Abstract
Functional placental insufficiency results in impaired feto-placental exchange, and subsequently in fetal growth restriction (FGR). We hypothesized that reductions in placental amino acid transporter activities in FGR pregnancies may be accompanied by abnormal expression of placental ammonia-handling enzymes. Term placentas were obtained from growth-restricted (N=11) and normal (N=17) human pregnancies, and examined for glutamate dehydrogenase (GDH), glutamine synthetase (GS) and glutaminase (GA) mRNA and protein expression. Northern and Western blots were normalized on human actin mRNA and protein expression. For GA, the presence of mRNA coding the kidney isoform, and the absence of mRNA coding the liver isoform of the enzyme were demonstrated in the human placenta. In FGR pregnancies, placental expression of GDH mRNA was reduced (P < 0.05) compared to normal pregnancies (1.576 ± 0.144 vs. 2.092 ± 0.177, respectively; mean ± SE), whereas GS and GA mRNA expresiion was not different between the two types of pregnancy. GDH protein expression were also reduced (P < 0.05) in FGR placentas compared to normal placentas (1.055 ± 0.079 vs. 1.322 ± 0.053, respectively; mean ± SE). The GS and GA protein expression was not different in FGR pregnancies. Our data indicate that in cases of FGR, glutamate-to-oxoglutarate transformation in the placenta is limited, yet glutamine synthesis from and decomposition to glutamate seems to be preserved. This may reflect down-regulation of GDH in response to decreased fetal liver output and reduced umbilical artery glutamate concentrations in human FGR pregnancies.
Keywords: ammonia, fetal growth restriction, glutamate dehydrogenase, glutaminase, glutamine synthetase, placenta
INTRODUCTION
The provision of amino acids to the fetus in support of growth and development requires a balance between uteroplacental uptake, metabolism and transport, the degree of which varies considerably between amino acid types [1]. Furthermore, for glutamine and glutamate, there is an interorgan exchange between the placenta and fetal liver [2] that exists throughout most of gestation. Provision of glutamine to the fetus results from the combination of uterine uptake and transfer coupled with placental production [2]. There is considerable fetal hepatic uptake of glutamine, with approximately 50% exiting the fetal liver after conversion to glutamate [3]. Hepatic production is the primary source of fetal glutamate, as well as determining the supply to the placenta [2]. Of the glutamate perfusing the placenta, approximately 90% is extracted by the placenta [4]. Placental glutamate extraction, coupled with placental ammonia production associated with branched-chain amino acid transamination [5], completes the interorgan cyclicity of glutamine/glutamate during pregnancy.
The shuttling of ammonia as the amino group is enzymatically regulated by glutamine synthetase (GS) catalyzing glutamine synthesis from glutamate; glutaminase (GA) catalyzing glutamine decomposition to glutamate; as well as by glutamate dehydrogenase (GDH) catalyzing glutamate decomposition to oxoglutarate. In mammals, two forms of mitochondrial GA are present. The liver type GA is encoded on chromosome 12, and expressed in postnatal liver, brain and pancreas, whereas the kidney type GA was assigned to chromosome 2, and its expression was reported in kidney, brain, pancreas, heart, lung, and placenta [6].
Fetal growth restriction (FGR) complicates approximately 8% of all pregnancies, often resulting from functional placental insufficiency, placing the infant at significant risk for morbidity and mortality [7–9]. Both the fetus and the placenta are involved, with profound systemic changes in placental and fetal metabolism [10]. Fetal concentrations of most amino acids are reduced in FGR pregnancies [11–13], and this reduction has been related to altered placental amino acid transport systems [14]. Evidence of altered amino acid metabolism is provided by reports of elevated neonatal blood ammonia concentrations in small for gestational age infants [15], as well as by increased urinary ammonia output during the early neonatal period [16]. However, before delivery, we have found reduced ammonia concentrations in both arterial and venous umbilical blood [17]. The limited information available [18,19] on the expression of enzymes involved in ammonia metabolism and detoxification in the human placenta does not allow an explanation for these variable observations.
Ammonia is produced by deamination within both the fetus and the placenta. When compared to the mother, the human [20–22], rat [23] and sheep [5,24,25] fetus is exposed to greater ammonia concentrations. In contrast to the sheep [25], in humans umbilical arterial ammonia concentrations are greater than umbilical vein concentrations [22], inferring greater ammonia production by the fetus rather than the placenta. Batshaw and Brusilow [15] reported hyperammonemia in low birthweight and small for gestational age human infants (0–3 days of age). Interestingly, the umbilical arterio-venous ammonia concentration difference was reduced in FGR pregnancies [17]. Virtually all ammonia generated by the fetal liver comes from glutamine deamination into glutamate [3], and almost all fetal glutamine originates from the placenta [2]. Since fetal glutamate/glutamine metabolism may be impacted by placental expression of GDH, GS, and GA, these enzymes may potentially influence ammonia levels in the fetus.
Therefore, it was our objective to examine placental mRNA and protein relative abundance of the three enzymes related to the feto-maternal ammonia interchange: GDH [E.C. 1.4.1.3], GS [E.C. 6.3.1.2], and GA [E.C. 3.5.1.2] in normal and FGR pregnancies at term.
