Abstract
A decade of aggressive researches on carbon nanotubes (CNTs) has paved way for extending these unique nanomaterials into a wide range of applications. In the relatively new arena of nanobiotechnology, a vast majority of applications are based on CNTs, ranging from miniaturized biosensors to organ regeneration. Nevertheless, the complexity of biological systems poses a significant challenge in developing CNT-based tissue engineering applications. This review focuses on the recent developments of CNT-based tissue engineering, where the interaction between living cells/tissues and the nanotubes have been transformed into a variety of novel techniques. This integration has already resulted in a revaluation of tissue engineering and organ regeneration techniques. Some of the new treatments that were not possible previously become reachable now. Because of the advent of surface chemistry, the CNT’s biocompatibility has been significantly improved, making it possible to serve as tissue scaffolding materials to enhance the organ regeneration. The superior mechanic strength and chemical inert also makes it ideal for blood compatible applications, especially for cardiopulmonary bypass surgery. The applications of CNTs in these cardiovascular surgeries led to a remarkable improvement in mechanical strength of implanted catheters and reduced thrombogenecity after surgery. Moreover, the functionalized CNTs have been extensively explored for in vivo targeted drug or gene delivery, which could potentially improve the efficiency of many cancer treatments. However, just like other nanomaterials, the cytotoxicity of CNTs has not been well established. Hence, more extensive cytotoxic studies are warranted while converting the hydrophobic CNTs into biocompatible nanomaterials.
Keywords: carbon nanotube, nanobiotechnology, tissue engineering, gene delivery, drug delivery
Introduction
Carbon nanotubes (CNTs) are the graphite sheets rolled into cylindrical tubes of nanoscale diameter and length in nano or micro meter ranges. Based on the number of concentric cylinders of graphite sheets, CNTs are categorized into single- or multi-walled. CNTs possess some unique chemical, electrical, and mechanical properties. For example, they have a very high ratio of surface area over volume because of their diameter in a nano-scale. This property is attractive for biomedical applications, where a high aspect ratio is highly desired as more biomolecules can be loaded onto the nanotubes and interact with cells and tissues. Moreover, CNTs have a very high optical absorbance in the near-infrared (NIR) regime,1 allowing them to be visualized through the infrared fluorescence microscopy imaging. CNTs can also act as microwave absorbers,2 allowing microwave-based thermal drug release. In addition, CNTs are one of the strongest materials ever reported3 and also possess a very high thermal conductivity.4
CNTs are synthesized mainly by three methods: arc-discharge,5,6 laser ablation,7 and chemical vapor deposition (CVD).8 The first two methods utilize graphite rods as a source of carbon, while hydrocarbons like methane and ethylene are used in CVD. As CNTs are highly hydrophobic, the lack of solubility in physiological solutions is one of the main difficulties in integrating them into biological systems. Hence, a number of methods have been developed to solubilize CNTs through surface modification.9–11
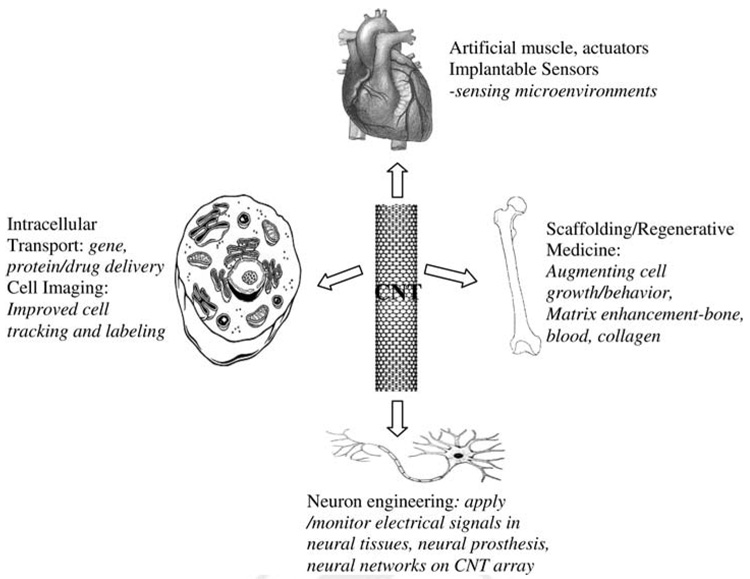
Since its discovery in 1991,12 both single- and multi-walled CNTs (SWCNT and MWCNT) have been exploited and evaluated for various applications. It has been of immense interest to apply these nanotubes to improve human health and disease treatment because of their aforementioned attractive chemical, electrical, and mechanical properties. Currently, the majority of studies on CNT’s biological applications revolve around the biosensor development in which the CNTs are integrated with biomolecules such as proteins, nucleic acids or carbohydrates and used as a sensor for ultra-sensitive disease diagnosis. Detailed reviews on these applications can be found elsewhere.13–16 However, of late, considerable efforts have been made to utilize CNTs as biocompatible carriers or biomaterials for tissue regeneration and in vivo gene and drug delivery (Figure 1). In such scenario, the CNTs are in constant interaction with living cells and tissues. It is, therefore, important to document these interactions so that new approaches and technologies can be developed and applied to medical science and disease treatment. This review intends to update the most recent developments of CNT-based tissue engineering. On the other hand, the interaction between CNTs and cells/tissues can have adverse effect on the environment, which has called for a great awareness of their potential risk to human health. Thus, the possible threats of CNTs to the living system and environment are also discussed here briefly.
Figure 1.
A schematic diagram showing potential applications of CNTs in tissue engineering.
CNT-Mediated Tissue Scaffolding for Organ Regeneration
Constant searches for new materials with increased mechanical stability and chemical inertness has resulted in the development of various biomaterials that are commercialized for clinical applications. Following the same trend, CNT has been explored for growing a variety of tissues or organs both in vitro and in vivo. We will discuss these perspectives in the following several sections.
Biocompatibility of CNT
The proposed biomedical applications of the CNT range from implantable glucose sensors17 to artificial muscles.18 It has been shown that CNT can behave as actuators that can be converted into fuel cells by which the energy required for pumping the blood through the heart can be generated continuously.18 But, the most important parameter that must be determined before exploring any in vivo applications of the CNT is its biocompatibility. Many studies have been launched to characterize the CNT’s biocompatibility. Most of these studies were performed in vitro. For example, Garibladi et al examined the cultivation of H9c2(2–1) cells, a rat cardiac muscle cell line, in the presence of insoluble SWCNTs at a concentration of 0.2 mg/mL.19 They found that the cell viability is unaffected in short term (1–3 days), but a longer incubation indicates a significant reduction in cell viability.
The effect of CNT on the formation of extracellular matrix (ECM) has also been characterized. One of the major components of ECM is collagen. The collagen is produced initially as alpha polypeptide chains and then is self-assembled into a triple helix.20 It possesses a high tensile strength and can impart resistance to tensile and shear loads in tissues. Hence, collagen has been widely used in the regenerative medicine. It can be assumed that the incorporation of CNT with collagen has the potential of improving the mechanical and electrical properties of these biomaterials. Such improved properties are highly desired in various soft-and hard-tissue applications, such as blood vessels or cartilages. Also, the presence of nanotubes can create an electrically conductive matrix along with the cardiac muscle and neural tissues, where electrical signals are propagated. Mac-Donald et al prepared CNT-collagen composite and examined its biocompatibility.21 In their experiment, the single-walled carbon nanotubes (SWCNTs) were functionalized to carry carboxyl group on both their tips and sidewalls by nitric and sulfuric acid treatment. After functionalization, the nanotubes were mixed with rat aortic smooth muscle cells in a suitable culture medium containing acid stabilized bovine collagen. SWCNT concentrations from 0.2 to 2 wt % were used in these experiments. It was found that the presence of SWCNTs do not alter the collagen gel formation. By confocal microscopy and vital staining of the cells embedded within collagen constructs, it is confirmed that cell viability is uniformly high throughout the collagen matrix and the cell morphology is unaffected by the presence of CNT in the matrix. Though it is unknown how SWCNT interacts with collagens, this work points toward developing a modified collagen matrix without any major effect on gel compaction, cell viability, or cell proliferation. In yet another study, carboxyl functionalized SWCNT were incorporated into alginate gel and subjected to in vivo biocompatibility studies in rat by subcutaneous implantation. This study resulted in mild tissue response but no adverse effects.22
A recent article highlighted the possible correlation between the CNT scaffolds and focal adhesion kinase (FAK) expression by the cells controlling the cell viability when such scaffolds are used for in vitro cell culture.23 It was found that the HeLa cells grown on multi-walled carbon nanotubes (MWCNTs) or SWCNTs differ in their viability and the cell morphology. For cells cultured on SWCNTs, they are wide-spread on the substrate and the FAK expression is higher as compared with MWCNTs. FAK is a nonreceptor cytoplasmic tyrosine kinase involved in signaling pathways and regulates cytoskeletal organization, cell adhesion, migration, survival and proliferation.24 A further account of the in vivo cytotoxicity studies of CNTs will be discussed later in this review.
CNT used as blood compatible biomaterials
Most of the currently available biomaterials that are in contact with blood are not blood- compatible. Most often, nonspecific binding of certain plasma proteins on the surface of biomaterial mediates thrombosis or blood clotting through a cascade of reactions.25 Usually, when any material comes in contact with blood, the adsorption of plasma proteins occurs nonspecifically and this adsorbed layer interacts with blood cells like platelets and red blood cells (RBC) leading to intracellular signaling, platelet activation and blood clotting. Ideally, the biomaterials used in blood should be chemically inert and should not promote any thrombosis or blood clotting. CNT can be a potential material for these applications, as it is chemically inert and able to tolerate weariness because of its incredible mechanical strength. To explore the application of CNTs in blood, Meng et al investigated a possibility of the integration of CNT into polyurethane.26 They found that both tensile strength and elongation capability of the polyurethane can be enhanced significantly by co-precipitating MWCNTs into the polyurethane matrix. In the modified matrix, the platelets showed relatively lower level of adhesion owing to the presence of CNTs. Their experiment demonstrated that MWCNT-polyurethane leads to a reduction in thrombosis and also, the hemolysis index, (a measure of disruption of red blood cells).26 Hence, such a composite may be able to find its application in cardiovascular surgery and in other blood contact environments.
