinte.164.10.1092. [DOI] [PubMed] [Google Scholar]
- 7.Friedenreich CM. Review of anthropometric factors and breast cancer risk. Eur J Cancer Prev. 2001 Feb;10(1):15–32. doi: 10.1097/00008469-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hsing AW, Gao YT, Chua S, Jr, Deng J, Stanczyk FZ. Insulin resistance and prostate cancer risk. J Natl Cancer Inst. 2003 Jan 1;95(1):67–71. doi: 10.1093/jnci/95.1.67. [DOI] [PubMed] [Google Scholar]
- 9.La Vecchia C, Negri E, Franceschi S, D'Avanzo B, Boyle P. A case-control study of diabetes mellitus and cancer risk. Br J Cancer. 1994 Nov;70(5):950–3. doi: 10.1038/bjc.1994.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsen TI, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. Br J Cancer. 2001 Feb 2;84(3):417–22. doi: 10.1054/bjoc.2000.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thygesen LC, Gronbaek M, Johansen C, Fuchs CS, Willett WC, Giovannucci E. Prospective weight change and colon cancer risk in male US health professionals. Int J Cancer. 2008 Sep 1;123(5):1160–5. doi: 10.1002/ijc.23612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trevisan M, Liu J, Muti P, Misciagna G, Menotti A, Fucci F. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol Biomarkers Prev. 2001 Sep;10(9):937–41. [PubMed] [Google Scholar]
- 13.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. Jama. 2005 Jan 12;293(2):194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 14.Colangelo LA, Gapstur SM, Gann PH, Dyer AR, Liu K. Colorectal cancer mortality and factors related to the insulin resistance syndrome. Cancer Epidemiol Biomarkers Prev. 2002 Apr;11(4):385–91. [PubMed] [Google Scholar]
- 15.Blair SN, Kampert JB, Kohl HW, 3rd, Barlow CE, Macera CA, Paffenbarger RS, Jr, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. Jama. 1996 Jul 17;276(3):205–10. [PubMed] [Google Scholar]
- 16.Blair SN, Kohl HW, 3rd, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. Jama. 1989 Nov 3;262(17):2395–401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 17.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005 Jan;45(1):142–61. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 18.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J. 1959 Jun;10(6):675–88. [PubMed] [Google Scholar]
- 19.Pollock ML, Bohannon RL, Cooper KH, Ayres JJ, Ward A, White SR, et al. A comparative analysis of four protocols for maximal treadmill stress testing. Am Heart J. 1976 Jul;92(1):39–46. doi: 10.1016/s0002-8703(76)80401-2. [DOI] [PubMed] [Google Scholar]
- 20.American College of Sports Medicine. ACSM's Guidelines For Exercise Testing And Prescription. 7th. Philadelphia: Lippincott Williams and Wilkins; 2005. p. 2005. [Google Scholar]
- 21.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005 Oct 25;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR. The metabolic syndrome and risk of incident colorectal cancer. Cancer. 2006 Jul 1;107(1):28–36. doi: 10.1002/cncr.21950. [DOI] [PubMed] [Google Scholar]
- 23.Sturmer T, Buring JE, Lee IM, Gaziano JM, Glynn RJ. Metabolic abnormalities and risk for colorectal cancer in the physicians' health study. Cancer Epidemiol Biomarkers Prev. 2006 Dec;15(12):2391–7. doi: 10.1158/1055-9965.EPI-06-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. 2005 Jul 26;112(4):505–12. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 25.Hsing AW, Sakoda LC, Chua S., Jr Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007 Sep;86(3):s843–57. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 26.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003 Apr 24;348(17):1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 27.Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer. 2003 May 10;104(6):669–76. doi: 10.1002/ijc.10974. [DOI] [PubMed] [Google Scholar]
- 28.Lahmann PH, Hoffmann K, Allen N, van Gils CH, Khaw KT, Tehard B, et al. Body size and breast cancer risk: findings from the European Prospective Investigation into Cancer And Nutrition (EPIC) Int J Cancer. 2004 Sep 20;111(5):762–71. doi: 10.1002/ijc.20315. [DOI] [PubMed] [Google Scholar]
- 29.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001 Nov;131(11 Suppl):3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 30.Koohestani N, Tran TT, Lee W, Wolever TM, Bruce WR. Insulin resistance and promotion of aberrant crypt foci in the colons of rats on a high-fat diet. Nutr Cancer. 1997;29(1):69–76. doi: 10.1080/01635589709514604. [DOI] [PubMed] [Google Scholar]
- 31.Moschos SJ, Mantzoros CS. The role of the IGF system in cancer: from basic to clinical studies and clinical applications. Oncology. 2002;63(4):317–32. doi: 10.1159/000066230. [DOI] [PubMed] [Google Scholar]