MATERIALS AND METHODS
Study group
This study was approved in advance by the Committee of Ethics, Medical University of Bialystok, Bialystok, Poland. The placentas were collected in the Delivery Ward of the Medical University Hospital, Bialystok, Poland, from 28 term (38 to 41 weeks of gestational age) human singleton pregnancies: 11 FGR pregnancies (the study group), and 17 normal pregnancies (controls) after uncomplicated vaginal delivery of normal neonates. The mothers gave no own or family history of inborn errors of metabolism. At least three biometric and Doppler ultrasound assessments in the second and third trimesters were performed. In the FGR group, the fetus met current criteria of the disorder [26]. Moreover, all normal newborns had birthweights above the 10th centile of the birthweight appropriate for their gestational age, and all FGR newborns had birthweights below the 10th centile of their gestational age [27]. Details for the study groups are presented in Table 1.
Table 1.
Details of study groups.
| FGR | Normal | P value | |
|---|---|---|---|
| Birthweight (g) | 2463.6 ± 124.9 | 3550.0 ± 108.0 | P < 0.001 |
| Gestational age (wk) | 38.6 ± 0.5 | 40.2 ± 0.2 | P < 0.05 |
| Maternal age (yr) | 28.3 ± 1.5 | 30.8 ± 1.3 | NS |
Values are means ± SE. FGR pregnancies (N = 11) versus normal pregnancies as controls (N = 17). NS – not significant.
Tissue collection
Placentas were collected immediately postpartum, placed on ice and perfused with chilled 0.25 M sucrose to remove remnants of blood until a clear perfusate was obtained [28]. Placentas were sampled just below the surface of the chorionic plate. Collected tissues were snap frozen in liquid N2, and stored at −80 °C until RNA and protein isolation. Additional placental tissue was prepared for immunohistochemical studies by fixing 3–5 mm sections in cold 4% buffered paraformaldehyde. After washing in PBS and dehydrating in a series of graded alcohols, sections were embedded in paraffin and stored at −20 °C.
RNA isolation and cDNA generation
Total cellular RNA (tcRNA) was isolated from tissue samples (0.05–0.2 g) according to a modified method described by Chomczynski and Mackey [29], using Tri-Reagent and 1-bromo-3-chloropropane (BCP; Molecular Research Center, Inc., Cincinnati, OH), followed by isopropanol-ethanol purification. For each mRNA of interest, human-specific polymerase chain reaction (PCR) primers were designed to allow generation of appropriate cDNAs for use in Northern hybridization analyses. Human GDH antisense (5′ TGT ATG CCA AGC CAG AGT G 3′) and sense (5′ CAG GTG AGC GGG AGA TGT C 3′) primers were designed to amplify an 855 nucleotide (nt) fragment (NM_005271, nt 780-1634). Antisense (5′ GTT GGA GTG GGA TGA AGA A 3′) and sense (5′ TGG AAG AGT TGC CTG AGT G 3′) primers were used to amplify a 1016 nt (NM_002065, nt 280-1295) human GS cDNA. For the kidney isoform (AF_223943, nt 499-1521) of GA, antisense (5′ CAG CAC ATC ATA CCC ATA ACA 3′) and sense (5′ TAC AGC ACT CAA ATC TAC AGG 3′) primers were used to generate a 1023 nt cDNA, whereas for the liver isoform (AF_223944, nt 1352-2086) the antisense (5′ TTT TTG GTG GTT ATG GAT TAC 3′) and sense (5′ GGC GAG AGT GTG CTG AGT GCT 3′) primers generated a 755 nt cDNA. A cDNA encoding human γ-actin, and its use in Northern hybridizations, has already been described [30]. Total cellular RNA was reverse-transcribed using SuperScript II Reverse Transcriptase (Invitrogen Corp., Carlsbad, CA), after optimization (optimal annealing temperature was 58 °C for all primers), PCR was performed under stringent conditions using Taq DNA Polymerase or Platinium Pfx DNA Polymerase in a Robocycler Gradient 40 thermal cycler (Stratagene, La Jolla, CA). The accumulation of the reaction product was confirmed by electrophoresis through 1.2% agarose gels, and human liver poly (A)+ RNA was used as a positive control to determine the presence of kidney and/or liver isoforms of GA mRNA in human placenta. The resulting cDNA were purified, polished and ligated into pPCR Script Amp SK (+) cloning vector and transformed into the XL10 Gold Kan Ultracompetent Cells using the PCR-Script Amp Cloning kit (Stratagene, La Jolla, CA). Positive colonies were selected using X-gal and IPTG, cultured, and plasmid DNA was isolated using QIAprep Spin Maxi Prep kits (Qiagen, Valencia, CA). Authenticity of the cDNA was verified by nt sequencing (Macromolecular Resources, Colorado State University).
Northern hybridization
The expression of placental mRNA was determined using Northern hybridization, as previously described [30,31]. Twenty-five μg tcRNA was electrophoresed through 1.2% agarose gels (0.66 M formaldehyde). The RNA was transferred to nylon membranes using 20 × SSC buffer (0.3 M NaCl and 30 mM Na3C6H5O7) overnight at room temperature, rinsed and UV cross-linked. Nonspecific binding was blocked by incubating membranes in hybridization buffer containing 20 × SSPE (3 M NaCl, 0.2 M NaH2PO4, and 20 mM ethylenediaminetetraacetic acid, EDTA; pH 7.7), 50% formamide, 10% dextran sulfate, 0.1% sodium dodecyl sulphate (SDS) and 100 μg/mL denaturated herring sperm DNA at 42 °C for 5 h. The hybridization probes for GDH, GS and GA mRNA were prepared using reverse primers and [α-32P] dCTP for PCR. Radiolabelled actin cDNA was prepared using DECAprime labelling kit with exonuclease-free Klenow fragment of DNA polymerase and [α-32P] dCTP. Membranes were hybridized at 42 °C overnight with radiolabelled cDNA (1–5 × 108 cpm/μg cDNA), and washed in 2 × SSC/0.1% SDS at 25 °C for 20 min, then at 42 °C for 20 min until low background activity was registered, and twice for 20 min each at 60 °C. Membranes were exposed to a phosphor image screen for 72 h, scanned with a Storm Analyzer (Molecular Dynamics/Amersham Biosciences, Piscataway, NJ) and then stripped with 0.5% SDS for 30 min before rehybridization with the other radiolabelled cDNA. Resulting band intensities were determined densitometrically through Image Quant (Molecular Dynamics), and normalized on the intensity of the actin signal (ratio of mRNA of interest to actin mRNA), which was determined not to vary between control and FGR placentas.