With similar goals, Endo et al fabricated a nanocomposite-based micro catheter through melt extrusion utilizing high-purity CNTs as filler and nylon as a matrix.27 In their experiment, pellets of Nylon-12 and 10% (wt) MWCNTs were mixed using a conventional mixer, compounded into pieces and fed into a conventional twin-screw extruder in the temperature range of 250–280°C to fabricate the micro catheter with diameters of few millimeters. CNTs were observed to uniformly distribute in the matrix on the inner or outer surfaces of the catheter. The elastic modulus of the micro catheter was increased by about 50% because of the incorporation of MWCNT. It was found that the CNT-based catheter has a lower reactivity with the blood and in vivo thrombus formation in artery was largely depressed. It was also observed that comparative size of fibril and blood cell is smaller for the CNT-based catheter. Thus, they confirmed that nanotube modified nylon biomaterial has improved mechanical strength and reduced thrombogenicity (better antithrombotic property). These catheters will be valuable for use in cardiopulmonary bypass, vascular dilating devices, intra-vascular catheters, and during hemodialysis. However, the long-term efficacy of such modified catheters after implantation is yet to be determined.
CNT for bone regeneration
Developing biocompatible scaffold materials that can support the growth and proliferation of osteoblast and thereby increment to replace the bone tissues still remains as a major challenge for biomedical engineers. Bone tissue is a natural composite of collagen fibers and hydroxylapatite crystals. Osteoblasts proliferate on the bone surface and secrete matrix proteins.28 They mineralize the matrix by producing hydroxylapatite. Currently, most artificial bone scaffolds used in bone graft are made up of polymers and peptide fibers. However, these materials have relatively low strength and are often susceptible to immune rejection. CNT can be a promising material for casting bone scaffolds because of its high mechanical strength, flexibility, elasticity, and low density. As aforementioned the CNT is one of the strongest materials on the earth. If incorporated, they can improve the mechanical properties of the bone scaffolds. In a scaffold used for bone graft, the growth of hydroxylapatite crystals depends on the ability of the scaffold to attract calcium ions and initiate the crystallization process. The idea was to inject the CNT into a bone fracture for supporting the growth of new tissues to heal the fracture.29 This idea has been examined by Haddon’s group.29,30 They functionalized SWCNTs with phosphonates. The poly amino benzene sulfonic acid (PABS) was deposited as a film on solid substrate and CaCl2 and Na2HPO4 were added simultaneously on to it. This resulted in the nucleation and crystallization of hydroxy apatite crystals on the SWCNTs. They found that calcium ions were attracted to the nanotubes because of the negatively-charged functional groups introduced on the CNT surface. They also tested the bone formation on CNTs with different functional groups on its surface. These nanotubes included SWCNTs and MWCNTs, acid-treated SWCNTs, and polyethylene glycol (PEG) or PABS coated SWCNTs. The CNT is highly hydrophobic without any chemical modification. The acidic treatment generates carboxyl groups on both the tips and sidewalls of the CNTs. To test the bone formation on these nanotubes, the untreated nanotubes were dispersed in ethanol by sonication and the treated nanotubes were dissolved in water. The dissolved or dispensed nanotubes were then sprayed onto preheated glass cover slips. After air drying, these cover slips were sterilized by UV irradiation. The cultivation of bone-forming cells, osteosarcoma ROS 17/2.8 on these CNT modified cover slips revealed that surface-charged nanotubes inhibited the growth and the proliferation of osteoblasts, whereas surface-neutralized nanotubes promotes the cell growth and the formation of the mineralized bone. They also discovered that the presence of CNTs in the scaffold improves the cell adhesion, which is crucial for cell growth, proliferation, differentiation, and migration within the scaffold. Studies with CAL-72 osteoblast-like cells demonstrated that the presence of vertically aligned CNTs in the scaffold significantly influences cell growth, morphology, and orientation by steering toward a nanopatterning.31 Yet another method of nucleation and growth of hydroxylapatite by using MWCNT was demonstrated by Liao et al.32 Developing strategies and studying the mechanism of biomineralization with CNTs can aid in the advancement of nanocomposite production to be used for hard tissue (bone/teeth) repair and replacement.
Extensive studies on a CNT-reinforced porous polyurethane nanocomposite scaffold for osteoblast growth and mineralization was performed by Jell et al.33 The matrix did not cause any cytotoxicity on SaOS-2 osteoblast cells. Apart from improving the physical properties, because of the changes in surface chemistries and nanoscale architectures, it was found that there is an increased level of production of vascular endothelial growth factor—a potent angiogenic factor— in proportion to the CNT loading in the scaffold. This also highlights on the ability of CNT modified scaffolds to influence the cellular behavior.
Another application of CNTs in bone engineering is to enhance the mechanical properties of some biomaterials that have been currently used in bone regeneration. For example, polymethyl methacrylate (PMMA) is a common polymer material for bone cement and dental prostheses. It has been found that the properties of bone cement can be improved remarkably by incorporating CNTs into the PMMA polymer. Marrs et al have investigated the mechanical properties of the MWCNT (0–10 wt %) incorporated PMMA cement.34 They found that the incorporation of MWCNT favorably alters the static and fatigue mechanical properties of this acrylic bone cement. They also discovered that the augmentation of the bone cement with the nanotubes offers thermal benefits and improves the longevity of the implants. Usually, the temperature in the bone-cement interface inside the body is elevated leading to hyperthermia based destruction of bone adjacent to cement mantle.35 The presence of MWCNTs in the bone cement can significantly reduce the high temperatures at cement-bone interfaces during in vivo polymerization. This can be attributed to the high thermal conductivity of the carbon nanotubes.34
It has also been found that the elevated surface roughness due to the presence of CNTs in the bone cement may be responsible for the increase in the osteoblastic cell proliferation and differentiation when they are cultured on polystyrene.36 A similar study to evaluate the effect of CNTs in the matrices suggests that the surface roughness of the MWCNT-poly(carbonate)-urethane constructs elevates with an increase at the CNT levels.37 This rising in the roughness can lead to a higher adsorption of the proteins like fibronectin on the composite to enhance the adhesion of cells onto the surface of the matrices.
Most of the aforementioned studies on bone regeneration were carried out utilizing in vitro cell culture models. In vivo studies have also been performed to explore the effect of CNTs on bone formation and the bone-tissue compatibility.38–40 For example, Usui et al. implanted MWCNT particles into mouse skull subperiosteum and tibial bones.38 They found that the MWCNTs did not cause any major inflammatory reaction. Moreover, incorporation of recombinant human bone morphogenic protein-2 (rhBMP-2) with the MWCNTs implanted into the bone can reportedly accelerate the new bone formation. This study also demonstrated that the MWCNTs did not inhibit the bone repair in mouse and during the 4 weeks study period, the nanotubes become integrated into the bone tissue, whereas, in the control mice implanted with graphite particles, bone repair is completely inhibited, but the actual mechanisms leading to such differences are yet to be investigated. Studies by Wang et al also demonstrated that the biocompatibility of CNTs for bone regeneration.39 Their experimental results indicate that the MWCNT-polycarbosilane composites instilled into rat femur leads to only an insignificant inflammation in 4 weeks, and the newly formed bone is remodeled around the nanotubes. Similarly, using MWCNT-chitosan composite scaffold, the evolution of the skeletal muscle cell line C2C12 toward an osteoblastic lineage and the in vivo ectopic formation of bone tissue in presence of rhBMP-2 (by implanting the CNT-based scaffolds adsorbed with rhBMP-2 in muscle tissue) has been studied.40
CNT for neuron regeneration
Neuron regeneration after brain injury is considered to be a very difficult task.41 Reestablishing intricate connection between neurons for brain signal pathways has been investigated aggressively. As CNTs are electrically conductive42 and possesses diameters less than 100 nm and aspect ratio (height to diameter) close to that of nerve fibers,30 they are potential candidates for neuron regrowth or remodeling. Mattson et al first reported the growth of neurons on 4-hydroxynonenal (4-HNE) modified MWCNTs deposited on polyethylene amine coated cover slips.43 They found that embryonic rat hippocampal neurons are able to attach to the nanotube’s surface and the presence of 4-HNE promotes the neurite outgrowth and the neurite branching as compared with the unmodified nanotubes which support only the neurite outgrowth. CNTs thus open up the possibility of incorporating one or more bioactive molecules on their surface to develop a regulated neuron network on selected matrices. In another study, Lovat et al showed the possibility of using MWCNTs as potential devices to improve neural signal transfer.44 These MWCNTs were functionalized with pyrrollidone groups on both tips and sidewalls for improving the solubility. The functionalized nanotubes were dissolved in dimethylformamide and deposited on the glass surface. After solvent evaporation, the MWCNTs were defunctionalized by placing the glass in an oven at 350°C under nitrogen atmosphere for 15 min. A stable layer of purified nonfunctionalized nanotubes is then obtained by this procedure. Afterwards, the hippocampal neurons are inoculated and grow on the glass surface and monitored for the occurrence of spontaneous post synaptic currents (PSC) by single-cell patch-clamp recordings. It was found that the cultured neural network is discontinuously distributed on dispersed nanotube film, and CNT substrates increase the spontaneous synaptic activity and firing of hippocampal neurons, though there was not any significant change in the action potential of these neuron cells.
In another study, Hu et al investigated the effect of CNT’s surface charge on the regulation of neurite outgrowth, length and branching.45 They prepared the SWCNT graft copolymers by reacting the carboxyl-functionalized CNTs with oxalyl chloride to form an acyl chloride intermediate that was further reacted with branched polyethyleneimine (PEI), forming the graft copolymer of SWCNT-PEI. This graft is positively charged at physiological pH. The modified nanotubes can be readily dispersed in water by sonication and spray onto the glass cover slips for growing neuron cells. This group tested the proliferation of hippocampal neuron cells on the nanotube-coated glass cover slips and found that neurons grown on these cover slips show more branched neurites as compared with the control substrates. The viability and long-term growth of neuron cells on the CNT surface were also characterized and investigated using NG1108-15 neuronal cells.46 The study suggests that neuron cells can maintain a high viability on the surface of SWCNTs for 10 days.
Brain is composed of thousands of neuronal types inter-connected with specificity to form precisely arranged neural circuits essential for normal function of the nervous system. But how the neuronal circuits are established is still unclear. One of the methods to explore such neuronal circuits is to employ the CNT as substrate to grow the neuron cells. A number of groups have attempted to grow neurons on the solid substrates containing CNTs, but most of these works do not highlight whether the neurons can be stimulated via CNTs. A recent study hinted a possibility of stimulating single or multiple synaptic pathways in cultured neuron networks via a CNT platform.47 These studies indicates that the currents elicited from neurons cultured on the SWCNT surface behave similar to those grown on glass substrates, indicating no influence of CNTs on the electro-physiological characteristics of the neuron cells. For example, on solid substrates like quartz, CNTs can be patterned as islands using polydimethylsiloxane (PDMS) stencil covering and CVD technology.48 On such substrates, the neurons can be self-wired each other through axons and dendrites to form neural networks.49 It was found that neuron cells, with an optimized cell seeding, can grow with a specific geometry on a quartz substrate fabricated with CNT islands. It was also observed that compact connections among the pairs of the neuron cell clusters occur spontaneously through a single, nonadherent straight bundle composed of axons and dendrites. Even though some models were proposed for the stimulation of the neuronal culture via the SWCNT layer on the glass substrate, the interpretative complications of the voltage-clamp data are still open for more aggressively designed experiments and associated simulations.
Another approach to utilize CNTs for promoting the formation of neuron network is to directly add CNTs to the cell culture medium. Ni et al. explored this possibility.50 To incorporate the CNTs in the hippocampal neuronal cultures, open-end carboxyl functionalized SWCNTs were modified with poly aminobezene sulphonic acid (PABA) or poly ethylene glycol (PEG). These CNTs were then used to cast graft polymers. They found that although neuron cells grow well in the presence of SWCNT, there is a decrease in the number of neuritis per neuron formed in the culture. They also discovered that the SWCNT graft copolymers modulate the neurite outgrowth by increasing their length. This point toward the fact that CNTs can be used at the local site of nerve injury and enhance the outgrowth of selected neurites, thereby increasing the neural network in the brain. Another study with CNT incorporated neuron cell culture was reported by Matsumoto et al.51 Using MWCNTs coated with neurotrophin, they studied the differentiation and survival of neurons on the CNTs. To use CNTs to enhance the neuron cell growth and formation of neurites, they covalently immobilized neurotrophins like nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) modified with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) on amine functionalized MWCNTs. The neurite out-growth of embryonic chick Dorsal Root Ganglion neurons was found to be promoted by the incorporation nanotubes in the neuron cell culture medium. These results are very encouraging, as it may pave the way to achieve the goal of regenerating the neural network in a certain pattern which can function as desired under in vivo conditions.
Apart from those cells mentioned earlier, attempts are also being made for directing the growth of stem cells using substrates modified with CNTs. For example, differentiation of the mouse neural stem cells (NSC) on SWCNT composite had been explored.52 Inclusion of SWCNT in the composite imparts structural flexibility and chemical stability, without any adverse effect on the differentiation of NSCs. Similarly, experiments with mesenchymal stem cells reveal that CNT monolayer patterns on the solid substrates help in controlling the adhesion and shape of the stem cells. A cysteamine self-assembled monolayer coated with SWCNT monolayer exhibits about 60% increase in cell spreading as compared with the control which devoid of the nanotubes.53 Apart from directed cell growth, such substrates could also find applications in nanoscale biological or chemical sensors.
CNT as a Carrier for Selective Delivery of Biomolecules to Cells and Tissues
Another promising application of CNTs in tissue engineering is the development of new drug and gene delivery systems. For example, cancer treatment represents an enormous challenge for drug delivery in tissue engineering. Because most anti-cancer drugs are harmful to normal tissues as well, it is highly desired to develop a targeted drug delivery system that can distinguish a cancer cell from healthy cells while delivering the drugs.54 CNTs are excellent candidates for developing such a system.
To use as a cargo for in vivo biomolecule delivery, the CNTs need to be chemically modified so that the biomolecules of interest can be readily loaded inside or outside the tubes, which can then be released from the tubes upon reaching the target cells or tissues. CNTs can be functionalized in many different ways depending on their terminal applications. The functionalization helps to integrate various biomolecules such as proteins, nucleic acids, carbohydrates, and other organic chemicals to sidewalls or open-ends of the CNTs. The ability to incorporate drugs or genes into functionalized CNTs demonstrates a new era in pharmacotherapy for selective delivery of drugs or genes to cells and tissues.
CNT for gene delivery
The conventional gene delivery mainly depends on viral vectors. This approach can potentially induce undesirable immune responses, inflammation and even oncogenic effects.55 Hence, a variety of nonviral vectors such as liposomes, cationic lipids, polymers have been developed to replace the viral vector-mediated gene delivery.56 CNT-mediated gene delivery is a relatively new technique in this category. This approach relies on the interaction between CNTs and nucleic acids. It has been shown that CNTs can interact directly with DNA through Van der Waals and hydrophobic forces.57 A number of studies revealed that DNA can wrap around the CNTs, and thereby assists in the dispersion of the nanotubes in a liquid phase.58,59 In addition to this, various methods are reported to covalently attach either single or double stranded DNA to the CNTs.60,61 The large surface area of the CNT allows more DNA to associate tightly and condense with the nanotubes. Hence, the possibility of utilizing the CNTs as biological cargos for gene delivery is under extensive investigations.
Different approaches have been attempted in CNT-based gene delivery. One was developed by Singh et al.62 In their approach, both SWCNTs and MWCNTs were functionalized with ammonium or lysine to carry positive charges on the surface of the nanotubes. The mixing of the positively-charged CNTs with negatively-charged DNA can lead to the formation of DNA-CNT complexes through the static electronic forces formed between them. The change in ion strength that occurs when these complexes enter into the cells results in the release of DNAs from the nanotubes and its translocation into nuclei for gene expression. Their experimental results suggest that gene delivery made by MWCNT is more efficient than by SWCNT. They found that the ammonia functionalized MWCNTs are able to transfect the cells with a higher degree of DNA condensation as compared with SWCNT. Although in vitro transfection of CNT-plasmid DNA was found to be successful, the determination of optimal transfection conditions, the mechanism of cellular entry as well as the roles of charge density and tube dimension in transfection efficiency are yet to be elucidated.
Cai et al63 developed another approach that utilizes vertically aligned CNTs for gene delivery. Vertically aligned CNTs can be grown using the plasma-chemical vapor deposition (PCVD) technique. These nanotubes are characterized by the presence of ferromagnetic catalytic nickel particles on their tips and hence, are responsive to magnetic agitation. It has been shown that the short nanotubes (<2 µm) with an aspect ratio of 2.9 are magnetically drivable, whereas the longer ones (>15 µm) with an aspect ratio of 0.7 are nonresponsive. These nanotubes can be covalently linked to DNA through EDC coupling mechanism, in which the carboxylic groups formed on the functionalized nanotubes are used to react with primary amine groups in the DNA efficiently. The EDC coupling creates a CNT-DNA complex in which the DNAs are attached directly to the nanotube surface. To deliver these DNAs into cells such as mouse cortical neuron cells, a rotating magnetic field was applied by placing a magnet beneath the cell culture dishes or flasks, which helps to drive/spread the DNA-nanotube complexes toward the magnet, allowing the tight contact of these complexes with the cells that are attached to the bottom of the cell culture dishes. This technique is termed as nanospearing. It has been observed that the use of rotating field of the magnet significantly improves the gene transfection efficiency. Their experimental result indicates that an efficient DNA delivery can be achieved to both dividing and nondividing cells. The nondividing cells such as neuron cells and primary B cells are usually very hard to transfect. It has been found that the transduction efficiency of this approach is quite comparable to those of viral-based techniques. It is also important to assess the impact of CNT-based nanospearing on cellular homeostasis and cytotoxicity. They further investigated the biocompatibilty of CNT nanospearing on primary B-cell culture.64 Carboxyl functionalized CNTs were conjugated with poly–L-Lysine and then incubated with a plasmid DNA to condense with it. Upon subjecting to magnetic nanospearing, the gene was expressed in the cells, whereas there was no detectable increase in cell size. Moreover, neither expression of specific surface markers like CD71 nor any other nonspecific activation of primary B-lymphocytes can be observed in these cells. Therefore, magnetic nanospearing approach provides an alternative way for gene delivery.
On the other hand, CNT-based RNA delivery has also been explored.65–67 Initial studies using RNA polymer, i.e. poly(rU), revealed that RNA can bind to the CNT surface through the pi-stacking and hydrophobic interactions much similar to the case of plasmid DNA.68,69 Thus, RNA can be delivered into cells through their spontaneous absorption onto the CNT surface. However, the success in RNA delivery requires a systematic study of targeting, delivery, release and biological functions of the CNT-nucleic acid conjugate apart from the cytotoxic studies. In this direction, Dai’s group performed gene silencing studies with CNT covalently conjugated siRNA.66 To load RNA onto CNTs, the nanotubes are functionalized first by adsorbing phospholipids (PLs) grafted onto amine-terminated polyethylene glycol (PEG; PL-PEG2000-NH2). The CNT-RNA complexes can then be formed by conjugating the thiol-modified siRNA to the amine groups of PEG on the sidewalls of CNTs through disulfide linkages. It has been shown that the delivery of siRNA into cells can be achieved by simply incubating the cells with the CNT-siRNA conjugates. This method resulted in RNA transfection efficiency which is comparable with those of liposome-based RNA transfection approaches. Experimental results further revealed that there was about 90% of gene silencing in T-cells with 3 days of incubation with CNTs. The application of these CNT-siRNA complexes in human primary cells (peripheral blood mononuclear cells) resulted in a 60% of knock down.