Western immunoblot analysis
Samples of placental tissue (0.05–0.2 g) were pulverized in liquid N2, and sonicated on ice in 0.48 M Tris (pH 7.4), 10 mM EGTA, 10 mM EDTA, 0.1 mM PMSF, and Sigma protease inhibitor cocktail (P8340). Supernatant was collected by centrifugation, and the protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA). Twenty-five μg aliquots of protein were electrophoresed through 4–12% bis-tris denaturating gels in MOPS-SDS running buffer (Invitrogen Corp., Carlsbad, CA), as previously described [31]. After electrophoresis, proteins were transferred to nitrocellulose membranes, and the membranes blocked with non-fat dry milk TBST buffer (5% NFDM, 10 mM Tris-HCl, 150 mM NaCl, and 0.1% Tween 20, pH 8.0) at 37 °C for 2 h. Membranes were then incubated with rabbit anti-GDH (1:5,000), rabbit anti-GS (1:1,000), rabbit anti-GA (1:2,000) antisera or for normalization with mouse anti-actin (clone C4 mouse IgG1 kappa light chain antibody against chicken gizzard actin 1:10,000) antiserum at 4 °C overnight, then rinsed in TBS (10 mM Tris-HCl pH 8.0, 150 mM NaCl, pH 8.0). Subsequently, membranes were incubated with anti-rabbit IgG (1:80 000) or anti-mouse IgG (1:20 000) horseradish peroxidase-conjugated secondary antibody for 2 h, and rinsed in TBS. Following incubation in Supersignal West Femto Maximum Sensitivity Substrate (Pierce Chemical Co., Rockford, IL), the membranes were exposed to X-ray film. Protein quantification was accomplished using ImageQuant (Molecular Dynamics). The membranes were stripped of antibodies (Restore Western Blot Stripping Buffer, Pierce Chemical Co., Rockford, IL) before being incubated with the other primary antisera in a sequential fashion.
Immunohistochemistry
Immunohistochemical localization of GDH, GS and GA in term placental tissue was completed using an immunoperoxidase procedure (ABC Elite Kit, Vector Laboratories, Inc., Burlingame, CA) as previously described by this laboratory [30]. Briefly, 6 μm sections were deparaffinized, rehydrated in a graded ethanol series, endogenous peroxidase activity was quenched with three 30-minute incubations in 3% H2O2, and nonspecific binding was blocked by incubating the sections in 2% normal goat serum for 45 min. The sections were then incubated with primary antisera (see above) overnight at 4 °C, washed in PBS, and incubated with biotinylated goat antirabbit IgG antiserum and avidin-peroxidase conjugate, and diaminobenzidine tetrahydrochloride was used as the color substrate. The primary antibody was replaced with normal goat serum as the negative control.
Statistical analysis
Statistical differences between FGR and normal placentas were determined by Mann-Whitney test. After normal distributions of data were observed, the differences were re-confirmed using Student’s t-test for unpaired observations. Data are presented as mean ± standard error (SE) of mean as well as percentiles for comparison. P < 0.05 was considered statistically significant.
RESULTS
Placentas were collected from 17 normal and 11 FGR pregnancies following uncomplicated vaginal delivery (Table 1). While average gestational age varied by 1.6 weeks (P < 0.05) at delivery, FGR infants weighed 30.6% less (P < 0.001). These placentas were then processed for mRNA and protein expression analysis, as well as immunohistochemical localization of GDH, GS, and GA.
Reverse transcriptase-PCR was used to determine if the human placenta expressed either the liver or kidney isoform of GA. Amplicons derived from the kidney isoform of GA were identified for the term placenta (Fig. 1), whereas mRNA encoding the liver isoform of GA appears to be absent (Fig. 1). Further analysis of GA mRNA focused on the kidney isoform.
Figure 1.
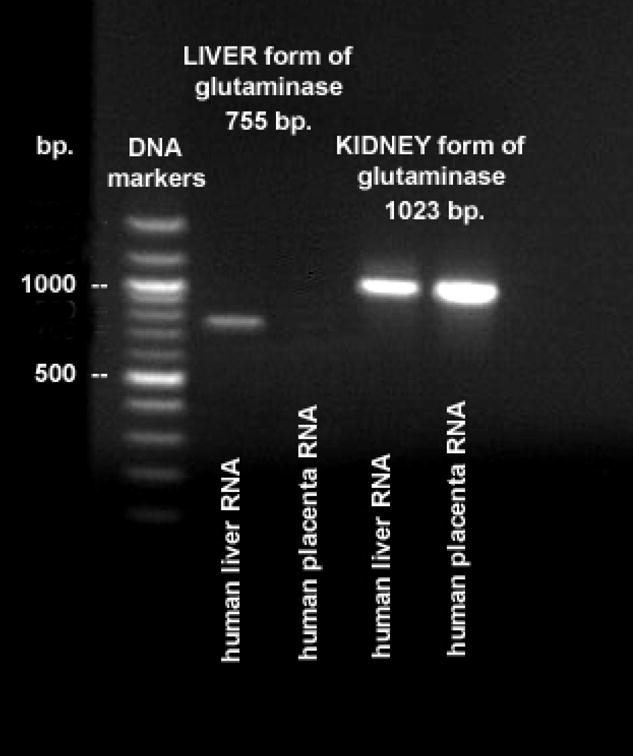
Electrophoretic separation of RT-PCR amplicons using primers specific for the liver (755 bp.) and kidney (1023 bp.) isoforms of glutaminase (GA) using normal term human placenta tcRNA as the template. Human liver poly (A)+ RNA was used as a positive control. Note the absence of amplification of a liver GA isoform from normal term human placenta tcRNA.