Most gene delivery studies with CNT reported so far are based on in vitro cell cultures. A summary of both in vivo and in vitro transfection studies with CNTs are reviewed recently by Lacerda et al.70 Even though significant progresses have been made in the applicability of the CNTs for in vitro gene transfection, the mechanism behind the DNA or RNA release from the CNTs are yet to be elucidated, which considerably impairs the further improvement in gene delivery efficiency by this method. Also, the application of CNTs for in vivo gene delivery has not been extensively studied, partly owing to the toxicity and nonbiodegradability of the CNTs. Furthermore, the elimination or exclusion of CNTs from the human bodies after administration needs to be well established before they can be used for in vivo gene delivery.
CNT for protein transportation
The ability to transport a protein of interest into living cells is another important application of CNTs in tissue engineering. In general, a protein of interest can either be spontaneously adsorbed or covalently bound to the CNTs and such modified nanotubes can then serve as transporters to deliver the protein into living cells. However, it is of particular importance that the CNT-bound protein remains biologically active during its conjugation with nanotubes. This has been extensively investigated. Pantarotto et al. have characterized the immunogenicity of SWCNT bound peptides.71,72 They covalently coupled the peptide, a neutralizing B cell epitope from the Foot Mouth Disease Virus, with amino-functionalized SWCNTs by chemoselective ligation using N-succinimidyl 3-maleimidopropionate derivatization. The intraperitoneal administration of CNT-bound epitope to the mice elicited a strong antibody response against the peptide, whereas no antibody was produced against the CNT back-bone alone. This experiment shows that the CNT-bound peptides retain their appropriate secondary conformation necessary for eliciting an immune response. This study suggests a promising utility of CNT for vaccine delivery and thus replacing or complementing the existing vaccine carriers.
Moreover, CNT is found to facilitate the transportation of proteins into living cells. It has been well documented that CNT-bound proteins tend to be internalized readily by cells through endocytosis pathways. Kam et al have shown that CNT-bound proteins can traverse across the cell membrane and be internalized, whereas naked proteins cannot travel across effectively into cells.73 They tested three different proteins: bovine serum albumin (66 kDa), proteinA (42 kDa), and human immunoglobulin (150 kDa). These proteins were pre-labeled with fluorescent dyes and were noncovalently attached to the carboxyl-functionalized SWCNT by mixing the proteins with nanotubes. After protein attachment, the CNTs were added to the cell culture medium. Both nonadhesive cells like HL-60 (human leukemia cell line) and Jurkat cells and adhesive cells such as HeLa and NIH-3T3 were tested for CNT-based protein delivery. This study revealed that the naked proteins in the medium are unable to traverse across the cell membrane, whereas the CNT-bound proteins are readily internalized by the cells. Further tests divulged that the cellular uptake and transportation of large conjugates such as the immunoglobulin (150 kDa, 7–8 nm) was less efficient as compared with the smaller ones having molecular weight less than 80 kDa. They also found that the internalization process does not affect the cell viability and proliferation. By staining the cellular endosomal vesicle compartments with the red FM-64 dyes, they showed that the CNT-protein conjugates are taken up by the cells through endocytosis pathways. To intensify the endosomal release of CNT-bound proteins, they used chloroquine. Chloroquine can increase the pH inside endosomes. It can be added directly to the cell culture medium after exposing the cells to the CNT-bound proteins. This leads to an osmotic shock resulting in swelling and rupture of endosomal compartment. They observed a uniform distribution of fluorescent dye-labeled CNT-bound proteins in the entire cytoplasm, whereas only few discrete spots were found inside the cells in the absence of chloroquine.
The protein attachment and transport by CNTs were further studied by Dai’s group for selective cancer cell destruction by hyperthermia therapy.74 As mentioned earlier, the SWCNT exhibits a strong absorption in the near-infrared region (NIR) regime (>700 nm), whereas cells and tissues are highly transparent in this range. Thus, continuous NIR irradiation of CNTs will result in heating the cells if the CNTs are internalized inside the cells, resulting thermal destruction of the cells. To selectively deliver the CNTs into cancer cells, SWCNTs were first be functionalized with phospholipids and then antibodies against the folate receptors present in the HeLa cells (human cervical cancer cells). It has been shown that the mixing of the antibody-bound CNTs with cells can lead to the internalization of the CNTs exclusively to the cancer cells. Upon exposure to continuous NIR radiation (808 nm laser), the cells are killed because of the excessive local heating generated from a strong photo absorption of the SWCNTs internalized in the cancer cells, whereas normal cells remain unaffected.74 Such a strategy can be further modified for targeted delivery of chemotherapeutic drugs exclusively to cancer cells by introducing tumor marker specific antibody and drug onto the CNTs.75 Extensive reviews on strategies and use of CNTs for cancer therapy as well as for other drug design, discovery and delivery has been published recently.76–80
Monitoring CNT Within Living Cells and Tissues
The visualization of CNTs within living cells or tissues is crucial to understand their behavior or their interaction with cells and tissues. To date, a variety of approaches have been developed. Among these approaches, the use of a fluorescent dye shows tremendous merits, as it offers a high contrast and specificity when microscopy is used for visualization. Direct visualization of CNTs can be accomplished by the interaction of the hydrophobic sidewalls of the nanotubes with long chained aliphatic fluorescent molecules81 so that a fluorescent dye can be conjugated to the nanotubes. According to Didenko et al.’s study, individual SWCNT can be visualized by fluorescent polymer wrapping, where the nanotubes are suspended in water with the help of detergent such as sodium dodecylbenzene sulfonate (SDS) and labeled with poly(vinylpyrrolidone)-1300 Alexa fluorescent dye conjugates.82 Similarly, SDS suspended SWCNTs can be labeled with hydrophobic red fluorescent dye PHK26 and visualized through the immunofluorescence microscopy.83
On the other hand, the modification of the SWCNT’s surface by compounds like poly(10,11-dihydro-10,11-disila-5,10,10,11,11-pentamethyl-5H-dibenz[b,f]azepine-2,8-diyl) will enable the nanotubes to be distinctly visualized through immunofluorescence microscopy.84 Using this approach Sirdeshmukh and Panchapakesan investigated the interaction between antibodies and nanotubes.85 In their experiment, nanotubes are labeled with a fluorescent dye (dihexyloxacarbocyanine iodide) by directly agitating SWCNTs with SDS and dye. Upon excitation at 488 nm, the dye attached to the CNTs emits fluorescence to visualize the nanotubes. The labeled nanotubes can be further mixed with polyclonal goat anti-mouse IgG Alexa 546 conjugates (emitting at 543 nm). The co-localization of these two different fluorescent dyes helps the visualization of the interaction between the nanotubes and the antibodies. Fluorescein isothiocyanate (FITC) has also been adopted for labeling the CNTs to determine the translocation of the nanotubes across the cell membrane.86 Quantum-dot (QD), a relatively newer fluorescent nanomaterial, has also been explored for visualization of CNTs within living cells. To attach the QDs to the CNTs, the QDs needs to be first functionalized with mercaptoacetic acid, and then mix with SWCNT suspension in the presence of SDS.87 The functionalized QDs can arrange themselves on the sidewalls of SWCNTs by virtue of electrostatic interaction between zinc ions in their shell and electronegative oxygen atoms/anions in the sulfonate group of the surfactant, sodium-dodecyl-sulfonate, leading to the fluorescence visualization of SWCNTs.
CNT can also be imaged through the NIR microscopy. As mentioned earlier, semiconducting SWCNTs show a band gap fluorescence emission in the NIR region at wavelengths characteristic to the specific structure.1 Hence, NIR microscopy can be used for monitoring the appearance of the nanotubes within living cells. For example, the presence of CNTs in the larvae of Drosophila melanogastrer (fruit flies) was determined by NIR imaging.88 One of the major advantages of this technique is that the accumulations of nanotubes in the tissues can be imaged nondestructively. For biomedical applications, methods relying on NIR spectrum have advantages, because most cells and tissues are transparent in the NIR range and thus, offer greater depth of light penetration and reduced background fluorescence.
Cytotoxicity of CNT
The cytotoxicity and long-term impact of CNTs on human health need to be characterized very carefully before applying them in vivo. It has been well known that nanoparticles can create reactive forms of oxygen that damages the cells. Cells can defend themselves by producing anti-oxidants when they encounter low concentrations of nanoparticles. It has been shown that cells can become inflamed or die when the concentrations of nanoparticles increase.89 More detailed accounts on cytotoxicity and pathogenicity of nanoparticles have been reviewed recently.89,90 Just like most other nanoparticles, CNTs also possess a small size and large surface area. Therefore, it is likely that they will evoke the generation of reactive oxygen species (ROS) when used in vivo.
Recently, a number of groups have come out with reports on CNT’s toxicity studies. These studies were either performed under in vitro conditions with cell lines91–93 or in vivo with animal models.88,94–96 Nevertheless, the results reported by these studies are contradictory in nature, with some indicating CNTs are highly toxic and others showing lack of any toxic effects. For example, Cheng et al. reported that cell exposure to SWCNTs could induce a significant hatching delay of zera fish embryos.97 Another study shows that the treatment of the HEK 293 cells with SWCNTs can lead to time and dose dependent reduction in cell viability.98 Where as, experiments performed on the HL-60 cells revealed that SWCNTs up to 25 µg/mL have no effect on cell proliferation and viability if the cells are exposed to CNTs for a short time period such as 2 h.73 In vivo and in vitro studies by Muller et al. with epithelial cells pointed out the possible adverse effects of MWCNTs.99 Some of the recent reviews have extensively discussed about the biocompatibility and toxicity of CNTs in particular.100,101 The discrepancy in the cytotoxicity/biocompatibility studies of CNTs can be due in part to various factors such as the size, the nature (soluble or insoluble), and the way of functionalization, the concentration and exposure time of CNTs used in vitro or in vivo. Compared with the solubilized CNTs, the exposure to the hydrophobic CNTs are particularly toxic to the cells and tissues. It was pointed out in the previous section that the CNTs are now being used even along with various stem cells. Though successful stem cell differentiations were reported, it is also interesting to note that severe DNA damages were observed in certain stem cells. For example, studies by Zhu et al using MWCNTs on mouse embryonic stem cells (ESC) revealed that the CNTs can accumulate and induce apoptosis and activate tumor suppressor protein p53 within 2 h exposure.102 It was also observed that the MWCNTs can increase the mutation frequency at least by twofolds in the mouse ESCs. Moreover, pharyngeal aspiration of SWCNTs can induce robust acute inflammatory reaction with the very early onset of a fibrinogenic response and formation of granulomas in mice, as reported by Shvedova et al.95 Even after two months of aspiration, the presence of the nanotubes was observed in the pulmonary granulamatous lesion. Salvador-Morales et al. further confirmed that both SWCNTs and MWCNTs can activate the human serum complement system via classical pathways.103 Yet another pulmonary toxicity study of CNTs in mouse model was reported by Chou et al recently.104 In their study, it was found that intratracheal administration of 0.5 mg of SWCNTs into 8 week old male ICR mice results in alveolar macrophage activation, various chronic inflammatory responses and severe pulmonary granuloma formation. Activation of transcription factors like NF-κβ as well as AP-1 were found in SWCNT treated macrophages, resulting in oxidative stress and leading to associated innate and adaptive immune responses.
Another aspect of the CNT cytotoxicty studies involves examining the clearance of CNTs from the body after their introduction. Studies have been performed using both soluble and insoluble CNTs in moue models. Singh et al. investigated the tissue biodistribution and clearance of soluble CNTs.96 They conjugated ammonium functionalized SWCNTs or MWCNTs with radioactive metal Indium-111 (111In) using diethylene triamine pentaacetic acid (DTPA) as chelating agent. The radioactively labeled CNTs were administered to the mice intravenously and the presence of the CNTs in various organs/tissues was monitored by measuring the radioactivity of the 111In. It was found that both SWCNTs and MWCNTs follow a rapid first order clearance from the blood compartment through renal excretion route without any toxic side effects or mortality. This study suggested that soluble CNTs are safe for use in vivo. Another pilot toxicology study using functionalized SWCNT (noncovalently PEGylated SWCNT as well as oxidized covalently PEGylated SWCNTs) injected into the blood stream of mice did not indicate any evidence of acute or chronic toxicity over 4 months.105
It is true that drawing a general conclusion about the safe use of CNTs in vivo is difficult because most studies are conducted based on varying concentrations and different conditions leading to contradictory results.106 Obviously, the functionalization or solubilization of CNTs cannot be a single and affirmative solution for preventing the CNT cytotoxicity. The presence of modified CNTs can be hazardous in various routes. For example, a study conducted by Roberts et al. using CNTs on Daphnia magna, an aquatic invertebrate used widely for regulatory environmental testing, demonstrate that the CNTs can be modified during the digestion process by the organism and rendered it insoluble back into the environment.107 This means that the nanotubes can be soluble and stable before they are taken up by organisms, but their interaction with biological systems can quickly convert the nanotubes into an insoluble form. Leaking of the nanotubes from implanted CNT-based sensors and scaffolds and its accumulation in the body can have serious and wide spread adverse effects which have not been characterized extensively so far. The modification of the CNT surface coating by Daphnia magna reinstates the need for developing better methods for functionalization and solubilization of the CNTs. Otherwise the use of CNTs may result in unanticipated effects on the tissue or organism exposed.
Remarks
The incredible pace of advancement of nanobiotechnology has created tremendous enthusiasm among the academia and industries for the use of CNTs for tissue engineering applications. However, the surface chemistry of CNTs and their reactivity with biological systems are yet to be completely understood. For most technical applications, CNTs of high purity are required, but contaminants in production and the batch to batch repeatability in a large-scale production are still a big challenge in large scale CNT synthesis. The major contaminants along with CNTs include amorphous or graphitic carbon, fullerenes and metal catalyst residues. Further-more, because of their geometry and hydrophobic surface, CNTs have a tendency to form agglomerates and thus usually exist in bundles, which needs to be isolated by different treatments. Hence, the immediate studies must focus on its surface chemistry before CNTs can become one of the major biomaterials for use in tissue engineering such as organ regeneration. As aforementioned, the surface of CNTs is not active in chemistry and does not bear any biological activity. Thus, CNT itself is not a biomaterial and cannot be used directly in tissue engineering. Various surface modifications are required to make it biocompatible or supporting cell or tissue growing. Although a variety of techniques have been developed as mentioned earlier, it is still lacking of a standardized approach for surface modification. Furthermore, it is highly desired to develop a simple approach to modify the CNT surface so that it can be readily utilized in tissue engineering. Conventional purification methods adopt very harsh conditions such as using strong acids or organic solvents. For example, strong acid treatment is always used to create carboxyl groups on the surface of CNTs for further functionalization. However, the location and number of carboxyl groups formed on the surface of CNTs after the acidic treatment is always unknown and is hard to control. Also, the quality of the treatment varies batch by batch. This brings lot of uncertainties in in-vivo applications. Thus, a controllable approach needs to be established for surface modification of CNTs.
Moreover, various agents used to disperse CNTs are cytotoxic, making them less effective in tissue engineering. Diverse purification strategies reported, but most of them change the surface of the CNTs and hence, the way in which the biological system responds to its presence. It is not only the surface modification, but trapping molecules inside the nanotubes also can change the properties. Reports indicate that unpurified SWCNTs can stimulate the free radical generation in human keratinocytes and bronchial epithelial cells108 whereas many reports with MWCNTs and functionalized SWCNTs demonstrated that they can act as excellent free radical scavengers.109 The existence of contaminants and dispersant agents complicates the interpretation of results of toxicological studies on CNTs. So far no preclinical therapeutic efficacy data exist for the use of CNTs to treat any disease. Because the research as well as our knowledge on CNTs is on infancy, the existing hype around the CNTs can lead to unrealistic hopes and misrepresentation. As there are various contradicting results reported on varying experimental setups, the cytotoxic effects of nanotubes need to be studied in detail before the technological advancement in tissue engineering/biomedical application of the nanotubes can be commercialized.
Table 1.
A Summary of the Representative In Vitro and In Vivo Studies Performed with Carbon Nanotubes
| Application / Study | Type of CNT | Biological System | Reference |
|---|---|---|---|
| Biocompatibility / cytotoxicity | |||
| In vivo, biodistribution and clearance | SWCNT functionalized with DTPA (soluble) | Mice, intravenously | Singh et al.96 |
| In vivo, biodistribution | SWCNT (insoluble) | Drosophila, oral administration | Leeuw et al88 |
| In vivo, pulmonary toxicity | SWCNT (insoluble) | Mice, intrapharangeal instillation | Li et al.,94 Shvedova et al.95 |
| In vitro, complement activation | SWCNT dispersed with TritonX-100 | Rabbit erythrocytes, human serum/plasma | Salvador-Morales et al.103 |
| In vitro, interaction with mammalian cells | SWCNT | Rat aortic smooth muscle cells, Human epithelial like cell (HeLa), Human leukemia cell line (HL-60) | Raja et al.,92 Yehia et al.,93 Cui et al.91 |
| In vitro, issues with viability assays | SWCNT (insoluble) | Alveolar epithelial cell line, alveolar macrophage cell line, endothelial cell line | Worle-Knirsch et al.106 |
| In vitro, neurite outgrowth | SWCNT Functionalized with PABS or PEG (soluble) | Rat hippocampal neuronal culture in presence of media containing CNTs | Ni et al.50 |
| Scaffolds for tissue engineering | |||
| In vitro, bone regeneration biomaterial | SWCNT and MWCNT | Rat Osteosarcoma cell line | Zanello et al.30 |
| Vertically aligned CNTs | Human Osteosarcoma cell line CAL-72 | Giannona et al.31 | |
| In vitro, tissue engineering scaffold | —COOH functionalized SWCNT along with collagen | Rat aortic smooth muscle cells | MacDonald et al.21 |
| In vivo, tissue engineering scaffold | —COOH functionalized SWCNT with alginate | Rat, subcutaneous implantation | Kawaguchi et al.22 |
| In vitro, Neuron regeneration | MWCNT (insoluble) and MWCNT coated with 4-hydroxynonenal | Primary hippocampal cell culture from rat embryo | Mattson et al.43 |
| MWCNT coated with neutrotrophin | Chick embryonic DRG neuron | Matsumoto et al.51 | |
| SWCNT functionalized with polyethyleneimine | Rat hippocampal neuronal culture | Hu et al.45 | |
| In vitro, neuronal electrical signal transfer | SWCNT | Rat hippocampal neuronal culture | Mazzatenta et al.47 |
| MWCNT functionalized | Rat hippocampal neuronal culture | Lovat et al.44 | |
| In vitro, blood compatible biomaterial | MWCNT (insoluble) along with polyurethane | Human platelet suspension | Meng et al.26 |
| In vivo, blood compatible biomaterial | MWCNT along with Nylon | Dog, subclavian artery and subclavian vein | Endo et al.27 |
| Gene delivery to tissues | |||
| In vitro, DNA delivery and near infra red heating for cell destruction | SWCNT, functionalized with PEG and phospholipids (soluble) | HeLa cells | Kam et al.74 |
| In vitro, plasmid DNA delivery by magnetic spearing | —COOH functionalized CNT | Primary B-cells, Primary neurons | Cai et al.63,64 |
| In vivo, dsDNA | —COOH functionalized SWCNT | Mice tumor tissue | Zhang et al.67 |
| In vitro, RNA poly(rU) | SWCNT | Human breast cancer MCF7 cell line | Lu et al.68 |
| In vivo, siRNA delivery for suppressing tumor growth | Functionalized SWCNT | Mice tumor tissue as well as in vitro using tumor cell lines | Zhang et al67 |
| Targeting and drug delivery to tissues | |||
| In vivo, protein for immunization (peptide from foot and mouth disease virus) | SWCNT (soluble) | Mice, intraperitoneal | Pantarotto et al.72 |
| In vitro, various sized proteins like streptavidin, albumin, immunoglobulin | —COOH functionalized SWCNT (soluble) | Adhesive cell lines (HeLa, NIH-3T3) and non adhesive cell lines (HL-60, Jurkat) lines | Kam et al73 |
| In vivo, protein (antibody) as well as radiometalion chelates for tumor cell targeting and destruction | Functionalized SWCNT (soluble) | Mice, intravenous | McDevitt et al.75 |
Acknowledgment
This work is partly supported by NIH grant EB006378-01 and Arkansas Biosciences Institute grant 0402-27504-21-726. The authors thank Ms. Lakshmi Kannan for her assistance in manuscript preparation.