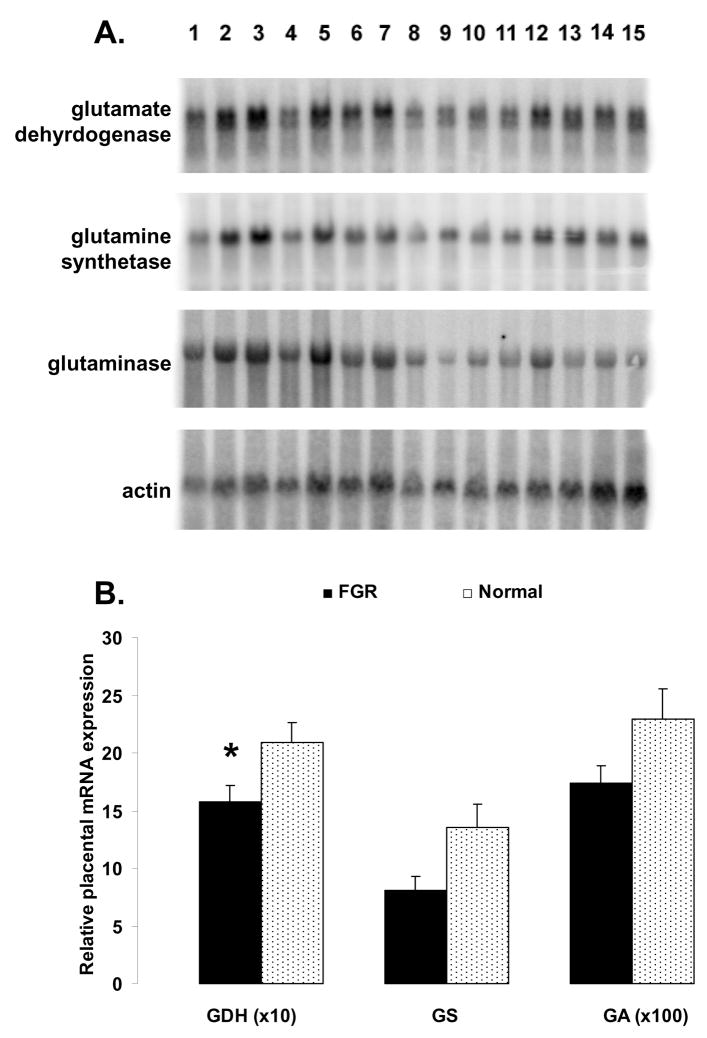
In FGR pregnancies (Fig. 2), placental expression of GDH mRNA was reduced (P < 0.05) compared to normal placenta expression (1.58 ± 0.14 vs. 2.09 ± 0.18, respectively). Placental GS and GA mRNA expression was also less in FGR pregnancies (Fig. 2), although the differences were not sufficient to meet statistical significance (GS: 8.04 ± 1.30 vs 13.50 ± 2.15, P < 0.06; GA: 0.17 ± 0.015 vs 0.23 ± 0.026, P = 0.12; compare Table 2).
Figure 2.
Panel A: Northern blots of glutamate dehydrogenase (GDH), glutamine synthetase (GS), glutaminase (GA), and human γ-actin in placental tissue from patients 1 to 15 (randomly interlaced numbers: 1–3, 5–11, and 13–15 – Normal; numbers: 4, 12, and 14 – FGR). Panel B: Effect of FGR (N=11) on placental expression of GDH, GS and GA mRNA, as compared to normal term placenta controls (N=17). To correspond to graph units, values of GDH mRNA concentrations were multiplied by a factor of 10, and values of GA mRNA concentrations by a factor of 100. Values presented are the means ± SE and * represents P<0.05 for FGR vs. Normal.
Table 2.
RNA and protein abundance in FGR and Normal pregnancy groups. The differences calculated using Mann-Whitney test.
| Pregnancy | Percentiles | P value | |||
|---|---|---|---|---|---|
| 25 | 50 | 75 | |||
| GDH RNA | FGR | 1,210007 | 1,46603 | 1,716775 | P < 0.05 |
| Normal | 1,511009 | 1,946792 | 2,502407 | ||
| GS RNA | FGR | 8,979369 | 9,749276 | 11,53168 | NS |
| Normal | 6,065287 | 8,682345 | 16,85973 | ||
| GA RNA | FGR | 0,131264 | 0,147133 | 0,220199 | NS |
| Normal | 0,151154 | 0,218799 | 0,271684 | ||
| GDH protein | FGR | 0,871564 | 1,023467 | 1,148489 | P < 0.05 |
| Normal | 1,174461 | 1,242331 | 1,335169 | ||
| GS protein | FGR | 0,988432 | 1,119201 | 1,279232 | NS |
| Normal | 1,063893 | 1,204152 | 1,299537 | ||
| FGR | 1,633144 | 1,730401 | 1,832569 | NS | |
| GA protein | Normal | 1,396295 | 1,605021 | 1,884731 | |
FGR pregnancies (N = 11) versus normal pregnancies as controls (N = 17). NS – not significant. See text for details.
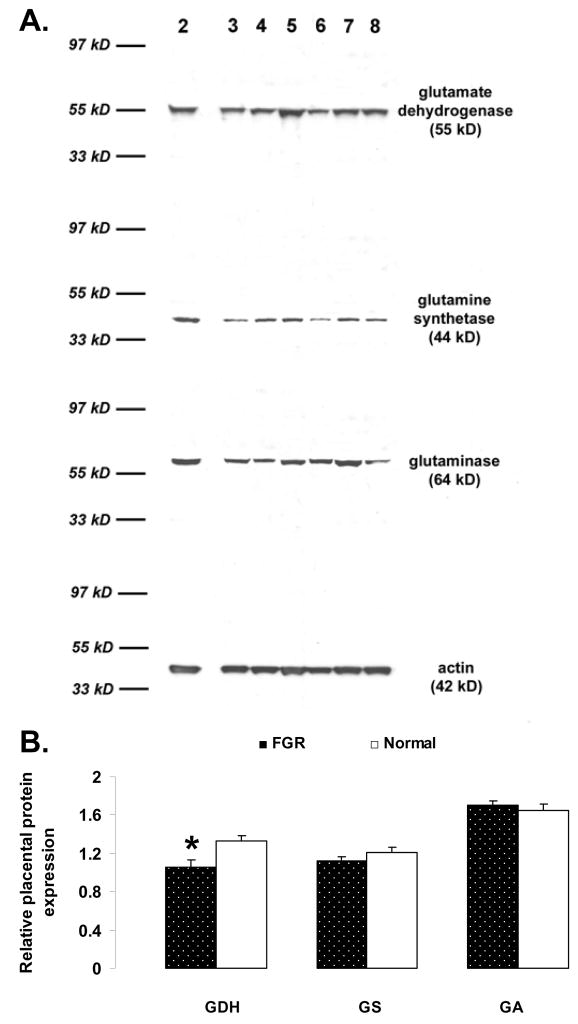
To determine if the changes in placental GDH, GS and GA mRNA expression were reflected in changes in protein expression, placental samples were subjected to immunoblot analysis (Fig. 3). Similar to the mRNA relative abundance, GDH (subunit mol weight 55 kD) relative abundance was reduced (P < 0.05) in FGR placentas compared to normal placentas (1.06 ± 0.08 vs. 1.32 ± 0.05; Fig. 4). By contrast, the GS (subunit mol weight 44 kD) and GA (subunit mol weight 64 kD) protein relative abundance was not reduced in FGR pregnancies (GS: 1.13 ± 0.04 vs 1.20 ± 0.05; GA: 1.71 ± 0.04 vs. 1.64 ± 0.07; FGR vs. normal, respectively; Fig. 3; compare Table 2).
Figure 3.
Panel A: Representative Western immunoblots of glutamate dehydrogenase (GDH), glutamine synthetase (GS), glutaminase (GA), and actin in placental tissue from patients 2 to 8 (numbers: 2, 3, and 5 to 8 – Normal placenta samples; number: 4 – FGR). Panel B: Effect of FGR (N=11) on placental protein expression of GDH, GS and GA, as compared to normal term placenta controls (N=17). Values presented are the means ± SE and * represents P < 0.05 for FGR vs. Normal.
Figure 4.
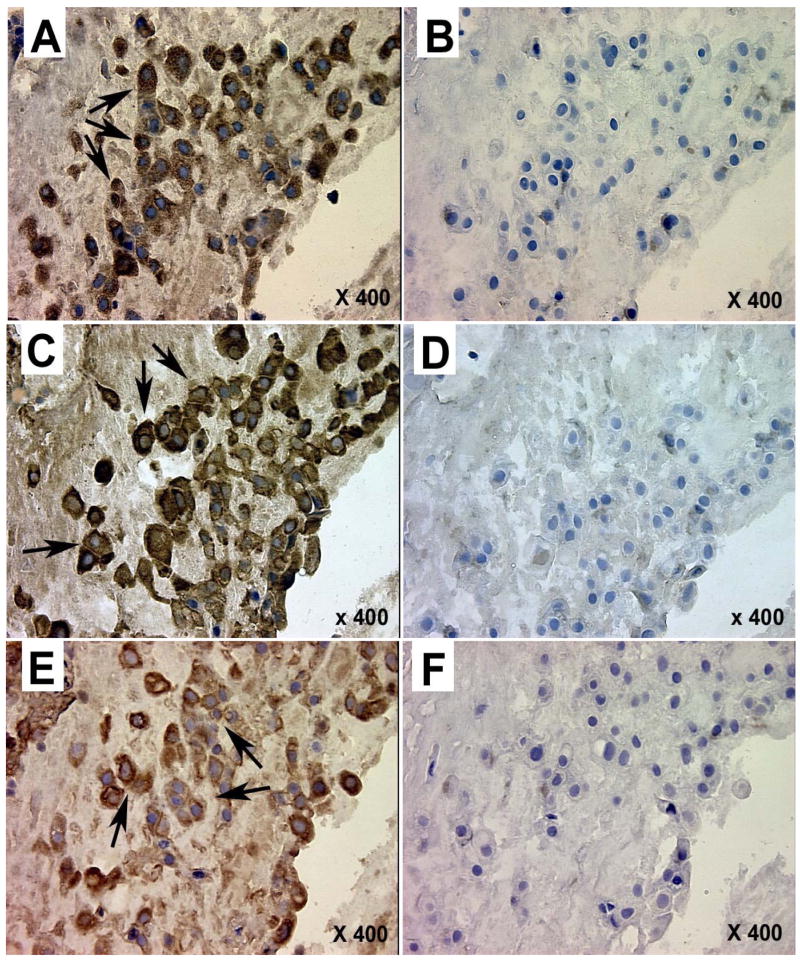
Photomicrographs illustrating tissue localization of glutamate dehydrogenase (A, B), glutamine synthetase (C, D), and glutaminase (E, F) in sequential sections of the same placental fragment from a normal pregnancy. A, C, E – staining with primary antisera, B, D, F – normal rabbit serum controls. Arrows mark positive staining for cytotrophoblast cells (Langhans cells) grouped in columns within stem villi near the chorionic plate.