Notation
- BDNF
brain-derived neurotrophic factor
- CNT
carbon nanotube
- CVD
chemical vapor deposition
- DTPA
diethylene triamine pentaacetic acid
- ECM
extracellular matrix
- EDC
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride
- ESC
embryonic stem cell
- FAK
focal adhesion kinase
- FITC
Fluorescein isothiocyanate
- MWCNT
multi-walled carbon nanotube
- NGF
nerve growth factor
- NIR
near-infrared
- NSC
mouse neural stem cell
- PABA
poly aminobezene sulphonic acid
- PABS
poly amino benzene sulfonic acid
- PCVD
plasma-chemical vapor deposition
- PDMS
polydimethylsiloxane
- PEG
polyethylene glycol
- PEI
polyethyleneimine
- PL
phospholipids
- PMMA
polymethyl methacrylate
- PSC
post synaptic currents
- QD
Quantum-dot
- RBC
red blood cell
- ROS
reactive oxygen species
- SDS
sodium dodecylbenzene sulfonate
- SWCNT
single-walled carbon nanotube
Literature Cited
- 1.O’Connell MJ, Bachilo SM, Huffman CB, Moore VC, Strano MS, Haroz EH, Rialon KL, Boul PJ, Noon WH, Kittrell C, Ma J, Hauge RH, Weisman RB, Smalley RE. Band gap fluorescence from individual single-walled carbon nanotubes. Science. 2002;297:593–596. doi: 10.1126/science.1072631. [DOI] [PubMed] [Google Scholar]
- 2.Sharon M, Pradhan D, Zacharia R, Puri V. Application of carbon nanomaterial as a microwave absorber. J Nanosci Nanotechnol. 2005;5:2117–2120. doi: 10.1166/jnn.2005.186. [DOI] [PubMed] [Google Scholar]
- 3.Yu M-F, Files BS, Arepalli S, Ruoff RS. Tensile loading of ropes of single wall carbon nanotubes and their mechanical properties. Phys Rev Lett. 2000;84:5552. doi: 10.1103/PhysRevLett.84.5552. [DOI] [PubMed] [Google Scholar]
- 4.Motoo F, Xing Z, Huaqing X, Hiroki A, Koji T, Tatsuya I, Hidekazu A, Tetsuo S. Measuring the thermal conductivity of a single carbon nanotube. Phys Rev Lett. 2005;95:065502. doi: 10.1103/PhysRevLett.95.065502. [DOI] [PubMed] [Google Scholar]
- 5.Ebbesen TW, Ajayan PM. Large-scale synthesis of carbon nanotubes. Nature. 1992;358:220–222. [Google Scholar]
- 6.Thess A, Lee R, Nikolaev P, Dai H, Petit P, Robert J, Xu C, Lee YH, Kim SG, Rinzler AG, Colbert DT, Scuseria GE, Tomanek D, Fischer JE, Smalley RE. Crystalline ropes of metallic carbon nanotubes. Science. 1996;273:483–487. doi: 10.1126/science.273.5274.483. [DOI] [PubMed] [Google Scholar]
- 7.Kuo TF, Chi CC, Lin IN. Synthesis of carbon nanotubes by laser ablation of graphites at room temperature. Jpn J Appl Phys. 2001;40:7147–7150. [Google Scholar]
- 8.Varadan VK, Xie J. Large-scale synthesis of multi-walled carbon nanotubes by microwave CVD. Smart Mater Struct. 2002;11:610–616. [Google Scholar]
- 9.Banerjee S, Kahn MG, Wong SS. Rational chemical strategies for carbon nanotube functionalization. Chemistry. 2003;9:1898–1908. doi: 10.1002/chem.200204618. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Musameh M, Lin Y. Solubilization of carbon nanotubes by nafion toward the preparation of amperometric biosensors. J Am Chem Soc. 2003;125:2408–2409. doi: 10.1021/ja028951v. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Iqbal Z, Malhotra SV. Functionalization of carbon nanotubes with amines and enzymes. Chem Phys Lett. 2005;402:96–101. [Google Scholar]
- 12.Iijima S. Helical microtubules of graphitic carbon. Nature. 1991;354:56–58. [Google Scholar]
- 13.Veetil JV, Ye K. Development of immunosensors using carbon nanotubes. Biotechnol Prog. 2007;23:517–531. doi: 10.1021/bp0602395. [DOI] [PubMed] [Google Scholar]
- 14.Cui D. Advances and prospects on biomolecules functionalized carbon nanotubes. J Nanosci Nanotechnol. 2007;7:1298–1314. doi: 10.1166/jnn.2007.654. [DOI] [PubMed] [Google Scholar]
- 15.Balasubramanian K, Burghard M. Biosensors based on carbon nanotubes. Anal Bioanal Chem. 2006;385:452–468. doi: 10.1007/s00216-006-0314-8. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Yantasee W, Wang J. Carbon nanotubes (CNTs) for the development of electrochemical biosensors. Front Biosci. 2005;10:492–505. doi: 10.2741/1545. [DOI] [PubMed] [Google Scholar]
- 17.Ziegler KJ. Developing implantable optical biosensors. Trends Biotechnol. 2005;23:440–444. doi: 10.1016/j.tibtech.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Ebron VH, Yang Z, Seyer DJ, Kozlov ME, Oh J, Xie H, Razal J, Hall LJ, Ferraris JP, Macdiarmid AG, Baughman RH. Fuel-powered artificial muscles. Science. 2006;311:1580–1583. doi: 10.1126/science.1120182. [DOI] [PubMed] [Google Scholar]
- 19.Silvano G, Claudio B, Valter B, Giorgio G, Claudio N. Carbon nanotube biocompatibility with cardiac muscle cells. Nanotechnology. 2006;17:391–397. [Google Scholar]
- 20.Gentleman E, Lay AN, Dickerson DA, Nauman EA, Livesay GA, Dee KC. Mechanical characterization of collagen fibers and scaffolds for tissue engineering. Biomaterials. 2003;24:3805–3813. doi: 10.1016/s0142-9612(03)00206-0. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald RA, Laurenzi BF, Viswanathan G, Ajayan PM, Stegemann JP. Collagen-carbon nanotube composite materials as scaffolds in tissue engineering. J Biomed Mater Res A. 2005;74:489–496. doi: 10.1002/jbm.a.30386. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi M, Fukushima T, Hayakawa T, Nakashima N, Inoue Y, Takeda S, Okamura K, Taniguchi K. Preparation of carbon nanotube-alginate nanocomposite gel for tissue engineering. Dent Mater J. 2006;25:719–725. doi: 10.4012/dmj.25.719. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Wang X, Lu Q, Fu C. Influence of carbon nanotube scaffolds on human cervical carcinoma HeLa cell viability and focal adhesion kinase expression. Carbon. 2008;46:453–460. [Google Scholar]
- 24.Parsons JT, Martin KH, Slack JK, Taylor JM, Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene. 2000;19:5606–5613. doi: 10.1038/sj.onc.1203877. [DOI] [PubMed] [Google Scholar]
- 25.Janvier G, Baquey C, Roth C, Benillan N, Belisle S, Hardy J-F. Extracorporeal circulation, hemocompatibility, and biomaterials. Ann Thorac Surg. 1996;62:1926–1934. doi: 10.1016/s0003-4975(96)00942-3. [DOI] [PubMed] [Google Scholar]
- 26.Meng J, Kong H, Xu HY, Song L, Wang CY, Xie SS. Improving the blood compatibility of polyurethane using carbon nanotubes as fillers and its implications to cardiovascular surgery. J Biomed Mater Res A. 2005;74:208–214. doi: 10.1002/jbm.a.30315. [DOI] [PubMed] [Google Scholar]
- 27.Endo M, Koyama S, Matsuda Y, Hayashi T, Kim YA. Thrombogenicity and blood coagulation of a microcatheter prepared from carbon nanotube-nylon-based composite. Nano Lett. 2005;5:101–115. doi: 10.1021/nl0482635. [DOI] [PubMed] [Google Scholar]
- 28.Mann S, Weiner S. Biomineralization: structural questions at all length scales. J Struct Biol. 1999;126:179–181. doi: 10.1006/jsbi.1999.4131. [DOI] [PubMed] [Google Scholar]
- 29.Zhao B, Hu H, Mandal SK, Haddon RC. A bone mimic based on the self-assembly of hydroxyapatite on chemically functionalized single-walled carbon nanotubes. 2005;17:3235–3241. [Google Scholar]
- 30.Zanello LP, Zhao B, Hu H, Haddon RC. Bone cell proliferation on carbon nanotubes. Nano Lett. 2006;6:562–567. doi: 10.1021/nl051861e. [DOI] [PubMed] [Google Scholar]
- 31.Giannona S, Firkowska I, Rojas-Chapana J, Giersig M. Vertically aligned carbon nanotubes as cytocompatible material for enhanced adhesion and proliferation of osteoblast-like cells. J Nanosci Nanotechnol. 2007;7:1679–1683. doi: 10.1166/jnn.2007.454. [DOI] [PubMed] [Google Scholar]
- 32.Liao S, Xu G, Wang W, Watari F, Cui F, Ramakrishna S, Chan CK. Self-assembly of nano-hydroxyapatite on multi-walled carbon nanotubes. Acta Biomater. 2007;3:669–675. doi: 10.1016/j.actbio.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Jell G, Verdejo R, Safinia L, Shaffer MSP, Stevens MM, Bismarck A. Carbon nanotube-enhanced polyurethane scaffolds fabricated by thermally induced phase separation. J Mater Chem. 2008;18:1865–1872. [Google Scholar]
- 34.Marrs B, Andrews R, Rantell T, Pienkowski D. Augmentation of acrylic bone cement with multiwall carbon nanotubes. J Biomed Mater Res A. 2006;77:269–276. doi: 10.1002/jbm.a.30651. [DOI] [PubMed] [Google Scholar]
- 35.Kim P, Shi L, Majumdar A, McEuen PL. Thermal transport measurements of individual multiwalled nanotubes. Phys Rev Lett. 2001;87:215502. doi: 10.1103/PhysRevLett.87.215502. [DOI] [PubMed] [Google Scholar]
- 36.Hatano K, Inoue H, Kojo T, Matsunaga T, Tsujisawa T, Uchiyama C, Uchida Y. Effect of surface roughness on proliferation and alkaline phosphatase expression of rat calvarial cells cultured on polystyrene—materials science in catalysis. Bone. 1999;25:439–445. doi: 10.1016/s8756-3282(99)00192-1. [DOI] [PubMed] [Google Scholar]
- 37.Khang D, Kim SY, Liu-Snyder P, Palmore GT, Durbin SM, Webster TJ. Enhanced fibronectin adsorption on carbon nanotube/poly(carbonate) urethane: independent role of surface nano-roughness and associated surface energy. Biomaterials. 2007;28:4756–4768. doi: 10.1016/j.biomaterials.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 38.Usui Y, Aoki K, Narita N, Murakami N, Nakamura I, Nakamura K, Ishigaki N, Yamazaki H, Horiuchi H, Kato H, Taruta S, Kim YA, Endo M, Saito N. Carbon nanotubes with high bone-tissue compatibility and bone-formation acceleration effects. Small. 2008;4:240–246. doi: 10.1002/smll.200700670. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Watari F, Omori M, Liao S, Zhu Y, Yokoyama A, Uo M, Kimura H, Ohkubo A. Mechanical properties and biological behavior of carbon nanotube/polycarbosilane composites for implant materials. J Biomed Mater Res B Appl Biomater. 2007;82:223–230. doi: 10.1002/jbm.b.30724. [DOI] [PubMed] [Google Scholar]
- 40.Abarrategi A, Gutiérrez MC, Moreno-Vicente C, Hortigüela MJ, Ramos V, López-Lacomba JL, Ferrer ML, del Monte F. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials. 2008;29:94–102. doi: 10.1016/j.biomaterials.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Günther KHZ. Proteomics of traumatic brain injury and regeneration. Proteomics Clin Appl. 2007;1:1362–1372. doi: 10.1002/prca.200700420. [DOI] [PubMed] [Google Scholar]
- 42.Rao AM, Richter E, Bandow S, Chase B, Eklund PC, Williams KA, Fang S, Subbaswamy KR, Menon M, Thess A, Smalley RE, Dresselhaus G, Dresselhaus MS. Diameter-selective raman scattering from vibrational modes in carbon nanotubes. Science. 1997;275:187–191. doi: 10.1126/science.275.5297.187. [DOI] [PubMed] [Google Scholar]
- 43.Mattson MP, Haddon RC, Rao AM. Molecular functionalization of carbon nanotubes and use as substrates for neuronal growth. J Mol Neurosci. 2000;14:175–182. doi: 10.1385/JMN:14:3:175. [DOI] [PubMed] [Google Scholar]
- 44.Lovat V, Pantarotto D, Lagostena L, Cacciari B, Grandolfo M, Righi M, Spalluto G, Prato M, Ballerini L. Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 2005;5:1107–1110. doi: 10.1021/nl050637m. [DOI] [PubMed] [Google Scholar]
- 45.Hu H, Ni Y, Mandal SK, Montana V, Zhao B, Haddon RC, Parpura V. Polyethyleneimine functionalized single-walled carbon nanotubes as a substrate for neuronal growth. J Phys Chem B. 2005;109:4285–4289. doi: 10.1021/jp0441137. [DOI] [PubMed] [Google Scholar]
- 46.Gheith MK, Sinami VA, Wicksted JP, Matts RL, Kotov NA. Single-walled carbon nanotube polyelectrolyte multilayers and freestanding films as a biocompatible platform for neuroprosthetic implants. Adv Mater. 2005;17:2663–2670. [Google Scholar]
- 47.Mazzatenta A, Giugliano M, Campidelli S, Gambazzi L, Businaro L, Markram H, Prato M, Ballerini L. Interfacing neurons with carbon nanotubes: electrical signal transfer and synaptic stimulation in cultured brain circuits. J Neurosci. 2007;27:6931–6936. doi: 10.1523/JNEUROSCI.1051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gabay T, Jakobs E, Ben-Jacob E, Hanein Y. Engineered self-organization of neural networks using carbon nanotube clusters. Phys A: Stat Mech Appl. 2005;350:611–621. [Google Scholar]
- 49.Sorkin R, Gabay T, Blinder P, Baranes D, Ben-Jacob E, Hanein Y. Compact self-wiring in cultured neural networks. J Neural Eng. 2006;3:95–101. doi: 10.1088/1741-2560/3/2/003. [DOI] [PubMed] [Google Scholar]
- 50.Ni Y, Hu H, Malarkey EB, Zhao B, Montana V, Haddon RC, Parpura V. Chemically functionalized water soluble single-walled carbon nanotubes modulate neurite outgrowth. J Nanosci Nanotechnol. 2005;5:1707–1712. doi: 10.1166/jnn.2005.189. [DOI] [PubMed] [Google Scholar]
- 51.Matsumoto K, Sato C, Naka Y, Kitazawa A, Whitby RL, Shimizu N. Neurite outgrowths of neurons with neurotrophin-coated carbon nanotubes. J Biosci Bioeng. 2007;103:216–220. doi: 10.1263/jbb.103.216. [DOI] [PubMed] [Google Scholar]
- 52.Jan E, Kotov NA. Successful differentiation of mouse neural stem cells on layer-by-layer assembled single-walled carbon nanotube composite. Nano Lett. 2007;7:1123–1128. doi: 10.1021/nl0620132. [DOI] [PubMed] [Google Scholar]
- 53.Park SY, Park SY, Namgung S, Kim B, Im J, Kim JY, Sun K, Lee KB, Nam JM, Park Y, Hong S. Carbon nanotube mono-layer patterns for directed growth of mesenchymal stem cells. Adv Mater. 2007;19:2530–2534. [Google Scholar]
- 54.Jin S, Ye K. Nanoparticle-mediated drug delivery and gene therapy. Biotechnol Prog. 2007;23:32–41. doi: 10.1021/bp060348j. [DOI] [PubMed] [Google Scholar]
- 55.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 56.Davis ME. Non-viral gene delivery systems. Curr Opin Biotechnol. 2002;13:128–131. doi: 10.1016/s0958-1669(02)00294-x. [DOI] [PubMed] [Google Scholar]
- 57.Gao H, Kong Y. Simulation of DNA-nanotube interactions. Annu Rev Mater Res. 2004;124:123–150. [Google Scholar]
- 58.Rajendra J, Baxendale M, DitRap LG, Rodger A. Flow linear dichroism to probe binding of aromatic molecules and DNA to single-walled carbon nanotubes. J Am Chem Soc. 2004;126:11182–11188. doi: 10.1021/ja048720j. [DOI] [PubMed] [Google Scholar]
- 59.Zheng M, Jagota A, Semke ED, Diner BA, McLean RS, Lustig SR, Richardson RE, Tassi NG. DNA-assisted dispersion and separation of carbon nanotubes. Nat Mater. 2003;2:338–342. doi: 10.1038/nmat877. [DOI] [PubMed] [Google Scholar]
- 60.Yang W, Moghaddam MJ, Taylor S, Bojarski B, Wieczorek L, Herrmann J, McCall MJ. Single-walled carbon nanotubes with DNA recognition. Chem Phys Lett. 2007;443:169–172. [Google Scholar]
- 61.Ramanathan T, Fisher FT, Ruoff RS, Brinson LC. Amino-functionalized carbon nanotubes for binding to polymers and biological systems. Chem Mater. 2005;17:1290–1295. [Google Scholar]
- 62.Singh R, Pantarotto D, McCarthy D, Chaloin O, Hoebeke J, Partidos CD, Briand JP, Prato M, Bianco A, Kostarelos K. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: toward the construction of nanotube-based gene delivery vectors. J Am Chem Soc. 2005;127:4388–4396. doi: 10.1021/ja0441561. [DOI] [PubMed] [Google Scholar]
- 63.Cai D, Mataraza JM, Qin ZH, Huang Z, Huang J, Chiles TC, Carnahan D, Kempa K, Ren Z. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nat Methods. 2005;2:449–454. doi: 10.1038/nmeth761. [DOI] [PubMed] [Google Scholar]
- 64.Cai D, Doughty CA, Potocky TB, Dufort FJ, Huang Z, Blair D, Kempa K, Ren ZF, Chiles TC. Carbon nanotube-mediated delivery of nucleic acids does not result in non-specific activation of B lymphocytes. Nanotechnology. 2007;18:365101–365111. [Google Scholar]
- 65.Kateb B, Van Handel M, Zhang L, Bronikowski MJ, Manohara H, Badie B. Internalization of MWCNTs by microglia: possible application in immunotherapy of brain tumors. Neuroimage. 2007;37 Suppl 1:S9–S17. doi: 10.1016/j.neuroimage.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 66.Liu Z, Winters M, Holodniy M, Dai H. siRNA delivery into human T cells and primary cells with carbon-nanotube transporters. Angew Chem Int Ed Engl. 2007;46:2023–2027. doi: 10.1002/anie.200604295. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Z, Yang X, Zhang Y, Zeng B, Wang S, Zhu T, Roden RB, Chen Y, Yang R. Delivery of telomerase reverse transcriptase small interfering RNA in complex with positively charged single-walled carbon nanotubes suppresses tumor growth. Clin Cancer Res. 2006;12:4933–4939. doi: 10.1158/1078-0432.CCR-05-2831. [DOI] [PubMed] [Google Scholar]
- 68.Lu Q, Moore JM, Huang G, Mount AS, Rao AM, Larcom LL, Ke PC. RNA polymer translocation with single-walled carbon nanotubes. Nano Lett. 2004;4:2473–2477. [Google Scholar]
- 69.Rahul R, Janet L, Qi L, Gayatri K, Katherine OF, William CF, Apparao MR, Pu Chun K. Single-molecule fluorescence microscopy and Raman spectroscopy studies of RNA bound carbon nanotubes. 2004;85:4228–4230. [Google Scholar]
- 70.Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotube cell translocation and delivery of nucleic acids in vitro and in vivo. J Mater Chem. 2008;18:17–22. [Google Scholar]