Immunohistochemical localization of GDH, GS, and GA were performed with both FGR and normal placentas (Fig. 4). Positive staining for GDH, GS, and GA was predominantly identified in large cytotrophoblast cells (Langhans cells), grouped in columns within stem villi originating close to the chorionic plate.
DISCUSSION
Throughout most of gestation, there is an interorgan exchange of glutamine and glutamate between the placenta and fetal liver [2,3]. Little or no glutamate is transported from the placenta to the fetus, whereas there is a large production of glutamate by the fetal liver released into the fetal circulation. Approximately 90% of the glutamate delivered from the fetus is extracted by the placenta, an extraction rate unique to glutamate [4]. In the placenta, only about 6% of fetus-derived glutamate is reconverted to glutamine; most of the glutamate undergoes oxidative deamination with concomitant production of CO2 and ammonia by the action of GDH. It is thought that via this reaction placental GDH regulates NADPH supply for progesterone synthesis in the placenta [32].
In our present study, we examined the expression of GDH, GS and GA in placental tissue harvested at term from normal and FGR pregnancies. While relative abundance of GS and GA mRNA and protein was not different between normal and FGR placenta, GDH mRNA and protein relative abundance was significantly reduced in FGR placenta. The lack of effect on GS and GA expression suggests that both enzymes maintain their function, and placental glutamine synthesis as well as decomposition may be preserved in human FGR placenta. Interestingly, we determined that the GA isoform present in human placenta was the kidney isoform of GA. This finding confirms an earlier report of Aledo et al. [6] who examined human tissue specific expression of the two types of GA in different organs. Furthermore, all three enzymes were predominantly localized by immunohistochemistry to columns of large cytotrophoblast cells within stem villi. GS has been previously localized to villous cytotrophoblast and mesenchyme layers [19], in agreement with our observations for all three enzymes.
The significant reductions in GDH mRNA and protein expression in term FGR placenta may have resulted from a reduction in substrate concentration. Fetal amino acid concentrations are often reported to be diminished in FGR pregnancies [11,12], and both in vitro and in vivo derived evidence demonstrates reduced capacity for amino acid transport in FGR pregnancies [14,33–35]. Therefore, reductions in fetal arterial glutamate concentrations may inhibit GDH expression in a substrate-dependent manner. Such a regulation of mitochondrial glutamine/glutamate metabolism has been reported for cultured proximal tubule-like cell lines [36].
An alternate explanation for the reduction in placental GDH expression in our FGR placentas is the observed reduction in fetal concentrations of the essential amino acid leucine [11,12]. Leucine stimulates GDH in a concentration-dependent manner in nerve tissue [37], therefore the reported reductions in fetal leucine concentrations may be tied to reductions in GDH expression. However, it should be noted that while the earlier studies [11,12] report reductions in umbilical leucine concentrations, Paolini et al. [35], examining well-defined FGR pregnancies, with significantly lower umbilical venous O2 saturation, O2 content and pH, did not find a difference in umbilical leucine concentrations. Consequently, it appears that fetal amino acid concentrations in FGR pregnancies may well be impacted by the severity of growth restriction. This concept is supported by recent studies in sheep, in which fetal amino acid concentrations were reduced in moderate FGR, as compared to controls, whereas in severe FGR pregnancies amino acid concentrations tended to be higher when compared to controls [38]. The differences in fetal amino acid concentrations between moderate and severe FGR pregnancies may be a reflection of altered fetal metabolic status.
As noted earlier, our previous finding of significant reductions in fetal ammonia concentrations at delivery [17] is in contrast with the earlier report of elevated concentrations of ammonia in low birthweight infants in their first days of life [15]. In many ways these studies are not comparable, for instance due to limited oxygen and substrate provision before birth and improved oxygen and substrate provision after birth. Yet, the contrasting results may again reflect the degree of growth restriction and the impact on fetal amino acid concentrations. In our current study, birthweight was reduced by 30.6%, a more moderate growth restriction when compared to the studies by Batshaw and Brusilow [15] and Paolini et al. [35]. Consequently one might speculate that in our pregnancies fetal amino acid concentrations were reduced, and the reduction in placental GDH possibly coupled with reductions in fetal liver GA might be tied to lower fetal ammonia concentrations. While the present study cannot define the origin or direct cause of abnormal fetal ammonia concentrations in growth restricted pregnancies, it does provide new information on the functioning of the feto-placental glutamine-glutamate shuttle.
Acknowledgments
Authors are thankful to Dr. Norman P. Curthoys from the Department of Biochemistry and Molecular Biology, Colorado State University, for providing rabbit anti-GA Ab, and to Dr. Kurt Benirschke from Department of Pathology, University of California-San Diego Medical Center, San Diego, CA, for his evaluation of immunohistochemical staining.
GRANTS
Fulbright Commission sponsored Dr. Marcin Jozwik’s post-doc research fellowship at Colorado State University during this study. Study was partly supported by State Committee for Scientific Research, Warsaw, Poland, grants # 4 P05E 08616 and # 2 P05E 061 27 and by National Institutes of Health grant HD43089.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Battaglia FC, Regnault TRH. Placental transport and metabolism of amino acids. Placenta. 2001;22:145–161. doi: 10.1053/plac.2000.0612. [DOI] [PubMed] [Google Scholar]
- 2.Battaglia FC. Glutamine and glutamate exchange between the fetal liver and the placenta. J Nutr. 2000;130 (4S Suppl):974S–977S. doi: 10.1093/jn/130.4.974S. [DOI] [PubMed] [Google Scholar]
- 3.Vaughn P, Lobo C, Battaglia FC, Fennessey PV, Wilkening RB, Meschia G. Glutamine-glutamate exchange between placenta and fetal liver. Am J Physiol. 1995;268:E705–E711. doi: 10.1152/ajpendo.1995.268.4.E705. [DOI] [PubMed] [Google Scholar]
- 4.Moores RR, Jr, Vaughn PR, Battaglia FC, Fennessey PV, Wilkening RB, Meschia G. Glutamate metabolism in the fetus and placenta of late gestation sheep. Am J Physiol. 1994;267:R89–R96. doi: 10.1152/ajpregu.1994.267.1.R89. [DOI] [PubMed] [Google Scholar]
- 5.Jozwik M, Teng C, Battaglia FC, Meschia G. Fetal supply of amino acids and amino nitrogen after maternal infusion of amino acids in pregnant sheep. Am J Obstet Gynecol. 1999;180:447–453. doi: 10.1016/s0002-9378(99)70230-9. [DOI] [PubMed] [Google Scholar]
- 6.Aledo JC, Gómez-Fabre PM, Olalla L, Márquez J. Identification of two human glutaminase loci and tissue-specific expression of the two related genes. Mamm Genome. 2000;11:1107–1110. doi: 10.1007/s003350010190. [DOI] [PubMed] [Google Scholar]
- 7.Brar HS, Rutherford SE. Classification of intrauterine growth retardation. Semin Perinatol. 1988;12:2–10. [PubMed] [Google Scholar]
- 8.Gray PH, O’Callaghan MJ, Harvey JM, Burke CJ, Payton DJ. Placental pathology and neurodevelopment of the infant with growth restriction. Dev Med Child Neurol. 1999;41:16–20. doi: 10.1017/s0012162299000043. [DOI] [PubMed] [Google Scholar]
- 9.Pollack RN, Divon MY. Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol. 1992;35:99–107. doi: 10.1097/00003081-199203000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Cetin I, Radaelli T, Taricco E, Giovannini N, Alvino G, Pardi G. The endocrine and metabolic profile of the growth-retarded fetus. J Pediatr Endocrinol Metab. 2001;14 (Suppl 6):1497–1505. [PubMed] [Google Scholar]
- 11.Cetin I, Marconi AM, Bozzetti P, Sereni LP, Corbetta C, Pardi G, Battaglia FC. Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am J Obstet Gynecol. 1988;158:120–126. doi: 10.1016/0002-9378(88)90792-2. [DOI] [PubMed] [Google Scholar]
- 12.Cetin I, Ronzoni S, Marconi AM, Perugino G, Corbetta C, Battaglia FC, Pardi G. Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am J Obstet Gynecol. 1996;174:1575–1583. doi: 10.1016/s0002-9378(96)70609-9. [DOI] [PubMed] [Google Scholar]
- 13.Economides DL, Nicolaides KH, Gahl WA, Bernardini I, Evans MI. Plasma amino acids in appropriate- and small-for-gestational-age fetuses. Am J Obstet Gynecol. 1989;161:1219–1227. doi: 10.1016/0002-9378(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 14.Regnault TRH, Friedman JE, Wilkening RB, Anthony RV, Hay WW., Jr Fetoplacental transport and utilization of amino acids in IUGR – a review. Placenta. 2005;26:S52–S62. doi: 10.1016/j.placenta.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Batshaw ML, Brusilow SW. Asymptomatic hyperammonemia in low birthweight infants. Pediatr Res. 1978;12:221–224. doi: 10.1203/00006450-197803000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe HM, Sokol RJ, Dombrowski MP, Bottoms SF, Norman GS. Increased neonatal urinary ammonia: a marker for in utero caloric deprivation? Am J Perinatol. 1989;6:4–7. doi: 10.1055/s-2007-999533. [DOI] [PubMed] [Google Scholar]
- 17.Pietrzycki B, Jozwik M, Jozwik M, Chojnowski M, Jozwik M. Ammonia concentration in human arterial and venous umbilical blood in cases of intrauterine growth restriction (IUGR) (Abstract P9) Fetal Diagn Ther. 2003;18 (Suppl 1):S42. [Google Scholar]
- 18.Makarewicz W, Swierczynski J. Phosphate-dependent glutaminase in the human term placental mitochondria. Biochem Med Metab Biol. 1988;39:273–278. doi: 10.1016/0885-4505(88)90085-0. [DOI] [PubMed] [Google Scholar]