- 71.Pantarotto D, Partidos CD, Graff R, Hoebeke J, Briand JP, Prato M, Bianco A. Synthesis, structural characterization, and immunological properties of carbon nanotubes functionalized with peptides. J Am Chem Soc. 2003;125:6160–6164. doi: 10.1021/ja034342r. [DOI] [PubMed] [Google Scholar]
- 72.Pantarotto D, Partidos CD, Hoebeke J, Brown F, Kramer E, Briand JP, Muller S, Prato M, Bianco A. Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem Biol. 2003;10:961–966. doi: 10.1016/j.chembiol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Kam NW, Dai H. Carbon nanotubes as intracellular protein transporters: generality and biological functionality. J Am Chem Soc. 2005;127:6021–6026. doi: 10.1021/ja050062v. [DOI] [PubMed] [Google Scholar]
- 74.Kam NW, O’Connell M, Wisdom JA, Dai H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc Natl Acad Sci USA. 2005;102:11600–11605. doi: 10.1073/pnas.0502680102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McDevitt MR, Chattopadhyay D, Kappel BJ, Jaggi JS, Schiffman SR, Antczak C, Njardarson JT, Brentjens R, Scheinberg DA. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J Nucl Med. 2007;48:1180–1189. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- 76.Prato M, Kostarelos K, Bianco A. Functionalized carbon nanotubes in drug design and discovery. Acc Chem Res. 2008;41:60–68. doi: 10.1021/ar700089b. [DOI] [PubMed] [Google Scholar]
- 77.Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr Opin Chem Biol. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Klumpp C, Kostarelos K, Prato M, Bianco A. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics. Biochim Biophys Acta—Biomembranes. 2006;1758:404–412. doi: 10.1016/j.bbamem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 79.Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotubes as nanomedicines: from toxicology to pharmacology. Adv Drug Deliv Rev. 2006;58:1460–1470. doi: 10.1016/j.addr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 80.Bianco A, Kostarelos K, Prato M. Opportunities and challenges of carbon-based nanomaterials for cancer therapy. Expert Opin Drug Deliv. 2008;5:331–342. doi: 10.1517/17425247.5.3.331. [DOI] [PubMed] [Google Scholar]
- 81.Rohit P, Washburn S, Richard S, Richard EC, Michael RF. Visualization of individual carbon nanotubes with fluorescence microscopy using conventional fluorophores. Appl Phys Lett. 2003;83:1219–1221. [Google Scholar]
- 82.Didenko VV, Moore VC, Baskin DS, Smalley RE. Visualization of individual single-walled carbon nanotubes by fluorescent polymer wrapping. Nano Lett. 2005;5:1563–1567. doi: 10.1021/nl050840h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rajat D, Matteo P. Dynamics of individual single-walled carbon nanotubes in water by real-time visualization. Phys Rev Lett. 2006;96:246104. doi: 10.1103/PhysRevLett.96.246104. [DOI] [PubMed] [Google Scholar]
- 84.Otobe K, Nakao H, Hayashi H, Nihey F, Yudasaka M, Iijima S. Fluorescence visualization of carbon nanotubes by modification with silicon-based polymer. Nano Lett. 2002;2:1157–1160. [Google Scholar]
- 85.Sirdeshmukh RT, Teker K, Panchapakesan B. Proceedings of International Conference on MEMS, NANO and Smart Systems (ICMENS’04) Canada: Alberta; 2004. Functionalization of carbon nanotubes with antibodies for breast cancer detection applications; pp. 48–53. [Google Scholar]
- 86.Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun (Camb) 2004;1:16–17. doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]
- 87.Chaudhary S, Kim JH, Singh KV, Ozkan M. Fluorescence microscopy visualization of single-walled carbon nanotubes using semiconductor nanocrystals. Nano Lett. 2004;4:2415–2419. [Google Scholar]
- 88.Leeuw TK, Reith RM, Simonette RA, Harden ME, Cherukuri P, Tsyboulski DA, Beckingham KM, Weisman RB. Single-walled carbon nanotubes in the intact organism: near-IR imaging and biocompatibility studies in Drosophila. Nano Lett. 2007;7:2650–2654. doi: 10.1021/nl0710452. [DOI] [PubMed] [Google Scholar]
- 89.Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 90.Buzea C, Blandino IIP, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–MR172. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 91.Cui D, Tian F, Coyer SR, Wang J, Pan B, Gao F, He R, Zhang Y. Effects of antisense-Myc-conjugated single-walled carbon nanotubes on HL-60 cells. J Nanosci Nanotechnol. 2007;7:1639–1646. doi: 10.1166/jnn.2007.348. [DOI] [PubMed] [Google Scholar]
- 92.Raja PM, Connolley J, Ganesan GP, Ci L, Ajayan PM, Nalamasu O, Thompson DM. Impact of carbon nanotube exposure, dosage and aggregation on smooth muscle cells. Toxicol Lett. 2007;169:51–63. doi: 10.1016/j.toxlet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Yehia HN, Draper RK, Mikoryak C, Walker EK, Bajaj P, Musselman IH, Daigrepont MC, Dieckmann GR, Pantano P. Single-walled carbon nanotube interactions with HeLa cells. J Nanobiotechnol. 2007;5:8. doi: 10.1186/1477-3155-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Z, Hulderman T, Salmen R, Chapman R, Leonard SS, Young SH, Shvedova A, Luster MI, Simeonova PP. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environ Health Perspect. 2007;115:377–382. doi: 10.1289/ehp.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D, Hubbs AF, Antonini J, Evans DE, Ku BK, Ramsey D, Maynard A, Kagan VE, Castranova V, Baron P. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 96.Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc Natl Acad Sci USA. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheng J, Flahaut E, Cheng SH. Effect of carbon nanotubes on developing zebrafish (Danio rerio) embryos. Environ Toxicol Chem. 2007;26:708–716. doi: 10.1897/06-272r.1. [DOI] [PubMed] [Google Scholar]
- 98.Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett. 2005;155:73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 99.Muller J, Decordier I, Hoet PH, Lombaert N, Thomassen L, Huaux F, Lison D, Kirsch-Volders M. Clastogenic and aneugenic effects of multi-wall carbon nanotubes in epithelial cells. Carcinogenesis. 2008;29:427–433. doi: 10.1093/carcin/bgm243. [DOI] [PubMed] [Google Scholar]
- 100.Fiorito S, Serafino A, Andreola F, Togna A, Togna G. Toxicity and biocompatibility of carbon nanoparticles. J Nanosci Nanotechnol. 2006;6:591–599. doi: 10.1166/jnn.2006.125. [DOI] [PubMed] [Google Scholar]
- 101.Kolosnjaj J, Szwarc H, Moussa F. Toxicity studies of carbon nanotubes. In: Chan WCW, editor. Bio-Applications of Nanoparticles. Springer; 2007. pp. 181–204. [DOI] [PubMed] [Google Scholar]
- 102.Zhu L, Chang DW, Dai L, Hong Y. DNA damage induced by multiwalled carbon nanotubes in mouse embryonic stem cells. Nano Lett. 2007;7:3592–3597. doi: 10.1021/nl071303v. [DOI] [PubMed] [Google Scholar]
- 103.Salvador-Morales C, Flahaut E, Sim E, Sloan J, Green ML, Sim RB. Complement activation and protein adsorption by carbon nanotubes. Mol Immunol. 2006;43:193–201. doi: 10.1016/j.molimm.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 104.Chou CC, Hsiao HY, Hong QS, Chen CH, Peng YW, Chen HW, Yang PC. Single-walled carbon nanotubes can induce pulmonary injury in mouse model. Nano Lett. 2008;8:437–445. doi: 10.1021/nl0723634. [DOI] [PubMed] [Google Scholar]
- 105.Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NWS, Chu P, Liu Z, Sun X, Dai H, Gambhir SS. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- 106.Worle-Knirsch JM, Pulskamp K, Krug HF. Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett. 2006;6:1261–1268. doi: 10.1021/nl060177c. [DOI] [PubMed] [Google Scholar]
- 107.Roberts AP, Mount AS, Seda B, Souther J, Qiao R, Lin S, Ke PC, Rao AM, Klaine SJ. In vivo biomodification of lipid-coated carbon nanotubes by Daphnia magna. Environ Sci Technol. 2007;41:3025–3029. doi: 10.1021/es062572a. [DOI] [PubMed] [Google Scholar]
- 108.Shvedova A, Castranova V, Kisin E, Schwegler-Berry D, Murray A, Gandelsman V, Maynard A, Baron P. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66:1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 109.Fenoglio I, Tomatis M, Lison D, Muller J, Fonseca A, Nagy JB, Fubini B. Reactivity of carbon nanotubes: free radical generation or scavenging activity? Free Radic Biol Med. 2006;40:1227–1233. doi: 10.1016/j.freeradbiomed.2005.11.010. [DOI] [PubMed] [Google Scholar]