- 19.DeMarco V, McCain MD, Strauss D, Chakrabarti R, Neu J. Characterization of glutamine synthetase transcript, protein, and enzyme activity in the human placenta. Placenta. 1997;18:541–545. doi: 10.1016/0143-4004(77)90008-x. [DOI] [PubMed] [Google Scholar]
- 20.Colombo JP, Peheim E, Kretschmer R, Dauwalder H, Sidiropoulos D. Plasma ammonia concentrations in newborns and children. Clin Chim Acta. 1984;138:283–291. doi: 10.1016/0009-8981(84)90135-9. [DOI] [PubMed] [Google Scholar]
- 21.DeSanto J, Nagomi W, Lietchy EA, Lemons JA. Blood ammonia concentration in cord during pregnancy. Early Hum Dev. 1993;33:1–8. doi: 10.1016/0378-3782(93)90168-t. [DOI] [PubMed] [Google Scholar]
- 22.Jozwik M, Jozwik M, Pietrzycki B, Chojnowski M, Teng C, Jozwik M, Battaglia FC. Maternal and fetal blood ammonia concentrations in normal term human pregnancies. Biol Neonate. 2005;87:38–43. doi: 10.1159/000081702. [DOI] [PubMed] [Google Scholar]
- 23.Garcia MV, Martin-Barrientos J, Medina JM. Maternal-fetal relationship in ammonia metabolism during late gestation in the rat. Biol Neonate. 1988;53:315–320. doi: 10.1159/000242807. [DOI] [PubMed] [Google Scholar]
- 24.Holzman IR, Lemons JA, Meschia G, Battaglia FC. Ammonia production by the pregnant uterus. Proc Soc Exp Biol Med. 1977;156:27–30. doi: 10.3181/00379727-156-39868. [DOI] [PubMed] [Google Scholar]
- 25.Jozwik M, Teng C, Meschia G, Battaglia FC. Contribution of branched-chain amino acids to uteroplacental ammonia production in sheep. Biol Reprod. 1999;61:792–796. doi: 10.1095/biolreprod61.3.792. [DOI] [PubMed] [Google Scholar]
- 26.Galan HL, Ferrazzi E, Hobbins JC. Intrauterine growth restriction (IUGR): biometric and Doppler assessment. Prenat Diagn. 2002;22:331–337. doi: 10.1002/pd.311. [DOI] [PubMed] [Google Scholar]
- 27.Brzozowska I. Physical development parameters of newborn infants in Poland. Probl Med Wieku Rozwoj. 1973;3:83–98. [PubMed] [Google Scholar]
- 28.Jozwik M, Glowacka D, Popowicz J. Hexosamine synthesis in human placenta in vitro. Am J Obstet Gynecol. 1967;99:258–261. doi: 10.1016/0002-9378(67)90329-8. [DOI] [PubMed] [Google Scholar]
- 29.Chomczynski P, Mackey K. Substitution of chloroform by bromo-chloropropane in the single-step method of RNA isolation. Anal Biochem. 1995;225:163–164. doi: 10.1006/abio.1995.1126. [DOI] [PubMed] [Google Scholar]
- 30.Kappes SM, Warren WC, Pratt SL, Liang R, Anthony RV. Quantification and cellular localization of ovine placental lactogen messenger ribonucleic acid expression during mid- and late gestation. Endocrinology. 1992;131:2829–2838. doi: 10.1210/endo.131.6.1446621. [DOI] [PubMed] [Google Scholar]
- 31.Regnault TRH, Orbus RJ, de Vrijer B, Davidsen M, Limesand SW, Galan HL, Wilkening RB, Anthony RV. Placental expression of VEGF, PlGF and their receptors in a model of placental insufficiency-intrauterine growth restriction (PI-IUGR) Placenta. 2002;23:132–144. doi: 10.1053/plac.2001.0757. [DOI] [PubMed] [Google Scholar]
- 32.Klimek J, Makarewicz W, Swierczynski J, Bossy-Bukato G, Zalewski L. Mitochondrial glutamine and glutamate metabolism in human placenta and its possible link with progesterone biosynthesis. Trophoblast Res. 1993;7:77–86. [Google Scholar]
- 33.Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Norberg S, Powell TL, Jansson T. Intrauterine growth restriction is associated with a reduced activity of placental taurine transporters. Pediatr Res. 1998;44:233–238. doi: 10.1203/00006450-199808000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Paolini CL, Marconi AM, Ronzoni S, Di Noio M, Fennessey PV, Pardi G, Battaglia FC. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metabol. 2001;86:5427–5432. doi: 10.1210/jcem.86.11.8036. [DOI] [PubMed] [Google Scholar]
- 36.Welbourne T, Nissim I. Regulation of mitochondrial glutamine/glutamate metabolism by glutamate transport: studies with 15N. Am J Physiol Cell Physiol. 2001;280:C1151–C1159. doi: 10.1152/ajpcell.2001.280.5.C1151. [DOI] [PubMed] [Google Scholar]
- 37.Plaitakis A, Zaganas I. Regulation of human glutamate dehydrogenases: Implications for glutamate, ammonia and energy metabolism in brain. J Neurosci Res. 2001;66:899–908. doi: 10.1002/jnr.10054. [DOI] [PubMed] [Google Scholar]
- 38.de Vrijer B, Regnault TRH, Wilkening RB, Meschia G, Battaglia FC. Placental uptake and transport of ACP, a neutral nonmetabolizable amino acid, in an ovine model of fetal growth restriction. Am J Physiol Endocrinol Metab. 2004;287:E1114–E1124. doi: 10.1152/ajpendo.00259.2004. [DOI] [PubMed] [Google Scholar]